Submitted:
01 January 2024
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
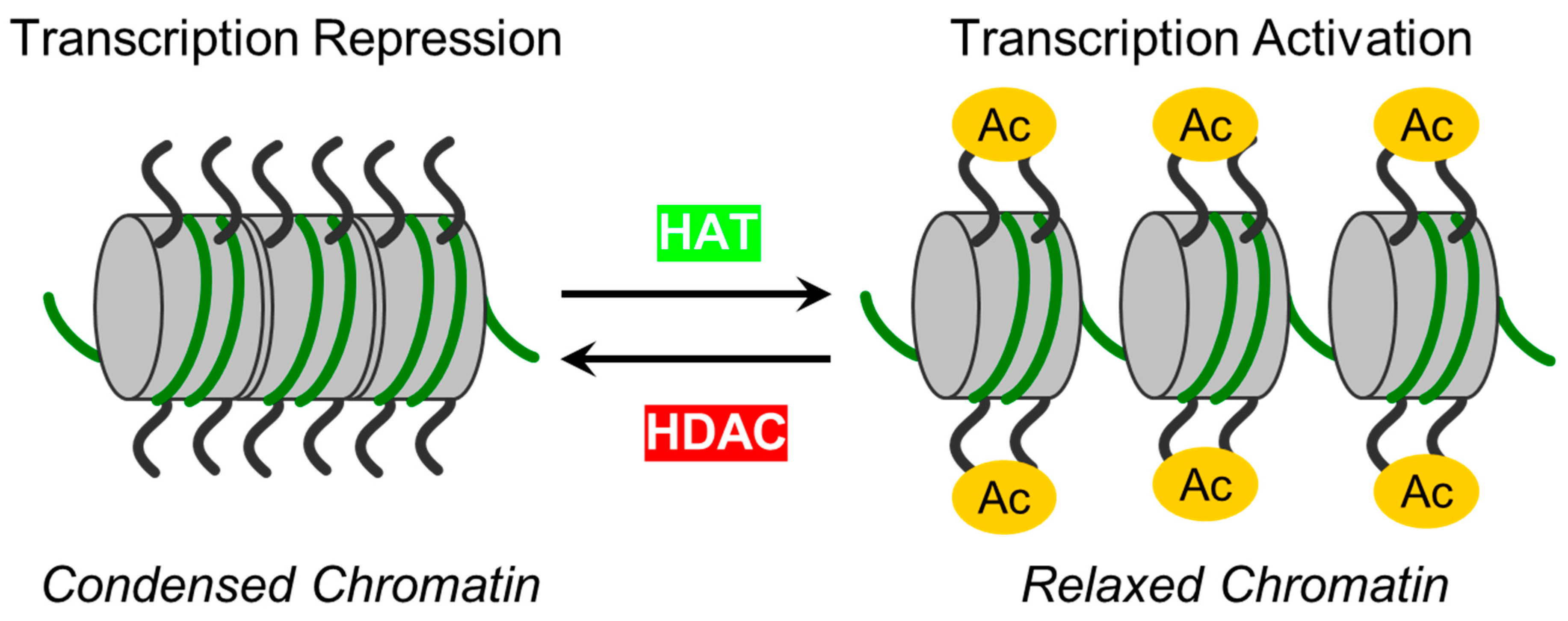
2. Zinc-dependent HDACs: Classification, functions, regulations and modulations
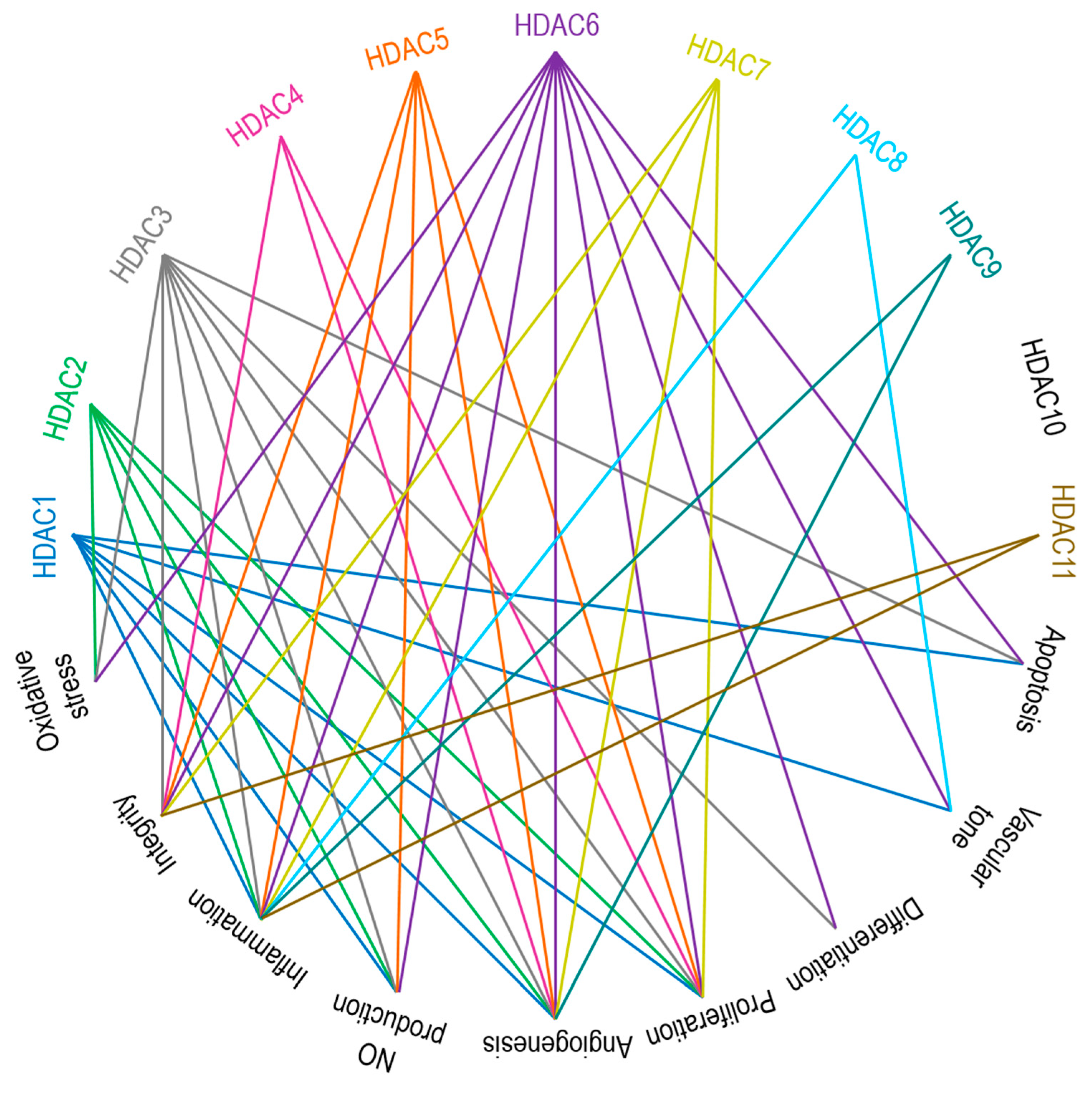
3. Zinc-Dependent HDACs in Endothelial Function
3.1. Class I HDACs: HDAC1, HDAC2, HDAC3, and HDAC8.
3.2. Class IIa HDACs: HDAC4, HDAC5, HDAC7, and HDAC9
3.3. Class IIb HDACs: HDAC6 and HDAC10
3.4. Class IV HDAC: HDAC11
4. EC-mediated central signaling cascades and their regulation by zinc-dependent HDACs
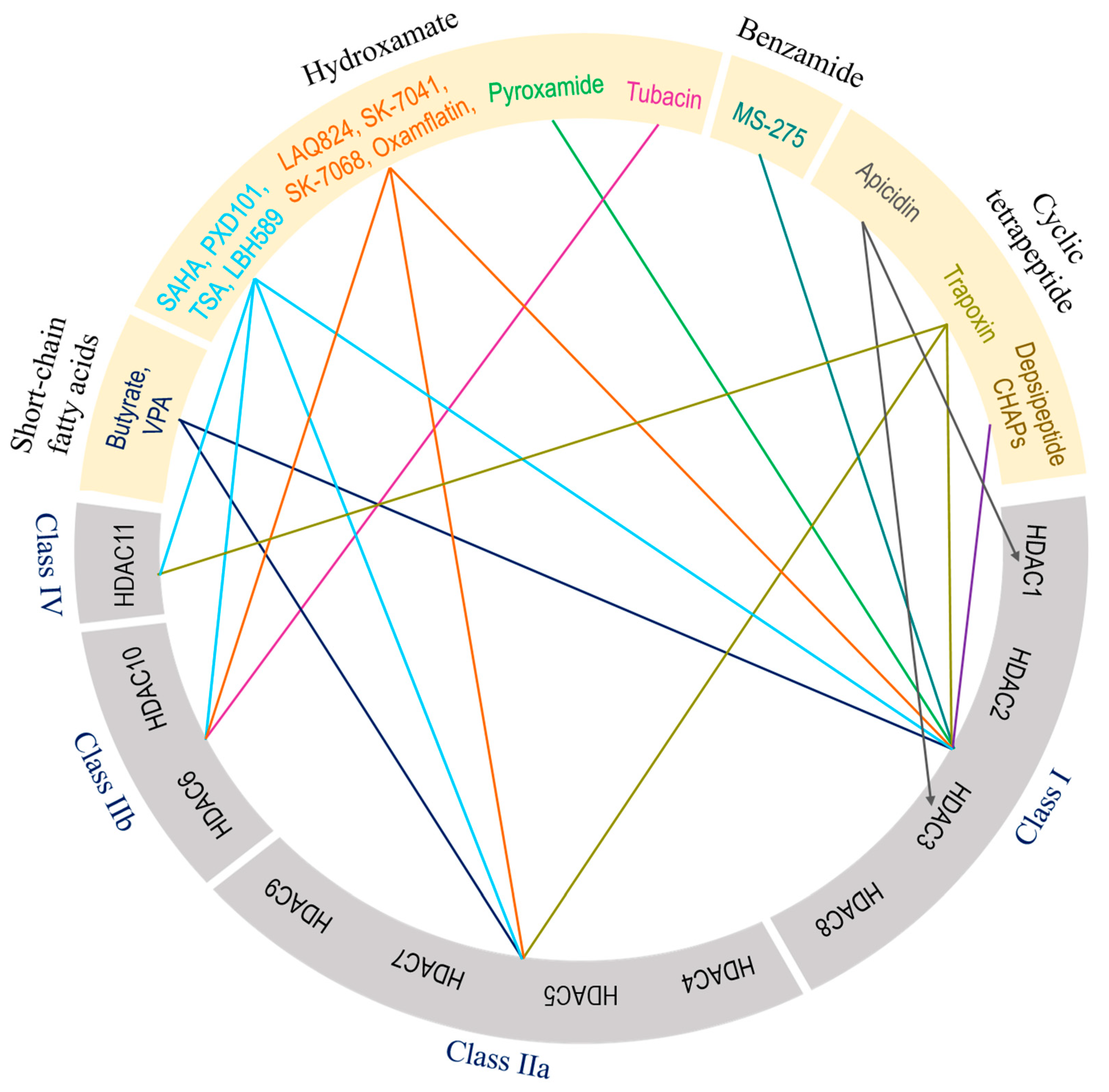
5. Therapeutic targeting of zinc-dependent HDACs in lung injury.
6. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mokrá, D. Acute lung injury - from pathophysiology to treatment. Physiol Res. 2020, 69, S353–S366. [Google Scholar] [CrossRef] [PubMed]
- Long, M.E.; Mallampalli, R.K.; Horowitz, J.C. Pathogenesis of pneumonia and acute lung injury. Clin Sci (Lond). 2022, 136, 747–769. [Google Scholar] [CrossRef] [PubMed]
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N Engl J Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef]
- Cusack, R.; Bos, L.D.; Povoa, P.; Martin-Loeches, I. Endothelial dysfunction triggers acute respiratory distress syndrome in patients with sepsis: a narrative review. Front Med (Lausanne). 2023, 10, 1203827. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012, 307, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, K.P.; Hudson, L.D.; Goodman, R.B.; Hough, C.L.; Lanken, P.N.; Hyzy, R.; Thompson, B.T.; Ancukiewicz, M.; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006, 354, 1671–1684. [Google Scholar] [CrossRef]
- Wiedemann, H.P.; Wheeler, A.P.; Bernard, G.R.; Thompson, B.T.; Hayden, D.; deBoisblanc, B.; Connors, A.F. Jr.; Hite, R.D.; Harabin, A.L.; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006, 354, 2564–2575. [Google Scholar] [CrossRef] [PubMed]
- Arcaro, G.; Zenere, B.M.; Travia, D.; Zenti, M.G.; Covi, G.; Lechi, A.; Muggeo, M. Non-invasive detection of early endothelial dysfunction in hypercholesterolaemic subjects. Atherosclerosis. 1995, 114, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Maniatis, N.A.; Kotanidou, A.; Catravas, J.D.; Orfanos, S.E. Endothelial pathomechanisms in acute lung injury. Vascul Pharmacol. 2008, 49, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.G.; Kotanidou, A.; Dimopoulou, I.; Orfanos, S.E. Endothelial damage in acute respiratory distress syndrome. Int J Mol Sci. 2020, 21, 8793. [Google Scholar] [CrossRef]
- Romero, M.J.; Yue, Q.; Singla, B.; Hamacher, J.; Sridhar, S.; Moseley, A.S.; Song, C.; Mraheil, M.A.; Fischer, B.; Zeitlinger, M.; Chakraborty, T.; Fulton, D.; Gan, L.; Annex, B.H.; Csanyi, G.; Eaton, D.C.; Lucas, R. Direct endothelial ENaC activation mitigates vasculopathy induced by SARS-CoV2 spike protein. Front Immunol. 2023, 14, 1241448. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.W.; Ilyas, I.; Weng, J.P. Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol Sin. 2023, 44, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Huertas, A.; Guignabert, C.; Barberà, J.A.; Bärtsch, P.; Bhattacharya, J.; Bhattacharya, S.; Bonsignore, M.R.; Dewachter, L.; Dinh-Xuan, A.T.; Dorfmüller, P.; Gladwin, M.T.; Humbert, M.; Kotsimbos, T.; Vassilakopoulos, T.; Sanchez, O.; Savale, L.; Testa, U.; Wilkins, M.R. Pulmonary vascular endothelium: The orchestra conductor in respiratory diseases: Highlights from basic research to therapy. Eur Respir J. 2018, 51, 1700745. [Google Scholar] [CrossRef] [PubMed]
- Simmons, S.; Erfinanda, L.; Bartz, C.; Kuebler, W.M. Novel mechanisms regulating endothelial barrier function in the pulmonary microcirculation. J Physiol. 2019, 597, 997–1021. [Google Scholar] [CrossRef] [PubMed]
- Borek, I.; Birnhuber, A.; Voelkel, N.F.; Marsh, L.M.; Kwapiszewska, G. The vascular perspective on acute and chronic lung disease. J Clin Invest. 2023, 133, e170502. [Google Scholar] [CrossRef]
- Mehta, D.; Malik, A.B. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef] [PubMed]
- Dudek, S.M.; Garcia, J.G. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol (1985). 2001, 91, 1487–1500. [Google Scholar] [CrossRef] [PubMed]
- Lampugnani, M.G.; Corada, M.; Caveda, L.; Breviario, F.; Ayalon, O.; Geiger, B.; Dejana, E. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol. 1995, 129, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E.; Orsenigo, F.; Lampugnani, M.G. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008, 121, 2115–2122. [Google Scholar] [CrossRef]
- Aslam, M.; Gündüz, D.; Troidl, C.; Heger, J.; Hamm, C.W.; Schulz, R. Purinergic Regulation of Endothelial Barrier Function. Int J Mol Sci. 2021, 22, 1207. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, X.; Sun, X.; Hu, W.; Miao, Q.R. The role of histone protein acetylation in regulating endothelial function. Front Cell Dev Biol. 2021, 9, 672447. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E.; Wang, N.; Stamenovic, D. Tensegrity, cellular biophysics, and the mechanics of living systems. Rep Prog Phys. 2014, 77, 046603. [Google Scholar] [CrossRef] [PubMed]
- Widdicombe, J. Functional morphology and physiology of pulmonary rapidly adapting receptors (RARs). Anat Rec A Discov Mol Cell Evol Biol. 2003, 270, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.F.; Nelson, C.M.; Tan, J.L.; Chen, C.S. Cadherins, RhoA, and Rac1 are differentially required for stretch-mediated proliferation in endothelial versus smooth muscle cells. Circ Res. 2007, 101, e44–52. [Google Scholar] [CrossRef] [PubMed]
- Tzima, E.; Irani-Tehrani, M.; Kiosses, W.B.; Dejana, E.; Schultz, D.A.; Engelhardt, B.; Cao, G.; DeLisser, H.; Schwartz, M.A. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005, 437, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wu, D.; Birukov, K.G. Mechanosensing and mechanoregulation of endothelial cell functions. Compr Physiol. 2019, 9, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, L.; Kovacs-Kasa, A.; Verin, A.D.; Fulton, D.; Lucas, R.; Su, Y. Histone deacetylases in vascular permeability and remodeling associated with acute lung injury. Vessel Plus. 2018, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Chiu, J.J. Atherosclerosis and flow: roles of epigenetic modulation in vascular endothelium. J Biomed Sci. 2019, 26, 56. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Liu, B.; Fu, T.; Liu, Y.; Li, B.; Li, N.; Geng, Q. The role of histone deacetylases in acute lung injury-friend or foe. Int J Mol Sci. 2023, 24, 7876. [Google Scholar] [CrossRef]
- Shen, Z.; Bei, Y.; Lin, H.; Wei, T.; Dai, Y.; Hu, Y.; Zhang, C.; Dai, H. The role of class IIa histone deacetylases in regulating endothelial function. Front Physiol. 2023, 14, 1091794. [Google Scholar] [CrossRef]
- Zhang, H.N.; Dai, Y.; Zhang, C.H.; Omondi, A.M.; Ghosh, A.; Khanra, I.; Chakraborty, M.; Yu, X.B.; Liang, J. Sirtuins family as a target in endothelial cell dysfunction: implications for vascular ageing. Biogerontology. 2020, 21, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Bahl, S.; Seto, E. Regulation of histone deacetylase activities and functions by phosphorylation and its physiological relevance. Cell Mol Life Sci. 2021, 78, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Eom, G.H.; Kook, H. Posttranslational modifications of histone deacetylases: implications for cardiovascular diseases. Pharmacol Ther. 2014, 143, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Seidel, C.; Schnekenburger, M.; Dicato, M.; Diederich, M. Histone deacetylase 6 in health and disease. Epigenomics. 2015, 7, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Iaconelli, J.; Xuan, L.; Karmacharya, R. HDAC6 modulates signaling pathways relevant to synaptic biology and neuronal differentiation in human stem-cell-derived neurons. Int J Mol Sci. 2019, 20, 1605. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Kawaguchi, Y.; Lai, C.H.; Kovacs, J.J.; Higashimoto, Y.; Appella, E.; Yao, T.P. MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. EMBO J. 2002, 21, 6236–6245. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Adhikari, N.; Amin, S.A.; Jha, T. Histone deacetylase 8 (HDAC8) and its inhibitors with selectivity to other isoforms: An overview. Eur J Med Chem. 2019, 164, 214–240. [Google Scholar] [CrossRef]
- Kumar, S.; Attrish, D.; Srivastava, A.; Banerjee, J.; Tripathi, M.; Chandra, P.S.; Dixit, A.B. Non-histone substrates of histone deacetylases as potential therapeutic targets in epilepsy. Expert Opin Ther Targets. 2021, 25, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W. The Zinc-dependent HDACs: Non-histone substrates and catalytic deacylation beyond deacetylation. Mini Rev Med Chem. 2022, 22, 2478–2485. [Google Scholar] [CrossRef]
