1. Introduction
Gastric cancer is a major problem influencing life expectancy due to its aggressive nature [
1]. It is associated with poor prognosis dependent on tumour stage at presentation [
2]. According to GLOBOCAN 2020, gastric cancer is the fifth most common cancer and the fourth leading cause of mortality worldwide [
3]. The outcome of GC and GEJ adenocarcinoma following curative resection alone is predominantly dismal, which points to the necessity of application of perioperative chemotherapy [
4,
5]. This approach in western patients with locally advanced, primary resectable GC and GEJ cancer has been gold standard since both the MAGIC trial and the French FNCLCC/FFCD 97033 study [
4,
5]. The most effective type of chemotherapy, as reported by Al-Batran S. et al in Lancet 2019, is the FLOT regimen, which induces more tumour responses than other regimens and improves the margin free resection rate. FLOT is superior to ECF/ECX with respect to complete pathological response (15% vs 6%) and median overall survival (50 months vs 35 months) [
6]. As approximately 10%-15% of patients fail to respond to this treatment, it is vital to conduct studies on the application of biomarkers in GC and GEJ cancer [
6]. There are currently no biomarkers enabling prediction of therapeutic efficacy, real-time tumour dynamic or identifying patients at increased risk of a poor pathological response. In non-responding patients to neoadjuvant chemotherapy such markers could allow clinicians to apply a more individualized approach e.g. avoiding exposition to the potential toxicity of unnecessary chemotherapy, thus improving the quality of life and making it possible to perform earlier surgery as well as reduce the cost of treatment.
Scientific research over the last decade has explained the fundamental molecular mechanisms and provided conclusive evidence that inflammation is now established as a hallmark of cancer. The tumour microenvironment, which includes inflammatory cells or inflammatory mediators such as cytokines, chemokines, growth factors, prostaglandins and stromal activation, plays a decisive role in various stages of tumour development, including initiation, malignant transformation, promotion, invasion as well as formation of metastases [
7,
8,
9]. Pro-inflammatory cytokines (IL-1, IL-6, IL-8, TNF-α) are responsible for the promotion of metastasis and cachexia, while anti-inflammatory immunosuppressive cytokines such as in IL-10 are reported to be a marker of higher stage of the disease [
10,
11]. Interleukin-6 (IL-6) and interleukin-8 (IL-8) are important pro-angiogenic factors in gastric cancer through induction of vascular endothelial growth factor (VEGF) [
12,
13]. Both IL-1 and IL-6 are involved in the growth of neoplastic cells in gastric cancer and the metastasis formation [
14,
15]. In addition to pro-inflammatory cytokines, activation of the coagulation and angiogenesis systems are believed to be factors associated with the development of cancer [
16]. Activated platelets are the source of VEGF, which is responsible for the promotion of neoangiogenesis. Lymphocytes participate in both humoral and cellular anti-tumour immune response [
17].
In numerous studies on patients with various types of cancer, the lymphocyte to monocyte ratio (LMR), neutrophil to lymphocyte ratio (NLR), and platelet to lymphocyte ratio (PLR) were assessed as prognostic markers for overall survival (OS), disease-free survival (DFS) and progression-free survival (PFS). The study by Lian L. et al. conducted on patients with primary operable gastric cancer showed that preoperative low levels of PLR and NLR were correlated with better clinicopathological features, including a lower depth of tumour invasion, fewer lymph node metastases and an early stage cancer based on the TNM classification according to the AJCC [
18].
Additionally, low level of leukocytes and lymphocytes prior to systemic adjuvant therapy, was a predictor of poor outcome in response to this treatment [
19]. Patients with primary metastatic gastric cancer undergoing palliative systemic treatment who had a low NLR level before treatment had statistically significantly better disease control rate (DCR), longer progression-free survival (PFS), and longer overall survival compared to patients with initially high NLR level [
19,
20]. Arigami T. et al developed a new scoring system (F-NLR) based on fibrinogen concentration (F) and NLR ratio as a predictive and prognostic factor to chemotherapy or chemoradiotherapy in patients with advanced gastric cancer. Higher F-NLR values were significantly more frequent in the subgroup of patients with disease progression during treatment [
21].
In 1869, during the autopsy of a patient with metastatic cancer, Thomas Ashworth first observed that cells similar to those of the primary tumour were present in peripheral blood. These cells are circulating tumour cells (CTCs): rare cancer cells released from the tumour into the bloodstream, which are thought to play a key role in cancer metastasis. Many studies have shown the identification of CTCs in patients with various types of cancer and their usefulness as a marker of response to systemic treatment [
22].
In view of the above considerations, we selected the parameters as potential predictive factors of neoadjuvant chemotherapy in patients with locally advanced GC and GEJ cancer.
2. Materials and Methods
We conducted this prospective study in the Maria Sklodowska-Curie National Research Institute of Oncology, in Warsaw, Poland in order to identify serum biomarkers of early response to NAC from collected biomaterial. The trial was performed in accordance with the principles of the Declaration of Helsinki and the protocol was approved by the Local Bioethics Committee at the Maria Sklodowska-Curie National Research Institute of Oncology (MSCNRIO) in Warsaw (approval number 51/2016/2017). A total of 71 patients and 15 healthy volunteers signed informed consent.
2.1. Inclusion and Exclusion Criteria
The main eligibility criteria included: written informed consent for participation in the trial, patients with histopathologically confirmed GC or GEJ adenocarcinoma of a clinical stage cT2-T4/cN-any or cT-any/cN+, ECOG (Eastern Cooperative Oncology Group) performance status 2, adequate liver, kidney and hematologic function, age 18 years old. The main exclusion criteria were the following: evidence of distant metastasis, history of other primary malignancies, prior chemotherapy or radiotherapy, active or documented prior autoimmune or inflammatory disorder, current or prior use of immunosuppressive medication or corticosteroids exceeding 10 mg/day of prednisone or its equivalent, allergy to iodine contrast agent, concomitant disease (coronary heart disease, arrhythmia, stroke) preventing administration of chemotherapy according to protocol, pregnancy, breastfeeding.
