1. Introduction
Laryngeal squamous cell carcinoma (LSCC) represents a significant portion, approximately 4.5%, of all malignancies, making it the most prevalent form of head and neck cancer in males [
1,
2]. The variability in tumor characteristics, clinical behavior, and prognosis is closely tied to the anatomical site affected, including the glottis, supraglottis, or subglottis regions [
3]. Squamous cell carcinoma constitutes a substantial majority, accounting for 85% to 95% of all laryngeal cancers. Alarming statistics from 2018 in the United States alone reported around 13,150 newly diagnosed cases of laryngeal cancer, leading to approximately 3,710 deaths [
4]. The incidence ratio between males and females is notably high at 3.9:1 [
5]. While racial predilection is not overtly evident, race emerges as an independent prognostic factor. African American patients, in particular, present at a younger age and exhibit lower overall survival rates compared to their Caucasian, Hispanic, and Asian counterparts [
6]. This disparity underscores the intricate interplay of genetic and environmental factors in the development and progression of LSCC. Tobacco and alcohol use stand out as primary causative factors for laryngeal cancer, with risk escalating in proportion to the intensity and duration of consumption. Interestingly, after cessation of tobacco or alcohol use, it takes approximately 15 years for the risk levels to normalize, emphasizing the long-term impact of these habits [
1].
The management of LSCC involves a nuanced approach, with treatment options contingent on the location and stage of the disease [
7]. Organ preservation strategies, including radiotherapy [
8,
9] and transoral laser microsurgery (TLM) [
10,
11], as well as open horizontal partial laryngectomy or definitive surgery (total laryngectomy) [
12], are employed. Surgical interventions, whether diagnostic or therapeutic, play a pivotal role in providing critical insights into tumor biology and guiding the subsequent course of treatment.
Despite advances in understanding various human tumor types, the precise role and contribution of members of the Chaperone System (CS), such as heat shock proteins (Hsps), in the carcinogenic process of the larynx, especially the vocal cords, remain to be firmly established [
13,
18]. This pilot study embarks on an immunomorphological evaluation of tissue levels and topography of specific Hsps (Hsp10, Hsp27, Hsp60, and Hsp90) utilizing immunohistochemistry. The study meticulously quantifies the presence of these molecules in vocal cord tissue during stages of dysplasia and malignancy. The primary objective is to establish a robust platform for further investigations into the mechanisms underlying the participation of Hsps in laryngeal carcinogenesis. The study aims to ascertain whether and how the quantitative levels of these CS components influence the progression of tissue lesions.
Anticipating that the results will shed light on the potential role of Hsps as biomarkers, the study seeks to provide valuable insights for diagnosis, prognostication, and patient monitoring. The implications of this research extend beyond the laboratory, holding the promise of more personalized and effective approaches to managing laryngeal squamous cell carcinoma. The quest for biomarkers and a deeper understanding of the molecular intricacies involved paves the way for advancements in the field of head and neck oncology, offering hope for improved outcomes and enhanced patient care.
2. Materials and Methods
2.1. Sample Collection
The vocal cord tissue samples utilized in this study were sourced from the archives of the "Ospedali Riuniti VILLA SOFIA-CERVELLO" hospital in Palermo. These samples, collected over two-year spanning from 2020 to 2022, were meticulously categorized into three distinct groups to facilitate a comprehensive morphological analysis. The stratification of these tissue specimens aimed to capture the spectrum of conditions within the vocal cords, providing valuable insights into the molecular and cellular changes associated with health, dysplasia, and squamous cell carcinoma (SCC).
The first group denoted as the Healthy group, comprised ten tissue specimens sourced from both male and female individuals with ages ranging from 48 to 70 years. These specimens were characterized by their normal physiological status, serving as a baseline for comparison against the other groups. The inclusion of both genders and a varied age range within this group ensured a representative sample reflecting the diversity of the general population.
The second group labeled Dysplasia, encompassed ten tissue samples collected from individuals exhibiting dysplastic changes in their vocal cord tissue. Within this group, six males and four females, aged between 45 and 78 years, were represented. Dysplasia, a condition marked by abnormal cellular growth and differentiation, provides a critical intermediary stage in the progression toward more severe pathologies such as cancer. Analyzing this group allowed for a nuanced understanding of the morphological alterations associated with the precancerous state.
The third group, Squamous Cell Carcinoma (SCC), comprised ten tissue specimens from individuals diagnosed with squamous cell carcinoma of the vocal cords. This group featured an equal distribution of five males and five females, aged between 68 and 82 years, and exhibited a state of moderate differentiation.
The comprehensive collection of vocal cord tissue samples over the specified time frame and their meticulous categorization into Healthy, Dysplasia, and SCC groups provided a robust foundation for the morphological study. This stratified approach ensured that the analysis could capture the dynamic changes occurring at different stages of vocal cord pathology. The utilization of archived samples from a reputable hospital further adds credibility to the study, as the samples are likely to be well-preserved and accurately documented.
In summary, the vocal cord tissue samples collected from the archives of "Ospedali Riuniti VILLA SOFIA-CERVELLO" hospital in Palermo form a diverse and representative dataset, allowing for a comprehensive investigation into the morphological variations associated with health, dysplasia, and squamous cell carcinoma. The categorization into distinct groups based on health status and the inclusion of age and gender diversity within each group enhances the capacity of the study to uncover meaningful insights into the intricate processes underlying vocal cord pathologies.