- Dunaway, L.S.; Pollock, J.S. HDAC1: an environmental sensor regulating endothelial function. Cardiovasc Res. 2022, 118, 1885–1903. [Google Scholar] [CrossRef]
- Yao, H.; Rahman, I. Role of histone deacetylase 2 in epigenetics and cellular senescence: implications in lung inflammaging and COPD. Am J Physiol Lung Cell Mol Physiol. 2012, 303, L557–L566. [Google Scholar] [CrossRef]
- Pandey, D.; Hori, D.; Kim, J.H.; Bergman, Y.; Berkowitz, D.E.; Romer, L.H. NEDDylation promotes endothelial dysfunction: a role for HDAC2. J Mol Cell Cardiol. 2015, 81, 18–22. [Google Scholar] [CrossRef]
- Ziesché, E.; Kettner-Buhrow, D.; Weber, A.; Wittwer, T.; Jurida, L.; Soelch, J.; Müller, H.; Newel, D.; Kronich, P.; Schneider, H.; Dittrich-Breiholz, O.; Bhaskara, S.; Hiebert, S.W.; Hottiger, M.O.; Li, H.; Burstein, E.; Schmitz, M.L.; Kracht, M. The coactivator role of histone deacetylase 3 in IL-1-signaling involves deacetylation of p65 NF-κB. Nucleic Acids Res. 2013, 41, 90–109. [Google Scholar] [CrossRef] [PubMed]
- Zampetaki, A.; Zeng, L.; Margariti, A.; Xiao, Q.; Li, H.; Zhang, Z.; Pepe, A.E.; Wang, G.; Habi, O.; deFalco, E.; Cockerill, G.; Mason, J.C.; Hu, Y.; Xu, Q. Histone deacetylase 3 is critical in endothelial survival and atherosclerosis development in response to disturbed flow. Circulation. 2010, 121, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, Q.; Adrianto, I.; Wu, X.; Glassbrook, J.; Khalasawi, N.; Yin, C.; Yi, Q.; Dong, Z.; Geissmann, F.; Zhou, L.; Mi, Q.S. Histone deacetylase 3 controls lung alveolar macrophage development and homeostasis. Nat Commun. 2020, 11, 3822. [Google Scholar] [CrossRef]
- Kee, H.J.; Ryu, Y.; Seok, Y.M.; Choi, S.Y.; Sun, S.; Kim, G.R.; Jeong, M.H. Selective inhibition of histone deacetylase 8 improves vascular hypertrophy, relaxation, and inflammation in angiotensin II hypertensive mice. Clin Hypertens. 2019, 25, 13. [Google Scholar] [CrossRef]
- Hou, F.; Wei, W.; Qin, X.; Liang, J.; Han, S.; Han, A.; Kong, Q. The posttranslational modification of HDAC4 in cell biology: Mechanisms and potential targets. J Cell Biochem. 2020, 121, 930–937. [Google Scholar] [CrossRef]
- Ding, Q.; Shao, C.; Rose, P.; Zhu, Y.Z. Epigenetics and vascular senescence-potential new therapeutic targets? Front Pharmacol. 2020, 11, 535395. [Google Scholar] [CrossRef] [PubMed]
- Litke, C.; Bading, H.; Mauceri, D. Histone deacetylase 4 shapes neuronal morphology via a mechanism involving regulation of expression of vascular endothelial growth factor D. J Biol Chem. 2018, 293, 8196–8207. [Google Scholar] [CrossRef]
- Turpaev, K.T. Transcription factor KLF2 and its role in the regulation of inflammatory processes. Biochemistry (Mosc). 2020, 85, 54–67. [Google Scholar] [CrossRef]
- Margariti, A.; Zampetaki, A.; Xiao, Q.; Zhou, B.; Karamariti, E.; Martin, D.; Yin, X.; Mayr, M.; Li, H.; Zhang, Z.; De Falco, E.; Hu, Y.; Cockerill, G.; Xu, Q.; Zeng, L. Histone deacetylase 7 controls endothelial cell growth through modulation of beta-catenin. Circ Res. 2010, 106, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.; Parra, M.; Verdin, E.; Bassel-Duby, R.; Olson, E.N. Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc Natl Acad Sci U S A. 2008, 105, 7738–7743. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Young, B.D.; Li, S.; Qi, X.; Richardson, J.A.; Olson, E.N. Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell. 2006, 126, 321–334. [Google Scholar] [CrossRef]
- Gao, C.; Ho, C.C.; Reineke, E.; Lam, M.; Cheng, X.; Stanya, K.J.; Liu, Y.; Chakraborty, S.; Shih, H.M.; Kao, H.Y. Histone deacetylase 7 promotes PML sumoylation and is essential for PML nuclear body formation. Mol Cell Biol. 2008, 28, 5658–5667. [Google Scholar] [CrossRef] [PubMed]
- Turtoi, A.; Mottet, D.; Matheus, N.; Dumont, B.; Peixoto, P.; Hennequière, V.; Deroanne, C.; Colige, A.; De Pauw, E.; Bellahcène, A.; Castronovo, V. The angiogenesis suppressor gene AKAP12 is under the epigenetic control of HDAC7 in endothelial cells. Angiogenesis. 2012, 15, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Kasotakis, G.; Kintsurashvili, E.; Galvan, M.D.; Graham, C.; Purves, J.T.; Agarwal, S.; Corcoran, D.L.; Sullenger, B.A.; Palmer, S.M.; Remick, D.G. Histone deacetylase 7 inhibition in a murine model of gram-negative pneumonia-induced acute lung injury. Shock. 2020, 53, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Schiano, C.; Benincasa, G.; Franzese, M.; Della Mura, N.; Pane, K.; Salvatore, M.; Napoli, C. Epigenetic-sensitive pathways in personalized therapy of major cardiovascular diseases. Pharmacol Ther. 2020, 210, 107514. [Google Scholar] [CrossRef] [PubMed]
- Gal, J.; Chen, J.; Na, D.Y.; Tichacek, L.; Barnett, K.R.; Zhu, H. The acetylation of Lysine-376 of G3BP1 regulates RNA binding and stress granule dynamics. Mol Cell Biol. 2019, 39, e00052–19. [Google Scholar] [CrossRef] [PubMed]
- Leucker, T.M.; Nomura, Y.; Kim, J.H.; Bhatta, A.; Wang, V.; Wecker, A.; Jandu, S.; Santhanam, L.; Berkowitz, D.; Romer, L.; Pandey, D. Cystathionine γ-lyase protects vascular endothelium: a role for inhibition of histone deacetylase 6. Am J Physiol Heart Circ Physiol. 2017, 312, H711–H720. [Google Scholar] [CrossRef]
- Kaluza, D.; Kroll, J.; Gesierich, S.; Yao, T.P.; Boon, R.A.; Hergenreider, E.; Tjwa, M.; Rössig, L.; Seto, E.; Augustin, H.G.; Zeiher, A.M.; Dimmeler, S.; Urbich, C. Class IIb HDAC6 regulates endothelial cell migration and angiogenesis by deacetylation of cortactin. EMBO J. 2011, 30, 4142–4156. [Google Scholar] [CrossRef]
- Nomura, Y.; Nakano, M.; Woo Sung, H.; Han, M.; Pandey, D. Inhibition of HDAC6 Activity Protects against endothelial dysfunction and atherogenesis in vivo: A role for HDAC6 neddylation. Front Physiol. 2021, 12, 675724. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, S.H.; Choi, M.C.; Lee, J.; Oh, D.Y.; Im, S.A.; Bang, Y.J.; Kim, T.Y. Class II histone deacetylases play pivotal roles in heat shock protein 90-mediated proteasomal degradation of vascular endothelial growth factor receptors. Biochem Biophys Res Commun. 2008, 368, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Ye, D.; Zhu, S.; Jia, W.; Lu, C.; Wang, G.; Guo, X.; Yu, Y.; Wu, C.; Kang, J. HDAC10 promotes angiogenesis in endothelial cells through the PTPN22/ERK axis. Oncotarget. 2017, 8, 61338–61349. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Tan, R.; Sun, M.; Yuan, L.; Ruiz, M.; Dupuis, J.; Hu, Q.; Zhu, L. MiR-1249 on endothelial extracellular vesicles mediates cigarette smoke-induced pulmonary hypertension by inhibiting HDAC10 (Histone Deacetylase 10)-NFκB (Nuclear Factor κB)-CaSR (Calcium-Sensing Receptor) cascade. Hypertension. 2022, 79, 2721–2732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ge, J. Proteinase-activated receptor-2 modulates Ve-Cadherin expression to affect human vascular endothelial barrier function. J Cell Biochem. 2017, 118, 4587–4593. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Jin, Z.; Lv, X.; Zheng, Z.; Gao, H.; Deng, Y.; Liu, Y.; Chen, L.; Wang, W.; He, J.; Gu, J.; Lin, R. Hydroxytyrosol acetate inhibits vascular endothelial cell pyroptosis via the HDAC11 signaling pathway in atherosclerosis. Front Pharmacol. 2021, 12, 656272. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Jin, Z.; Zheng, Z.; Lv, X.; Ren, L.; Yang, J.; Chen, D.; Wang, B.; Yang, W.; Chen, L.; Wang, W.; Gu, J.; Lin, R. HDAC11 promotes both NLRP3/caspase-1/GSDMD and caspase-3/GSDME pathways causing pyroptosis via ERG in vascular endothelial cells. Cell Death Discov. 2022, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Porter, N.J.; Christianson, D.W. Structure, mechanism, and inhibition of the zinc-dependent histone deacetylases. Curr Opin Struct Biol. 2019, 59, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Shvedunova, M.; Akhtar, A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat Rev Mol Cell Biol. 2022, 23, 329–349. [Google Scholar] [CrossRef]
- Schator, D.; Gomez-Valero, L.; Buchrieser, C.; Rolando, M. Patho-epigenetics: histone deacetylases as targets of pathogens and therapeutics. Microlife. 2021, 2, uqab013. [Google Scholar] [CrossRef]
- Yoshida, M.; Kudo, N.; Kosono, S.; Ito, A. Chemical and structural biology of protein lysine deacetylases. Proc Jpn Acad Ser B Phys Biol Sci. 2017, 93, 297–321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.C.; Wang, Z.; Zhao, T.Y. The important role of histone deacetylases in modulating vascular physiology and arteriosclerosis. Atherosclerosis. 2020, 303, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ito, M.; Elliott, W.M.; Cosio, B.; Caramori, G.; Kon, O.M.; Barczyk, A.; Hayashi, S.; Adcock, I.M.; Hogg, J.C.; Barnes, P.J. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005, 352, 1967–1976. [Google Scholar] [CrossRef]