2.2. Patient Treatment and Procedure
Clinical stage at baseline was evaluated by oesophagogastroduodenoscopy (OGD), computed tomography (CT) or magnetic resonance imaging (MRI) scan of the chest, abdomen and pelvis and physical examination. Diagnostic laparoscopy was not performed in any patient as the Polish standards of care state, that it is recommended, but not mandatory. FLOT administration consisted of four preoperative and four postoperative cycles; during each 2-week cycle we administered docetaxel 50 mg/m2 on day 1, oxaliplatin 85 mg/m2 on day 1, leucovorin 200 mg/m2 on day 1, and 5-FU 2600 mg/m2 as 24-h infusion on day 1. Patients were assessed according to their medical history, physical examination, weight, ECOG performance status, complete blood count, blood chemical tests, CEA, CA125, CA19.9, d-dimer, fibrinogen at baseline and before the start of every cycle. CTCs and IL-1β, IL-6, IL-8, IL-10 were measured in a pilot group of 40 patients at baseline and before the start of C2 and C3. We graded adverse events according to CTCAE 4.03 (Common Terminology Criteria for Adverse Events v4.03) before each cycle and we used granulocyte colony-stimulating factor (G-CSF) for primary prophylaxis of febrile neutropenia. Chemotherapy was continued according to protocol unless written informed consent was withdrawn, unacceptable toxicity occurred or progression of the disease was observed. In order to confirm the absence of progression of disease or occurrence of metastases, CT or MRI scan of the chest, abdomen and pelvis was performed between 2 and 4 weeks following the completion of the last cycle of preoperative chemotherapy. Tumour response was determined according to the Response Evaluation Criteria in Solid Tumours 1.1 (RECIST v1.1). Surgery was scheduled for 4-6 weeks following the completion of the last cycle of chemotherapy. Pathological tumour regression (TRG) of the primary tumour to NAC was evaluated according to Becker classification, which classifies pathologic response as follows: TGR1a: no residual tumour/tumour bed, TGR1b: <10% residual tumour/tumour bed, TGR2: 10-50% residual tumour/tumour bed, TGR3: >50% residual tumour/tumour bed.
2.3. Biochemical Analysis
Venous blood collection VACUETTE® was performed using the VACUETTE® system. The Sysmex XN-550 haematology analyser was used for analysis of differential white blood cell count following the manufacturer’s protocol. The lymphocyte-to-monocyte ratio (LMR) was calculated by dividing an absolute count of lymphocytes (109/l) by an absolute count of monocytes (109/l). The platelet-to-lymphocyte ratio (PLR) was calculated by dividing an absolute count of platelets (109/l) by an absolute count of lymphocytes (109/l). The neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing an absolute count of neutrophils (109/l) by an absolute count of lymphocytes (109/l). Plasma fibrinogen (F) was determined from blood plasma collected on sodium edetate (EDTA) using the Clauss method with Fibrinogen-C XL reagent in the ACL TOP 500 (WERFEN) coagulation analyser according to the manufacturer’s recommendations. F-NLR score was based on plasma fibrinogen (F) and NLR. Patients with hyperfibrinogenemia (>400 mg/dl) and high NLR (>3.0) received 2 points. Patients with only one of the above-mentioned abnormalities in biochemical parameters received 1 point, while those with fibrinogen concentration < 400 mg/dl and low NLR (<3.0) received 0 points. D-dimer was determined by enzyme-linked immunosorbent assay (ELISA) from citrated plasma using VIDAS D-Dimer Exclusion reagents by VIDAS system according manufacturer’s recommendations. The tumour marker levels (CEA, CA125, CA19.9) were determined by electrochemiluminescence with Roche kits in the Cobas E601 system. The cut-off point for the markers were set according to the manufacturer’s recommendations. The serum concentration of IL-1β, IL-6, IL-8 and IL-10 were determined by using an enzyme-linked immunosorbent assay (ELISA) with R&D Systems (Minneapolis, MN, USA) according to manufacturer’s recommendations.
2.4. Molecular Detection of Circulating Tumour Cells (CTCs)
Molecular detection of CTCs was performed by assessing the mRNA expression of tumour-associated markers (CEA, CK19, survivin). The VACUETTE® system was used for venous blood collection. A 2.5 ml sample of peripheral venous blood from all of the patients and healthy volunteers was collected into PAXgene Blood RNA tubes (Qiagen, Hilden, Germany). Micro-centrifuge was used for purification and isolation of peripheral blood mononuclear cells (PBMCs). The RNA isolation was performed using the PAXgene Blood RNA Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions and the QIAcube automatic nucleic acid isolation apparatus (Qiagen, Hilden, Germany). The amount of RNA was measured using a Quantus Fluorometer (Promega, Madison, Wisconsin, USA). The measurement was made by using a fluorescent RNA-specific dye-QuantiFluor RNA System (Promega Madison, Wisconsin, USA). The amount of RNA was expressed in ng/µl. A sprectrophotometric test was performed in order to check the purity of the isolated RNA, with absorbance measured at 260 and 280 nm. The NanoDrop ND2000 device was used for the measurement (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Then on the basis of ratio of A260 and A280, the instrument determined the degree of RNA purity. RNA was considered sufficiently purified material for further analysis if this ratio was approximately 2. Reverse transcription reactions were performed with the SuperScript IV VILO kit Master Mix with ezDNase enzyme (Thermo Fisher Sci- entific, Waltham, Massachusetts, USA). Measurement of the expression of reference genes (TBP, HPRT, SDHA, YWHAZ, HPRT, GAPDH, ZNF410) and marker genes (CK19, CEA, survivin) was performed using the real-time polymerase chain reaction method (Real-Time PCR, qPCR), it is presented in
Appendix A (
Table A1). Quantitative PCR reaction was performed using the ABI PRISM 7500 Applied Biosystems 7500 Fast Real-Time PCR System instrument (Applied Biosystems, Carlsbad, USA). The reaction mixture consisted of TaqMan® Gene Expression Master Mix 38 (Thermo Fisher Scientific, Waltham, Massachusetts, USA), TaqMan® probes specific for selected genes (Thermo Fisher Scientific, Waltham, Massachusetts, USA) and cDNA matrix. Based on the qualitative assessment, three reference genes were selected and served as internal controls for further studies. The three selected genes were: TBP, HPRT and ZNF410. These genes were characterized by the highest stability of all the tested genes and showed constant expression in both patients and healthy volunteers. Where possible, quantitative analysis of the expression of CK19 and CEA marker genes and survivin was performed. The expression value was calculated according to the comparative method. The value of the relative expression levels allowed to estimate the changes in the expression of selected marker genes in patients with gastric cancer as compared to healthy volunteers.
2.5. Statistical Analysis
Statistical analysis was performed in R software (version 4.1.2). Age was described with median and range, levels of IL-6 were described with mean and standard deviation or median and interquartile range, depending on distribution normality. Categorical variables were presented as absolute frequency and proportion of the group. Distribution normality was verified with Shapiro-Wilk test, accompanied by skewness and kurtosis. Variance homogeneity was assessed with Levene’s test. Comparisons between prognosis groups were performed with t-Student test, Mann-Whitney U test, one-way Anova analysis and Kruskal-Wallis test, as appropriate. Post-hoc multiple comparison was conducted Dunn test with Bonferroni adjustment. Receiver operating characteristic (ROC) analysis was prepared in order to identify parameters with high potential to predict prognosis group. Optimal thresholds were calculated with Youden index.
3. Results
Between January 2018 and November 2019 a total of 71 patients signed informed consent and started treatment. However, the final data analysis was conducted on 61 patients at the age of 30-77 (median 63 years, 52.5% male and 47.5% female). Two patients did not meet inclusion criteria, as they were not primary resectable. Five patients who had received partial preoperative chemotherapy failed to report to the centre again and there was no further contact with them and three patients did not consent to gastrectomy. Full pre-operative treatment of four cycles of FLOT regimen was administered to 93.4% (57) patients. CTCs and ILs were measured in a pilot group of 40 patients. Baseline characteristics of the patients, surgical and pathology results of treatment are presented in
Appendix A (
Table A2 and
Table A3).