2.2. Immunohistochemical Analysis
The immunohistochemical investigations (IHC) were performed on 5-micron thick tissue sections, obtained with a microtome from paraffin blocks. The resulting sections were subjected to deparaffinization, which consisted of immersing the sections in xylene for 30 min at 60 °C. Subsequently, the sections were rehydrated in a descending scale of alcohol at 23 °C (steps in alcohol 100% for 10 min, alcohol 95% for 5 min, alcohol 80% for 5 min, alcohol 50% for 5 min) and lastly rinsing with distilled water for 5 min. Then, the sections were incubated for 8 min in 350 mL of saline solution, Sodium Citrate (Sodium Citrate Buffer pH 6) at 80 °C to allow for the unmasking of antigens that may have been masked by fixation and paraffin embedding. Subsequently, to avoid the detachment of the sections from the slide, the sections were immersed in acetone at -20 °C for 5 min. All subsequent reactions were conducted at room temperature (23 °C), using the Immunoperoxidase Secondary Detection System (Millipore, Burlington, MA, USA & Canada, cat. N DAB-500.). In the next step, the sections were rinsed with PBS (Phosphate Buffered Saline pH 7.4) for 5 min, followed by incubation of the sections with 3% hydrogen peroxide for the inhibition of endogenous peroxidase for 5 min. After rinsing with PBS for 5 min, the non-specific sites were blocked by using a protein-blocker reagent (blue-colored reagent) for 5 min. Subsequently, the sections were incubated with the primary antibodies anti-Hsp10 (rabbit anti-Hsp10 polyclonal antibody, D-8, Santa Cruz Biotechnology, Dallas, TX, USA, cat. N: sc-20958, dilution 1:200), anti-Hsp27 (mouse anti-Hsp27 monoclonal antibody, F-4; Santa Cruz Biotechnology, cat N: sc-13132, dilution 1:200), anti-Hsp60 (rabbit polyclonal anti-Hsp60 antibody, Abcam, Cambridge, CB2 0AX, UK, Cat. No. ab46798, dilution 1:400), and anti-Hsp90 (mouse anti-Hsp90 monoclonal antibody, clone F-8, Santa Cruz Biotechnology, Cat. No. sc-13119, dilution 1:200), overnight at 4 °C. For all reactions, the appropriate negative controls were also carried out by adding only PBS to the sections instead of the primary antibody. After the incubation with the primary antibody, the samples were rinsed with PBS for 30 seconds with Rinse Buffer 1x and incubated with secondary antibody (Secondary antibodies: biotinylated goat anti-mouse IgG, Cat. No. 20775, Millipore Burlington, MA, USA & Canada, cat. N DAB-500) drops for 10 min at room temperature. After subsequent rinsing with PBS for 5 min, the reaction continued with incubation with Streptavidin HRP (Streptavidin HRP: Diluted in Tris Buffered Saline, Cat. No. 2774, Millipore Burlington, MA, USA & Canada, cat. N DAB-500) drops for 10 min and, after further washing in PBS for 5 min, the samples were incubated with an adequate volume of Chromogen Reagent and for the nuclear blue counterstaining, after another buffer rinse, stained with the Hematoxylin Counter Stain solution for 1 min at room temperature. Finally, the slides were mounted with coverslips using the permanent mount (Vecta Mount, H-5000, Vector Laboratories, Inc., Burlingame, CA, USA). Sections were observed with an optical microscope (Microscope Axioscope 5/7 KMAT, Carl Zeiss) connected to a digital camera (Microscopy Camera Axiocam 208 color, Carl Zeiss). All evaluations were performed at 400X magnification and repeated for 10 High-Power Fields (HPF).
2.3. Statistical Analysis
The statistical analysis was performed using GraphPad Prism 4.0 software (GraphPad Inc, San Diego, CA, USA) employing One-way ANOVA analysis to determine the presence of statistically significant differences. All data were reported as mean ± standard deviation, and the threshold for statistical significance was set at p≤0.05.
3. Results
3.1. Hsp10
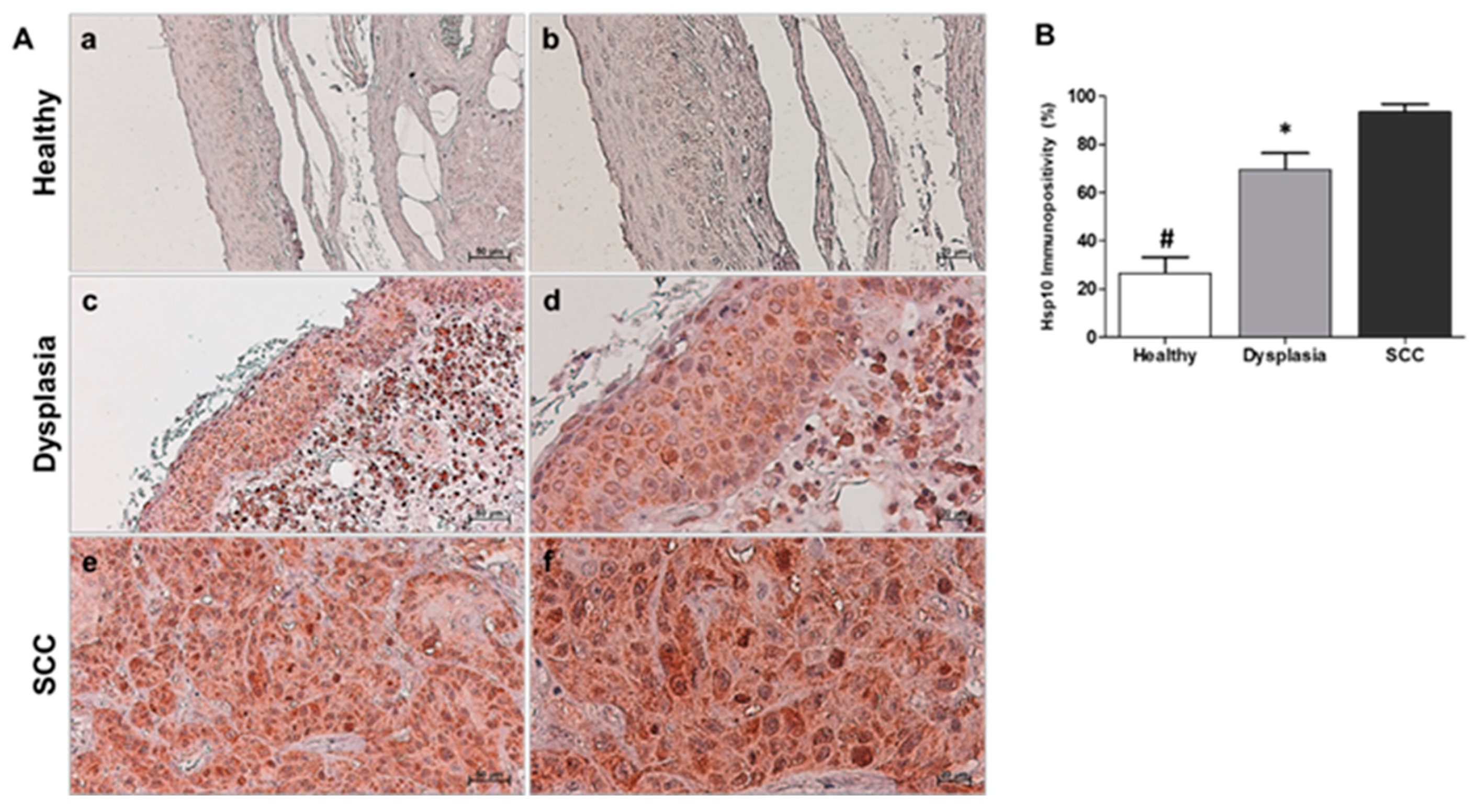
The results of our immunohistochemical assessment of Hsp10 experiments have shown that healthy tissue has an average tissue level of 26.5% ± 6.5, with a cytoplasmic granular immunolocalization. However, the dysplastic tissue group exhibited higher tissue levels, with a percentage of 69.5% ± 7 and a cytoplasmic and sometimes nuclear localization (
Figure 1A). The tumor tissue group showed even higher tissue levels, consisting of 93.5% ± 3, with cytoplasmic and nuclear localization (also seen in
Figure 1A). From our data, we can conclude that there is a gradual increase in Hsp10 tissue expression levels, progressing from healthy tissue to dysplastic tissue and tumor tissue (
Figure 1B). Through statistical analysis, we have discovered a significant difference (p<0.01) between the healthy tissue group (Healthy) and the dysplastic (Dysplasia) and squamous cell carcinoma group (SCC), as well as between the dysplastic group and squamous carcinoma group (
Figure 1B).
