Submitted:
28 December 2023
Posted:
29 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Research methodology
3. Results and Discussion
4. Conclusions
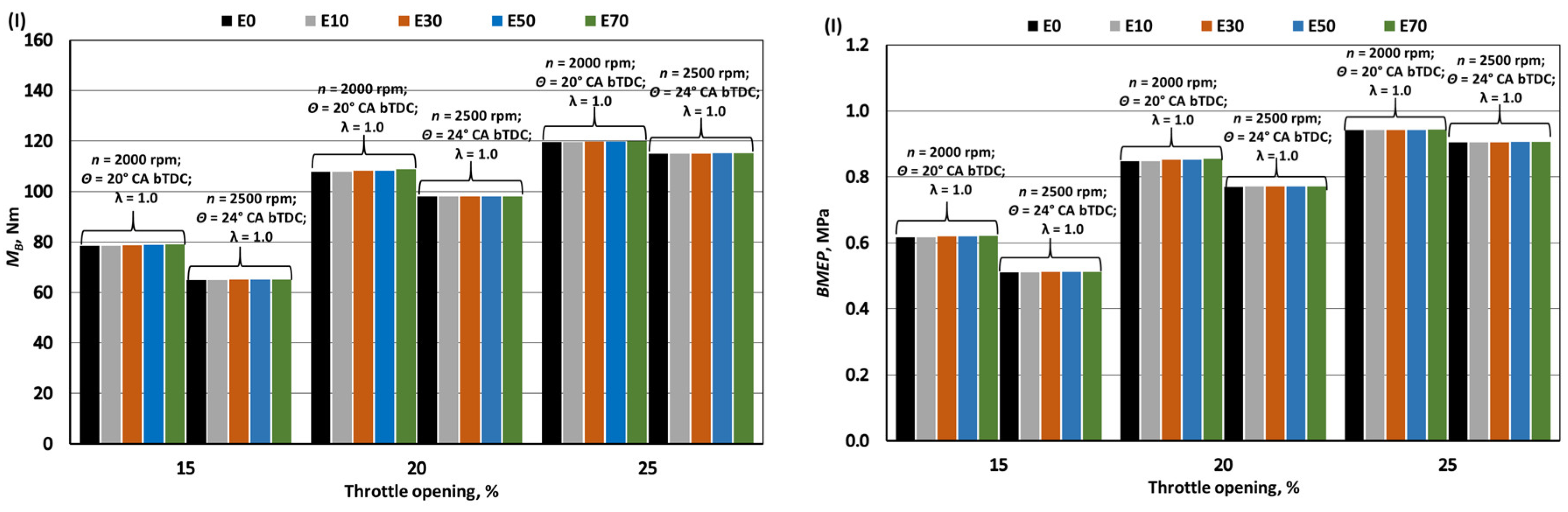
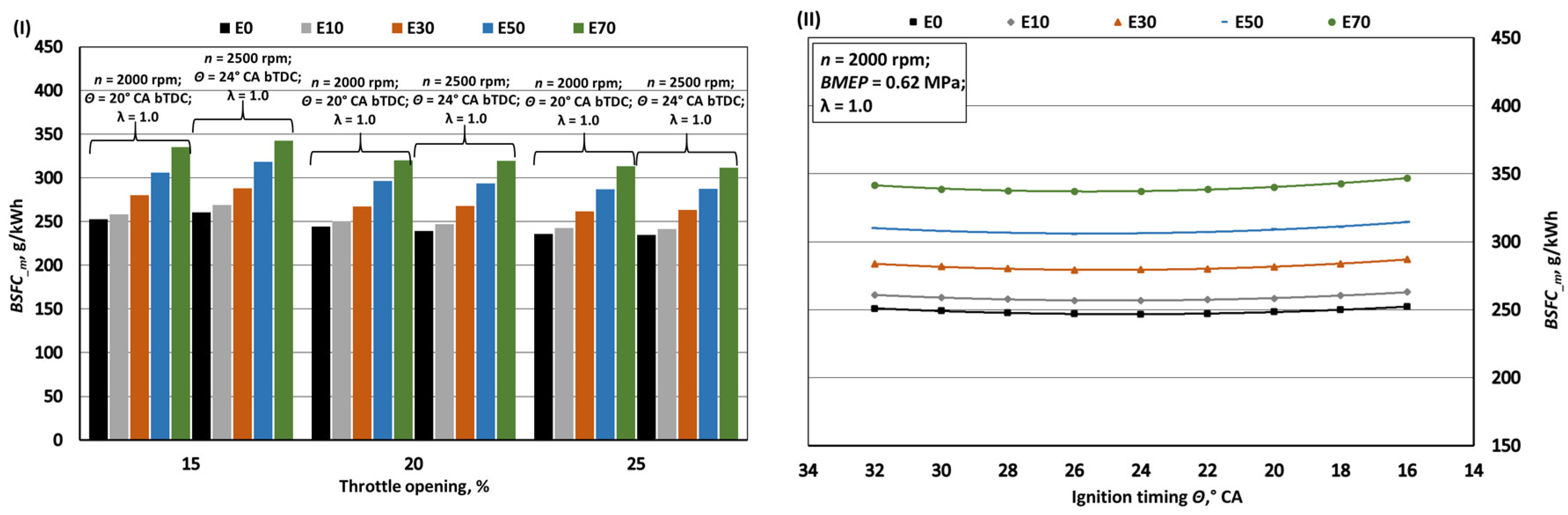
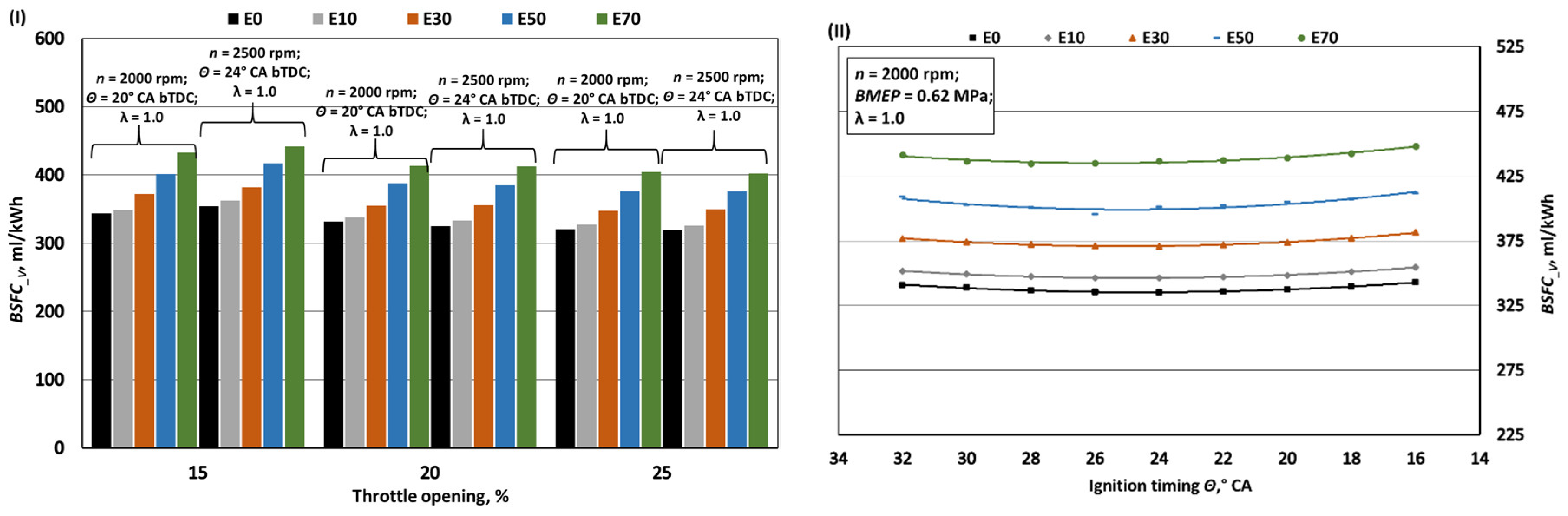
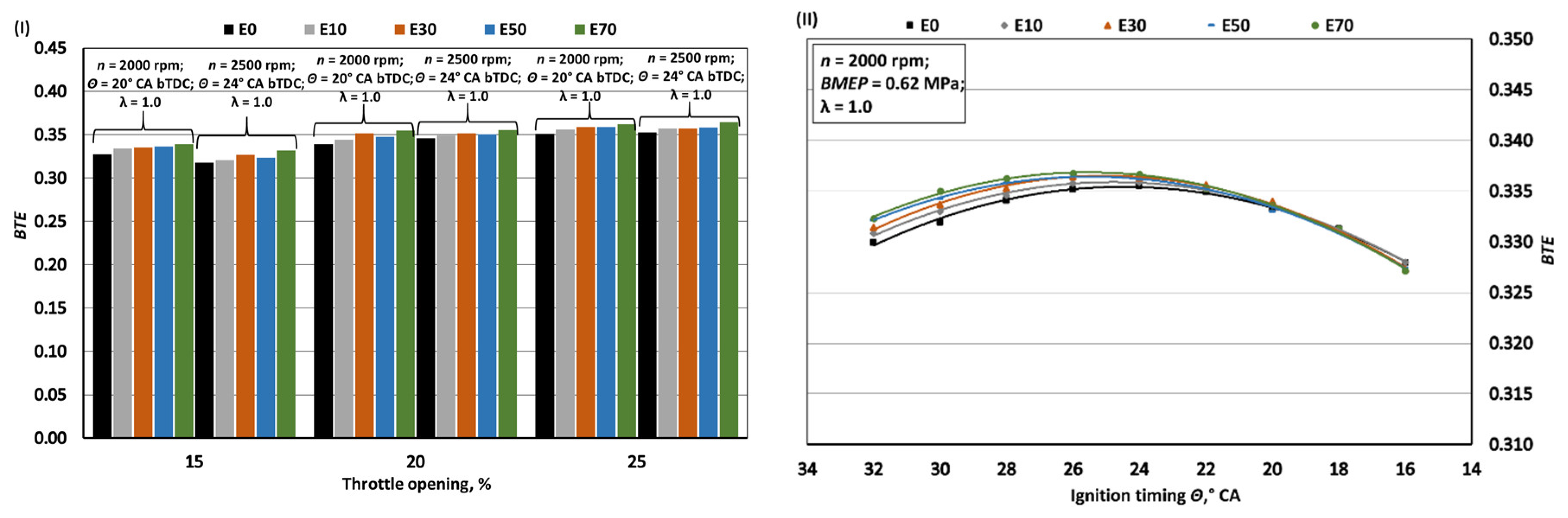
- When bioethanol concentration in gasoline blend reaches 70%, the engine's MB and BMEP increase by approximately 1%. This rise is influenced by factors like a 27.1% reduction in LHV, a 27.6% increase in fuel mass per 1 kg of air, and improved volumetric efficiency due to lower intake mixture temperatures from bioethanol's higher latent heat of evaporation. This also enhances BTE by around 1.7% by aiding hydrocarbon oxidation. However, bioethanol's lower LHV causes a 26% increase in BSFC_m and a 22.5% increase in BSFC_V compared to pure gasoline (E0).