- Ito, K.; Caramori, G.; Lim, S.; Oates, T.; Chung, K.F.; Barnes, P.J.; Adcock, I.M. Expression and activity of histone deacetylases in human asthmatic airways. Am J Respir Crit Care Med. 2002, 166, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Kee, H.J.; Eom, G.H.; Joung, H.; Shin, S.; Kim, J.R.; Cho, Y.K.; Choe, N.; Sim, B.W.; Jo, D.; Jeong, M.H.; Kim, K.K.; Seo, J.S.; Kook, H. Activation of histone deacetylase 2 by inducible heat shock protein 70 in cardiac hypertrophy. Circ Res. 2008, 103, 1259–1269. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kee, H.J.; Jin, L.; Ryu, Y.; Sun, S.; Kim, G.R.; Jeong, M.H. Inhibition of class IIa histone deacetylase activity by gallic acid, sulforaphane, TMP269, and panobinostat. Biomed Pharmacother. 2018, 101, 145–154. [Google Scholar] [CrossRef]
- Yoon, S.; Eom, G.H. HDAC and HDAC inhibitor: from cancer to cardiovascular diseases. Chonnam Med J. 2016, 52, 1–11. [Google Scholar] [CrossRef]
- Ganesan. Targeting the Zinc-dependent histone deacetylases (HDACs) for drug discovery chemical epigenetics, 2020, 33. ISBN: 978-3-030-42981-2.
- Neugebauer, R.C.; Sippl, W.; Jung, M. Inhibitors of NAD+ dependent histone deacetylases (sirtuins). Curr Pharm Des. 2008, 14, 562–573. [Google Scholar] [CrossRef]
- Agarwal, R.; Pattarawat, P.; Duff, M.R. .; Wang, H.R.; Baudry, J.; Smith, J.C. Structure-based discovery of selective histone deacetylase (HDAC) 3 and 4 inhibitors. bioRxiv, 2022; 05.31.494169. [Google Scholar] [CrossRef]
- Milazzo, G.; Mercatelli, D.; Di Muzio, G.; Triboli, L.; De Rosa, P.; Perini, G.; Giorgi, F.M. Histone deacetylases (HDACs): evolution, specificity, role in transcriptional complexes, and pharmacological actionability. Genes (Basel). 2020, 11, 556. [Google Scholar] [CrossRef]
- Delcuve, G.P.; Khan, D.H.; Davie, J.R. Roles of histone deacetylases in epigenetic regulation: emerging paradigms from studies with inhibitors. Clin Epigenetics. 2012, 4, 5. [Google Scholar] [CrossRef]
- Emiliani, S.; Fischle, W.; Van Lint, C.; Al-Abed, Y.; Verdin, E. Characterization of a human RPD3 ortholog, HDAC3. Proc Natl Acad Sci USA. 1998, 95, 2795–2800. [Google Scholar] [CrossRef]
- Taunton, J.; Hassig, C.A.; Schreiber, S.L. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science. 1996, 272, 408–411. [Google Scholar] [CrossRef] [PubMed]
- de Ruijter, A.J.; van Gennip, A.H.; Caron, H.N.; Kemp, S.; van Kuilenburg, A.B. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003, 370, 737–749. [Google Scholar] [CrossRef]
- Bradley, E.W.; Carpio, L.R.; van Wijnen, A.J.; McGee-Lawrence, M.E.; Westendorf, J.J. Histone deacetylases in bone development and skeletal disorders. Physiol Rev. 2015, 95, 1359–1381. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.A.; Hagelkruys, A.; Seiser, C. Transcription and beyond: the role of mammalian class I lysine deacetylases. Chromosoma. 2014, 123, 67–78. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, J.S. A short guide to histone deacetylases including recent progress on class II enzymes. Exp Mol Med. 2020, 52, 204–212. [Google Scholar] [CrossRef]
- Bertos, N.R.; Wang, A.H.; Yang, X.J. Class II histone deacetylases: structure, function, and regulation. Biochem Cell Biol. 2001, 79, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E.; Dequiedt, F.; Kasler, H.G. Class II histone deacetylases: versatile regulators. Trends Genet. 2003, 19, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Grozinger, C.M.; Schreiber, S.L. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc Natl Acad Sci U S A. 2000, 97, 7835–7840. [Google Scholar] [CrossRef]
- Di Giorgio, E.; Brancolini, C. Regulation of class IIa HDAC activities: it is not only matter of subcellular localization. Epigenomics. 2016, 8, 251–269. [Google Scholar] [CrossRef]
- Lahm, A.; Paolini, C.; Pallaoro, M.; Nardi, M.C.; Jones, P.; Neddermann, P.; Sambucini, S.; Bottomley, M.J.; Lo Surdo, P.; Carfí, A.; Koch, U.; De Francesco, R.; Steinkühler, C.; Gallinari, P. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc Natl Acad Sci U S A. 2007, 104, 17335–17340. [Google Scholar] [CrossRef] [PubMed]
- Parra, M. Class IIa HDACs - new insights into their functions in physiology and pathology. FEBS J. 2015, 282, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Das Gupta, K.; Shakespear, M.R.; Curson, J.E.B.; Murthy, A.M.V.; Iyer, A.; Hodson, M.P.; Ramnath, D.; Tillu, V.A.; von Pein, J.B.; Reid, R.C.; Tunny, K.; Hohenhaus, D.M.; Moradi, S.V.; Kelly, G.M.; Kobayashi, T.; Gunter, J.H.; Stevenson, A.J.; Xu, W.; Luo, L.; Jones, A.; Johnston, W.A.; Blumenthal, A.; Alexandrov, K.; Collins, B.M.; Stow, J.L.; Fairlie, D.P.; Sweet, M.J. Class IIa Histone deacetylases drive toll-like receptor-inducible glycolysis and macrophage inflammatory responses via pyruvate kinase M2. Cell Rep. 2020, 30, 2712–2728.e8. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.D.; Cai, R.; Bhatia, U.; Asselbergs, F.A.; Song, C.; Terry, R.; Trogani, N.; Widmer, R.; Atadja, P.; Cohen, D. Isolation and characterization of a novel class II histone deacetylase, HDAC10. J Biol Chem. 2002, 277, 6656–6666. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, A.R.; Yao, T.P. Molecular cloning and characterization of a novel histone deacetylase HDAC10. J Biol Chem. 2002, 277, 3350–3356. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.J.; Liu, J.; Bertos, N.R.; Yang, X.J. Identification of HDAC10, a novel class II human histone deacetylase containing a leucine-rich domain. Nucleic Acids Res. 2002, 30, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Álvarez, Y.; Suelves, M. HDAC11: a multifaceted histone deacetylase with proficient fatty deacylase activity and its roles in physiological processes. FEBS J. 2022, 289, 2771–2792. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Sun, L.; Aramsangtienchai, P.; Spiegelman, N.A.; Zhang, X.; Huang, W.; Seto, E.; Lin, H. HDAC11 regulates type I interferon signaling through defatty-acylation of SHMT2. Proc Natl Acad Sci U S A. 2019, 116, 5487–5492. [Google Scholar] [CrossRef] [PubMed]
- Kee, H.J.; Kook, H. Roles and targets of class I and IIa histone deacetylases in cardiac hypertrophy. J Biomed Biotechnol. 2011, 2011, 928326. [Google Scholar] [CrossRef]
- Dzreyan, V.A.; Demyanenko, S.V. The role of post-translational protein acetylation and deacetylation in the apoptosis of neurons of the peripheral nervous system. Biochem. Moscow Suppl. Ser. A 2023, 17, 249–263. [Google Scholar] [CrossRef]
- Ali, I.; Conrad, R.J.; Verdin, E.; Ott, M. Lysine acetylation goes global: from epigenetics to metabolism and therapeutics. Chem Rev. 2018, 118, 1216–1252. [Google Scholar] [CrossRef]
- Millán-Zambrano, G.; Burton, A.; Bannister, A.J.; Schneider, R. Histone post-translational modifications - cause and consequence of genome function. Nat Rev Genet. 2022, 23, 563–580. [Google Scholar] [CrossRef]
- Chen, H.P.; Zhao, Y.T.; Zhao, T.C. Histone deacetylases and mechanisms of regulation of gene expression. Crit Rev Oncog. 2015, 20, 35–47. [Google Scholar] [CrossRef]
- King, J.; Patel, M.; Chandrasekaran, S. Metabolism, HDACs, and HDAC inhibitors: A systems biology perspective. Metabolites. 2021, 11, 792. [Google Scholar] [CrossRef]
- Chen, X.; He, Y.; Fu, W.; Sahebkar, A.; Tan, Y.; Xu, S.; Li, H. Histone deacetylases (HDACs) and atherosclerosis: a mechanistic and pharmacological review. Front Cell Dev Biol. 2020, 8, 581015. [Google Scholar] [CrossRef]
- Cross, D.; Drury, R.; Hill, J.; Pollard, A.J. Epigenetics in Sepsis: Understanding its role in endothelial dysfunction, immunosuppression, and potential therapeutics. Front Immunol. 2019, 10, 1363. [Google Scholar] [CrossRef]
- Zemskov, E.A.; Gross, C.M.; Aggarwal, S.; Zemskova, M.A.; Wu, X.; Gu, C.; Wang, T.; Tang, H.; Black, S.M. NF-κB-dependent repression of Sox18 transcription factor requires the epigenetic regulators histone deacetylases 1 and 2 in acute lung injury. Front Physiol. 2022, 13, 947537. [Google Scholar] [CrossRef]
- Kashio, T.; Shirakura, K.; Kinoshita, M.; Morita, M.; Ishiba, R.; Muraoka, K.; Kanbara, T.; Tanaka, M.; Funatsu, R.; Hino, N.; Koyama, S.; Suzuki, R.; Yoshioka, Y.; Aoshi, T.; Doi, T.; Okada, Y. HDAC inhibitor, MS-275, increases vascular permeability by suppressing Robo4 expression in endothelial cells. Tissue Barriers. 2021, 9, 1911195. [Google Scholar] [CrossRef]
- Rolando, M.; Stefani, C.; Doye, A.; Acosta, M.I.; Visvikis, O.; Yevick, H.G.; Buchrieser, C.; Mettouchi, A.; Bassereau, P.; Lemichez, E. Contractile actin cables induced by Bacillus anthracis lethal toxin depend on the histone acetylation machinery. Cytoskeleton (Hoboken). 2015, 72, 542–556. [Google Scholar] [CrossRef]
- Pandey, D.; Sikka, G.; Bergman, Y.; Kim, J.H.; Ryoo, S.; Romer, L.; Berkowitz, D. Transcriptional regulation of endothelial arginase 2 by histone deacetylase 2. Arterioscler Thromb Vasc Biol. 2014, 34, 1556–1566. [Google Scholar] [CrossRef]
