Background
Global Impact of Dementia: Dementia, a condition characterized by the deterioration of cognitive functioning beyond the expected age-related decline, poses a significant global health challenge. The World Health Organization estimates that 55 million individuals worldwide are currently living with dementia, with an additional 10 million new cases diagnosed annually [
https://www.who.int/news-room/fact-sheets/detail/dementia#:~:text=Key%20facts,%2Dand%20middle%2Dincome%20countries]. This condition not only affects memory but also impacts comprehension, calculation, language, and judgment. Psychiatric manifestations, including changes in mood and behaviour, often accompany cognitive decline. Globally, dementia ranks as the seventh leading cause of death and contributes significantly to disability among the elderly. The ramifications of dementia extend beyond individual suffering, encompassing broader societal and public health implications (Nichols et al., 2022).
Types of Dementia: The Royal College of Psychiatrists identifies several subtypes of dementia, including Alzheimer's disease, vascular dementia, dementia with Lewy bodies, Parkinson's disease dementia, and frontotemporal dementia [
https://www.rcpsych.ac.uk/improving-care/nccmh/service-design-and-development/dementia]. Alzheimer's disease, responsible for 60% of all dementia cases, is marked by a gradual onset of memory impairments associated with tau deposits affecting neurotransmission. Vascular dementia, linked to disrupted blood supply in the brain, presents memory loss, language difficulties, and physical problems. Dementia with Lewy bodies results from Lewy body deposits, leading to memory problems, confusion, and visual hallucinations. Frontotemporal dementia, arising from insults to the frontal areas of the brain, manifests with marked personality changes, behavioural alterations, and language difficulties.
Introduction to Mild Cognitive Impairment: Mild Cognitive Impairment (MCI) represents a less severe cognitive decline than dementia. Individuals with MCI remain functional, and experience milder memory impairment compared to dementia. Peterson et al.'s (Petersen et al., 1999) diagnostic criteria for MCI include memory complaints, normal daily activities, normal general cognitive functioning, abnormal memory based on age, and the absence of clinical signs of dementia. A global perspective on MCI and dementia reveals alarming trends. Li et al. (GBD 2016 Dementia Collaborators, 2019, 1990–2019) reported a significant increase in the incidence and prevalence of Alzheimer's disease and other forms of dementia. The Lancet study (Nichols et al., 2022) forecasting dementia prevalence by 2050 predicts a rise from 57.4 million cases in 2019 to 152.8 million cases. Efforts to address MCI and dementia are imperative given the high progression rates from MCI to Alzheimer's disease. Diabetes, prediabetes, and Metabolic Syndrome (MetS) were all linked to elevated risks of MCI progressing to dementia (Pal et al., 2018).
Advancement to dementia in individuals affected by diabetes, prediabetes, and metabolic syndrome: The global prevalence of diabetes increased significantly from 108 million in 1980 to 422 million in 2014, with a higher rise in low- and middle-income countries [
https://www.who.int/news-room/fact-sheets/detail/diabetes]. Diabetes is a leading cause of blindness, kidney failure, heart attacks, stroke, and lower limb amputation, contributing to an estimated 2 million deaths in 2019. Between 2000 and 2019, there was a 3% increase in diabetes mortality rates by age. People with Type 1 diabetes face a higher risk of dementia, with a 93% increased likelihood according to a study. Older adults with type 1 diabetes, hospitalized for blood sugar extremes, are at elevated dementia risk, especially when experiencing both highs and lows. High blood sugar levels, common in type 2 diabetes, are strongly correlated with Alzheimer's disease, leading to increased beta-amyloid protein, a key Alzheimer's marker. Early-stage type 2 diabetes is associated with brain dysfunction, insulin resistance, and impaired glucose use for normal brain function. Individuals with Type 2 diabetes show accelerated cognitive decline, particularly in executive function and processing speed, with earlier onset increasing dementia risk. The impact of diabetes on the brain is linked to the blood protein hemoglobin A1C (HbA1C), affecting memory function and hippocampal size. The gene for amyloid precursor protein (APP), involved in Alzheimer's, also affects the insulin pathway disrupted in diabetes, suggesting a potential therapeutic target for both diseases. Dementia prevention (Ngandu et al., 2015) includes adopting a healthy lifestyle with a balanced diet, regular exercise, maintaining normal body weight, while the management of diabetes involves a combination of diet, physical activity, medication, and regular screening.
Assessment Guidelines: The National Institute for Clinical Excellence (NICE) (Overview | Dementia: assessment, management and support for people living with dementia and their carers | Guidance | NICE, 2018) provides guidelines for assessing cognitive impairment. History-taking, physical examination, blood and urine tests, and cognitive testing with validated tools are recommended for non-specialists. Specialists are advised to consider subtype-specific diagnosis, neuropsychological testing, and imaging to differentiate dementia subtypes.
Neuroimaging for Diagnosis and Screening: Neuroimaging plays a crucial role in differentiating dementia subtypes. Various tools, including fluorodeoxyglucose-positron emission tomography (FDG-PET) and magnetic resonance imaging (MRI), aid in identifying specific patterns associated with Alzheimer's disease, frontotemporal dementia, and vascular dementia. Functional Near-Infrared Spectroscopy (fNIRS) emerges as a non-invasive and cost-effective alternative to traditional imaging methods (Pinti et al., 2020). While sharing similarities with functional MRI (fMRI) in measuring hemodynamic responses, fNIRS surpasses fMRI in portability, allowing assessments in naturalistic environments. Its application extends to psychiatry research, providing valuable insights into various psychiatric disorders, including schizophrenia, depression, bipolar disorder, and ADHD. fNIRS has proven instrumental in detecting characteristic changes in cerebral cortex hemodynamics associated with psychiatric disorders. Studies have shown alterations in frontal activation for schizophrenia, changes in oxyhemoglobin concentration for ADHD, and distinctive patterns for panic disorder, obsessive-compulsive disorder, and depression.
Normal brain function relies on regulated cerebral blood flow (CBF), a process compromised in vascular dementia, particularly subcortical ischemic dementia affecting cognitive abilities in the elderly (Zhao et al., 2023),(Zhao et al., 2022),(Li et al., 2021). This condition can result from cerebral microangiopathy e.g. in diabetes, leading to increased vessel stiffness and a presumed reduction in slow spontaneous oscillations (Lee et al., 2000),(Zhao et al., 2022),(Li et al., 2013). While the longitudinal recovery of CBF regulation in these cases remains understudied, cerebrovascular reactivity (CVR) – the change in CBF in response to vasoactive stimuli – could provide insights into the pathogenesis of CBF dysfunction. Existing literature uses stimuli like acetazolamide and carbon dioxide, but these have systemic effects (Fierstra et al., 2013). Transcranial electrical stimulation (tES) offers a promising approach to evoke CBF (Zheng et al., 2011) which may be partly effected by the autonomic responses (Arora and Dutta, 2022),(Rodrigues et al., 2022). However, the role of autonomic function in regulating CBF is not fully understood, and the contribution of the baroreflex in CBF regulation is debated (Ogoh and Tarumi, 2019). Recent studies combining genetic engineering in animal models and mathematical analysis of cardiovascular signals in humans reveal insights into the interplay between the arterial baroreceptor reflex (baroreflex) and arousal (Silvani et al., 2015). Mild baroreceptor stimulation, especially under anesthesia, may inhibit cortical arousal, while significant increases or decreases in baroreflex activation induce arousal in both animals and humans under normal physiological conditions. Additionally, cardiovascular changes during autonomic arousals and transitions between wakefulness and sleep involve adjustments in the baroreflex set point and its equilibrium with central autonomic commands that may be modulated with tES. While acute increases in systemic blood pressure trigger peripheral vasodilation via baroreflex, cerebral vasculature may need to constrict to protect the blood-brain barrier (Ogoh and Tarumi, 2019). Here, regional differences in autonomic outflow between systemic and cerebral blood vessels are possible. Additionally, the impact of direct (autonomic regulation) and indirect (systemic blood pressure regulation) baroreflex influences on CBF may vary. In hypertensive patients, sympathetic nerve activation leads to cerebral vasoconstriction, unlike in normotensive individuals. The complex interplay of arterial baroreflex with other factors like cardiac output, respiratory chemoreflex, and physiological conditions such as cardiovascular disease, complicates understanding CBF regulation. Despite challenges in identifying its role, the arterial baroreflex's significance in maintaining adequate CBF, especially in disease conditions, should be acknowledged and investigated.
Effect of the sympathetic nervous system on cerebral oxygenation in patients with type 2 diabetes [by Seyyed Alireza Hosseini Kakhak]: Diabetes mellitus (T2DM) is the fourth cause of death among non-communicable diseases and accounts for 2 million deaths annually worldwide. The prevalence of diabetes is increasing dramatically, especially in low- and middle-income countries than in high-income countries. (
https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases).
T2DM is associated with serious microvascular or macrovascular complications in many organs and systems that lead to a cluster of diseases including heart disease and stroke, peripheral arterial disease, retinopathy, nephropathy, peripheral neuropathy, and lower-extremity amputations (Deshpande et al., 2008). Cardiovascular disease (CVD), where the coronary and blood vessels are negatively affected, is the main cause of death in patients with T2DM (
https://diabetes.org/about-diabetes/complications/cardiovascular-disease)
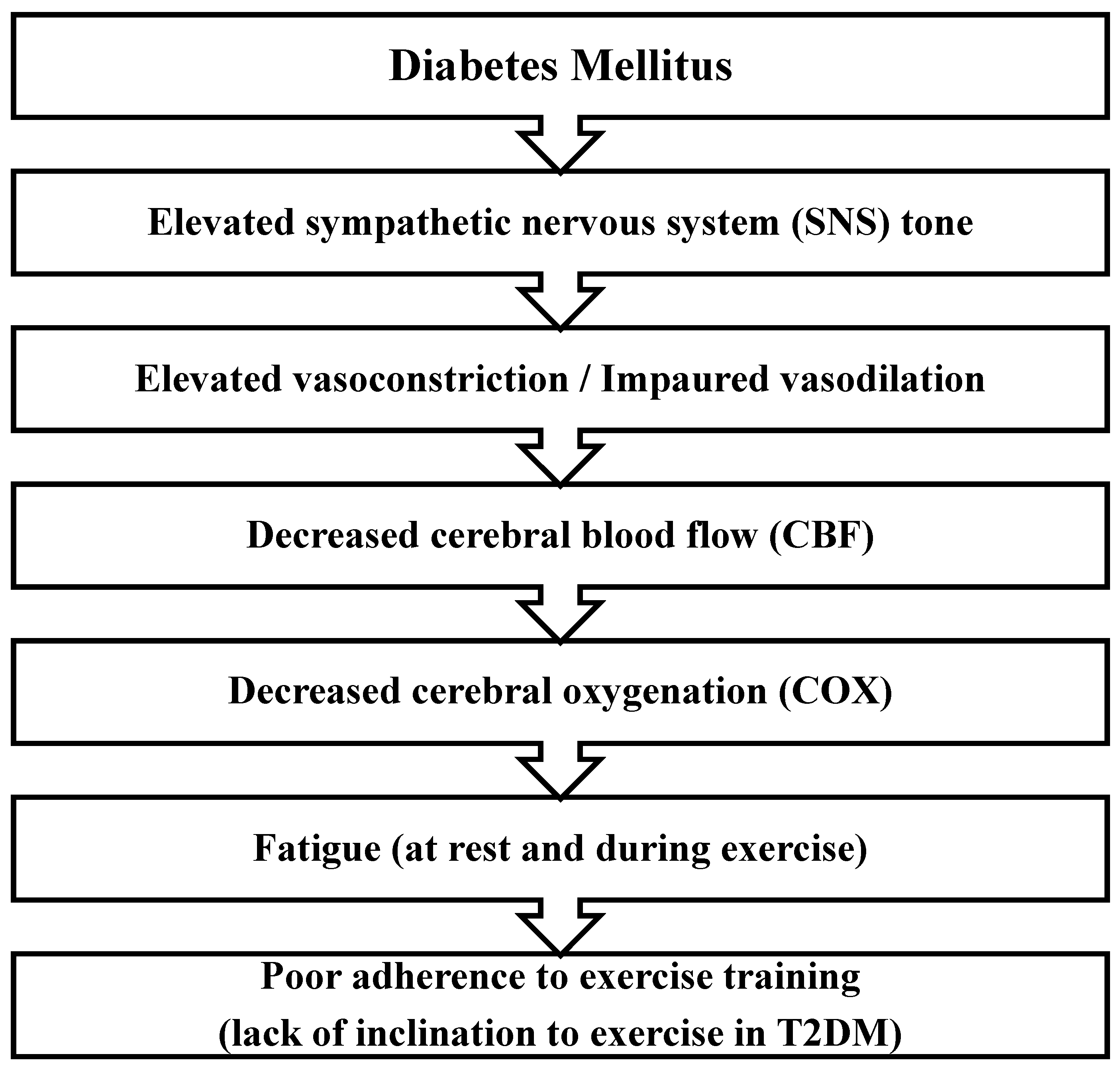
A lesser-known aspect of cardiovascular disorders associated with T2DM is the condition in which T2DM leads to increased sympathetic nervous system (SNS) activity – see
Figure 1. Therefore, although exercise is known as a valid, effective, non-expensive, and side-effect-free modality in the management and treatment of T2DM, patients feel more effort, difficulty, and trouble during exercise, and this may lead to the unwillingness of these patients to exercise and show poor adherence to exercise (Doneddu et al., 2020).
