Submitted:
31 December 2023
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. The Family of Nuclear Factor (Erythroid 2)-Like (NRF) Transcription Factors
1.2. Deciphering the transcriptional NRF2 -regulated target genes
1.3. NRF2- driven response to oxidative stress and drug metabolizing
1.4. NRF2 signaling pathway in cancer
| Underlying molecular mechanism | Cellular effect | Reference |
|---|---|---|
| Transporter pumps (ABC proteins, SERCA, V-ATPase) |
These proteins exhibit elevated expression levels in chemo-resistant cancer cells and play a role in the development of drug resistance. | [124,125,126] |
| Oncogenes EGFR |
The overexpression of EGFR triggers the activation of NF-κB and STAT3, which subsequently leads to the development of chemo-resistance and unfavorable treatment outcomes. |
[127] |
| KRAS | Oncogenic KRAS promotes drug resistance via upregulation of the cell protective stress response gene, NRF-2, at the transcriptional level. | [128] |
| (PI3K)/Akt | AKT involves in apoptosis, migration, and proliferation. | [129] |
| NF-кB | Following activation, NF-κB translocates to the nucleus, elevating the expression of BCL-2, BCL-XL, XIAP, survivin, and AKT, thereby contributing to accelerated tumorigenesis, increased aggressiveness, drug resistance, and induction of EMT. | [130] |
| ERKs |
ERKs are recognized for their role as activators of various transcription factors, including ETS Like1, along with downstream protein kinases. These factors are closely linked to processes such as cell proliferation, drug resistance, and apoptosis. | [131] |
| Oncogenic Viruses |
Viral onco-proteins contribute to chemo-resistance through multiple mechanisms, including the regulation of cellular transporters and drug targets, modulation of signaling pathways involved in drug-induced cell death responses, and activation of pathways that counteract the effects of drugs. | [120] |
| Rb |
Oncogenic p53 causes chemo-resistance of cancer cells by increasing the expression of MDR-1. | [132,133,134,135,136,137] |
| CKIs |
These mechanisms involve inducing cell cycle arrest and activating DNA repair processes | [138,139,140] |
| PTEN |
Increase apoptosis, regulating cell cycle progression. | [141,142] |
| BRCA1 |
Reduction of cell proliferation, migration, survival and cell size, Regulating transcription, cell cycle checkpoint, DNA repair, and apoptosis. |
[143,144,145,146] |
|
Mitochondrial alteration SERCA |
Bcl-2 and Bcl-xL contribute to heightened drug resistance, while reducing their expression enhances the cytotoxic impact of cisplatin and gemcitabine. Moreover, the level of survivin expression was found to be linked to the degree of cisplatin resistance in gasteric cancer cells. |
[147] |
| V-ATPase |
Somatic mutations occurring in the mitochondrial genome (mtDNA) of cancer cells lead to impaired mitochondrial function, which in turn contributes to the development of chemo-resistance. | [148] |
| DNA repair |
BER and NER can confer the resistance to chemo drugs that target DNA. RAD51, a crucial participant in homologous recombination during double-strand break (DSB) repair, being overexpressed, serves as a marker for resistance to Cisplatin (CDDP) in non-small cell lung cancer (NSCLC). Similarly, elevated expression of ERCC1, a component of the nucleotide excision repair (NER) pathway, is associated with resistance to CDDP in both human hepatocellular carcinoma (HCC) cell lines and specimens. | [149,150,151] |
|
Autophagy |
In tamoxifen-resistance breast cancer cells, SAHA, as a HDAC inhibitor, can induce autophagic cell death and reduce tumor growth. Despite the challenges about the anticancer and pro-survival function of autophagy, in vitro and in vivo research has been more confirmed that autophagy could be considered as a facilitator of cancer chemo-resistance. In NSCLC cells, autophagy inhibition using Chloroquine, before paclitaxel treatment, prevents drug resistance. |
[14,152,153] |
| UPR |
CSCs and actively dividing tumor cells might exploit distinct branches of the UPR to reinforce their pre-existing mechanisms of chemo-resistance. Notably, the suppression of all three UPR branches—GRp78, ATF6, ATF4, and XBP1s—has shown a correlation with the restoration of sensitivity in chemotherapy-resistant cancer cells. | [24,116,117,118,119] |
| EMT |
EMT has been identified as a promoter of chemo-resistance against the DNA alkylating agent cyclophosphamide and the DNA synthesis inhibitor gemcitabine. Specifically, the attenuation of Snail or Twist has been linked to increased sensitivity to chemotherapy. | [154] |
| Cancer stemness |
Cancer stem cells (CSCs) resist chemotherapy by increasing the levels of P-glycoprotein, ABCG2, BCL-2, and survivin. Recent findings highlight NRF2's role in preserving stemness, intensifying tumorigenicity, and initiating chemo-resistance within CSCs. |
[155,156] |
| Regulatory redox network |
The mechanisms of ROS-mediated acquired chemo-resistance include autophagy, ER stress, overcoming cell cycle arrest, and enhancing epithelial to mesenchymal transition or cancer stem-like cells. Numerous chemotherapy agents, including cisplatin, doxorubicin, etoposide, paclitaxel, and bortezomib, induce cancer cell death by elevating ROS levels. Adjusting intracellular antioxidant levels holds potential therapeutic benefits but can be complex. While antioxidants may impede chemotherapy efficacy by scavenging ROS, they can also trigger chemotherapy-related toxicity, highlighting a delicate balance. | [12,120,157] |
2. Redox regulatory network involved in the induction of autophagy/UPR and tumor chemo-resistance
2.1. The cross talk between different arms of UPR-autophagy in drug resistance and cancer cell survival
2.1.1. Interaction among IRE1/XBP1s, IRE1/TRAF2/ASK1/JNK, and Autophagy
2.1.2. The role of IRE1/XBP1s arm of UPR and autophagy in drug resistance and cancer cell survival
2.1.3. Cross-talk between the PERK/eukaryotic translation initial factor 2α (eIF2α)/ ATF4/CHOP axis and autophagy
2.1.4. The role of PERK arm of UPR and autophagy in drug resistance and cancer cell survival
2.1.5. Interplay between ATF6 and autophagy
2.1.6. The role of ATF6 arm of UPR/ autophagy in drug resistance and cancer cell survival
2.1.6. Other pathways involved in ER stress-induced autophagy
3. NRF2 controls UPR and proteostasis
3.1. NRF2 modulates autophagy
4. Evidence show overexpression of NRF2 promotes post-initiation stages of cancer
4.1. NRF2 as double-edged sword in cancers
4.2. Targeting of Nrf2 Signaling to fight Chemo-resistance: NRF2 inhibitors
| Classification and origins |
Compound | Structure | Dose and time | Mechanism | Model and effect | Refs. |
|---|---|---|---|---|---|---|
|
Brucea javanica Plant |
Brusatol (Bru) | 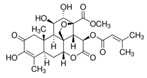 |
2 mg/kg, five times for 16 days via intraperitoneally | ↓ NRF2/GSH axis | ↓tumor mass ↓NRF2, SLC7A11, GCLC, and GCLM expression Six-to-eight-week-old NSG mice |
[298] |
|
Flavonoid |
Luteolin (Lut) | 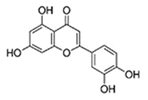 |
40 mg/kg BW/day; 14 days |
Unclear | ↓NRF2 protein levels in mouse liver and intestine (C57BL/6, Male, 6 weak old) | [293] |
| Flavonoid | Apigenin (Api) | 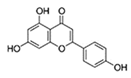 |
In vivo: 50 mg/kg BW/day; every 3 days for 7 times |
PI3K/Akt pathway: ↓p-Akt | ↓tumor size in male BALB/c nude mice (aged 5 weeks) that were implanted with BEL-7402 cells; ↓level of NRF2 protein | [294] |
| Flavonoid | Chrysin (Chry) | 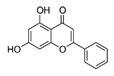 |
40 and 80 mg/kg/day by oral gavage; once a day, 5 times per week | ↓ERK/NRF2 signaling pathway | ↓Tumor size of mice (male BALB/c athymic nude mice, 4–6 weeks) ↓translocation of NRF2 into the nucleus and ↓ expression of (HO-1) and NQO-1 |
[295] |
| Flavonoid | Wogonin (Wog) | 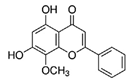 |
40 mg/kg intravenously, once every other day for 30 days | ↓NF-κB/NRF2 pathway | NOD/SCID immunodeficient mice (aged 5–6 weeks) ↓ nuclear NF-κB p65, p-Stat3 and NRF2 expression and ↓phosphorylation of IKKα and IκBα ↓NF-κB p65 and NRF2 expression in spleen |
[296] |
| A traditional Chinese medicine | triptolide | 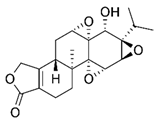 |
0.25 mg/kg, by intraperitoneal every other day for 10 days | transcriptional regulation of NRF2 |
↓Tumor growth and weight C57BL/6 mice (6–8 weeks, male) ↓NRF2 and downstream genes Gclc and Gclm increases the chemosensitivity of xenograft tumors to epirubicin |
[299] |
| An alkaloid | Trigonelline (Trig) | 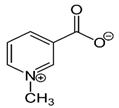 |
0.02 mg/kg intraperitoneally for 21 days | ↓ a nuclear level of activated NRF2 protein |
↓tumor growth and weight 8-week-old female SCID–beige mice ↑responsiveness of various cell lines to both anticancer drugs and apoptosis induced by TRAIL. |
[300] |
| Vitamin derivative | all-trans-retinoic acid |  |
10 µM, 48h 40 mg/kg, three times weekly via intraperitoneally |
↓NRF2/POMP axis | ↓cell viability, purified CD138+ plasma cells from patients with myeloma ↓tumor growth 6-week-old non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice |
[261] |
|
Aniline-based compound |
IM3829 (4-(2-Cyclohex-ylethoxy) aniline) | 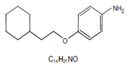 |
5mg/kg/day), intraperitoneally for 4 days | ↓NRF2-binding activity and expression of NRF2 target genes, increase ROS accumulation in irradiated cell |
↓tumor growth, without changes in body weight, Five-week, female, athymic BALB/c nude mice |
[301] |
| A probe molecule that binds to Nrf2 | ML385 | 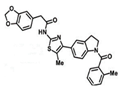 |
30 mg/kg daily Monday to Friday), intraperitoneally for 3 weeks | blocks NRF2 transcriptional activity | ↓tumor growth, athymic nude mice, ↓NRF2, NQO1, and ABCG2 expression | [302] |
| A chemical substance | ARE expression modulator 1 (AEM1) | 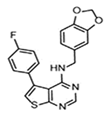 |
50 mg/kg, BW; twice a day for 10 days |
Unclear | ↓Tumor growth of mice which were implanted with A549 cells (Nude mice, Male, 6 wk old) | [303] |
| A febrifugine derivatives | halofuginone | 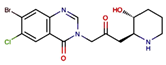 |
0.25 mg/kg, every day intraperitoneally | Inducing a cellular amino acid starvation response that repressed protein level of NRF2 |
↓tumor growth, 6–8-week-old male nude mice, enhances the anticancer effects of cisplatin. without severe toxicity |
[304] |
| Corticosteroid drug | Clobetasol propionate | 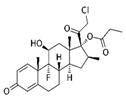 |
CP (0.5 or 1 mg/kg, n = 5 per group) were intraperitoneally injected every 2 days (3 days per week) for 40 days. |
↓NRF2 in a GR and GSK3-dependent manner | Balb/c-nu mice (6–8 weeks). ↓NRF2 and the expression of its key targets in KEAP1 mutant NSCLCs and induction of oxidative stress. ↓tumor growth and shrinkage of tumor size |
[305] |
| Purified from Streptomyces sp. 3728-17 strain |
K-563 | 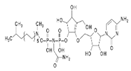 |
100 mg/kg, subcutaneously to the mice twice a day for 1 day |
↓Keap1/NRF2 pathway | Male severe combined immunodeficient (SCID) mice, 5 weeks’ old ↓expressions of Keap1/NRF2 pathway Targeted genes (HMOX1, GCLC, GCLM,AKR1C1, ME1, NQO1, and TXNRD1) |
[306] |
| Anti-tumor drug | Camptothecin | 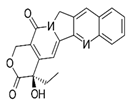 |
CPT (3 mg/ kg body weight) were intraperitoneally (IP) injected twice a week for a total of three times. | ↓NRF2–ARE pathway activity | ↓tumor growth BALB/Cnu/nu mice (4–6 weeks, male). Sensitization of a variety of cancer cells and a xenograft hepatocellular carcinoma model to chemotherapeutic drugs | [307] |
| Type 2 diabetes drug | Metformin | 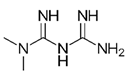 |
200 μg/mL, diluted in drinking water and administered for day 22 | ↓NRF2, HO-1, KI-67 and PCNA expression |
↓tumor growth, Female BALB/C nude mice (6-8 weeks of age) enhancing the anti-cancer effect of EGCG on NSCLC xenografts |
[308] |
| Cardiac glycoside drug | Digoxin | 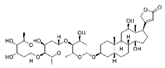 |
0.1 mg/kg, daily, i.g for 24 days | ↓Activity of NRF2 through suppressing PI3k/Akt signaling pathway |
↓tumor growth In female BALB/c nude mice (aged 6 weeks, weighing 18 ± 2 g), the resistance to gemcitabine was effectively reversed by inhibiting NRF2 in SW1990/Gem and Pac-1/Gem cells. |
[30] |
4.3. Clinical Overview
5. Conclusion
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-MA | 3-Methyladenine |
| 5-FU | 5-Fluorouracil |
| ABC | Advanced Breast Cancer |
| ABCCs | Multidrug resistance (MDR)-associated proteins |
| ABCG2 | Breast cancer resistance protein |
| AEM | Are Expression Modulator |
| AKRs | Aldo-Keto Reductases |
| AKT | Protein kinase B |
| ALDH1 | Aldehyde Dehydrogenase 1 |
| AMPK | Adenosine monophosphate-activated protein kinase |
| AP-1 | Activator protein 1 |
| ARE | Antioxidant Response Element |
| ARS | Antioxidants and redox signaling |
| ASK1 | Apoptosis-signal regulating kinase 1 |
| ATF | Activating Transcription Factor |
| ATG | Autophagy-related protein |
| ATRA | All-Trans-Retinoic Acid |
| BACH1 | BTB and CNC homology 1 |
| BCRP/ABCG2 Breast cancer resistance protein | |
| BECN | Encoding beclin |
| BRAF | B-Raf proto-oncogene |
| BRG-1 | Brahma-Related Gene 1 |
| bZIP | Basic Region/Leucine Zipper |
| CALCOCO2 | Calcium-Binding and Coiled-Coil Domain-Containing Protein 2 |
| CBP | cAMP -binding protein |
| CBRs | Carbonyl Reductases |
| CDDP | Cisplatin |
| CHD6 | Chromodomain Helicase Dna-Binding Protein 6 |
| CHOP | Homologous protein |
| cIPA | Cellular inhibitors of apoptosis |
| CML | Chronic Myeloid Leukemia |
| CNC | Cap ‘N’ Collar |
| CNC-bZIP | Collar Basic Region Leucine Zipper |
| COX-2 | Cyclooxygenase-2 |
