Submitted:
26 December 2023
Posted:
28 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Initial Management during the First 72 Hours
2.1. Fluid Resuscitation
2.1.1. Goal-Directed Therapy (GDT)
2.1.2. Fluid Type
2.1.3. Fluid Rate and Volume
2.1.4. Fluid Therapy Duration
2.2. Pain Control
2.2.1. Opioids
| Reference | Participants (N) | Aggressive Resuscitation | Non-Aggressive Resuscitation | Effect of Aggressive Resuscitation |
|---|---|---|---|---|
| Mato et al. [29], 2009 | Severe AP lesser than 72 hr onset (76) | 10 – 15 ml/kg/hr | 5 – 10 ml/kg/hr | Harmful, more sepsis, mortality, mechanical ventilation, and ACS. |
| Mato et al. [30], 2010 | Severe AP lesser than 24 hr onset (115) | Rapid hemodilution with goal hematocrit < 35% at 48 hr | Slow hemodilution with goal hematocrit > 35% at 48 hr | Harmful, more sepsis, and mortality |
| Wu et al. [31], 2011 | Any severity AP (40) | GDT with 20ml/kg bolus →1.5 or 3ml/kg/hr of LR or NS |
LR or NS adjusted by physician | Similar in SIRS and CRP at 24 hr |
| Buxbaum et al. [8], 2017 | Predicted mild AP (60) | 20ml/kg bolus over 2 hr → 3ml/kg/hr infusion of LR |
10ml/kg bolus over 2hr → 1.5ml/kg/hr infusion of LR |
Beneficial, more clinical improvement, and less persistent SIRS and hemoconcentration |
| At timepoint (12, 24, 36 hours) If hematocrit, BUN, or creatinine increased, 20ml/kg bolus → 3ml/kg/hr infusion If labs were decreased and pain relived, 1.5ml/kg/hr infusion and start diet | ||||
| Cuéllar-Monterrubio et al. [35], 2020 | Any severity AP, more than 24hr onset (88) | 20ml/kg bolus → 3ml/kg/hr (first 24 hr) → 30ml/hr (next 24 hr) of HS |
20ml/kg bolus (only if hypovolemia) → 1.5ml/kg/hr (first 24 hr) → 30ml/hr (next 24 hr) of HS |
No benefit, no difference in persistent SIRS, pancreatic necrosis, respiratory complications, AKI and LOS |
| De-Madaria et al. [7], 2022 | Mild AP, lesser than 24hr onset (249) | 20ml/kg bolus → 3ml/kg/hr infusion of LR |
10ml/kg bolus (only if hypovolemia) → 1.5ml/kg/hr infusion of LR |
Harmful, mor fluid overload |
| At timepoint (3,12, 24, 48, 72 hours) If hypovolemia → 20ml/kg bolus → 3ml/kg/hr If normovolemia → 1.5ml/kg/hr If fluid overload → decrease or stop |
At timepoint (3,12, 24, 48, 72 hours) If hypovolemia → 10ml/kg bolus → 1.5 ml/kg/hr If normovolemia → 1.5ml/kg/hr If fluid overload → decrease or stop |
|||
2.2.2. NSAIDs
2.2.3. Epidural Analgesia
2.3. Nutritional Support
2.3.1. When to Start Oral Feeding
2.3.2. Route of Tube Feeding
2.4. Prophylactic Antibiotic Use
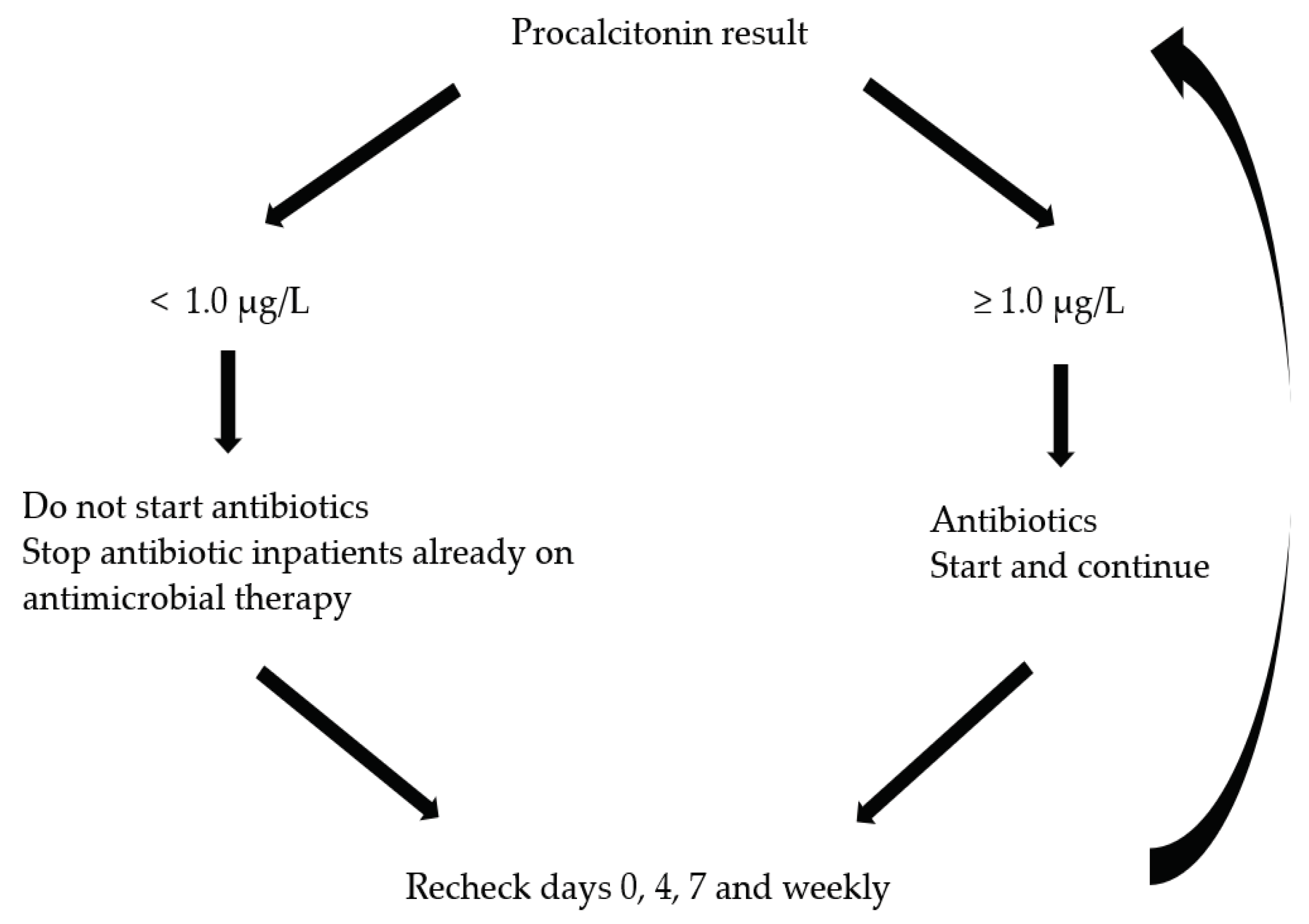
2.4.1. Procalcitonin-Guided Antibiotic Use
2.5. ERCP Role in Gallstone Pancreatitis
2.6. Other therapeutic interventions
2.6.1. Low Molecular Weight Heparin (LMWH)
2.6.2. Protease Inhibitors
3. Convalescent Treatment
3.1. Cholecystectomy in Gallstone AP
3.1.1. Timing of Cholecystectomy
3.2. Alcohol Intervention in Alcoholic AP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mederos, M.A.; Reber, H.A.; Girgis, M.D. Acute pancreatitis: A review. JAMA 2021, 325, 382–390. [CrossRef]
