Submitted:
25 December 2023
Posted:
26 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Detection and screening based on ultraviolet spectroscopy
3. Detection and screening based on visible light spectrum
4. Detection and screening based on fluorescence spectroscopy
4.1. Detection and screening based on conventional strategies of fluorescence spectroscopy
4.2. Detection and screening based on fluorescence spectroscopy using biosensors
5. Detection screening based on other technologies
6. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moragues T, Arguijo D, Beneyton T, Modavi C, Simutis K, Abate AR, et al. Droplet-based microfluidics. Nature Reviews Methods Primers. 2023, 3, 32. [Google Scholar] [CrossRef]
- Chiu, F.; Stavrakis, S. High-throughput droplet-based microfluidics for directed evolution of enzymes. Electrophoresis. 2019, 40. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yang, G.; Ma, F. Fluorescence coupling strategies in fluorescence-activated droplet sorting (FADS) for ultrahigh-throughput screening of enzymes, metabolites, and antibodies. Biotechnology advances. 2023, 66, 108173. [Google Scholar] [CrossRef] [PubMed]
- Muretta JM, Rajasekaran D, Blat Y, Little S, Myers M, Nair C, et al. HTS driven by fluorescence lifetime detection of FRET identifies activators and inhibitors of cardiac myosin. SLAS discovery : advancing life sciences R & D. 2023, 28, 223–232. [Google Scholar] [CrossRef]
- Sesen, M.; Alan, T.; Neild, A. Droplet control technologies for microfluidic high throughput screening (μHTS). Lab on a chip. 2017, 17, 2372–2394. [Google Scholar] [CrossRef] [PubMed]
- Rao C, Huisman DH, Vieira HM, Frodyma DE, Neilsen BK, Chakraborty B, et al. A Gene Expression High-Throughput Screen (GE-HTS) for Coordinated Detection of Functionally Similar Effectors in Cancer. Cancers. 2020, 12. [Google Scholar] [CrossRef]
- Yang D, Yu Z, Zheng M, Yang W, Liu Z, Zhou J, et al. Artificial intelligence-accelerated high-throughput screening of antibiotic combinations on a microfluidic combinatorial droplet system. Lab on a chip. 2023, 23, 3961–3977. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature. 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Miansari, M.; Friend, J.R. Acoustic Nanofluidics via Room-Temperature Lithium Niobate Bonding: A Platform for Actuation and Manipulation of Nanoconfined Fluids and Particles. Advanced Functional Materials. 2016, 26. [Google Scholar] [CrossRef]
- Zhang, B.; wu, W.; Zhao, Q.; Yan, S. Geometric optimization of double layered microchannel with grooves array for enabling nanoparticle manipulation. Physics of Fluids. 2023, 35, 062009. [Google Scholar] [CrossRef]
- Zeng, W.; Guo, L.; Xu, S.; Chen, J.; Zhou, J. High-Throughput Screening Technology in Industrial Biotechnology. Trends in biotechnology. 2020, 38, 888–906. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-L.; Lee, P.-Y.; Yang, C.-L.; Huang, W.-Y.; Lin, Y.-S. Recent Advances in Applications of Droplet Microfluidics. Micromachines. 2015, 6, 1249–1271. [Google Scholar] [CrossRef]