3.2. Hsp27
The immunopositivity of Hsp27 appeared granular in the cytoplasm in all samples of healthy tissue group with an average percentage of 30.5% ± 4, whereas in dysplastic tissue group, it was observed in the cytoplasm diffusely with an average percentage of 66% ± 5.5. Finally, squamous carcinoma tissue group, the Hsp27 immunopositivity exhibited a significantly higher value of 92.5% ± 4 and was localized in the cytoplasm and occasionally in the nucleus (
Figure 2A). The statistical analysis conducted on the normal tissue, dysplastic tissue, and squamous carcinoma tissue (
Figure 2B) revealed a progressive elevation in the tissue levels of Hsp27 with a significant difference (p<0.01) between the healthy tissue group and the dysplastic and squamous carcinoma group, and between the dysplastic group and squamous carcinoma group (
Figure 2B).
3.3. Hsp60
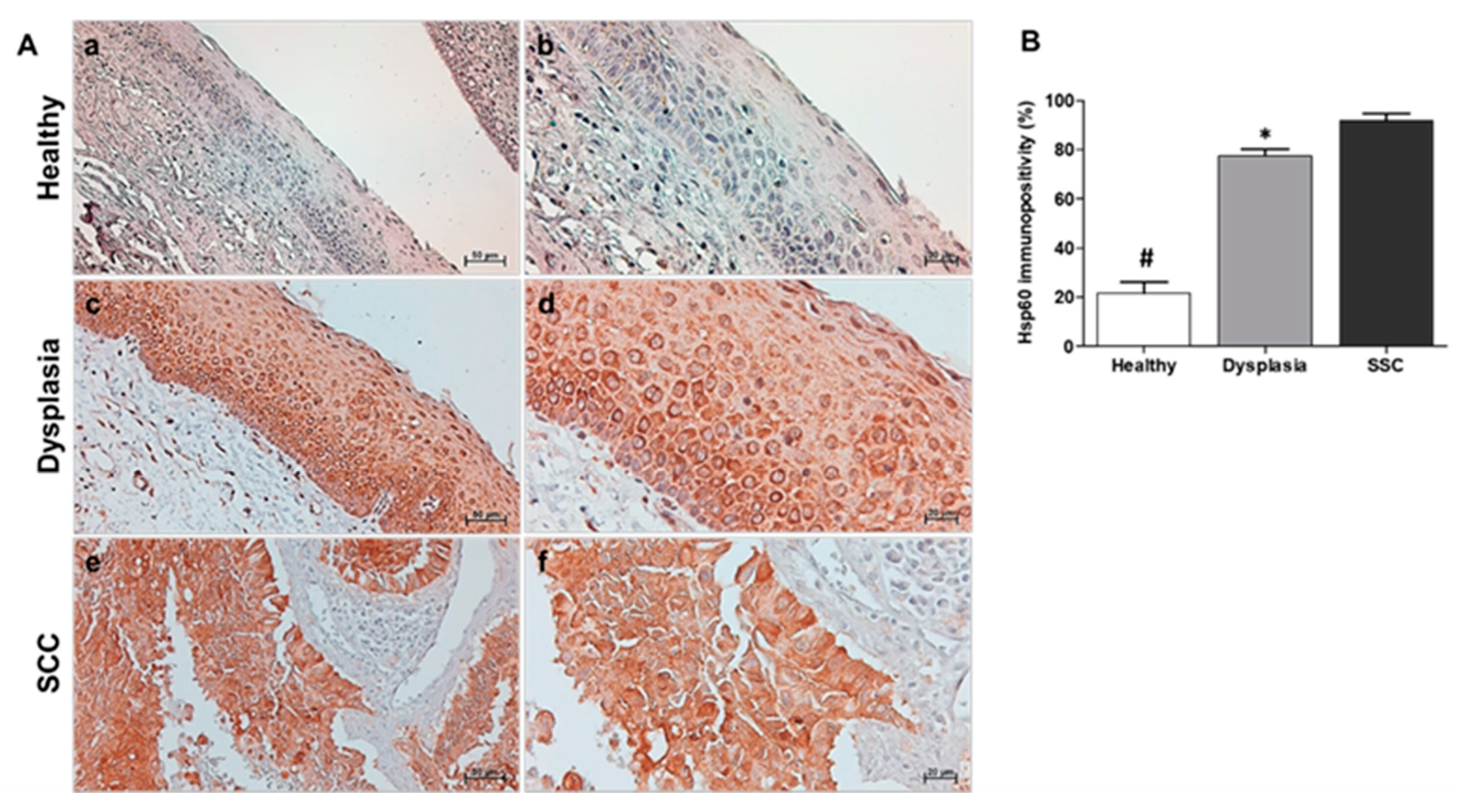
In the healthy tissue group, Hsp60 immunopositivity was observed to be cytoplasmic and granular, with an average percentage of 22% ± 4.5. However, in the dysplastic tissue group, the Hsp60 percentage immunopositivity was 78% ± 2.5, while in the squamous carcinoma group, it was 92% ± 3. The immunolocalization was predominantly diffuse and cytoplasmic (
Figure 3A). Statistical analysis revealed a significant increase in the tissue expression levels of Hsp60 observed during the transition from normal to dysplastic tissue and neoplastic tissue groups. A statistically significant difference (p<0.01) was detected between the healthy tissue group and the dysplasia and carcinoma groups (
Figure 3B).