In support of this claim, our studies show that during exercise, SNS tone dramatically increased in patients with T2DM, which results in elevated vasoconstriction, which means these patients have limited vasodilatory capacity (Roberto et al., 2019). During exercise, cerebral blood flow (CBF) and hence cerebral oxygenation (COX) increases in normal and healthy people. Any condition that impairs CBF may lead to fatigue. It has been shown that during exercise training, CBF is impaired in T2DM which may be because of SNS hyperactivity. This event leads to a decrease in COX and therefore induction of fatigue. In line with this evidence, we found that in patients with T2DM, concurrent performing a mental task (MT) and metaboreflex could not enhance COX in the same amount of healthy people. So, COX can be a limiting factor in physical performance in T2DM patients (Doneddu et al., 2020).
Brain and muscle oxygenation changes after 2-month exercise in sedentary older adults with diabetes [by Fei Zhao, Mancheung Cheung]: Abnormalities in the muscle metaboreflex contribute to reduced exercise tolerance and increased cardiovascular risk (Gama et al., 2021),(Nesti et al., 2020). Although exercise training is known to benefit neurocardiovascular function, its impact on the muscle metaboreflex is still debated. Some studies suggest that exercise training enhances the sensitization of muscle metabolically afferents and improves neurocardiovascular responses to muscle metaboreflex activation, while others report no significant changes. Possible mechanisms for improvement include heightened sensitivity of channels and receptors, increased antioxidant capacity, reduced metabolite accumulation, improved functional sympatholysis, and enhanced muscle perfusion. Therefore, there is a need to explore the dose-response relationship of different exercise components and modalities in individuals with both intact and impaired muscle metaboreflex, as well as investigate specific mechanisms underlying metaboreflex improvements in T2DM (Nesti et al., 2020). Our 2-month moderate-intensity exercise intervention clinical trial was pre-registered on ClinicalTrials.gov (NCT04626453 and NCT04812288). It included two groups: an Intervention group comprising older sedentary adults with type 2 diabetes (T2DM) and a control group consisting of healthy older adults, encompassing both active and sedentary individuals.
Multidomain Interventions for Cognitive Enhancement: Ngandu et al. (Ngandu et al., 2015) trial emphasized the importance of addressing modifiable risk factors for dementia and the multidomain intervention involving diet, exercise, cognitive training, and vascular risk monitoring on cognitive performance in older individuals at risk from the general population to enhance or preserve cognitive performance and its implications for older individuals at risk. However, irregularities in the muscle metaboreflex contribute to reduced exercise tolerance in T2DM (Doneddu et al., 2020),(Kim et al., 2015).
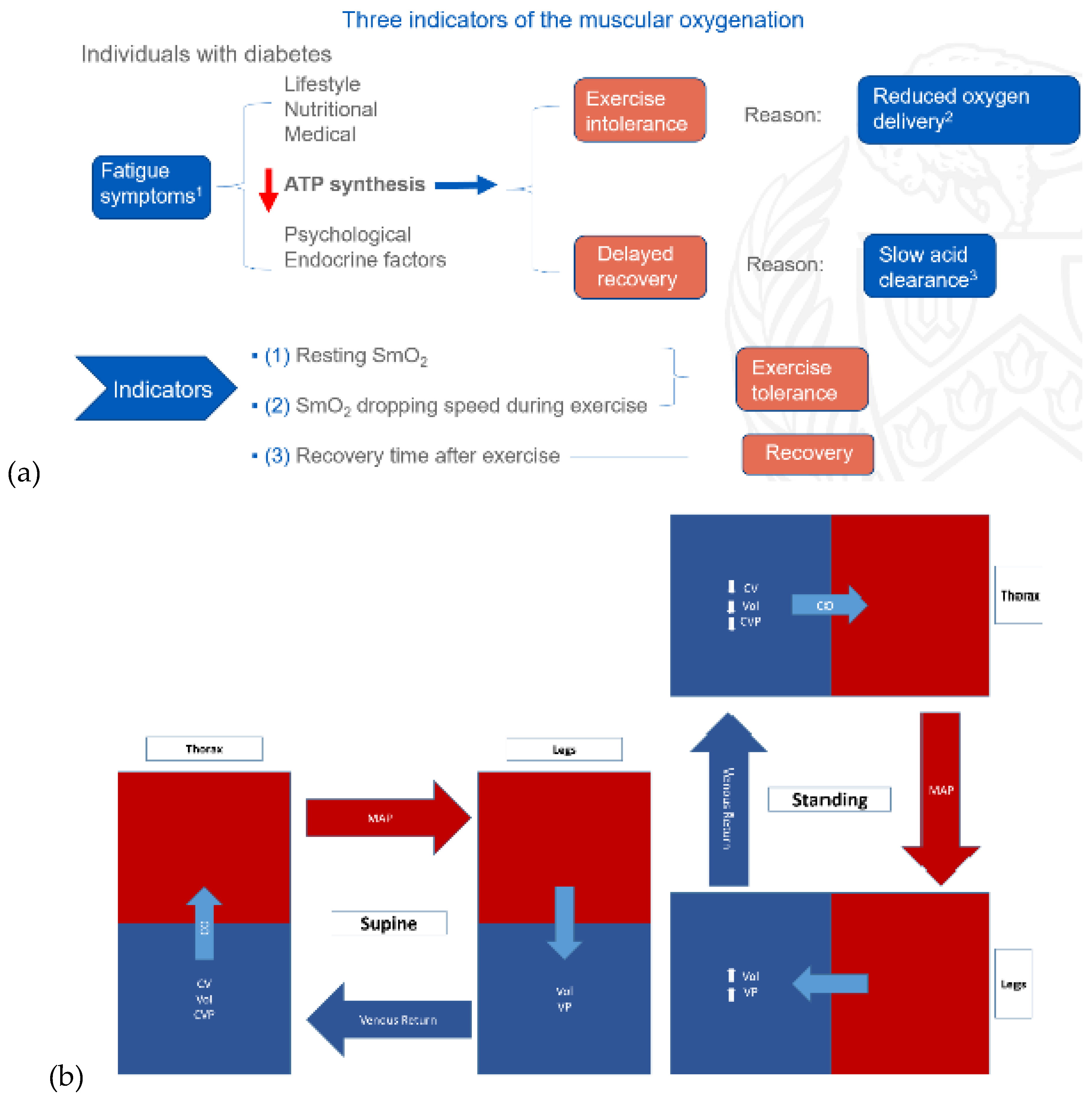
Exercise Intervention for Type 2 Diabetes-Related Cognitive Impairment: We conducted a clinical trial (NCT04626453 and NCT04812288) targeting cognitive impairment in individuals with type 2 diabetes through a tailored aerobic and resistance exercise regimen aligned with the recommendations of the American College of Sports Medicine (Zhao et al., 2019). We highlighted the design of a 2-month individualized progressive moderate-intensity exercise intervention, including prospective registration at ClinicalTrials.gov and the involvement of two groups: an Intervention group comprising older sedentary adults with type 2 diabetes and a Control group consisting of healthy older adults. This study aimed to investigate the impact of a 2-month moderate-intensity aerobic-and-resistance exercise on various health parameters in sedentary older adults with T2DM. The participants underwent personalized progressive exercise, with assessments before and after the intervention. Results showed positive changes in glucose attached hemoglobin (HbA1C), cognitive and physical performance, as well as brain and muscle oxygenation. The exercise program contributed to improved basic executive function, lower extremity function, and endurance in the diabetes group. Brain overactivation reduced, and differences between the diabetes group and control groups decreased by 40% in cognitive and physical tests. The study suggests that this exercise regimen could be beneficial for individuals with compromised oxidative capacity, such as those with cardiovascular diseases or other cognitive illnesses. The exercise effects on the muscles were monitored using the following optical neuroimaging measures (Zhao et al., 2019) – see
Figure 2.
Resting SmO2 (Tissue Oxygen Saturation at Rest): Resting SmO2 refers to the baseline level of tissue oxygen saturation in a muscle or specific tissue region while an individual is at rest. SmO2 is a measure of the percentage of oxygenated hemoglobin in the total hemoglobin present in the tissue. Resting SmO2 provides a baseline measurement before any physical activity or exercise, offering insight into the oxygenation status of the tissue under normal, non-stressful conditions.
SmO2 Drop During Exercise: SmO2 drop during exercise refers to the gradual decline or "drop" in tissue oxygen saturation observed during sustained physical activity. As an individual engages in exercise, there is an increased demand for oxygen by working muscles. SmO2 drop reflects the gradual decrease in tissue oxygen saturation as oxygen is utilized by muscles for energy production. Monitoring SmO2 drop during exercise helps assess how well oxygen delivery matches the oxygen consumption requirements during the activity.
SmO2 Recovery After Exercise: SmO2 recovery after exercise refers to the rate at which tissue oxygen saturation returns to its baseline level following the cessation of physical activity. After exercise, the body continues to consume oxygen to restore energy reserves and clear accumulated metabolic byproducts. SmO2 recovery is a measure of how efficiently the tissue regains its baseline oxygen saturation level, reflecting the ability of the cardiovascular and respiratory systems to meet the post-exercise oxygen demands and support recovery. Faster recovery may indicate better oxygen utilization efficiency (Wang et al., 2006).
How Functional Electrical Stimulation Can Facilitate Venous Return During Exercise Intervention? During dynamic exercise, rhythmic contractions of skeletal muscles compress intramuscular veins, transferring kinetic energy to venous blood and aiding its return to the heart (Miller et al., 2005). The skeletal muscle pump is highly effective, emptying over 40% of intramuscular blood volume with a single contraction. Venous outflow primarily occurs during the concentric phase of contraction, emphasizing the role of increased intramuscular pressure as a significant energy source for blood returning to the heart during exercise – see
Figure 2b. Mancheung Cheung’s MS thesis studied hemodynamics resulting from calf muscle activity in healthy humans through biophysical modeling and experiments using frequency domain near-infrared spectroscopy (Cheung, n.d.). Functional Electrical Stimulation (FES) can induce contractions in leg muscles, artificially restoring the body's ability to pump blood from the legs to the heart. This activates the muscle pump, enhancing both superficial and deep vein pumping – see
Figure 2c. FES-induced leg muscle contractions have been linked to increased cardiac output, stroke volume, and venous return, leading to higher ventricular filling and end-diastolic volume. This, in turn, triggers the Frank-Starling effect, resulting in elevated stroke volume and arterial blood pressure. Studies demonstrate that FES-induced contractions in leg muscles help maintain stable blood pressure during positional changes, which is crucial for individuals with tetraplegia, known for lower blood pressure (Chao and Cheing, 2005). Also, attaining an optimal blood pressure is vital (Schenk et al., 2021), as cerebral autoregulation may be compromised below 60mmHg, which may be facilitated with FES (Dutta et al., 2021).