- Amirifar L, Besanjideh M, Nasiri R, Shamloo A, Nasrollahi F, de Barros NR, et al. Droplet-based microfluidics in biomedical applications. Biofabrication. 2022, 14. [Google Scholar] [CrossRef]
- Weitong, Q.; Guangyu, Y. Research and application progress of microdroplets high throughput screening methods. synthetic biology. 2023, 4, 966–979. [Google Scholar] [CrossRef]
- Sun G, Qu L, Azi F, Liu Y, Li J, Lv X, et al. Recent progress in high-throughput droplet screening and sorting for bioanalysis. Biosensors & bioelectronics. 2023, 225, 115107. [Google Scholar] [CrossRef]
- Hansen, S.K.; Jamali, B.; Hubbuch, J. Selective high throughput protein quantification based on UV absorption spectra. Biotechnology and bioengineering. 2013, 110, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Duncombe TA, Ponti A, Seebeck FP, Dittrich PS. UV-Vis Spectra-Activated Droplet Sorting for Label-Free Chemical Identification and Collection of Droplets. Analytical chemistry. 2021, 93, 13008–13013. [Google Scholar] [CrossRef] [PubMed]
- Medcalf, E.J.; Gantz, M.; Kaminski, T.S.; Hollfelder, F. Ultra-High-Throughput Absorbance-Activated Droplet Sorting for Enzyme Screening at Kilohertz Frequencies. Analytical chemistry. 2023, 95, 4597–4604. [Google Scholar] [CrossRef]
- Baret JC, Miller OJ, Taly V, Ryckelynck M, El-Harrak A, Frenz L, et al. Fluorescence-activated droplet sorting (FADS): efficient microfluidic cell sorting based on enzymatic activity. Lab on a chip. 2009, 9, 1850–1858. [Google Scholar] [CrossRef]
- Wang BL, Ghaderi A, Zhou H, Agresti J, Weitz DA, Fink GR, et al. Microfluidic high-throughput culturing of single cells for selection based on extracellular metabolite production or consumption. Nature biotechnology. 2014, 32, 473–478. [Google Scholar] [CrossRef]
- Gielen, F.; Hours, R.; Emond, S.; Fischlechner, M.; Schell, U.; Hollfelder, F. Ultrahigh-throughput–directed enzyme evolution by absorbance-activated droplet sorting (AADS). Proceedings of the National Academy of Sciences. 2016, 113, E7383–E7389. [Google Scholar] [CrossRef]
- Chen J, Vestergaard M, Jensen TG, Shen J, Dufva M, Solem C, et al. Finding the Needle in the Haystack-the Use of Microfluidic Droplet Technology to Identify Vitamin-Secreting Lactic Acid Bacteria. mBio. 2017, 8. [Google Scholar] [CrossRef]
- Beneyton, T.; Thomas, S.; Griffiths, A.D.; Nicaud, J.M.; Drevelle, A.; Rossignol, T. Droplet-based microfluidic high-throughput screening of heterologous enzymes secreted by the yeast Yarrowia lipolytica. Microbial cell factories. 2017, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen J, Vestergaard M, Jensen T, Shen J, Dufva M, Solem C, et al. Finding the Needle in the Haystack—the Use of Microfluidic Droplet Technology to Identify Vitamin-Secreting Lactic Acid Bacteria. mBio. 2017, 8, e00526–17. [Google Scholar] [CrossRef]