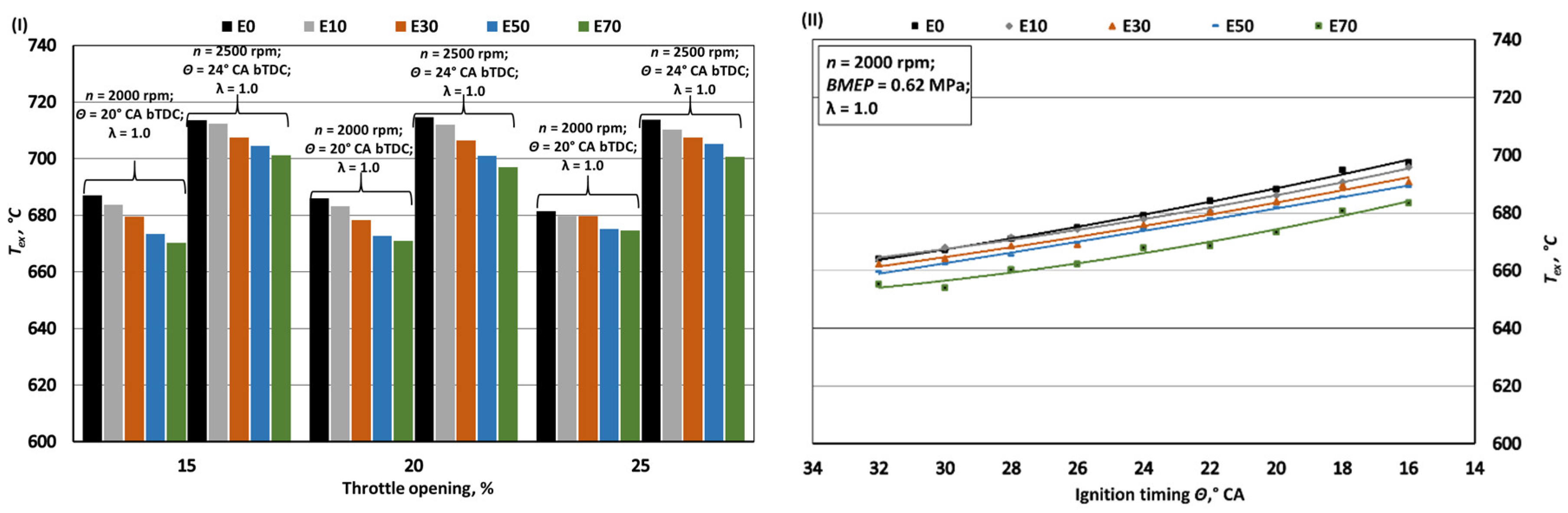
- The exhaust temperature of E70 decreases by approximately 1.9% due to lower initial combustion temperatures resulting from enhanced intake mixture cooling and the lower flame temperature of bioethanol. While bioethanol has a faster burning rate than gasoline, it experiences a longer ignition delay phase. To achieve maximum BTE and minimum BSFC, the ignition timing for E70 needs to be advanced by around 2° CA compared to the optimal timing for E0.
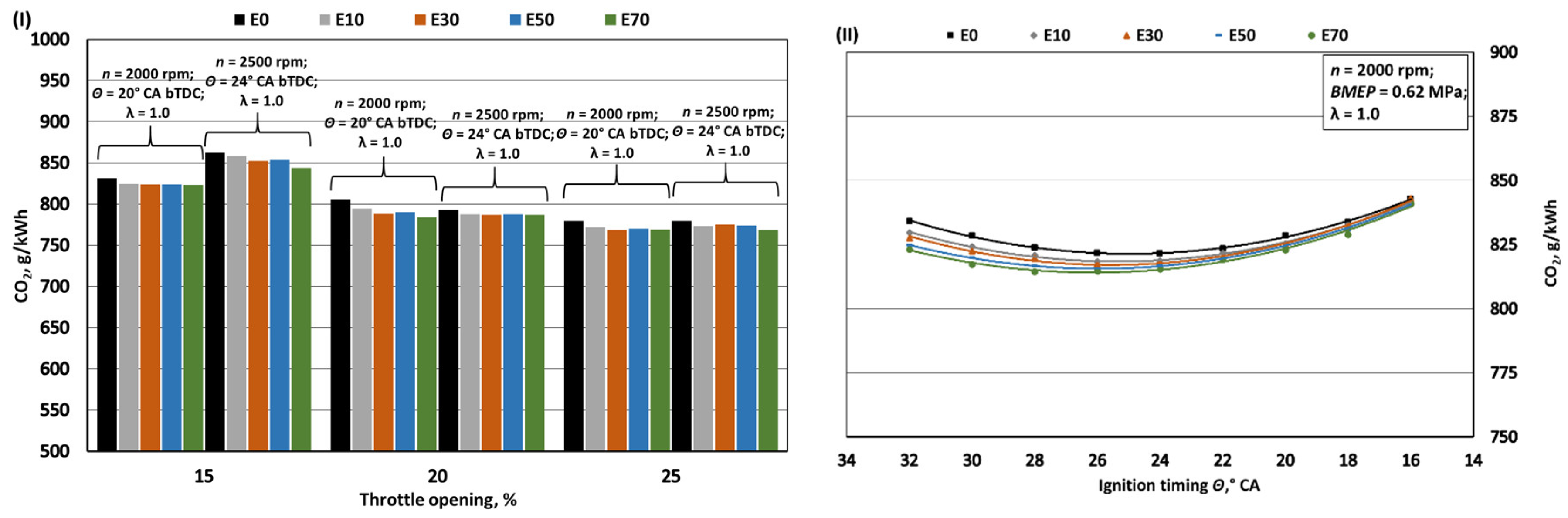
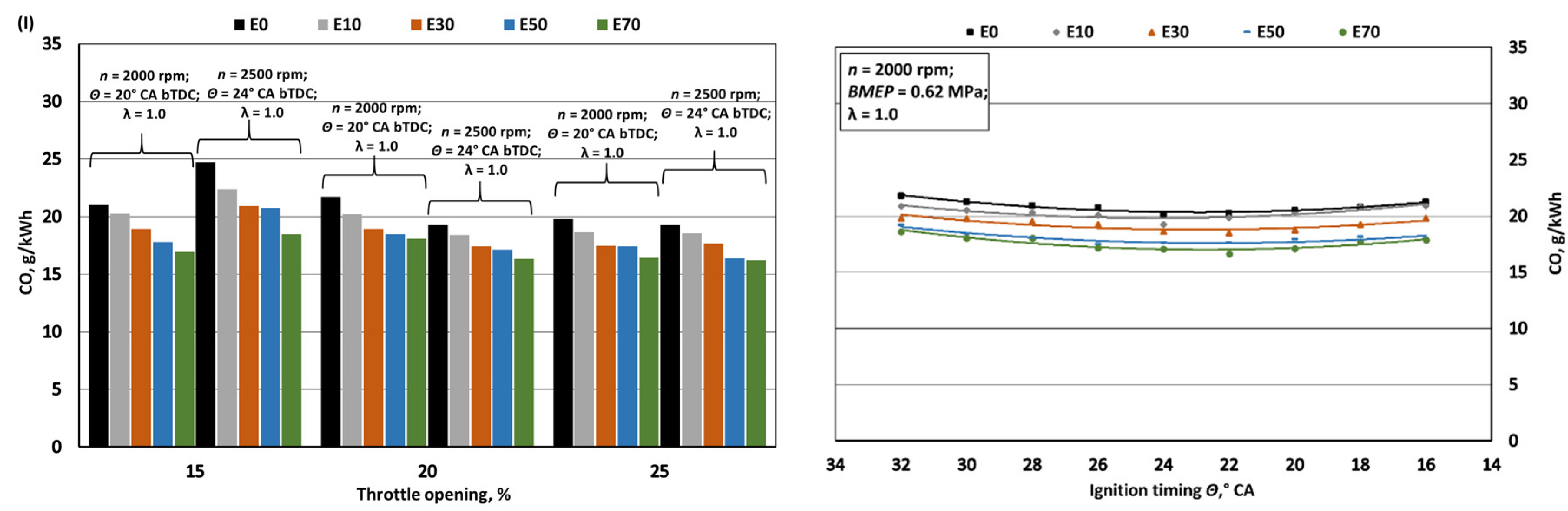
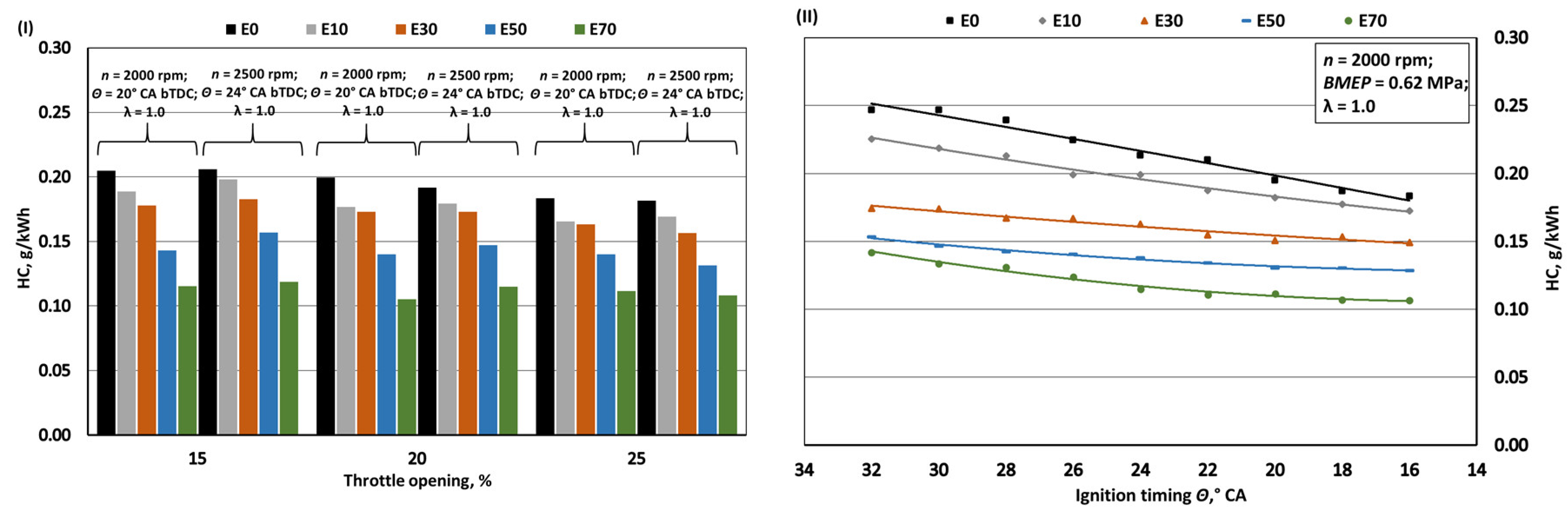
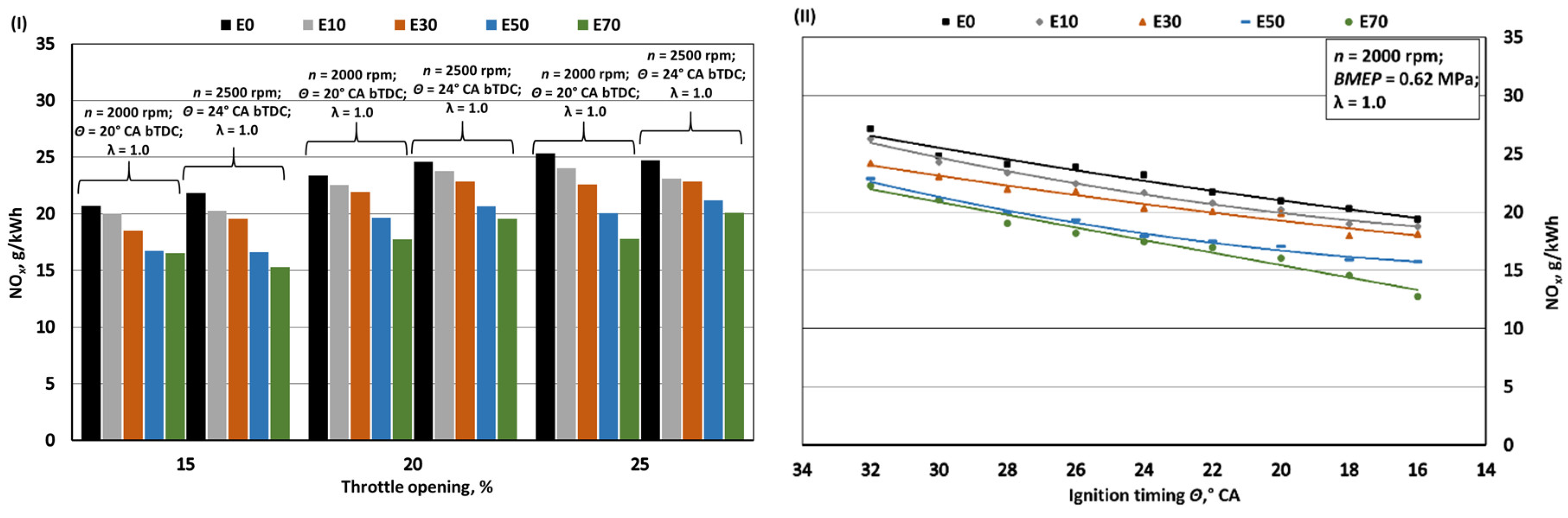
- Increasing bioethanol concentration in gasoline blends leads to a reduction in greenhouse gas emissions, particularly CO2 and NOx. The decrease in specific CO2 emissions when bioethanol concentration reaches 70% is approximately 1.1%, despite a 24.8% reduction in the fuel's C/H