- Krishna, S.G.; Kamboj, A.K.; Hart, P.A.; Hinton, A.; Conwell, D.L. The changing epidemiology of acute pancreatitis hospitalizations: A decade of trends and the impact of chronic pancreatitis. Pancreas 2017, 46, 482–488. [CrossRef]
- de-Madaria, E.; Buxbaum, J.L. Advances in the management of acute pancreatitis. Nat Rev Gastroenterol Hepatol 2023, 20, 691–692. [CrossRef]
- Lee, P.J.; Papachristou, G.I. New insights into acute pancreatitis. 2019, 16, 479–496. [CrossRef]
- Lee, A.; Ko, C.; Buitrago, C.; Hiramoto, B.; Hilson, L.; et al. Lactated ringers vs normal saline resuscitation for mild acute pancreatitis: A randomized trial. 2021, 160, 955–957.e954. [CrossRef]
- Myburgh, J.A.; Finfer, S.; Bellomo, R.; Billot, L.; Cass, A.; Gattas, D.; Glass, P.; Lipman, J.; Liu, B.; McArthur, C. et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012, 367, 1901–1911. [CrossRef]
- de-Madaria, E.; Buxbaum, J.L.; Maisonneuve, P.; Garcia Garcia de Paredes, A.; Zapater, P.; Guilabert, L.; Vaillo-Rocamora, A.; Rodriguez-Gandia, M.A.; Donate-Ortega, J.; Lozada-Hernandez, E.E. et al. Aggressive or moderate fluid resuscitation in acute pancreatitis. N Engl J Med 2022, 387, 989–1000. [CrossRef]
- Buxbaum, J.L.; Quezada, M.; Da, B.; Jani, N.; Lane, C.; Mwengela, D.; Kelly, T.; Jhun, P.; Dhanireddy, K.; Laine, L. Early aggressive hydration hastens clinical improvement in mild acute pancreatitis. Am J Gastroenterol 2017, 112, 797–803. [CrossRef]
- de-Madaria, E.; Soler-Sala, G.; Sanchez-Paya, J.; Lopez-Font, I.; Martinez, J.; Gomez-Escolar, L.; Sempere, L.; Sanchez-Fortun, C.; Perez-Mateo, M. Influence of fluid therapy on the prognosis of acute pancreatitis: A prospective cohort study. Am J Gastroenterol 2011, 106, 1843–1850. [CrossRef]
- De Waele, J.J.; Leppaniemi, A.K. Intra-abdominal hypertension in acute pancreatitis. World J Surg 2009, 33, 1128–1133. [CrossRef]
- Wilms, H.; Mittal, A.; Haydock, M.D.; van den Heever, M.; Devaud, M.; Windsor, J.A. A systematic review of goal directed fluid therapy: Rating of evidence for goals and monitoring methods. J Crit Care 2014, 29, 204–209. [CrossRef]
- Crockett, S.D.; Wani, S.; Gardner, T.B.; Falck-Ytter, Y.; Barkun, A.N.; American Gastroenterological Association Institute Clinical Guidelines, C. American gastroenterological association institute guideline on initial management of acute pancreatitis. Gastroenterology 2018, 154, 1096–1101. [CrossRef]
- Lee, S.H.; Choe, J.W.; Cheon, Y.K.; Choi, M.; Jung, M.K.; Jang, D.K.; Jo, J.H.; Lee, J.M.; Kim, E.J.; Han, S.Y. et al. Revised clinical practice guidelines of the korean pancreatobiliary association for acute pancreatitis. Gut Liver 2023, 17, 34–48. [CrossRef]
- Yokoe, M.; Takada, T.; Mayumi, T.; Yoshida, M.; Isaji, S.; Wada, K.; Itoi, T.; Sata, N.; Gabata, T.; Igarashi, H. et al. Japanese guidelines for the management of acute pancreatitis: Japanese guidelines 2015. J Hepatobiliary Pancreat Sci 2015, 22, 405–432. [CrossRef]