3.4. Hsp90
The evaluation of tissue expression levels of Hsp90 in the examined samples exhibited an average percentage of 22% ± 5 in the healthy tissue group, 60% ± 7 in the dysplastic tissue group, and 90% ± 4 in the squamous carcinoma tissue group. Immunopositivity was predominantly localized within the cytoplasm across all samples, except for healthy tissues, where it was primarily observed in the basal layers of the epithelium (
Figure 4A). Through comprehensive statistical analysis encompassing healthy tissues, dysplastic tissues, and squamous carcinoma tissues (
Figure 4B), a progressive increase in Hsp90 expression values was observed. Notably, a statistically significant difference (p<0.01) was discerned between the healthy group versus the dysplasia and squamous carcinoma groups.
4. Discussion
In our morphological study, we sought to substantiate or refute the hypotheses we had posited by assessing the tissue levels of four heat shock proteins, namely Hsp10, Hsp27, Hsp60, and Hsp90, as well as scrutinizing the immunopositivity localization for each protein across various stages of differentiation within the carcinogenic vocal cord mucosa, juxtaposed with healthy tissue samples. Cells heavily rely on heat shock proteins for critical tasks essential to maintaining cellular homeostasis. Consequently, these proteins are ubiquitously present and fulfill indispensable roles, encompassing the facilitation of proper protein folding, translocation, degradation, and elimination of aberrant protein aggregates [
19]. These multifaceted functions render them as chaperonins. Notably, their involvement transcends these primary duties and extends into other domains, including DNA replication [
20], gene expression regulation [
19,
20], modulation of apoptotic and autophagic processes, and participation in cellular senescence [
21]. Remarkably, our study unveiled an unexpected association between these proteins and the intricate processes underpinning vocal cord carcinogenesis [
22]. The findings of this analyzed protein pool align with existing scientific evidence that supports Hsps playing a role in the oncogenic process and its progression [
23].
In several recent studies, the implication of heat shock proteins in various mechanisms underlying the onset of different types of tumors has been hypothesized and, at times, confirmed [
24,
25,
26]. Gastric, hepatic, breast, ovarian, lung, pancreatic, colorectal, squamous cell of tongue, glioma, and osteosarcoma cancers are among the many tumor variants in which the involvement of heat shock proteins has been highlighted. This implication of heat shock proteins has also been found in laryngeal squamous cell carcinoma [
27,
28,
29,
30].
Heat shock proteins seem to be involved in the carcinogenesis process either directly or indirectly. Due to the hostile tumor microenvironment, cells overexpress hsp genes in response to altered oxygen levels, nutrient and metabolite deficiencies, and significant pH shifts. [
31]. One of the indirect mechanisms that are commonly recognized involves cancer cells releasing extracellular vesicles that contain Hsp27 and Hsp90 into the tumor environment. These heat shock proteins interact with plasma receptors on cancer cells, triggering internal signaling pathways that promote the epithelial-mesenchymal transition (EMT) process. EMT is a significant characteristic of cancer [
32]. In addition, Hsps play a pivotal role in the regulation of extracellular matrix (ECM) remodeling and the modulation of its stiffness through their interactions with and control over a multitude of extracellular client proteins. Notably, Hsp90a, and in certain instances, Hsp60, have been identified as indispensable factors in driving the invasiveness and fostering the development of metastases in conditions such as fibrosarcoma, esophageal squamous cell carcinoma, and breast cancer cells [
33]. Additionally, Hsps can indirectly contribute to carcinogenesis through epigenetic interactions involving hsp receptors and translation factors such as hsf-1. Moreover, a noteworthy category of cellular pathways in which these molecules participate in the carcinogenesis process involves a set of post-translational modifications, including sumoylation, methylation, and phosphorylation, which their domains, such as AKT domain or C-terminal domain, and their receptors undergo [
34]. Moreover, another avenue through which Hsps promote tumor growth and impede therapeutic intervention lies in their role in fostering resistance to chemotherapy and radiation. Indeed, empirical observations have indicated that when tumor cells are subjected to treatments such as ionizing radiation or alkylating agents, the Hsps family actively undermines the efficacy of these therapies by deploying defense mechanisms designed to safeguard the structural integrity of the cell and the integrity of its DNA [
35,
36].
The Hsp10-Hsp60 complex is crucial in regulating intracellular pathways related to cellular proliferation and apoptosis, and it is known that malfunctioning of this complex can result in malignant cell transformation [
37,
38,
39]. Furthermore, Hsp60 has been found to exhibit pro-angiogenic and pro-metastatic properties [
40,
41]. Hsp27 also looks to be implicated in cellular apoptosis, and its involvement in this process plays a pivotal role in cancer regulation development, progression, and metastasis [
42]. Additionally, Hsp27 has been identified as a contributor to the suppression of cellular senescence, enabling tumor cells to acquire an immortal phenotype [
43]. Therefore, dysregulation of Hsp90 can have significant oncogenic implications, as it is involved in numerous processes that drive cell proliferation. It is crucial to recognize that Hsp90 has a fundamental function in tumor transformation. It regulates cell adhesion processes that are critical for the development of metastatic capabilities. Additionally, it promotes neo-angiogenesis, which is the foundation of tumor growth and increases the potential for metastasis [
44,
45,
46].