| CP | Clobetasol Propionate |
| CPT | Camptothecin |
| CQ | Chloroquine |
| CREB | cAMP-response element binding protein |
| CSCs | Cancer Steam Cells |
| CTX | Cytotoxic Chemotherapy |
| CYPs | Cytochrome P450s |
| DAPK1 | Death-Associated Kinase 1 |
| DMSO | Dimethyl sulfoxide |
| EBP | Enhancer Binding Protein |
| EGCG | Epigallocatechin-3-gallate |
| eIF2a | Initiation Factor 2-Alpha |
| EMT | Epithelial-Mesenchymal Transition |
| ER | Endoplasmic Reticulum |
| ERAD | ER-associated degradation |
| ERRα | Estrogen-Related Receptor A |
| FOXO-1 | Anti-apoptotic forkhead box O-1 |
| G6PD | Glucose 6-phosphate dehydrogenase |
| GA | Golgi apparatus |
| GAA | Acid α-glucosidase |
| GABARAPL1Gamma-aminobutyric acid receptor-associated protein-like 1 | |
| GCL | Glutamate-Cysteine Ligase |
| GCLC | Glutamate-Cysteine Ligase Catalytic |
| GCLM | Glutamate-Cysteine Ligase Modulator |
| GR | Glutathione reductase |
| GRP78 | Glucose-Regulated Protein 78 |
| GSCs | Glioma Stem Cells |
| GSH | Glutathione |
| GSK3 | Glycogen synthase kinase-3 |
| GSS | Glutathione synthetize |
| GSSG | Oxidized glutathione |
| GST | Glutathione S-Transferase |
| HCC | Hepatocellular Carcinoma |
| HO | Heme Oxygenase |
| IL | Interleukin |
| iNOS | Induced Nitric Oxide Synthase |
| IR | Irradiation |
| IRE1 | Inositol-requiring enzyme1 |
| JNK | Jun NH2-terminal kinase |
| KEAP1Kelch-Like-Ech-Associated Protein 1 | |
| KIR | Keap1-Interacting Region |
| LC3B | light chain 3B |
| MAPK | Mitogen-activated protein kinase |
| MDR | Multidrug resistance-associated proteins |
| MMP-9 | Metalloproteinase-9 |
| MRP1 | Multidrug-resistance-associated protein-1 |
| MSCs | Mesenchymal Stem Cells |
| mTOR | Mammalian target of rapamycin |
| mTORC | Mammalian target of rapamycin complex |
| NADPH | Adenine Dinucleotide Phosphate |
| Neh | Nrf2-ECH homology |
| NFE2 | Nuclear factor erythroid-derived 2 |
| NF-E2 | Nuclear Factor-Erythroid 2 |
| NFE2L2 | NFE2 like BZIP Transcription Factor 2 |
| NQO1 | Nad(P)H:Quinine Oxidoreductase 1 |
| Nrf2 | Nuclear Related Factor 2 |
| OX | Electrophiles |
| PDI | Protein Disulfide Isomerase |
| PERK | Protein kinase RNA-like ER kinase |
| PGD | Phosphogluconate Dehydrogenase |
| PHGDH | Phosphoglycerate Dehydrogenase |
| PI3K | Phosphoinositide 3-Kinase |
| POMP | Proteasome Maturation Protein |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| PPP | Pentose Phosphate Pathway |
| PSAT1 | Phosphoserine Aminotransferase-1 |
| RAC3 | Receptor-Associated Co-Activator 3 |
| Rb | Retinoblastoma |
| RIDD | Regulated Ire1 Dependent Decay |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| ROS/RS | Reactive Oxygen and Nitrogen Products |
| RXRα | Retinoid X receptor alpha |
| SERCA | Calcium transport ATPase |
| SHMT2 | Serine Hydroxymethyltransferase-2 |
| shRNAs | Small hairpin RNA |
| Simva | Simvastatin |
| Sirt6 | Sirtuin 6 |
| SLC7A11 | Solute carrier 7A11 |
| sMAF | Small musculoaponeurotic fibrosarcoma |
| SMRT | Silencing mediator for retinoid and thyroid hormone receptor |
| SOD | Superoxide Dismutase |
| SP1 | Specificity Protein 1 |
| TFs | Transcription Factors |
| TGF-β | Transforming growth factor |
| TKT | Transketolase |
| TME | Tumor microenvironment |
| TMZ | Temozolomide |
| TNBC | Triple-Negative Breast Cancer |
| TNF-α | Tumor Necrosis Factor |
| TRAF2 | Tumor Necrosis Factor Receptor-Associated Factor-2 |
| TRB3 | Tribbles-Related Protein3 |
| Trx | Thioredoxin |
| TSC2 | Tuberous Sclerosis Complex 2 |
| UGT | Udp-Glucuronosyltransferase |
| ULK | Unc-51-Like Kinase |
| UPR | Unfolded Protein Response |
| VEGF | Vascular Endothelial Growth Factor |
| XBP1 | X-Box-Binding Protein-1 |
| β-TrCP | β-transducin repeat-containing E3 ubiquitin protein ligase |
References
- Bracci, L.; Schiavoni, G.; Sistigu, A.; Belardelli, F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death & Differentiation 2014, 21, 15–25. [Google Scholar]
- Bailly, C.; Thuru, X.; Quesnel, B.J.N.c. Combined cytotoxic chemotherapy and immunotherapy of cancer: modern times. NAR cancer 2020, 2, zcaa002. [Google Scholar] [CrossRef]
- Malone, E.; Maltese, M.; Coady, L.; Hammond, L.; Silva, N.; Gullo, G.; Crown, J.J.A.o.O. Use and clinical impact of conventional cytotoxic chemotherapy (CTx) subsequent to immunotherapy in metastatic melanoma. Annals of Oncology 2016, 27, vi396. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: a brief review. Advanced pharmaceutical bulletin 2017, 7, 339. [Google Scholar] [CrossRef] [PubMed]
- Davodabadi, F.; Sajjadi, S.F.; Sarhadi, M.; Mirghasemi, S.; Hezaveh, M.N.; Khosravi, S.; Andani, M.K.; Cordani, M.; Basiri, M.; Ghavami, S. Cancer chemotherapy resistance: Mechanisms and recent breakthrough in targeted drug delivery. European Journal of Pharmacology 2023, 958, 176013. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resistance 2019, 2, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Brasseur, K.; Gévry, N.; Asselin, E. Chemoresistance and targeted therapies in ovarian and endometrial cancers. Oncotarget 2017, 8, 4008. [Google Scholar] [CrossRef]
- Lu, C.; Shervington, A. Chemoresistance in gliomas. Molecular and cellular biochemistry 2008, 312, 71–80. [Google Scholar] [CrossRef]
- Shojaei, S.; Koleini, N.; Samiei, E.; Aghaei, M.; Cole, L.K.; Alizadeh, J.; Islam, M.I.; Vosoughi, A.r.; Albokashy, M.; Butterfield, Y. Simvastatin increases temozolomide-induced cell death by targeting the fusion of autophagosomes and lysosomes. The FEBS journal 2020, 287, 1005–1034. [Google Scholar] [CrossRef]
- Shojaei, S.; Alizadeh, J.; Thliveris, J.; Koleini, N.; Kardami, E.; Hatch, G.M.; Xu, F.; Hombach-Klonisch, S.; Klonisch, T.; Ghavami, S. Statins: a new approach to combat temozolomide chemoresistance in glioblastoma. Journal of Investigative Medicine 2018, 66, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Badana, A.K.; Malla, R. Reactive oxygen species: a key constituent in cancer survival. Biomarker insights 2018, 13, 1177271918755391. [Google Scholar] [CrossRef]
- Kim, E.-K.; Jang, M.; Song, M.-J.; Kim, D.; Kim, Y.; Jang, H.H. Redox-mediated mechanism of chemoresistance in cancer cells. Antioxidants 2019, 8, 471. [Google Scholar] [CrossRef]
- Mishra, J.; Bhatti, G.K.; Sehrawat, A.; Singh, C.; Singh, A.; Reddy, A.P.; Reddy, P.H.; Bhatti, J.S. Modulating autophagy and mitophagy as a promising therapeutic approach in neurodegenerative disorders. Life Sciences 2022, 121153. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Choudhury, D.; Das, A.; Mukherjee, D.D.; Dasgupta, M.; Bandopadhyay, S.; Chakrabarti, G. Autophagy inhibition with chloroquine reverts paclitaxel resistance and attenuates metastatic potential in human nonsmall lung adenocarcinoma A549 cells via ROS mediated modulation of β-catenin pathway. Apoptosis 2019, 24, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, J.; Kochan, M.M.; Stewart, V.D.; Drewnik, D.A.; Hannila, S.S.; Ghavami, S. Inhibition of Autophagy Flux Promotes Secretion of Chondroitin Sulfate Proteoglycans in Primary Rat Astrocytes. Mol Neurobiol 2021, 58, 6077–6091. [Google Scholar] [CrossRef]
- Mokarram, P.; Albokashy, M.; Zarghooni, M.; Moosavi, M.A.; Sepehri, Z.; Chen, Q.M.; Hudecki, A.; Sargazi, A.; Alizadeh, J.; Moghadam, A.R. New frontiers in the treatment of colorectal cancer: autophagy and the unfolded protein response as promising targets. Autophagy 2017, 13, 781–819. [Google Scholar] [CrossRef]
- Siri, M.; Behrouj, H.; Dastghaib, S.; Zamani, M.; Likus, W.; Rezaie, S.; Hudecki, J.; Khazayel, S.; Los, M.J.; Mokarram, P.; et al. Casein Kinase-1-Alpha Inhibitor (D4476) Sensitizes Microsatellite Instable Colorectal Cancer Cells to 5-Fluorouracil via Authophagy Flux Inhibition. Arch Immunol Ther Exp (Warsz) 2021, 69, 26. [Google Scholar] [CrossRef]
- Peixoto, P.; Grandvallet, C.; Feugeas, J.-P.; Guittaut, M.; Hervouet, E. Epigenetic control of autophagy in cancer cells: a key process for cancer-related phenotypes. Cells 2019, 8, 1656. [Google Scholar] [CrossRef]
- da Silva Rosa, S.C.; Martens, M.D.; Field, J.T.; Nguyen, L.; Kereliuk, S.M.; Hai, Y.; Chapman, D.; Diehl-Jones, W.; Aliani, M.; West, A.R.; et al. BNIP3L/Nix-induced mitochondrial fission, mitophagy, and impaired myocyte glucose uptake are abrogated by PRKA/PKA phosphorylation. Autophagy 2021, 17, 2257–2272. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Karsli-Uzunbas, G.; Poillet-Perez, L.; Sawant, A.; Hu, Z.S.; Zhao, Y.; Moore, D.; Hu, W.; White, E. Autophagy promotes mammalian survival by suppressing oxidative stress and p53. Genes & development 2020, 34, 688–700. [Google Scholar]
- Ravanan, P.; Srikumar, I.F.; Talwar, P. Autophagy: The spotlight for cellular stress responses. Life sciences 2017, 188, 53–67. [Google Scholar] [CrossRef]
- Dastghaib, S.; Shojaei, S.; Mostafavi-Pour, Z.; Sharma, P.; Patterson, J.B.; Samali, A.; Mokarram, P.; Ghavami, S. Simvastatin induces unfolded protein response and enhances temozolomide-induced cell death in glioblastoma cells. Cells 2020, 9, 2339. [Google Scholar] [CrossRef] [PubMed]
- McGrath, E.P.; Logue, S.E.; Mnich, K.; Deegan, S.; Jäger, R.; Gorman, A.M.; Samali, A. The unfolded protein response in breast cancer. Cancers 2018, 10, 344. [Google Scholar] [CrossRef]
- Madden, E.; Logue, S.E.; Healy, S.J.; Manie, S.; Samali, A. The role of the unfolded protein response in cancer progression: From oncogenesis to chemoresistance. Biology of the Cell 2019, 111, 1–17. [Google Scholar] [CrossRef]
- Siwecka, N.; Rozpędek, W.; Pytel, D.; Wawrzynkiewicz, A.; Dziki, A.; Dziki, Ł.; Diehl, J.A.; Majsterek, I. Dual role of endoplasmic reticulum stress-mediated unfolded protein response signaling pathway in carcinogenesis. International journal of molecular sciences 2019, 20, 4354. [Google Scholar] [CrossRef]
- McCarthy, N.; Dolgikh, N.; Logue, S.; Patterson, J.B.; Zeng, Q.; Gorman, A.M.; Samali, A.; Fulda, S. The IRE1 and PERK arms of the unfolded protein response promote survival of rhabdomyosarcoma cells. Cancer Letters 2020, 490, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Vrishni, S.; Singh, B.K.; Rahman, I.; Kakkar, P. Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free radical research 2010, 44, 1267–1288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Eguchi, S.; Alam, A.; Ma, D. The role of nuclear factor-erythroid 2 related factor 2 (Nrf-2) in the protection against lung injury. American Journal of Physiology-Lung Cellular and Molecular Physiology 2017, 312, L155–L162. [Google Scholar] [CrossRef]
- Niture, S.K.; Jaiswal, A.K. Nrf2-induced antiapoptotic Bcl-xL protein enhances cell survival and drug resistance. Free Radical Biology and Medicine 2013, 57, 119–131. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Y.; Yang, M.; Wang, K.; Liu, Y.; Zhang, M.; Yang, Y.; Jin, C.; Wang, R.; Hu, R. Digoxin sensitizes gemcitabine-resistant pancreatic cancer cells to gemcitabine via inhibiting Nrf2 signaling pathway. Redox biology 2019, 22, 101131. [Google Scholar] [CrossRef]
- Tazehkand, A.P.; Akbarzadeh, M.; Velaie, K.; Sadeghi, M.R.; Samadi, N. The role of Her2-Nrf2 axis in induction of oxaliplatin resistance in colon cancer cells. Biomedicine & Pharmacotherapy 2018, 103, 755–766. [Google Scholar]
- Wang, X.-J.; Sun, Z.; Villeneuve, N.F.; Zhang, S.; Zhao, F.; Li, Y.; Chen, W.; Yi, X.; Zheng, W.; Wondrak, G.T. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 2008, 29, 1235–1243. [Google Scholar] [CrossRef]
- de la Vega, M.R.; Dodson, M.; Chapman, E.; Zhang, D.D. NRF2-targeted therapeutics: New targets and modes of NRF2 regulation. Current opinion in toxicology 2016, 1, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Villeneuve, N.F.; Sun, Z.; Wong, P.K.; Zhang, D.D. Dual roles of Nrf2 in cancer. Pharmacological research 2008, 58, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Azami, N.; Hamzehlou, S.; Farahani, M.V.; Hushmandi, K.; Ashrafizadeh, M. Nrf2 Signaling Pathway in Chemoprotection and Doxorubicin Resistance: Potential Application in Drug Discovery. Antioxidants 2021, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Kalthoff, S.; Ehmer, U.; Freiberg, N.; Manns, M.P.; Strassburg, C.P. Interaction between oxidative stress sensor Nrf2 and xenobiotic-activated aryl hydrocarbon receptor in the regulation of the human phase II detoxifying UDP-glucuronosyltransferase 1A10. Journal of Biological Chemistry 2010, 285, 5993–6002. [Google Scholar] [CrossRef]
- Shelton, P.; Jaiswal, A.K. The transcription factor NF-E2-related factor 2 (Nrf2): a protooncogene? The FASEB Journal 2013, 27, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Song, M.-Y.; Kim, E.-H. Role of Oxidative Stress and Nrf2/KEAP1 Signaling in Colorectal Cancer: Mechanisms and Therapeutic Perspectives with Phytochemicals. Antioxidants 2021, 10, 743. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a transcription factor for stress response and beyond. International journal of molecular sciences 2020, 21, 4777. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radical Biology and Medicine 2015, 88, 108–146. [Google Scholar] [CrossRef]
- Wang, H.; Liu, K.; Geng, M.; Gao, P.; Wu, X.; Hai, Y.; Li, Y.; Li, Y.; Luo, L.; Hayes, J.D. RXRα inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer research 2013, 73, 3097–3108. [Google Scholar] [CrossRef]
- Marchev, A.S.; Dimitrova, P.A.; Burns, A.J.; Kostov, R.V.; Dinkova-Kostova, A.T.; Georgiev, M.I.J.A.o.t.N.Y.A.o.S. Oxidative stress and chronic inflammation in osteoarthritis: can NRF2 counteract these partners in crime? Annals of the New York Academy of Sciences 2017, 1401, 114–135. [Google Scholar] [CrossRef]