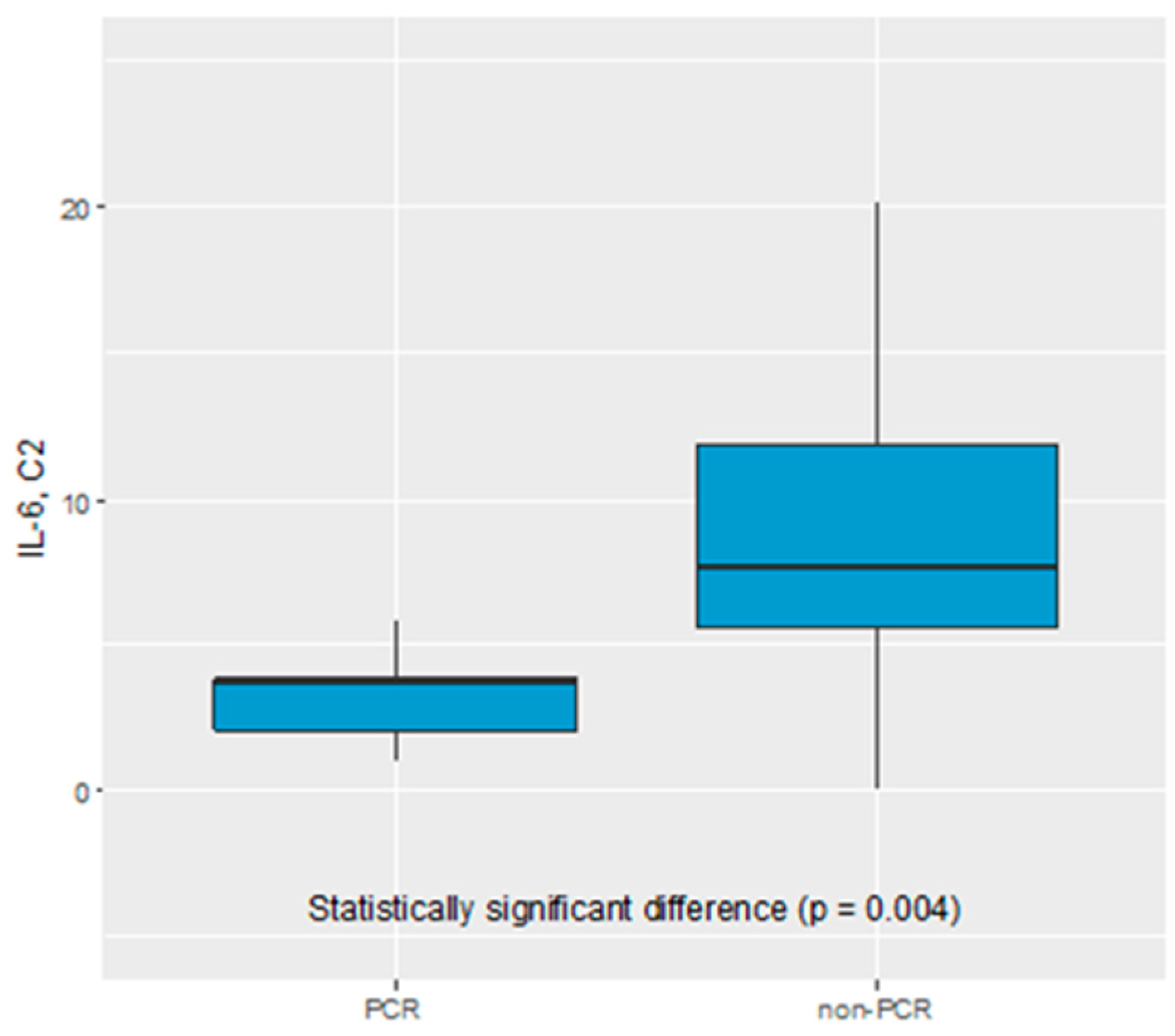
We did not find any statistical significance of CEA, CA19.9, CA125, IL-1β, IL-8, IL-10, F-NLR, LMR, NLR, PLR, CTCs as predictive biomarker of early response to NAC. Only IL-6 serum level was found to be a potential biomarker of pathological response to NAC. The IL-6 serum level before C2 of chemotherapy was significantly elevated in non-pCR vs complete pathological response (pCR) group (7.63 pg/mL vs 3.71 pg/mL, p=0.004), see
Figure 1.
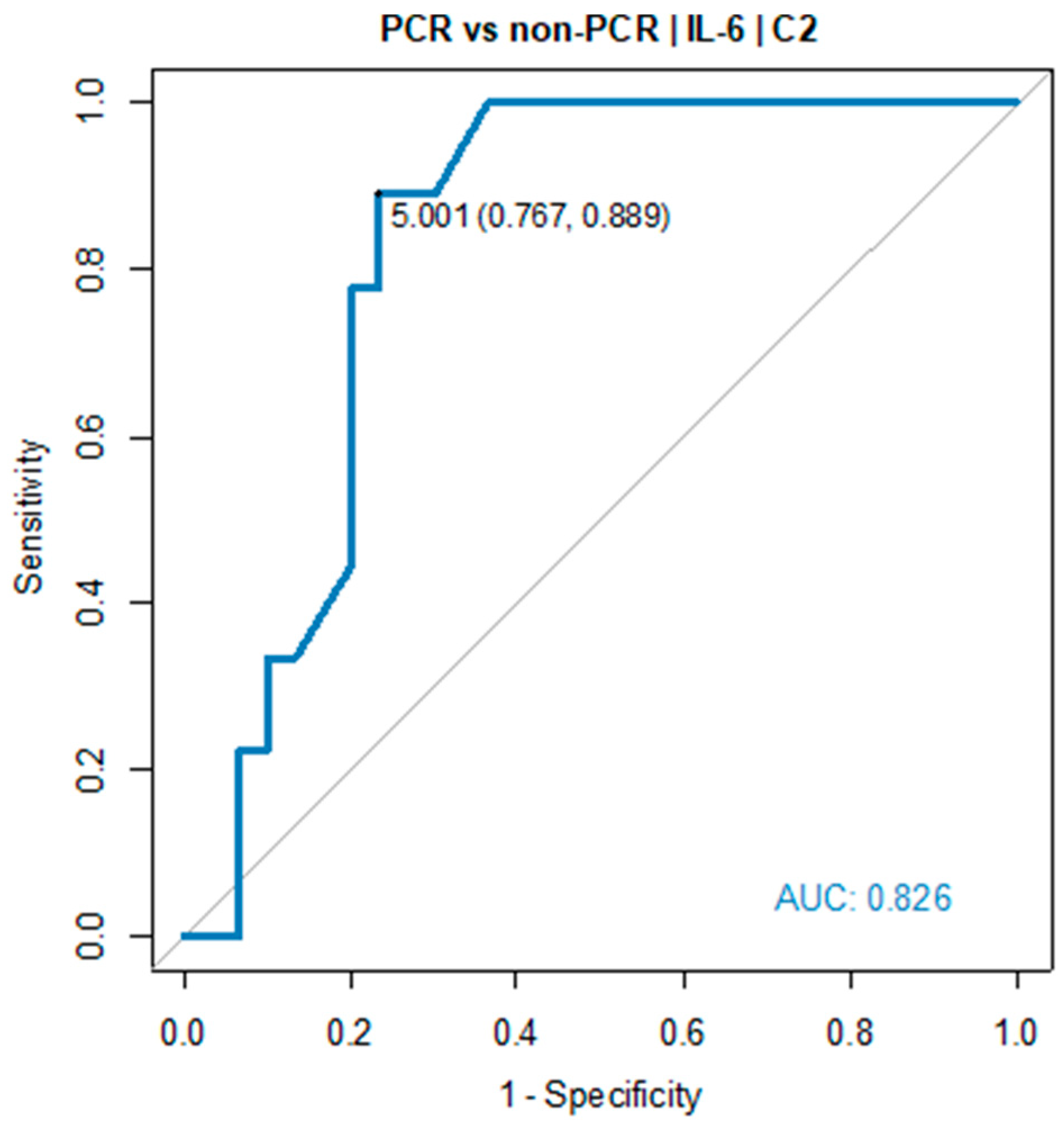
Receiver operating characteristic (ROC) curve showed the predictive power of IL-6. The optimal threshold for diagnosing pCR was 5.0 pg/mL (AUC=0.826, 95% CI: 0.698-0.954, p=0.001), see
Table 1 and
Figure 2.