The examined molecules in this study are all involved in metabolic processes that enable atypical cells to deviate from normal physiological processes. The findings of this study, combined with previous research, confirm a connection between heightened levels of heat shock proteins and disorders marked by cellular imbalance and loss of differentiation. The immunohistochemical analysis of the study detected Hsps in the vocal cord epithelium across all three morphological states (healthy, dysplastic, and squamous carcinoma tissue), with a gradual rise in tissue levels from healthy to dysplastic and cancerous forms. The gradual and consistent increase in Hsps levels is most likely a result of changes in both intra- and extracellular conditions. In healthy tissues, Hsps are found at normal levels. However, in dysplasia, there is an increase in Hsps, which suggests changes in the structure of the cell and a greater need for protein expression. Cancerous tissues exhibit even higher levels of Hsps, indicating a complete disruption of cellular homeostasis and altered cellular differentiation. Our analysis also revealed a shift in the distribution of Hsps within cells, which is consistent with previous research showing elevated levels of Hsps in many cancer pathologies. Furthermore, research has demonstrated that Hsps change their localization, moving from mitochondria to the cytoplasm and sometimes even the cellular membrane [
47,
48,
49]. Our study revealed that Hsp10 and Hsp27 were localized in the cytoplasm, whereas Hsp60 and Hsp90 were present in both the cytoplasm and nucleus, with Hsp10 showing nuclear localization. These findings, in line with the literature, support the role of Hsps in the development of vocal cord tissue cancer. Thus, their involvement in the processes of tissue remodeling, altered cell differentiation, angiogenesis, and apoptosis was confirmed.
5. Conclusions
The pilot study expounded upon in this report lays the groundwork by furnishing essential histopathological data, serving as a pivotal springboard for an exhaustive exploration into the intricate mechanisms governing the involvement of heat shock proteins (Hsps), specifically Hsp10, Hsp27, Hsp60, and Hsp90, in the progression of squamous cell carcinoma affecting the vocal cords. A discernible correlation has been identified between the levels of these four chaperones and the sequential advancement of malignancy, tracing its trajectory from dysplasia to full-fledged cancer.
The potential significance of these biomolecular assessments goes beyond mere observation, presenting a promising avenue for their expeditious application as indispensable biomarkers in the realms of diagnostic procedures, prognostic evaluations, and continuous patient monitoring. This reservoir of scientific evidence concurrently establishes a robust foundation, paving the way for subsequent analyses poised to validate the earlier-reported outcomes. The envisaged substantiation not only augurs well for the credibility of the initial findings but also instills confidence in the prospective classification of the heat shock protein family as pivotal biomarkers, significantly contributing to the nuanced landscape of pathological evaluations and clinical diagnoses.
The data gleaned from this study propels our understanding of the dynamic interplay between heat shock proteins and the progression of vocal cord squamous cell carcinoma. The observed correlation provides a tangible link between the levels of these proteins and the evolving stages of malignancy. This nuanced insight positions heat shock proteins as potential indicators of disease severity and progression.
Furthermore, the implications extend to the clinical arena, where the identified biomarkers hold promise for refining diagnostic accuracy, prognostic assessments, and the development of targeted therapeutic strategies. As these findings are integrated into the broader scope of cancer research, the scientific community is poised to unlock new dimensions in the comprehension and management of squamous cell carcinoma, potentially paving the way for more personalized and effective treatment modalities.
In conclusion, the pilot study serves as a crucial cornerstone in unraveling the intricacies of heat shock protein involvement in vocal cord squamous cell carcinoma. The correlation established here sets the stage for a more comprehensive exploration and application of these biomarkers in clinical settings, fostering advancements that may revolutionize the landscape of cancer diagnostics and treatment.
Author Contributions
Conceptualization, Rappa F. and Intili G.; methodology, Intili G.; software, Rappa F.; validation, Rappa F.; formal analysis, Rappa F..; investigation, Intili G., Fucarino A, Modica MD, Lentini L.; resources, Pitruzzella A., Lentini L and Modica M.D.; data curation, Rappa F., Intili G.; writing—original draft preparation, Intili G., Calabrò F.; writing—review and editing, Rappa F., Intili G., Fucarino A.; visualization, Rappa F. and Pitruzzella A.; supervision, Rappa F., and Pitruzzella A.; project administration Rappa F. and Pitruzzella A.; funding acquisition, Pitruzzella A,. All authors have read and agreed to the published version of the manuscript.
Funding
This research received fundings from the University of Palermo (Italy), and the Euro-Mediterranean Institute of Science and Technology (Palermo, Italy).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
We are grateful to Alberto J.L. Macario, Everly Conway de Macario, Fabio Bucchieri, and Francesco Cappello for their critical revision and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferlay, J.; Parkin, D.M.; Steliarova-Foucher, E. Estimates of Cancer Incidence and Mortality in Europe in 2008. Eur J Cancer 2010, 46, . [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2018, 68, . [CrossRef]
- Smith, A.H.; Handley, M.A.; Wood, R. Epidemiological Evidence Indicates Asbestos Causes Laryngeal Cancer. Journal of Occupational Medicine 1990, 32, . [CrossRef]
- Hirvikoski, P.; Virtaniemi, J.; Kumpulainen, E.; Johansson, R.; Kosma, V.M. Supraglottic and Glottic Carcinomas: Clinically and Biologically Distinct Entities? Eur J Cancer 2002, 38, . [CrossRef]
- Miller, K.D.; Goding Sauer, A.; Ortiz, A.P.; Fedewa, S.A.; Pinheiro, P.S.; Tortolero-Luna, G.; Martinez-Tyson, D.; Jemal, A.; Siegel, R.L. Cancer Statistics for Hispanics/Latinos, 2018. CA Cancer J Clin 2018, 68, . [CrossRef]
- Shin, J.Y.; Truong, M.T. Racial Disparities in Laryngeal Cancer Treatment and Outcome: A Population-Based Analysis of 24,069 Patients. Laryngoscope 2015, 125, . [CrossRef]
- Hashibe, M.; Brennan, P.; Benhamou, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Maso, L.D.; Daudt, A.W.; Fabianova, E.; Wünsch-Filho, V.; et al. Alcohol Drinking in Never Users of Tobacco, Cigarette Smoking in Never Drinkers, and the Risk of Head and Neck Cancer: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst 2007, 99, . [CrossRef]
- Marioni, G.; Marchese-Ragona, R.; Cartei, G.; Marchese, F.; Staffieri, A. Current Opinion in Diagnosis and Treatment of Laryngeal Carcinoma. Cancer Treat Rev 2006, 32, . [CrossRef]
- Hendriksma, M.; Van Ruler, M.A.P.; Verbist, B.M.; De Jong, M.A.; Langeveld, T.P.M.; Van Benthem, P.P.G.; Sjögren, E. V. Survival and Prognostic Factors for Outcome after Radiotherapy for T2 Glottic Carcinoma. Cancers (Basel) 2019, 11, . [CrossRef]
- Lucioni, M.; Marioni, G.; Bertolin, A.; Giacomelli, L.; Rizzotto, G. Glottic Laser Surgery: Outcomes According to 2007 ELS Classification. European Archives of Oto-Rhino-Laryngology 2011, 268, . [CrossRef]
- Ansarin, M.; Cattaneo, A.; Santoro, L.; Massaro, M.A.; Zorzi, S.F.; Grosso, E.; Preda, L.; Alterio, D. Laser Surgery of Early Glottic Cancer in Elderly. Acta Otorhinolaryngol Ital 2010, 30. PMCID: PMC3008153; PMID: 21253281.