- Liu, P.; Kerins, M.J.; Tian, W.; Neupane, D.; Zhang, D.D.; Ooi, A. Differential and overlapping targets of the transcriptional regulators NRF1, NRF2, and NRF3 in human cells. Journal of Biological Chemistry 2019, 294, 18131–18149. [Google Scholar] [CrossRef]
- Paladino, S.; Conte, A.; Caggiano, R.; Pierantoni, G.M.; Faraonio, R. Nrf2 pathway in age-related neurological disorders: insights into MicroRNAs. Cellular Physiology and Biochemistry 2018, 47, 1951–1976. [Google Scholar] [CrossRef]
- Wang, R.; Liang, L.; Matsumoto, M.; Iwata, K.; Umemura, A.; He, F. Reactive Oxygen Species and NRF2 Signaling, Friends or Foes in Cancer? Biomolecules 2023, 13, 353. [Google Scholar] [CrossRef]
- Bauer, A.K.; Cho, H.-Y.; Miller-DeGraff, L.; Walker, C.; Helms, K.; Fostel, J.; Yamamoto, M.; Kleeberger, S.R. Targeted deletion of Nrf2 reduces urethane-induced lung tumor development in mice. PloS one 2011, 6, e26590. [Google Scholar] [CrossRef]
- Towers, C.G.; Fitzwalter, B.E.; Regan, D.; Goodspeed, A.; Morgan, M.J.; Liu, C.-W.; Gustafson, D.L.; Thorburn, A. Cancer cells upregulate NRF2 signaling to adapt to autophagy inhibition. Developmental cell 2019, 50, 690–703. e696. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, H.; O'Connor, T.; Katsuoka, F.; Engel, J.D.; Yamamoto, M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene 2002, 294, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sykiotis, G.P.; Bohmann, D. Stress-activated cap'n'collar transcription factors in aging and human disease. Science signaling 2010, 3, re3–re3. [Google Scholar] [CrossRef] [PubMed]
- Luk, A.D.W.; Yang, X.; Alcasabas, A.P.; Hao, R.C.; Chan, K.W.; Lee, P.P.; Yang, J.; Chan, G.C.F.; So, J.C.C.; Yang, W.J.B.j.o.h. NF-E2 mutation as a novel cause for inherited thrombocytopenia. British Journal of Haematology 2020, 189, e41. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.K.; Lee, C.S.; Young, P.; Beskow, A.; Chan, J.Y.; Deshaies, R.J. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Molecular cell 2010, 38, 17–28. [Google Scholar] [CrossRef]
- Kim, H.M.; Han, J.W.; Chan, J.Y. Nuclear factor erythroid-2 like 1 (NFE2L1): structure, function and regulation. Gene 2016, 584, 17–25. [Google Scholar] [CrossRef]
- Ibrahim, L.; Mesgarzadeh, J.; Xu, I.; Powers, E.T.; Wiseman, R.L.; Bollong, M.J. Defining the Functional Targets of Cap ‘n’collar Transcription Factors NRF1, NRF2, and NRF3. Antioxidants 2020, 9, 1025. [Google Scholar] [CrossRef]
- Bhawe, K.; Roy, D. Interplay between NRF1, E2F4 and MYC transcription factors regulating common target genes contributes to cancer development and progression. Cellular Oncology 2018, 41, 465–484. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, T.; Zhou, W.; Zhang, Y.; Xu, G.; Xu, Q.; Li, S.; Gao, Y.; Wang, Z.; Xu, J. Long noncoding RNA LINC01132 enhances immunosuppression and therapy resistance via NRF1/DPP4 axis in hepatocellular carcinoma. Journal of Experimental & Clinical Cancer Research 2022, 41, 270. [Google Scholar]
- Sekine, H.; Motohashi, H. Roles of CNC Transcription Factors NRF1 and NRF2 in Cancer. Cancers 2021, 13, 541. [Google Scholar] [CrossRef]
- Chowdhury, A.M.A.; Katoh, H.; Hatanaka, A.; Iwanari, H.; Nakamura, N.; Hamakubo, T.; Natsume, T.; Waku, T.; Kobayashi, A. Multiple regulatory mechanisms of the biological function of NRF3 (NFE2L3) control cancer cell proliferation. Scientific reports 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Immonen, A.; Haapasaari, K.-M.; Skarp, S.; Karihtala, P.; Teppo, H.-R. NRF3 Decreases during Melanoma Carcinogenesis and Is an Independent Prognostic Marker in Melanoma. Oxidative medicine and cellular longevity 2022, 2022. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, Z.; Chen, Q.; Pan, Y.; Lu, H.; Zhang, H.; Yu, Y.; Dai, Y. NRF3 suppresses breast cancer cell metastasis and cell proliferation and is a favorable predictor of survival in breast cancer. OncoTargets and therapy 2019, 12, 3019. [Google Scholar] [CrossRef]
- Kobayashi, A.; Waku, T. New addiction to the NRF2-related factor NRF3 in cancer cells: ubiquitin-independent proteolysis through the 20S proteasome. Cancer Science 2020, 111, 6–14. [Google Scholar] [CrossRef]
- Kobayashi, A. Roles of NRF3 in the Hallmarks of Cancer: Proteasomal Inactivation of Tumor Suppressors. Cancers 2020, 12, 2681. [Google Scholar] [CrossRef]
- Cloer, E.W.; Goldfarb, D.; Schrank, T.P.; Weissman, B.E.; Major, M.B. NRF2 activation in cancer: from DNA to protein. Cancer research 2019, 79, 889–898. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional regulation by Nrf2. Antioxidants & redox signaling 2018, 29, 1727–1745. [Google Scholar]
- Katsuoka, F.; Yamamoto, M. Small Maf proteins (MafF, MafG, MafK): history, structure and function. Gene 2016, 586, 197–205. [Google Scholar] [CrossRef]
- Inouye, S.; Kubo, T.; Miyamoto, T.; Iyoda, T.; Okita, N.; Akagi, R. Heat shock-induced heme oxygenase-1 expression in a mouse hepatoma cell line is dependent on HSF1 and modified by NRF2 and BACH1. Genes to Cells 2022, 27, 719–730. [Google Scholar] [CrossRef]
- Mafra, D.; Alvarenga, L.; Cardozo, L.F.; Stockler-Pinto, M.B.; Nakao, L.S.; Stenvinkel, P.; Shiels, P.G. Inhibiting BTB domain and CNC homolog 1 (Bach1) as an alternative to increase Nrf2 activation in chronic diseases. Biochimica et Biophysica Acta (BBA)-General Subjects 2022, 1866, 130129. [Google Scholar] [CrossRef]
- Warnatz, H.-J.; Schmidt, D.; Manke, T.; Piccini, I.; Sultan, M.; Borodina, T.; Balzereit, D.; Wruck, W.; Soldatov, A.; Vingron, M. The BTB and CNC homology 1 (BACH1) target genes are involved in the oxidative stress response and in control of the cell cycle. Journal of Biological Chemistry 2011, 286, 23521–23532. [Google Scholar] [CrossRef]
- Paramasivan, P.; Kankia, I.H.; Langdon, S.P.; Deeni, Y.Y. Emerging role of nuclear factor erythroid 2-related factor 2 in the mechanism of action and resistance to anticancer therapies. Cancer Drug Resistance 2019, 2, 490–515. [Google Scholar] [CrossRef]
- Sarcinelli, C.; Dragic, H.; Piecyk, M.; Barbet, V.; Duret, C.; Barthelaix, A.; Ferraro-Peyret, C.; Fauvre, J.; Renno, T.; Chaveroux, C. ATF4-dependent NRF2 transcriptional regulation promotes antioxidant protection during endoplasmic reticulum stress. Cancers 2020, 12, 569. [Google Scholar] [CrossRef]
- Chung, S.; Kim, S.; Son, M.; Kim, M.; Koh, E.S.; Shin, S.J.; Park, C.W.; Kim, H.-S. Inhibition of p300/CBP-associated factor attenuates renal tubulointerstitial fibrosis through modulation of NF-kB and Nrf2. International journal of molecular sciences 2019, 20, 1554. [Google Scholar] [CrossRef]
- Sánchez-Ortega, M.; Carrera, A.C.; Garrido, A. Role of NRF2 in Lung Cancer. Cells 2021, 10, 1879. [Google Scholar] [CrossRef]
- Rao, J.; Qian, X.; Li, G.; Pan, X.; Zhang, C.; Zhang, F.; Zhai, Y.; Wang, X.; Lu, L. ATF3-Mediated NRF2/HO-1 Signaling regulates TLR4 innate immune responses in mouse liver ischemia/reperfusion injury. American Journal of Transplantation 2015, 15, 76–87. [Google Scholar] [CrossRef]
- Kratschmar, D.V.; Calabrese, D.; Walsh, J.; Lister, A.; Birk, J.; Appenzeller-Herzog, C.; Moulin, P.; Goldring, C.E.; Odermatt, A. Suppression of the Nrf2-dependent antioxidant response by glucocorticoids and 11β-HSD1-mediated glucocorticoid activation in hepatic cells. PloS one 2012, 7, e36774. [Google Scholar] [CrossRef]
- Namani, A.; Li, Y.; Wang, X.J.; Tang, X. Modulation of NRF2 signaling pathway by nuclear receptors: implications for cancer. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 2014, 1843, 1875–1885. [Google Scholar] [CrossRef]
- Chen, Q.M.; Maltagliati, A.J. Nrf2 at the heart of oxidative stress and cardiac protection. Physiological Genomics 2018, 50, 77–97. [Google Scholar] [CrossRef]
- Robertson, H.; Dinkova-Kostova, A.T.; Hayes, J.D. Nrf2 and the ambiguous consequences of its activation during initiation and the subsequent stages of tumourigenesis. Cancers 2020, 12, 3609. [Google Scholar] [CrossRef]
- Wu, K.C.; Cui, J.Y.; Klaassen, C.D. Effect of graded Nrf2 activation on phase-I and-II drug metabolizing enzymes and transporters in mouse liver. PloS one 2012, 7, e39006. [Google Scholar] [CrossRef]
- Miao, W.; Hu, L.; Scrivens, P.J.; Batist, G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. Journal of Biological Chemistry 2005, 280, 20340–20348. [Google Scholar] [CrossRef]
- Shen, G.; Kong, A.N. Nrf2 plays an important role in coordinated regulation of Phase II drug metabolism enzymes and Phase III drug transporters. Biopharmaceutics & drug disposition 2009, 30, 345–355. [Google Scholar]
- Wang, X.J.; Li, Y.; Luo, L.; Wang, H.; Chi, Z.; Xin, A.; Li, X.; Wu, J.; Tang, X. Oxaliplatin activates the Keap1/Nrf2 antioxidant system conferring protection against the cytotoxicity of anticancer drugs. Free Radical Biology and Medicine 2014, 70, 68–77. [Google Scholar] [CrossRef]
- Fu, Z.D.; Selwyn, F.P.; Cui, J.Y.; Klaassen, C.D. RNA sequencing quantification of xenobiotic-processing genes in various sections of the intestine in comparison to the liver of male mice. Drug Metabolism and Disposition 2016, 44, 842–856. [Google Scholar] [CrossRef]
- Kohalmy, K.; Vrzal, R. Regulation of phase II biotransformation enzymes by steroid hormones. Current Drug Metabolism 2011, 12, 104–123. [Google Scholar] [CrossRef]
- Jaramillo, A.C.; Saig, F.A.; Cloos, J.; Jansen, G.; Peters, G.J. How to overcome ATP-binding cassette drug efflux transporter-mediated drug resistance? Cancer Drug Resistance 2018, 1, 6–29. [Google Scholar] [CrossRef]
- Mani, M.; Khaghani, S.; Mohammadi, T.G.; Zamani, Z.; Azadmanesh, K.; Meshkani, R.; Pasalar, P.; Mostafavi, E. Activation of Nrf2-antioxidant response element mediated glutamate cysteine ligase expression in hepatoma cell line by homocysteine. Hepatitis monthly 2013, 13. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Meierjohann, S. NRF2-dependent stress defense in tumor antioxidant control and immune evasion. Pigment Cell & Melanoma Research 2021, 34, 268–279. [Google Scholar]
- Dovinova, I.; Kvandova, M.; Balis, P.; Gresova, L.; Majzunova, M.; Horakova, L.; Chan, J.Y.; Barancik, M. The Role of Nrf2 and PPARγ in the Improvement of Oxidative Stress in Hypertension and Cardiovascular Diseases. Physiological Research 2020, 69. [Google Scholar] [CrossRef]
- Giudice, A.; Montella, M. Activation of the Nrf2–ARE signaling pathway: a promising strategy in cancer prevention. Bioessays 2006, 28, 169–181. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Paluszczak, J.; Baer-Dubowska, W. The Nrf2-ARE signaling pathway: an update on its regulation and possible role in cancer prevention and treatment. Pharmacological reports 2017, 69, 393–402. [Google Scholar] [CrossRef]
- Kamiya, T.; Courtney, M.; Laukkanen, M.O. redox-activated signal transduction pathways mediating cellular functions in inflammation, Differentiation, Degeneration, Transformation, and Death. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, J.; Cao, M.; Zhao, Z.; Cao, B.; Yu, S. The potential roles of Nrf2/Keap1 signaling in anticancer drug interactions. Current Research in Pharmacology and Drug Discovery 2021, 2, 100028. [Google Scholar] [CrossRef]
- Gallorini, M.; Carradori, S.; Panieri, E.; Sova, M.; Saso, L. Modulation of NRF2: biological dualism in cancer, targets and possible therapeutic applications. Antioxidants and Redox Signaling 2023. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.-J.; Cheng, X.-D.; Zhang, J.; Zhang, W.-D. Dual roles and therapeutic potential of Keap1-Nrf2 pathway in pancreatic cancer: a systematic review. Cell Communication and Signaling 2019, 17, 1–15. [Google Scholar] [CrossRef]
- Telkoparan-Akillilar, P.; Panieri, E.; Cevik, D.; Suzen, S.; Saso, L. Therapeutic targeting of the NRF2 signaling pathway in cancer. Molecules 2021, 26, 1417. [Google Scholar] [CrossRef]
- Hu, T.; Pan, C.; Zhang, T.; Ni, M.; Wang, W.; Zhang, S.; Chen, Y.; Wang, J.; Fang, Q. Nrf2 overexpression increases the resistance of acute myeloid leukemia to cytarabine by inhibiting replication factor C4. Cancer Gene Therapy 2022, 29, 1773–1790. [Google Scholar] [CrossRef]
- Chartoumpekis, D.V.; Wakabayashi, N.; Kensler, T.W. Keap1/Nrf2 pathway in the frontiers of cancer and non-cancer cell metabolism. Biochemical Society Transactions 2015, 43, 639–644. [Google Scholar] [CrossRef]
- Mitsuishi, Y.; Taguchi, K.; Kawatani, Y.; Shibata, T.; Nukiwa, T.; Aburatani, H.; Yamamoto, M.; Motohashi, H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer cell 2012, 22, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Kleeberger, S.; Bream, J.; Fallon, P.; Kensler, T.; Yamamoto, M.; Reddy, S. Genetic disruption of the Nrf2 compromises cell-cycle progression by impairing GSH-induced redox signaling. Oncogene 2008, 27, 5821–5832. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nature Reviews Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Wang, H.; Liu, K.; Chi, Z.; Zhou, X.; Ren, G.; Zhou, R.; Li, Y.; Tang, X.; Wang, X.J. Interplay of MKP-1 and Nrf2 drives tumor growth and drug resistance in non-small cell lung cancer. Aging (Albany NY) 2019, 11, 11329. [Google Scholar] [CrossRef]
- Niture, S.K.; Jaiswal, A.K. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. Journal of Biological Chemistry 2012, 287, 9873–9886. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Yun, S.-M.; Song, M.-Y.; Jung, K.; Kim, E.-H. Cyanidin chloride induces apoptosis by inhibiting NF-κB signaling through activation of Nrf2 in colorectal cancer cells. Antioxidants 2020, 9, 285. [Google Scholar] [CrossRef]