ratio. However, it's important to note that increased BSFC has a negative impact in this context. The reduction in CO2 emissions, while not substantial, should be considered alongside the fact that bioethanol is a renewable fuel, with E100 having approximately 60% lower CO2 emissions during its life cycle compared to E0. With the introduction of bioethanol, NOx emissions decrease due to the lower combustion temperature, resulting in reductions of approximately 4.5%, 8.8%, 18.2%, and 23.5% when switching from E0 to E10, E30, E50, and E70, respectively. However, it's crucial to limit ignition timing as increasing it leads to higher NOx emissions. The oxygen content in bioethanol reduces the emission of incomplete combustion products, resulting in decreases of approximately 3.8%, 8.9%, 13.7%, and 16.7% in CO emissions and 8.2%, 19.7%, 32.0%, and 43.5% in HC emissions when transitioning from E0 to E10, E30, E50, and E70, respectively. Advancing the ignition timing by +2° CA, which optimizes BTE, reduces specific CO emissions but increases HC emissions as combustion initiates before the air-fuel mixture is fully mixed.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A/F | Air to fuel ratio |
| BMEP | Brake mean effective pressure |
| BSFC_m | Specific fuel mass consumption |
| BSFC_V | Specific fuel volume consumption |
| bTDC | Before top dead center |
| BTE | Break thermal efficiency |
| C | Carbon |
| C/H | Carbon-to-hydrogen ratio |
| CA | Crank angle |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| E | Ethanol |
| ECU | Electronic control unit |
| H | Hydrogen |
| HC | Hydrocarbons |
| LHV | Lower heating value |
| MB | Brake torque |
| n | Engine speed |
| NOx | Nitrogen oxides |
| O | Oxygen |
| Tex | Exhaust Temperature |
| Θ | Ignition timing |
| λ (lambda) | Excess air ratio |
References
- UNFCCC The Paris Agreement.; Paris, November 29 2018.