- Abatemarco J, Sarhan MF, Wagner JM, Lin JL, Liu L, Hassouneh W, et al. RNA-aptamers-in-droplets (RAPID) high-throughput screening for secretory phenotypes. Nature communications. 2017, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, P.; Samborski, A.; Ostaszewski, R.; Garstecki, P. Evaluation of droplet-based microfluidic platforms as a convenient tool for lipases and esterases assays. Preparative biochemistry & biotechnology. 2019, 49, 727–734. [Google Scholar] [CrossRef]
- Terekhov SS, Smirnov IV, Malakhova MV, Samoilov AE, Manolov AI, Nazarov AS, et al. Ultrahigh-throughput functional profiling of microbiota communities. Proceedings of the National Academy of Sciences of the United States of America. 2018, 115, 9551–9556. [Google Scholar] [CrossRef]
- Lyu F, Pan M, Patil S, Wang J-H, Matin AC, Andrews JR, et al. Phenotyping antibiotic resistance with single-cell resolution for the detection of heteroresistance. Sensors and Actuators B: Chemical. 2018, 270, 396–404. [Google Scholar] [CrossRef]
- Cao, X.; Luo, Z.; Zeng, W.; Xu, S.; Zhao, L.; Zhou, J. Enhanced avermectin production by Streptomyces avermitilis ATCC 31267 using high-throughput screening aided by fluorescence-activated cell sorting. Applied Microbiology and Biotechnology. 2018, 102, 703–712. [Google Scholar] [CrossRef]
- Zhang, W.; Fu, J.; Wang, Y.; Zhang, X.; Li, J. Enhanced visible-light photocatalytic activity of ZnS/BiOBr/graphene oxide ternary composite. Journal of Physics and Chemistry of Solids. 2019, 127, 19–27. [Google Scholar] [CrossRef]
- Qiang W, Ling-ran F, Luo W, Han-guang L, Lin W, Ya Z, et al. Mutation Breeding of Lycopene-Producing Strain Blakeslea Trispora by a Novel Atmospheric and Room Temperature Plasma (ARTP). Applied Biochemistry and Biotechnology. 2014, 174, 452–460. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, P.; Chen, J.; Du, G.; Li, H.; Zhou, J. Characterization of mutants of a tyrosine ammonia-lyase from Rhodotorula glutinis. Appl Microbiol Biotechnol. 2016, 100, 10443–10452. [Google Scholar] [CrossRef]
- Mendoza LD, Rodriguez JA, Leclaire J, Buono G, Fotiadu F, Carrière F, et al. An ultraviolet spectrophotometric assay for the screening of sn-2-specific lipases using 1,3-O-dioleoyl-2-O-α-eleostearoyl-sn-glycerol as substrate. Journal of lipid research. 2012, 53, 185–194. [Google Scholar] [CrossRef]
- Ye Lijuan, Wang Jiamin, Wang Lu, Cao Yi. Cell-based high throughput screening of a-amino acid ester hydrolase variants. Microbiology. 2011, 39, 0264–0271. [Google Scholar]
- Tian, H.; Yu, B.; Ai, L.; Yu, H.; Chen, C. A high-throughput system for screening high diacetyl-producing lactic acid bacteria in fermented milk in 96-well microplates. Journal of Food Measurement and Characterization. 2020, 14, 548–556. [Google Scholar] [CrossRef]
- Xiaodan, C.; Qun, F.; Zhaolun, F. Ultraviolet detection capillary electrophoresis system based on composite microfluidic chip. 2004, 25, 1231–1234. 2004, 25, 1231–1234. [Google Scholar]