- Hori, D.; Nomura, Y.; Nakano, M.; Han, M.; Bhatta, A.; Chen, K.; Akiyoshi, K.; Pandey, D. Endothelial-specific overexpression of histone deacetylase 2 protects mice against endothelial dysfunction and atherosclerosis. Cell Physiol Biochem. 2020, 54, 947–958. [Google Scholar] [CrossRef]
- Hou, Q.; Hu, K.; Liu, X.; Quan, J.; Liu, Z. HADC regulates the diabetic vascular endothelial dysfunction by targetting MnSOD. Biosci Rep. 2018, 38, BSR20181042. [Google Scholar] [CrossRef]
- Bedenbender, K.; Scheller, N.; Fischer, S.; Leiting, S.; Preissner, K.T.; Schmeck, B.T.; Vollmeister, E. Inflammation-mediated deacetylation of the ribonuclease 1 promoter via histone deacetylase 2 in endothelial cells. FASEB J. 2019, 33, 9017–9029. [Google Scholar] [CrossRef]
- Garcia, J.G.; Liu, F.; Verin, A.D.; Birukova, A.; Dechert, M.A.; Gerthoffer, W.T.; Bamberg, J.R.; English, D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001, 108, 689–701. [Google Scholar] [CrossRef]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; Spiegel, S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009, 325, 1254–1257. [Google Scholar] [CrossRef]
- Joshi, A.D.; Barabutis, N.; Birmpas, C.; Dimitropoulou, C.; Thangjam, G.; Cherian-Shaw, M.; Dennison, J.; Catravas, J.D. Histone deacetylase inhibitors prevent pulmonary endothelial hyperpermeability and acute lung injury by regulating heat shock protein 90 function. Am J Physiol Lung Cell Mol Physiol. 2015, 309, L1410–L1419. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, Z.; Zhang, F.; Huang, L.; Xing, C.; Liu, N.; Xu, Y.; Wang, X. HDAC3 inhibition prevents oxygen glucose deprivation/reoxygenation-induced transendothelial permeability by elevating PPARγ activity in vitro. J Neurochem. 2019, 149, 298–310. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, F.; Yu, Z.; Guo, S.; Liu, N.; Jiang, Y.; Lo, E.H.; Xu, Y.; Wang, X. HDAC3 inhibition prevents blood-brain barrier permeability through Nrf2 activation in type 2 diabetes male mice. J Neuroinflammation. 2019, 16, 103. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Huang, S.; Chen, G.; Sun, J.; Chen, Y.; Wang, N.; Dong, Y.; Shen, E.; Hu, Z.; Gong, W.; Jin, L.; Cong, W. Histone deacetylase 3 inhibition alleviates type 2 diabetes mellitus-induced endothelial dysfunction via Nrf2. Cell Commun Signal. 2021, 19, 35. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Sorensen, K.E.; Gooch, V.M.; Spiegelhalter, D.J.; Miller, O.I.; Sullivan, I.D.; Lloyd, J.K.; Deanfield, J.E. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992, 340, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shang, C.; Wang, B.; Wang, G.; Jin, Z.; Yao, F.; Yue, Z.; Bai, L.; Wang, R.; Zhao, S.; Liu, E.; Wang, W. HDAC3 inhibitor suppresses endothelial-to-mesenchymal transition via modulating inflammatory response in atherosclerosis. Biochem Pharmacol. 2021, 192, 114716. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, C.I.; Lin, T.E.; Lim, S.H.; Zhou, J.; Tseng, Y.C.; Chien, S.; Chiu, J.J. Role of histone deacetylases in transcription factor regulation and cell cycle modulation in endothelial cells in response to disturbed flow. Proc Natl Acad Sci U S A. 2012, 109, 1967–1972. [Google Scholar] [CrossRef] [PubMed]
- Waltregny, D.; Glénisson, W.; Tran, S.L.; North, B.J.; Verdin, E.; Colige, A.; Castronovo, V. Histone deacetylase HDAC8 associates with smooth muscle alpha-actin and is essential for smooth muscle cell contractility. FASEB J. 2005, 19, 966–968. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Cleary, R.A.; Wang, R.; Gannon, O.J.; Seto, E.; Tang, D.D. Histone deacetylase 8 regulates cortactin deacetylation and contraction in smooth muscle tissues. Am J Physiol Cell Physiol. 2014, 307, C288–C295. [Google Scholar] [CrossRef] [PubMed]
- Bandela, M.; Belvitch, P.; Garcia, J.G.N.; Dudek, S.M. Cortactin in lung cell function and disease. Int J Mol Sci. 2022, 23, 4606. [Google Scholar] [CrossRef]
- Martin, M.; Kettmann, R.; Dequiedt, F. Class IIa histone deacetylases: regulating the regulators. Oncogene. 2007, 26, 5450–5467. [Google Scholar] [CrossRef]
- Martin, M.; Kettmann, R.; Dequiedt, F. Class IIa histone deacetylases: conducting development and differentiation. Int J Dev Biol. 2009, 53, 291–301. [Google Scholar] [CrossRef]
- Kovacs-Kasa, A.; Kovacs, L.; Cherian-Shaw, M.; Patel, V.; Meadows, M.L.; Fulton, D.J.; Su, Y.; Verin, A.D. Inhibition of Class IIa HDACs improves endothelial barrier function in endotoxin-induced acute lung injury. J Cell Physiol. 2021, 236, 2893–2905. [Google Scholar] [CrossRef]
- Lobera, M.; Madauss, K.P.; Pohlhaus, D.T.; Wright, Q.G.; Trocha. M.; Schmidt, D.R.; Baloglu, E.; Trump, R.P.; Head, M.S.; Hofmann, G.A.; et al. Selective class IIa histone deacetylase inhibition via a non-chelating zinc-binding group. Nat Chem Biol. 2013, 9, 319–325. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Yuan, H.; Liu, Y.; Wen, X. Effects of dynamic changes in histone acetylation and deacetylase activity on pulmonary fibrosis. Int Immunopharmacol. 2017, 52, 272–280. [Google Scholar] [CrossRef]
- Yang, D.; Xiao, C.; Long, F.; Su, Z.; Jia, W.; Qin, M.; Huang, M.; Wu, W.; Suguro, R.; Liu, X.; Zhu, Y. HDAC4 regulates vascular inflammation via activation of autophagy. Cardiovasc Res. 2018, 114, 1016–1028. [Google Scholar] [CrossRef]
- Chen, M.; Cheng, H.; Chen, X.; Gu, J.; Su, W.; Cai, G.; Yan, Y.; Wang, C.; Xia, X.; Zhang, K.; Zhang, M.; Jiang, H.; Chen, Y.; Yao, L. The activation of histone deacetylases 4 prevented endothelial dysfunction: A crucial mechanism of HuangqiGuizhiWuwu Decoction in improving microcirculation dysfunction in diabetes. J Ethnopharmacol. 2023, 307, 116240. [Google Scholar] [CrossRef]
- Liu, Z.M.; Wang, X.; Li, C.X.; Liu, X.Y.; Guo, X.J.; Li, Y.; Chen, Y.L.; Ye, H.X.; Chen, H.S. SP1 promotes HDAC4 expression and inhibits HMGB1 expression to reduce intestinal barrier dysfunction, oxidative stress, and inflammatory response after sepsis. J Innate Immun. 2022, 14, 366–379. [Google Scholar] [CrossRef]
- Hu, K.; Huang, M.J.; Ling, S.; Li, Y.X.; Cao, X.Y.; Chen, Y.F.; Lei, J.M.; Fu, W.Z.; Tan, B.F. LncRNA CASC11 upregulation promotes HDAC4 to alleviate oxidized low-density lipoprotein-induced injury of cardiac microvascular endothelial cells. Kaohsiung J Med Sci. 2023. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, X.; Li, Q.; Zhou, S.M.; Hu, B.; Hu, G.W.; Niu, X.; Guo, S.C.; Wang, Y.; Deng, Z.F. Role of phosphorylated HDAC4 in stroke-induced angiogenesis. Biomed Res Int. 2017, 2017, 2957538. [Google Scholar] [CrossRef]
- Schader, T.; Löwe, O.; Reschke, C.; Malacarne, P.; Hahner, F.; Müller, N.; Gajos-Draus, A.; Backs, J.; Schröder, K. Oxidation of HDAC4 by Nox4-derived H2O2 maintains tube formation by endothelial cells. Redox Biol. 2020, 36, 101669. [Google Scholar] [CrossRef]
- Hrgovic, I.; Doll, M.; Pinter, A.; Kaufmann, R.; Kippenberger, S.; Meissner, M. Histone deacetylase inhibitors interfere with angiogenesis by decreasing endothelial VEGFR-2 protein half-life in part via a VE-cadherin-dependent mechanism. Exp Dermatol. 2017, 26, 194–201. [Google Scholar] [CrossRef]
- Zecchin, A.; Pattarini, L.; Gutierrez, M.I.; Mano, M.; Mai, A.; Valente, S.; Myers, M.P.; Pantano, S.; Giacca, M. Reversible acetylation regulates vascular endothelial growth factor receptor-2 activity. J Mol Cell Biol. 2014, 6, 116–127. [Google Scholar] [CrossRef]
- Urbich, C.; Rössig, L.; Kaluza, D.; Potente, M.; Boeckel, J.N.; Knau, A.; Diehl, F.; Geng, J.G.; Hofmann, W.K.; Zeiher, A.M.; Dimmeler, S. HDAC5 is a repressor of angiogenesis and determines the angiogenic gene expression pattern of endothelial cells. Blood. 2009, 113, 5669–5679. [Google Scholar] [CrossRef]
- Tsou, P.S.; Wren, J.D.; Amin, M.A.; Schiopu, E.; Fox, D.A.; Khanna, D.; Sawalha, A.H. Histone deacetylase 5 is overexpressed in scleroderma endothelial cells and impairs Angiogenesis via repression of proangiogenic factors. Arthritis Rheumatol. 2016, 68, 2975–2985. [Google Scholar] [CrossRef]
- Jiang, Z.; Tan, J.; Yuan, Y.; Shen, J.; Chen, Y. Semaglutide ameliorates lipopolysaccharide-induced acute lung injury through inhibiting HDAC5-mediated activation of NF-κB signaling pathway. Hum Exp Toxicol. 2022, 41, 9603271221125931. [Google Scholar] [CrossRef]
- Wang, Y.; Abrol, R.; Mak, J.Y.W.; Das Gupta, K.; Ramnath, D.; Karunakaran, D.; Fairlie, D.P.; Sweet, M.J. Histone deacetylase 7: a signalling hub controlling development, inflammation, metabolism and disease. FEBS J. 2023, 290, 2805–2832. [Google Scholar] [CrossRef]
- Hsu, A.; Duan, Q.; McMahon, S.; Huang, Y.; Wood, S.A.; Gray, N.S.; Wang, B.; Bruneau, B.G.; Haldar, S.M. Salt-inducible kinase 1 maintains HDAC7 stability to promote pathologic cardiac remodeling. J Clin Invest. 2020, 130, 2966–2977. [Google Scholar] [CrossRef]