- European Environment Agency. Transport and Environment Report 2022: Digitalisation in the Mobility System : Challenges and Opportunities.; Publications Office: LU, 2022. [Google Scholar]
- Campbell, P.; Zhang, Y.; Yan, F.; Lu, Z.; Streets, D. Impacts of Transportation Sector Emissions on Future U.S. Air Quality in a Changing Climate. Part II: Air Quality Projections and the Interplay between Emissions and Climate Change. Environ. Pollut. 2018, 238, 918–930. [Google Scholar] [CrossRef]
- Xin, Y.; Xing, X.; Li, X.; Hong, H. A Biomass–Solar Hybrid Gasification System by Solar Pyrolysis and PV– Solid Oxide Electrolysis Cell for Sustainable Fuel Production. Appl. Energy 2024, 356, 122419. [Google Scholar] [CrossRef]
- Gupta, P.; Protim Das, P.; Mubarak, M.; Shaija, A. Performance and Emission Analysis of Single Cylinder SI Engine Using Bioethanol-Gasoline Blend Produced from Salvinia Molesta. IOP Conf. Ser. Mater. Sci. Eng. 2018, 297, 012005. [Google Scholar] [CrossRef]
- Puricelli, S.; Cardellini, G.; Casadei, S.; Faedo, D.; Van Den Oever, A.E.M.; Grosso, M. A Review on Biofuels for Light-Duty Vehicles in Europe. Renew. Sustain. Energy Rev. 2021, 137, 110398. [Google Scholar] [CrossRef]
- Shere, A.; Subramanian, K.A. Effects of Hydrogen and EGR on Energy Efficiency Improvement with Ultra Low Emissions in a Common Rail Direct Injection Compression Ignition Engine Fueled with Dimethyl Ether (DME) under HCCI Mode. Int. J. Hydrog. Energy 2024, 52, 1447–1474. [Google Scholar] [CrossRef]
- Yang, Z.; Du, Y.; Geng, Q.; He, G. Energy Loss and Comprehensive Performance Analysis of a Novel High-Efficiency Hybrid Cycle Hydrogen-Gasoline Rotary Engine under off-Design Conditions. Energy Convers. Manag. 2022, 267, 115942. [Google Scholar] [CrossRef]
- Li, J.; Xiong, F.; Fan, M.; Chen, Z. The Role of Nonfood Bioethanol Production in Neutralizing China’s Transport Carbon Emissions: An Integrated Life Cycle Environmental-Economic Assessment. Energy Sustain. Dev. 2022, 70, 68–77. [Google Scholar] [CrossRef]
- Holmatov, B.; Schyns, J.F.; Krol, M.S.; Gerbens-Leenes, P.W.; Hoekstra, A.Y. Can Crop Residues Provide Fuel for Future Transport? Limited Global Residue Bioethanol Potentials and Large Associated Land, Water and Carbon Footprints. Renew. Sustain. Energy Rev. 2021, 149, 111417. [Google Scholar] [CrossRef]
- Mohammed Shahinsha, N.; John, J.A.; Singh, K.; Kumar, N. Reduction in Automotive Emissions by Ethanol Blending in SI Engine: A Review. Mater. Today Proc. 2023, S2214785323018801. [Google Scholar] [CrossRef]
- Milovanoff, A.; Posen, I.D.; Saville, B.A.; MacLean, H.L. Well-to-Wheel Greenhouse Gas Implications of Mid-Level Ethanol Blend Deployment in Canada’s Light-Duty Fleet. Renew. Sustain. Energy Rev. 2020, 131, 110012. [Google Scholar] [CrossRef]
- Najafi, G.; Ghobadian, B.; Yusaf, T.; Safieddin Ardebili, S.M.; Mamat, R. Optimization of Performance and Exhaust Emission Parameters of a SI (Spark Ignition) Engine with Gasoline–Ethanol Blended Fuels Using Response Surface Methodology. Energy 2015, 90, 1815–1829. [Google Scholar] [CrossRef]
- Park, S.H.; Cha, J.; Kim, H.J.; Lee, C.S. Effect of Early Injection Strategy on Spray Atomization and Emission Reduction Characteristics in Bioethanol Blended Diesel Fueled Engine. Energy 2012, 39, 375–387. [Google Scholar] [CrossRef]
- Butkus, A.; Pukalskas, S. THE RESEARCH INTO THE INFLUENCE OF ECOLOGICAL PETROL ADDITIVES IN THE AUTOMOBILE LABORATORY. TRANSPORT 2004, 19, 24–27. [Google Scholar] [CrossRef]
- Pikūnas, A.; Pukalskas, S.; Grabys, J. Influence of Composition of Gasoline-Ethanol Blends on Parameters of Internal Combustion Engines. 2003.