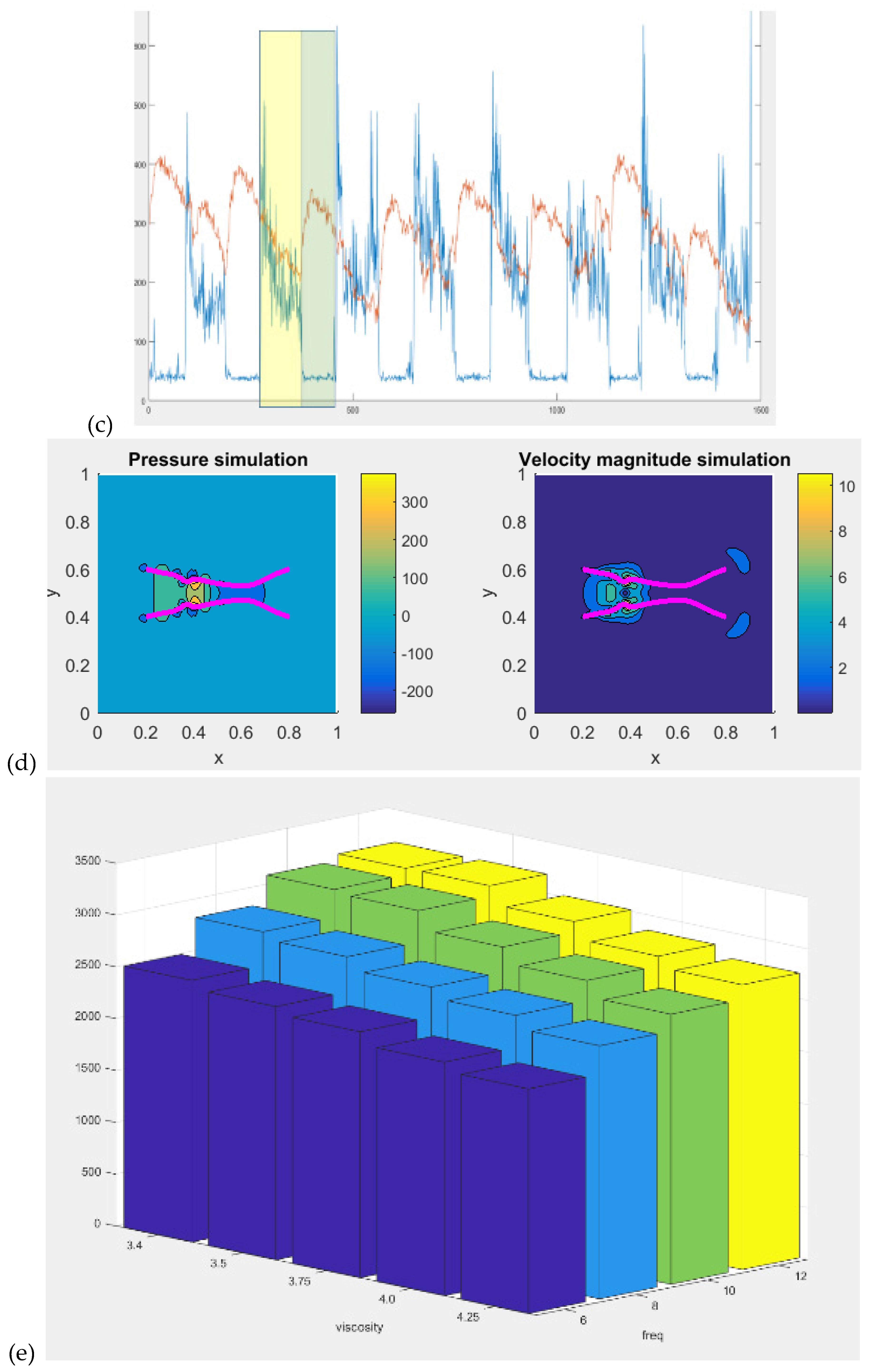
We conducted muscle-fluid-structure interaction simulations to model muscle forces driving flexible structures representing blood vessel walls (Cheung, n.d.). A simplified muscle model combining length-tension and force-velocity profiles was implemented. The immersed boundary (IB) method facilitated fluid-structure interactions with a uniform computational grid. The study focused on the biophysical relationship between different muscle activation frequencies and blood viscosity, affecting blood vessel end velocity. The investigation is relevant to electrical stimulation's impact on femoral vein velocity, crucial for understanding its effects in aging populations. For our IB modeling, we utilize a dynamic viscosity range of 3.4 to 4.25 N*s/m^2, reflecting high shear conditions relevant to muscle stimulation. This choice is justified by simulating venous return during FES-assisted walking, where muscle contraction increases blood flow speed, resembling arterial flow more than venous. Additionally, most research data falls within the 3 to 4.5 viscosity range. Higher shear viscosity remains relatively stable, unlike lower shear viscosity. Parameters like muscle stimulation frequency and total time were adjusted.
Figure 2d displays pressure and velocity during simulated blood vessel contraction waves, and data from the vessel end are saved for analysis. Notably, a decrease in the first peak of blood vessel end velocity is observed with increasing viscosity and decreasing pulsatile frequency – see
Figure 2e. Consequently, to achieve and maintain a specific target blood vessel end velocity (flow rate), adjustments to the FES pulsatile frequency should be tailored to the subject's specific blood viscosity in case of large differences which may be crucial in subjects with T2DM (Sun et al., 2022).
In whole-body exercises, it's crucial to consider the difference in muscle mass recruitment compared to isolated calf contraction (Miller et al., 2005). The addition of quadriceps contraction can minimize respiratory-related fluctuations in limb venous return by increasing total blood inflow, reducing venous vessel compliance, and improving the effectiveness of the calf muscle pump. During whole-body exercise, addition of active expiration to diaphragmatic breathing, even at low workloads, alters the within-breath pattern of blood flow modulation and reduces net femoral venous blood flow (see the Appendix of (Miller et al., 2005)).
Practicality and Feasibility of Individualized Home-Based Exercise Programs: We found (Zhao et al., 2023) a remarkable completion rate (89.14%) observed during the 2-month moderate intensity individualized and progressive exercise program, highlighting its practicality, feasibility, and excellent tolerance, particularly during the COVID-19 pandemic. This is postulated to be successful due to individualization which addressed the metaboreflex limitations (Doneddu et al., 2020) highlighted in the previous section. Participants in the study followed a combined exercise regimen, adhering to guidelines from the American College of Sports Medicine (ACSM). To determine the baseline exercise intensity, we let participants choose a comfortable ankle weight for knee flexion and extension and tested the maximum number for both movements. The program included progressive resistance exercises and brisk walking six days a week. Participants chose one rest day each week and were encouraged to engage in 20-minute sessions of both resistance and aerobic exercises. The resistance exercises focused on major muscle groups, reduced to four movements, and included ankle weights for knee extension and flexion, chair-stand, and heel raises. For the resistance exercises, participants performed 3-4 sets with a 30-second break between sets. The intensity and progression were personalized, and the number of repetitions increased gradually. Walking sessions, considered moderate-intensity exercise, were determined based on heart rate reserve (HRR) calculations and participants' maximum walking speed from the 6 Minute Walking Test. The walking duration ranged from 8-15 minutes per session.
The study incorporated safety measures, including individualized resting times and indoor walking to prevent tripping hazards. Participants received biweekly phone calls to monitor safety, satisfaction, and adherence. The adherence rate for the 2-month exercise program was high, with participants achieving a mean score of 89.14%. The exercise program aimed to enhance or preserve cognitive performance and physical well-being in older adults with T2DM.
Physiological Changes and Brain Response: Examining the Zhao et al. (Zhao et al., 2019) study, we found significant decreases in muscle oxygen saturation during specific tasks and post-intervention changes in prefrontal activation (Zhao et al., 2022). The design of the exercise regimen, tailored to individual exercise tolerance capacities, likely contributed to the effectiveness of the short-duration program. We postulated based on the operational modal analysis of the fNIRS signals (Zhao et al., 2023) that the cerebral effects were partly subserved by the exercise effects on the autonomic nervous system (Hautala et al., 2009). The autonomic nervous system, part of the peripheral nervous system, regulates involuntary physiological processes such as heart rate, blood pressure, respiration, digestion, and sexual arousal (Karemaker, 2017). It comprises three divisions: sympathetic, parasympathetic, and enteric. Both the sympathetic and parasympathetic systems have afferent and efferent fibers for sensory input and motor output to the central nervous system (CNS). Typically, their motor pathways involve a two-neuron series, with a preganglionic neuron originating in the CNS and a postganglionic neuron in the periphery that innervates target tissues. Our study (Zhao et al., 2023) did not find a significant improvement in the muscle oxygenation recovery (Zhao et al., 2019) after the physical tasks, which leads to the postulate on post-exercise ischemia where the sympathetic activity to the heart may stay elevated (O’Leary, 1993). Nevertheless, it is also postulated that the heart rate can decrease due to an upsurge in parasympathetic outflow (O’Leary, 1993). Here, the available evidence (Koep et al., 2022) suggests that cerebral sympathetic nerve activity behaves differently from peripheral circulation, influenced in part by changes in intracranial pressure and cerebral blood volume. Sympathetic activation's effects on CBF are more intricate compared to those in peripheral circulation, varying based on the specific region and receptor involved. The cerebral circulation exhibits unique compensatory responses and interactions with parasympathetic nerve activity. Here, elevated cerebral sympathetic nerve activity may serve the functional purpose of preventing hyperperfusion injury in humans (Koep et al., 2022) that may be dysfunctional in T2DM (Zhao et al., 2023).
We analyzed task-related hemodynamic brain responses suggesting higher cognitive effort and a surge in cerebral blood flow the T2DM subjects (Zhao et al., 2023).
