1. Introduction
Recently genome analysis has revealed a series of genes, enzymes, and receptor proteins responsible for various diseases, thereby providing further understanding of life phenomena. Biopharmaceuticals such as antibody drugs, nucleic acid drugs, and gene therapy drugs will undoubtedly play a pivotal role in future pharmaceuticals. Nevertheless, natural organic compounds are still in high demand as lead compounds for drug discovery and as tools for biological research [1-2]. However, natural organic compounds that show promising activity often require structural modification due to insufficient supply from nature, lack of chemical stability, or toxicity. In such cases, chemical synthetic supply is essential. On the other hand, in recent years, medium-sized molecules with molecular weights ranging from 500 to 2000 have been attracting attention as drug discovery leads because they can potentially address the gap between small molecules and macromolecules. In addition, they have broad adaptability and high specificity for in vivo targets such as protein-protein interactions. Synthesis of such molecular-sized natural products requires convergent synthetic strategies that divide them into segments, which are later combined. Therefore, the development of simple method for the synthesis of segments will eventually lead to a shortening the synthetic process. In this regard, establishment of synthetic methods for functionalizable segments is important. Although a number of precise synthetic reactions have already been developed for the accurate and efficient synthesis of organic molecules with complex structures, further development of various methodologies is required. We have focused on bicyclic 𝛾-lactones as one such segment, and have investigated their usefulness as well as simple synthetic methods. In this review, the Lewis acid template Diels-Alder reaction is discussed as a method for the construction of bicyclic 𝛾-lactones.
2. Template Effects in the Diels-Alder Reaction
Although it has been more than 90 years since the first Diels-Alder reaction was reported, Diels-Alder reaction is still regarded as one of the most important reactions in precise organic synthesis [
3]. Many rules for the stereoselectivity were discovered and theories on the mechanism were developed, thus the Diels-Alder reaction has progressed along with the development of organic chemistry. This is not only interesting in terms of principle and theory, but also attractive in terms of practicality and versatility. The products can be predicted based on predictions supported by theory, and reactions can actually proceed with a high degree of reliability. In synthetic studies of natural products with complicated structures, we often encounter extremely slow reaction rates or significantly low yields in organic reactions of substrates that have many functional groups. In the Diels-Alder reaction, however, the reaction proceeds as long as the diene or the dienophile is multi-functionalized or medium-sized molecules, if the diene and dienophile are in close proximity and the spatial environment is favorable. Therefore, the Diels-Alder reaction is easily incorporated into the synthetic plan from the viewpoint of reaction reliability.
As for the stereoselectivity of the intermolecular Diels-Alder reaction, regioselectivity, diastereoselectivity and enantioselectivity as well as endo-exo selectivity can be problematic, whereas the intramolecular Diels-Alder reaction is expected to solve the regio- and diastereoselectivity problems[4-7]. For these reasons, attempts have been made to expand the intermolecular Diels-Alder reaction to an intramolecular Diels-Alder reaction by temporarily tethering diene and dienophile using various chemical species, taking advantage of the advantages of the intramolecular Diels-Alder reaction. Chemical species used as tethers include boron, silicon, magnesium, and aluminum etc. In many cases, heating is required due to the presence of intramolecular strain energy. Therefore, chemical species tolerating heat with high oxophilicity are preferable. On the other hand, the diene and dienophile-tethered Diels-Alder reaction is not suitable for catalytic system. Here several tethered chemical species are mentioned in turn.
2.1. Silicon-tethered Diels-Alder Reaction
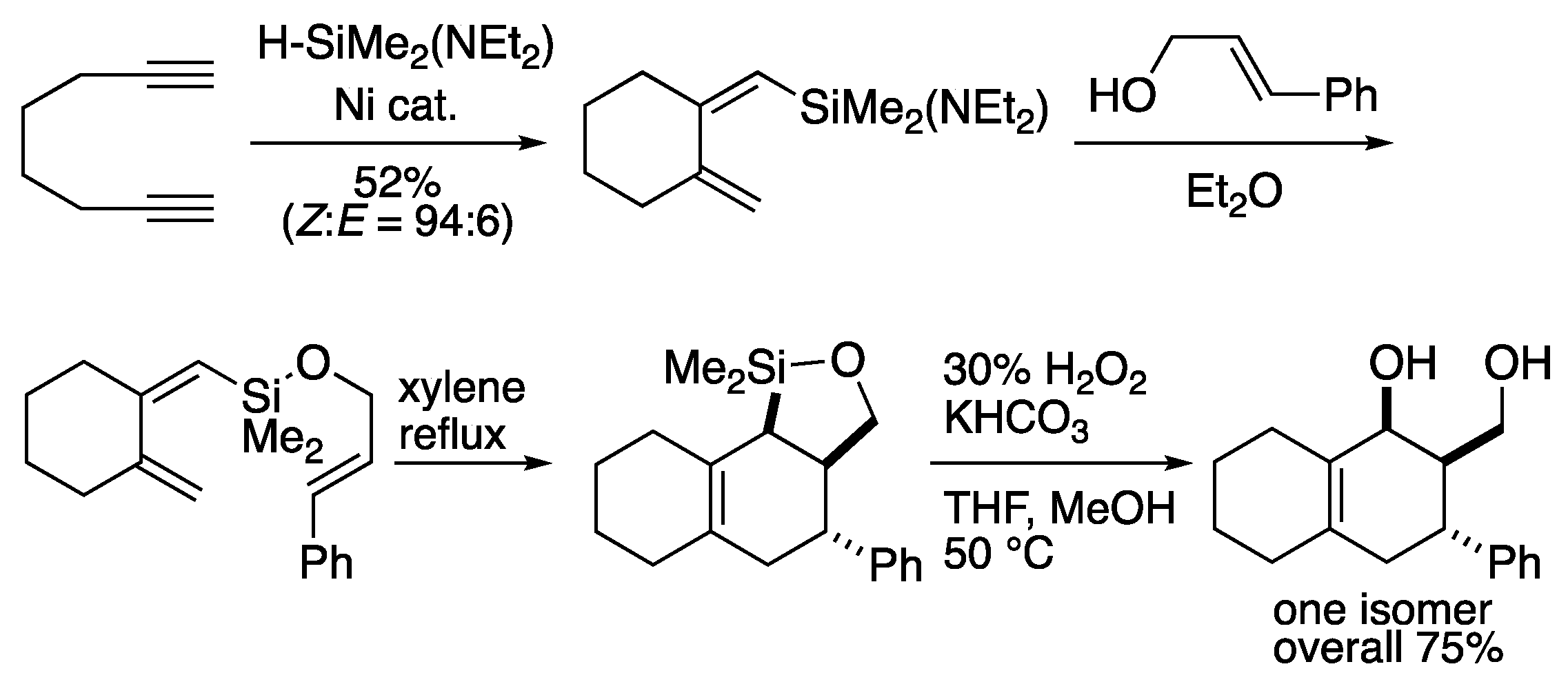
Silicon has often been used as a chemical species for temporal tethers since Tamao and Ito
et al. reported the pioneering Diels-Alder reaction using silicon as a tether in 1989 (
Scheme 1) [
8]. They prepared dienylsilane using a nickel catalyst, followed by coupling with allylic alcohol to afford silicon-tethered compound. Thermal Diels-Alder reaction of silicon-tethered compound proceeded in a highly stereoselective manner to give bicyclic compounds in an overall yield of 75% with high efficiency
via Tamao oxidation.
Stork and co-workers reported the thermal Diels-Alder reaction of silicon-tethered substrates using various alkenylsilanes [9, 10]. They comprehensively investigated the reaction of geometric isomers with polysubstituted alkenylsilanes and found that various cyclohexene derivatives were obtained (
Scheme 2). The products could be further derivatized by TBAF treatment or Tamao oxidation. In this case, the regioselectivity of the functional group on the cyclohexene was completely controlled, indicating the usefulness of the template Diels-Alder reaction.
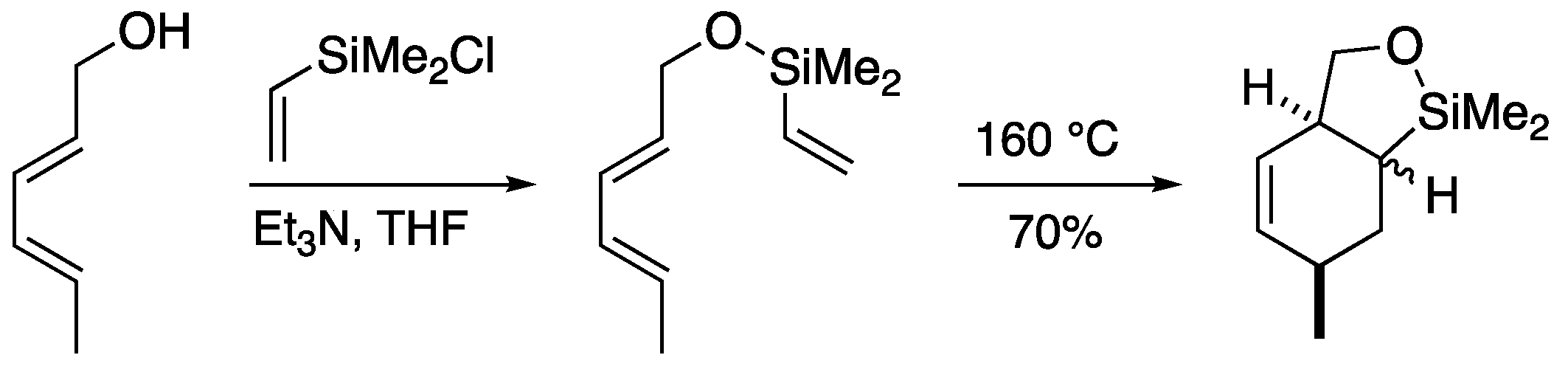
On the other hand, Shea
et al. reported the Diels-Alder reaction of dienylsilyl ether intermediate under thermal condition, which afforded
exo-adduct in 98% yield as a single product (
Scheme 3) [11, 12]. The reaction of dialkoxysilanes was also studied by Craig [
13] , Fortin [
14], and others [
15], and a review article is available for reference [
16].
2.2. Magnesium-tethered and aluminum-tethered Diels-Alder Reaction
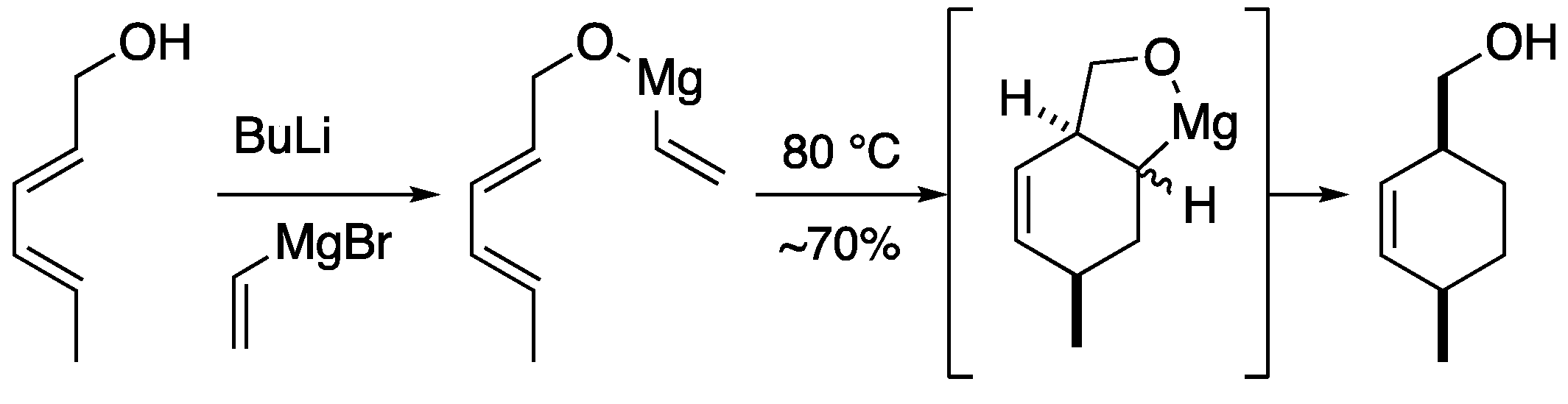
Magnesium and aluminum can also be temporary tethers (
Scheme 4). [
17] Stork
et al. reported a magnesium-tethered Diels-Alder reaction in which the substrate was prepared from vinylmagnesium bromide and allylic alcohol using butyllithium. The magnesium-tethered intermediate underwent a thermal Diels-Alder reaction to afford the cyclohexene derivative in good yield. It is interesting to note that the thermal Diels-Alder reaction proceeded at lower temperatures than the aforementioned silicon-tethered molecules, although the dienophile is considered the formal vinyl anion in this case. They also found that the Diels-Alder reaction proceeds similarly with aluminum as the tether.