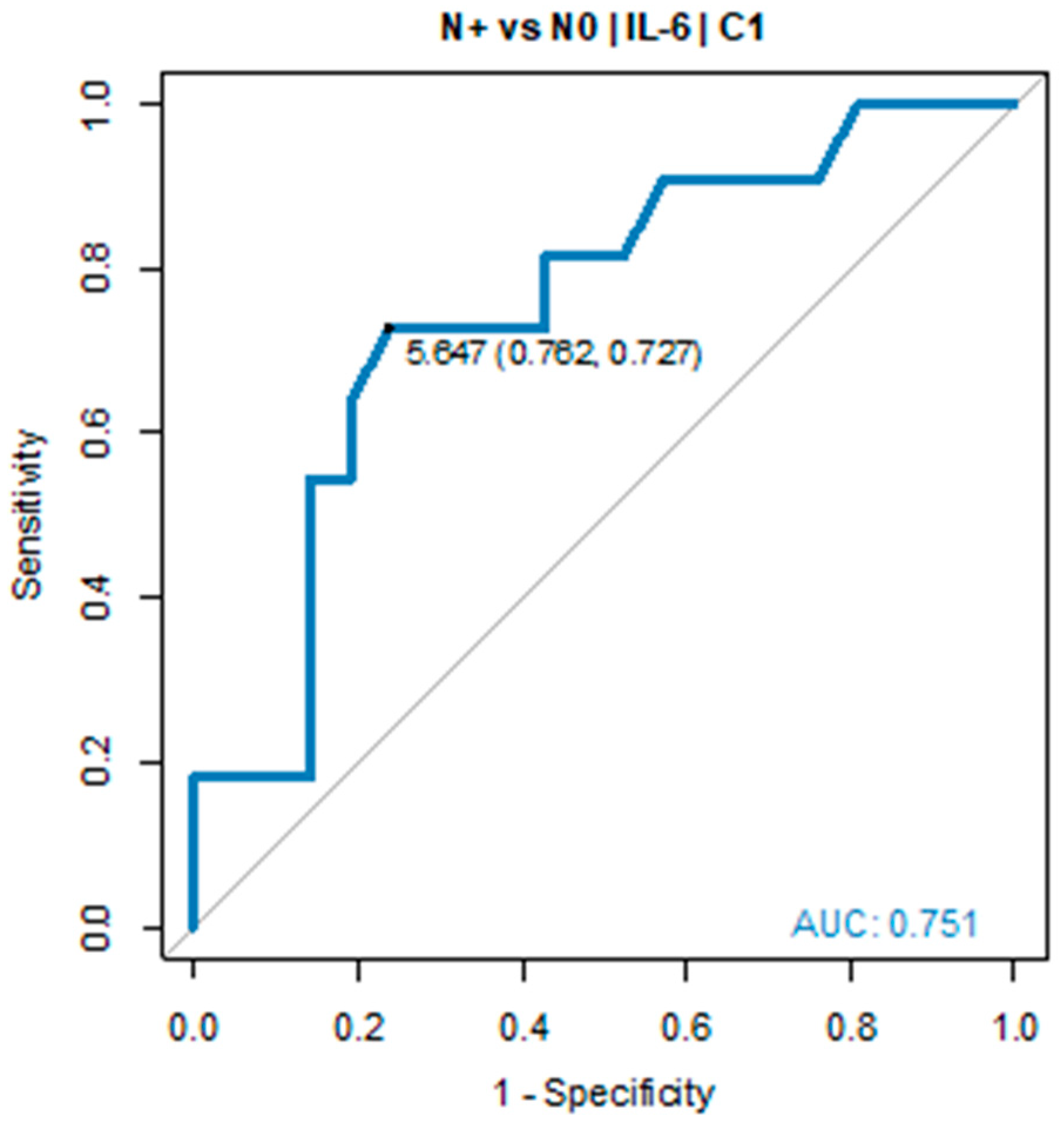
In all patients with IL-6 serum levels below 5.0 pg/mL in C2, tumour regression TRG1a/1b according to Becker classification was detected in postoperative histopathological specimens. Due to the small sample size, the pCR group was defined as TGR-1a/1b and ypN0. A similar relationship was found in ypN0 vs ypN+ group. The IL-6 serum level before C1 of chemotherapy was significantly elevated in ypN+ vs ypN0 (7.69 pg/mL vs 2.89 pg/mL, p = 0.022). ROC curve showed the predictive power of IL-6. The optimal threshold for diagnosing ypN0 was 5.0 pg/mL (AUC = 0.751, 95% CI: 0.568-0.934, p = 0.017), see
Table 2 and
Figure 3.