- Succo, G.; Peretti, G.; Piazza, C.; Remacle, M.; Eckel, H.E.; Chevalier, D.; Simo, R.; Hantzakos, A.G.; Rizzotto, G.; Lucioni, M.; et al. Open Partial Horizontal Laryngectomies: A Proposal for Classification by the Working Committee on Nomenclature of the European Laryngological Society. European Archives of Oto-Rhino-Laryngology 2014, 271, . [CrossRef]
- Zhai, L.L.; Xie, Q.; Zhou, C.H.; Huang, D.W.; Tang, Z.G.; Ju, T.F. Overexpressed HSPA2 Correlates with Tumor Angiogenesis and Unfavorable Prognosis in Pancreatic Carcinoma. Pancreatology 2017, 17, . [CrossRef]
- Garg, M.; Kanojia, D.; Seth, A.; Kumar, R.; Gupta, A.; Surolia, A.; Suri, A. Heat-Shock Protein 70-2 (HSP70-2) Expression in Bladder Urothelial Carcinoma Is Associated with Tumour Progression and Promotes Migration and Invasion. Eur J Cancer 2010, 46, . [CrossRef]
- Scieglinska, D.; Gogler-Piglowska, A.; Butkiewicz, D.; Chekan, M.; Malusecka, E.; Harasim, J.; Habryka, A.; Krawczyk, Z. HSPA2 Is Expressed in Human Tumors and Correlates with Clinical Features in Non-Small Cell Lung Carcinoma Patients. Anticancer Res 2014, 34. PMID: 24922646.
- Zhang, H.; Chen, W.; Duan, C.J.; Zhang, C.F. Overexpression of HSPA2 Is Correlated with Poor Prognosis in Esophageal Squamous Cell Carcinoma. World J Surg Oncol 2013, 11, . [CrossRef]
- Jalbout, M.; Bouaouina, N.; Gargouri, J.; Corbex, M.; Ahmed, S. Ben; Chouchane, L. Polymorphism of the Stress Protein HSP70-2 Gene Is Associated with the Susceptibility to the Nasopharyngeal Carcinoma. Cancer Lett 2003, 193, . [CrossRef]
- Fu, Y.; Zhao, H.; Li, X.S.; Kang, H.R.; Ma, J.X.; Yao, F.F.; Du, N. Expression of HSPA2 in Human Hepatocellular Carcinoma and Its Clinical Significance. Tumor Biology 2014, 35, . [CrossRef]
- Saini J, Sharma PK. Clinical, Prognostic and Therapeutic Significance of Heat Shock Proteins in Cancer. Curr Drug Targets. 2018;19(13):1478-1490. [CrossRef]
- Zheng G, Zhang Z, Liu H, Xiong Y, Luo L, Jia X, Peng C, Zhang Q, Li N, Gu Y, Lu M, Song Y, Pan H, Liu J, Liu W, He Z. HSP27-Mediated Extracellular and Intracellular Signaling Pathways Synergistically Confer Chemoresistance in Squamous Cell Carcinoma of Tongue. Clin Cancer Res. 2018 Mar 1;24(5):1163-1175. Epub 2017 Dec 15. [CrossRef]
- Seclì L, Fusella F, Avalle L, Brancaccio M. The dark-side of the outside: how extracellular heat shock proteins promote cancer. Cell Mol Life Sci. 2021 May;78(9):4069-4083. Epub 2021 Feb 5. [CrossRef]
- Wang X, An D, Wang X, Liu X, Li B. Extracellular Hsp90α clinically correlates with tumor malignancy and promotes migration and invasion in esophageal squamous cell carcinoma. Onco Targets Ther. 2019 Feb 11;12:1119-1128Retraction in: Onco Targets Ther. 2020 Jul 29;13:7353. [CrossRef]
- Campanella, C.; Rappa, F.; Sciumè, C.; Marino Gammazza, A.; Barone, R.; Bucchieri, F.; David, S.; Curcurù, G.; Caruso Bavisotto, C.; Pitruzzella, A.; et al. Heat Shock Protein 60 Levels in Tissue and Circulating Exosomes in Human Large Bowel Cancer before and after Ablative Surgery. Cancer 2015, 121, . [CrossRef]
- Rappa, F.; Farina, F.; Zummo, G.; David, S.; Campanella, C.; Carini, F.; Tomasello, G.; Damiani, P.; Cappello, F.; De Macario, E.C.; et al. HSP-Molecular Chaperones in Cancer Biogenesis and Tumor Therapy: An Overview. Anticancer Res 2012, 32.