2.3. Boron-tethered Diels-Alder Reaction
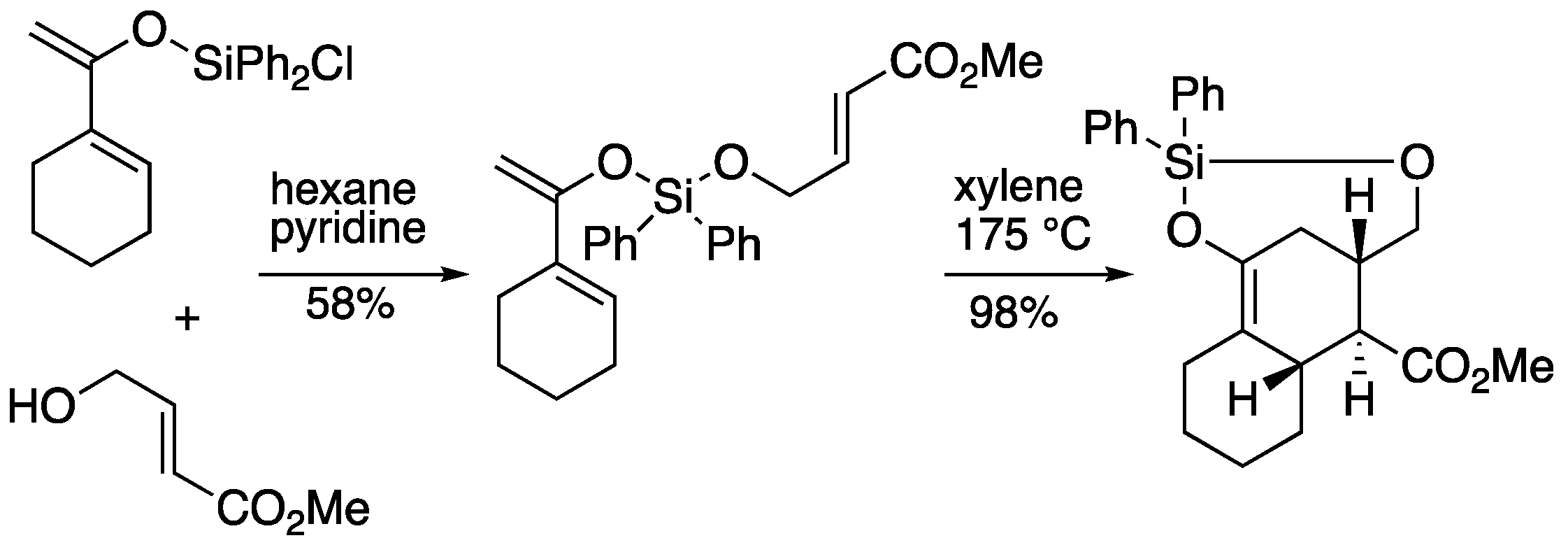
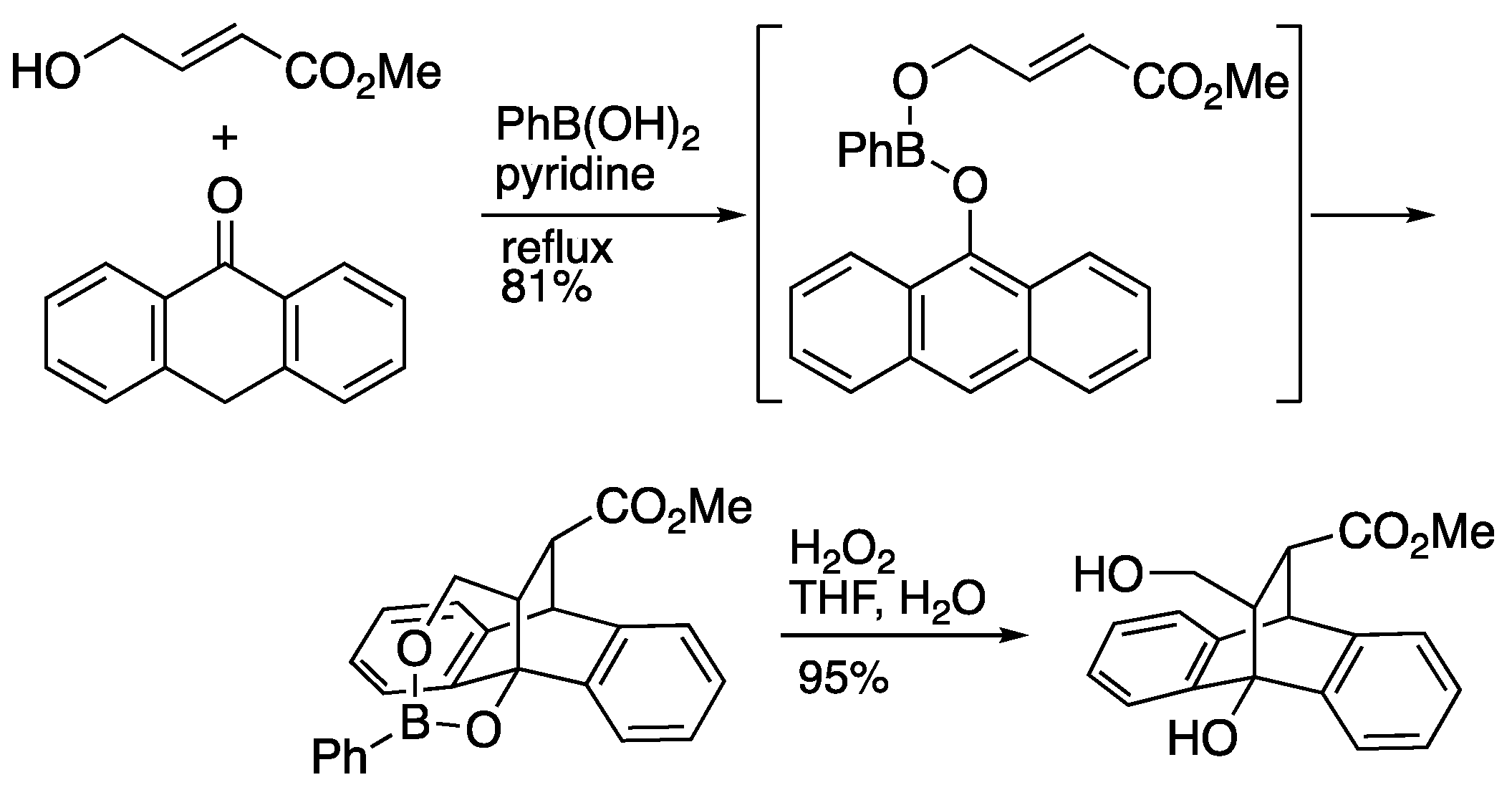
Boronic acids are known as temporary tethers. Narasaka and Iwasawa
et al. reported the Diels-Alder reaction of anthrone with 4-hydroxy-2-butenoate using phenylboronic acid as a template (
Scheme 5) [
18]. In this reaction, an enol-type boronic ester was prepared by dehydration, yielding Diels-Alder adduct in 81% yield. In contrast, when anthrone was subjected to base-catalyzed Diels-Alder reaction of acrylates without phenylboronic acid, the opposite regioisomer was obtained, indicating that the phenylboronic acid template completely controls the regioselectivity.
3. Synthesis of bicyclic 𝛾-lactone by intramolecular Diels-Alder reaction
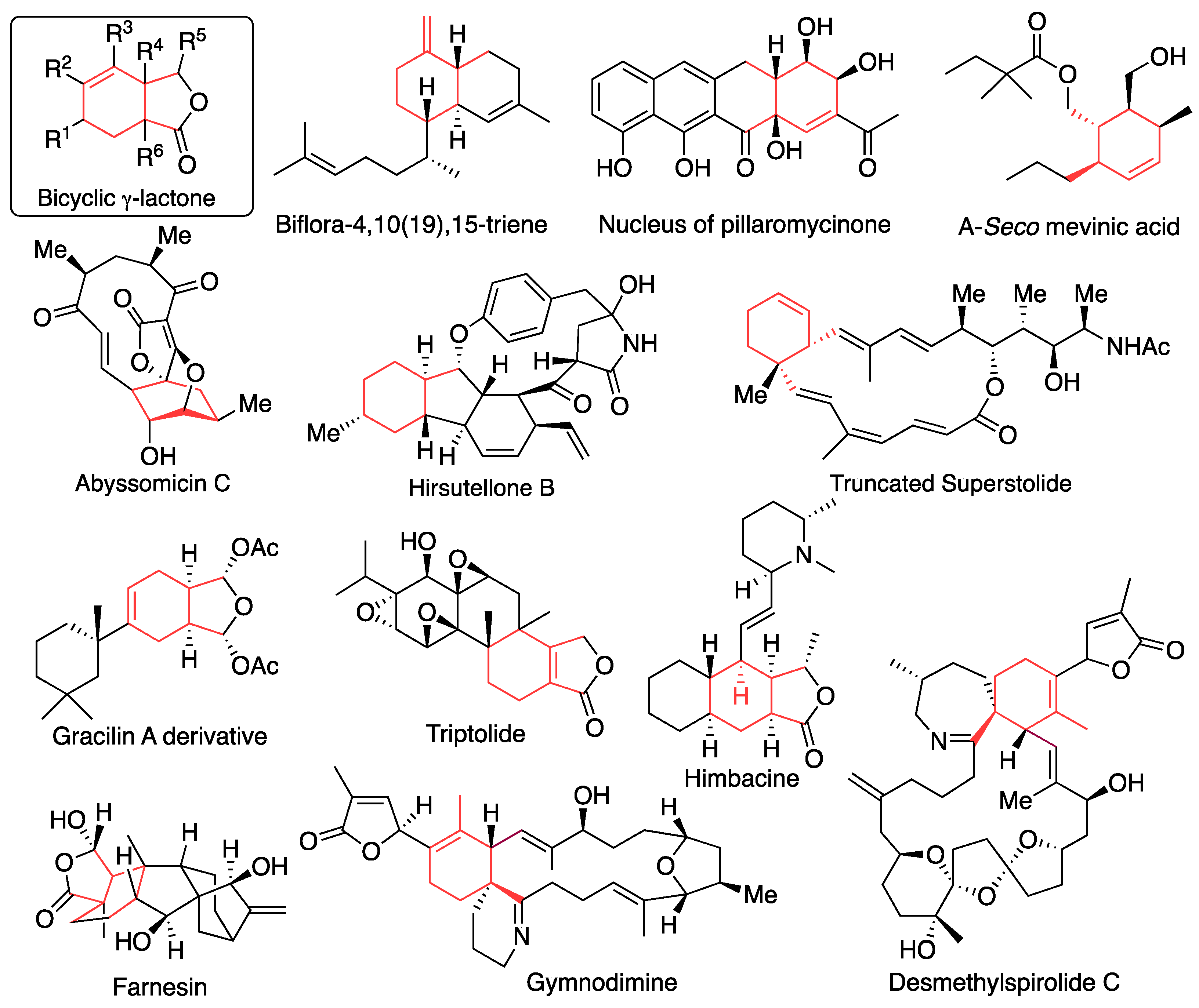
Substituted bicyclic 𝛾-lactone with intramolecular unsaturated bonds has a high potential for the key intermediate of natural product synthesis due to its moderate functional groups. Representatives of the synthesis of natural products and natural product-related substances, in which bicyclic 𝛾-lactone was used as a key intermediate, are as follows (
Figure 1); total synthesis of biflora-4,10(19),15-trien by Grieco [
19], synthesis of nucleus of pillaromycinone by White [20, 21], formal synthesis of A-seco mevinic acid by Potier [
22], total synthesis of abyssomicin C by Nicolaou [23-25], synthesis of core of abyssomicin C by Maier [
26], synthesis of core of hirsutellones by Roush [
27], synthesis of truncated superstolide by Jin [
28], synthesis of simplified derivative of gracilin A by Romo [
29], formal synthesis of triptolide by Sherburn [
30], synthetic approach of himbacine by Sherburn [
31], total synthesis of farnesin by Gao [
32], synthesis of spirocyclic core of gymnodimime and desmethylspirolide by Landais [
33]. We also reported synthetic approach of spirolide upper fragment [
34].