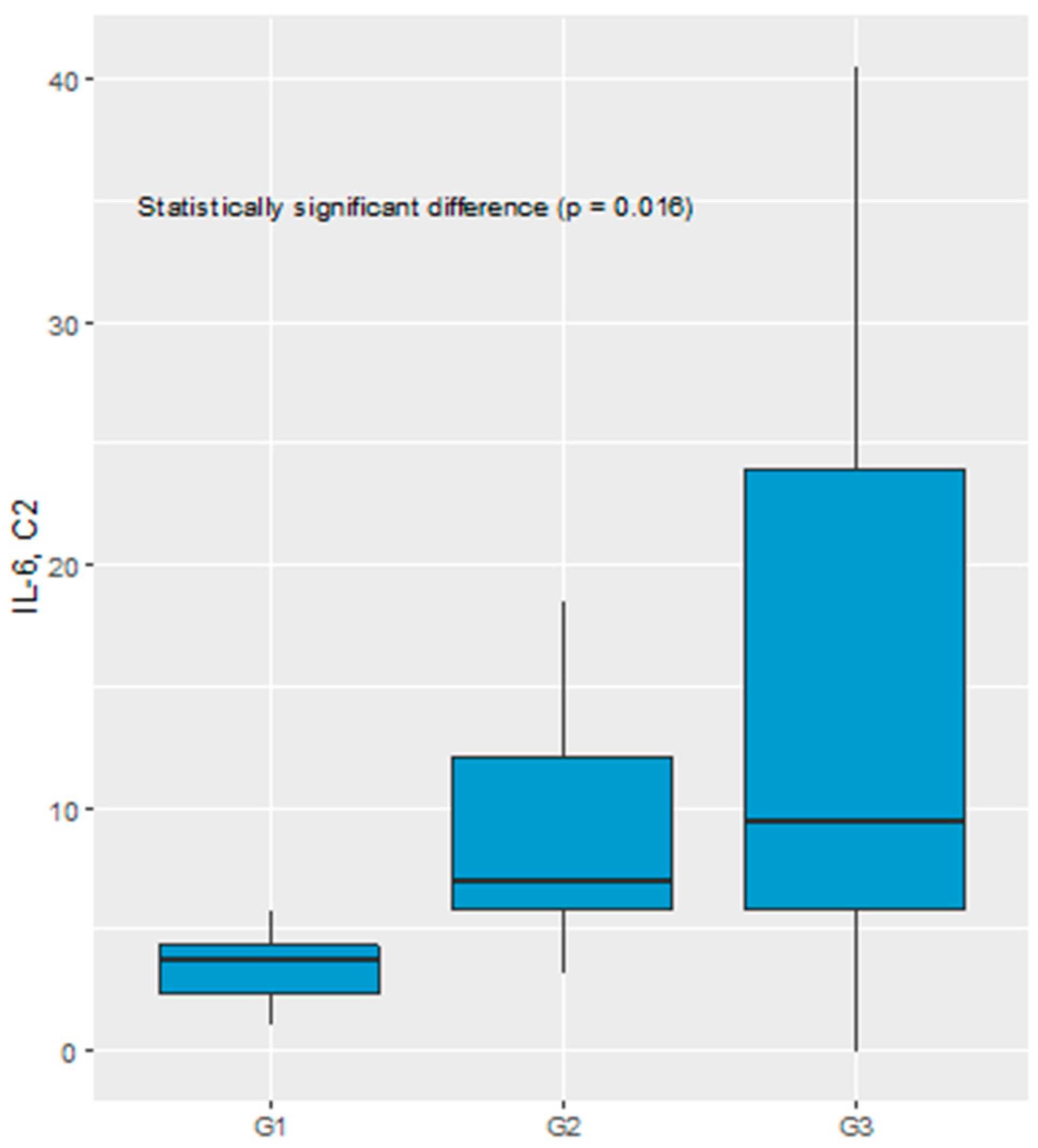
Significant difference in the IL-6 serum level before C2 of chemotherapy was recognized comparing TGR1, TGR2 and TGR3 groups (3.76 pg/mL vs 7.07 pg/mL vs 9.43 pg/mL, respectively, p = 0.016), see
Figure 4.
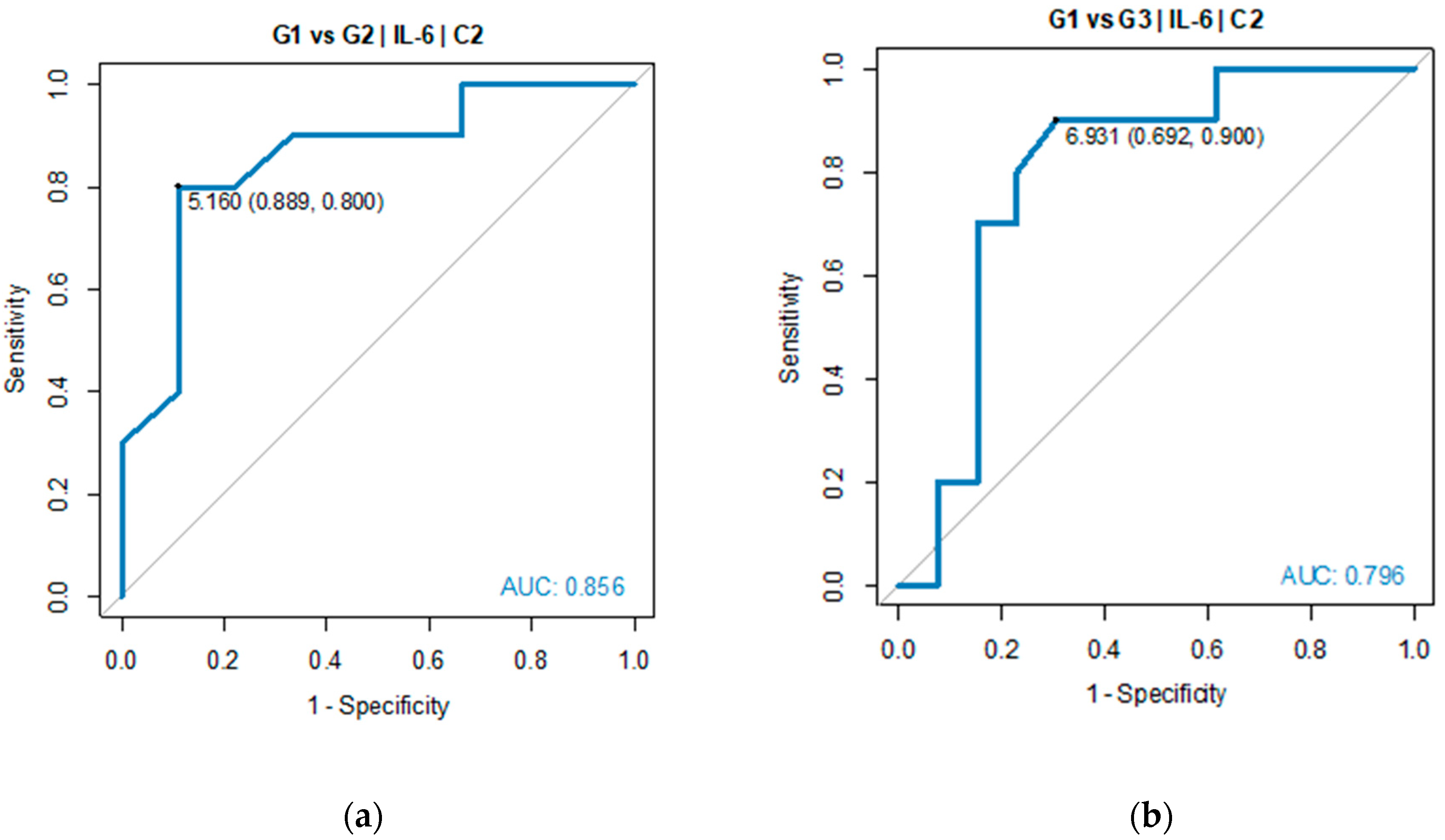
Pairwise comparisons indicated TGR1 and TGR3 as the groups with significant difference in IL-6 serum level. ROC analysis was performed to verify predictive power of IL-6 for diagnosing TGR groups against each of two other groups. Good diagnostic quality was identified when IL-6 was used to differentiate TGR1 from TGR2 and TGR1 from TGR3. The optimal threshold for diagnosing TGR1 vs TGR2 was 5.16 pg/mL (AUC=0.856, 95% CI: 0.674-1.000, p=0.005). The optimal threshold for diagnosing TGR1 vs TGR3 was 6.93 pg/mL (AUC=0.796, 95% CI: 0.596-0.997, p=0.004), see
Table 3 and
Figure 5.