- Wu, B.U.; Bakker, O.J.; Papachristou, G.I.; Besselink, M.G.; Repas, K.; van Santvoort, H.C.; Muddana, V.; Singh, V.K.; Whitcomb, D.C.; Gooszen, H.G. et al. Blood urea nitrogen in the early assessment of acute pancreatitis: An international validation study. Arch Intern Med 2011, 171, 669–676. [CrossRef]
- Wu, B.U.; Johannes, R.S.; Sun, X.; Conwell, D.L.; Banks, P.A. Early changes in blood urea nitrogen predict mortality in acute pancreatitis. Gastroenterology 2009, 137, 129–135. [CrossRef]
- Brown, A.; Baillargeon, J.D.; Hughes, M.D.; Banks, P.A. Can fluid resuscitation prevent pancreatic necrosis in severe acute pancreatitis? Pancreatology 2002, 2, 104–107. [CrossRef]
- Jin, T.; Li, L.; Deng, L.; Wen, S.; Zhang, R.; Shi, N.; Zhu, P.; Lan, L.; Lin, Z.; Jiang, K. et al. Hemoconcentration is associated with early faster fluid rate and increased risk of persistent organ failure in acute pancreatitis patients. JGH Open 2020, 4, 684–691. [CrossRef]
- Crosignani, A.; Spina, S.; Marrazzo, F.; Cimbanassi, S.; Malbrain, M.; Van Regenmortel, N.; Fumagalli, R.; Langer, T. Intravenous fluid therapy in patients with severe acute pancreatitis admitted to the intensive care unit: A narrative review. Ann Intensive Care 2022, 12, 98. [CrossRef]
- Jin, T.; Li, L.; Zhu, P.; Deng, L.; Zhang, X.; Hu, C.; Shi, N.; Zhang, R.; Tan, Q.; Chen, C. et al. Optimising fluid requirements after initial resuscitation: A pilot study evaluating mini-fluid challenge and passive leg raising test in patients with predicted severe acute pancreatitis. Pancreatology 2022, 22, 894–901. [CrossRef]
- Zhou, S.; Buitrago, C.; Foong, A.; Lee, V.; Dawit, L.; Hiramoto, B.; Chang, P.; Schilperoort, H.; Lee, A.; de-Madaria, E. et al. Comprehensive meta-analysis of randomized controlled trials of lactated ringer's versus normal saline for acute pancreatitis. Pancreatology 2021, 21, 1405–1410. [CrossRef]
- Semler, M.W.; Self, W.H.; Wanderer, J.P.; Ehrenfeld, J.M.; Wang, L.; Byrne, D.W.; Stollings, J.L.; Kumar, A.B.; Hughes, C.G.; Hernandez, A. et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med 2018, 378, 829–839. [CrossRef]
- Self, W.H.; Semler, M.W.; Wanderer, J.P.; Wang, L.; Byrne, D.W.; Collins, S.P.; Slovis, C.M.; Lindsell, C.J.; Ehrenfeld, J.M.; Siew, E.D. et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med 2018, 378, 819–828. [CrossRef]
- de-Madaria, E.; Herrera-Marante, I.; Gonzalez-Camacho, V.; Bonjoch, L.; Quesada-Vazquez, N.; Almenta-Saavedra, I.; Miralles-Macia, C.; Acevedo-Piedra, N.G.; Roger-Ibanez, M.; Sanchez-Marin, C. et al. Fluid resuscitation with lactated ringer's solution vs normal saline in acute pancreatitis: A triple-blind, randomized, controlled trial. United European Gastroenterol J 2018, 6, 63–72. [CrossRef]
- Zhao, G.; Zhang, J.G.; Wu, H.S.; Tao, J.; Qin, Q.; Deng, S.C.; Liu, Y.; Liu, L.; Wang, B.; Tian, K. et al. Effects of different resuscitation fluid on severe acute pancreatitis. World J Gastroenterol 2013, 19, 2044–2052. [CrossRef]
