1. Introduction
In the thermodynamic study of irreversible processes, the rate of entropy generation,
, is a fundamental quantity. It may be expressed as the sum of the products of flux
and its associated thermodynamic force
, or
. For a device that involves energy conversion—such as a heat engine, the description requires at least two flux-force pairs, such that
Here, is the spontaneous heat flux (), while the velocity flux is the driven flux (). In order to satisfy the second law, we must have .
For a generic heat engine, in simultaneous contact with two heat reservoirs at temperatures
, let
and
respectively denote the thermal flux entering and exiting the engine. The power output of the device is given by:
where
F is the external load and
is the velocity flux. The overall rate of entropy generation is
In order to express the above in the canonical form, we use (
2) to rewrite
and thus identify the following two flux-force pairs:
The description of irreversible processes is further enriched by the assuming small magnitudes of the forces and proposing linear flux-force relations:
, where
are the Onsager coefficients which obey
where we assume Onsager reciprocity,
. Now, it is also well known that there is no unique choice of the flux-force pairs to express the rate of entropy generation. Thus, for the thermal flux
, one may choose the cold flux
. Accordingly, the modified force
. Other authors have proposed using a mean thermal flux over the hot and cold fluxes [
1,
2]. The mean flux also plays a role in the linear-irreversible framework for coupled autonomous machines [
3]. In these previous approaches, two fluxes in general describe the rate of entropy generation, as in Eq. (
1). However, under the so-called strong coupling condition,
, we have the result that
is directly proportional to
. In this case, only one flux is adequate to describe the rate of entropy generation.
Motivated by the above observation, in this paper, we develop an approach to describe the rate of entropy generation in terms of a
single effective flux
and its corresponding generalized force
. Now, in the case of a single effective flux that describes our engine, we let
. Further, we keep the thermodynamic description within a linear framework, which implies a linear flux-force relation,
, where
is a suitable transport coefficient. So, the rate of entropy generation is effectively expressed as:
The applicability of the second law requires that
[
4]. The exact form of
depends on details of the model, in particular the way irreversibilities are treated within the model. In this paper, we apply our formalism to the Constant Properties Model of a thermoelectric generator (TEG), whuch provides a paradigmatic model for such a heat engine. In this case, internal and external irreversibilities can be formulated relatively easily [
5,
6,
7,
8]. Under various approximations whereby one or the other kind of irreversibilities can be neglecred, we identify the corresponding effective flux along with the transport coefficient
for each case.
The paper is organized as follows. In Section II, we outline the effective flux approach and develop the basic expressions for power output. In Section III, we describe the so-called Constant Properties Model of a TEG. In Section IV, we determine the effective thermal flux in a TEG with an internal irreversibility only, while Section V deals exclusively with an external irreversibility. Section VI is devoted to TEG with both internal and external irreversibilities. Finally, in Section VII, we conclude with a discussion of our results.
2. The effective flux approach
In the proposed scheme, we assume the validity of Eqs. (
3) and (
8). It is convenient to define the ratio
. Then, we can express Eq. (
3) as:
Now, to assign a specific form to the effective flux, we note that , where the equality implies a vanishing power output. The effective flux is bounded by the given hot and cold fluxes: . More generally, we demand that is a homogeneous function of and , so that we can express , where is to be determined as a function of .
Thus, Eq. (
8) can be written as:
From Eqs. (
9) and (
10), we can write:
Finally, power output can be expressed as:
. Upon using Eq. (
11), we obtain
Note that apart from
,
P is expressed as a universal expression in terms of
[
9,
10]. The actual expression for
P as followed under different models dictates the form taken by the function
, which in turn determines the effective flux
. We shall illustrate these cases using the TEG model as described in the following.