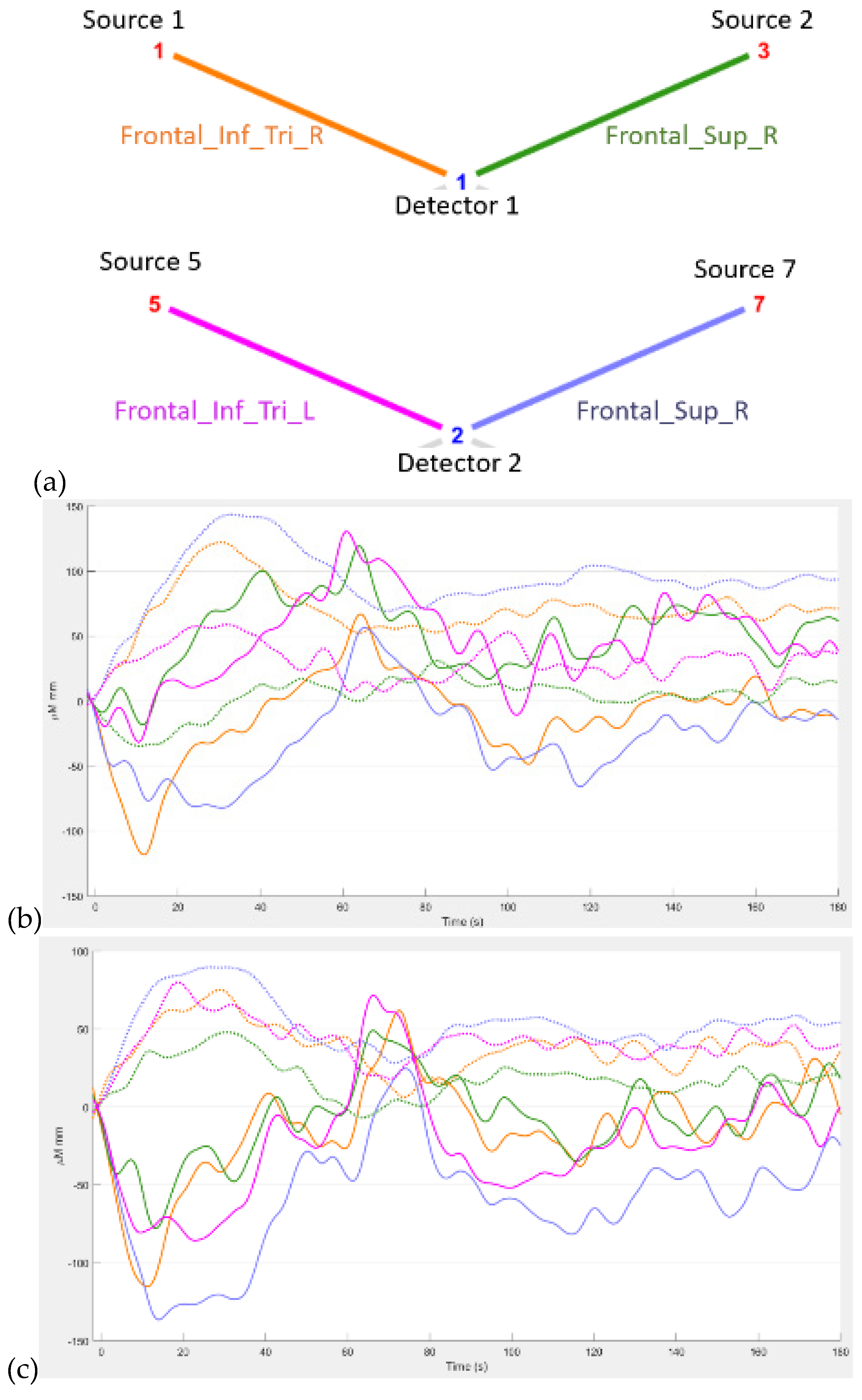
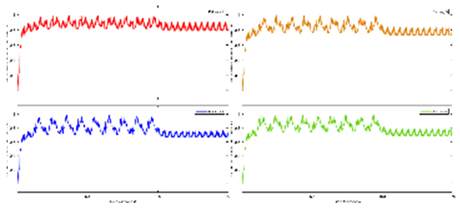
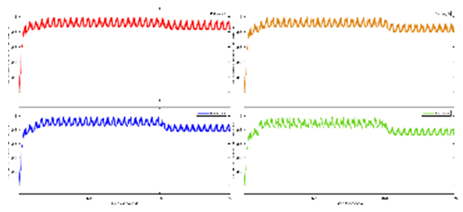
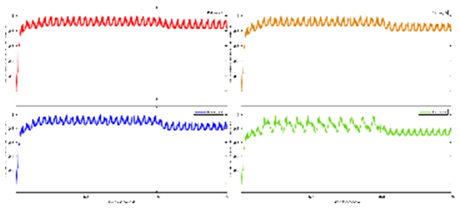
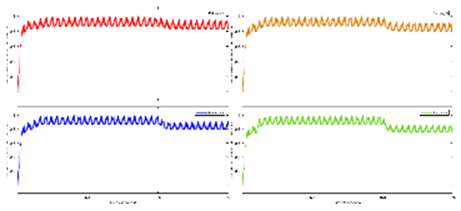
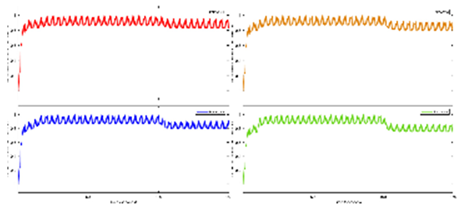
Figure 3 shows the results from our study (Zhao et al., 2023) where the difference between the hemodynamic response functions (HRFs) of the healthy controls and T2DM intervention group illustrates the mental stress (due to cognitive task) related early “surge” in cerebral blood flow in T2DM with solid line showing oxyhemoglobin concentration changes (
Figure 3d,e). In contrast, the healthy controls have an “initial dip” in the cognitive task related oxyhemoglobin concentration changes (
Figure 3c,d). Here, it is postulated that elevated levels of circulating epinephrine in T2DM (Yufu et al., 2014) enhanced the blood pressure responses to mental stress and systemic blood pressure regulation through the arterial baroreflex influencing cerebral blood flow and cerebral function (Ogoh and Tarumi, 2019). Then, our exercise regimen resulted in a reduced “surge” in oxyhemoglobin concentration changes (Figures 3e versus 3d) during medium-difficulty cognitive tasks, indicating a positive effect on cognitive function and possibly better systemic blood pressure regulation through the arterial baroreflex. Also, our results provided evidence of pathological changes in the brain among older adults with T2DM reflected in the very low frequency hemodynamic oscillations (Zhao et al., 2022) that can be linked to neural sympathetic and metabolic activity (Saleem et al., 2016). Indeed, a published study (Saleem et al., 2016) showed that sympathetic activity significantly influences very-low-frequency fluctuations in cerebral blood flow. Saleem and colleagues (Saleem et al., 2016) investigated three-way interaction effects between CBF, blood pressure and end-tidal CO2 (PetCO2) using wavelet phase synchronization index (PSI) change scores that were postulated to indicate sympathetic blockade treatment response varied based on frequency and whether PSI values were corrected for PetCO2. Here, they found that sympathetic blockade increased PSI for frequency components ≤0.03 Hz while placebo administration did not impact PSI values. The results suggested that very-low-frequency CBF dynamics involve nonlinear and nonstationary interactions between blood pressure and PetCO2, as well as frequency-dependent interplay with the sympathetic nervous system. Moreover, very-low-frequency fluctuations in CBF can be linked to cerebrospinal fluid pulsatility (Gruszecki et al., 2018). While similar studies have been conducted in frail older adults and those with mild cognitive impairment (Ogoh and Tarumi, 2019), our research (Zhao et al., 2022) focused on older adults with T2DM. Regarding cognitive performance, our research (Zhao et al., 2022) also compared brain activation between T2DM and healthy older adults during cognitive tasks. Brain overactivation was observed in older adults with T2DM and the choice of cognitive tests influenced the brain regions exhibiting overactivation (Zhao et al., 2022). The study highlighted the potential compensatory role of brain overactivation and the positive impact of exercise on mitigating these effects. Understanding brain mechanisms during cognitive tasks enhances the knowledge of exercise interventions for cognitive health.
In summary, our research (Zhao et al., 2022) demonstrated the beneficial effects of a 2-month individualized progressive exercise program on cognitive function and muscular oxidative capacity in older adults with T2DM. The findings contribute to the understanding of moderate intensity exercise interventions for cognitive health and highlight the importance of individualized exercise regimens to address the metaboreflex related reduced exercise tolerance in T2DM (Kim et al., 2015),(Nesti et al., 2020). We also highlight the potential for monitoring intervention effects and personalizing exercise regimens based on individual brain responses, paving the way for more targeted and effective exercise interventions.
Proposed non-invasive electrical stimulation for priming cerebral hemodynamics (by Yashika Arora, Marcel Stefanski, Anirban Dutta)
In the systematic review by Machado and colleagues (Machado et al., 2019) and meta-analysis of 22 studies involving 393 participants, the effects of transcranial Direct Current Stimulation (tDCS) on exercise performance were examined. Weak evidence suggested a significant positive effect of anodal tDCS (a-tDCS) over the motor cortex (M1) on time to exhaustion (TTE) in cycling, but results were influenced by a single study. No significant effects were found for cathodal tDCS (c-tDCS) on TTE. For isometric muscle strength, no significant effects were observed for a-tDCS applied before or during exercise. Mixed results were reported for isokinetic muscle strength. A quantitative synthesis indicated a significant improvement in cycling performance with a-tDCS over M1, but caution is advised due to the influence of a single study. Commercial tDCS devices for exercise performance were not addressed in peer-reviewed studies, raising safety and efficacy concerns. Methodological aspects, including individual variability and optimal tDCS parameters, need further exploration in future research.
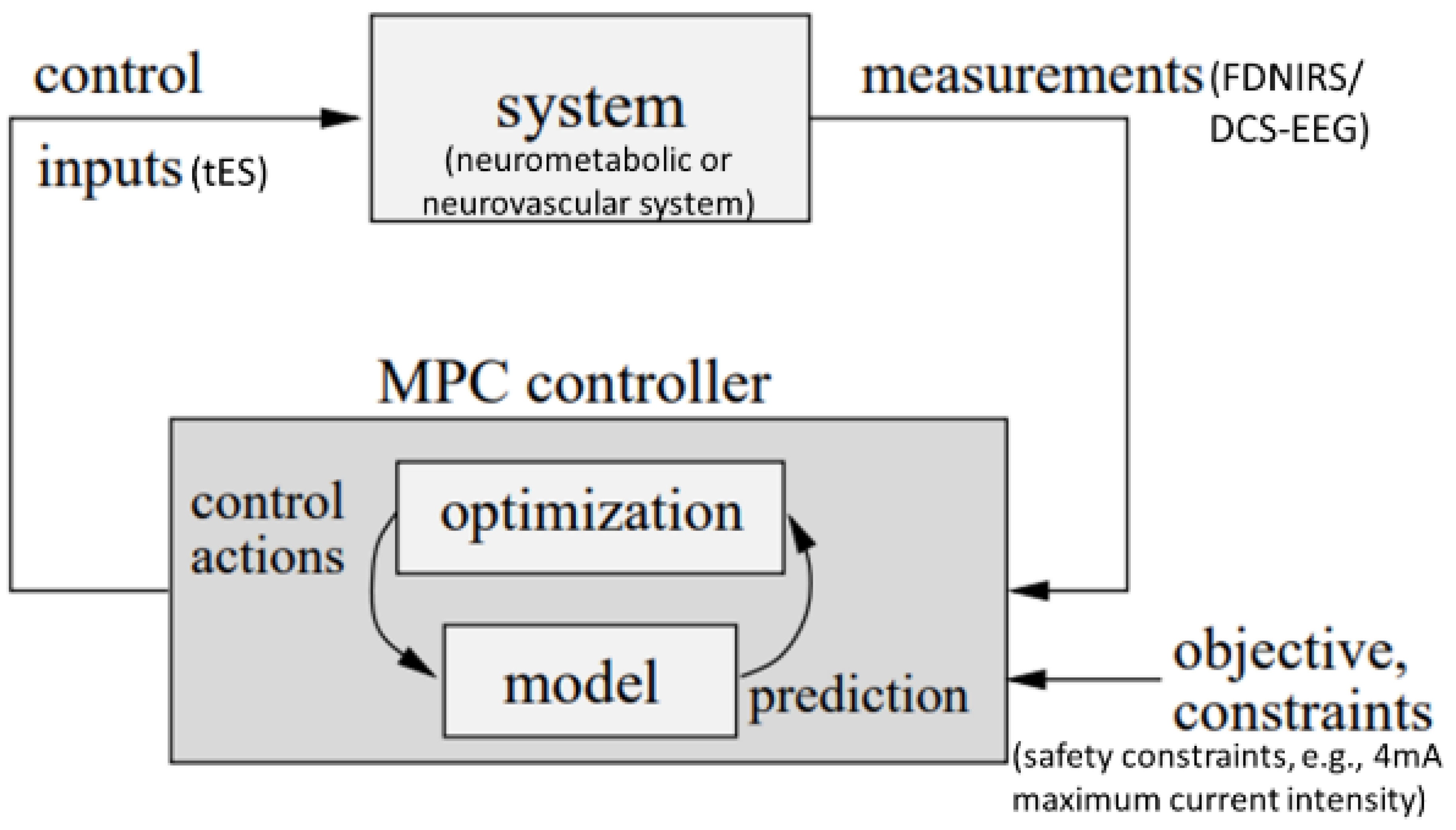
In our opinion, transcranial electrical stimulation (tES) can improve exercise performance when individually customized for priming hemodynamic response (Dutta, 2015),(Guhathakurta and Dutta, 2016),(Arora et al., 2021),(Arora and Dutta, 2022) to address detrimental cerebral effects of metaboreflex (Roberto et al., 2019),(Doneddu et al., 2020) during exercise interventions. Then, neuromuscular electrical stimulation can also have beneficial cerebral hemodynamic effect (Dutta et al., 2021) during exercise interventions. Different forms of tES have varying current profiles. For tES, transcranial direct current stimulation (tDCS) uses a monophasic, non-oscillating baseline, while transcranial alternating current stimulation (tACS) involves rhythmically reversing the electron flow. Other methods include transcranial oscillating current stimulation (tOCS), using a direct component to guide oscillations, and transcranial random noise stimulation (tRNS), injecting alternating current with bounded stochasticity (Guleyupoglu et al., 2013),(Paulus, 2011). Neurovascular modulation occurs in various stimulation protocols, with mechanisms not fully understood. As tES affects blood vessels through neuronal or non-neuronal cells, a deeper understanding of signaling pathways is crucial. tACS, unique in manipulating intrinsic oscillations, may hold promise for treating vascular dementia (Antal and Paulus, 2013),(Helfrich et al., 2014) when applied with a human in the loop approach (Arora and Dutta, 2022) – see
Figure 4.