- Du, X.J.; Hu, W.M.; Xia, Q.; Huang, Z.W.; Chen, G.Y.; Jin, X.D.; Xue, P.; Lu, H.M.; Ke, N.W.; Zhang, Z.D. et al. Hydroxyethyl starch resuscitation reduces the risk of intra-abdominal hypertension in severe acute pancreatitis. Pancreas 2011, 40, 1220–1225. [CrossRef]
- Ma, Y.; Yan, T.; Xu, F.; Ding, J.; Yang, B.; Ma, Q.; Wu, Z.; Lyu, J.; Wang, Z. Infusion of human albumin on acute pancreatitis therapy: New tricks for old dog? Front Pharmacol 2022, 13, 842108. [CrossRef]
- Working Group, I.A.P.A.P.A.A.P.G. Iap/apa evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013, 13, e1–e15. [CrossRef]
- Mao, E.Q.; Tang, Y.Q.; Fei, J.; Qin, S.; Wu, J.; Li, L.; Min, D.; Zhang, S.D. Fluid therapy for severe acute pancreatitis in acute response stage. Chin Med J (Engl) 2009, 122, 169–173. [CrossRef]
- Mao, E.Q.; Fei, J.; Peng, Y.B.; Huang, J.; Tang, Y.Q.; Zhang, S.D. Rapid hemodilution is associated with increased sepsis and mortality among patients with severe acute pancreatitis. Chin Med J (Engl) 2010, 123, 1639–1644. [CrossRef]
- Wu, B.U.; Hwang, J.Q.; Gardner, T.H.; Repas, K.; Delee, R.; Yu, S.; Smith, B.; Banks, P.A.; Conwell, D.L. Lactated ringer's solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011, 9, 710–717 e711. [CrossRef]
- Yaowmaneerat, T.; Sirinawasatien, A. Update on the strategy for intravenous fluid treatment in acute pancreatitis. World J Gastrointest Pharmacol Ther 2023, 14, 22–32. [CrossRef]
- Gülen, B.; Dur, A.; Serinken, M.; Karcioğlu, Ö.; Sönmez, E. Pain treatment in patients with acute pancreatitis: A randomized controlled trial. 2016, 27, 192–196. [CrossRef]
- Basurto Ona, X.; Rigau Comas, D.; Urrútia, G. Opioids for acute pancreatitis pain. 2013, 2013. [CrossRef]
- Cuellar-Monterrubio, J.E.; Monreal-Robles, R.; Gonzalez-Moreno, E.I.; Borjas-Almaguer, O.D.; Herrera-Elizondo, J.L.; Garcia-Compean, D.; Maldonado-Garza, H.J.; Gonzalez-Gonzalez, J.A. Nonaggressive versus aggressive intravenous fluid therapy in acute pancreatitis with more than 24 hours from disease onset: A randomized controlled trial. Pancreas 2020, 49, 579–583. [CrossRef]
- Saini, M.; Samanta, J.; Kumar, A.; Choudhury, A.; Dhar, J.; Jafra, A.; Chauhan, R.; Muktesh, G.; Gupta, P.; Gupta, V. et al. Buprenorphine versus diclofenac for pain relief in acute pancreatitis: A double-blinded randomized controlled trial. Clin Gastroenterol Hepatol 2023. [CrossRef]
- Jabaudon, M.; Genevrier, A.; Jaber, S.; Windisch, O.; Bulyez, S.; et al. Thoracic epidural analgesia in intensive care unit patients with acute pancreatitis: The epipan multicenter randomized controlled trial. 2023, 27. [CrossRef]
- Louie, B.E.; Noseworthy, T.; Hailey, D.; Gramlich, L.M.; Jacobs, P.; Warnock, G.L. 2004 maclean-mueller prize enteral or parenteral nutrition for severe pancreatitis: A randomized controlled trial and health technology assessment. Can J Surg 2005, 48, 298–306.
- Al-Omran, M.; Albalawi, Z.H.; Tashkandi, M.F.; Al-Ansary, L.A. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev 2010, 2010, CD002837. [CrossRef]