- Ottevaere H, Van Overmeire S, Albero J, Nieradko L, Desmet G, Gorecki C, et al. Plastic light coupler for absorbance detection in silicon microfluidic channels. Microfluidics and Nanofluidics. 2015, 18, 559–568. [Google Scholar] [CrossRef]
- Hassan, S.U.; Nightingale, A.M.; Niu, X. Micromachined optical flow cell for sensitive measurement of droplets in tubing. Biomedical microdevices. 2018, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Samlali, K.; Vo, P.Q.N.; Shih, S.C.C. An integrated droplet-digital microfluidic system for on-demand droplet creation, mixing, incubation, and sorting. Lab on a chip. 2019, 19, 524–535. [Google Scholar] [CrossRef]
- Maceiczyk, R.M.; Hess, D.; Chiu, F.W.Y.; Stavrakis, S.; deMello, A.J. Differential detection photothermal spectroscopy: towards ultra-fast and sensitive label-free detection in picoliter & femtoliter droplets. Lab on a chip. 2017, 17, 3654–3663. [Google Scholar] [CrossRef] [PubMed]
- Gielen, F.; Hours, R.; Emond, S.; Fischlechner, M.; Schell, U.; Hollfelder, F. Ultrahigh-throughput–directed enzyme evolution by absorbance-activated droplet sorting (AADS). Proceedings of the National Academy of Sciences 2016, 113, E7383–E7389. [Google Scholar] [CrossRef]
- Wagner, J.; Liu, L.; Yuan, S.-F.; Venkataraman, M.; Abate, A.; Alper, H. A comparative analysis of single cell and droplet-based FACS for improving production phenotypes: Riboflavin overproduction in Yarrowia lipolytica. Metabolic Engineering. 2018, 47. [Google Scholar] [CrossRef]
- Kim HS, Hsu S-C, Han SI, Thapa HR, Guzman A, Browne DR, et al. High-throughput droplet microfluidics screening platform for selecting fast-growing and high lipid-producing microalgae from a mutant library. 2017, 1. [CrossRef]
- Choi JW, Vasamsetti BMK, Kim KW, Seo SH, Lee DH, Chang SI, et al. Analysis of ribonuclease activity in sub-nanoliter droplets by label-free fluorescence measurements. The Analyst. 2017, 142, 2610–2616. [Google Scholar] [CrossRef] [PubMed]
- Hardiman, E.; Gibbs, M.; Reeves, R.; Bergquist, P. Directed Evolution of a Thermophilic β-glucosidase for Cellulosic Bioethanol Production. Applied Biochemistry and Biotechnology. 2010, 161, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Xie, Y.; Huang, C.; Feng, Y.; Yang, G. An Improved Single Cell Ultrahigh Throughput Screening Method Based on In Vitro Compartmentalization. PloS one. 2014, 9, e89785. [Google Scholar] [CrossRef] [PubMed]
- Tu, R.; Martinez, R.; Prodanovic, R.; Klein, M.; Schwaneberg, U. A Flow Cytometry–Based Screening System for Directed Evolution of Proteases. SLAS Discovery. 2011, 16, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Agresti JJ, Antipov E, Abate AR, Ahn K, Rowat AC, Baret JC, et al. Ultrahigh-throughput screening in drop-based microfluidics for directed evolution. Proceedings of the National Academy of Sciences of the United States of America. 2010, 107, 4004–4009. [Google Scholar] [CrossRef]