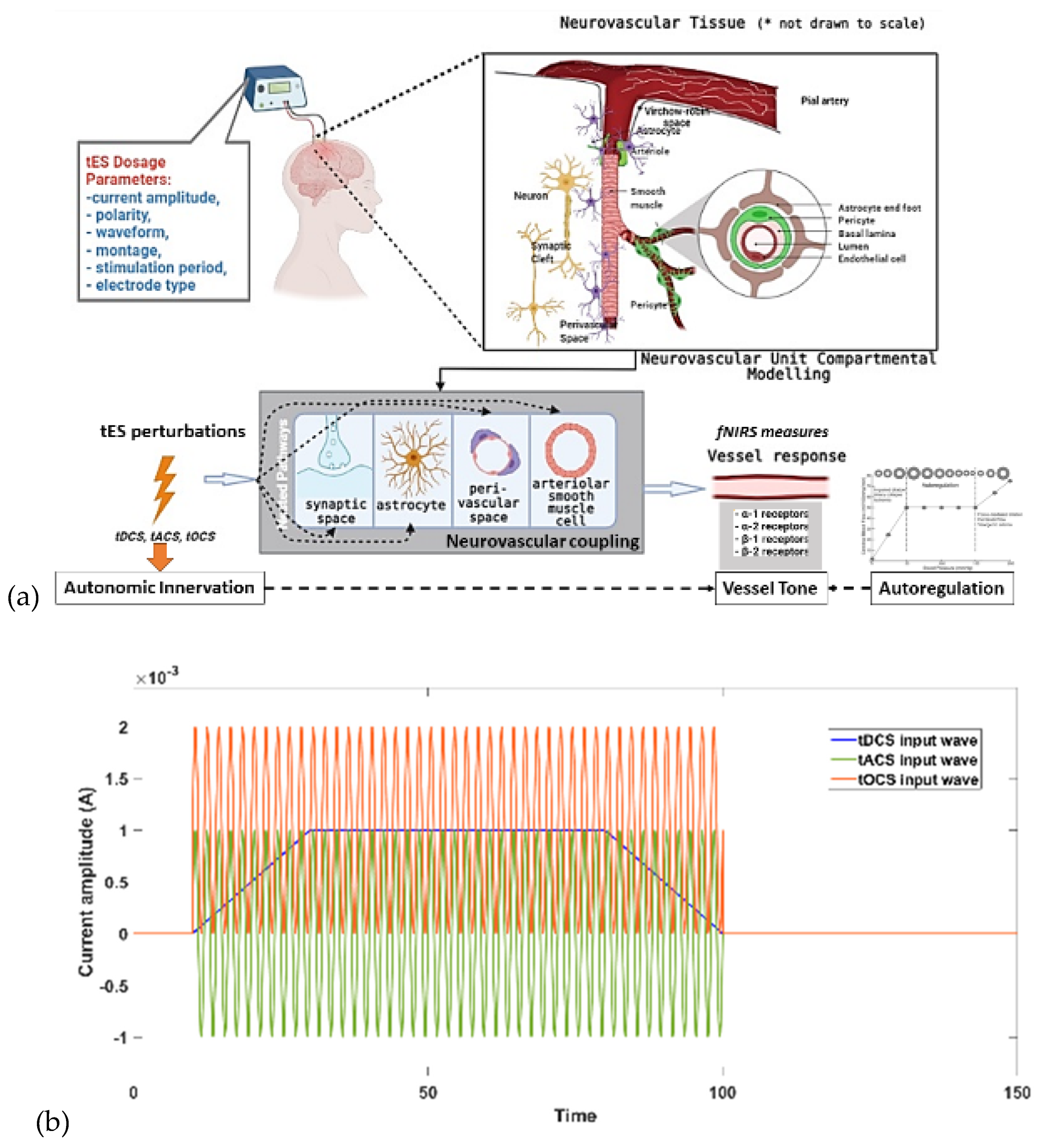
In this chapter, we present our physiological modelling approach (Arora et al., 2021),(Arora and Dutta, 2022) based on the physiology of neurovascular tissues for assessing the vascular response to electric fields generated by tES through various pathways in neurovascular unit (NVU). Our studies (Arora et al., 2021),(Arora and Dutta, 2022) presented a physiological model that incorporated the NVU components, including vascular smooth muscle, perivascular space, synaptic space, and astrocyte cell. The model aimed to capture the effects of transcranial electrical stimulation (tES), specifically transcranial alternating current stimulation (tACS), on direct and indirect vascular responses. Four nested NVU compartmental pathways were proposed, allowing the simulation of tES-induced vessel volume responses.
The tES current density, acting as an input pulse, perturbed state variables in each NVU compartment. The study considered four simulated pathways for vessel response modulation: synaptic potassium, astrocytic membrane potential, perivascular potassium currents, and voltage-gated ion channels on smooth muscle cells. These pathways were designed to simulate vessel oscillations within the frequency range <0.2 Hz, controlled by nonlinear calcium dynamics. Modal analysis (Rogers, 2000),(Modal Analysis - 1st Edition, n.d.), a technique commonly used in structural and fluid mechanics (Modal Analysis for Damage Detection in Structures | Semantic Scholar, n.d.), was applied to derive the characteristic dynamics of the NVU model. Modal analysis involves determining the system's natural frequencies, mode shapes, and damping factors, allowing the development of a mathematical model describing the system's behaviour. While modal analysis is traditionally used in engineering fields, the study applied this approach to analyse the NVU model, specifically focusing on evaluating neurovascular coupling modes induced by tACS. Then, the design of controls is imperative to modify the natural behaviour of interconnected synchronous generators in NVU systems. Despite the inherent nonlinearity of NVU systems, accurately predicting oscillations around an operating point is possible through linearized system models (Arora et al., 2021). This justifies the application of linear control theory for the design of oscillation controls (Rogers, 2000).
fNIRS of transcranial electrical stimulation effects on hemodynamics: The examination of transcranial electrical stimulation (tES) effects, both immediate and prolonged, is an area of interest of our neuroscientific research (Arora et al., 2021),(Dutta et al., 2015),(Sood et al., 2016). We examined an fMRI-tES dataset (Arora and Dutta, 2023) with a TR of 3.36 seconds, revealing similar finite impulse response hemodynamic response function “FIR HRF” model (with RH, TTP, and FWHM using the rsHRF toolbox (Wu et al., 2021)) in anodal and sham tDCS conditions at the electrode locations FC5 and PZ ROIs, but differing in the electrode location FP2 ROI. This discrepancy may be linked to local cortical inhibitory circuits, perivascular nerves, and astrocyte stimulation (Arora and Dutta, 2023). Here, our prior computational analyses proposed direct perivascular nerve and astrocyte stimulation during tDCS onset (Arora et al., 2021),(Arora and Dutta, 2022). Our prior findings also indicate perivascular space changes (
https://www.ismrm.org/workshops/2022/Neuromodulation/program.php), that may also raise safety concerns for higher intensity 4 mA stimulation especially in pathological tissues. Then, tES is postulated to also impact blood-brain barrier permeability, influencing neuronal function (Shin et al., 2020), that also raises safety concerns. Then, transcranial direct current stimulation (tDCS) and transcranial alternating current stimulation (tACS) differ in current profiles, with different therapeutic and safety implications. Indeed, short-duration tDCS can have physiological effects, impacting autonomic and hemodynamic response (Arora and Dutta, 2022), which may have better therapeutic implications than longer duration. Here, we suggest short duration ON-OFF tDCS time series (see
Figure 1 in (Dutta, 2015)) acting as slow transcranial oscillating-current stimulation (tOCS) (Arora and Dutta, 2022) may act through superficial nerves, noradrenergic axons, and efferent innervation to evoke beneficial hemodynamic response. Then, sympathoexcitation, reflected in pupil dilation (Arora and Dutta, 2022), may impact glucose regulation via noradrenaline's impact on cellular energy processes (Hertz et al., 2010). However, the correlation between fNIRS total haemoglobin (blood volume) changes and pupil dilation (Arora and Dutta, 2022) needs further investigation since pupil diameter has been shown to be inversely correlated with cortical hemodynamics during rest (and non-REM sleep) while when the mouse is alert, moving, or stimulated (as well as during REM sleep) positive correlations were found between pupil diameter and blood volume. Therefore, we postulate that the correlation analysis of pupil dilation with fNIRS total haemoglobin (blood volume) changes can provide insights into the LC-NA related effects of tES (Vanneste et al., 2020). Also, a mechanistic understanding of glucose-neurovascular tissue interaction during tES is crucial (Arora and Dutta, 2022). Here, tES sympathoexcitation may affect smooth muscle cells, particularly in arterioles with specific oscillatory frequencies, which is amenable to frequency domain modal analysis (Arora and Dutta, 2022).
Understanding the modulatory effects of tES on blood vessels necessitates exploring multiple pathways within the neurovascular unit (NVU). Unravelling these signalling pathways is crucial for comprehending tES effects on neurons and blood vessels to developing therapeutics, as discussed by Arora et al. (Arora et al., 2021) – see
Figure 5a. The stimulation evoked cerebral blood flow (CBF) changes depends on multiple pathways including sympathetic vascular tone (stimulus related norepinephrine released from sympathetic efferent nerves modulates vascular tone (Sheng and Zhu, 2018)) against which the neurovascular coupling need to act to dilate the blood vessels (Arora and Dutta, 2022). It has been shown that long-term administration of the noradrenaline (norepinephrine) reuptake inhibitor reboxetine (RBX) extended the effects of anodal tDCS on long-term potentiation-like plasticity for over 24 hours (Kuo et al., 2017). It also transformed cathodal tDCS-induced long-term depression-like plasticity into facilitation for 120 minutes. Here, tES effects on autonomic innervation (Arora and Dutta, 2022) and activation of noradrenergic receptors can have stimulatory effects on both energy-requiring and energy-yielding processes (Hertz et al., 2010). Specifically, tES electric field distribution (Khadka and Bikson, 2020) and its activating function (Arora and Dutta, 2022) can affect the three nerve arrangements in the human cerebral arteries (Bleys et al., 1996): (a) paravascular nerve bundles outside the tunica adventitia; (b) a meshwork-like perivascular plexus in the outer or middle adventitial zone; and (c) a deep intrinsic perivascular plexus at the adventitial–medial border, oriented transversely. Then, different subtypes of adrenoceptors often activate distinct processes, and the stimulation can allow for simultaneous enhancement of oxidative metabolism and/or glycogenolysis, e.g., in T2DM (Arora and Dutta, 2022). Unlike classical mechanisms, these effects may enable the stimulation of energy metabolism without preceding decreases in ATP. Stimulation of glycogenolysis is noteworthy, as it is considered an integral part of glucose breakdown via a significant 'glucose-glycogen shunt.' While an increase in mitochondrial Ca2+ has been observed in astrocytes, the direct stimulation of oxidative metabolism by elevated intracellular calcium has been extensively studied in muscle and liver. This includes the direct stimulation of mitochondrial dehydrogenases and oxidative phosphorylation, contributing to the understanding of noradrenaline's impact on cellular energy processes (Hertz et al., 2010). Indeed, neurovascular coupling itself may be modulated (Dutta, 2021a) since noradrenaline release from locus coeruleus axons induces vessel tone in arteriolar smooth muscle and contractile capillary pericytes (Korte et al., 2023). This vessel tone enables neuronal activity to trigger vasodilation, enhancing local cerebral blood flow – see
Figure 5a. In the brain, a significant portion of vascular resistance is in capillaries, and locus coeruleus axons release noradrenaline closer to pericytes than to arterioles. Then, the cerebral adrenoreceptor distribution exhibits heterogeneity, indicating region-specific autonomic regulation of CBF (Koep et al., 2022). The cerebral circulation features unique compensatory responses, involving chemo- and autoregulatory mechanisms, and interactions with parasympathetic nerve activity. This interplay between sympathetic and parasympathetic reflexes ensures optimal perfusion of CBF in response to changing perfusion pressures, aiming to optimize oxygen and nutrient delivery to the brain while maintaining blood volume and intracranial pressure. Here, tES effects on the autonomic innervation of the cranial circulation can include a predominant sympathetic component from the superior cervical ganglion and a cranial parasympathetic component that passes through the pterygopalatine (sphenopalatine) and otic ganglion. Then, Claassen and colleagues (Claassen et al., 2021) discusses mechanisms contributing to myogenic responses, focusing on the regulation of vascular tone, intravascular pressure distribution in the brain, and autoregulation of CBF. Key factors include the sensitivity of vascular diameter to changes in cellular membrane potential, influenced by voltage-dependent calcium channels (e.g., CaV2.1) and large conductance potassium channels (BKCa). Mechanotransducers are proposed as sensors for pressure changes, leading to depolarization of vascular muscle and increased intracellular Ca2+. This, in turn, activates contractile proteins, resulting in vasoconstriction. Local release of Ca2+ sparks can activate BKCa, inducing hyperpolarization and limiting vasoconstriction. Therefore, in our opinion, modulation of the vessel tone needs to well-coordinated with the neurovascular coupling related effects for adequate CBF response to task and stimulation related metabolic needs (which may be compromised in diabetes (Zhao et al., 2023)) that may be facilitated with tES – see
Figure 5a.