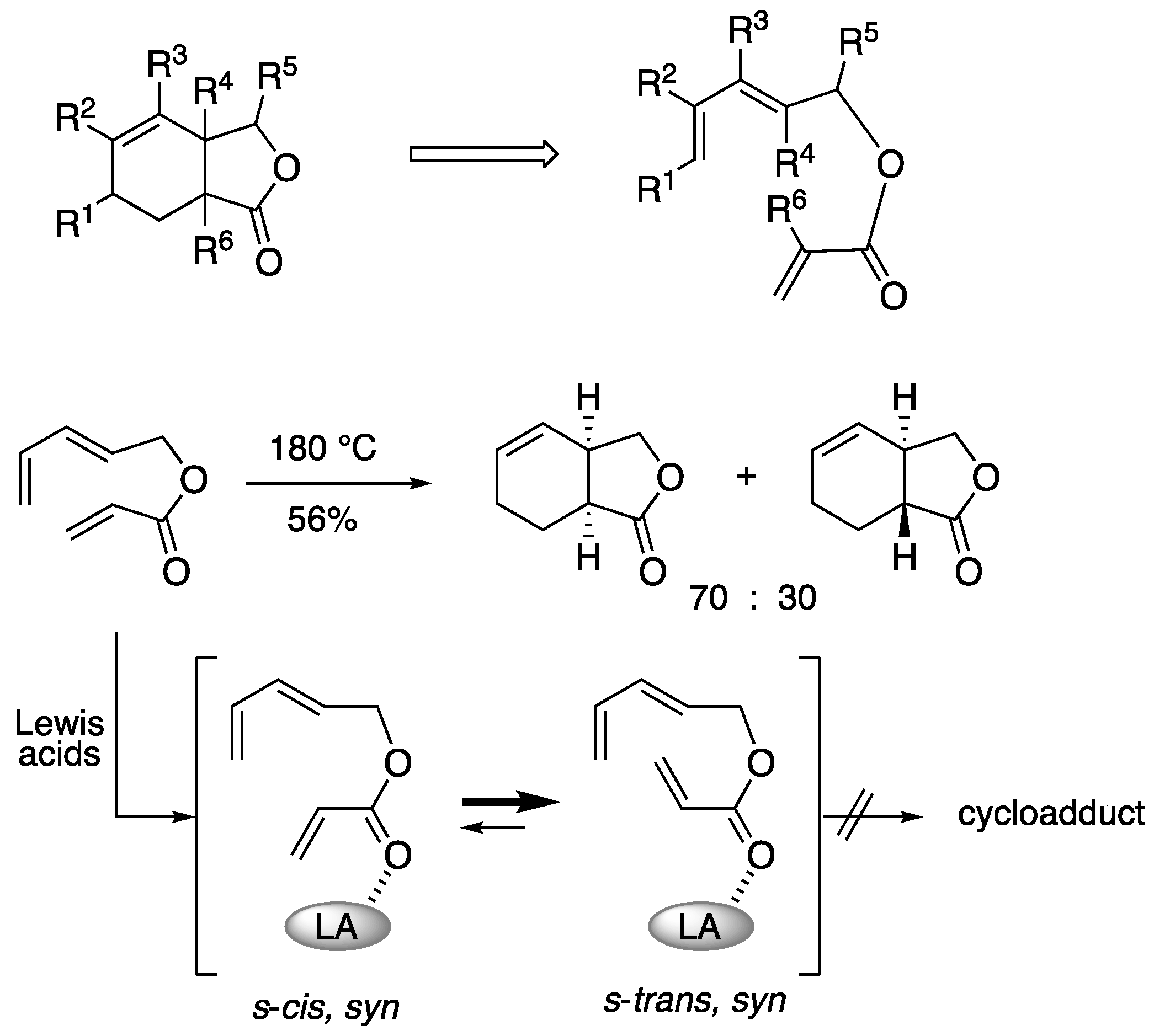
The functionalized bicyclic 𝛾-lactones are thought to be easily obtained by intramolecular Diels-Alder of the corresponding triene esters, but the stereoselective construction is not as straightforward as one might expect. This is because the sp2 carbon of the internal carbonyl carbon causes steric distortion during cyclization of the triene ester. The steric distortion of intramolecular reactions prevails over the secondary orbital interactions, and the general
endo rule cannot be adapted. In fact, this thermal Diels-Alder reaction gives a mixture of
cis- and
trans-fused rings in moderate yields (
Scheme 6) [
35]. On the other hand, it is known that activation of such triene esters with Lewis acid does not efficiently promote the Diels-Alder reaction. It is explained by the fact that when Lewis acid coordinates to the carbonyl group, the
s-trans-syn coordination is preferred over the
s-cis-syn coordination, and this coordination is unfavorable to the progress of the Diels-Alder reaction [
36]. Therefore, the construction of such bicyclic lactones by the catalytic Diels-Alder reaction with Lewis acid requires ingenuity. One solution to this problem is to use Lewis acid templates.
3. Lewis acid template-mediated Diels-Alder reaction.
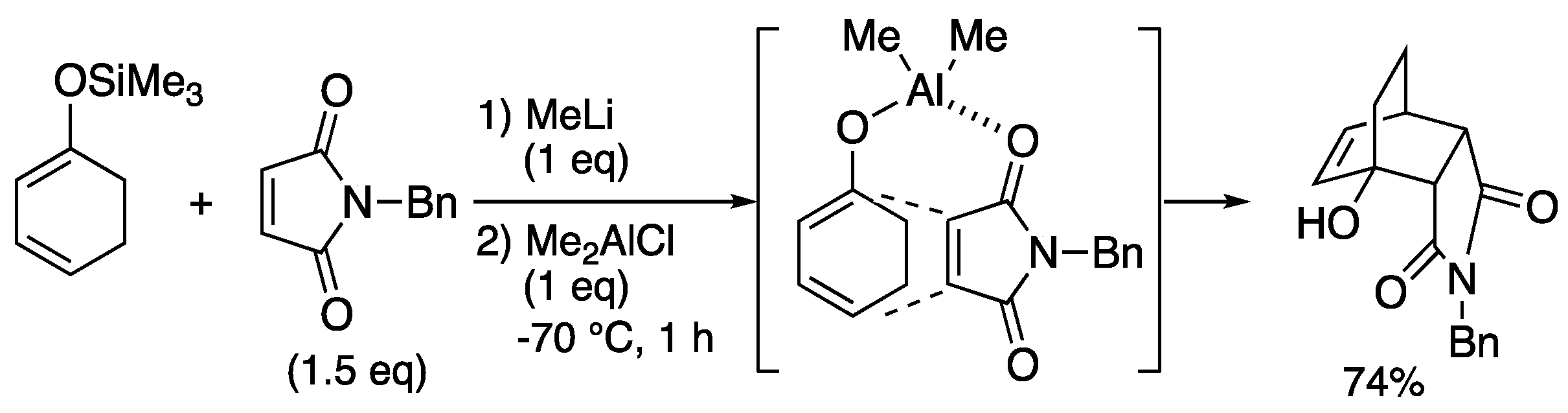
In 1997, Bienayé reported the pioneering works for Diels-Alder reaction using Lewis acid template of succinimide and cyclohexadiene derivatives (
Scheme 7) [37, 38]. The cyclohexadienylsilyl ether was lithiated, followed by the addition of dimethylaluminum chloride to form an alkoxyaluminum complex. Further addition of succinimide activated the dienophile and simultaneously brought the substrates closer together. Thus Diels-Alder reaction proceeded efficiently even at low temperatures to afford the corresponding tricyclic product in 74% yield.
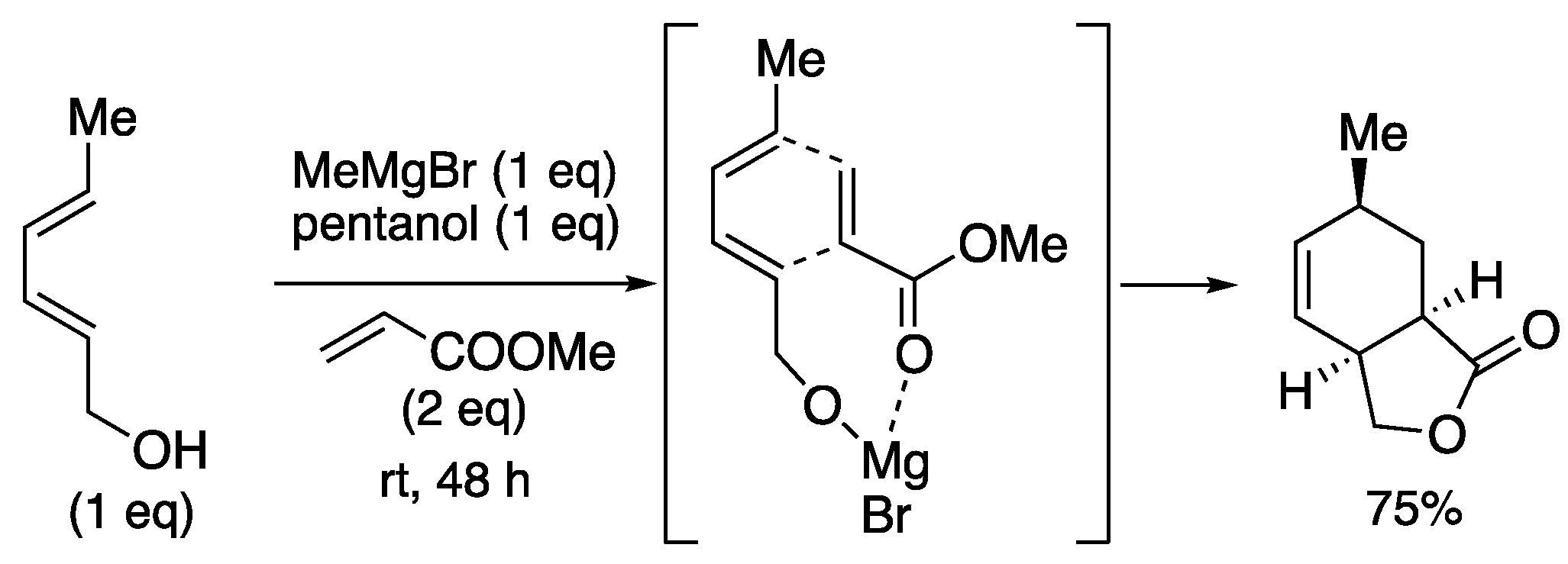
Ward and co-workers demonstrated a reaction using magnesium as a Lewis acid template (
Scheme 8) [
39]. The magnesium salt caused bimolecular proximity and activation of the dienophile, leading to the Diels-Alder reaction. Spontaneous lactonization occurs in the reaction system, and bicyclic lactone was produced in 75% yield.
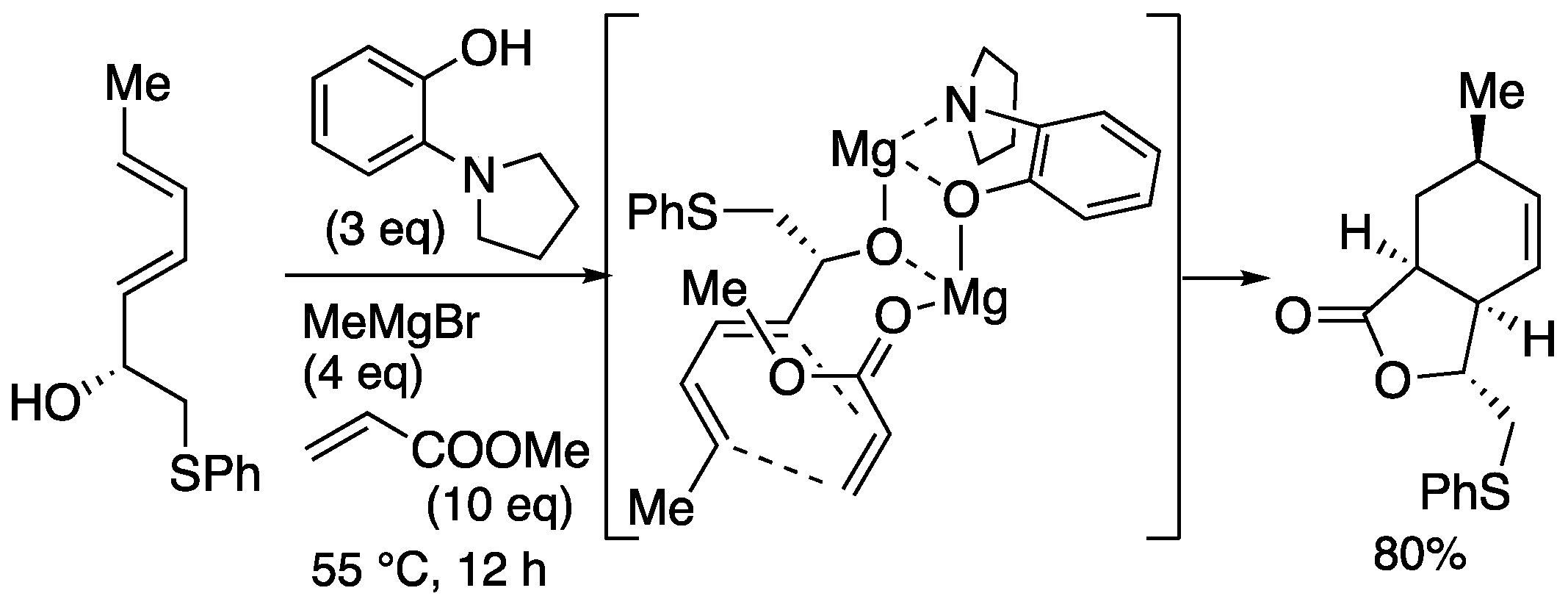
Nicolaou's group modified Ward's method using a template prepared from a 2-aminophenol derivative and Grignard reagent. In this case, bicyclic lactone was stereoselectively constructed by coordination to a secondary hydroxyl group (
Scheme 9) [
24]. Using the bicyclic lactone as a key intermediate, they achieved the total synthesis of the aforementioned abyssomicin
C A similar reaction was also reported by Georgiadis
et al. [
40].