- Su, Y.T.; Gao, C.; Liu, Y.; Guo, S.; Wang, A.; Wang, B.; Erdjument-Bromage, H.; Miyagi, M.; Tempst, P.; Kao, H.Y. Monoubiquitination of filamin B regulates vascular endothelial growth factor-mediated trafficking of histone deacetylase 7. Mol Cell Biol. 2013, 33, 1546–1560. [Google Scholar] [CrossRef]
- Yang, J.; Moraga, A.; Xu, J.; Zhao, Y.; Luo, P.; Lao, K.H.; Margariti, A.; Zhao, Q.; Ding, W.; Wang, G.; Zhang, M.; Zheng, L.; Zhang, Z.; Hu, Y.; Wang, W.; Shen, L.; Smith, A.; Shah, A.M.; Wang, Q.; Zeng, L. A histone deacetylase 7-derived peptide promotes vascular regeneration via facilitating 14-3-3γ phosphorylation. Stem Cells. 2020, 38, 556–573. [Google Scholar] [CrossRef]
- Martin, M.; Geudens, I.; Bruyr, J.; Potente, M.; Bleuart, A.; Lebrun, M.; Simonis, N.; Deroanne, C.; Twizere, J.C.; Soubeyran, P.; Peixoto, P.; Mottet, D.; Janssens, V.; Hofmann, W.K.; Claes, F.; Carmeliet, P.; Kettmann, R.; Gerhardt, H.; Dequiedt, F. PP2A regulatory subunit Bα controls endothelial contractility and vessel lumen integrity via regulation of HDAC7. EMBO J. 2013, 32, 2491–503. [Google Scholar] [CrossRef]
- Parra, M.; Mahmoudi, T.; Verdin, E. Myosin phosphatase dephosphorylates HDAC7, controls its nucleocytoplasmic shuttling, and inhibits apoptosis in thymocytes. Genes Dev. 2007, 21, 638–643. [Google Scholar] [CrossRef]
- Kovacs-Kasa, A.; Gorshkov, B.A.; Kim, K.M.; Kumar, S.; Black, S.M.; Fulton, D.J.; Dimitropoulou, C.; Catravas, J.D.; Verin, A.D. The protective role of MLCP-mediated ERM dephosphorylation in endotoxin-induced lung injury in vitro and in vivo. Sci Rep. 2016, 6, 39018. [Google Scholar] [CrossRef]
- Kim, K.M.; Csortos, C.; Czikora, I.; Fulton, D.; Umapathy, N.S.; Olah, G.; Verin, A.D. Molecular characterization of myosin phosphatase in endothelium. J Cell Physiol. 2012, 227, 1701–1708. [Google Scholar] [CrossRef]
- Guo, K.; Ma, Z.; Zhang, Y.; Han, L.; Shao, C.; Feng, Y.; Gao, F.; Di, S.; Zhang, Z.; Zhang, J.; Tabbò, F.; Ekman, S.; Suda, K.; Cappuzzo, F.; Han, J.; Li, X.; Yan, X. HDAC7 promotes NSCLC proliferation and metastasis via stabilization by deubiquitinase USP10 and activation of β-catenin-FGF18 pathway. J Exp Clin Cancer Res. 2022, 41, 91. [Google Scholar] [CrossRef]
- Shi, W.; Wei, X.; Wang, Z.; Han, H.; Fu, Y.; Liu, J.; Zhang, Y.; Guo, J.; Dong, C.; Zhou, D.; Zhou, Q.; Chen, Y.; Yi, F. HDAC9 exacerbates endothelial injury in cerebral ischaemia/reperfusion injury. J Cell Mol Med. 2016, 20, 1139–1149. [Google Scholar] [CrossRef]
- Lu, S.; Li, H.; Li, K.; Fan, X.D. HDAC9 promotes brain ischemic injury by provoking IκBα/NF-κB and MAPKs signaling pathways. Biochem Biophys Res Commun. 2018, 503, 1322–1329. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Cheng, Q.; Wu, Y. Aberrant expression and regulatory role of histone deacetylase 9 in vascular endothelial cell injury in intracranial aneurysm. Biomol Biomed. 2023. [Google Scholar] [CrossRef]
- Brancolini, C.; Di Giorgio, E.; Formisano, L.; Gagliano, T. Quis custodiet ipsos custodes (who controls the controllers)? two decades of studies on HDAC9. Life (Basel). 2021, 11, 90. [Google Scholar] [CrossRef]
- Joo, E.E.; Yamada, K.M. Post-polymerization crosstalk between the actin cytoskeleton and microtubule network. Bioarchitecture. 2016, 6, 53–59. [Google Scholar] [CrossRef]
- Valenzuela-Fernández, A.; Cabrero, J.R.; Serrador, J.M.; Sánchez-Madrid, F. HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol. 2008, 18, 291–297. [Google Scholar] [CrossRef]
- Karki, P.; Birukova, A.A. Microtubules as major regulators of endothelial function: Implication for lung injury. Front Physiol. 2021, 12, 758313. [Google Scholar] [CrossRef]
- Yu, J.; Ma, M.; Ma, Z.; Fu, J. HDAC6 inhibition prevents TNF-α-induced caspase 3 activation in lung endothelial cell and maintains cell-cell junctions. Oncotarget. 2016, 7, 54714–54722. [Google Scholar] [CrossRef]
- Smith, Q.; Macklin, B.; Chan, X.Y.; Jones, H.; Trempel, M.; Yoder, M.C.; Gerecht, S. Differential HDAC6 activity modulates ciliogenesis and subsequent mechanosensing of endothelial cells derived from pluripotent stem cells. Cell Rep. 2018, 24, 895–908.e6. [Google Scholar] [CrossRef]
- Gao, Y.S.; Hubbert, C.C.; Lu, J.; Lee, Y.S.; Lee, J.Y.; Yao, T.P. Histone deacetylase 6 regulates growth factor-induced actin remodeling and endocytosis. Mol Cell Biol. 2007, 27, 8637–8647. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, Z.; Zhang, Y.; Yong, S.; Salas-Burgos, A.; Koomen, J.; Olashaw, N.; Koomen, J.; Olashaw, N.; Parsons, J.T.; Yang, X.J.; Dent, S.R.; Yao, T.P.; Lane, W.S.; Seto, E. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell. 2007, 27, 197–213. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Caron, C.; Matthias, G.; Hess, D.; Khochbin, S.; Matthias, P. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 2003, 22, 1168–1179. [Google Scholar] [CrossRef]
- Yu, J.; Ma, Z.; Shetty, S.; Ma, M.; Fu, J. Selective HDAC6 inhibition prevents TNF-α-induced lung endothelial cell barrier disruption and endotoxin-induced pulmonary edema. Am J Physiol Lung Cell Mol Physiol. 2016, 311, L39–L47. [Google Scholar] [CrossRef]
- Zilberman, Y.; Ballestrem, C.; Carramusa, L.; Mazitschek, R.; Khochbin, S.; Bershadsky, A. Regulation of microtubule dynamics by inhibition of the tubulin deacetylase HDAC6. J Cell Sci. 2009, 122, 3531–3541. [Google Scholar] [CrossRef]
- Li, D.; Xie, S.; Ren, Y.; Huo, L.; Gao, J.; Cui, D.; Liu, M.; Zhou, J. Microtubule-associated deacetylase HDAC6 promotes angiogenesis by regulating cell migration in an EB1-dependent manner. Protein Cell. 2011, 2, 150–160. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Zhang, W.J.; Chang, S. HDAC6 inhibition: a significant potential regulator and therapeutic option to translate into clinical practice in renal transplantation. Front Immunol. 2023, 14, 1168848. [Google Scholar] [CrossRef]
- Saito, S.; Lasky, J.A.; Guo, W.; Nguyen, H.; Mai, A.; Danchuk, S.; Sullivan, D.E.; Shan, B. Pharmacological inhibition of HDAC6 attenuates endothelial barrier dysfunction induced by thrombin. Biochem Biophys Res Commun. 2011, 408, 630–634. [Google Scholar] [CrossRef]
- Gorshkov, B.A.; Zemskova, M.A.; Verin, A.D.; Bogatcheva, N.V. Taxol alleviates 2-methoxyestradiol-induced endothelial permeability. Vascul Pharmacol. 2012, 56, 56–63. [Google Scholar] [CrossRef]
- Borgas, D.; Chambers, E.; Newton, J.; Ko, J.; Rivera, S.; Rounds, S.; Lu, Q. Cigarette smoke disrupted lung endothelial barrier integrity and increased susceptibility to acute lung injury via histone deacetylase 6. Am J Respir Cell Mol Biol. 2016, 54, 683–696. [Google Scholar] [CrossRef]
- Karki, P.; Ke, Y.; Tian, Y.; Ohmura, T.; Sitikov, A.; Sarich, N.; Montgomery, C.P.; Birukova, A.A. Staphylococcus aureus-induced endothelial permeability and inflammation are mediated by microtubule destabilization. J Biol Chem. 2019, 294, 3369–3384. [Google Scholar] [CrossRef]
- Kuhlmann, N.; Wroblowski, S.; Knyphausen, P.; de Boor, S.; Brenig, J.; Zienert, A.Y.; Meyer-Teschendorf, K.; Praefcke, G.J.K.; Nolte, H.; Krüger, M.; Schacherl, M.; Baumann, U.; James, L.C.; Chin, J.W.; Lammers, M. Structural and mechanistic insights into the regulation of the fundamental rho regulator RhoGDIα by lysine acetylation. J Biol Chem. 2016, 291, 5484–5499. [Google Scholar] [CrossRef]
- Menden, H.; Xia, S.; Mabry, S.M.; Noel-MacDonnell, J.; Rajasingh, J.; Ye, S.Q.; Sampath, V. Histone deacetylase 6 regulates endothelial MyD88-dependent canonical TLR signaling, lung inflammation, and alveolar remodeling in the developing lung. Am J Physiol Lung Cell Mol Physiol. 2019, 317, L332–L346. [Google Scholar] [CrossRef]
- Zhang, H.P.; Wang, L.; Fu, J.J.; Fan, T.; Wang, Z.L.; Wang, G. Association between histone hyperacetylation status in memory T lymphocytes and allergen-induced eosinophilic airway inflammation. Respirology. 2016, 21, 850–857. [Google Scholar] [CrossRef]
- Liao, W.; Sun, J.; Liu, W.; Li, W.; Jia, J.; Ou, F.; Su, K.; Zheng, Y.; Zhang, Z.; Sun, Y. HDAC10 upregulation contributes to interleukin 1β-mediated inflammatory activation of synovium-derived mesenchymal stem cells in temporomandibular joint. J Cell Physiol. 2019, 234, 12646–12662. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, S.; Zhang, L.; Xiao, H.; Gan, H.; Chen, H.; Zhai, X.; Liang, P.; Zhao, J.; Li, Y. Histone deacetylation 10 alleviates inflammation after intracerebral hemorrhage via the PTPN22/NLRP3 pathway in rats. Neuroscience. 2020, 432, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Shi, H.; Zhang, D.; Wang, C.; Zhao, F.; Li, L.; Xu, Z.; Jiang, J.; Li, J. Nebulized inhalation of LPAE-HDAC10 inhibits acetylation-mediated ROS/NF-κB pathway for silicosis treatment. J Control Release. 2023, 364, 618–631. [Google Scholar] [CrossRef]