- Mas, G.; Man, N.; Nakata, Y.; Martinez-Caja, C.; Karl, D.; Beckedorff, F.; Tamiro, F.; Chen, C.; Duffort, S.; Itonaga, H. The SWI/SNF chromatin-remodeling subunit DPF2 facilitates NRF2-dependent antiinflammatory and antioxidant gene expression. The Journal of clinical investigation 2023, 133. [Google Scholar] [CrossRef]
- Chowdhry, S.; Nazmy, M.H.; Meakin, P.J.; Dinkova-Kostova, A.T.; Walsh, S.V.; Tsujita, T.; Dillon, J.F.; Ashford, M.L.; Hayes, J.D. Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radical Biology and Medicine 2010, 48, 357–371. [Google Scholar] [CrossRef]
- Belgorosky, D.; Girouard, J.; Langle, Y.V.; Hamelin-Morrissete, J.; Marino, L.; Agüero, E.I.; Malagrino, H.; Reyes-Moreno, C.; Eiján, A.M. Relevance of iNOS expression in tumor growth and maintenance of cancer stem cells in a bladder cancer model. Journal of Molecular Medicine 2020, 98, 1615–1627. [Google Scholar] [CrossRef]
- Umesalma, S.; Sudhandiran, G. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-κB, iNOS, COX-2, TNF-α, and IL-6 in 1, 2-dimethylhydrazine-induced rat colon carcinogenesis. Basic & clinical pharmacology & toxicology 2010, 107, 650–655. [Google Scholar]
- Paul, S.; Modak, D.; Chattaraj, S.; Nandi, D.; Sarkar, A.; Roy, J.; Chaudhuri, T.K.; Bhattacharjee, S. Aloe vera gel homogenate shows anti-inflammatory activity through lysosomal membrane stabilization and downregulation of TNF-α and Cox-2 gene expressions in inflammatory arthritic animals. Future Journal of Pharmaceutical Sciences 2021, 7, 1–8. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Z.; Tang, M.; Xing, C.; Chen, H.; Zheng, K.; Zhao, Z.; Zhou, S.; Zhao, A.Z.; Li, F. Endogenous production of ω-3 polyunsaturated fatty acids mitigates cisplatin-induced myelosuppression by regulating NRF2-MDM2-p53 signaling pathway. Free Radical Biology and Medicine 2023, 201, 14–25. [Google Scholar] [CrossRef] [PubMed]
- You, A.; Nam, C.-w.; Wakabayashi, N.; Yamamoto, M.; Kensler, T.W.; Kwak, M.-K. Transcription factor Nrf2 maintains the basal expression of Mdm2: an implication of the regulation of p53 signaling by Nrf2. Archives of biochemistry and biophysics 2011, 507, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Wu, J.; Dodson, M.; Rojo de la Vega, E.M.; Ning, Y.; Zhang, Z.; Yao, M.; Zhang, D.D.; Xu, C.; Yi, X. ABCF2, an Nrf2 target gene, contributes to cisplatin resistance in ovarian cancer cells. Molecular carcinogenesis 2017, 56, 1543–1553. [Google Scholar] [CrossRef]
- Orozco-Morales, M.; Hernández-Pedro, N.Y.; Barrios-Bernal, P.; Arrieta, O.; Ruiz-Godoy, L.M.; Aschner, M.; Santamaría, A.; Colín-González, A.L.J.A.-C.D. S-allylcysteine induces cytotoxic effects in two human lung cancer cell lines via induction of oxidative damage, downregulation of Nrf2 and NF-κB, and apoptosis. Anti-Cancer Drugs 2021, 32, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Murakami, K.; Nawamaki, M.; Kashiwakura, I. Effects of Nrf2 knockdown on the properties of irradiated cell conditioned medium from A549 human lung cancer cells. Biomedical reports 2018, 8, 461–465. [Google Scholar] [CrossRef]
- Wiel, C.; Le Gal, K.; Ibrahim, M.X.; Jahangir, C.A.; Kashif, M.; Yao, H.; Ziegler, D.V.; Xu, X.; Ghosh, T.; Mondal, T. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell 2019, 178, 330–345. e322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, H.-J.; Bao, Q.-C.; Wang, L.; Guo, T.-K.; Chen, W.-L.; Xu, L.-L.; Zhou, H.-S.; Bian, J.-L.; Yang, Y.-R. NRF2 promotes breast cancer cell proliferation and metastasis by increasing RhoA/ROCK pathway signal transduction. Oncotarget 2016, 7, 73593. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.C.; Yang, F.; Thorne, R.F.; Zhu, B.K.; Hersey, P.; Zhang, X.D. Human melanoma cells under endoplasmic reticulum stress acquire resistance to microtubule-targeting drugs through XBP-1-mediated activation of Akt. Neoplasia 2009, 11, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Zhai, W.L.; Yang, H.Y.; Jin, H.; Zhang, Q.X. Induction of ER stress protects gastric cancer cells against apoptosis induced by cisplatin and doxorubicin through activation of p38 MAPK. Biochemical and biophysical research communications 2011, 406, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Rauh, M.; Buchfelder, M.; Eyupoglu, I.Y.; Savaskan, N. The oxido-metabolic driver ATF4 enhances temozolamide chemo-resistance in human gliomas. Oncotarget 2017, 8, 51164. [Google Scholar] [CrossRef]
- Salaroglio, I.C.; Panada, E.; Moiso, E.; Buondonno, I.; Provero, P.; Rubinstein, M.; Kopecka, J.; Riganti, C. PERK induces resistance to cell death elicited by endoplasmic reticulum stress and chemotherapy. Molecular cancer 2017, 16, 1–13. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y.; Hua, H.; Li, M.; Luo, T.; Xu, L.; Wang, R.; Liu, D.; Zhang, Y.; Jiang, Y. Blockade of GRP78 sensitizes breast cancer cells to microtubules-interfering agents that induce the unfolded protein response. Journal of cellular and molecular medicine 2009, 13, 3888–3897. [Google Scholar] [CrossRef]
- Kim, J.K.; Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Han, X.; Fernando, P.M.D.J.; Oh, M.C.; Park, J.E.; Shilnikova, K.; Boo, S.J. Endoplasmic reticulum stress induces 5-fluorouracil resistance in human colon cancer cells. Environmental toxicology and pharmacology 2016, 44, 128–133. [Google Scholar] [CrossRef]
- Nahand, J.S.; Rabiei, N.; Fathazam, R.; Taghizadieh, M.; Ebrahimi, M.S.; Mahjoubin-Tehran, M.; Baghi, H.B.; Khatami, A.; Abbasi-Kolli, M.; Mirzaei, H.R. Oncogenic viruses and chemoresistance: What do we know? Pharmacological Research 2021, 105730. [Google Scholar] [CrossRef]
- Tao, W.; Wang, N.; Ruan, J.; Cheng, X.; Fan, L.; Zhang, P.; Lu, C.; Hu, Y.; Che, C.; Sun, D. Enhanced ROS-boosted phototherapy against pancreatic cancer via Nrf2-mediated stress-defense pathway suppression and ferroptosis induction. ACS Applied Materials & Interfaces 2022, 14, 6404–6416. [Google Scholar]
- Fu, D.; Wang, C.; Yu, L.; Yu, R. Induction of ferroptosis by ATF3 elevation alleviates cisplatin resistance in gastric cancer by restraining Nrf2/Keap1/xCT signaling. Cellular & molecular biology letters 2021, 26, 26. [Google Scholar]
- Moon, E.J.; Giaccia, A. Dual roles of NRF2 in tumor prevention and progression: possible implications in cancer treatment. Free Radical Biology and Medicine 2015, 79, 292–299. [Google Scholar] [CrossRef]
- Almasi, S.; El Hiani, Y.J.C. Exploring the therapeutic potential of membrane transport proteins: Focus on cancer and chemoresistance. Cancers 2020, 12, 1624. [Google Scholar] [CrossRef]
- Cole, S.; Bhardwaj, G.; Gerlach, J.; Mackie, J.; Grant, C.; Almquist, K.; Stewart, A.; Kurz, E.; Duncan, A.; Deeley, R.G.J.S. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science signaling 1992, 258, 1650–1654. [Google Scholar] [CrossRef]
- Deeley, R.G.; Cole, S.P.J.F.l. Substrate recognition and transport by multidrug resistance protein 1 (ABCC1). FEBS letters 2006, 580, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Fatemian, T.; Hoque Chowdhury, E. Targeting oncogenes and tumor suppressors genes to mitigate chemoresistance. Current cancer drug targets 2014, 14, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Wang, S.; Moghaddam, S.J.; Ooi, A.; Chapman, E.; Wong, P.K.; Zhang, D.D. Oncogenic KRAS confers chemoresistance by upregulating NRF2. Cancer research 2014, 74, 7430–7441. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-S.; Du, J.; Fan, Y.-J.; Liu, F.-J.; Cao, L.-L.; Liang, N.; Xu, D.-G.; Zhang, J.-D. Activation of endoplasmic reticulum stress promotes autophagy and apoptosis and reverses chemoresistance of human small cell lung cancer cells by inhibiting the PI3K/AKT/mTOR signaling pathway. Oncotarget 2016, 7, 76827. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guan, Z.; Liang, L.; Cheng, Y.; Zhou, J.; Li, J.; Xu, Y. NF-κB signaling plays irreplaceable roles in cisplatin-induced bladder cancer chemoresistance and tumor progression. International journal of oncology 2016, 48, 225–234. [Google Scholar] [CrossRef]
- Dong, Q.; Fu, L.; Zhao, Y.; Tan, S.; Wang, E. Derlin-1 overexpression confers poor prognosis in muscle invasive bladder cancer and contributes to chemoresistance and invasion through PI3K/AKT and ERK/MMP signaling. Oncotarget 2017, 8, 17059. [Google Scholar] [CrossRef] [PubMed]
- Almasan, A.; Yin, Y.; Kelly, R.E.; Lee, E.; Bradley, A.; Li, W.; Bertino, J.R.; Wahl, G.M. Deficiency of retinoblastoma protein leads to inappropriate S-phase entry, activation of E2F-responsive genes, and apoptosis. Proceedings of the National Academy of Sciences 1995, 92, 5436–5440. [Google Scholar] [CrossRef]
- Knudsen, K.E.; Booth, D.; Naderi, S.; Sever-Chroneos, Z.; Fribourg, A.F.; Hunton, I.C.; Feramisco, J.R.; Wang, J.Y.; Knudsen, E.S. RB-dependent S-phase response to DNA damage. Molecular and cellular biology 2000, 20, 7751–7763. [Google Scholar] [CrossRef]
- Sharma, A.; Comstock, C.E.; Knudsen, E.S.; Cao, K.H.; Hess-Wilson, J.K.; Morey, L.M.; Barrera, J.; Knudsen, K.E. Retinoblastoma tumor suppressor status is a critical determinant of therapeutic response in prostate cancer cells. Cancer research 2007, 67, 6192–6203. [Google Scholar] [CrossRef] [PubMed]
- Bosco, E.E.; Wang, Y.; Xu, H.; Zilfou, J.T.; Knudsen, K.E.; Aronow, B.J.; Lowe, S.W.; Knudsen, E.S. The retinoblastoma tumor suppressor modifies the therapeutic response of breast cancer. The Journal of clinical investigation 2007, 117, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Derenzini, M.; Donati, G.; Mazzini, G.; Montanaro, L.; Vici, M.; Ceccarelli, C.; Santini, D.; Taffurelli, M.; Treré, D. Loss of retinoblastoma tumor suppressor protein makes human breast cancer cells more sensitive to antimetabolite exposure. Clinical cancer research 2008, 14, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Stengel, K.R.; Dean, J.L.; Seeley, S.L.; Mayhew, C.N.; Knudsen, E.S. RB status governs differential sensitivity to cytotoxic and molecularly-targeted therapeutic agents. Cell cycle 2008, 7, 1095–1103. [Google Scholar] [CrossRef]
- Chow, L.; Wang, X.; Kwong, D.; Sham, J.; Tsao, S.; Nicholls, J.M. Effect of p16INK4a on chemosensitivity in nasopharyngeal carcinoma cells. International journal of oncology 2000, 17, 135–175. [Google Scholar] [CrossRef]
- Barboule, N.; Chadebech, P.; Baldin, V.; Vidal, S.; Valette, A. Involvement of p21 in mitotic exit after paclitaxel treatment in MCF-7 breast adenocarcinoma cell line. Oncogene 1997, 15, 2867–2875. [Google Scholar] [CrossRef]
- Yu, D.; Jing, T.; Liu, B.; Yao, J.; Tan, M.; McDonnell, T.J.; Hung, M.-C. Overexpression of ErbB2 blocks Taxol-induced apoptosis by upregulation of p21Cip1, which inhibits p34Cdc2 kinase. Molecular cell 1998, 2, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.J.; Han, J.; Kim, S.J.; Lee, M.J.; Ju, X.; Lee, Y.L.; Son, J.H.; Cui, J.; Jang, Y.; Chung, W.J.O.R. PTEN/AKT signaling mediates chemoresistance in refractory acute myeloid leukemia through enhanced glycolysis. Oncology Reports 2019, 42, 2149–2158. [Google Scholar] [CrossRef]
- Singh, M.; Chaudhry, P.; Fabi, F.; Asselin, E.J.B.c. Cisplatin-induced caspase activation mediates PTEN cleavage in ovarian cancer cells: a potential mechanism of chemoresistance. BMC cancer 2013, 13, 1–9. [Google Scholar] [CrossRef]
- Narod, S.A.; Foulkes, W.D. BRCA1 and BRCA2: 1994 and beyond. Nature Reviews Cancer 2004, 4, 665–676. [Google Scholar] [CrossRef]
- Dapic, V.; Monteiro, A.N. Functional implications of BRCA1 for early detection, prevention, and treatment of breast cancer. Critical Reviews™ in Eukaryotic Gene Expression 2006, 16. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.-X. BRCA1: cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic acids research 2006, 34, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-H.; Yu, H.; Deng, C.-X. A requirement for breast-cancer-associated gene 1 (BRCA1) in the spindle checkpoint. Proceedings of the National Academy of Sciences 2004, 101, 17108–17113. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wei, J.; Qian, X.; Ding, Y.; Yu, L.; Liu, B. Gambogic acid, a potent inhibitor of survivin, reverses docetaxel resistance in gastric cancer cells. Cancer letters 2008, 262, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Jones, A.; Fassone, E.; Sweeney, M.; Lebiedzinska, M.; Suski, J.; Wieckowski, M.; Tajeddine, N.; Hargreaves, I.; Yasukawa, T. PGC-1β mediates adaptive chemoresistance associated with mitochondrial DNA mutations. Oncogene 2013, 32, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Liang, W.; Liu, J.; Zhang, L.; Wei, J.; Yang, J.; Zhang, Y.; Huang, Z. Autophagy-mediating microRNAs in cancer chemoresistance. Cell biology and toxicology 2020, 1–20. [Google Scholar] [CrossRef]
- Takenaka, T.; Yoshino, I.; Kouso, H.; Ohba, T.; Yohena, T.; Osoegawa, A.; Shoji, F.; Maehara, Y. Combined evaluation of Rad51 and ERCC1 expressions for sensitivity to platinum agents in non-small cell lung cancer. International journal of cancer 2007, 121, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Shirabe, K.; Morita, K.; Umeda, K.; Kayashima, H.; Uchiyama, H.; Soejima, Y.; Taketomi, A.; Maehara, Y. Evaluation of ERCC1 expression for cisplatin sensitivity in human hepatocellular carcinoma. Annals of surgical oncology 2011, 18, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Dastghaib, S.; Hajiahmadi, S.; Seyfoori, A.; Amereh, M.; Zamani, M.; Shahsavari, Z.; Shojaei, S.; Akbari, M.; Mokarram, P.; Ghavami, S. Role of apoptosis, autophagy, and the unfolded protein response in glioblastoma chemoresistance. In Glioblastoma Resistance to Chemotherapy: Molecular Mechanisms and Innovative Reversal Strategies; Elsevier: 2021; pp. 201-242.