The major factors involved in the design of tES dosage are: current amplitude, waveform, polarity, duration, montage and electrode specifications (Knotkova et al., 2019) as depicted in
Figure 5(b). These factors are crucial in neuromodulating specific characteristics. For instance, tOCS has been shown to facilitate corticospinal excitability phase-independently both on and off-line, similar to tDCS (Bergmann et al., 2009). Meanwhile, tACS was more likely to entrain neuronal activity while blocking sensory input (Vieira et al., 2020). To comprehend the mechanistic aspects of tES techniques on hemodynamics, we used our mathematical model (Arora et al., 2021) based on the physiology of neurovascular tissue for evaluating the vascular response through various paths that are susceptible to the electric fields generated by tES as shown in
Figure 5(a). Our simulation model was constructed with four compartments, drawing from existing literature: synaptic space, astrocyte space, perivascular space, and arteriole smooth muscle cell space. To simulate the vessel volume response within the physiological model, we designed four nested neurovascular unit (NVU) compartmental pathways. Each state variable in these pathways could be influenced by the transcranial electrical stimulation (tES) current density, acting as the input pulse. The simulations of the model considered different tES-induced perturbations: synaptic potassium release from active neurons for Pathway 1, astrocytic transmembrane current for Pathway 2, perivascular potassium concentration for Pathway 3, and voltage-gated ion channel current on the smooth muscle cells (SMC) for Pathway 4. Detailed information regarding the implementation and analysis can be found in our publication (Arora et al., 2021).
Physiologically detailed models published earlier (Arora et al., 2021) were executed using the 'ode23tb' solver in Simulink (MathWorks, Inc., USA). These models simulated oscillations ranging from 0 to 0.2 Hz, generated by nonlinear calcium dynamics in response to transcranial direct current stimulation (tDCS) perturbations. Subsequently, we subjected the four nested neurovascular unit (NVU) compartmental pathways to perturbations from transcranial oscillating current stimulation (tOCS), tDCS, and transcranial alternating current stimulation (tACS) at varying frequencies (0.1 Hz to 10 Hz), conducting a sensitivity analysis for blood vessel diameter changes. Model simulations for different transcranial electrical stimulation (tES) pulses are available in