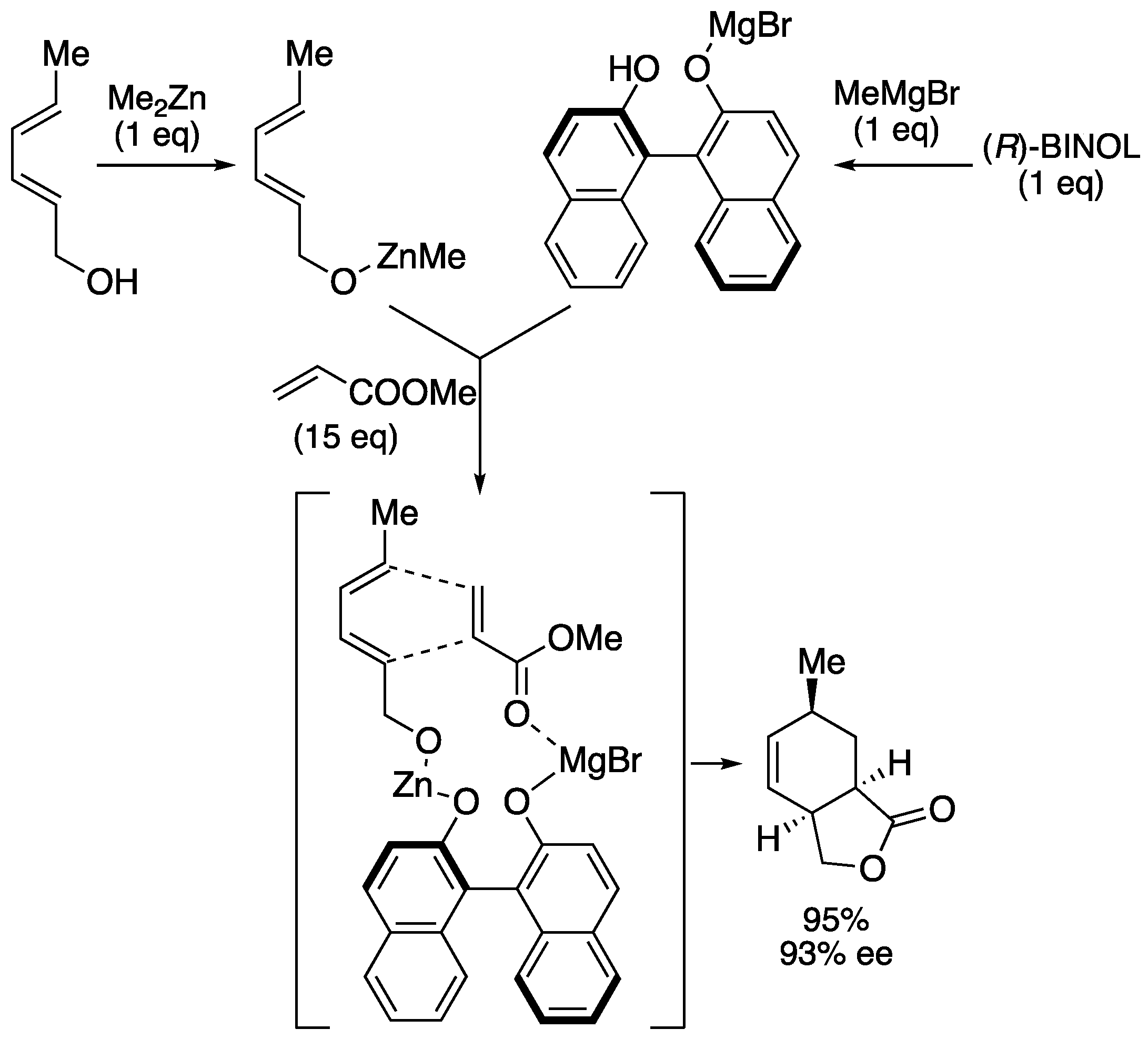
On the other hand, Ward and co-workers reported enantioselective variant of a Lewis acid template Diels-Alder reaction using optically active BINOL (
Scheme 10) [41, 42]. In this particular case, the coordination of the diene and dienophile components to a bimetallic Lewis acid template derived from BINOL and dimethylzinc, and methylmagnesium bromide effectively controlled the Diels-Alder reaction to generate bicyclic lactone with high regioselectivity, stereoselectivity, and enantioselectivity in excellent yield.
Notably all of these Lewis acid template reactions require stoichiometric quantity of reagents to achieve an acceptable conversion and the tethered Diels-Alder reaction with low catalyst loading had remained unexplored.
4. Development of Catalytic Chiral Lewis Acid Template Diels-Alder Reaction
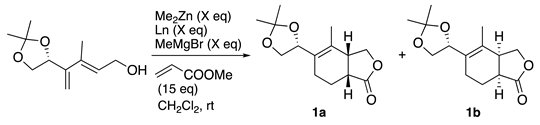
The authors were interested in the reaction reported by Ward in the course of natural product synthesis, and investigated the reaction with chiral dienols [
43]. The reaction using (
R)-BINOL as ligand gave highly stereoselective bicyclic lactones at a diastereomeric ratio of 4.8:1 (
Table 1, entry 1). In the reaction using (S)-BINOL, the selectivity ratio was almost reversed, indicating that the stereochemistry of the chiral center of the substrate does not significantly affect the formation ratio and that the reaction proceeds by reagent control (entry 2). Considering the effect of acidity, we next used 3,3'-dibromo-1,1'-bi-2-naphthol (3,3'-Br
2-BINOL), which gave almost the same result as those of BINOL (entry 3). On the other hand, when 5,5',6,6',7,7',8,8'-octahydro-1,1'-bi-2-naphthol (
H8-BINOL) was used, the selectivity was improved to 10:1 (entry 4). It should be noted that the reaction mediated by 0.5 equiv of bimetallic complex with 5 was completed within 2 h to afford 3a in excellent yield and high diastereoselectivity, which were similar to those observed for the stoichiometric reaction (entry 5). Encouraged by this result, we examined the reaction at lower catalyst loading. Consequently, the use of even 0.2 equivalent of the catalyst was found to effectively promote the high asymmetric induction (entry 6), whereas lowering the catalyst loading of 0.5 to 0.1 equivalent slightly diminished the yield and selectivity (entry 7).
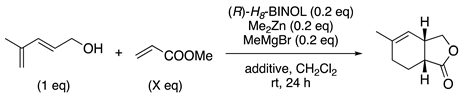
The catalytic reaction of achiral substrates was shown in
Table 2. When 4-methyl-2,4-pentadienol was reacted with methyl acrylate using 0.2 equiv of bimetallic complex, the optical yield was 52% ee, although the yield was good (entry 1). We investigated the solvent, temperature, combination of metals other than magnesium and zinc, and type of acrylic ester, but all efforts were fruitless. However, we found that the addition of molecular sieves 4 Å as an additive dramatically improved the optical yield (entry 2). Furthermore, it was turned out that decreasing the amount of acrylic ester markedly improved the enantioselectivity (entries 3-5). The same or slightly higher yields were obtained when the reaction was performed with molecular sieves at 5 Å (entry 6).
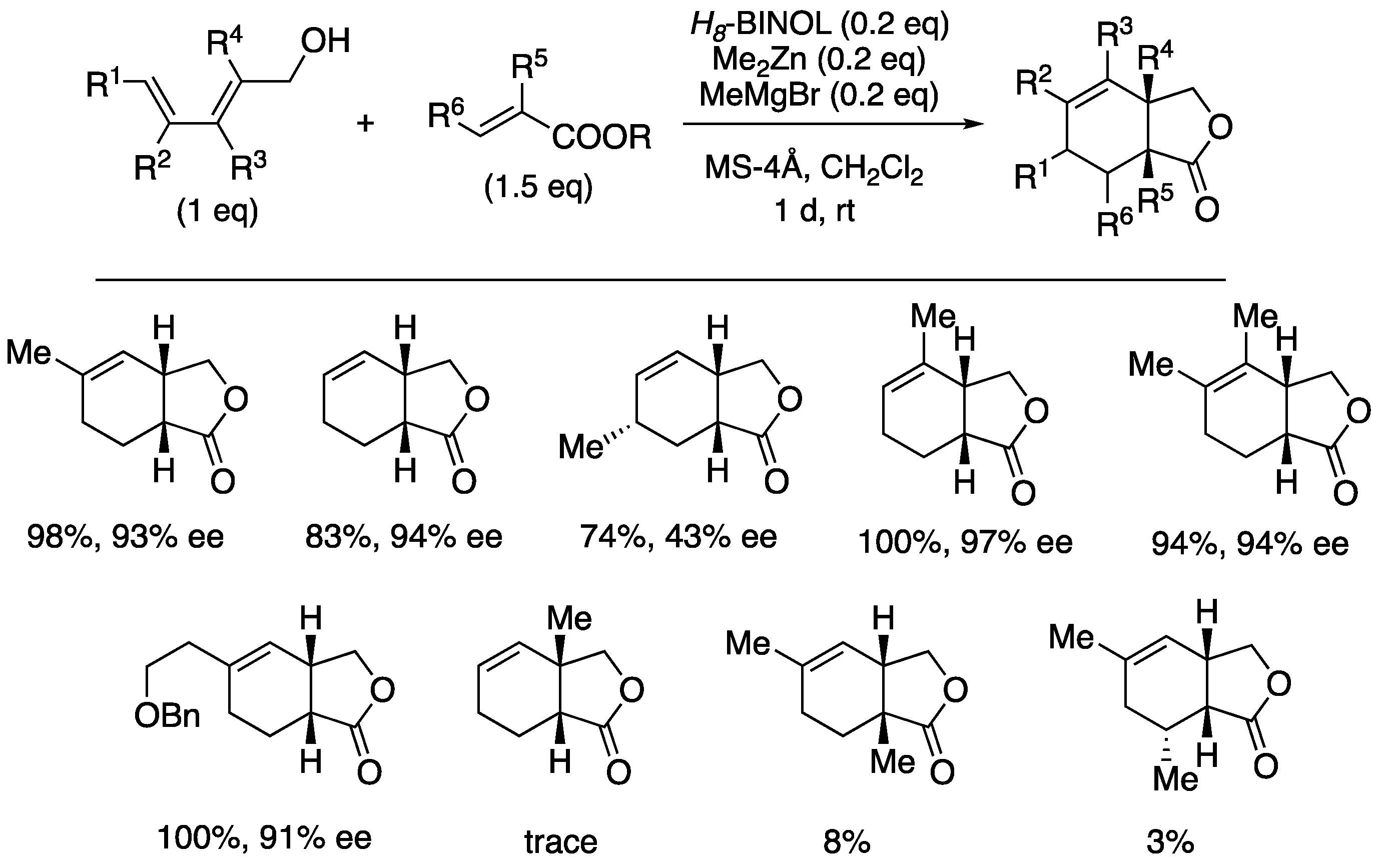
The generality of this protocol was next explored (
Scheme 11). It was found that the Lewis acid template-catalyzed reaction is applicable to various 2,4-pentadienol substituents, and both yield and optical yield are high, especially with substituents (R
3 and R
2) at 3 and 4 positions. On the other hand, the reaction of 2,4-hexadienol 6c exhibited the poor enantioselectivity, although the yield was acceptable. Furthermore, 2-methyl-2,4-pentadienol was found to be a less reactive substrate possibly for steric reasons.