- Turner, P.; Mamo, G.; Karlsson, E.N. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microbial cell factories. 2007, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Nakkharat, P.; Haltrich, D. Purification and characterisation of an intracellular enzyme with beta-glucosidase and beta-galactosidase activity from the thermophilic fungus Talaromyces thermophilus CBS 236. 58. Journal of biotechnology. 2006, 123, 304–313. [Google Scholar] [CrossRef]
- Huebner A, Olguin LF, Bratton D, Whyte G, Huck WT, de Mello AJ, et al. Development of quantitative cell-based enzyme assays in microdroplets. Analytical chemistry. 2008, 80, 3890–3896. [Google Scholar] [CrossRef]
- Varadarajan, N.; Rodriguez, S.; Hwang, B.Y.; Georgiou, G.; Iverson, B.L. Highly active and selective endopeptidases with programmed substrate specificities. Nature chemical biology. 2008, 4, 290–294. [Google Scholar] [CrossRef]
- Mizukami, S.; Watanabe, S.; Hori, Y.; Kikuchi, K. Covalent protein labeling based on noncatalytic beta-lactamase and a designed FRET substrate. Journal of the American Chemical Society. 2009, 131, 5016–5017. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Babiak, P.; Reymond, J.-L. Low background FRET-substrates for lipases and esterases suitable for high-throughput screening under basic (pH 11) conditions. Organic & Biomolecular Chemistry. 2006, 4, 1746–1754. [Google Scholar] [CrossRef]
- Hammar P, Angermayr SA, Sjostrom SL, van der Meer J, Hellingwerf KJ, Hudson EP, et al. Single-cell screening of photosynthetic growth and lactate production by cyanobacteria. Biotechnology for biofuels. 2015, 8, 193. [Google Scholar] [CrossRef]
- Abalde-Cela, S.; Gould, A.; Liu, X.; Kazamia, E.; Smith, A.G.; Abell, C. High-throughput detection of ethanol-producing cyanobacteria in a microdroplet platform. Journal of the Royal Society, Interface. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Ostafe, R.; Prodanović, R.; Lloyd Ung, W.; Weitz, D.A.; Fischer, R.J.B. A high-throughput cellulase screening system based on droplet microfluidics. Biomicrofluidics 2014, 8, 041102. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.C.; Pretorius, I.S.; Paulsen, I.T. Synthetic Evolution of Metabolic Productivity Using Biosensors. Trends in biotechnology. 2016, 34, 371–381. [Google Scholar] [CrossRef]
- Mengchu, S.; Liangyu, L.; Xiaolin, S.; Xinxiao, S.; Jia, W.; Qipeng, Y. Fluorescence detection-based high-throughput screening systems and devices facilitate cell factories constructio. synthetic biology. 2023, 4, 947–965. [Google Scholar] [CrossRef]
- Cheng, F.; Tang, X.L.; Kardashliev, T. Transcription Factor-Based Biosensors in High-Throughput Screening: Advances and Applications. Biotechnology journal. 2018, 13, e1700648. [Google Scholar] [CrossRef]
- Siedler, S.; Stahlhut, S.G.; Malla, S.; Maury, J.; Neves, A.R. Novel biosensors based on flavonoid-responsive transcriptional regulators introduced into Escherichia coli. Metabolic Engineering. 2014, 21, 2–8. [Google Scholar] [CrossRef]