- Gao, L.; Cueto, M.A.; Asselbergs, F.; Atadja, P. Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. J Biol Chem. 2002, 277, 25748–25755. [Google Scholar] [CrossRef]
- Lu, D.; Ma, Z.; Huang, D.; Zhang, J.; Li, J.; Zhi, P.; Zhang, L.; Feng, Y.; Ge, X.; Zhai, J.; Jiang, M.; Zhou, X.; Simone, C.B. 2nd.; Neal, J.W.; Patel, S.R.; Yan, X.; Hu, Y.; Wang, J. Clinicopathological characteristics and prognostic significance of HDAC11 protein expression in non-small cell lung cancer: a retrospective study. Transl Lung Cancer Res. 2022, 11, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xie, C.; Chen, Q.; Zhuang, S. HDAC11, an emerging therapeutic target for metabolic disorders. Front Endocrinol (Lausanne). 2022, 13, 989305. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Csak, T.; Kodys, K.; Catalano, D.; Ambade, A.; Furi, I.; Lowe, P.; Cho, Y.; Iracheta-Vellve, A.; Szabo, G. Alcohol-induced miR-155 and HDAC11 inhibit negative regulators of the TLR4 pathway and lead to increased LPS responsiveness of Kupffer cells in alcoholic liver disease. J Leukoc Biol. 2017, 102, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhong, M.; Qi, Z.; Shen, F.; Zhao, Q.; Wu, L.; Huang, Y.; Tsang, S.Y.; Yao, X. Histone deacetylase inhibitors relax mouse aorta partly through their inhibitory action on L-type Ca2+ channels. J Pharmacol Exp Ther. 2017, 363, 211–220. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Liu, B.; Geng, B.; Li, N.; Geng, Q. The role of HDAC3 and its inhibitors in regulation of oxidative stress and chronic diseases. Cell Death Discov. 2023, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Reichert, N.; Choukrallah, M.A.; Matthias, P. Multiple roles of class I HDACs in proliferation, differentiation, and development. Cell Mol Life Sci. 2012, 69, 2173–2187. [Google Scholar] [CrossRef] [PubMed]
- Telles, E.; Seto, E. Modulation of cell cycle regulators by HDACs. Front Biosci (Schol Ed). 2012, 4, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, M.J.; Tangutur, A.D.; Manyam, R.R. Epigenetic modulation and understanding of HDAC inhibitors in cancer therapy. Life Sci. 2021, 277, 119504. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Shu, B.; Zhang, Y.; Wang, M. Endothelial response to pathophysiological stress. arterioscler thromb vasc biol. 2019, 39, e233–e243. [Google Scholar] [CrossRef]
- Dzobo, K.E.; Hanford, K.M.L.; Kroon, J. Vascular metabolism as driver of Atherosclerosis: Linking endothelial metabolism to inflammation. Immunometabolism. 2021, 3, e210020. [Google Scholar] [CrossRef]
- Cheng, H.T.; Hung, W.C. Inhibition of proliferation, sprouting, tube formation and Tie2 signaling of lymphatic endothelial cells by the histone deacetylase inhibitor SAHA. Oncol Rep. 2013, 30, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Sun, M.; Ramchandran, R.; Raj, J.U. IGF-1 signaling in neonatal hypoxia-induced pulmonary hypertension: Role of epigenetic regulation. Vascul Pharmacol. 2015, 73, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, Y.; Loberg, A.; Tahara, S.M.; Malik, P.; Kalra, V.K. activated transcription factor 3 in association with histone deacetylase 6 negatively regulates microRNA 199a2 transcription by chromatin remodeling and reduces endothelin-1 expression. Mol Cell Biol. 2016, 36, 2838–2854. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, G.; Su, F.; Cai, Y.; Shi, L.; Meng, Y.; Liu, Z.; Sun, J.; Wang, M.; Qian, M.; Wang, Z.; Xu, X.; Cheng, Y.X.; Zhu, W.G.; Liu, B. HDAC8 cooperates with SMAD3/4 complex to suppress SIRT7 and promote cell survival and migration. Nucleic Acids Res. 2020, 48, 2912–2923. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.F.; Lu, J.Y.; Zhang, Y.J.; Zhang, L.X.; Lu, G.D.; Xie, Z.J.; Cheng, M.B.; Shen, Y.F.; Zhang, Y. C/EBPα negatively regulates SIRT7 expression via recruiting HDAC3 to the upstream-promoter of hepatocellular carcinoma cells. Biochim Biophys Acta. 2016, 1859, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Kaur, P.; Singh, P.; Singh, S.; Munshi, A. Differential molecular mechanistic behavior of HDACs in cancer progression. Med Oncol. 2022, 39, 171. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Gustafsson, A.B. Role of apoptosis in cardiovascular disease. Apoptosis. 2009, 14, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Tricot, O.; Mallat, Z.; Heymes, C.; Belmin, J.; Lesèche, G.; Tedgui, A. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation. 2000, 101, 2450–2453. [Google Scholar] [CrossRef] [PubMed]
- Bombeli, T.; Schwartz, B.R.; Harlan, J.M. Endothelial cells undergoing apoptosis become proadhesive for nonactivated platelets. Blood. 1999, 93, 3831–3838. [Google Scholar] [CrossRef] [PubMed]
- Cancel, L.M.; Tarbell, J.M. The role of apoptosis in LDL transport through cultured endothelial cell monolayers. Atherosclerosis. 2010, 208, 335–341. [Google Scholar] [CrossRef]
- Tajadura, V.; Hansen, M.H.; Smith, J.; Charles, H.; Rickman, M.; Farrell-Dillon, K.; Claro, V.; Warboys, C.; Ferro, A. β-catenin promotes endothelial survival by regulating eNOS activity and flow-dependent anti-apoptotic gene expression. Cell Death Dis. 2020, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Peng, K.; Wu, Q.; Wang, Y.; Fan, X.; Zhang, D.M.; Passerini, A.G.; Sun, C. HDAC1 and 2 regulate endothelial VCAM-1 expression and atherogenesis by suppressing methylation of the GATA6 promoter. Theranostics. 2021, 11, 5605–5619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, N.; Du, J.; Zhang, H.; Zhang, C. MicroRNA-200b-3p promotes endothelial cell apoptosis by targeting HDAC4 in atherosclerosis. BMC Cardiovasc Disord. 2021, 21, 172. [Google Scholar] [CrossRef] [PubMed]
- Shakespear, M.R.; Halili, M.A.; Irvine, K.M.; Fairlie, D.P.; Sweet, M.J. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 2011, 32, 335–343. [Google Scholar] [CrossRef]
- Dai, Y.; Wei, T.; Shen, Z.; Bei, Y.; Lin, H.; Dai, H. Classical HDACs in the regulation of neuroinflammation. Neurochem Int. 2021, 150, 105182. [Google Scholar] [CrossRef]
- Rajendrasozhan, S.; Yang, S.R.; Edirisinghe, I.; Yao, H.; Adenuga, D.; Rahman, I. Deacetylases and NF-kappaB in redox regulation of cigarette smoke-induced lung inflammation: epigenetics in pathogenesis of COPD. Antioxid Redox Signal. 2008, 10, 799–811. [Google Scholar] [CrossRef]
- Hu, Y.; Suliman, B.A. Roles of HDACs in the responses of innate immune cells and as targets in inflammatory diseases. Adv Exp Med Biol. 2017, 1024, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Gatla, H.R.; Muniraj, N.; Thevkar, P.; Yavvari, S.; Sukhavasi, S.; Makena, M.R. Regulation of chemokines and cytokines by histone deacetylases and an update on histone decetylase inhibitors in human diseases. Int J Mol Sci. 2019, 20, 1110. [Google Scholar] [CrossRef]
- Luan, Y.; Liu, H.; Luan, Y.; Yang, Y.; Yang, J.; Ren, K.D. New insight in HDACs: Potential therapeutic targets for the treatment of Atherosclerosis. Front Pharmacol. 2022, 13, 863677. [Google Scholar] [CrossRef]
- Kulthinee, S.; Yano, N.; Zhuang, S.; Wang, L.; Zhao, T.C. Critical functions of histone deacetylases (HDACs) in modulating inflammation associated with cardiovascular diseases. Pathophysiology. 2022, 29, 471–485. [Google Scholar] [CrossRef]
- Lucas, R.; Verin, A.D.; Black, S.M.; Catravas, J.D. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem Pharmacol. 2009, 77, 1763–1772. [Google Scholar] [CrossRef]
- He, M.; Zhang, B.; Wei, X.; Wang, Z.; Fan, B.; Du, P.; Zhang, Y.; Jian, W.; Chen, L.; Wang, L.; Fang, H.; Li, X.; Wang, P.A.; Yi, F. HDAC4/5-HMGB1 signalling mediated by NADPH oxidase activity contributes to cerebral ischaemia/reperfusion injury. J Cell Mol Med. 2013, 17, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, Z.; Liu, J. Role of HDACs in normal and malignant hematopoiesis. Mol Cancer. 2020, 19, 5, Erratum in: Mol Cancer. 2020, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Bertrand, P. Inside HDACs with more selective HDAC inhibitors. Eur J Med Chem. 2016, 121, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Bieliauskas, A.V.; Pflum, M.K. Isoform-selective histone deacetylase inhibitors. Chem Soc Rev. 2008, 37, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, F.; Chen, X.; Wang, J.; Zhao, Y.; Li, Y.; He, B. Zinc-dependent deacetylase (HDAC) inhibitors with different zinc binding groups. Curr Top Med Chem. 2019, 19, 223–241. [Google Scholar] [CrossRef]
- Li, Y.; Lin, S.; Gu, Z.; Chen, L.; He, B. Zinc-dependent deacetylases (HDACs) as potential targets for treating Alzheimer's disease. Bioorg Med Chem Lett. 2022, 76, 129015. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Wang, K.; Zhao, Y.; Yuan, S.; He, Z.; Zhang, J. Targeting histone deacetylases for heart diseases. Bioorg Chem. 2023, 138, 106601. [Google Scholar] [CrossRef] [PubMed]