ROC analysis did not show significant outcome of using IL-6 as prognosis parameter for TGR2 vs TGR3 groups (p > 0.05), which means IL-6 had good quality to predict that patients belonged to TGR1 group, while its quality to distinguish TGR2 from TGR3 groups was not proved.
4. Discussion
Gastric cancer treatment no longer involves surgery alone but over the past decade has become multimodality treatment. Most notably, the MAGIC trial and the French FNCLCC/FFCD 97033 trial demonstrated a significant survival benefit of perioperative treatment, which is currently a gold standard in western population [
4,
5,
6]. Response to neoadjuvant chemotherapy constitutes a substantial prognostic factor for disease-free survival and overall survival [
23,
24,
25]. As was demonstrated by the University of Texas MD Anderson Cancer Center, ypStage provides reasonable survival prediction based on TNM grouping, whereas clinical stage is not useful [
26]. In patients with tumour downstaging, disease-free survival and overall survival are longer than in patients without response to preoperative chemotherapy, and the best outcome is observed in patients with pathologic complete tumour regression [
23,
24,
25]. Unfortunately, the response rate after NAC remains limited [
4,
5,
6]. Moreover, there are currently no biomarkers enabling an individual prediction of therapeutic efficacy, real-time tumour dynamic or identifying patients at increased risk of a poor pathologic response. In non-responding patients to neoadjuvant chemotherapy such markers could allow to determine the optimal balance between the risks and benefits of avoiding NAC in patients with locally advanced GC or GEJ cancer. In our prospective, single-institution trial, we showed that elevated level of IL-6 prior to start of treatment and C2 might be a predictor of pathologic response to neoadjuvant chemotherapy. Patients who were not experiencing pathologic complete response (PCR) had statistically significant higher serum level in C2 than non-PCR. Similarly, node-positive (ypN+) patients had statistically significant higher serum level before start of treatment than node-negative (ypN0) patients. Multivariate analysis by Smyth E. et al demonstrated that the presence of lymph node metastases was the only factor independently predictive of overall survival in patients after NAC [
23]. The latest data presented by Athauda A. et al confirmed that lymph node status in the resection specimen is the single most important determiner of survival [
25].
Our prospective, pilot study is the first analysis of the utility of IL-6 as predictive biomarker of early response to NAC.
IL-6 is a key immunomodulatory cytokine, which is involved in the orchestration of the innate and acquired immune system and plays an important role in the regulation from various homeostatic to pathological processes such as immune disease and cancers [
27]. Studies by Kai H. et al and Ito R. et al show that IL-6 is involved in the growth of gastric cancer cells and the formation of metastases [
14,
15]. IL-6 is an important pro-angiogenic factor in gastric cancer through the induction of the VEGF [
28]. Significant correlation was observed between the serum concentration of IL-6 and the tumour stage, depth of tumour invasion, lymphatic invasion, venous invasion as well as lymph node metastasis [
29,
30]. Increasing data suggest that IL-6 plays a crucial role in the modulation of the function and activity of tumour-associated immune cells [
31]. IL-6 is a cancer-associated fibroblast (CAFs) specific secretory protein and a contributor to the dynamic crosstalk between tumour cells and microenvironment, which is essential for tumour growth, invasion and metastases. Epithelial-mesenchymal transition (EMT) of gastric cancer cells is induced by CAF-secreted IL-6. CAF-secreted IL-6 activates the Janus kinase (JAK) 1-signal transducer and activator of transcription 3 signal transduction (STAT) pathway in GC cell lines. The aberrantly hyperactivated IL-6/JAK/STAT3 pathway is generally associated with a poor clinical prognosis [
32,
33,
34]. In vitro and in vivo studies showed that CAF-secreted IL-6 is a very important contributor of chemoresistance in GC. The interaction of CAFs with tumour cells may induce a more aggressive phenotype of cancer cells and confer 5-fluorouracyl resistance to gastric cancer cell lines through the inhibition of apoptosis [
35]. This is extremely important as 5-fluorouracyl is the main cytostatic agent widely used in both perioperative and palliative treatment [
4,
5,
6].
In light of the above data, our study results are of clinical importance. If the results are confirmed in a larger group of patients, the measurement of IL-6 serum level prior to start of treatment and prior to administration of cycle 2 of neoadjuvant chemotherapy will enable quick identification of ypN+ and non-PCR patients with a poor prognosis. If the effect of IL-6 on inducing resistance to chemotherapy is also taken into consideration, it will be the basis for testing the efficacy of combination of perioperative chemotherapy with IL-6 receptor inhibition [
36]. Currently there is ongoing EMPOWER (NCT04333706) clinical trial of the combination of sarilumab (IL-6R inhibitor) plus capecitabine in triple negative breast cancer patients in stage I-III with high-risk residual disease [
37].
5. Conclusions
The above data suggest that IL-6 may be a predictive biomarker of pathologic response to neoadjuvant chemotherapy in patients with GC and GEJ cancer. The results were obtained on a small group of patients and currently cannot be used in everyday clinical practice. Confirmation of the results on a larger group of patients seems to be essential from clinical point of view, bearing in mind that the IL-6 plays a significant role in gastric cancer biology, particularly in metastasis formation and mechanism of chemotherapeutic resistance.
Author Contributions
The following statements should be used Conceptualization, Katarzyna Marcisz-Grzanka and Lucjan Wyrwicz; methodology: Katarzyna Marcisz-Grzanka, Lucjan Wyrwicz, Andrzej Tysarowski and Malgorzta Fuksiewicz; software, Katarzyna Marcisz-Grzanka; validation, Aleksandra Nowak, Andrzej Tysarowski and Malgorzta Fuksiewicz; formal analysis, Katarzyna Marcisz-Grzanka; data curation, Katarzyna Marcisz-Grzanka; investigation, Katarzyna Marcisz-Grzanka, Beata Kotowicz, Aleksandra Nowak, Mariola Winiarek, Maria Kowalska, Andrzej Tysarowski, Tomasz Olesinski, Jakub Palucki, Urszula Sulkowska and Agnieszka Kolasinska-Cwikla; resources, Katarzyna Marcisz-Grzanka, Beata Kotowicz, Aleksandra Nowak, Mariola Winiarek, Maria Kowalska, Andrzej Tysarowski, Tomasz Olesinski, Jakub Palucki, Urszula Sulkowska and Agnieszka Kolasinska-Cwikla; writing–original draft preparation, Katarzyna Marcisz-Grzanka; writing–review and editing, Katarzyna Marcisz-Grzanka, Lucjan Wyrwicz, Andrzej Tysarowski and Beata Kotowicz; supervision, Lucjan Wyrwicz; project administration, Katarzyna Marcisz-Grzanka; funding acquisition, Katarzyna Marcisz-Grzanka and Lucjan Wyrwicz. All authors have read and agreed to the published version of the manuscript.