- Tu, R.; Li, L.; Yuan, H.; He, R.; Wang, Q. Biosensor-enabled droplet microfluidic system for the rapid screening of 3-dehydroshikimic acid produced in Escherichia coli. Journal of industrial microbiology & biotechnology. 2020, 47, 1155–1160. [Google Scholar] [CrossRef]
- Paige JS, Wu KY, Jaffrey SR. RNA mimics of green fluorescent protein. Science (New York, NY). 2011, 333, 642–646. [CrossRef]
- Zhang, K.; Yang, Q.; Huang, W.; Wang, K.; Zhu, X.; Xie, M. Detection of HIV-1 ribonuclease H activity in single-cell by using RNA mimics green fluorescent protein based biosensor. Sensors and Actuators B: Chemical. 2019, 281, 439–444. [Google Scholar] [CrossRef]
- Lim, H.G.; Jang, S.; Jang, S.; Seo, S.W.; Jung, G.Y. Design and optimization of genetically encoded biosensors for high-throughput screening of chemicals. Current Opinion in Biotechnology. 2018, 54, 18–25. [Google Scholar] [CrossRef]
- Cheng F, Kardashliev T, Pitzler C, Shehzad A, Lue H, Bernhagen J, et al. A Competitive Flow Cytometry Screening System for Directed Evolution of Therapeutic Enzyme. ACS synthetic biology. 2015, 4, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Laohakunakorn N, Grasemann L, Lavickova B, Michielin G, Shahein A, Swank Z, et al. Bottom-Up Construction of Complex Biomolecular Systems With Cell-Free Synthetic Biology. Frontiers in bioengineering and biotechnology. 2020, 8, 213. [Google Scholar] [CrossRef]
- Lu, Y. Cell-free synthetic biology: Engineering in an open world. Synthetic and Systems Biotechnology. 2017, 2, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Rodionov, D.A.; Vitreschak, A.G.; Mironov, A.A.; Gelfand, M.S. Comparative genomics of the methionine metabolism in Gram-positive bacteria: a variety of regulatory systems. Nucleic acids research. 2004, 32, 3340–3353. [Google Scholar] [CrossRef]
- Shin, J.; Noireaux, V. An E.coli cell-free expression toolbox: application to synthetic gene circuits and artificial cells. ACS synthetic biology. 2012, 1, 29–41. [Google Scholar] [CrossRef]
- Tabuchi, T.; Yokobayashi, Y. High-throughput screening of cell-free riboswitches by fluorescence-activated droplet sorting. Nucleic acids research. 2022, 50, 3535–3550. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, D.; Nikoomanzar, A.; Paegel, B.M.; Chaput, J.C. Fluorescence-Activated Droplet Sorting for Single-Cell Directed Evolution. ACS synthetic biology. 2019, 8, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, Y.; Xu, Q.; Sun, X.; Meng, F. Recent Advances on Sorting Methods of High-Throughput Droplet-Based Microfluidics in Enzyme Directed Evolution. Frontiers in Chemistry. 2021, 6, 666867. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Lu, Y.; Ding, Y.; Li, L.; Zhang, F.; Wu, Q. Droplet-based microfluidics for dose–response assay of enzyme inhibitors by electrochemical method. Analytica chimica acta. 2013, 796, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Gasilova, N.; Yu, Q.; Qiao, L.; Girault, H.H. On-chip spyhole mass spectrometry for droplet-based microfluidics. Angewandte Chemie (International ed in English). 2014, 53, 4408–4412. [Google Scholar] [CrossRef]