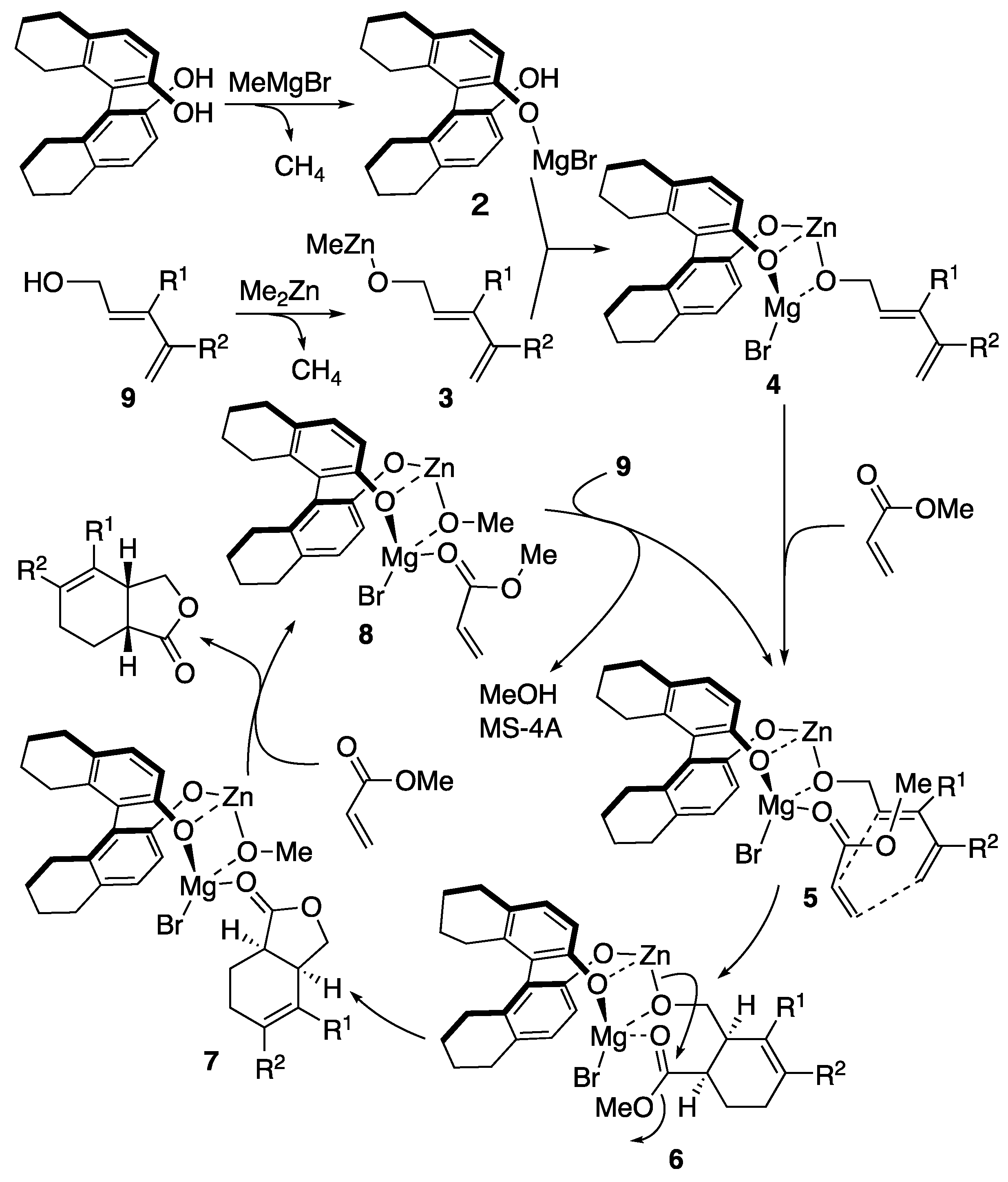
A plausible mechanism for the catalytic Diels-Alder reaction is depicted in
Scheme 12. In the catalytic cycle of the reaction, coordination of magnesium octahydrobinaphthoate
2 with zinc dienolate
3 would initially form bimetallic Lewis acid
4, which reacts with methyl acrylate to afford intermediate
5. The tethered intermediate
5 undergoes Diels-Alder reaction in endo-mode (
6), followed by lactonization (
7). The ligand exchange of
7 with methyl acrylate would release 𝛾-lactone product. The second exchange of the resulting complex
8 by dienol
9 regenerates
5 and releases methanol. The molecular sieves would play an important role for the capture of the methanol to accelerate the catalytic cycle. MS-4Å or MS-5Å is requisite for the catalytic reaction, possibly because molecular sieves play an important role for the capture of methanol, which is generated in the course of lactonization, to accelerate the catalytic cycle. The reason for the higher catalytic efficiency by
H8-BINOL than BINOL is not clear, but larger dihedral angle of
H8-BINOL may play an important role for smooth ligand exchange in the catalytic cycle.
5. Development to the hetero Diels-Alder reaction
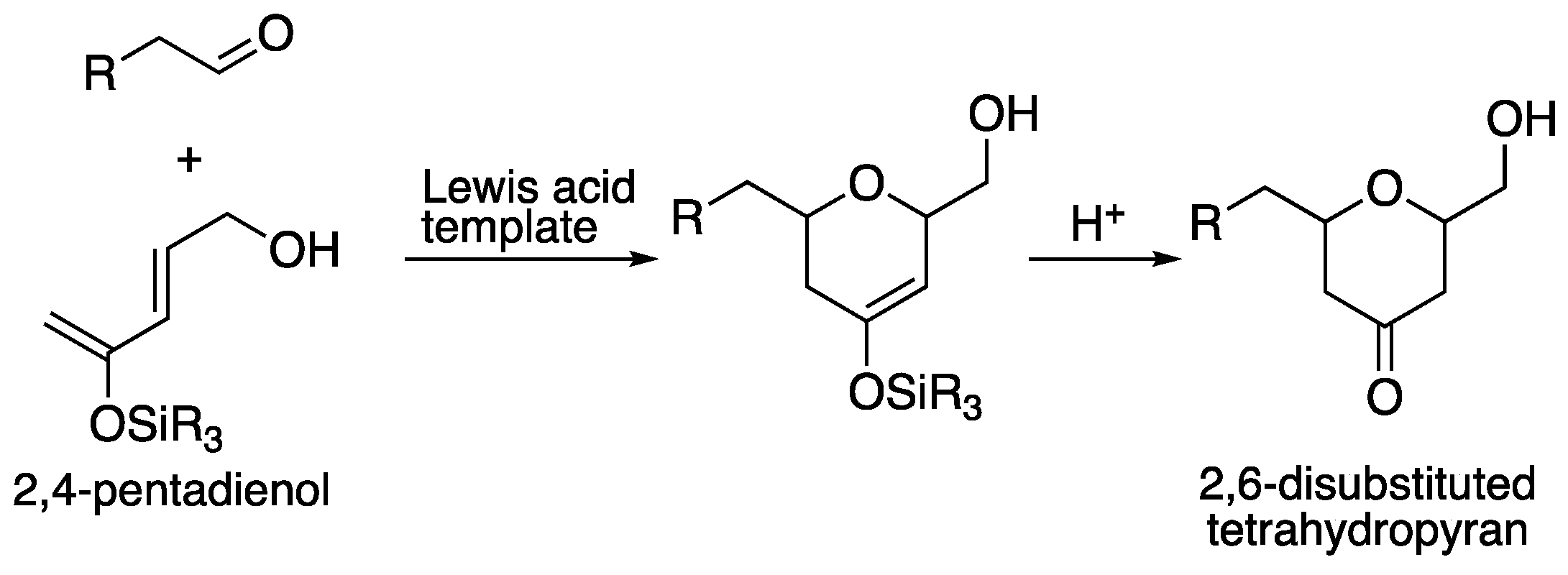
Next our efforts were focused on hetero Diels-Alder reaction. The hetero-Diels-Alder (HDA) reactions are reliable for the stereoselective construction of tetrahydropyrans, and effective asymmetric HDA reactions providing the dialkylated tetrahydropyrans have been developed [44, 45]. On the other hand, 2,6-disubstituted tetrahydropyrans are substructures found in many biologically active natural products such as bryostatins and exiguolides, and the establishment of stereoselective synthetic methods would be useful (
Scheme 13).
The substrates used as dienes are often four-carbon butadiene derivatives such as Kitahara-Danishefsky diene or Rawal diene, and five-carbon chain. There are few successful examples of asymmetric hetero Diels-Alder reactions using 5-carbon or larger diene derivatives as substrates. The following are some examples of asymmetric hetero-Diels-Alder reactions yielding 2,6-disubstituted products.
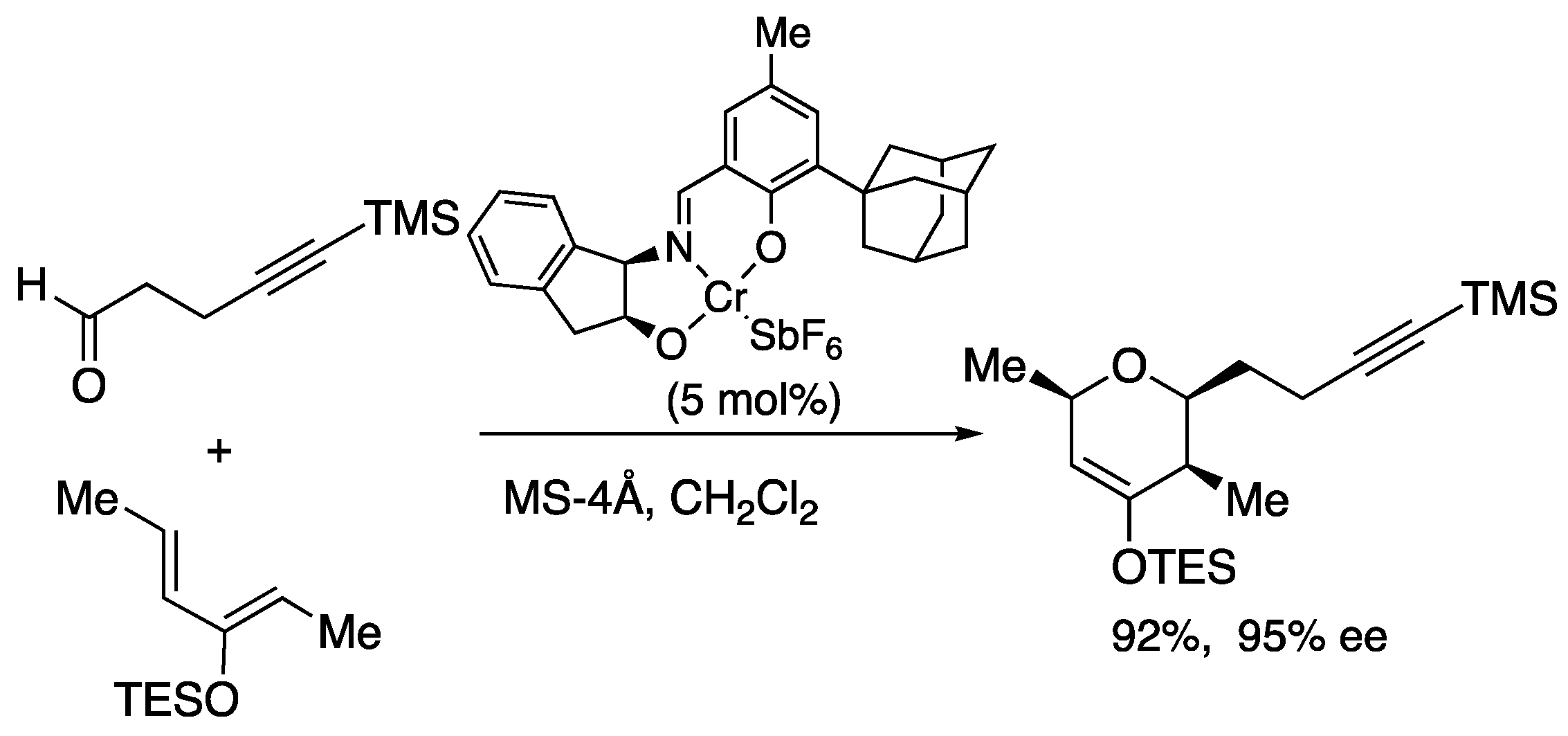
In 1999, Jacobsen reported a remarkable catalytic hetero-Diels-Alder reaction with an asymmetric chromium catalyst (
Scheme 14) [46, 47]. In this case, the tridentate Schiff base chromium catalyst facilitates hetero-Diels-Alder reaction providing dihydropyran compounds in excellent yield, selectivity, and versatility.
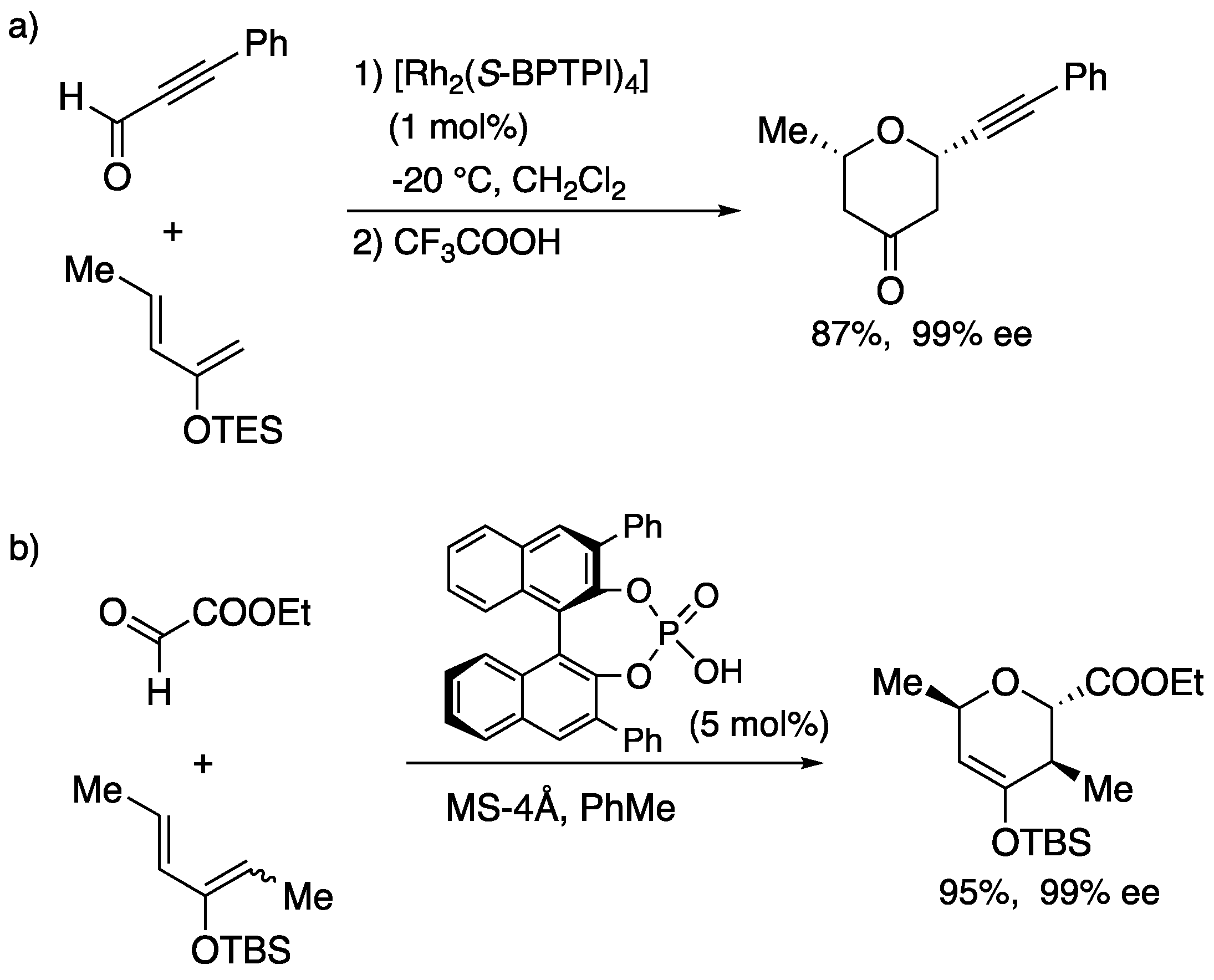
Other asymmetric hetero-Diels Alder reaction was reported by Anada-Hashimoto (
Scheme 15a). The rhodium-catalyzed reaction afforded tetrahydropyranone in 87% yield with excellent enantioselectivity after acidic treatment [
48]. On the other hand, Terada disclosed asymmetric hetero-Diels Alder reaction catalyzed by chiral Brønsted acid (
Scheme 15b) [
49]. In this case, chiral dihydropyran was obtained in 95% yield with 99% ee.