Funding
This academic clinical study research was financed by the statutory funds of the Maria Sklodowska-Curie National Research Institute of Oncology (MSCNRIO), Warsaw, Poland (grant number SN/GW24/2017 on 13 February 2017). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Institutional Review Board Statement
The trial was performed in accordance with the principles of the Declaration of Helsinki and protocol was approved by the Local Bioethics Committee at the Maria Sklodowska-Curie National Research Institute of Oncology (MSCNRIO) in Warsaw (approval number 51/2016/2017 on 5 January 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.” OR “Patient consent was waived due to REASON (please provide a detailed justification).” OR “Not applicable.” for studies not involving humans. You might also choose to exclude this statement if the study did not involve humans.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This academic clinical study research was financed by the statutory funds of the Maria Sklodowska-Curie National Research Institute of Oncology (MSCNRIO), Warsaw, Poland (grant number SN/GW24/2017 on 13 February 2017).
Conflicts of Interest
Katarzyna Marcisz-Grzanka, Beata Kotowicz, Aleksandra Nowak, Mariola Winiarek, Malgorzata Fuksiewicz, Maria Kowalska, Andrzej Tysarowski, Tomasz Olesinski, Jakub Palucki, Urszula Sulkowska, Agnieszka Kolasinska-Cwikla declare no conflict of interest. Lucjan Wyrwicz: consulting fees - MSD, AstraZeneca; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events - MSD, AstraZeneca; payment for expert testimony - MSD, AstraZeneca.
Appendix A
Table A1.
Reference genes selected for optimization.A1.
Table A1.
Reference genes selected for optimization.A1.
| Gene |
Name |
Protein Function |
| TBP |
TATA binding protein |
Transcription initiator–binds to a specific
DNA sequence–the TATA box |
| HPRT |
Hypoxanthine-guanine
phosphoribosyltransferase |
An enzyme involved in the metabolism of purines, allowing its recovery from degraded DNA for the re-synthesis of nucleotides |
| GAPDH |
Glyceraldehyde 3-phosphate
dehydrogenase |
An enzyme involved in glycolysis–converts glucose into carbon molecules and energy |
| SDHA |
Succinate dehydrogenase
complex, subunit A |
Mitochondrial respiratory chain complex–responsible for the transformation of succinate into fumarate |
| YWHAZ |
Monooxygenase/tryptophan
5-monooxygenase activation
protein zeta |
It is a regulator of cell apoptotic pathways–it takes part in metabolism and regulates the cell cycle |
| HMBS |
Hydroxymethylbilane
synthase |
An enzyme involved in the production of heme |
| ZNF410 |
Zinc fi
nger protein 410 |
Transcription factor |
Table A2.
Baseline characteristics of the treatment group.A2.
Table A2.
Baseline characteristics of the treatment group.A2.
| Factor |
Value |
| Age (years) |
| Median |
63 (30-77) |
| <60 |
20 (33%) |
| 60-69 |
30 (49%) |
| ≥70 |
11 (18%) |
| Sex |
| Male |
32 (52.5%) |
| Female |
29 (47.5%) |
| ECOG |
| 0 |
11 (18%) |
| 1 |
50 (82%) |
| Location of tumour |
| GEJ |
14 (23%) |
| Stomach |
47 (77%) |
| cT-stage |
| T1 |
1 (2%) |
| T2 |
28 (46%) |
| T3 |
27 (44%) |
| T4 |
5 (8%) |
| cN-stage |
| N0 |
30 (49%) |
| N1 |
11 (18%) |
| N2 |
11 (18%) |
| N3 |
9 (15%) |
| N+ |
30 (49%) |
| N- |
31 (51%) |
| TNM according to AJCC – the 8th edition |
| IIA |
27 (44%) |
| IIB |
18 (30%) |
| IIIA |
5 (8%) |
| IIIB |
7 (11%) |
| IIIC |
4 (7%) |
| Lauren’s type |
| Diffuse |
17 (28%) |
| Intestinal |
23 (38%) |
| Mixed |
12 (19%) |
| Not evaluable according to Lauren |
9 (15%) |
| Signed ring cell/poorly cohesive |
22 (36%) |
| Grading according to WHO |
| G1 |
1 (2%) |
| G2 |
21 (34%) |
| G3 |
28 (46%) |
| Not evaluable |
11 (18%) |
Table A3.
Surgical and pathology results of treatment.A3.
Table A3.
Surgical and pathology results of treatment.A3.
| Factor |
Value |
| Surgery |
| Tumour curative surgery R0–margin free |
52 (85%) |
| Tumour surgery R1 |
1 (2%) |
| Palliative surgery |
5 (8%) |
| No surgery |
3 (5%) |
| Histopathological tumour regression according to Becker classification |
| Complete–TRG1a |
7 (11%) |
| Subtotal–TRG1b |
6 (10%) |
| Complete or subtotal–TRG1a/b |
13 (21%) |
| Partial–TRG2 |
14 (23%) |
| Minimal or none–TRG3 |
26 (43%) |
| Palliative surgery–not evaluated TGR |
5 (8%) |
| Tumour stage (ypT) |
| Tx |
7 (11%) |
| T1 |
11 (18%) |
| T2 |
9 (15%) |
| T3 |
23 (38%) |
| T4 |
3 (5%) |
| ypT no available |
8 (13%) |
| Nodal status (ypN) |
| N0 |
34 (56%) |
| N1 |
5 (8%) |
| N2 |
6 (10%) |
| N3 |
8 (13%) |
| ypN no available |
8 (13%) |
| Lymphovascular invasion–LVI |
| Yes |
20 (33%) |
| No |
32 (52%) |
| N/A |
9 (15%) |
| Perineural invasion–PNI |
| Yes |
7 (11%) |
| No |
45 (74%) |
| N/A |
9 (15%) |
References
- Chau, I.; Norman, A.R.; Cunningham, D.; Waters, J.S.; Oates, J.; Ross, P.J. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer-pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004, 22(12), 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, R.T.; Murray, T.; Bolden, S.; Wingo., P.A. Cancer statistics. CA Cancer J Clin. 2000, 50(1), 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71(3), 209–249. [CrossRef]
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van De Velde CJ, Nicolson M, et al. MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006, 355(1), 11–20. [CrossRef]
- Ychou M, Boige V, Pignon JP, Conroy T, Bouch´e O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011, 1715(1721), 21444866–21444866. [CrossRef]
- Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. RD; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised. Lancet. 2019, 393(10184), 1948–1957. [CrossRef]
- Chai, E.Z.; Siveen, K.S.; Shanmugam, M.K.; Arfuso, F.; Sethi, G. Analysis of the intricate relationship between chronic inflammation and cancer. Biochem J. 2015, 468(1), 1–15. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature. 2002, 420(6917), 860–867. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140(6), 883–899. [Google Scholar] [CrossRef] [PubMed]
- Myers, JS. Proinflammatory cytokines and sickness behavior: implications for depression and cancer-related symptoms. Oncol Nurs Forum. 2008, 35(5), 802–807. [Google Scholar] [CrossRef] [PubMed]
- Szaflarska A, Szczepanik A, Siedlar M, Czupryna A, Sierzega M, Popiela T, et al. Preoperative plasma level of IL-10 but not of proinflammatory cytokines is an independent prognostic factor in patients with gastric cancer. Anticancer Res. 2009, 29(12), 5005–5012.