- Lee, Y.J.; Won, A.J.; Lee, J.; Jung, J.H.; Yoon, S.; Lee, B.M.; Kim, H.S. Molecular mechanism of SAHA on regulation of autophagic cell death in tamoxifen-resistant MCF-7 breast cancer cells. International journal of medical sciences 2012, 9, 881. [Google Scholar] [CrossRef]
- Yeldag, G.; Rice, A.; del Río Hernández, A. Chemoresistance and the self-maintaining tumor microenvironment. Cancers 2018, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.-C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950. [Google Scholar] [CrossRef] [PubMed]
- Kahroba, H.; Shirmohamadi, M.; Hejazi, M.S.; Samadi, N. The Role of Nrf2 signaling in cancer stem cells: From stemness and self-renewal to tumorigenesis and chemoresistance. Life sciences 2019, 239, 116986. [Google Scholar] [CrossRef]
- Barrera, G.; Cucci, M.A.; Grattarola, M.; Dianzani, C.; Muzio, G.; Pizzimenti, S. Control of Oxidative Stress in Cancer Chemoresistance: Spotlight on Nrf2 Role. Antioxidants 2021, 10, 510. [Google Scholar] [CrossRef]
- Hetz, C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nature reviews Molecular cell biology 2012, 13, 89–102. [Google Scholar] [CrossRef]
- McMellen, A.; Yamamoto, T.M.; Qamar, L.; Sanders, B.E.; Nguyen, L.L.; Ortiz Chavez, D.; Bapat, J.; Berning, A.; Post, M.D.; Johnson, J. ATF6-mediated signaling contributes to PARP Inhibitor Resistance in Ovarian Cancer. Molecular Cancer Research 2023, 21, 3–13. [Google Scholar] [CrossRef]
- Shi, Z.; Yu, X.; Yuan, M.; Lv, W.; Feng, T.; Bai, R.; Zhong, H. Activation of the PERK-ATF4 pathway promotes chemo-resistance in colon cancer cells. Scientific reports 2019, 9, 3210. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, H.; Yang, Y.; Zhao, D.; Wen, Y.; Lv, C.; Qiu, H.; Wang, C. The regulation of miR-320a/XBP1 axis through LINC00963 for endoplasmic reticulum stress and autophagy in diffuse large B-cell lymphoma. Cancer cell international 2021, 21, 1–13. [Google Scholar] [CrossRef]
- Gifford, J.B.; Huang, W.; Zeleniak, A.E.; Hindoyan, A.; Wu, H.; Donahue, T.R.; Hill, R. Expression of GRP78, master regulator of the unfolded protein response, increases chemoresistance in pancreatic ductal adenocarcinoma. Molecular cancer therapeutics 2016, 15, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Dauer, P.; Sharma, N.S.; Gupta, V.K.; Nomura, A.; Dudeja, V.; Saluja, A.; Banerjee, S. GRP78-mediated antioxidant response and ABC transporter activity confers chemoresistance to pancreatic cancer cells. Molecular oncology 2018, 12, 1498–1512. [Google Scholar] [CrossRef] [PubMed]
- Lanzillotta, C.; Zuliani, I.; Tramutola, A.; Barone, E.; Blarzino, C.; Folgiero, V.; Caforio, M.; Valentini, D.; Villani, A.; Locatelli, F. Chronic PERK induction promotes Alzheimer-like neuropathology in Down syndrome: Insights for therapeutic intervention. Progress in Neurobiology 2021, 196, 101892. [Google Scholar] [CrossRef]
- Wei, R.; Zhao, Y.; Wang, J.; Yang, X.; Li, S.; Wang, Y.; Yang, X.; Fei, J.; Hao, X.; Zhao, Y. Tagitinin C induces ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal cancer cells. International journal of biological sciences 2021, 17, 2703. [Google Scholar] [CrossRef] [PubMed]
- Küper, A.; Baumann, J.; Göpelt, K.; Baumann, M.; Sänger, C.; Metzen, E.; Kranz, P.; Brockmeier, U. Overcoming hypoxia-induced resistance of pancreatic and lung tumor cells by disrupting the PERK-NRF2-HIF-axis. Cell Death & Disease 2021, 12, 82. [Google Scholar]
- Lanzillotta, C.; Zuliani, I.; Tramutola, A.; Abisambra, J.F.; Barone, E.; Perluigi, M.; Domenico, F.D. PERK inhibition promotes the rescue of protein translation and Nrf2-related antioxidant response: Molecular and cell biology/oxidative stress. Alzheimer's & Dementia 2020, 16, e041867. [Google Scholar]
- Ivanova, I.G.; Park, C.V.; Yemm, A.I.; Kenneth, N.S. PERK/eIF2α signaling inhibits HIF-induced gene expression during the unfolded protein response via YB1-dependent regulation of HIF1α translation. Nucleic acids research 2018, 46, 3878–3890. [Google Scholar] [CrossRef]
- Akman, M.; Belisario, D.C.; Salaroglio, I.C.; Kopecka, J.; Donadelli, M.; De Smaele, E.; Riganti, C. Hypoxia, endoplasmic reticulum stress and chemoresistance: dangerous liaisons. Journal of Experimental & Clinical Cancer Research 2021, 40, 28. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxidative medicine and cellular longevity 2016, 2016, 3164734–3164734. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: the bright side of the moon. Experimental & Molecular Medicine 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Digaleh, H.; Kiaei, M.; Khodagholi, F. Nrf2 and Nrf1 signaling and ER stress crosstalk: implication for proteasomal degradation and autophagy. Cellular and Molecular Life Sciences 2013, 70, 4681–4694. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zou, Z. Targeting autophagy to overcome drug resistance: further developments. Journal of hematology & oncology 2020, 13, 1–18. [Google Scholar]
- Eskelinen, E.-L. The dual role of autophagy in cancer. Current opinion in pharmacology 2011, 11, 294–300. [Google Scholar] [CrossRef]
- Al Dhaheri, Y.; Attoub, S.; Ramadan, G.; Arafat, K.; Bajbouj, K.; Karuvantevida, N.; AbuQamar, S.; Eid, A.; Iratni, R. Carnosol induces ROS-mediated beclin1-independent autophagy and apoptosis in triple negative breast cancer. PloS one 2014, 9, e109630. [Google Scholar] [CrossRef]
- Shin, D.; Kim, E.H.; Lee, J.; Roh, J.-L. RITA plus 3-MA overcomes chemoresistance of head and neck cancer cells via dual inhibition of autophagy and antioxidant systems. Redox Biology 2017, 13, 219–227. [Google Scholar] [CrossRef]
- Du, F.; Feng, Y.; Fang, J.; Yang, M. MicroRNA-143 enhances chemosensitivity of Quercetin through autophagy inhibition via target GABARAPL1 in gastric cancer cells. Biomedicine & Pharmacotherapy 2015, 74, 169–177. [Google Scholar]
- Poillet-Perez, L.; Despouy, G.; Delage-Mourroux, R.; Boyer-Guittaut, M. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biology 2015, 4, 184–192. [Google Scholar] [CrossRef]
- Chavez-Dominguez, R.; Perez-Medina, M.; Lopez-Gonzalez, J.S.; Galicia-Velasco, M.; Aguilar-Cazares, D. The Double-Edge Sword of Autophagy in Cancer: From Tumor Suppression to Pro-tumor Activity. Frontiers in oncology 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, J.; Chen, J.; Hu, Q.; Gu, C.; Lin, W.; Chen, G. Endoplasmic reticulum stress is associated with neuroprotection against apoptosis via autophagy activation in a rat model of subarachnoid hemorrhage. Neuroscience letters 2014, 563, 160–165. [Google Scholar] [CrossRef]
- Shimodaira, Y.; Takahashi, S.; Kinouchi, Y.; Endo, K.; Shiga, H.; Kakuta, Y.; Kuroha, M.; Shimosegawa, T. Modulation of endoplasmic reticulum (ER) stress-induced autophagy by C/EBP homologous protein (CHOP) and inositol-requiring enzyme 1α (IRE1α) in human colon cancer cells. Biochemical and biophysical research communications 2014, 445, 524–533. [Google Scholar] [CrossRef]
- Rubiolo, J.; López-Alonso, H.; Martínez, P.; Millán, A.; Cagide, E.; Vieytes, M.; Vega, F.; Botana, L. Yessotoxin induces ER-stress followed by autophagic cell death in glioma cells mediated by mTOR and BNIP3. Cellular signalling 2014, 26, 419–432. [Google Scholar] [CrossRef]
- Grandjean, J.M.; Madhavan, A.; Cech, L.; Seguinot, B.O.; Paxman, R.J.; Smith, E.; Scampavia, L.; Powers, E.T.; Cooley, C.B.; Plate, L. Pharmacologic IRE1/XBP1s activation confers targeted ER proteostasis reprogramming. Nature chemical biology 2020, 16, 1052–1061. [Google Scholar] [CrossRef]
- Hetz, C.; Martinon, F.; Rodriguez, D.; Glimcher, L.H. The unfolded protein response: integrating stress signals through the stress sensor IRE1α. Physiological reviews 2011, 91, 1219–1243. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, J.; Ma, Y.; Lu, L.; Ma, C.; Qin, P.; Gao, E.; Zuo, M.; Yang, J.; Yang, L. Electroacupuncture Pretreatment Mitigates Myocardial Ischemia/Reperfusion Injury via XBP1/GRP78/Akt Pathway. Frontiers in Cardiovascular Medicine 2021, 8. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, M.; Yu, Y.; Li, S.; Ma, C.; Zhang, J.; Jiang, Y.; Li, Y.; Zheng, X.; Zhang, L. BCAT1 binds the RNA-binding protein ZNF423 to activate autophagy via the IRE1-XBP-1-RIDD axis in hypoxic PASMCs. Cell death & disease 2020, 11, 1–16. [Google Scholar]
- Zhang, L.; Zhang, C.; Wang, A. Divergence and conservation of the major UPR branch IRE1-bZIP signaling pathway across eukaryotes. Scientific reports 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Park, S.-M.; Kang, T.-I.; So, J.-S. Roles of XBP1s in Transcriptional Regulation of Target Genes. Biomedicines 2021, 9, 791. [Google Scholar] [CrossRef]
- Gomez, B.P.; Riggins, R.B.; Shajahan, A.N.; Klimach, U.; Wang, A.; Crawford, A.C.; Zhu, Y.; Zwart, A.; Wang, M.; Clarke, R. Human X-box binding protein-1 confers both estrogen independence and antiestrogen resistance in breast cancer cell lines. The FASEB Journal 2007, 21, 4013–4027. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, W.; Niu, Q.; Sun, Y.; Meng, C.; Tan, L.; Song, C.; Qiu, X.; Liao, Y.; Ding, C. eIF2α-CHOP-BCl-2/JNK and IRE1α-XBP1/JNK signaling promote apoptosis and inflammation and support the proliferation of Newcastle disease virus. Cell death & disease 2019, 10, 1–15. [Google Scholar]
- Ogata, M.; Hino, S.-i.; Saito, A.; Morikawa, K.; Kondo, S.; Kanemoto, S.; Murakami, T.; Taniguchi, M.; Tanii, I.; Yoshinaga, K. Autophagy is activated for cell survival after endoplasmic ReticulumStress. Molecular and cellular biology 2006, 26, 9220–9231. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.-G.; Jiang, Z.-X.; Li, J.-H.; Zhou, Z.; Zhang, Q.-H. Spliced XBP1 promotes macrophage survival and autophagy by interacting with Beclin-1. Biochemical and biophysical research communications 2015, 463, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Margariti, A.; Li, H.; Chen, T.; Martin, D.; Vizcay-Barrena, G.; Alam, S.; Karamariti, E.; Xiao, Q.; Zampetaki, A.; Zhang, Z. XBP1 mRNA splicing triggers an autophagic response in endothelial cells through BECLIN-1 transcriptional activation. Journal of Biological Chemistry 2013, 288, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Piperi, C.; Adamopoulos, C.; Papavassiliou, A.G. XBP1: a pivotal transcriptional regulator of glucose and lipid metabolism. Trends in Endocrinology & Metabolism 2016, 27, 119–122. [Google Scholar]
- Suzuki, H.; Kanekura, K.; Levine, T.P.; Kohno, K.; Olkkonen, V.M.; Aiso, S.; Matsuoka, M. ALS-linked P56S-VAPB, an aggregated loss-of-function mutant of VAPB, predisposes motor neurons to ER stress-related death by inducing aggregation of co-expressed wild-type VAPB. Journal of neurochemistry 2009, 108, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Ghemrawi, R.; Khair, M. Endoplasmic reticulum stress and unfolded protein response in neurodegenerative diseases. International journal of molecular sciences 2020, 21, 6127. [Google Scholar] [CrossRef] [PubMed]