- Zhang, J.; Liu, J.; Kou, L.; Zhang, X.; Tan, T. Bioethanol Production from Cellulose Obtained from the Catalytic Hydro-Deoxygenation (Lignin-First Refined to Aviation Fuel) of Apple Wood. Fuel 2019, 250, 245–253. [Google Scholar] [CrossRef]
- Shakelly, N.; Pérez-Cardona, J.R.; Deng, S.; Maani, T.; Li, Z.; Sutherland, J.W. Comparative Life Cycle Assessment of Bioethanol Production from Different Generations of Biomass and Waste Feedstocks. Procedia CIRP 2023, 116, 630–635. [Google Scholar] [CrossRef]
- Renzi, M.; Bietresato, M.; Mazzetto, F. An Experimental Evaluation of the Performance of a SI Internal Combustion Engine for Agricultural Purposes Fuelled with Different Bioethanol Blends. Energy 2016, 115, 1069–1080. [Google Scholar] [CrossRef]
- Yusoff, M.N.A.M.; Zulkifli, N.W.M.; Masjuki, H.H.; Harith, M.H.; Syahir, A.Z.; Khuong, L.S.; Zaharin, M.S.M.; Alabdulkarem, A. Comparative Assessment of Ethanol and Isobutanol Addition in Gasoline on Engine Performance and Exhaust Emissions. J. Clean. Prod. 2018, 190, 483–495. [Google Scholar] [CrossRef]
- Zhai, H.; Frey, H.C.; Rouphail, N.M.; Gonçalves, G.A.; Farias, T.L. Comparison of Flexible Fuel Vehicle and Life-Cycle Fuel Consumption and Emissions of Selected Pollutants and Greenhouse Gases for Ethanol 85 Versus Gasoline. J. Air Waste Manag. Assoc. 2009, 59, 912–924. [Google Scholar] [CrossRef]
- Song, L.; Liu, T.; Fu, W.; Lin, Q. Experimental Study on Spray Characteristics of Ethanol-Aviation Kerosene Blended Fuel with a High-Pressure Common Rail Injection System. J. Energy Inst. 2018, 91, 203–213. [Google Scholar] [CrossRef]
- Pecho, P.; Hrúz, M.; Sitar, M.; Bugaj, M. Possibilities of Using Bioethanol as an Alternative Fuel in the Conditions of Jet Engines. Transp. Res. Procedia 2021, 59, 183–192. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, W.; Wang, J.; Rao, D.; Chen, X.; Ma, H.; Zeng, W. Study on the Laminar Burning Velocity of Ethanol/RP-3 Aviation Kerosene Premixed Flame. Combust. Flame 2022, 238, 111921. [Google Scholar] [CrossRef]
- Wang, X.; Guo, L.; Lv, J.; Li, M.; Huang, S.; Wang, Y.; Ma, X. Process Design, Modeling and Life Cycle Analysis of Energy Consumption and GHG Emission for Jet Fuel Production from Bioethanol in China. J. Clean. Prod. 2023, 389, 136027. [Google Scholar] [CrossRef]
- Göktaş, M.; Kemal Balki, M.; Sayin, C.; Canakci, M. An Evaluation of the Use of Alcohol Fuels in SI Engines in Terms of Performance, Emission and Combustion Characteristics: A Review. Fuel 2021, 286, 119425. [Google Scholar] [CrossRef]
- Mendiburu, A.Z.; Lauermann, C.H.; Hayashi, T.C.; Mariños, D.J.; Rodrigues Da Costa, R.B.; Coronado, C.J.R.; Roberts, J.J.; De Carvalho, J.A. Ethanol as a Renewable Biofuel: Combustion Characteristics and Application in Engines. Energy 2022, 257, 124688. [Google Scholar] [CrossRef]