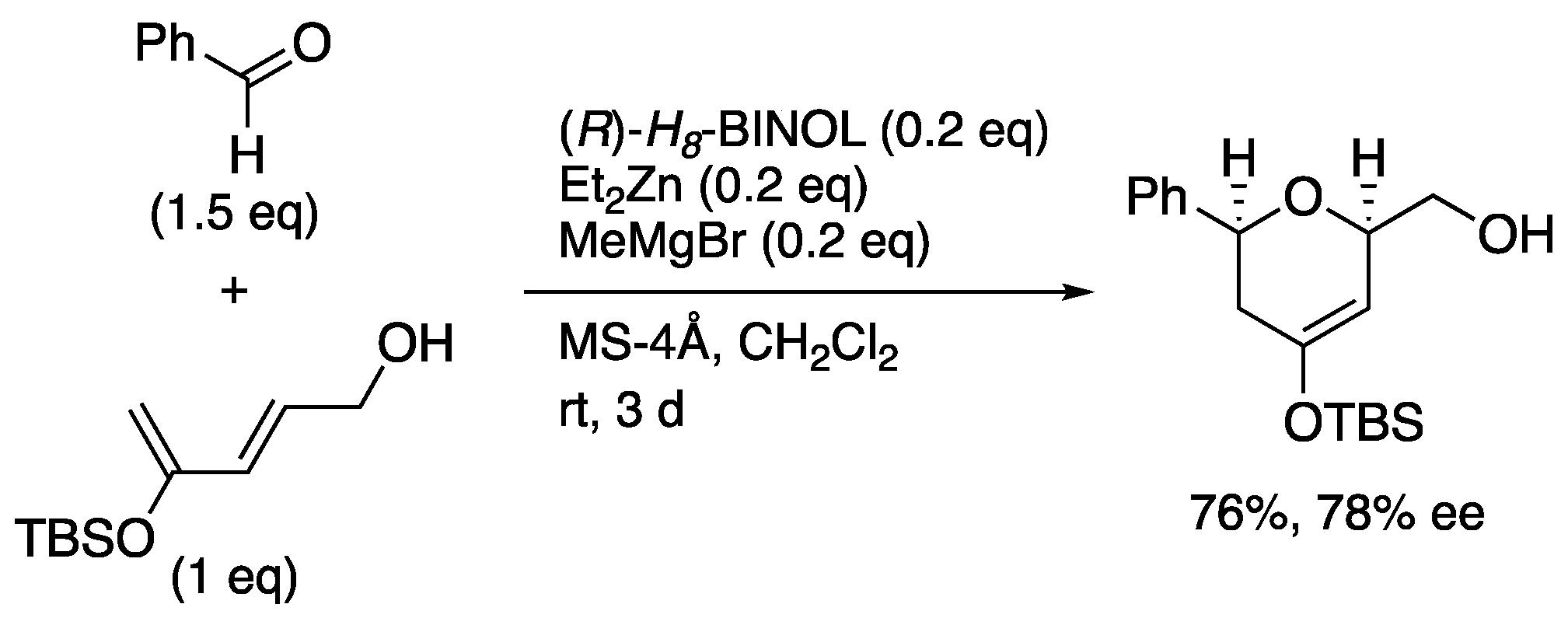
The authors investigated hetero-Diels-Alder reaction of 2,4-pentadienol and benzaldehyde using Lewis acid template (
Scheme 16) [
50]. After various investigations of the silyl group, Lewis acid, or biaryl ligands,
etc. were examined, it was found that the hetero-Diels-Alder reaction using
H8-BINOL afforded
cis-disubstituted 2,6-dihydropyran derivatives in 61% yield and 78% ee. The reaction using other aldehydes as substrates was also investigated.
6. Application of Lewis acid template-mediated Diels-Alder reaction to biological active natural products
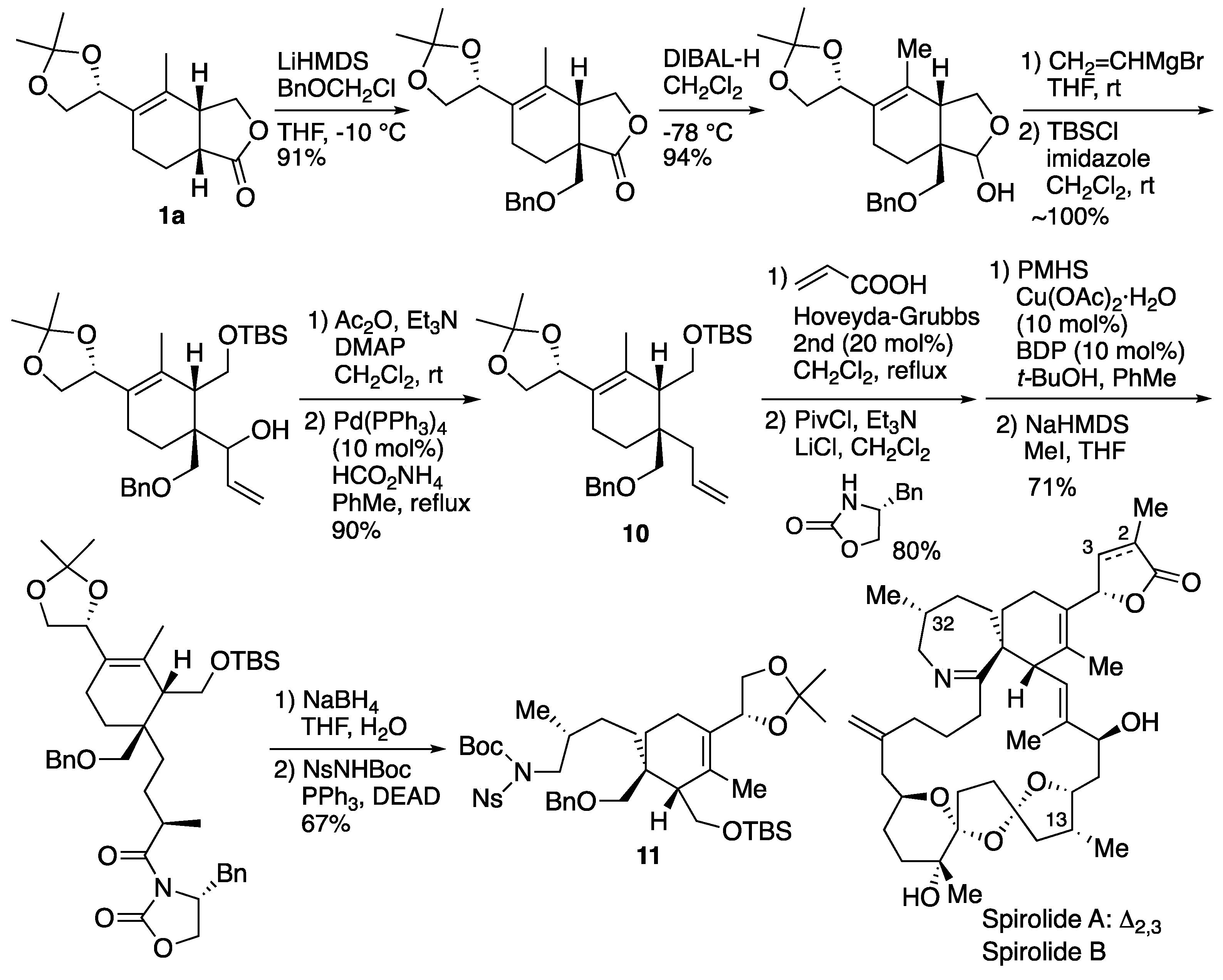
6.2. Ishihara's synthesis of upper segment of spirolide A and B
Since 1995, a number of marine natural products containing spiroimine moiety have been found as causative agents of food poisoning. Spirolides are marine toxins isolated from bivalves and share the same structure with natural products such as pinnatoxin and gymnodimine. In particular, 13-desmethylspyrrolide C is one of the most potent neurotoxins among non-peptide natural products. These natural products exhibit antagonist activity against nicotinic acetylcholine receptor (nAChR). Our synthetic studies of the spirocyclic core of spirolide A and B was commenced from compound
1a, which was available by Lewis acid template-catalyzed Diels-Alder reaction [
34]. A benzyloxymethyl group was stereoselectively introduced at the α-position of the lactone, which was then converted to lactol by DIBAL reduction (
Scheme 17). Next, the installation of vinyl group using the Grignard reagent, followed by TBS protection of the primary hydroxyl group, acetylation of the secondary hydroxyl group, and subsequent palladium-catalyzed reductive deacethoxylation afforded allylic product in good yields. The subsequent cross-metathesis was performed to introduce the oxazolidinone, followed by hydrogenation by Lipshutz 1,4-reduction and stereoselective methylation by Evans alkylation. Removal of the asymmetric auxiliary group and Fukuyama amine synthesis led to the synthesis of spirolide A and B upper segment
11.