supplementary material. This comprehensive approach considered the vascular effects of tES, incorporating both neuronal and non-neuronal mechanisms with distinct sensitivity levels. Notably, within the frequency range of 0.1 Hz to 10 Hz, we observed that vessel oscillations exhibited greater sensitivity to tOCS than to tACS, and entrainment effects were more pronounced at lower frequencies. Subsequently, modal analysis was performed on the physiological model, with detailed results presented in the following section.
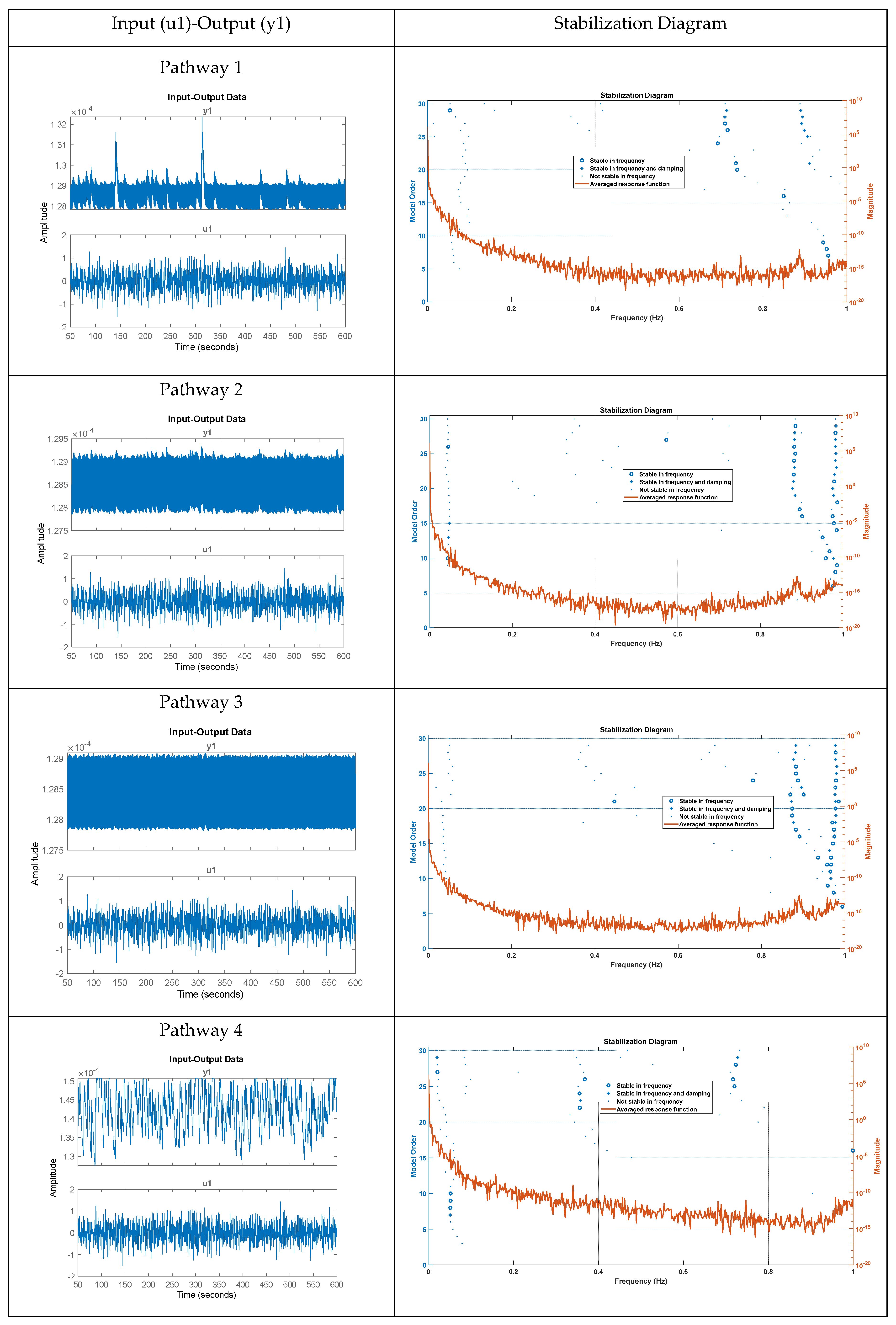
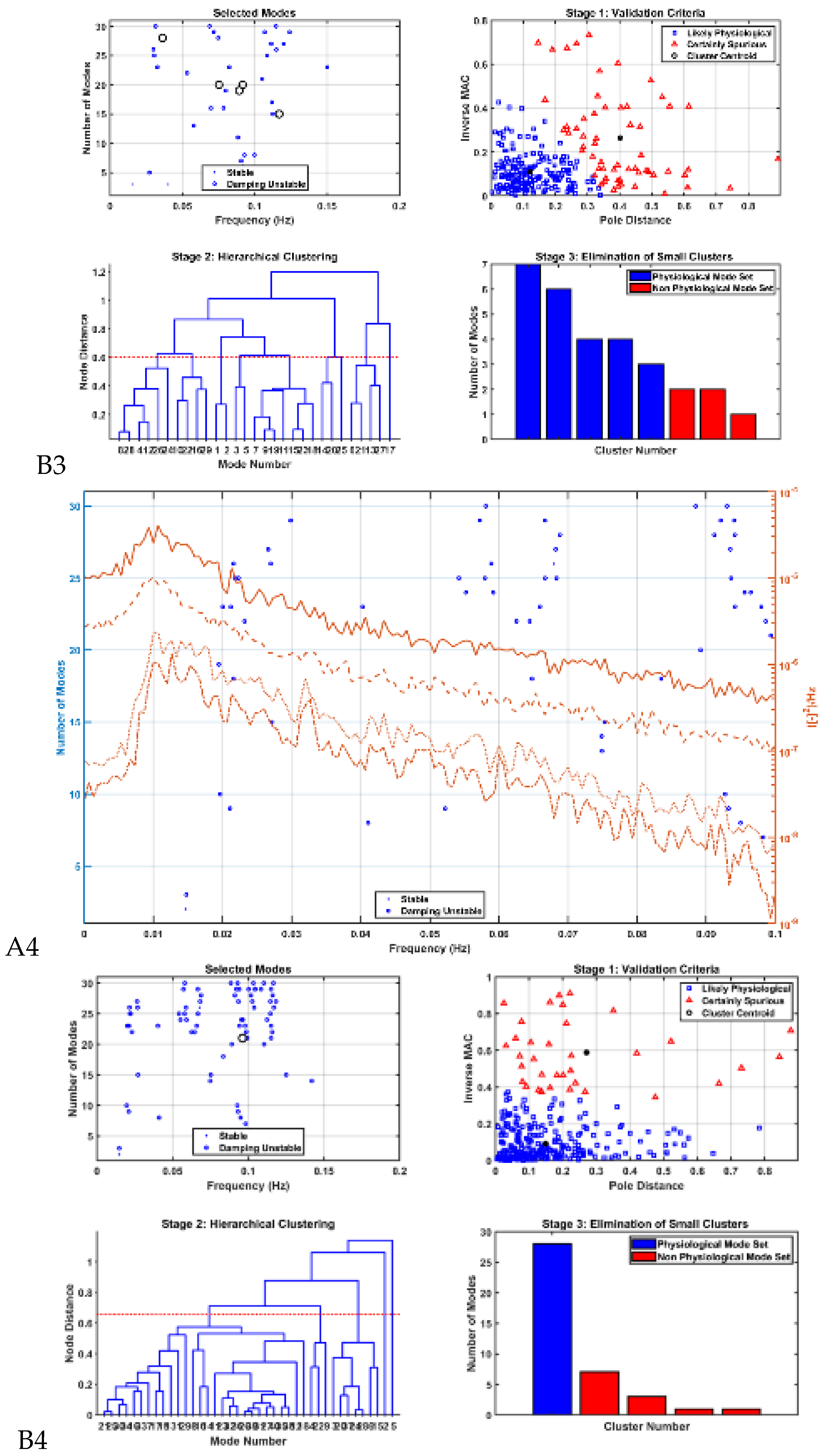
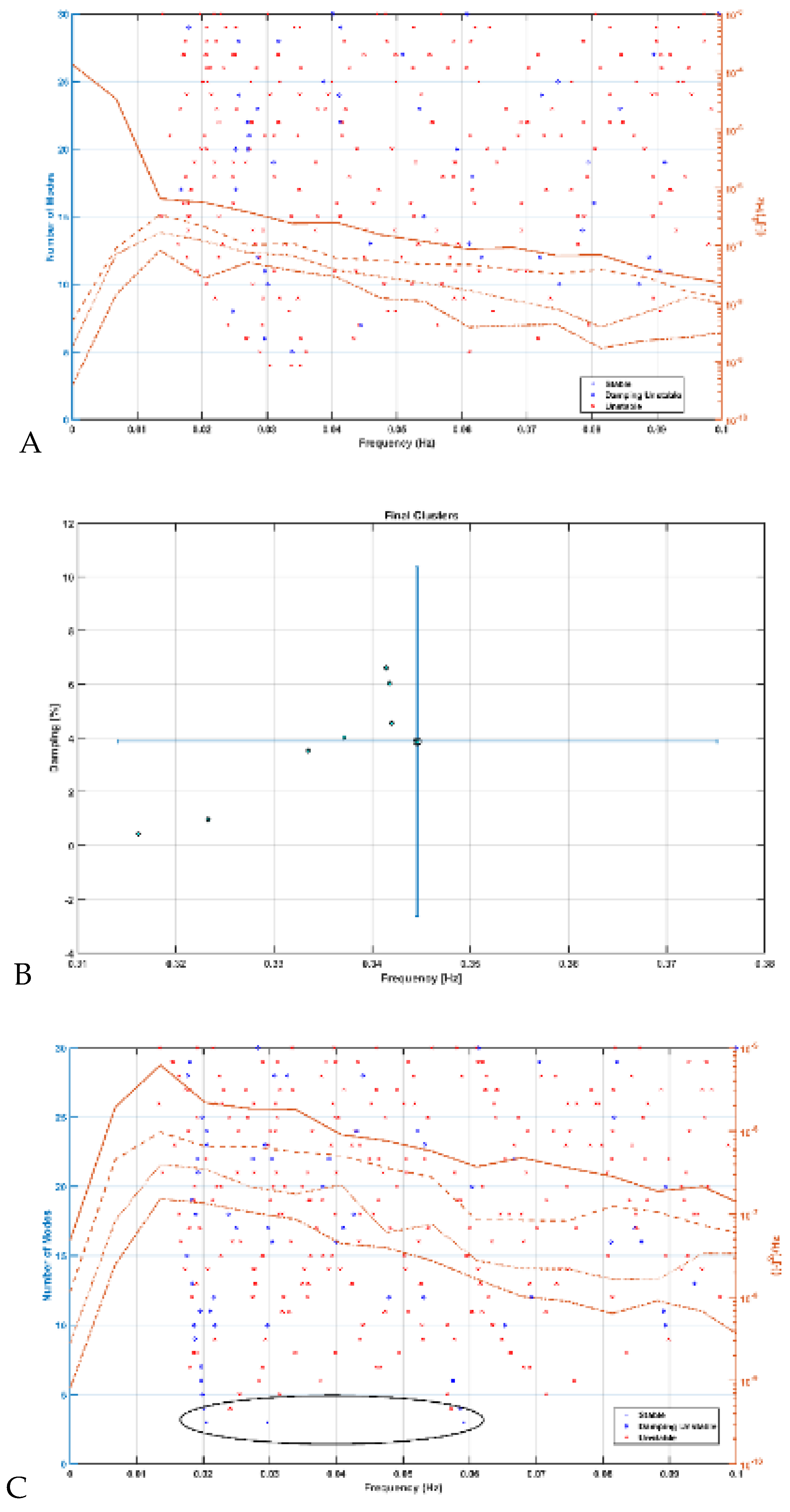
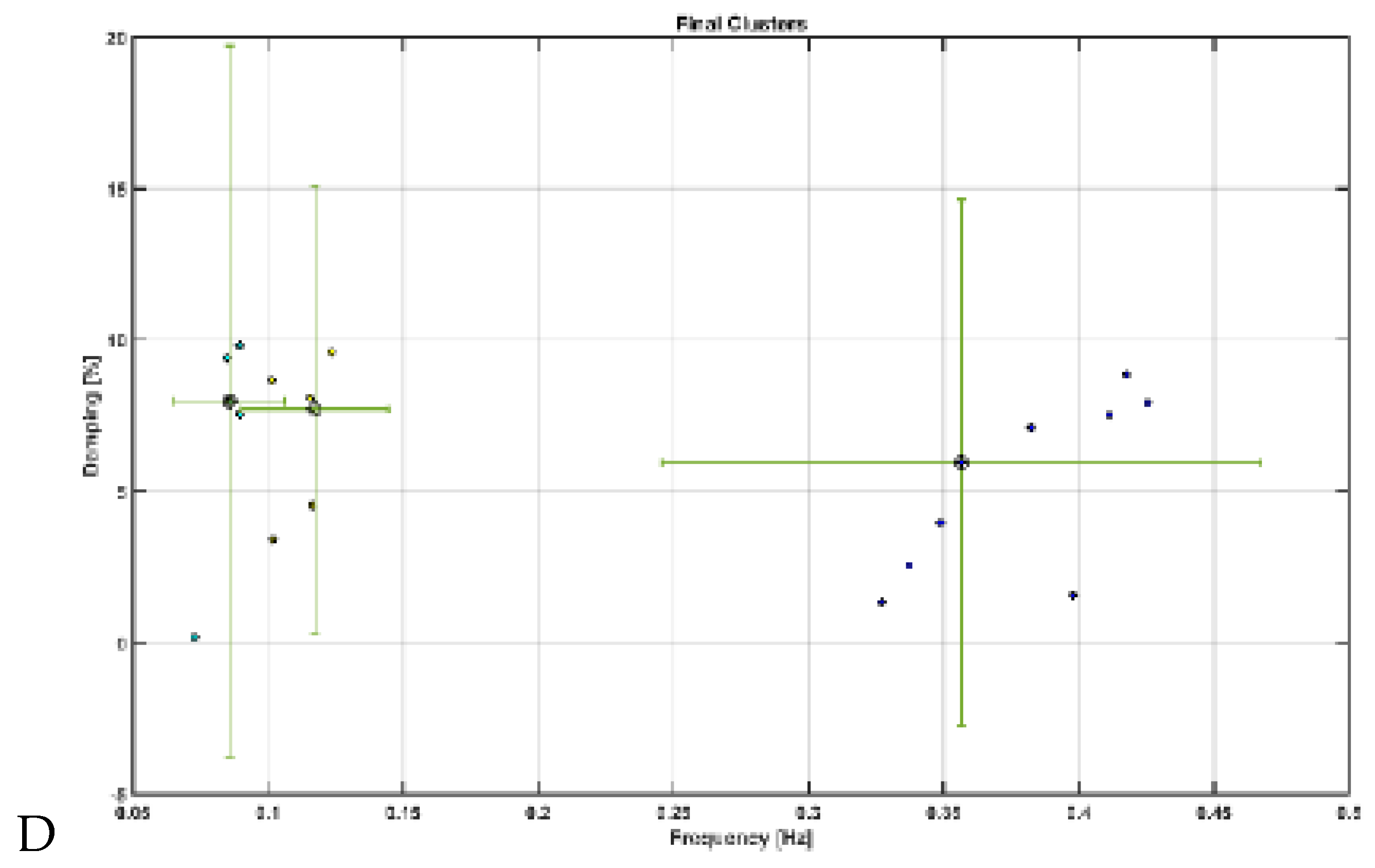
Modal analysis of transcranial electrical stimulation effects on hemodynamics: We applied ten random transcranial electrical stimulation (tES) perturbations, utilizing bandpass filtered (0.01-1 Hz) white noise inputs, to the four implemented physiologically constrained models. The input and output time series were recorded using the time-domain data object ('iddata' in MATLAB, MathWorks, Inc., USA). For modal analysis, we focused on the oscillatory component of the vessel response, excluding the initial 50 seconds of time series data. Modal analysis functions, including 'modalfrf,' 'modalfit,' and 'modalsd,' were applied to the data object to generate frequency-response functions, natural frequencies, and stabilization diagrams, respectively. The natural frequencies of the four system modes, determined from the measured frequency-response functions (frf) at frequencies (f) and a sample rate of 10 samples per second, were calculated using the peak-picking method. The linear model of the four physiologically detailed tES perturbation pathways was established using the Model Linearizer tool in Simulink (MathWorks, Inc., USA) linear analysis package. The damping ratio, natural frequency, and time constant of the poles were derived using the 'damp' function on the linear model system.
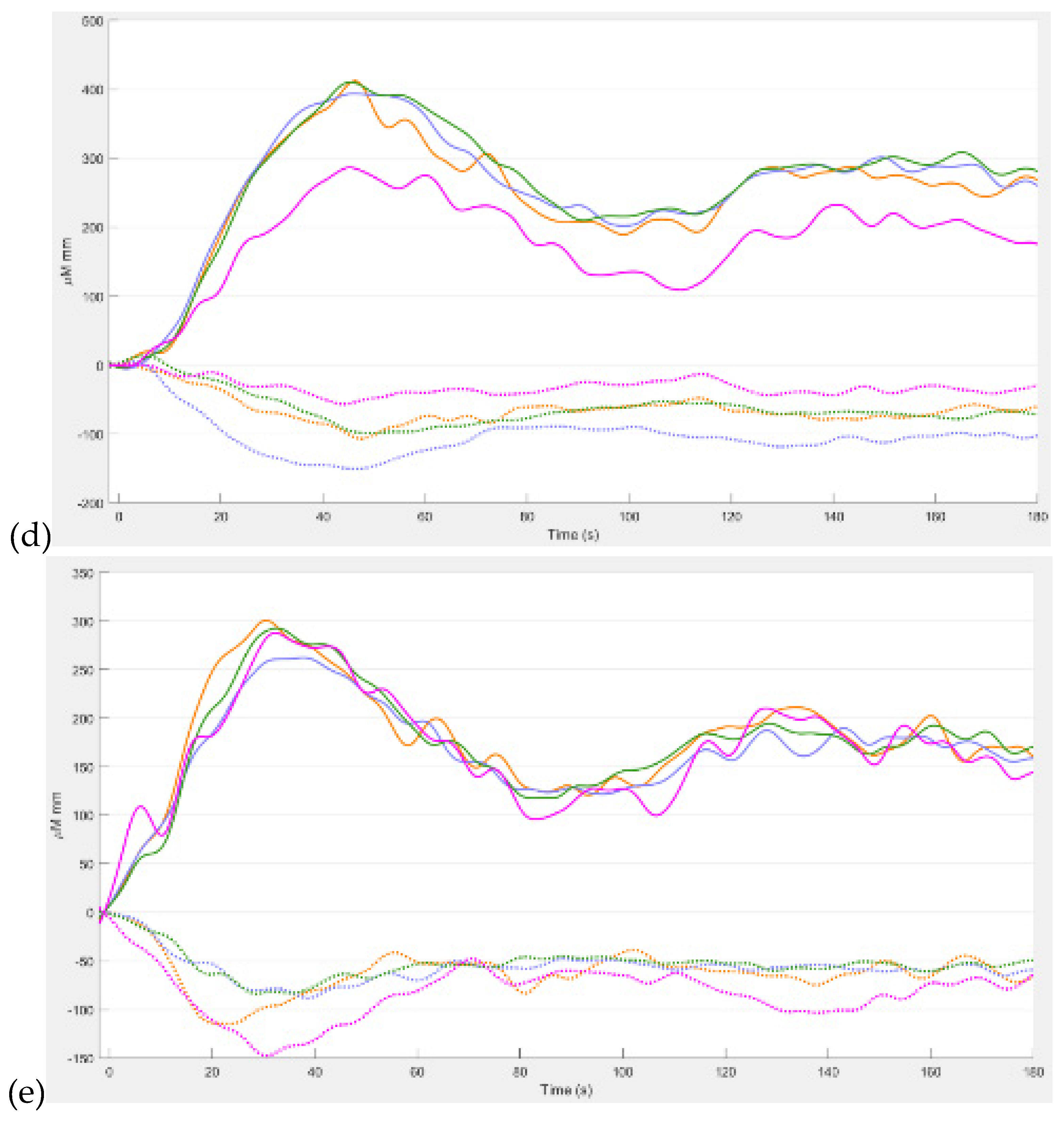
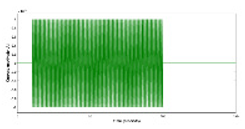
The input of transcranial electrical stimulation (tES) and the corresponding vessel responses for the proposed pathways are detailed in
Table 1. We subjected the four nested neurovascular unit (NVU) compartmental pathways to tOCS (combined tDCS and tACS) perturbations with varying frequencies (ranging from 0.1 Hz to 10 Hz) and direct current (DC) offsets (ranging from 0 to 2 mA). Subsequently, we conducted a sensitivity analysis to assess changes in blood vessel circumference.