- Vidal, R.L.; Figueroa, A.; Court, F.A.; Thielen, P.; Molina, C.; Wirth, C.; Caballero, B.; Kiffin, R.; Segura-Aguilar, J.; Cuervo, A.M. Targeting the UPR transcription factor XBP1 protects against Huntington's disease through the regulation of FoxO1 and autophagy. Human molecular genetics 2012, 21, 2245–2262. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lei, H.; Zhang, B.-G.; Xu, Z.-C.; Dong, C.; Hao, Y.-Q. c-Jun NH2-terminal kinase (JNK)/stress-activated protein kinase-associated protein 1 is a critical regulator for arthritis progression by meditating inflammation in mice model. International immunopharmacology 2020, 81, 106272. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, D.; Li, Y.; Qiao, H.; Shan, Z. Ketamine enhances autophagy and endoplasmic reticulum stress in rats and SV-HUC-1 cells via activating IRE1-TRAF2-ASK1-JNK pathway. Cell Cycle 2021, 1–16. [Google Scholar] [CrossRef]
- Wang, L.; Wang, P.; Dong, H.; Wang, S.; Chu, H.; Yan, W.; Zhang, X. Ulk1/FUNDC1 prevents nerve cells from hypoxia-induced apoptosis by promoting cell autophagy. Neurochemical research 2018, 43, 1539–1548. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Li, H.; Wei, C.; Mao, A.; Liu, W.; Pan, G. Polyphyllin D induces apoptosis and protective autophagy in breast cancer cells through JNK1-Bcl-2 pathway. Journal of Ethnopharmacology 2021, 114591. [Google Scholar] [CrossRef]
- Thorpe, J.A.; Schwarze, S.R. IRE1α controls cyclin A1 expression and promotes cell proliferation through XBP-1. Cell Stress and Chaperones 2010, 15, 497–508. [Google Scholar] [CrossRef]
- Gambella, M.; Rocci, A.; Passera, R.; Gay, F.; Omedè, P.; Crippa, C.; Corradini, P.; Romano, A.; Rossi, D.; Ladetto, M. High XBP1 expression is a marker of better outcome in multiple myeloma patients treated with bortezomib. Haematologica 2014, 99, e14. [Google Scholar] [CrossRef]
- Yang, W.; Xu, X.; Xu, M.; Zhou, J.; Xi, Z.; Guo, H.; Ming, J.; Huang, T. XBP1s Acts as a Tumor Suppressor to Inhibit the EMT Process and Metastasis of Papillary Thyroid Cancer. OncoTargets and therapy 2021, 14, 2339. [Google Scholar] [CrossRef]
- Mimura, N.; Fulciniti, M.; Gorgun, G.; Tai, Y.-T.; Cirstea, D.; Santo, L.; Hu, Y.; Fabre, C.; Minami, J.; Ohguchi, H. Blockade of XBP1 splicing by inhibition of IRE1α is a promising therapeutic option in multiple myeloma. Blood, The Journal of the American Society of Hematology 2012, 119, 5772–5781. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Iliopoulos, D.; Zhang, Q.; Tang, Q.; Greenblatt, M.B.; Hatziapostolou, M.; Lim, E.; Tam, W.L.; Ni, M.; Chen, Y. XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature 2014, 508, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Logue, S.E.; McGrath, E.P.; Cleary, P.; Greene, S.; Mnich, K.; Almanza, A.; Chevet, E.; Dwyer, R.M.; Oommen, A.; Legembre, P. Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy. Nature communications 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-A.; Fang, S.-U.; Su, C.-L.; Hsiao, C.-J.; Chang, C.-C.; Lin, Y.-F.; Cheng, C.-W. Silencing glucose-regulated protein 78 induced renal cell carcinoma cell line G1 cell-cycle arrest and resistance to conventional chemotherapy. In Proceedings of the Urologic Oncology: Seminars and Original Investigations, 2014; pp. 29. e21–29. e11. [Google Scholar]
- Raiter, A.; Lipovetsky, J.; Hyman, L.; Mugami, S.; Ben-Zur, T.; Yerushalmi, R. Chemotherapy Controls Metastasis Through Stimulatory Effects on GRP78 and Its Transcription Factor CREB3L1. Frontiers in oncology 2020, 10, 1500. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gronow, M.; Gopal, U.; Austin, R.C.; Pizzo, S.V. Glucose-regulated protein (GRP78) is an important cell surface receptor for viral invasion, cancers and neurological disorders. IUBMB life 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-y.; Yu, J.-y.; Leng, Y.-l.; Zhu, R.-r.; Liu, H.-x.; Wang, X.-y.; Yang, T.-t.; Guo, Y.-n.; Tang, J.-l.; Zhang, X.-c. MiR-181c sensitizes ovarian cancer cells to paclitaxel by targeting GRP78 through the PI3K/Akt pathway. Cancer Gene Therapy 2021, 1–14. [Google Scholar] [CrossRef]
- Suyama, K.; Watanabe, M.; Sakabe, K.; Okada, Y.; Matsuyama, D.; Kuroiwa, M.; Mochida, J. Overexpression of GRP78 protects glial cells from endoplasmic reticulum stress. Neuroscience letters 2011, 504, 271–276. [Google Scholar] [CrossRef]
- Elfiky, A.A.; Baghdady, A.M.; Ali, S.A.; Ahmed, M.I. GRP78 targeting: Hitting two birds with a stone. Life sciences 2020, 118317. [Google Scholar] [CrossRef]
- Yan, M.M.; Ni, J.D.; Song, D.; Ding, M.; Huang, J. Interplay between unfolded protein response and autophagy promotes tumor drug resistance. Oncology letters 2015, 10, 1959–1969. [Google Scholar] [CrossRef]
- Wang, Y.-c.; Li, X.; Shen, Y.; Lyu, J.; Sheng, H.; Paschen, W.; Yang, W. PERK (protein kinase RNA-like ER kinase) branch of the unfolded protein response confers neuroprotection in ischemic stroke by suppressing protein synthesis. Stroke 2020, 51, 1570–1577. [Google Scholar] [CrossRef]
- Teske, B.F.; Baird, T.D.; Wek, R.C. Methods for analyzing eIF2 kinases and translational control in the unfolded protein response. Methods in enzymology 2011, 490, 333–356. [Google Scholar]
- B'chir, W.; Chaveroux, C.; Carraro, V.; Averous, J.; Maurin, A.-C.; Jousse, C.; Muranishi, Y.; Parry, L.; Fafournoux, P.; Bruhat, A. Dual role for CHOP in the crosstalk between autophagy and apoptosis to determine cell fate in response to amino acid deprivation. Cellular signalling 2014, 26, 1385–1391. [Google Scholar] [CrossRef]
- Wang, J.; Kang, R.; Huang, H.; Xi, X.; Wang, B.; Wang, J.; Zhao, Z. Hepatitis C virus core protein activates autophagy through EIF2AK3 and ATF6 UPR pathway-mediated MAP1LC3B and ATG12 expression. Autophagy 2014, 10, 766–784. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Cheon, C.; Ko, S.-G. SH003 activates autophagic cell death by activating ATF4 and inhibiting G9a under hypoxia in gastric cancer cells. Cell death & disease 2020, 11, 1–14. [Google Scholar]
- Ma, X.-H.; Piao, S.-F.; Dey, S.; Mcafee, Q.; Karakousis, G.; Villanueva, J.; Hart, L.S.; Levi, S.; Hu, J.; Zhang, G.J.T.J.o.c.i. Targeting ER stress–induced autophagy overcomes BRAF inhibitor resistance in melanoma. The Journal of clinical investigation 2014, 124, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Tan, J.; Miao, Y.; Sun, Z.; Zhang, Q. Intermittent-hypoxia-induced autophagy activation through the ER-stress-related PERK/eIF2α/ATF4 pathway is a protective response to pancreatic β-cell apoptosis. Cellular Physiology and Biochemistry 2018, 51, 2955–2971. [Google Scholar] [CrossRef] [PubMed]
- Gorman, A.M.; Healy, S.J.; Jäger, R.; Samali, A. Stress management at the ER: regulators of ER stress-induced apoptosis. Pharmacology & therapeutics 2012, 134, 306–316. [Google Scholar]
- Wu, M.-Z.; Fu, T.; Chen, J.-X.; Lin, Y.-Y.; Yang, J.-E.; Zhuang, S.-M. LncRNA GOLGA2P10 is induced by PERK/ATF4/CHOP signaling and protects tumor cells from ER stress-induced apoptosis by regulating Bcl-2 family members. Cell death & disease 2020, 11, 1–12. [Google Scholar]
- Ohoka, N.; Yoshii, S.; Hattori, T.; Onozaki, K.; Hayashi, H. TRB3, a novel ER stress-inducible gene, is induced via ATF4–CHOP pathway and is involved in cell death. The EMBO journal 2005, 24, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Alers, S.; Löffler, A.S.; Wesselborg, S.; Stork, B.J.M.; biology, c. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Molecular and cellular biology 2012, 32, 2–11. [Google Scholar] [CrossRef]
- B’chir, W.; Maurin, A.-C.; Carraro, V.; Averous, J.; Jousse, C.; Muranishi, Y.; Parry, L.; Stepien, G.; Fafournoux, P.; Bruhat, A. The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic acids research 2013, 41, 7683–7699. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.E.; Eom, J.-I.; Jeung, H.-K.; Chung, H.; Kim, Y.R.; Kim, J.S.; Cheong, J.-W.; Min, Y.H. PERK/NRF2 and autophagy form a resistance mechanism against G9a inhibition in leukemia stem cells. Journal of Experimental & Clinical Cancer Research 2020, 39, 1–14. [Google Scholar]
- Cullinan, S.B.; Diehl, J.A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. Journal of Biological Chemistry 2004, 279, 20108–20117. [Google Scholar] [CrossRef] [PubMed]
- Bobrovnikova-Marjon, E.; Grigoriadou, C.; Pytel, D.; Zhang, F.; Ye, J.; Koumenis, C.; Cavener, D.; Diehl, J.A. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene 2010, 29, 3881–3895. [Google Scholar] [CrossRef]
- Krishnamoorthy, J.; Rajesh, K.; Mirzajani, F.; Kesoglidou, P.; Papadakis, A.; Koromilas, A.E. Evidence for eIF2α phosphorylation-independent effects of GSK2656157, a novel catalytic inhibitor of PERK with clinical implications. Cell Cycle 2014, 13, 801–806. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Bobrovnikova-Marjon, E.; Ji, X.; Liebhaber, S.A.; Diehl, J.A. PERK-dependent regulation of IAP translation during ER stress. Oncogene 2009, 28, 910–920. [Google Scholar] [CrossRef]
- Podszywalow-Bartnicka, P.; Cmoch, A.; Wolczyk, M.; Bugajski, L.; Tkaczyk, M.; Dadlez, M.; Nieborowska-Skorska, M.; Koromilas, A.E.; Skorski, T.; Piwocka, K. Increased phosphorylation of eIF2α in chronic myeloid leukemia cells stimulates secretion of matrix modifying enzymes. Oncotarget 2016, 7, 79706. [Google Scholar] [CrossRef]
- Kusio-Kobialka, M.; Podszywalow-Bartnicka, P.; Peidis, P.; Glodkowska-Mrowka, E.; Wolanin, K.; Leszak, G.; Seferynska, I.; Stoklosa, T.; Koromilas, A.E.; Piwocka, K. The PERK-eIF2α phosphorylation arm is a pro-survival pathway of BCR-ABL signaling and confers resistance to imatinib treatment in chronic myeloid leukemia cells. Cell Cycle 2012, 11, 4069–4078. [Google Scholar] [CrossRef]
- Fujimoto, A.; Kawana, K.; Taguchi, A.; Adachi, K.; Sato, M.; Nakamura, H.; Ogishima, J.; Yoshida, M.; Inoue, T.; Nishida, H. Inhibition of endoplasmic reticulum (ER) stress sensors sensitizes cancer stem-like cells to ER stress-mediated apoptosis. Oncotarget 2016, 7, 51854. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Hong, M.-Y.; Pei, J.; Gao, Y.-H.; Zheng, Y.; Xu, X. ER stress mediated-autophagy contributes to neurological dysfunction in traumatic brain injury via the ATF6 UPR signaling pathway. Molecular Medicine Reports 2021, 23, 1–1. [Google Scholar] [CrossRef]
- Bommiasamy, H.; Back, S.H.; Fagone, P.; Lee, K.; Meshinchi, S.; Vink, E.; Sriburi, R.; Frank, M.; Jackowski, S.; Kaufman, R.J. ATF6α induces XBP1-independent expansion of the endoplasmic reticulum. Journal of cell science 2009, 122, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Gade, P.; Ramachandran, G.; Maachani, U.B.; Rizzo, M.A.; Okada, T.; Prywes, R.; Cross, A.S.; Mori, K.; Kalvakolanu, D.V. An IFN-γ–stimulated ATF6–C/EBP-β–signaling pathway critical for the expression of Death Associated Protein Kinase 1 and induction of autophagy. Proceedings of the National Academy of Sciences 2012, 109, 10316–10321. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, S.; Dai, C.; Tang, S.; Yang, X.; Li, D.; Zhao, K.; Xiao, X. Quinocetone triggered ER stress-induced autophagy via ATF6/DAPK1-modulated mAtg9a trafficking. Cell biology and toxicology 2016, 32, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.M.; Ni, J.D.; Song, D.; Ding, M.; Huang, J.J.O.L. Interplay between unfolded protein response and autophagy promotes tumor drug resistance. Oncology Letters 2015, 10, 1959–1969. [Google Scholar] [CrossRef]