- von Knethen, A.; Brüne, B. Histone deacetylation inhibitors as therapy concept in sepsis. Int J Mol Sci. 2019, 20, 346. [Google Scholar] [CrossRef]
- Shanmugam, G.; Rakshit, S.; Sarkar, K. HDAC inhibitors: Targets for tumor therapy, immune modulation and lung diseases. Transl Oncol. 2022, 16, 101312. [Google Scholar] [CrossRef]
- Wu, S.Y.; Tang, S.E.; Ko, F.C.; Wu, G.C.; Huang, K.L.; Chu, S.J. Valproic acid attenuates acute lung injury induced by ischemia-reperfusion in rats. Anesthesiology. 2015, 122, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Kasotakis, G.; Galvan, M.D.; Osathanugrah, P.; Dharia, N.; Bufe, L.; Breed, Z.; Mizgerd, J.P.; Remick, D.G. Timing of valproic acid in acute lung injury: prevention is the best therapy? J Surg Res. 2017, 220, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Kasotakis, G.; Galvan, M.; King, E.; Sarkar, B.; Stucchi, A.; Mizgerd, J.P.; Burke, P.A.; Remick, D. Valproic acid mitigates the inflammatory response and prevents acute respiratory distress syndrome in a murine model of Escherichia coli pneumonia at the expense of bacterial clearance. J Trauma Acute Care Surg. 2017, 82, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Cheng, B.; Yang, L.; Li, G.; Yuan, Y.; Luo, G.; Shu, Z.; Jiang, H. LncRNA ZEB1-AS1 knockdown alleviates oxidative low-density lipoprotein-induced endothelial cell injury via the miR-590-5p/HDAC9 axis. Cent Eur J Immunol. 2021, 46, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.; Ulfhammer, E.; Magnusson, M.; Bergh, N.; Lunke, S.; El-Osta, A.; Medcalf, R.L.; Svensson, P.A.; Karlsson, L.; Jern, S. Role of histone acetylation in the stimulatory effect of valproic acid on vascular endothelial tissue-type plasminogen activator expression. PLoS One. 2012, 7, e31573. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Okada, M.; Mizuno, W.; Oda, M.; Ide, N.; Morita, T.; Hara, Y.; Yamawaki, H. HDAC4 mediates development of hypertension via vascular inflammation in spontaneous hypertensive rats. Am J Physiol Heart Circ Physiol. 2012, 302, H1894–904. [Google Scholar] [CrossRef] [PubMed]
- Granger, A.; Abdullah, I.; Huebner, F.; Stout, A.; Wang, T.; Huebner, T.; Epstein, J.A.; Gruber, P.J. Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J. 2008, 22, 3549–3560. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, J.; Malik, A.B.; Elias, H.K.; Rajasingh, S.; Simpson, A.D.; Sundivakkam, P.K.; Vogel, S.M.; Xuan, Y.T.; Dawn, B.; Rajasingh, J. Combinatorial therapy with acetylation and methylation modifiers attenuates lung vascular hyperpermeability in endotoxemia-induced mouse inflammatory lung injury. Am J Pathol. 2014, 184, 2237–2249. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, J.; Samanta, S.; Rajasingh, S.; Barani, B.; Xuan, Y.T.; Dawn, B.; Rajasingh, J. Epigenetic modifiers reduce inflammation and modulate macrophage phenotype during endotoxemia-induced acute lung injury. J Cell Sci. 2015, 128, 3094–3105. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.F.; Jiang, X.H.; Liu, H.; He, L.Y.; Luo, X.; Chen, F.C.; Tan, Y.L. miR-23b attenuates LPS-induced inflammatory responses in acute lung injury via inhibition of HDAC2. Biochem Genet. 2021, 59, 604–616. [Google Scholar] [CrossRef]
- Asare, Y.; Campbell-James, T.A.; Bokov, Y.; Yu, L.L.; Prestel, M.; El Bounkari, O.; Roth, S.; El Bounkari, O.; Roth, S.; Megens, R.T.A.; et al. Histone deacetylase 9 activates IKK to regulate atherosclerotic plaque vulnerability. Circ Res. 2020, 127, 811–823. [Google Scholar] [CrossRef]
- Illi, B.; Dello Russo, C.; Colussi, C.; Rosati, J.; Pallaoro, M.; Spallotta, F.; Rotili, D.; Spallotta, F.; Rotili, D.; Valente, S.; et al. Nitric oxide modulates chromatin folding in human endothelial cells via protein phosphatase 2A activation and class II histone deacetylases nuclear shuttling. Circ Res. 2008, 102, 51–58. [Google Scholar] [CrossRef]
- Isaacs, J.T.; Antony, L.; Dalrymple, S.L.; Brennen, W.N.; Gerber, S.; Hammers, H.; Wissing, M.; Kachhap, S.; Luo, J.; Xing, L.; Björk, P.; Olsson, A.; Björk, A.; Leanderson, T. Tasquinimod is an allosteric modulator of HDAC4 survival signaling within the compromised cancer microenvironment. Cancer Res. 2013, 73, 1386–1399. [Google Scholar] [CrossRef]
- Shen, F.; Hou, X.; Li, T.; Yu, J.; Chen, H.; Liu, N.; Qiu, A.; Zhuang, S. Pharmacological and genetic inhibition of HDAC4 alleviates renal injury and fibrosis in mice. Front Pharmacol. 2022, 13, 929334. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, X.; Shetty, S.; Hou, G.; Wang, Q.; Fu, J. HDAC6 inhibition blocks inflammatory signaling and caspase-1 activation in LPS-induced acute lung injury. Toxicol Appl Pharmacol. 2019, 370, 178–183. [Google Scholar] [CrossRef]
- Chi, Z.; Byeon, H.E.; Seo, E.; Nguyen, Q.T.; Lee, W.; Jeong, Y.; Choi, J.; Pandey, D.; Berkowitz, D.E.; Kim, J.H.; Lee, S.Y. Histone deacetylase 6 inhibitor tubastatin A attenuates angiotensin II-induced hypertension by preventing cystathionine γ-lyase protein degradation. Pharmacol Res. 2019, 146, 104281. [Google Scholar] [CrossRef]
- Zhou, B.; Zeng, S.; Li, N.; Yu, L.; Yang, G.; Yang, Y.; Zhang, X.; Fang, M.; Xia, J.; Xu, Y. Angiogenic factor with G patch and FHA domains 1 is a novel regulator of vascular injury. Arterioscler Thromb Vasc Biol. 2017, 37, 675–684. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, C.C.; Han, B.; Andrade, C.F.; Bai, X.; Uhlig, S.; Hubmayr, R.; Tsang, M.; Lodyga, M.; Keshavjee, S.; Slutsky, A.S.; Liu, M. DNA microarray analysis of gene expression in alveolar epithelial cells in response to TNF-alpha, LPS, and cyclic stretch. Physiol Genomics. 2004, 19, 331–342. [Google Scholar] [CrossRef]
- dos Santos, C.C.; Okutani, D.; Hu, P.; Han, B.; Crimi, E.; He, X.; Keshavjee, S.; Greenwood, C.; Slutsky, A.S.; Zhang, H.; Liu, M. Differential gene profiling in acute lung injury identifies injury-specific gene expression. Crit Care Med. 2008, 36, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Lynn, H.; Sun, X.; Casanova, N.; Gonzales-Garay, M.; Bime, C.; Garcia, J.G.N. Genomic and genetic approaches to deciphering acute respiratory distress syndrome risk and mortality. Antioxid Redox Signal. 2019, 31, 1027–1052. [Google Scholar] [CrossRef]
- Göttlicher, M.; Minucci, S.; Zhu, P.; Krämer, O.H.; Schimpf, A.; Giavara, S.; Sleeman, J.P.; Lo Coco, F.; Nervi, C.; Pelicci, P.G.; Heinzel, T. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001, 20, 6969–6978. [Google Scholar] [CrossRef] [PubMed]
- Sztajnkrycer, M.D. Valproic acid toxicity: overview and management. J Toxicol Clin Toxicol. 2002, 40, 789–801. [Google Scholar] [CrossRef]
- Verin, A.D. Letter to the Editor: "Histone deacetylase 7 inhibition in a murine model of Gram-negative Pneumonia-induced acute lung injury”. Shock. 53, 344-351, 2020. Shock. 2020, 53, 375. [Google Scholar] [CrossRef]
- Li, L.F.; Lee, C.S.; Lin, C.W.; Chen, N.H.; Chuang, L.P.; Hung, C.Y.; Liu, Y.Y. Trichostatin A attenuates ventilation-augmented epithelial-mesenchymal transition in mice with bleomycin-induced acute lung injury by suppressing the Akt pathway. PLoS One. 2017, 12, e0172571. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Li, L.; Fu, Z.J. Histone deacetylase inhibitors trichostatin A and suberoylanilide hydroxamic acid attenuate ventilator-induced lung injury. Pharmazie. 2014, 69, 55–59. [Google Scholar] [CrossRef]
- Ni, Y.F.; Wang, J.; Yan, X.L.; Tian, F.; Zhao, J.B.; Wang, Y.J.; Jiang, T. Histone deacetylase inhibitor, butyrate, attenuates lipopolysaccharide-induced acute lung injury in mice. Respir Res. 2010, 11, 33. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, S.; Wang, C.; Jiang, R.; Wan, J. Histone deacetylase inhibitors attenuate acute lung injury during cecal ligation and puncture-induced polymicrobial sepsis. World J Surg. 2010, 34, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Pooladanda, V.; Thatikonda, S.; Bale, S.; Pattnaik, B.; Sigalapalli, D.K.; Bathini, N.B.; Singh, S.B.; Godugu, C. Nimbolide protects against endotoxin-induced acute respiratory distress syndrome by inhibiting TNF-α mediated NF-κB and HDAC-3 nuclear translocation. Cell Death Dis. 2019, 10, 81. [Google Scholar] [CrossRef]
- Leus, N.G.; van der Wouden, P.E.; van den Bosch, T.; Hooghiemstra, W.T.R.; Ourailidou, M.E.; Kistemaker, L.E.; Bischoff, R.; Gosens, R.; Haisma, H.J.; Dekker, F.J. HDAC 3-selective inhibitor RGFP966 demonstrates anti-inflammatory properties in RAW 264.7 macrophages and mouse precision-cut lung slices by attenuating NF-κB p65 transcriptional activity. Biochem Pharmacol. 2016, 108, 58–74. [Google Scholar] [CrossRef]