We considered three model input waveforms as
In equation 1, we considered a sinusoidal amplitude (a) of 1 mA, a sinusoidal frequency (f) ranging from 0.1 to 10 Hz, and a DC offset (c) ranging from 0 to 2 times the amplitude for conducting a sensitivity analysis on the physiologically detailed mathematical model of the neurovascular unit (NVU). This analysis, facilitated by the Sensitivity Analyzer tool in MATLAB Simulink (MathWorks, Inc., USA), enabled the exploration of the transcranial electrical stimulation (tES) design space, identifying the most influential model parameters.
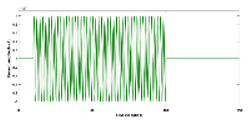
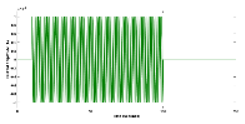
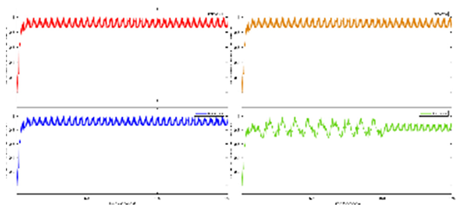
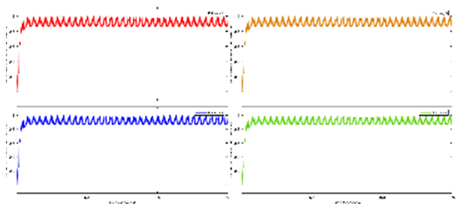
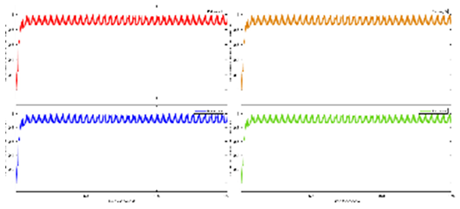
Figure 6 illustrates the impact of frequency and DC offset on the vessel response.
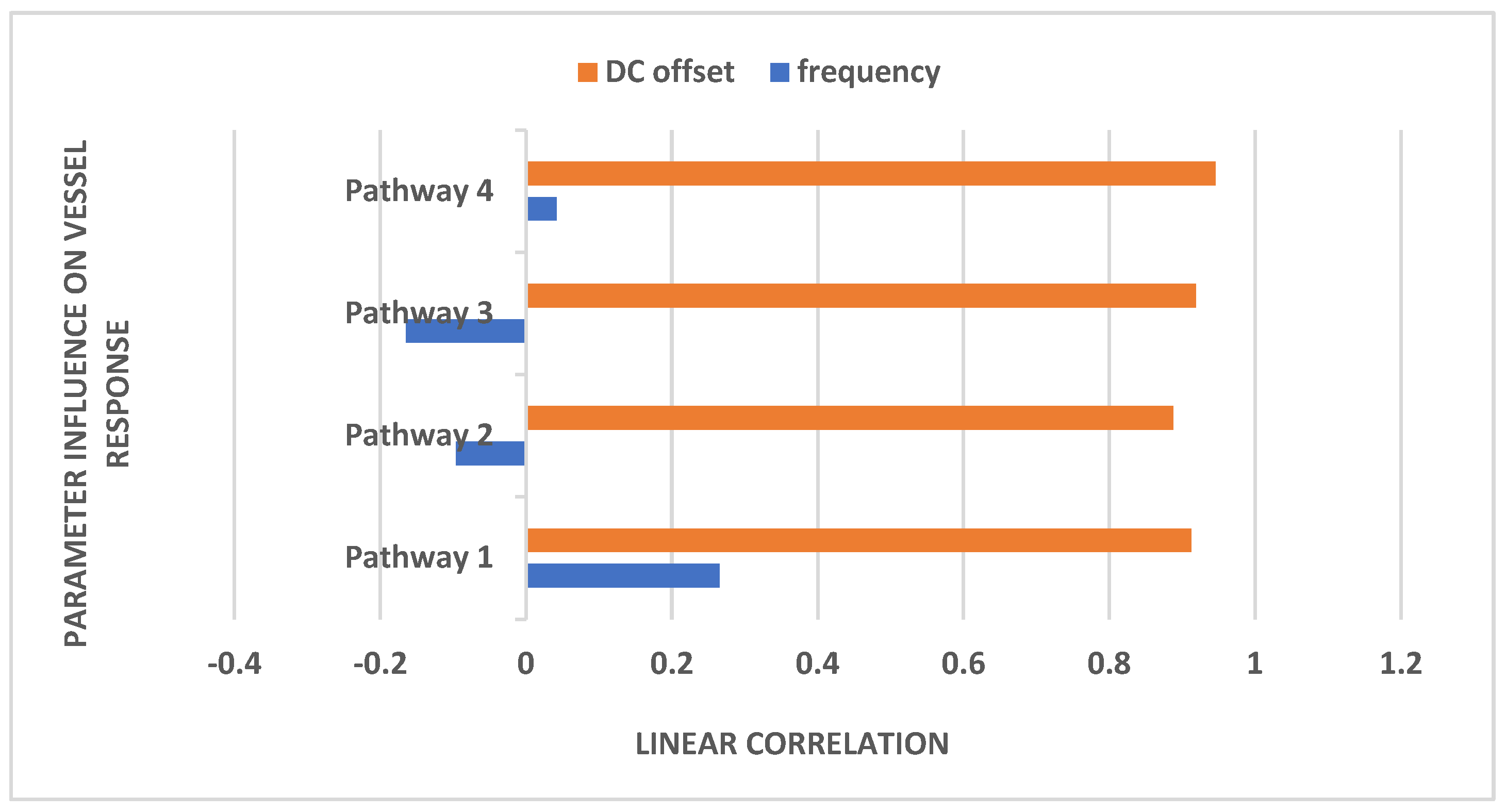
Mechanistic Insights: Table 2 presents the natural frequencies derived through modal analysis for the non-linear models of four physiologically detailed transcranial electrical stimulation (tES) perturbation pathways.
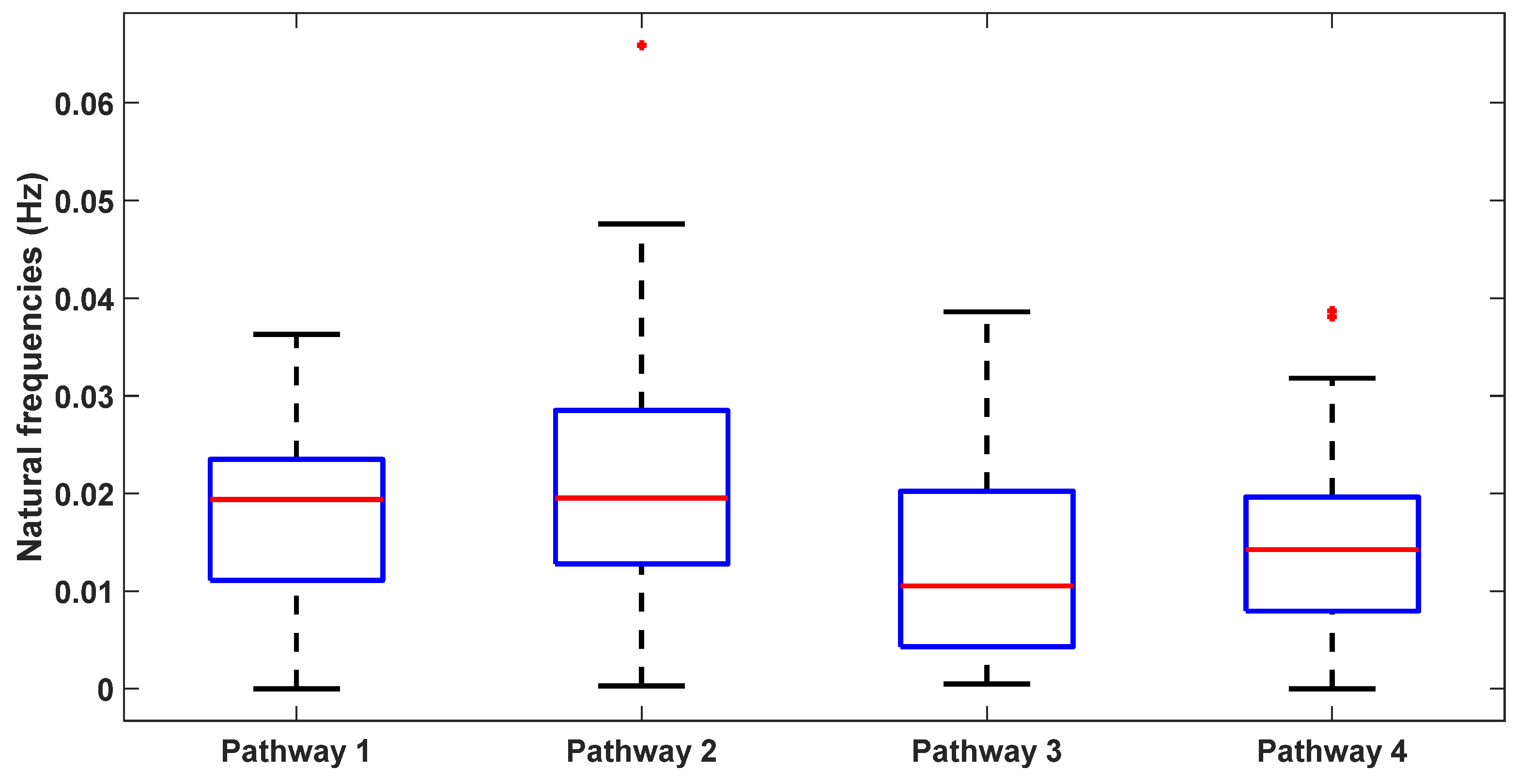
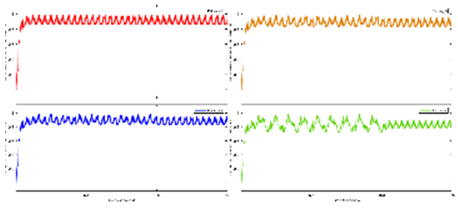
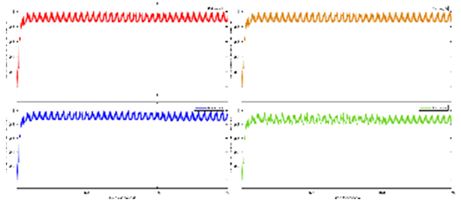
Figure 7 illustrates a boxplot representation of these frequencies. Additionally,
Figure 8 displays the stabilization diagram obtained for the first case, where the model input was bandpass filtered white noise with the default seed value, for all four pathways. Then,
Table 2 lists the system parameters associated with linearized model for the four pathways as follows. Pathway 1 involves tES perturbing the vessel response through the synaptic potassium pathway, Pathway 2 through the astrocytic pathway, Pathway 3 through the perivascular potassium pathway, and Pathway 4 through the smooth muscle cell (SMC) pathway.