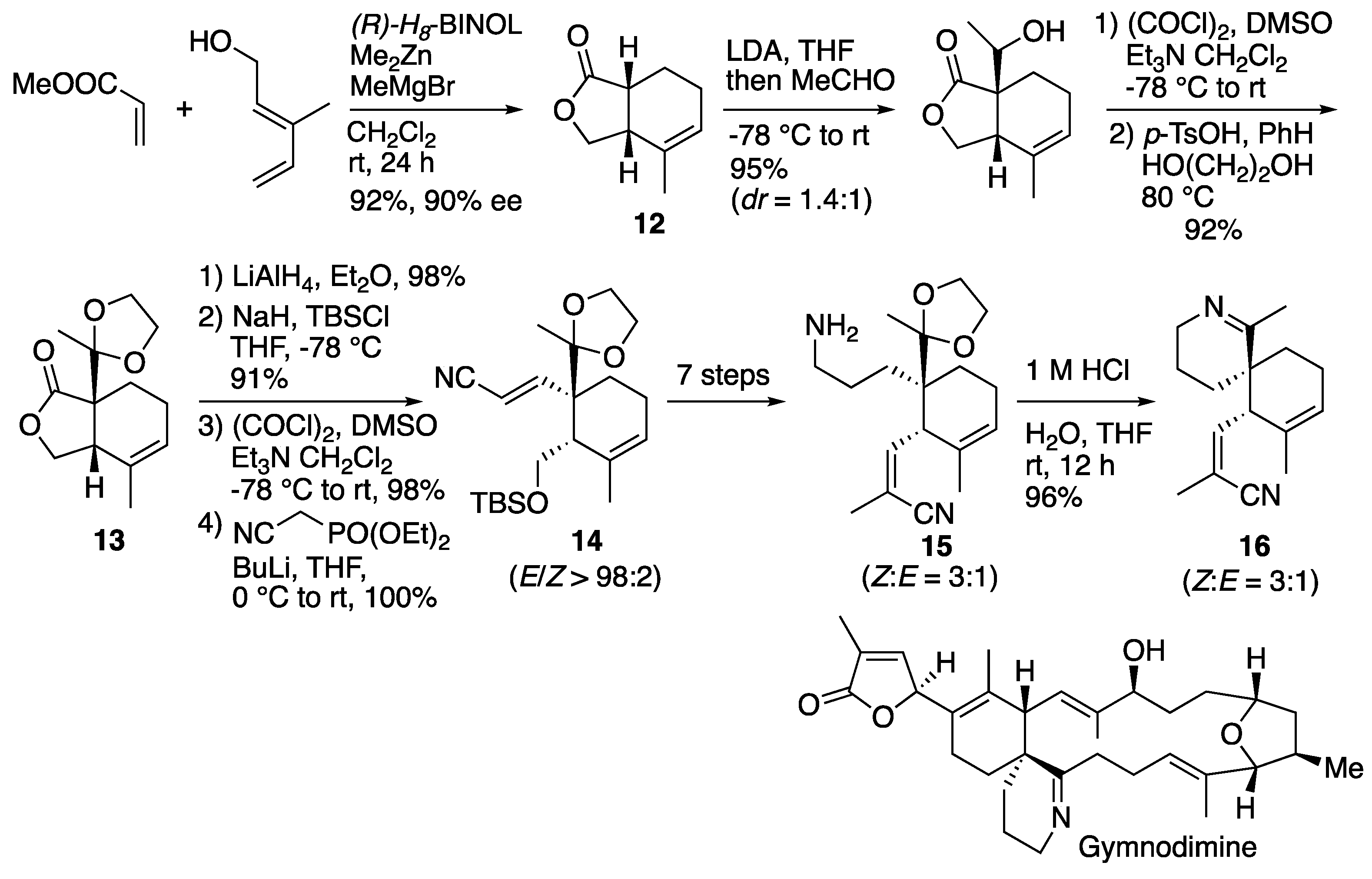
6.2. Landais's synthesis of spirocyclic core of demethylspirolide C and gymnodimine
Landais and co-workers also reported approach to natural products containing spiroimine, desmethylspirolide C and gymnodimine (
Scheme 18) [
33]. They prepared the bicyclic 𝛾-lactone
12 in good yield with high enantioselectivity by the Lewis acid template-catalyzed Diels-Alder reaction. The bicyclic product
12 was α-alkylated by aldol reaction, followed by oxidation and ketalization to afford
13. Lactone
13 was subjected to LiAlH
4 reduction to give diol, which was protected by TBS group, oxidation and Horner-Wadsworth-Emmons reaction to afford compound
14 in good yield. Compound
14 was converted in 7 steps to compound
15, which was exposed with HCl to furnish spirocyclic imine product
16 in excellent yield.
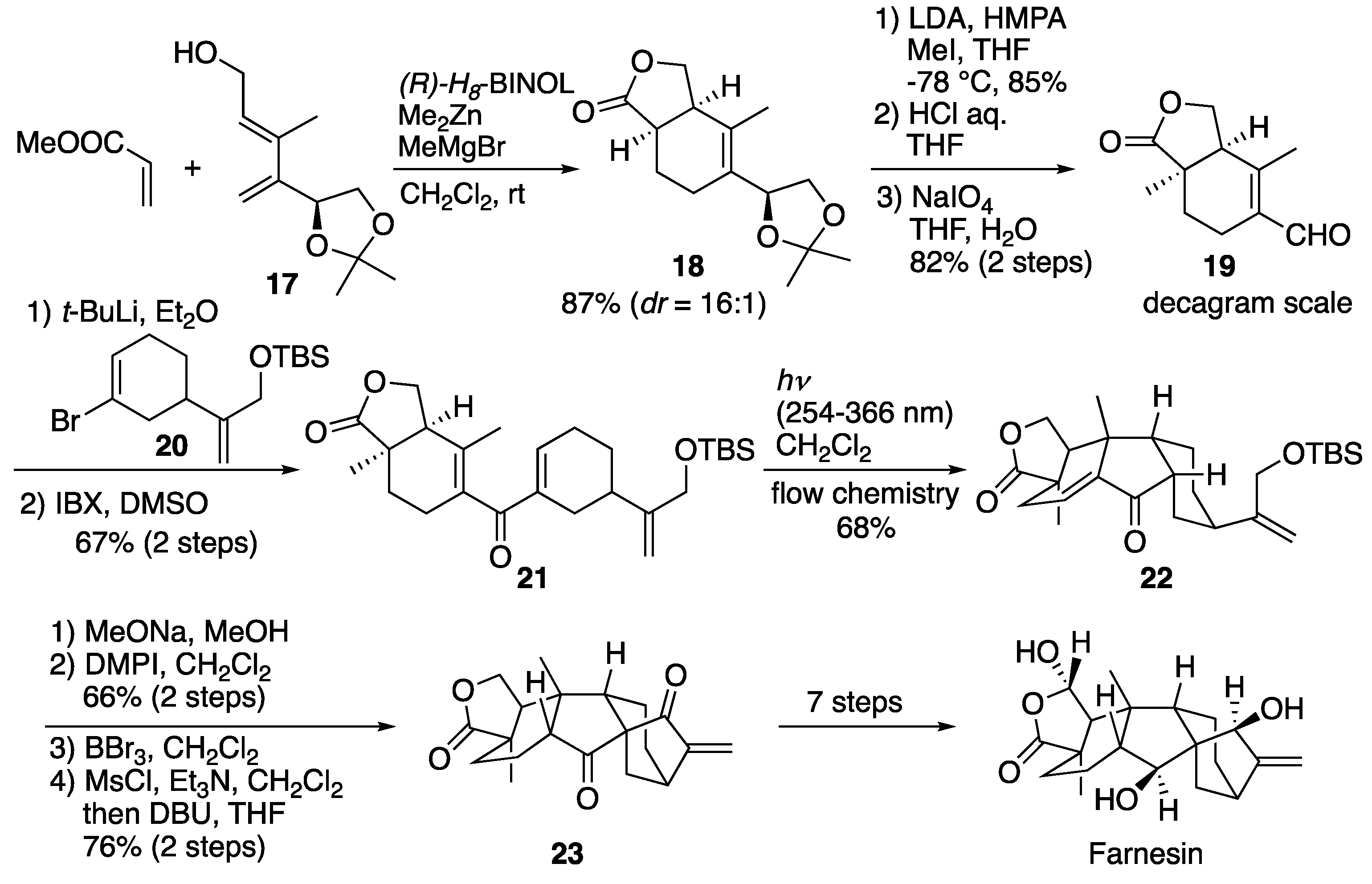
6.3. Gao's total synthesis of farnesin
In 2020 Gao
et al. reported the total synthesis of farnesin, which involves the photo-Nazarov and intramolecular aldol cyclization (
Scheme 19) [
32]. Their synthesis began with the construction of bicyclic 𝛾-lactone
18 by the Lewis acid template-catalyzed Diels-Alder reaction of
17 and methyl acrylate. Notably, the cycloaddition was scalable, generating the desired adduct in good yield with good diastereoselectivity. After α-methylation of
18, the acetonide group was transformed to aldehyde
19 via acidic treatment and subsequent oxidative cleavage. Coupling of aldehyde
19 with compound
20, followed by oxidation, furnished compound
21. Next, compound
21 was subjected to key photo-Nazarov reaction to provide complicated hydrofluorenone
22 as a single diastereomer. Compound
22 was transformed to pentacyclic product
23 via a SmI
2-promoted radical reaction. From compound
23, the total synthesis of farnesin was achieved by a seven-step transformation reaction.
Conclusion
In this paper, the Lewis acid template-mediated Diels-Alder reaction affording substituted bicyclic 𝛾-lactone was mentioned. The Diels-Alder reactions shown here are regarded as a useful and practical method, which allows simple access to valuable building blocks for the synthesis of complicated natural products. The author hopes that this account will help open up new strategies for the synthesis of biologically and pharmaceutically valuable substances.
Acknowledgments
I would like to express our sincere gratitude to Professor Susumi Hatayama (current Emeritus professor at Nagasaki university) for his useful advice. I would also like to thank to Yuki Watanabe, Shino Nakadachi, Yasunori Kawaguchi, Tomohiro Kurose, Yuka Ohzono, Yasuhiro Urayama, Kengo Oka, Tomohiro Baba, Fuma Usui, Keita Komine, and Hayato Fukuda for their tireless efforts in developing the results of this experiment. Part of this research was supported by JSPS KAKENHI Grant Numbers JP18K05126 and 23K04736. The author is grateful to Nagase Science and Technology Foundation for financial support. In addition, the author is also grateful to the Novartis foundation (Japan) for the promotion of science. This work was the result of using research equipment shared in MEXT project for promoting public utilization of advanced research infrastructure (program for supporting introduction of the new sharing system) Grant Number JPMXS0422500320.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Newman D. J.; Cragg G. M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770−803. [CrossRef]
- Atanasov, A. G.; Zotchev, S. B.; Dirsch, V. M. the International Natural Product Sciences Taskforce; Supuran, C. T. Nat. Rev. Drug Discov., 2021, 20, 200–216. [CrossRef]
- Nicolaou, K. C.; Snyder, S. A.; Montagnon T.; Vassilikogiannakis, G. The Diels–Alder Reaction in Total Synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668−1698. [CrossRef]
- Roush, W. R. Intramolecular Diels-Alder Reactions. Comprehensive Organic Synthesis; Trost, B. M., Fleming, I., Paquette, L. A., Eds.; Pergamon: Oxford, 1991; Vol. 5, pp. 513−550.
- Tanner, D.; Ascic, E. Intramolecular Diels-Alder Reactions. Comprehensive Organic Synthesis; 2nd edition; Knochel, P., Molander, G. A. Eds.; Elsevier: Oxford, 2014, Vol. 5, pp. 466−571.
- Craig, D. Stereochemical aspects of the intramolecular Diels–Alder reaction. Chem. Soc. Rev. 1987, 16, 187−238. [CrossRef]
- Fallis, A. G. The intramolecular Diels–Alder reaction: recent advances and synthetic applications. Can. J. Chem. 1984, 62, 183−234. [CrossRef]
- Tamao, K.; Kobayashi, K.; Ito, Y. An intramolecular Diels-Alder reaction of vinylsilanes. J. Am. Chem. Soc., 1989, 111, 6478−6480. [CrossRef]
- Stork G.; Chan, T. Y.; Breault, G. A.; The temporary silicon connection in the control of the regiochemistry of 4 + 2 cycloadditions. J. Am. Chem. Soc., 1992, 114, 7578−7559. [CrossRef]
- Sieburth, S. McN.; Fensterbank, L. An intramolecular Diels-Alder reaction of vinylsilanes. J. Org. Chem., 1992, 57, 5279−5281. [CrossRef]
- Shea, K.; Zandi, K. S.; Staab, A. J.; Carr, R. Disposable tethers in type 2 intramolecular Diels-Alder cycloaddition reactions. Applications in stereochemical control. Tetrahedron Lett., 1990, 31, 5885−5888. [CrossRef]
- Shea, K.; Staab, A. J.; Zandi, K. S. Pericyclic umpolung. Reversal of regioselectivity in the Diels-Alder reaction. Tetrahedron Lett., 1991, 32, 2715−2718. [CrossRef]
- Craig, D.; Reader, J. C. A novel strategy for regio- and stereocontrol in [4 + 2] cycloadditions. Intramolecular diels-alder reaction of a silyl acetal triene. Tetrahedron Lett., 1990, 31, 6585−6588. [CrossRef]
- Gillard, J. W.; Fortin, R.; Grimm, E. L.; Maillard, M.; Tjepkema, M.; Bernstein, M. A.; Glaser, R. The unsymmetrical silaketal as a neutral, removable tether for effecting intramolecular Diels-Alder reactions. Tetrahedron Lett., 1991, 32, 1145−1148. [CrossRef]
- L. Fensterbank, M. Malacria, S. McN. Sieburth, Intramolecular Reactions of Temporarily Silicon-Tethered Molecules. Synthesis, 1997, 813−854. [CrossRef]
- Bols, M.; Skrydstrup, T. Silicon-Tethered Reactions. Chem. Rev., 1995, 95, 1253-1277. [CrossRef]
- G. Stork, T. Y. Chan, Temporary Magnesium and Aluminum Connections in [4 + 2] Cycloadditions. J. Am. Chem. Soc., 1995, 117, 6595−6596. [CrossRef]
- K. Narasaka, S. Shimada, K. Osoda, N. Iwasawa, Phenylboronic Acid as a Template in the Diels-Alder Reaction, Synthesis, 1991, 1171−1172. [CrossRef]
- Grieco, P. A.; Ravi P. Nargund, R. P. Synthetic studies on diterpenes from a termite soldier: Total synthesis of (±)-biflora-4,10(19),15-triene. Tetrahedron Lett. 1986, 27, 4813−4816. [CrossRef]
- White, J. D.; Nolen, E. G., Jr.; Miller, C. H. Stereochemical transcription via the intramolecular Diels-Alder reaction. Enantioselective synthesis of the nucleus of (+)-pillaromycinone. J. Org. Chem. 1986, 51, 1150−1155 . [CrossRef]
- White, J. D.; Demnitz, F. W. J.; Xu, Q.; Martin, W. H. C. Synthesis of an Advanced Intermediate for (+)-Pillaromycinone. Staunton-Weinreb Annulation Revisited. Org. Lett. 2008, 10, 2833−2836. [CrossRef]
- Arseniyadis, S.; Brondi-Alves, R.; Yashunsky,D. V.; Potier, P.; Toupet, L.Formal total synthesis of an A-seco mevinic acid. Tetrahedron, 1997, 53, 1003−1014. [CrossRef]
- Nicolaou, K. C.; Chen, J. S. Abyssomicin C and atrop-Abysomicin C. Classics in Total Synthesis III: Further Targets, Strategies, Methods; Wiley-VCH: Weinheim, 2011; pp. 320−343.