- Rappa, F.; Pitruzzella, A.; Marino Gammazza, A.; Barone, R.; Mocciaro, E.; Tomasello, G.; Carini, F.; Farina, F.; Zummo, G.; Conway de Macario, E.; et al. Quantitative Patterns of Hsps in Tubular Adenoma Compared with Normal and Tumor Tissues Reveal the Value of Hsp10 and Hsp60 in Early Diagnosis of Large Bowel Cancer. Cell Stress Chaperones 2016, 21, . [CrossRef]
- Wu, J.; Liu, T.; Rios, Z.; Mei, Q.; Lin, X.; Cao, S. Heat Shock Proteins and Cancer. Trends Pharmacol Sci 2017, 38, . [CrossRef]
- Liu K, Huang J, Liu J, Li C, Kroemer G, Tang D, Kang R. HSP90 Mediates IFNγ-Induced Adaptive Resistance to Anti-PD-1 Immunotherapy. Cancer Res. 2022 May 16;82(10):2003-2018. [CrossRef]
- Sasame J, Ikegaya N, Kawazu M, Natsumeda M, Hayashi T, Isoda M, Satomi K, Tomiyama A, Oshima A, Honma H, Miyake Y, Takabayashi K, Nakamura T, Ueno T, Matsushita Y, Iwashita H, Kanemaru Y, Murata H, Ryo A, Terashima K, Yamanaka S, Fujii Y, Mano H, Komori T, Ichimura K, Cahill DP, Wakimoto H, Yamamoto T, Tateishi K. HSP90 Inhibition Overcomes Resistance to Molecular Targeted Therapy in BRAFV600E-mutant High-grade Glioma. Clin Cancer Res. 2022 Jun 1;28(11):2425-2439. [CrossRef]
- Dong L, Xue L, Zhang C, Li H, Cai Z, Guo R. HSP90 interacts with HMGCR and promotes the progression of hepatocellular carcinoma. Mol Med Rep. 2019 Jan;19(1):524-532. Epub 2018 Nov 19. [CrossRef]
- Zheng G, Zhang Z, Liu H, Xiong Y, Luo L, Jia X, Peng C, Zhang Q, Li N, Gu Y, Lu M, Song Y, Pan H, Liu J, Liu W, He Z. HSP27-Mediated Extracellular and Intracellular Signaling Pathways Synergistically Confer Chemoresistance in Squamous Cell Carcinoma of Tongue. Clin Cancer Res. 2018 Mar 1;24(5):1163-1175. Epub2017 Dec15. [CrossRef]
- Niu M, Zhang B, Li L, Su Z, Pu W, Zhao C, Wei L, Lian P, Lu R, Wang R, Wazir J, Gao Q, Song S, Wang H. Targeting HSP90 Inhibits Proliferation and Induces Apoptosis Through AKT1/ERK Pathway in Lung Cancer. Front Pharmacol. 2022 Jan 14;12:724192. [CrossRef]
- Seclì L, Fusella F, Avalle L, Brancaccio M. The dark-side of the outside: how extracellular heat shock proteins promote cancer. Cell Mol Life Sci. 2021 May;78(9):4069-4083. Epub 2021 Feb 5. PMID: 33544155. [CrossRef]
- Wang X, An D, Wang X, Liu X, Li B. Extracellular Hsp90α clinically correlates with tumor malignancy and promotes migration and invasion in esophageal squamous cell carcinoma. Onco Targets Ther. 2019 Feb 11;12:1119-1128. Retraction in: Onco Targets Ther. 2020 Jul 29;13:7353. PMID: 30809093. [CrossRef]
- Yang T, Ren C, Lu C, Qiao P, Han X, Wang L, Wang D, Lv S, Sun Y, Yu Z. Phosphorylation of HSF1 by PIM2 Induces PD-L1 Expression and Promotes Tumor Growth in Breast Cancer. Cancer Res. 2019 Oct 15;79(20):5233-5244; Epub 2019 Aug 13. [CrossRef]
- Sasame J, Ikegaya N, Kawazu M, Natsumeda M, Hayashi T, Isoda M, Satomi K, Tomiyama A, Oshima A, Honma H, Miyake Y, Takabayashi K, Nakamura T, Ueno T, Matsushita Y, Iwashita H, Kanemaru Y, Murata H, Ryo A, Terashima K, Yamanaka S, Fujii Y, Mano H, Komori T, Ichimura K, Cahill DP, Wakimoto H, Yamamoto T, Tateishi K. HSP90 Inhibition Overcomes Resistance to Molecular Targeted Therapy in BRAFV600E-mutant High-grade Glioma. Clin Cancer Res. 2022 Jun 1;28(11):2425-2439. [CrossRef]
- Liu K, Huang J, Liu J, Li C, Kroemer G, Tang D, Kang R. HSP90 Mediates IFNγ-Induced Adaptive Resistance to Anti-PD-1 Immunotherapy. Cancer Res. 2022 May 16;82(10):2003-2018. [CrossRef]
- Takayama, S.; Reed, J.C.; Homma, S. Heat-Shock Proteins as Regulators of Apoptosis. Oncogene 2003, 22, . [CrossRef]
- Czarnecka, A.M.; Campanella, C.; Zummo, G.; Cappello, F. Mitochondrial Chaperones in Cancer: From Molecular Biology to Clinical Diagnostics. Cancer Biol Ther 2006, 5, . [CrossRef]
- Wu, J.; Liu, T.; Rios, Z.; Mei, Q.; Lin, X.; Cao, S. Heat Shock Proteins and Cancer. Trends Pharmacol Sci 2017, 38, . [CrossRef]
- Tsai, Y.P.; Teng, S.C.; Wu, K.J. Direct Regulation of HSP60 Expression by C-MYC Induces Transformation. FEBS Lett 2008, 582, . [CrossRef]
- Lin, C.S.; He, P.J.; Hsu, W.T.; Wu, M.S.; Wu, C.J.; Shen, H.W.; Hwang, C.H.; Lai, Y.K.; Tsai, N.M.; Liao, K.W. Helicobacter Pylori-Derived Heat Shock Protein 60 Enhances Angiogenesis via a CXCR2-Mediated Signaling Pathway. Biochem Biophys Res Commun 2010, 397, . [CrossRef]
- Gibert, B.; Simon, S.; Dimitrova, V.; Diaz-Latoud, C.; Arrigo, A.P. Peptide Aptamers: Tools to Negatively or Positively Modulate HSPB1(27) Function. Philosophical Transactions of the Royal Society B: Biological Sciences 2013, 368, . [CrossRef]
- O’Callaghan-Sunol, C.; Gabai, V.L.; Sherman, M.Y. Hsp27 Modulates P53 Signaling and Suppresses Cellular Senescence. Cancer Res 2007, 67, . [CrossRef]
- Aoyagi, Y.; Fujita, N.; Tsuruo, T. Stabilization of Integrin-Linked Kinase by Binding to Hsp90. Biochem Biophys Res Commun 2005, 331, . [CrossRef]
- Ochel, H.J.; Schulte, T.W.; Nguyen, P.; Trepel, J.; Neckers, L. The Benzoquinone Ansamycin Geldanamycin Stimulates Proteolytic Degradation of Focal Adhesion Kinase. Mol Genet Metab 1999, 66, . [CrossRef]
- Wang, X.; Zhang, Y.; Zhao, Y.; Liang, Y.; Xiang, C.; Zhou, H.; Zhang, H.; Zhang, Q.; Qing, H.; Jiang, B.; et al. CD24 Promoted Cancer Cell Angiogenesis via Hsp90-Mediated STAT3/VEGF Signaling Pathway in Colorectal Cancer. Oncotarget 2016, 7, . [CrossRef]
- Campanella, C.; Rappa, F.; Sciumè, C.; Marino Gammazza, A.; Barone, R.; Bucchieri, F.; David, S.; Curcurù, G.; Caruso Bavisotto, C.; Pitruzzella, A.; et al. Heat Shock Protein 60 Levels in Tissue and Circulating Exosomes in Human Large Bowel Cancer before and after Ablative Surgery. Cancer 2015, 121, . [CrossRef]
- Rappa, F.; Farina, F.; Zummo, G.; David, S.; Campanella, C.; Carini, F.; Tomasello, G.; Damiani, P.; Cappello, F.; De Macario, E.C.; et al. HSP-Molecular Chaperones in Cancer Biogenesis and Tumor Therapy: An Overview. Anticancer Res 2012, 32; PMID: 23225410.