- Petrov, M.S.; Pylypchuk, R.D.; Uchugina, A.F. A systematic review on the timing of artificial nutrition in acute pancreatitis. Br J Nutr 2009, 101, 787–793. [CrossRef]
- Windsor, A.C.; Kanwar, S.; Li, A.G.; Barnes, E.; Guthrie, J.A.; Spark, J.I.; Welsh, F.; Guillou, P.J.; Reynolds, J.V. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut 1998, 42, 431–435. [CrossRef]
- Bakker, O.J.; van Brunschot, S.; van Santvoort, H.C.; Besselink, M.G.; Bollen, T.L.; Boermeester, M.A.; Dejong, C.H.; van Goor, H.; Bosscha, K.; Ahmed Ali, U. et al. Early versus on-demand nasoenteric tube feeding in acute pancreatitis. N Engl J Med 2014, 371, 1983–1993. [CrossRef]
- Ramírez-Maldonado, E.; López Gordo, S.; Pueyo, E.M.; Sánchez-García, A.; Mayol, S.; et al. Immediate oral refeeding in patients with mild and moderate acute pancreatitis: A multicenter, randomized controlled trial (padi trial). 2021, 274, 255–263. [CrossRef]
- Vege, S.S.; DiMagno, M.J.; Forsmark, C.E.; Martel, M.; Barkun, A.N. Initial medical treatment of acute pancreatitis: American gastroenterological association institute technical review. Gastroenterology 2018, 154, 1103–1139. [CrossRef]
- Werge, M.; Novovic, S.; Schmidt, P.N.; Gluud, L.L. Infection increases mortality in necrotizing pancreatitis: A systematic review and meta-analysis. Pancreatology 2016, 16, 698–707. [CrossRef]
- Wittau, M.; Mayer, B.; Scheele, J.; Henne-Bruns, D.; Dellinger, E.P.; et al. Systematic review and meta-analysis of antibiotic prophylaxis in severe acute pancreatitis. 2011, 46, 261–270. [CrossRef]
- Baltatzis, M.; Mason, J.M.; Chandrabalan, V.; Stathakis, P.; McIntyre, B.; et al. Antibiotic use in acute pancreatitis: An audit of current practice in a tertiary centre. 2016, 16, 946–951. [CrossRef]
- Baltatzis, M.; Mason, J.M.; Chandrabalan, V.; Stathakis, P.; McIntyre, B.; Jegatheeswaran, S.; Jamdar, S.; O'Reilly, D.A.; Siriwardena, A.K. Antibiotic use in acute pancreatitis: An audit of current practice in a tertiary centre. Pancreatology 2016, 16, 946–951. [CrossRef]
- Parniczky, A.; Lantos, T.; Toth, E.M.; Szakacs, Z.; Godi, S.; Hagendorn, R.; Illes, D.; Koncz, B.; Marta, K.; Miko, A. et al. Antibiotic therapy in acute pancreatitis: From global overuse to evidence based recommendations. Pancreatology 2019, 19, 488–499. [CrossRef]
- Siriwardena, A.K.; Jegatheeswaran, S.; Mason, J.M.; Baltatzis, M.; Sheen, A.J. et al. A procalcitonin-based algorithm to guide antibiotic use in patients with acute pancreatitis (procap): A single-centre, patient-blinded, randomised controlled trial. 2022, 7, 913–921. [CrossRef]
- Yadav, D.; Lowenfels, A.B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013, 144, 1252–1261. [CrossRef]
- Lerch, M.M.; Saluja, A.K.; Runzi, M.; Dawra, R.; Saluja, M.; Steer, M.L. Pancreatic duct obstruction triggers acute necrotizing pancreatitis in the opossum. Gastroenterology 1993, 104, 853–861. [CrossRef]
- Fan, S.T.; Lai, E.C.; Mok, F.P.; Lo, C.M.; Zheng, S.S.; Wong, J. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med 1993, 328, 228–232. [CrossRef]