- Pullagura, B.K.; Amarapalli, S.; Gundabala, V. Coupling electrohydrodynamics with photopolymerization for microfluidics-based generation of polyethylene glycol diacrylate (PEGDA) microparticles and hydrogels. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2021, 608, 125586. [Google Scholar] [CrossRef]
- Luo C, Yang X, Fu Q, Sun M, Ouyang Q, Chen Y, et al. Picoliter-volume aqueous droplets in oil: electrochemical detection and yeast cell electroporation. Electrophoresis. 2006, 27, 1977–1983. [Google Scholar] [CrossRef]
- Zhu, Y.; Fang, Q. Analytical detection techniques for droplet microfluidics--a review. Analytica chimica acta. 2013, 787, 24–35. [Google Scholar] [CrossRef]
- Goto H, Kanai Y, Yotsui A, Shimokihara S, Shitara S, Oyobiki R, et al. Microfluidic screening system based on boron-doped diamond electrodes and dielectrophoretic sorting for directed evolution of NAD(P)-dependent oxidoreductases. Lab on a chip. 2020, 20, 852–861. [Google Scholar] [CrossRef]
- Norris, J.L.; Porter, N.A.; Caprioli, R.M. Mass spectrometry of intracellular and membrane proteins using cleavable detergents. Analytical chemistry. 2003, 75, 6642–6647. [Google Scholar] [CrossRef]
- Heinemann J, Deng K, Shih SCC, Gao J, Adams PD, Singh AK, et al. On-chip integration of droplet microfluidics and nanostructure-initiator mass spectrometry for enzyme screening. Lab on a chip. 2017, 17, 323–331. [Google Scholar] [CrossRef]
- Holland-Moritz DA, Wismer MK, Mann BF, Farasat I, Devine P, Guetschow ED, et al. Mass Activated Droplet Sorting (MADS) Enables High-Throughput Screening of Enzymatic Reactions at Nanoliter Scale. Angewandte Chemie (International ed in English). 2020, 59, 4470–4477. [Google Scholar] [CrossRef]
- Willner, M.R.; McMillan, K.S.; Graham, D.; Vikesland, P.J.; Zagnoni, M. Surface-Enhanced Raman Scattering Based Microfluidics for Single-Cell Analysis. Analytical chemistry. 2018, 90, 12004–12010. [Google Scholar] [CrossRef] [PubMed]
- Wang X, Xin Y, Ren L, Sun Z, Zhu P, Ji Y, et al. Positive dielectrophoresis-based Raman-activated droplet sorting for culture-free and label-free screening of enzyme function in vivo. Science advances. 2020, 6, eabb3521. [Google Scholar] [CrossRef] [PubMed]
- Liu WW, Zhu Y. "Development and application of analytical detection techniques for droplet-based microfluidics"-A review. Analytica chimica acta. 2020, 1113, 66–84. [Google Scholar] [CrossRef]
- Swyer I, Soong R, Dryden MDM, Fey M, Maas WE, Simpson A, et al. Interfacing digital microfluidics with high-field nuclear magnetic resonance spectroscopy. Lab on a chip. 2016, 16, 4424–4435. [Google Scholar] [CrossRef] [PubMed]