- Kitadai Y, Haruma K, Sumii K, Yamamoto S, Ue T, Yokozaki H, et al. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am J Pathol. 1998, 152(1), 93–100.
- Huang SP, Wu MS, Shun CT, Wang HP, Lin MT, Kuo ML, et al. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci. 2004, 11(4), 517–527. [CrossRef]
- Kai H, Kitadai Y, Kodama M, Cho S, Kuroda T, Ito M, et al. Involvement of proinflammatory cytokines IL-1beta and IL-6 in progression of human gastric carcinoma. Anticancer Res. 2005, 25(2A), 709–713.
- Ito R, Yasui W, Kuniyasu H, Yokozaki H, Tahara E. Expression of interleukin-6 and its effect on the cell growth of gastric carcinoma cell lines. Jpn J Cancer Res. 1997, 88(10), 953–958. [CrossRef]
- Kim HK, Song KS, Park YS, Kang YH, Lee YJ, Lee KR, et al. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur J Cancer. 2003, 39(2), 184–191. Erratum in: Eur J Cancer. 2003, 39(17), 2569. [CrossRef]
- Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2015, 41(10), 971–978. [CrossRef]
- Lian L, Xia YY, Zhou C, Shen XM, Li XL, Han SG, et al. Application of platelet/lymphocyte and neutrophil/lymphocyte ratios in early diagnosis and prognostic prediction in patients with resectable gastric cancer. Cancer Biomark. 2015, 15(6), 899–907. [CrossRef]
- Ock, CY., Nam, AR., Lee, J. et al. Prognostic implication of antitumor immunity measured by the neutrophil–lymphocyte ratio and serum cytokines and angiogenic factors in gastric cancer. Gastric Cancer. 2017, 20, 254–262. [CrossRef]
- Cho IR, Park JC, Park CH, Jo JH, Lee HJ, Kim S, et al. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer. 2014, 17(4), 703–710. [CrossRef]
- Arigami T, Uenosono Y, Ishigami S, Okubo K, Kijima T, Yanagita S, et al. A Novel Scoring System Based on Fibrinogen and the Neutrophil-Lymphocyte Ratio as a Predictor of Chemotherapy Response and Prognosis in Patients with Advanced Gastric Cancer. Oncology. 2016, 90(4), 186–192. [CrossRef]
- Tsujiura M, Ichikawa D, Konishi H, Komatsu S, Shiozaki A, Otsuji E. Liquid biopsy of gastric cancer patients: circulating tumor cells and cell-free nucleic acids. World J Gastroenterol. 2014, 20(12), 3265–3286. [CrossRef]
- Smyth EC, Fassan M, Cunningham D, Allum WH, Okines AF, Lampis A, et al. Effect of Pathologic Tumor Response and Nodal Status on Survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J Clin Oncol. 2016, 34(23), 2721–2727. [CrossRef]
- Reim D, Novotny A, Friess H, Slotta-Huspenina J, Weichert W, Ott K, et al. Significance of tumour regression in lymph node metastases of gastric and gastrooesophageal junction adenocarcinomas. J Pathol Clin Res. 2020, 6(4), 263–272. [CrossRef]
- Athauda A, Nankivell M, Langer R, Pritchard S, Langley RE, Loga KV, et al. Pathological regression of primary tumour and metastatic lymph nodes following chemotherapy in resectable OG cancer: pooled analysis of two trials. Br J Cancer. 2023, 128(11), 2036–2043. [CrossRef]
- Ikoma N, Blum M, Estrella JS, Das P, Hofstetter WL, Fournier KF, et al. Evaluation of the American Joint Committee on Cancer 8th edition staging system for gastric cancer patients after preoperative therapy. Gastric Cancer. 2018, 21(1), 74–83. [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol. 2021, 33(3), 127–148. [Google Scholar] [CrossRef] [PubMed]
- Huang SP, Wu MS, Shun CT, Wang HP, Lin MT, Kuo ML, et al. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci. 2004, 11(4), 517–527. [CrossRef]
- Ashizawa T, Okada R, Suzuki Y, Takagi M, Yamazaki T, Sumi T, et al. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: role of IL-6 as a prognostic factor. Gastric Cancer. 2005, 8(2), 124–131. [CrossRef]
- Ikeguchi M, Hatada T, Yamamoto M, Miyake T, Matsunaga T, Fukumoto Y, et al. Serum interleukin-6 and -10 levels in patients with gastric cancer. Gastric Cancer. 2009, 12(2), 95–100. [CrossRef]
- Oya Y, Hayakawa Y, Koike K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020, 111(8), 2696–2707. [CrossRef]
- Wu X, Tao P, Zhou Q, Li J, Yu Z, Wang X, et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget. 2017, 8(13), 20741–20750. [CrossRef]
- Kinoshita H, Hirata Y, Nakagawa H, Sakamoto K, Hayakawa Y, Takahashi R, et al. Interleukin-6 mediates epithelial-stromal interactions and promotes gastric tumorigenesis. PLoS One. 2013, 8(4), e60914. P. [CrossRef]
- Karakasheva TA, Lin EW, Tang Q, Qiao E, Waldron TJ, Soni M, et al. IL-6 Mediates Cross-Talk between Tumor Cells and Activated Fibroblasts in the Tumor Microenvironment. Cancer Res. 2018, 78(17), 4957–4970. [CrossRef]
- Ham IH, Oh HJ, Jin H, Bae CA, Jeon SM, Choi KS, et al. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol Cancer. 2019, 18(1), 68. [CrossRef]
- Raskova M, Lacina L, Kejik Z, Venhauerova A, Skalickova M, Kolar M, et al. The Role of IL-6 in Cancer Cell Invasiveness and Metastasis-Overview and Therapeutic Opportunities. Cells. 2022, 11(22), 3698. [CrossRef]
- Jayachandran, P. A Dose Finding Phase 1 of Sarilumab Plus Capecitabine in HER2/Neu-Negative Metastatic Breast Cancer and a Single-arm, Historically-controlled Phase 2 Study of Sarilumab Plus Capecitabine in Stage I-III Triple Negative Breast Cancer With High-Risk Residual Disease (EMPOWER). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04333706 (accessed on 27 November 2023).
- GeneCards. Available online: https://www.genecards.org/ (accessed on 27 November 2023).
- UniProt. Available online: https://www.uniprot.org/ (accessed on 27 November 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).