- Nguyen, H.C.B.; Adlanmerini, M.; Hauck, A.K.; Lazar, M.A. Dichotomous engagement of HDAC3 activity governs inflammatory responses. Nature 2020, 584, 286–290. [Google Scholar] [CrossRef]
- Ning, L.; Rui, X.; Bo, W.; Qing, G. The critical roles of histone deacetylase 3 in the pathogenesis of solid organ injury. Cell Death Dis. 2021, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Lauffer, B.E.; Mintzer, R.; Fong, R.; Mukund, S.; Tam, C.; Zilberleyb, I.; Flicke, B.; Ritscher, A.; Fedorowicz, G.; Vallero, R.; et al. Histone deacetylase (HDAC) inhibitor kinetic rate constants correlate with cellular histone acetylation but not transcription and cell viability. J Biol Chem. 2013, 288, 26926–26943. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Jung, S.M.; Park, K.S.; Kim, K.J. Integrative analysis of lung molecular signatures reveals key drivers of idiopathic pulmonary fibrosis. BMC Pulm Med. 2021, 21, 404. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Park, K.S.; Kim, K.J. Integrative analysis of lung molecular signatures reveals key drivers of systemic sclerosis-associated interstitial lung disease. Ann Rheum Dis. 2022, 81, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Bergougnoux, A.; Petit, A.; Knabe, L.; Bribes, E.; Chiron, R.; De Sario, A.; Claustres, M.; Molinari, N.; Vachier, I.; Taulan-Cadars, M.; Bourdin, A. The HDAC inhibitor SAHA does not rescue CFTR membrane expression in Cystic Fibrosis. Int J Biochem Cell Biol. 2017, 88, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Histone deacetylase-2 and airway disease. Ther Adv Respir Dis. 2009, 3, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Role of HDAC2 in the pathophysiology of COPD. Annu Rev Physiol. 2009, 71, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Kee, H.J.; Kim, I.; Jeong, M.H. Zinc-dependent histone deacetylases: Potential therapeutic targets for arterial hypertension. Biochem Pharmacol. 2022, 202, 115111. [Google Scholar] [CrossRef]
- Su, Y.; Han, W.; Kovacs-Kasa, A.; Verin, A.D.; Kovacs, L. HDAC6 Activates ERK in airway and pulmonary vascular remodeling of chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2021, 65, 603–614. [Google Scholar] [CrossRef]
- Wang, Y.; Wallach, J.; Duane, S.; Wang, Y.; Wu, J.; Wang, J.; Adejare, A.; Ma, H. Developing selective histone deacetylases (HDACs) inhibitors through ebselen and analogs. Drug Des Devel Ther. 2017, 11, 1369–1382. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, R.; Sun, S. Drug Repurposing: Escitalopram attenuates acute lung injury by inhibiting the SIK2/ HDAC4/ NF-κB signaling cascade. Biochem Biophys Res Commun. 2022, 599, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Tu, T.; Schmull, S.; Han, Y.; Wang, W.; Li, H. Dual inhibition of HDAC and tyrosine kinase signaling pathways with CUDC-907 attenuates TGFβ1 induced lung and tumor fibrosis. Cell Death Dis. 2020, 11, 765. [Google Scholar] [CrossRef] [PubMed]
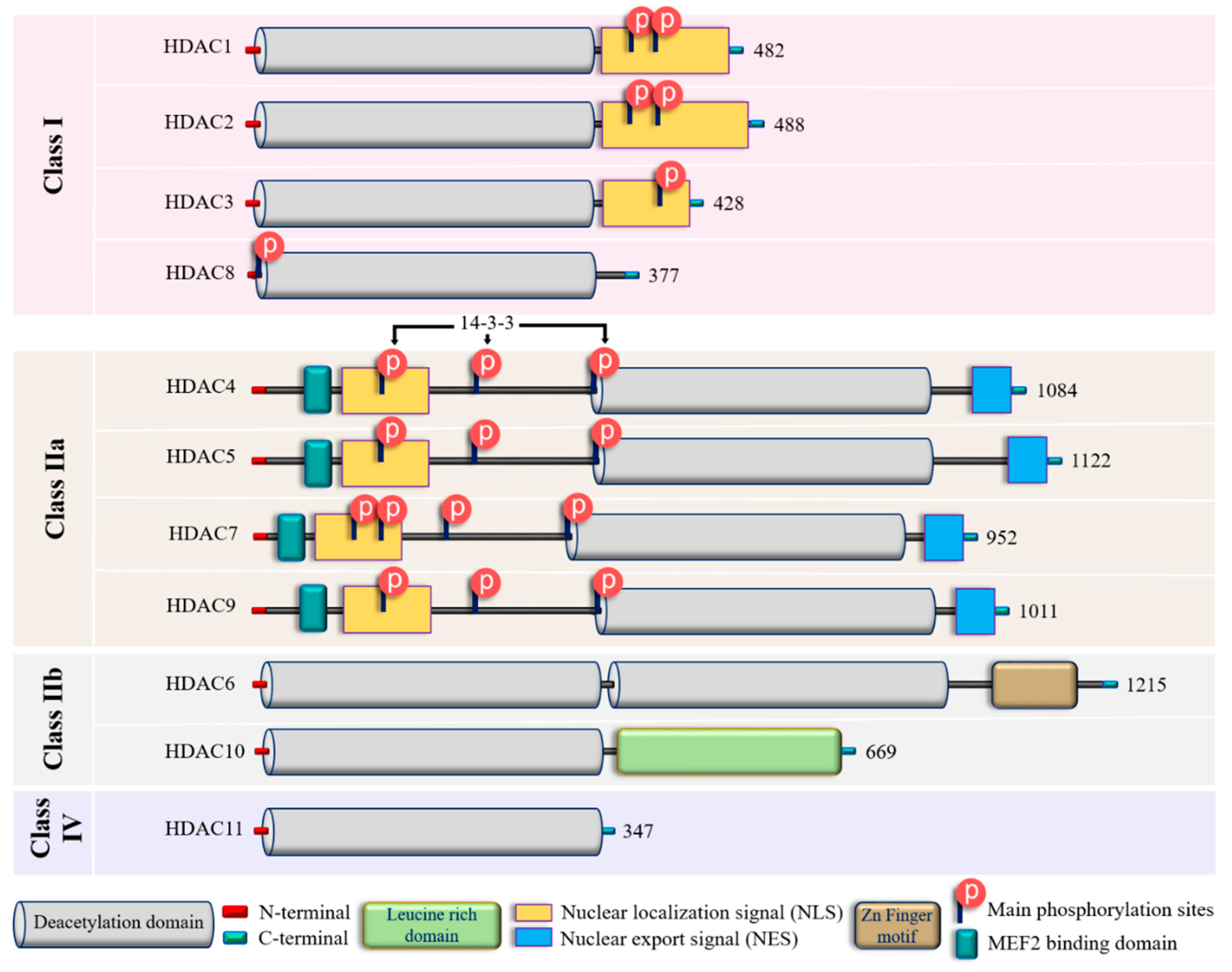
| Class | Isoform | Subcellular distribution | Preferential expression | A.A. length |
Non-histone substrates | Activities/functions in vasculature |
|---|---|---|---|---|---|---|
| Class I | HDAC1 | Nucleus | Ubiquitous | 482 | p53, SHP, MyoD, STAT3, E2F1, AMPK, NF-kB, RB1, CtIP, ATF4, SRF [36-39] | Facilitates the impact of external and environmental stimuli on ECs [40] |
| HDAC2 | Nucleus | Ubiquitous | 488 | YY1, BCL6, GCCR, STAT3 [37] | Protect against DNA damage response and the onset of cellular senescence [41], critical for vascular homeostasis and endothelial health [42] | |
| HDAC3 | Nucleus/ Cytoplasm |
Ubiquitous | 428 | YY1, SHP, p65, GATA1, MEF2D, STAT3, ATF4, SUMO-LXR [37-39,43] | Preserves endothelial integrity [44]; Controls lung alveolar macrophage development and homeostasis [45] | |
| HDAC8 | Nucleus/ Cytoplasm |
Ubiquitous | 377 | Actin, SMC3 [37]; KMT2D, NCOA3, TUBA1A [39] | Culprit in hypertension [46] | |
| Class IIa | HDAC4 | Nucleus/ Cytoplasm |
Heart, SM, Brain | 1084 | HP1, GATA1 [37]; SRF, ATF4, SUMO-LXR [38]; human transcription factor HIF-1α [39] | Regulates cellular senescence, apoptosis and autophagy, Acts as inflammatory mediator [47, 48], regulator of vascular endothelial growth factor D [49] |
| HDAC5 | Nucleus/ Cytoplasm |
Heart, SM, Brain | 1122 | HP1, SMAD7 [37]; p53 [39] | Controls activity of KLF2, KLF2 activation in ECs; induces eNOS expression resulting in vasodilation [50] | |
| HDAC7 | Nucleus/ Cytoplasm |
Heart, Placenta, Pancreas, SM | 952 | PLAG1, PLAG2 [37]; HIF-1α [38] | Suppresses EC proliferation [51], controls EC proliferation and migration [52], maintains vascular integrity in embryogenesis [53], promotes promyelocytic leukemia protein sumoylation [54], Promotes angiogenesis [55]; involves in E. coli-induced ALI [56] | |
| HDAC9 | Nucleus/ Cytoplasm |
SM, Brain | 1011 | NA | Inflammatory mediator [57] | |
| Class IIb | HDAC6 | Cytoplasm | Heart, Liver, Kidney, Pancreas | 1215 | HSP90, SHP, SMAD, α-tubulin [37], G3BP1 [58]; Survivin, AKT, β-catenin, Peroxiredoxin, MMP-9 [38]; p53, ERK1, human cortactin [39] | Crucial in EC function [59], Regulates EC migration and angiogenesis [60], Important in atherosclerosis [61] and HSP90-mediated VEGFR regulation [62] |
| HDAC10 | Cytoplasm | Liver, Spleen, Kidney | 669 | AKT, β-catenin, MMP-9 [38]; N-acetylputrescine, N8-acetylspermidine [39] |
Accelerates angiogenesis in EC via PTPN22/ERK axis [63], Pulmonary hypertension [64], Regulates HSP90-mediated VEGFR [62] | |
| Class IV | HDAC11 | Nucleus | Brain, Heart, SM, Kidney, & Testis | 347 | MyoD [38]; SHMT2 [39] | Compromises the vascular endothelial barrier function [65], Key player in atherosclerosis [66], Triggers caspase-mediated pathways (NLRP3/caspase-1/GSDMD; caspase-3/GSDME) causing pyroptosis [67] |
| Class | Inhibitor | Mode of action | Reference/s |
|---|---|---|---|
| Class I | Valproic acid | Attenuate parameters of lung injury like oxidative stress, apoptosis, and inflammation, enhance HO-1 activity (ALI) | [223] |
| Reduces levels of IL-6 and tumor necrosis factor (ALI) | [224] | ||
| Reduces neutrophil influx into lungs and local tissue destruction via decreasing myeloperoxidase activity. Ameliorate pulmonary as well as systemic inflammatory response (ARDS) | [225] | ||
| Antagonizes the inflammatory damage of vascular tissues | [226] | ||
| Inhibits VEGFR-2 protein expression in angiogenesis | [142] | ||
| Increases histone acetylation in thrombopoiesis | [227] | ||
| Sodium butyrate | Inhibits VEGFR-2 protein expression in angiogenesis | [142] | |
| Trichostatin A | Alleviates HDAC4-mediated vascular inflammatory responses in hypertension | [228] | |
| Prevents I/R injury-induced activation of gene programs that include cell death and vascular permeability | [229] | ||
| Inhibits VEGFR-2 protein expression in angiogenesis | [142] | ||
| Trichostatin A + 5-Aza 2-deoxycytidine |
inhibits the eNOS-Cav1-MLC2 signaling pathway and enhance acetylation of histone markers and improve EC permeability (ALI) | [230] | |
| Reduce inflammation and promote an anti-inflammatory M2 macrophage by inhibiting MAPK-HuR-TNF and activating STAT3-Bcl2 pathways (ALI) | [231] | ||
| miR-23b (HDAC2) | Reduces levels of IL-1β, IL-6, and TNF-α and inhibit HDAC2 (ALI) | [232] | |
| PCI34051 (HDAC8) |
Reduces blood pressure via attenuating a component of the RAS or modulating nitric oxide signaling pathways (Hypertension) | [46] | |
| Class IIa | Valproic acid | Same to Class I | - |
| Sodium butyrate | Same to Class I | - | |
| Trichostatin A | Same to Class I | - | |
| TMP 195 | Limits proinflammatory responses in Atherosclerosis | [233] | |
| MC1568 | Abolishes NO-induced formation of macromolecular complexes and regulates downstream gene expression | [234] | |
| Tasquinimod (HDAC4) |
Allosterically binds to HDAC4 and prevents HDAC4/nuclear receptor corepressor (N-CoR); HDAC3 complex formation, which inhibits HDAC4-regulated histone deacetylation and transcription thus reduces inflammation in Angiogenesis | [235]; [236] | |
| Class IIb | Sodium butyrate | Same to Class I | - |
| Trichostatin A | Same to Class I | - | |
| Class IIb (HDAC6) |
CAY10603 | Prevent ɑ-tubulin deacetylation, protects against inflammation, blocks IĸB phosphorylation, and reduce caspase-1 activation particularly in epithelial cells (ALI) | [237] |
| Tubastatin A | Inhibit angiotensin II-induced hypertension via protecting cystathionine γ-lyase protein degradation | [238] | |
| Class IV | Trichostatin A | Same to Class I | - |
| Hydroxytyrosol acetate | Inhibits pyroptosis by possible targeting of HDAC11 in TNF-α-induced HUVECs (Atherosclerosis) | [66] | |
| Quisinostat | Aggf1 regulates the pathophysiology of vascular endothelium therefore HDAC11 inhibitor restore expression of Aggf1 in vascular injury | [239] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