- Nicolaou, K. C.; Harrison, S. T. Total Synthesis of Abyssomicin C and atrop-Abyssomicin C. Angew. Chem., Int. Ed. 2006, 45, 3256−3260. [CrossRef]
- Nicolaou, K. C.; Harrison, S. T. Total Synthesis of Abyssomicin C, Atrop-abyssomicin C, and Abyssomicin D: Implications for Natural Origins of Atrop-abyssomicin C. J. Am. Chem. Soc. 2007, 129, 429−440. [CrossRef]
- Rath, J.-P.; Kinast, S.; Maier, M. E. Synthesis of the Fully Functionalized Core Structure of the Antibiotic Abyssomicin C. Org. Lett. 2005, 7, 3089–3092. [CrossRef]
- Halvorsen, G. T.; Roush, W. R. Stereoselective synthesis of the decahydrofluorene core of the hirsutellones. Tetrahedron Lett. 2011, 52, 2072-2075. [CrossRef]
- Chen, L.; Ahmed, K. B. R.; Huang, P.; Jin, Z. Design, Synthesis, and Biological Evaluation of Truncated Superstolide A. Angew. Chem., Int. Ed. 2013, 52, 3446−3449. [CrossRef]
- Abbasov, M. E., Alvariño, R., Chaheine, C. M. Alonso, E.; Sánchez, J. A.; Conner, M. L.; Alfonso, A.; Jaspars, M.; Botana, L. M. Romo, D. Simplified immunosuppressive and neuroprotective agents based on gracilin A. Nat. Chem. 2019, 11, 342–350. [CrossRef]
- Miller, N. A.; Willis, A. C.; Sherburn, M. S. Formal total synthesis of triptolide. Chem. Commun., 2008, 1226−1228. [CrossRef]
- Wong, L. S.-M.; Sherburn, M. S. IMDA-Radical Cyclization Approach to (+)-Himbacine, Org. Lett. 2003, 5, 3603–3606. [CrossRef]
- Que, Y.; Shao, H.; He, H.; Gao, S. Total Synthesis of Farnesin through an Excited-State Nazarov Reaction. Angew. Chem., Int. Ed. 2020, 59, 7444−7449. [CrossRef]
- Guthertz, A.; Lusseau, J.; Desvergnes, V.; Massip, S. ; Landais Y. An Approach towards the Synthesis of the Spiroimine Fragment of 13-Desmethylspirolide C and Gymnodimine A. Chem. Eur. J. 2019, 25, 1553−1560. [CrossRef]
- Ishihara, J.; Usui, F.; Kurose, T.; Baba, T.; Kawaguchi, Y.; Watanabe, Y.; Hatakeyama, S. Synthetic Studies on Spirolides A and B: Formation of the Upper Carbon Framework Based on a Lewis Acid Template-Catalyzed Diels–Alder Reaction. Chem. Eur. J. 2019, 25, 1543−1552. [CrossRef]
- Cayzer, T. N. ; Wong, L. S.-M.; Turner, P.; Paddon-Row, M. N.; Sherburn, M. S. Optimising Stereoselectivity in Intramolecular Diels-Alder Reactions of Pentadienyl Acrylates: Synthetic and Computational Investigations into the ‘Steric Directing Group’ Approach. Chem. Eur. J. 2002, 8, 739−750. [CrossRef]
- Smith, D. A.; Sakan, K.; Houk, K. N. Stereoselectivities of thermal and Lewis acid catalyzed intramolecular Diels-Alder reactions of internally activated dienophiles to form 5–11 membered rings. Tetrahedron Lett., 1986, 27, 4877−4880. [CrossRef]
- Bienaymé, H. Angew. Chem. Int. Ed. Engl. 1997, 36, 2670−2673. Enantioselective Diels–Alder Cycloaddition by Preorganization on a Chiral Lewis Acid Template . [CrossRef]
- Bienaymé, H.; Longeau, A. Internally Lewis acid-catalyzed Diels-Alder cycloadditions. Tetrahedron, 1997, 53, 9637−9646. [CrossRef]
- Ward, D. E.; Abaee, M. S. Intramolecular Diels−Alder Reaction by Self-Assembly of the Components on a Lewis Acid Template. Org. Lett. 2000, 2, 3937−3940. [CrossRef]
- Zografos, A. L.; Yiotakis, A.; Georgiadis, D. Rapid Access to the Tricyclic Spirotetronic Core of Abyssomicins. Org. Lett. 2005, 7, 4515–4518. [CrossRef]
- D. E. Ward, M. S. Souweha, Catalytic Enantioselective Diels−Alder Reaction by Self-Assembly of the Components on a Lewis Acid Template. Org. Lett., 2005, 7, 3533−3536. [CrossRef]
- Souweha, M. S.; Arab, A. ApSimon, M.; Fallis, A. G. Diene-Transmissive Cycloadditions: Control of Monocycloaddition by Self-Assembly on a Lewis Acid Template. Org. Lett. 2007, 9, 615–618 . [CrossRef]
- Ishihara, J.; Nakadachi, S. Watanabe, Y. Hatakeyama, S. Lewis Acid Template-Catalyzed Asymmetric Diels-Alder Reaction. J. Org. Chem., 2015, 80, 2037−2041. [CrossRef]
- Jørgensen, K. A. Catalytic Enantioselective Cycloaddition Reactions of Carbonyl Compounds. Cycloaddition Reactions in Organic Synthesis; Kobayashi, S., Jørgensen. K. A. Eds.; Wiley-VCH, Weinheim, 2002, pp. 151−185. [CrossRef]
- Ishihara, K.; Sakakura, A. Hetero-Diels-Alder Reactions. Comprehensive Organic Synthesis; 2nd edition; Knochel, P., Molander, G. A. Eds.; Elsevier: Oxford, 2014, Vol. 5, pp. 409−465.
- Dossetter, A. G.; Jamison, T. F.; Jacobsen, E. N. Highly Enantio- and DiastereoselectiveHetero-Diels-Alder Reactions Catalyzed by New Chiral Tridentate Chromium (iii) Catalysts. Angew. Chem., Int. Ed. 1999, 38, 2398−2400. [CrossRef]
- Thompson, C. F.; Jamison, T. F.; Jacobsen, E. N. FR901464: Total Synthesis, Proof of Structure, and Evaluation of Synthetic Analogues. J. Am. Chem. Soc. 2001, 123, 41, 9974–9983. [CrossRef]
- Anada, M.; Washio, T.; Shimada, N.; Kitagaki S.; Nakajima, M.; Shiro, M.; Hashimoto, S. A New Dirhodium(II) Carboxamidate Complex as a Chiral Lewis Acid Catalyst for Enantioselective Hetero-Diels–Alder Reactions. Angew. Chem. Int. Ed., 2004, 43, 2665−2668. [CrossRef]
- Momiyama, N.; Tabuse H.; Terada, M. Chiral Phosphoric Acid-Governed Anti-Diastereoselective and Enantioselective Hetero-Diels−Alder Reaction of Glyoxylate. J. Am. Chem. Soc., 2009, 131, 12882−12883. [CrossRef]
- Ishihara, J.; Ohzono, Y.; Oka, K.; Urayama, Y.; Hatakeyama, S. Bimetallic Lewis Acid Template-Mediated Enantioselective Hetero-Diels-Alder Reactions of 4-Siloxy-2,4-pentadienols. Heterocycles, 2019, 99, 1330−1341. [CrossRef]
Scheme 1.
Tamao-Ito’s pioneering intramolecular Diels-Alder reaction of dienylsilane.
Scheme 1.
Tamao-Ito’s pioneering intramolecular Diels-Alder reaction of dienylsilane.
Scheme 2.
Stork’s silicon-tether intramolecular Diels-Alder reaction.
Scheme 2.
Stork’s silicon-tether intramolecular Diels-Alder reaction.
Scheme 3.
Intramolecular Diels-Alder reaction of dialkoxysilane by Shea.
Scheme 3.
Intramolecular Diels-Alder reaction of dialkoxysilane by Shea.
Scheme 4.
Temporary magnesium connection in Diels-Alder reaction.
Scheme 4.
Temporary magnesium connection in Diels-Alder reaction.
Scheme 5.
Phenylboronic acid template Diels-Alder reaction.
Scheme 5.
Phenylboronic acid template Diels-Alder reaction.
Figure 1.
Representatives of the synthesis of natural products and natural product-related substances using bicyclic 𝛾-lactone as a key intermediate.
Figure 1.
Representatives of the synthesis of natural products and natural product-related substances using bicyclic 𝛾-lactone as a key intermediate.
Scheme 6.
Intramolecular Diels-Alder reaction of triene ester.
Scheme 6.
Intramolecular Diels-Alder reaction of triene ester.
Scheme 7.
Bienaye’s Lewis acid template-mediated Diels-Alder reaction.
Scheme 7.
Bienaye’s Lewis acid template-mediated Diels-Alder reaction.
Scheme 8.
Ward’s Lewis acid template-mediated Diels-Alder reaction.
Scheme 8.
Ward’s Lewis acid template-mediated Diels-Alder reaction.
Scheme 9.
Nicolaou’s Lewis acid template-mediated Diels-Alder reaction.
Scheme 9.
Nicolaou’s Lewis acid template-mediated Diels-Alder reaction.
Scheme 10.
Ward’s enantioselective Diels-Alder reaction.
Scheme 10.
Ward’s enantioselective Diels-Alder reaction.
Scheme 11.
Synthesis of bicyclic 𝛾-lactone by Lewis acid template Diels-Alder reaction.
Scheme 11.
Synthesis of bicyclic 𝛾-lactone by Lewis acid template Diels-Alder reaction.
Scheme 12.
Plausible mechanism of Lewis acid-catalyzed Diels-Alder reaction.
Scheme 12.
Plausible mechanism of Lewis acid-catalyzed Diels-Alder reaction.
Scheme 13.
General scheme of our hetero-Diels-Alder reaction.
Scheme 13.
General scheme of our hetero-Diels-Alder reaction.
Scheme 14.
Jacobsen’s asymmetric hetero-Diels-Alder reaction.
Scheme 14.
Jacobsen’s asymmetric hetero-Diels-Alder reaction.
Scheme 15.
Asymmetric hetero-Diels-Alder reaction providing 2,6-substituted dihydropyran derivatives.
Scheme 15.
Asymmetric hetero-Diels-Alder reaction providing 2,6-substituted dihydropyran derivatives.
Scheme 16.
Lewis acid template hetero-Diels-Alder reaction.
Scheme 16.
Lewis acid template hetero-Diels-Alder reaction.
Scheme 17.
Ishihara’s synthesis of upper fragment of spirolide A and B.
Scheme 17.
Ishihara’s synthesis of upper fragment of spirolide A and B.
Scheme 18.
Landais’s of spirocyclic core of gymnodimine.
Scheme 18.
Landais’s of spirocyclic core of gymnodimine.
Scheme 19.
Gao's total synthesis of farnesin.
Scheme 19.
Gao's total synthesis of farnesin.
Table 1.
Preparation of bicyclic 𝛾-lactone.
Table 1.
Preparation of bicyclic 𝛾-lactone.
| entry |
Ln |
X (eq) |
time |
yield |
ratio
(1a : 1b) |
| 1 |
(R)-BINOL |
1.0 |
3.5 h |
100% |
4.8 : 1 |
| 2 |
(S)-BINOL |
1.0 |
24 h |
80% |
1 : 3.7 |
| 3 |
(R)-3,3‘-Br2-BINOL |
1.0 |
20 h |
94% |
4.6 : 1 |
| 4 |
(R)-H8-BINOL |
1.0 |
24 h |
98% |
9.9 : 1 |
| 5 |
(R)-H8-BINOL |
0.5 |
2.5 h |
99% |
10 : 1 |
| 6 |
(R)-H8-BINOL |
0.2 |
4 h |
96% |
10 : 1 |
| 7 |
(R)-H8-BINOL |
0.1 |
24 h |
98% |
8 : 1 |
Table 2.
Asymmetric Diels-Alder reaction.
Table 2.
Asymmetric Diels-Alder reaction.
| entry |
acrylate ( x eq) |
additive |
yield |
% ee |
| 1 |
15 |
- |
83% |
52%ee |
| 2 |
15 |
MS 4 Å |
100% |
84%ee |
| 3 |
5 |
MS 4 Å |
100% |
88%ee |
| 4 |
2.5 |
MS 4 Å |
100% |
92%ee |
| 5 |
1.5 |
MS 4 Å |
98% |
93%ee |
| 6 |
1.5 |
MS 5 Å |
100% |
95%ee |
| 7 |
1.5 |
MS 13 X |
NR |
- |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).