- Higa, A.; Taouji, S.; Lhomond, S.; Jensen, D.; Fernandez-Zapico, M.E.; Simpson, J.C.; Pasquet, J.-M.; Schekman, R.; Chevet, E. Endoplasmic reticulum stress-activated transcription factor ATF6α requires the disulfide isomerase PDIA5 to modulate chemoresistance. Molecular and cellular biology 2014, 34, 1839–1849. [Google Scholar] [CrossRef]
- Tay, K.H.; Luan, Q.; Croft, A.; Jiang, C.C.; Jin, L.; Zhang, X.D.; Tseng, H.-Y. Sustained IRE1 and ATF6 signaling is important for survival of melanoma cells undergoing ER stress. Cellular signalling 2014, 26, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Min, H.-Y.; Pei, H.; Wei, X.; Sim, J.Y.; Park, S.-H.; Hwang, S.J.; Lee, H.-J.; Hong, S.; Shin, Y.K. The ATF6-EGF Pathway Mediates the Awakening of Slow-Cycling Chemoresistant Cells and Tumor Recurrence by Stimulating Tumor Angiogenesis. Cancers 2020, 12, 1772. [Google Scholar] [CrossRef]
- Schewe, D.M.; Aguirre-Ghiso, J.A. ATF6α-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proceedings of the National Academy of Sciences 2008, 105, 10519–10524. [Google Scholar] [CrossRef]
- Li, J.; Ni, M.; Lee, B.; Barron, E.; Hinton, D.; Lee, A. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death & Differentiation 2008, 15, 1460–1471. [Google Scholar]
- Rashid, M.-u.; Lorzadeh, S.; Gao, A.; Ghavami, S.; Coombs, K.M. PSMA2 knockdown impacts expression of proteins involved in immune and cellular stress responses in human lung cells. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 2023, 1869, 166617. [Google Scholar] [CrossRef]
- Cook, K.L.; Shajahan, A.N.; Wärri, A.; Jin, L.; Hilakivi-Clarke, L.A.; Clarke, R. Glucose-regulated protein 78 controls cross-talk between apoptosis and autophagy to determine antiestrogen responsiveness. Cancer research 2012, 72, 3337–3349. [Google Scholar] [CrossRef]
- Shimada, Y.; Kobayashi, H.; Kawagoe, S.; Aoki, K.; Kaneshiro, E.; Shimizu, H.; Eto, Y.; Ida, H.; Ohashi, T. Endoplasmic reticulum stress induces autophagy through activation of p38 MAPK in fibroblasts from Pompe disease patients carrying c. 546G> T mutation. Molecular genetics and metabolism 2011, 104, 566–573. [Google Scholar] [CrossRef]
- Verchot, J. The ER quality control and ER associated degradation machineries are vital for viral pathogenesis. Frontiers in plant science 2014, 5, 66. [Google Scholar] [CrossRef]
- Lemberg, M.K.; Strisovsky, K. Maintenance of organellar protein homeostasis by ER-associated degradation and related mechanisms. Molecular Cell 2021. [Google Scholar] [CrossRef]
- Cybulsky, A.V. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nature Reviews Nephrology 2017, 13, 681–696. [Google Scholar] [CrossRef]
- Ferro-Novick, S.; Reggiori, F.; Brodsky, J.L. ER-Phagy, ER homeostasis, and ER quality control: implications for disease. Trends in Biochemical Sciences 2021. [Google Scholar] [CrossRef]
- Peng, Y.; Guo, L.; Gu, A.; Shi, B.; Ren, Y.; Cong, J.; Yang, X. Electroacupuncture alleviates polycystic ovary syndrome-like symptoms through improving insulin resistance, mitochondrial dysfunction, and endoplasmic reticulum stress via enhancing autophagy in rats. Molecular Medicine 2020, 26, 1–13. [Google Scholar] [CrossRef]
- de la Vega, M.R.; Chapman, E.; Zhang, D.D. NRF2 and the hallmarks of cancer. Cancer cell 2018, 34, 21–43. [Google Scholar] [CrossRef]
- Pajares, M.; Cuadrado, A.; Rojo, A.I. Modulation of proteostasis by transcription factor NRF2 and impact in neurodegenerative diseases. Redox biology 2017, 11, 543–553. [Google Scholar] [CrossRef]
- Jiang, T.; Harder, B.; De La Vega, M.R.; Wong, P.K.; Chapman, E.; Zhang, D.D. p62 links autophagy and Nrf2 signaling. Free Radical Biology and Medicine 2015, 88, 199–204. [Google Scholar] [CrossRef]
- Lau, A.; Wang, X.-J.; Zhao, F.; Villeneuve, N.F.; Wu, T.; Jiang, T.; Sun, Z.; White, E.; Zhang, D.D. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Molecular and cellular biology 2010, 30, 3275–3285. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Zhang, D.; Hannink, M.; Arvisais, E.; Kaufman, R.J.; Diehl, J.A. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Molecular and cellular biology 2003, 23, 7198–7209. [Google Scholar] [CrossRef]
- Glover-Cutter, K.M.; Lin, S.; Blackwell, T.K. Integration of the unfolded protein and oxidative stress responses through SKN-1/Nrf. PLoS genetics 2013, 9, e1003701. [Google Scholar] [CrossRef]
- Zhu, Y.-p.; Zheng, Z.; Hu, S.; Ru, X.; Fan, Z.; Qiu, L.; Zhang, Y. Unification of opposites between two antioxidant transcription factors Nrf1 and Nrf2 in mediating distinct cellular responses to the endoplasmic reticulum stressor tunicamycin. Antioxidants 2020, 9, 4. [Google Scholar] [CrossRef]
- DeNicola, G.M.; Chen, P.-H.; Mullarky, E.; Sudderth, J.A.; Hu, Z.; Wu, D.; Tang, H.; Xie, Y.; Asara, J.M.; Huffman, K.E. Erratum: NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nature genetics 2016, 48, 473. [Google Scholar] [CrossRef]
- Li, B.; Fu, J.; Chen, P.; Ge, X.; Li, Y.; Kuiatse, I.; Wang, H.; Wang, H.; Zhang, X.; Orlowski, R.Z. The nuclear factor (erythroid-derived 2)-like 2 and proteasome maturation protein axis mediate bortezomib resistance in multiple myeloma. Journal of Biological Chemistry 2015, 290, 29854–29868. [Google Scholar] [CrossRef]
- Srivastava, R.; Fernández-Ginés, R.; Encinar, J.A.; Cuadrado, A.; Wells, G. The current status and future prospects for therapeutic targeting of KEAP1-NRF2 and β-TrCP-NRF2 interactions in cancer chemoresistance. Free Radical Biology and Medicine 2022. [Google Scholar] [CrossRef]
- Hajiahmadi, S.; Lorzadeh, S.; Iranpour, R.; Karima, S.; Rajabibazl, M.; Shahsavari, Z.; Ghavami, S. Temozolomide, simvastatin and acetylshikonin combination induces mitochondrial-dependent apoptosis in GBM cells, which is regulated by autophagy. Biology 2023, 12, 302. [Google Scholar] [CrossRef]
- Alizadeh, J.; da Silva Rosa, S.C.; Weng, X.; Jacobs, J.; Lorzadeh, S.; Ravandi, A.; Vitorino, R.; Pecic, S.; Zivkovic, A.; Stark, H. Ceramides and Ceramide Synthases in Cancer: Focus on Apoptosis and Autophagy. European Journal of Cell Biology 2023, 151337. [Google Scholar] [CrossRef]
- DeBlasi, J.M.; DeNicola, G.M. Dissecting the crosstalk between NRF2 signaling and metabolic processes in cancer. Cancers 2020, 12, 3023. [Google Scholar] [CrossRef] [PubMed]
- Behrooz, A.B.; Cordani, M.; Donadelli, M.; Ghavami, S. Metastatic outgrowth via the two-way interplay of autophagy and metabolism. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 2023, 166824. [Google Scholar] [CrossRef]
- Pajares, M.; Jiménez-Moreno, N.; García-Yagüe, Á.J.; Escoll, M.; de Ceballos, M.L.; Van Leuven, F.; Rábano, A.; Yamamoto, M.; Rojo, A.I.; Cuadrado, A. Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy 2016, 12, 1902–1916. [Google Scholar] [CrossRef]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.-S.; Ueno, I.; Sakamoto, A.; Tong, K.I. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nature cell biology 2010, 12, 213–223. [Google Scholar] [CrossRef]
- Bhatt, V.; Khayati, K.; Hu, Z.S.; Lee, A.; Kamran, W.; Su, X.; Guo, J.Y. Autophagy modulates lipid metabolism to maintain metabolic flexibility for Lkb1-deficient Kras-driven lung tumorigenesis. Genes & development 2019, 33, 150–165. [Google Scholar]
- Barzegar Behrooz, A.; Latifi-Navid, H.; da Silva Rosa, S.C.; Swiat, M.; Wiechec, E.; Vitorino, C.; Vitorino, R.; Jamalpoor, Z.; Ghavami, S. Integrating Multi-Omics Analysis for Enhanced Diagnosis and Treatment of Glioblastoma: A Comprehensive Data-Driven Approach. Cancers 2023, 15, 3158. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, J.; Kavoosi, M.; Singh, N.; Lorzadeh, S.; Ravandi, A.; Kidane, B.; Ahmed, N.; Mraiche, F.; Mowat, M.R.; Ghavami, S. Regulation of Autophagy via Carbohydrate and Lipid Metabolism in Cancer. Cancers 2023, 15, 2195. [Google Scholar] [CrossRef]
- Gundamaraju, R.; Lu, W.; Paul, M.K.; Jha, N.K.; Gupta, P.K.; Ojha, S.; Chattopadhyay, I.; Rao, P.V.; Ghavami, S. Autophagy and EMT in cancer and metastasis: Who controls whom? Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 2022, 1868, 166431. [Google Scholar] [CrossRef] [PubMed]
- Inami, Y.; Waguri, S.; Sakamoto, A.; Kouno, T.; Nakada, K.; Hino, O.; Watanabe, S.; Ando, J.; Iwadate, M.; Yamamoto, M. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. Journal of Cell Biology 2011, 193, 275–284. [Google Scholar] [CrossRef]
- Todoric, J.; Antonucci, L.; Di Caro, G.; Li, N.; Wu, X.; Lytle, N.K.; Dhar, D.; Banerjee, S.; Fagman, J.B.; Browne, C.D. Stress-activated NRF2-MDM2 cascade controls neoplastic progression in pancreas. Cancer cell 2017, 32, 824–839. e828. [Google Scholar] [CrossRef]
- Kageyama, S.; Sou, Y.-s.; Uemura, T.; Kametaka, S.; Saito, T.; Ishimura, R.; Kouno, T.; Bedford, L.; Mayer, R.J.; Lee, M.-S. Proteasome dysfunction activates autophagy and the Keap1-Nrf2 pathway. Journal of Biological Chemistry 2014, 289, 24944–24955. [Google Scholar] [CrossRef]
- Yadav, R.K.; Chae, S.-W.; Kim, H.-R.; Chae, H.J. Endoplasmic reticulum stress and cancer. Journal of cancer prevention 2014, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-N.; Kavianpour, S.; Zhang, T.; Zhang, X.; Nguyen, D.; Thombre, R.; He, L.; Wang, J. MARK2 phosphorylates eIF2α in response to proteotoxic stress. PLoS biology 2021, 19, e3001096. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Sayin, V.I.; Davidson, S.M.; Bauer, M.R.; Singh, S.X.; LeBoeuf, S.E.; Karakousi, T.R.; Ellis, D.C.; Bhutkar, A.; Sanchez-Rivera, F.J. Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nature medicine 2017, 23, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer cell 2020. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.C.; Hay, N. The pentose phosphate pathway and cancer. Trends in biochemical sciences 2014, 39, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Deblois, G.; Smith, H.W.; Tam, I.S.; Gravel, S.-P.; Caron, M.; Savage, P.; Labbé, D.P.; Bégin, L.R.; Tremblay, M.L.; Park, M. ERRα mediates metabolic adaptations driving lapatinib resistance in breast cancer. Nature communications 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Takamura, A.; Komatsu, M.; Hara, T.; Sakamoto, A.; Kishi, C.; Waguri, S.; Eishi, Y.; Hino, O.; Tanaka, K.; Mizushima, N. Autophagy-deficient mice develop multiple liver tumors. Genes & development 2011, 25, 795–800. [Google Scholar]
- Kroemer, G.; Mariño, G.; Levine, B. Autophagy and the integrated stress response. Molecular cell 2010, 40, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, X.; Contino, G.; Liesa, M.; Sahin, E.; Ying, H.; Bause, A.; Li, Y.; Stommel, J.M.; Dell'Antonio, G. Pancreatic cancers require autophagy for tumor growth. Genes & development 2011, 25, 717–729. [Google Scholar]