- van Santvoort, H.C.; Besselink, M.G.; de Vries, A.C.; Boermeester, M.A.; Fischer, K.; Bollen, T.L.; Cirkel, G.A.; Schaapherder, A.F.; Nieuwenhuijs, V.B.; van Goor, H. et al. Early endoscopic retrograde cholangiopancreatography in predicted severe acute biliary pancreatitis: A prospective multicenter study. Ann Surg 2009, 250, 68–75. [CrossRef]
- Hallensleben, N.D.; Stassen, P.M.C.; Schepers, N.J.; Besselink, M.G.; Anten, M.P.G.F.; et al. Patient selection for urgent endoscopic retrograde cholangio-pancreatography by endoscopic ultrasound in predicted severe acute biliary pancreatitis (apec-2): A multicentre prospective study. 2023, 72, 1534–1542. [CrossRef]
- Schepers, N.J.; L Hallensleben, N.D.; Besselink, M.G.; F Anten, M.-P.G.; Bollen, T.L.; et al. Urgent endoscopic retrograde cholangiopancreatography with sphincterotomy versus conservative treatment in predicted severe acute gallstone pancreatitis (apec): A multicentre randomised controlled trial. 2020, 396, 167. [CrossRef]
- Patil, B.; Meena, L.N.; Sharma, D.C.; Agarwal, G.; Dadhich, Y.; et al. Impact of low-molecular-weight heparin in the treatment of moderately severe and severe acute pancreatitis; a randomized, single blind, phase 3 control trial. 2022, 101. [CrossRef]
- Seta, T.; Noguchi, Y.; Shimada, T.; Shikata, S.; Fukui, T. Treatment of acute pancreatitis with protease inhibitors: A meta-analysis correspondence and requests for reprints to. 2004, 16, 1287–1293. [CrossRef]
- Wang, G.; Liu, Y.; Zhou, S.-F.; Qiu, P.; Xu, L.; et al. Effect of somatostatin, ulinastatin and gabexate on the treatment of severe acute pancreatitis. 2016. [CrossRef]
- Crockett, S.D.; Wani, S.; Gardner, T.B.; Falck-Ytter, Y.; Barkun, A.N.; et al. American gastroenterological association institute guideline on initial management of acute pancreatitis. 2018, 154, 1096–1101. [CrossRef]
- Boerma, D.; Rauws, E.A.; Keulemans, Y.C.; Janssen, I.M.; Bolwerk, C.J.; Timmer, R.; Boerma, E.J.; Obertop, H.; Huibregtse, K.; Gouma, D.J. Wait-and-see policy or laparoscopic cholecystectomy after endoscopic sphincterotomy for bile-duct stones: A randomised trial. Lancet 2002, 360, 761–765. [CrossRef]
- da Costa, D.W.; Bouwense, S.A.; Schepers, N.J.; Besselink, M.G.; van Santvoort, H.C.; van Brunschot, S.; Bakker, O.J.; Bollen, T.L.; Dejong, C.H.; van Goor, H. et al. Same-admission versus interval cholecystectomy for mild gallstone pancreatitis (poncho): A multicentre randomised controlled trial. Lancet 2015, 386, 1261–1268. [CrossRef]
- da Costa, D.W.; Dijksman, L.M.; Bouwense, S.A.; Schepers, N.J.; Besselink, M.G.; van Santvoort, H.C.; Boerma, D.; Gooszen, H.G.; Dijkgraaf, M.G.; Dutch Pancreatitis Study, G. Cost-effectiveness of same-admission versus interval cholecystectomy after mild gallstone pancreatitis in the poncho trial. Br J Surg 2016, 103, 1695–1703. [CrossRef]
- Hallensleben, N.D.; Timmerhuis, H.C.; Hollemans, R.A.; Pocornie, S.; Van Grinsven, J.; et al. Optimal timing of cholecystectomy after necrotising biliary pancreatitis. 2022, 71, 974–982. [CrossRef]