- Sanap, P.P.; Diwan, A.G.; Mahajan, Y.S. Ethanol Blending in Petrol: A Techno - Commercial Overview. Mater. Today Proc. 2023, S2214785323019508. [Google Scholar] [CrossRef]
- Verma, A.; Dugala, N.S.; Singh, S. Experimental Investigations on the Performance of SI Engine with Ethanol-Premium Gasoline Blends. Mater. Today Proc. 2022, 48, 1224–1231. [Google Scholar] [CrossRef]
- Inbanaathan, P.V.; Balasubramanian, D.; Nguyen, V.N.; Le, V.V.; Wae-Hayee, M.; R, R.; Veza, I.; Yukesh, N.; Kalam, M.A.; Sonthalia, A.; et al. Comprehensive Study on Using Hydrogen-Gasoline-Ethanol Blends as Flexible Fuels in an Existing Variable Speed SI Engine. Int. J. Hydrog. Energy 2023, 48, 39531–39552. [Google Scholar] [CrossRef]
- Suresh, D.; Porpatham, E. Influence of High Compression Ratio on the Performance of Ethanol-Gasoline Fuelled Lean Burn Spark Ignition Engine at Part Throttle Condition. Case Stud. Therm. Eng. 2024, 53, 103832. [Google Scholar] [CrossRef]
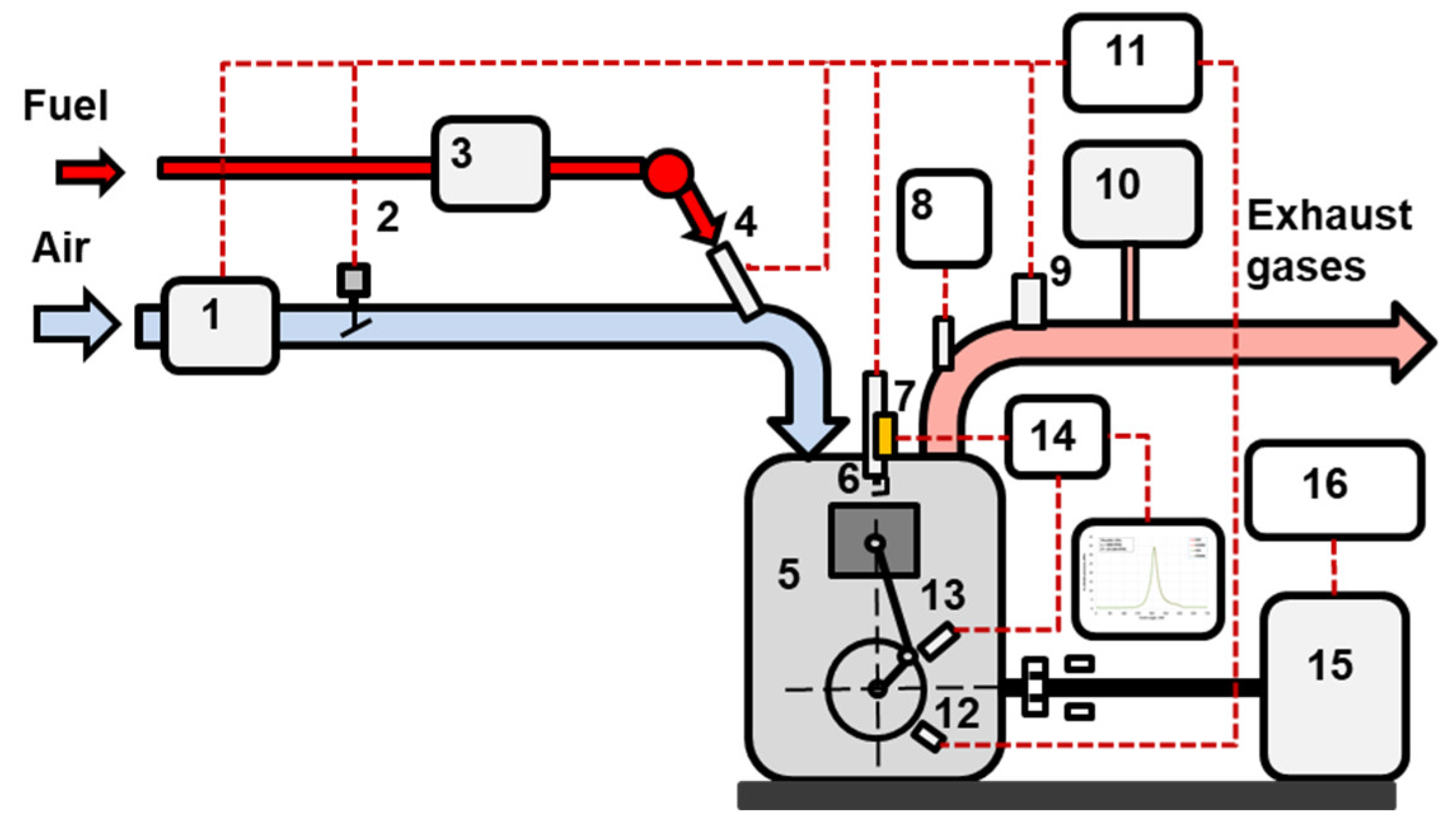
| Specifications of the Nissan HR 16DE engine | Value |
|---|---|
| Number of cylinders | 4 |
| Piston stroke | 83.6 mm |
| Cylinder bore | 78 mm |
| Number of valves per cylinder | 4 |
| Displacement | 1598 cm3 |
| Compression ratio | 10.7 |
| Nominal power | 84 kW at 6000 rpm |
| Maximum engine torque | 156 Nm at 4400 rpm |
| Indicator |
Fuel | |
|---|---|---|
| Gasoline (100%) | Bioethanol (100%) | |
| Chemical formula | CnH2n+2 (C4 … C12) | C2H5OH |
| Molecular weight | 100 … 105 | 46.07 |
| Elemental composition %: Carbon (C) Hydrogen (H) Oxygen (O) |
86.00 13.98 0.02 |
52.14 13.13 34.73 |
| C/H | 6.15 | 3.97 |
| Density (20°C), kg/m3 | 736 | 790 |
| Viscosity (40°C) (mm2/s) | 0.4 … 0.8 | 1.13 |
| Boiling point, °C | 27 … 225 | 78 |
| Latent heat of evaporation, kJ/kg | 364 | 840 |
| Auto-ignition temperature, °C | 257 | 422 |
| Laminar flame speed, cm/s | 51 | 63 |
| Adiabatic flame temperature, °C | 2307 | 2247 |
| Freezing point, °C | -40 | -114 |
| Stoichiometric air to fuel ratio (A/F), kg air/1 kg fuel |
14.84 | 9.10 |
| Flammability limits by volume in air, %: lower limit upper limit |
~ 0.6 ~ 8 |
~ 3.5 ~ 15 |
| Octane number | 88 … 98 | 109 |
| Lower heating value (mass) (LHV_m), MJ/kg | 43.5 | 27.0 |
| Mixture | E0 | E10 | E30 | E50 | E70 | ΔE70, % |
|---|---|---|---|---|---|---|
| Density, kg/m3 | 736.00 | 741.75 | 753.01 | 763.96 | 774.59 | 5.24 |
| C | 86.00 | 82.39 | 75.33 | 68.47 | 61.80 | -28.14 |
| H | 13.998 | 13.91 | 13.73 | 13.55 | 13.38 | -4.41 |
| O | 0.002 | 3.70 | 10.94 | 17.98 | 24.82 | 1240900 |
| C/H | 6.14 | 5.92 | 5.49 | 5.05 | 4.62 | -24.76 |
| A/F | 14.84 | 14.23 | 13.03 | 11.87 | 10.74 | -27.63 |
| LHV, MJ/kg | 43.50 | 41.74 | 38.30 | 34.96 | 31.71 | -27.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