- Rappa, F.; Pitruzzella, A.; Marino Gammazza, A.; Barone, R.; Mocciaro, E.; Tomasello, G.; Carini, F.; Farina, F.; Zummo, G.; Conway de Macario, E.; et al. Quantitative Patterns of Hsps in Tubular Adenoma Compared with Normal and Tumor Tissues Reveal the Value of Hsp10 and Hsp60 in Early Diagnosis of Large Bowel Cancer. Cell Stress Chaperones 2016, 21, . [CrossRef]
Figure 1.
(A): Hsp10 immunohistochemistry. Representative images of immunohistochemical results for Healthy (a,b), Dysplasia (c,d), and SCC (squamous cell carcinoma; e,f). (a,c,e): magnification 200×, scale bar 50 μm; (b,d,f): magnification 400×; scale bar 20 μm. (B): Statistical analysis of Hsp10 results. The histogram revealed a significant difference between the Healthy group and the Dysplasia and SCC groups (# p ≤ 0.01), as well as between the Dysplasia group and the SCC group (* p ≤ 0.01).
Figure 1.
(A): Hsp10 immunohistochemistry. Representative images of immunohistochemical results for Healthy (a,b), Dysplasia (c,d), and SCC (squamous cell carcinoma; e,f). (a,c,e): magnification 200×, scale bar 50 μm; (b,d,f): magnification 400×; scale bar 20 μm. (B): Statistical analysis of Hsp10 results. The histogram revealed a significant difference between the Healthy group and the Dysplasia and SCC groups (# p ≤ 0.01), as well as between the Dysplasia group and the SCC group (* p ≤ 0.01).
Figure 2.
(A): Hsp27 immunohistochemistry. Representative images of immunohistochemical results for Healthy (a,b), Dysplasia (c,d), and squamous cell carcinoma (SCC; e,f). (a,c,e): magnification 200×, scale bar 50 μm; (b,d,f): magnification 400×; scale bar 20 μm. (B): Statistical analysis of Hsp27 results. The histogram revealed a significant difference between the Healthy group and the Dysplasia and SCC groups (# p ≤ 0.01), as well as between the Dysplasia group and the SCC group (* p ≤ 0.01).
Figure 2.
(A): Hsp27 immunohistochemistry. Representative images of immunohistochemical results for Healthy (a,b), Dysplasia (c,d), and squamous cell carcinoma (SCC; e,f). (a,c,e): magnification 200×, scale bar 50 μm; (b,d,f): magnification 400×; scale bar 20 μm. (B): Statistical analysis of Hsp27 results. The histogram revealed a significant difference between the Healthy group and the Dysplasia and SCC groups (# p ≤ 0.01), as well as between the Dysplasia group and the SCC group (* p ≤ 0.01).
Figure 3.
(A): Hsp60 immunohistochemistry. Representative images of immunohistochemical results for Healthy (a,b), Dysplasia (c,d), and SCC (squamous cell carcinoma; (e,f). (a,c,e): magnification 200×, scale bar 50 μm; (b,d,f): magnification 400×; scale bar 20 μm. (B): Statistical analysis of Hsp60 results. The histogram revealed a significant difference between the Healthy group and the Dysplasia and SCC groups (# p ≤ 0.01), as well as between the Dysplasia group and the SCC group (* p ≤ 0.01).
Figure 3.
(A): Hsp60 immunohistochemistry. Representative images of immunohistochemical results for Healthy (a,b), Dysplasia (c,d), and SCC (squamous cell carcinoma; (e,f). (a,c,e): magnification 200×, scale bar 50 μm; (b,d,f): magnification 400×; scale bar 20 μm. (B): Statistical analysis of Hsp60 results. The histogram revealed a significant difference between the Healthy group and the Dysplasia and SCC groups (# p ≤ 0.01), as well as between the Dysplasia group and the SCC group (* p ≤ 0.01).
Figure 4.
(A): Hsp90 immunohistochemistry. Representative images of immunohistochemical results for Healthy (a,b), Dysplasia (c,d), and SCC (squamous cell carcinoma; e,f). (a,c,e): magnification 200×, scale bar 50 μm; (b,d,f): magnification 400×; scale bar 20 μm. (B): Statistical analysis of Hsp90 results. The histogram revealed a significant difference between the Healthy group and the Dysplasia and SCC groups (# p ≤ 0.01), as well as between the Dysplasia group and the SCC group (* p ≤ 0.01).
Figure 4.
(A): Hsp90 immunohistochemistry. Representative images of immunohistochemical results for Healthy (a,b), Dysplasia (c,d), and SCC (squamous cell carcinoma; e,f). (a,c,e): magnification 200×, scale bar 50 μm; (b,d,f): magnification 400×; scale bar 20 μm. (B): Statistical analysis of Hsp90 results. The histogram revealed a significant difference between the Healthy group and the Dysplasia and SCC groups (# p ≤ 0.01), as well as between the Dysplasia group and the SCC group (* p ≤ 0.01).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).