- Karsli-Uzunbas, G.; Guo, J.Y.; Price, S.; Teng, X.; Laddha, S.V.; Khor, S.; Kalaany, N.Y.; Jacks, T.; Chan, C.S.; Rabinowitz, J.D. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer discovery 2014, 4, 914–927. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, K.; Wang, H.; Dai, Y. Inhibition of autophagy by chloroquine enhances the antitumor efficacy of sorafenib in glioblastoma. Cellular and molecular neurobiology 2016, 36, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Chapman, E. The role of natural products in revealing NRF2 function. Natural product reports 2020, 37, 797–826. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, A.; Chandel, N.S. Targeting antioxidants for cancer therapy. Biochemical pharmacology 2014, 92, 90–101. [Google Scholar] [CrossRef]
- Mostafavi-Pour, Z.; Ramezani, F.; Keshavarzi, F.; Samadi, N. The role of quercetin and vitamin C in Nrf2-dependent oxidative stress production in breast cancer cells. Oncology letters 2017, 13, 1965–1973. [Google Scholar] [CrossRef]
- Ramezani, F.; Samadi, N.; Mostafavi-Pour, Z. Sequential therapy of breast cancer cell lines with vitamin C and quercetin improves the efficacy of chemotherapeutic drugs. Nutrition and cancer 2017, 69, 881–891. [Google Scholar] [CrossRef]
- Lin, H.; Qiao, Y.; Yang, H.; Nan, Q.; Qu, W.; Feng, F.; Liu, W.; Chen, Y.; Sun, H. Small molecular Nrf2 inhibitors as chemosensitizers for cancer therapy. Future medicinal chemistry.
- Olayanju, A.; Copple, I.M.; Bryan, H.K.; Edge, G.T.; Sison, R.L.; Wong, M.W.; Lai, Z.Q.; Lin, Z.X.; Dunn, K.; Sanderson, C.M.; et al. Brusatol provokes a rapid and transient inhibition of Nrf2 signaling and sensitizes mammalian cells to chemical toxicity-implications for therapeutic targeting of Nrf2. Free Radic Biol Med 2015, 78, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Chian, S.; Thapa, R.; Chi, Z.; Wang, X.J.; Tang, X. Luteolin inhibits the Nrf2 signaling pathway and tumor growth in vivo. Biochemical and Biophysical Research Communications 2014, 447, 602–608. [Google Scholar] [CrossRef]
- Gao, A.M.; Ke, Z.P.; Wang, J.N.; Yang, J.Y.; Chen, S.Y.; Chen, H. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis 2013, 34, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Sun, K.; Wang, X.; Pan, H.; Zhu, J.; Ji, X.; Li, X. Chrysin suppresses proliferation, migration, and invasion in glioblastoma cell lines via mediating the ERK/Nrf2 signaling pathway. Drug design, development and therapy 2018, 12, 721. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, X.; Zhang, Y.; Yang, L.; Liu, Y.; Huang, S.; Lu, L.; Kong, L.; Li, Z.; Guo, Q. Wogonin reversed resistant human myelogenous leukemia cells via inhibiting Nrf2 signaling by Stat3/NF-κB inactivation. Scientific reports 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Tang, X.; Fu, X.; Liu, Y.; Yu, D.; Cai, S.J.; Yang, C. Blockade of Glutathione Metabolism in IDH1-Mutated Glioma. Mol Cancer Ther 2020, 19, 221–230. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, Z.; Guo, R.; Ren, F. Nrf2 inhibitor, brusatol in combination with trastuzumab exerts synergistic antitumor activity in HER2-positive cancers by inhibiting Nrf2/HO-1 and HER2-AKT/ERK1/2 pathways. Oxidative medicine and cellular longevity 2020, 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, H.; Chen, F.; Lv, H.; Xu, Z.; Fu, J.; Hou, Y.; Xu, Y.; Pi, J. Triptolide enhances chemotherapeutic efficacy of antitumor drugs in non-small-cell lung cancer cells by inhibiting Nrf2-ARE activity. Toxicology and Applied Pharmacology 2018, 358, 1–9. [Google Scholar] [CrossRef]
- Arlt, A.; Sebens, S.; Krebs, S.; Geismann, C.; Grossmann, M.; Kruse, M.; Schreiber, S.; Schäfer, H. Inhibition of the Nrf2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity. Oncogene 2013, 32, 4825–4835. [Google Scholar] [CrossRef]
- Lee, S.; Lim, M.J.; Kim, M.H.; Yu, C.H.; Yun, Y.S.; Ahn, J.; Song, J.Y. An effective strategy for increasing the radiosensitivity of Human lung Cancer cells by blocking Nrf2-dependent antioxidant responses. Free Radic Biol Med 2012, 53, 807–816. [Google Scholar] [CrossRef]
- Singh, A.; Venkannagari, S.; Oh, K.H.; Zhang, Y.Q.; Rohde, J.M.; Liu, L.; Nimmagadda, S.; Sudini, K.; Brimacombe, K.R.; Gajghate, S.; et al. Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1-Deficient NSCLC Tumors. ACS Chem Biol 2016, 11, 3214–3225. [Google Scholar] [CrossRef]
- Bollong, M.J.; Yun, H.; Sherwood, L.; Woods, A.K.; Lairson, L.L.; Schultz, P.G. A small molecule inhibits deregulated NRF2 transcriptional activity in cancer. ACS chemical biology 2015, 10, 2193–2198. [Google Scholar] [CrossRef]
- Tsuchida, K.; Tsujita, T.; Hayashi, M.; Ojima, A.; Keleku-Lukwete, N.; Katsuoka, F.; Otsuki, A.; Kikuchi, H.; Oshima, Y.; Suzuki, M. Halofuginone enhances the chemo-sensitivity of cancer cells by suppressing NRF2 accumulation. Free Radical Biology and Medicine 2017, 103, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Jung, B.; Lee, S.; Yoo, H.; Shin, E.; Ko, H.; Chang, S.; Kim, S.; Jeon, S. A clinical drug library screen identifies clobetasol propionate as an NRF2 inhibitor with potential therapeutic efficacy in KEAP1 mutant lung cancer. Oncogene 2017, 36, 5285–5295. [Google Scholar] [CrossRef] [PubMed]
- Hori, R.; Yamaguchi, K.; Sato, H.; Watanabe, M.; Tsutsumi, K.; Iwamoto, S.; Abe, M.; Onodera, H.; Nakamura, S.; Nakai, R. The discovery and characterization of K-563, a novel inhibitor of the Keap1/Nrf2 pathway produced by Streptomyces sp. Cancer medicine 2019, 8, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, H.; Zhu, J.; Zhao, R.; Xue, P.; Zhang, Q.; Bud Nelson, M.; Qu, W.; Feng, B.; Pi, J. Camptothecin suppresses NRF2–ARE activity and sensitises hepatocellular carcinoma cells to anticancer drugs. British journal of cancer 2017, 117, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Jiao, Y.; Xue, J.; Zhang, Q.; Yang, H.; Xing, L.; Chen, G.; Wu, J.; Zhang, S.; Zhu, W. Metformin sensitizes non-small cell lung cancer cells to an epigallocatechin-3-gallate (EGCG) treatment by suppressing the Nrf2/HO-1 signaling pathway. International journal of biological sciences 2017, 13, 1560. [Google Scholar] [CrossRef]
- Keller, T.L.; Zocco, D.; Sundrud, M.S.; Hendrick, M.; Edenius, M.; Yum, J.; Kim, Y.J.; Lee, H.K.; Cortese, J.F.; Wirth, D.F.; et al. Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nat Chem Biol 2012, 8, 311–317. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Slocum, S.L.; Skoko, J.J.; Shin, S.; Kensler, T.W. When NRF2 talks, who's listening? Antioxid Redox Signal 2010, 13, 1649–1663. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: a Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol Rev 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Huang, M.T.; Shen, G.; Yuan, X.; Lin, W.; Khor, T.O.; Conney, A.H.; Kong, A.N. Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res 2006, 66, 8293–8296. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Iijima, K.; Miyamoto, M.; Nakahara, I.; Tanaka, H.; Ohtsuji, M.; Suzuki, T.; Kobayashi, A.; Yokota, J.; Sakiyama, T.; et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res 2008, 68, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Ohta, T.; Tong, K.I.; Kokubu, A.; Odogawa, R.; Tsuta, K.; Asamura, H.; Yamamoto, M.; Hirohashi, S. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proceedings of the National Academy of Sciences of the United States of America 2008, 105, 13568–13573. [Google Scholar] [CrossRef] [PubMed]
- Ki, S.H.; Cho, I.J.; Choi, D.W.; Kim, S.G. Glucocorticoid receptor (GR)-associated SMRT binding to C/EBPbeta TAD and Nrf2 Neh4/5: role of SMRT recruited to GR in GSTA2 gene repression. Mol Cell Biol 2005, 25, 4150–4165. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Hayes, J.D.; Henderson, C.J.; Wolf, C.R. Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. Proceedings of the National Academy of Sciences of the United States of America 2007, 104, 19589–19594. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, K.; Geng, M.; Gao, P.; Wu, X.; Hai, Y.; Li, Y.; Li, Y.; Luo, L.; Hayes, J.D.; et al. RXRα inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res 2013, 73, 3097–3108. [Google Scholar] [CrossRef] [PubMed]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid Med Cell Longev 2019, 2019, 9372182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Su, L.; Ye, Q.; Zhang, S.; Kung, H.; Jiang, F.; Jiang, G.; Miao, J.; Zhao, B. Discovery of a novel Nrf2 inhibitor that induces apoptosis of human acute myeloid leukemia cells. Oncotarget 2017, 8, 7625–7636. [Google Scholar] [CrossRef]
- Li, D.; Wang, H.; Ding, Y.; Zhang, Z.; Zheng, Z.; Dong, J.; Kim, H.; Meng, X.; Zhou, Q.; Zhou, J. Targeting the NRF-2/RHOA/ROCK signaling pathway with a novel aziridonin, YD0514, to suppress breast cancer progression and lung metastasis. Cancer letters 2018, 424, 97–108. [Google Scholar] [CrossRef]
- Hamzawy, M.; Abo-Youssef, A.; Malak, M.; Khalaf, M. Multiple targets of Nrf 2 inhibitor; trigonelline in combating urethane-induced lung cancer by caspase-executioner apoptosis, cGMP and limitation of cyclin D1 and Bcl2. European Review for Medical & Pharmacological Sciences 2022, 26. [Google Scholar]
- Rehman, M.U.; Rashid, S.; Arafah, A.; Qamar, W.; Alsaffar, R.M.; Ahmad, A.; Almatroudi, N.M.; Alqahtani, S.M.; Rashid, S.M.; Ahmad, S.B. Piperine regulates Nrf-2/Keap-1 signalling and exhibits anticancer effect in experimental colon carcinogenesis in Wistar rats. Biology 2020, 9, 302. [Google Scholar] [CrossRef]
- Khurana, N.; Sikka, S.C. Targeting crosstalk between Nrf-2, NF-κB and androgen receptor signaling in prostate cancer. Cancers 2018, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Meng, W.; Liu, Y.; Li, C.; Zhang, C.; Wang, P.; Almoallim, H.S.; Manikandan, V.; Guan, H. Elucidating the immunomodulatory effect of daidzein in Benzo (a) pyrene-Induced lung cancer mice model through modulation of proliferating cell nuclear antigen, NF-κB, CYP1A1, and NRF. Pharmacognosy Magazine 2022, 18, 193. [Google Scholar]
- Chen, P.; Fang, X.; Mao, H.; Lin, Y.; Li, B. Curcumin combination with miR-144-3p suppresses non-small cell lung cancer by regulating Nrf-2/HO-1 in vitro and vivo study. Journal of Biomaterials and Tissue Engineering 2019, 9, 476–484. [Google Scholar] [CrossRef]
- Yehia, R.; Saleh, S.; El Abhar, H.; Saad, A.S.; Schaalan, M. L-Carnosine protects against Oxaliplatin-induced peripheral neuropathy in colorectal cancer patients: A perspective on targeting Nrf-2 and NF-κB pathways. Toxicology and applied pharmacology 2019, 365, 41–50. [Google Scholar] [CrossRef]
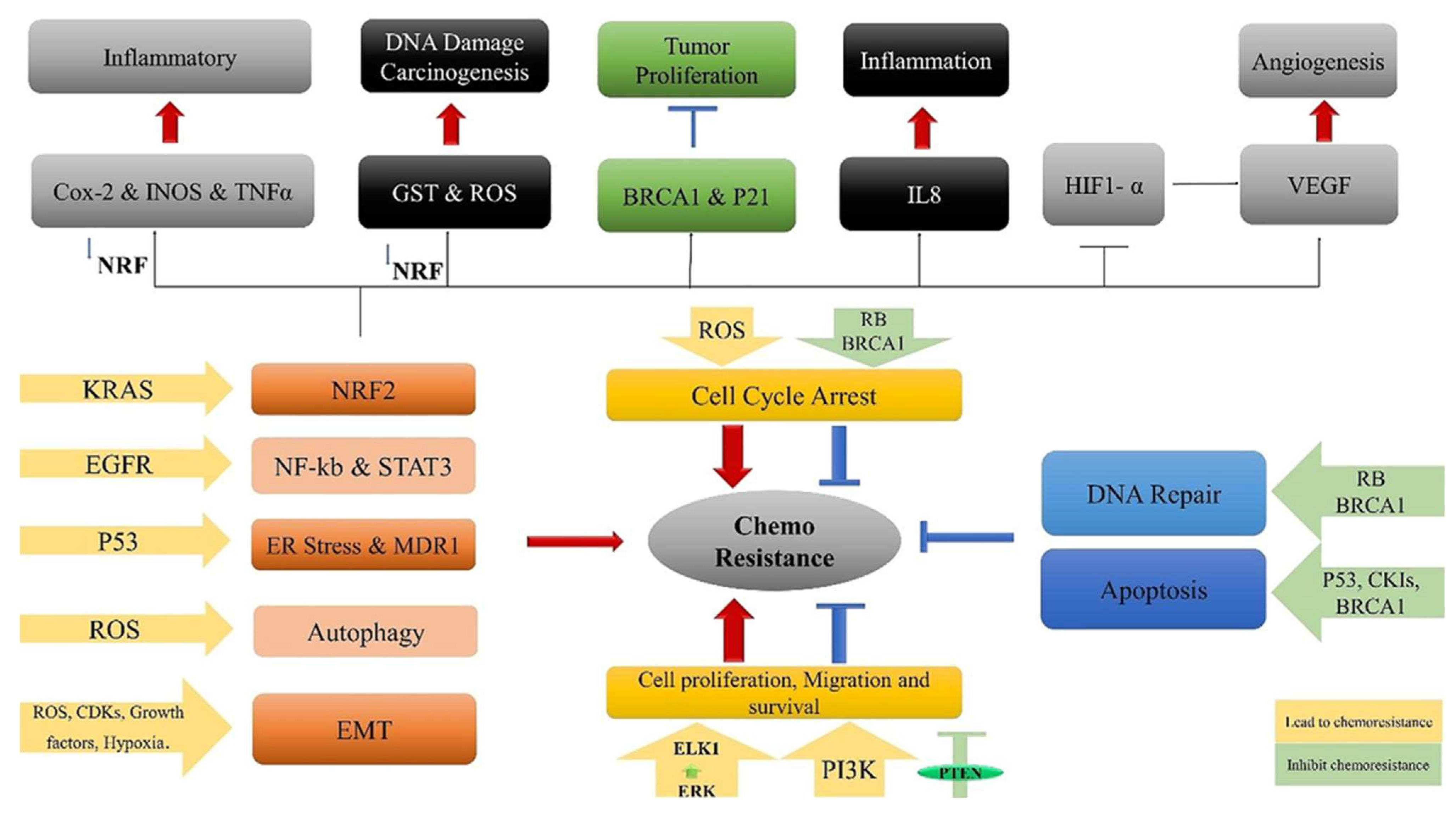
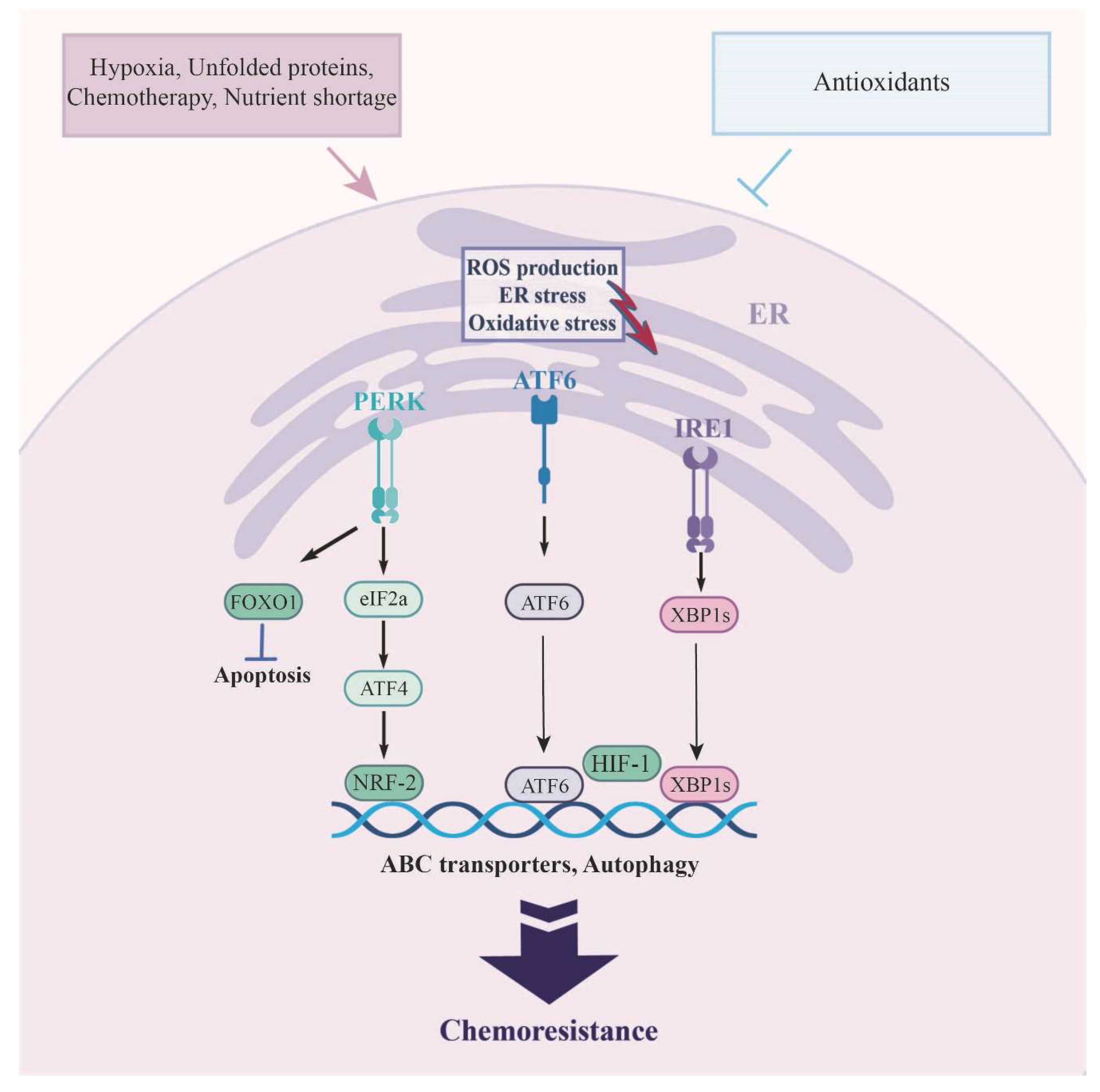
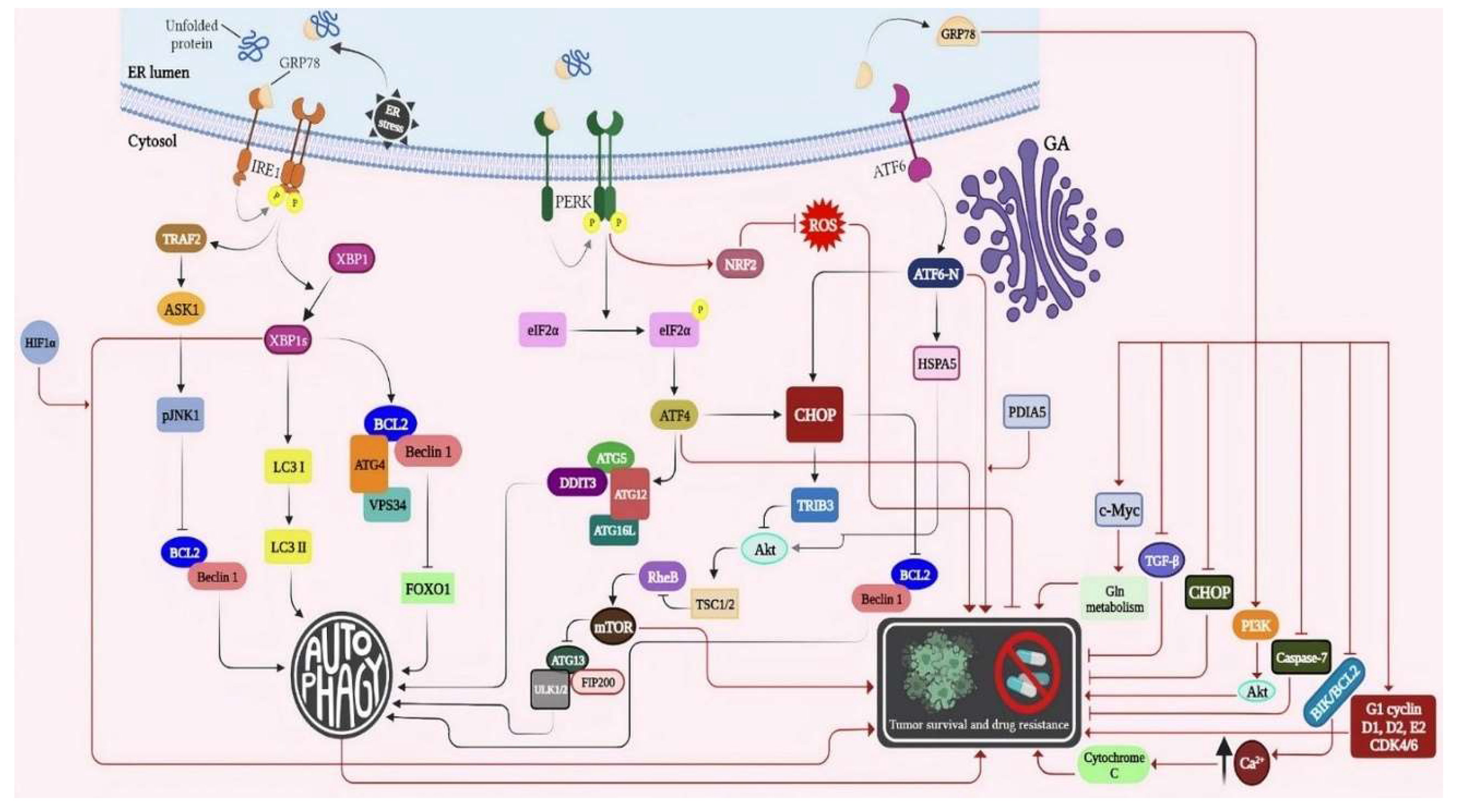
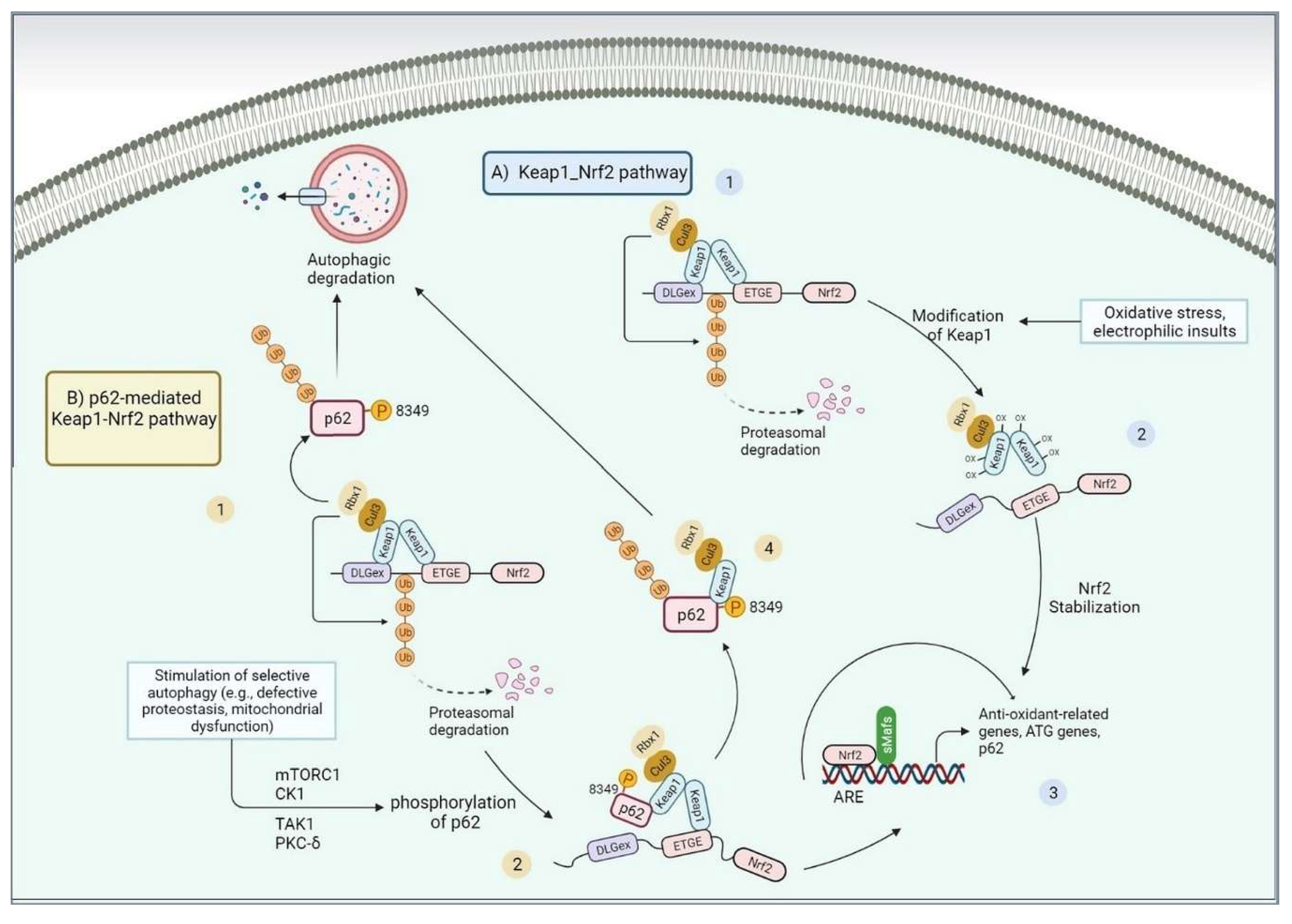
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