- Pelli, H.; Lappalainen-Lehto, R.; Piironen, A.; Sand, J.; Nordback, I. Risk factors for recurrent acute alcohol-associated pancreatitis: A prospective analysis. Scand J Gastroenterol 2008, 43, 614–621. [CrossRef]
- Nordback, I.; Pelli, H.; Lappalainen-Lehto, R.; Jarvinen, S.; Raty, S.; Sand, J. The recurrence of acute alcohol-associated pancreatitis can be reduced: A randomized controlled trial. Gastroenterology 2009, 136, 848–855. [CrossRef]
| Parameters and Target | Significance in GDT |
|---|---|
| HR < 120 /min | An elevated heart rate can indicate an imbalance between oxygen supply and demand, guiding therapeutic interventions in GDT. Persistent tachycardia might suggest inadequate resuscitation or ongoing inflammation. |
| MAP 65-90 mmHg | A consistent MAP is crucial for ensuring adequate blood flow to vital organs. In GDT, adjustments in fluid volume and vasopressor medications might be considered to maintain or achieve a target MAP, ensuring optimal organ perfusion. |
| CVP 8 – 12 cmH2O | It indicates the volume and filling status of the right atrium. In GDT, CVP is used to assess the patient's volume status and right-sided cardiac preload, guiding fluid management. |
| UO ≥ 0.5 ml/kg/hr | A decrease in UO is an early and sensitive indicator of reduced kidney perfusion. Maintaining adequate urine output is crucial in GDT as it provides valuable information on general tissue perfusion. |
| ScvO2 ≥ 70% | An indicator in assessing the adequacy of tissue oxygenation. A decrease in ScvO2 can suggest that tissue oxygen demand is exceeding supply. This could be due to decreased oxygen delivery (e.g., due to low cardiac output or hemoglobulin) or increased oxygen consumption (e.g., due to increased metabolic demand). |
| BUN < 25mg/dL | An elevated BUN has been useful prognostic biomarker of severe AP, reflecting acute renal injury in AP caused by decrease in circulatory volume and direct injury mechanisms, which is facilitated by the autodigestion and inflammatory cytokines [15,16]. Whereas a declining or normalized BUN level reflects recovery of renal perfusion and adequate resuscitation. |
| Hematocrit < 44% | Hemoconcentration (high hematocrit values) is linked with high fluid sequestration and increased viscosity which might contribute to impaired pancreatic microcirculation. Therefore, hematocrit has long been identified as a marker associated with the development of pancreatic necrosis and persistent organ failure [17,18]. Fluid rate adjustment can be guided by the biochemical targets of hematocrit of 35 – 44% at 12 and 24 hours after AP onset. |
| Lactate | Lactate level increases when aerobic cellular respiration is impaired with switch to anaerobic metabolism. Elevated lactate level has been considered as a well-recognized biomarker of tissue hypoxia/hypoperfusion in the critically ill patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