3. TEG model
Thermoelectricity is a non-equilibrium phenomenon, which can be studied within the framework of Onsager-Callen theory [
11,
12,
13,
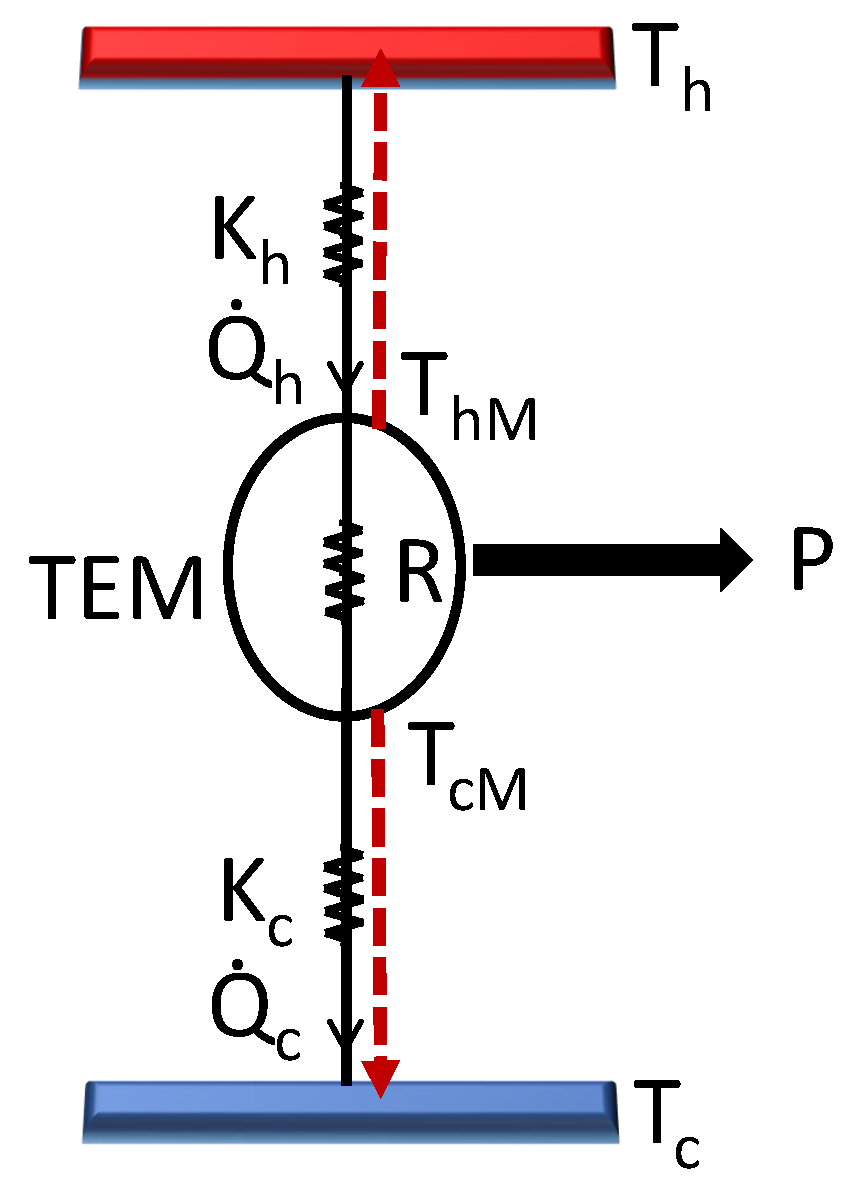
14]. The coupling between the gradients of temperature and electric potential gives rise to various thermoelectric effects. Consider the thermoelectric material (TEM) to be a one-dimensional substance of length
L with fixed values of internal resistance
R and Seebeck coefficient
. Let
I denote the constant current flowing through the TEM (see
Figure 1). Also, we work within the strong-coupling assumption [
15,
16], and so any heat leakage between the reservoirs is negligible. Then, based on Onsager formalism and Domenicali’s heat equation [
17], thermal fluxes at the end points of TEM are written as follows.
In the above equations, the first term corresponds to convective heat flow, where
is the local temperature of TEM at hot (cold) side. The second term is the Joule heat received by each reservoir. Assuming a Newtonian heat flow [
18,
19] between a reservoir and TEM, the hot and cold fluxes are also given by
where
and
are the heat transfer coefficients. From the equality of Eqs. (
13) and (
15), we get an expression for
, as:
Substituting this in Eq. (
15), we can write
Similarly, for the cold flux, we obtain:
Using the definition of
, we can express the electric current as function of
:
. Further, we verify that
I is a monotonic function of
. Thereby, we express power output in terms of the variable
, as in Eq. (
12).
4. TEG with internal irreversibility
In this case, the only source of irreversibility is the internal electrical resistance of the working substance. No dissipation is involved in thermal contacts with heat reservoirs. More precisely, we consider the limit
. This leads to the simplification:
and
. Also, we have considered bypass or heat leaks to be negligible. This is the so-called exoreversible model [
16,
20]. So, Eqs. (
17) and (
18) are simplified to
Now, expressing
I in terms of
by using Eq. (
19) and (20), we obtain
So, the power output can be expressed in the form
where we define the thermal conductance of TEM as [
5,
7]
Comparing the expressions of power in Eqs. (
12) and (
22), we identify
as the effective thermal conductivity, and
. So, the effective thermal flux
is given by:
Thus, the entropy generation in an exoreversible model may be effectively described by a thermal flux which is the arithmetic mean of the hot and cold fluxes.
5. TEG with external irreversibility
In this case, the sources of irreversibility are the heat exchangers having finite thermal conductances,
and
, on the hot and the cold side, respectively. Also, we assume that TEM has zero internal resistance (
). This is the so-called endoreversible approximation [
18,
19]. Then, the heat fluxes are simplified to
Again, from the above expressions, we obtain
I in terms of the variable
:
Therefore, the output power in terms of
is given by
where the contact thermal conductance is
Again, a comparison between Eqs. (
12) and (
27) suggests that
, or, in other words, the mean thermal flux is the geometric mean:
with an effective thermal conductivity as
From Eqs. (
22), (
27), and (
60), we see that for thermoelectric generator, the power output can be written in the form of Eq. (
12), where
is a constant specific to each irreversibility. More importantly, the effective heat flux has been found to be in the form of either the arithmetic mean or the geometric mean over the hot and cold fluxes. However, so far we have considered TEG with only one kind of irreversibility. In the following, we find specific configurations with both internal and external irreversibilities, and show instances of the effective heat flux in the form of generalized means.
7. Discussion
The main motivation for this work was to express the rate of entropy generation, for a steady-state heat engine under the tight-coupling assumption, in terms of a single effective flux. In the standard description of linear-irreversible engines within Onsager framework, the tight coupling assumption yields a single flux to describe the rate of entropy generation. This applies to steady-state engines at the
local level. However, a similar analysis has not been carried out when we scale up this description to
global level such as for thermoeletric generators where the length of the thermoelectric material is finite [
20]. It has been observed earlier, that thermodynamic forces in the global picture are discrete versions of the local forces. Secondly, the thermal fluxes involve quadratic terms which express Joule heating. This leads to nonlinear flux-force relations. Analogous to the tight coupling framework at the local level, we formulated entropy generation in terms of an effective thermal flux. We observed explicit forms of this flux in TEG, which is a homogeneous function of the hot and cold fluxes. In the simple case of only external (internal) irreversibility, we obtain the effective flux in the form of the arithmetic (geometric) mean of hot and cold fluxes.
For the endoreversible approximation, we chose Newton’s law which yields the geometric mean flux. We note that the form of the mean depends on the specific heat transfer law. If instead of Newton’s law, we choose inverse-temperature law of linear irreversible thermodynamics, then we have
where
are the heat transfer coefficients. The above fluxes are to be respectively matched with
and
, from Eqs. (
13) and (14). Thus, we obtain
Therefore, thermal fluxes entering and exiting the thermoelectric material are as follows [
23,
24].
Now, by using Eqs. (
57) and (58), we express
I in terms of
, as
The output power in terms of
is as follows.
where
and
. From Eqs. (
12) and (
60), we have
, which defines the so-called weighted quadratic mean:
with an effective thermal conductance of
. We also note that as the cold (hot) contact approches the reversible limit, implying
or
, the effective heat flux goes to the limit
.
An analogous effective framework was proposed for discrete heat engines running in finite cycle period
[
10]. We briefly summarize the important features of this framework. Let the total work extracted be given as
and total entropy produced is
. The average power is defined as
and the average rate of entropy production:
. Again, we work in the strong coupling regime. Instead of effective flux, we postulate an effective heat flowing through the system
in time
. This leads to defining an average effective flux as
. Now, the total rate of entropy production is considered as quadratic function of the effective flux.
In the case of discrete heat devices, the effective heat
may be taken in the form of a homogeneous function of
and
. In contrast, for the case of autonomous devices, we note that it is the effective
flux that is given in terms of the hot and cold fluxes exchanged with the reservoirs. Despite this difference, the form of the objective function, for instance, the power output is given by a similar expression in the formulation for discrete engines [
10] and the present framework. Thus for the discrete models, we have
where
and so the efficiency,
. Further, the effective heat is given by
. The formulation proposed in this paper applicable to scaled-up steady state engines. Using the model example of TEG, we are able to analyze different types of irreversibilities which yield specific forms of the effective fluxes. This leads to a simplified description of these models for engines under tight-coupling condition. A similar analysis may be carried out for thermoelectric cooler or pump.