- Hale, W.; Rossetto, G.; Greenhalgh, R.; Finch, G.; Utz, M. High-resolution nuclear magnetic resonance spectroscopy in microfluidic droplets. Lab on a chip. 2018, 18, 3018–3024. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Mudrik, J.M.; Wheeler, A.R. A guiding light: spectroscopy on digital microfluidic devices using in-plane optical fibre waveguides. Anal Bioanal Chem. 2015, 407, 7467–7475. [Google Scholar] [CrossRef]
- Chen, J.; Vestergaard, M.; Jensen, T.G.; Jing, S.; Tg, J. Finding the Needle in the Haystack—the Use of Microfluidic Droplet Technology to Identify Vitamin-Secreting Lactic Acid Bacteria. mBio. 2017, 8, e00526–17. [Google Scholar] [CrossRef] [PubMed]
- Kim HS, Hsu SC, Han SI, Thapa HR, Guzman AR, Browne DR, et al. High-throughput droplet microfluidics screening platform for selecting fast-growing and high lipid-producing microalgae from a mutant library. Plant Direct. 2017, 1, e00011. [Google Scholar] [CrossRef]
- Qiao Y, Zhao X, Zhu J, Tu R, Dong L, Wang L, et al. Fluorescence-activated droplet sorting of lipolytic microorganisms using a compact optical system. Lab Chip. 2017, 18, 190–196. [Google Scholar]
- Xu, J.G.; Huang, M.S.; Wang, H.F.; Fang, Q. Forming a Large-Scale Droplet Array in a Microcage Array Chip for High-Throughput Screening. Anal Chem. 2019, 91, 10757–10763. [Google Scholar] [CrossRef] [PubMed]
- 93. Qiao Y, Hu R, Chen D, Wang L, Wang Z, Yu H, et al. Fluorescence-activated droplet sorting of PET degrading microorganisms. J Hazard Mater. 2022, 424 Pt B, 127417.
- Zhang, G.; Chen, Y.; Li, Q.; Zhou, J.; Li, J.; Du, G. Growth-coupled evolution and high-throughput screening assisted rapid enhancement for amylase-producing Bacillus licheniformis. Bioresour Technol. 2021, 337, 125467. [Google Scholar] [CrossRef] [PubMed]
- Prodanovic, R.; Ung, W.L.; Durdic, K.I.; Fischer, R.; Weitz, D.A.; Ostafe, R. A High-Throughput Screening System Based on Droplet Microfluidics for Glucose Oxidase Gene Libraries. Molecules. 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zeng, W.; Xu, S.; Zhou, J. Fluorescence-activated droplet sorting for enhanced pyruvic acid accumulation by Candida glabrata. Bioresour Technol. 2020, 318, 124258. [Google Scholar] [CrossRef]
- Steyer, D.J.; Kennedy, R.T. High-Throughput Nanoelectrospray Ionization-Mass Spectrometry Analysis of Microfluidic Droplet Samples. Anal Chem. 2019, 91, 6645–6651. [Google Scholar] [CrossRef]
- Diefenbach XW, Farasat I, Guetschow ED, Welch CJ, Kennedy RT, Sun S, et al. Enabling Biocatalysis by High-Throughput Protein Engineering Using Droplet Microfluidics Coupled to Mass Spectrometry. ACS Omega. 2018, 3, 1498–1508. [Google Scholar] [CrossRef]
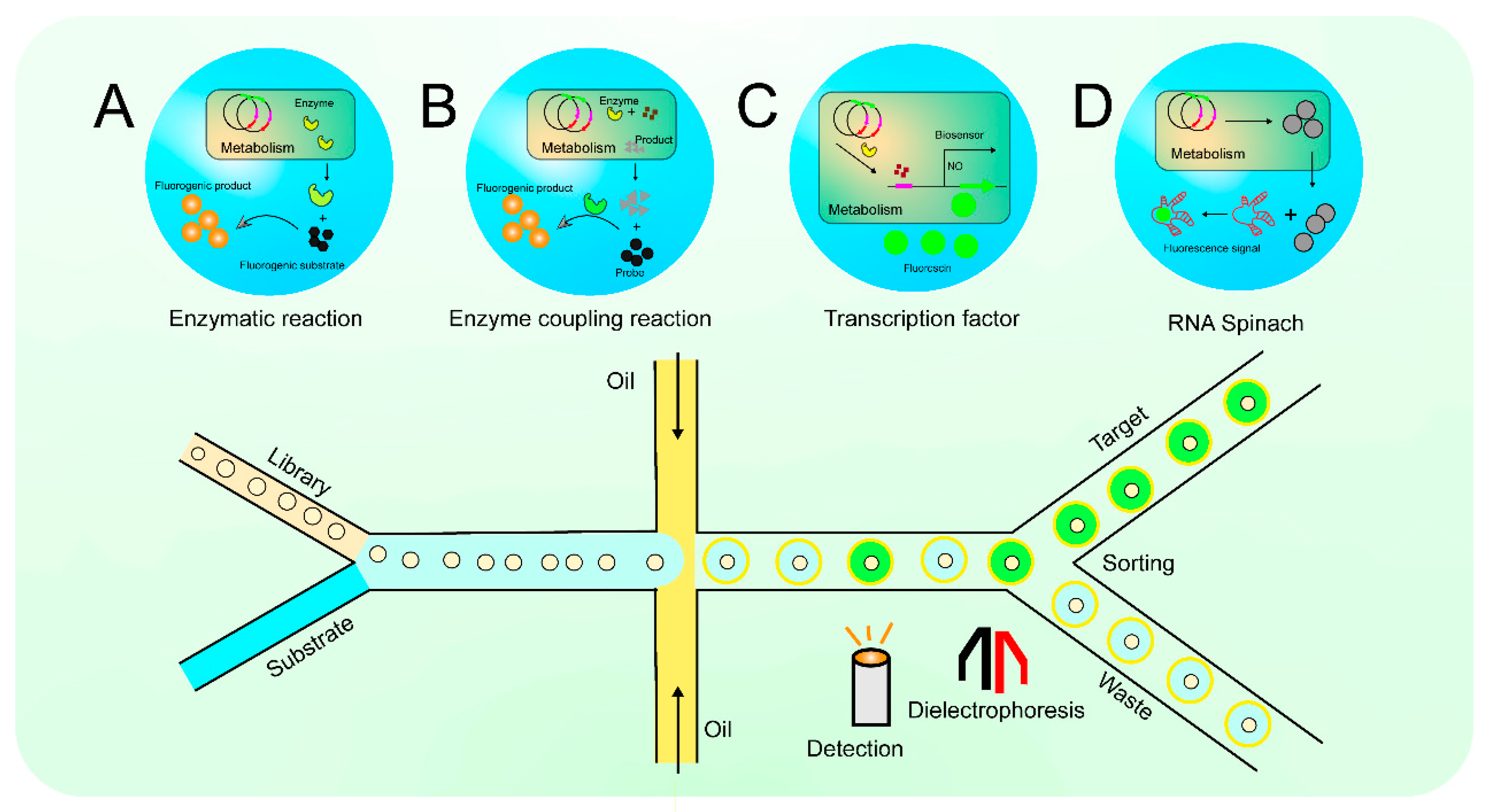
| Spectrum | Screening analysis strategies | Screening target | Specific applications | References. |
| Ultraviolet light | Addition of metabolite dyes | - | A planar microfluidic spectroscopy detection system was proposed, which could continuously analyze and determine thymol blue of the staining at 180-890 nm, and realize high-throughput detection of the corresponding stained species | (88) |
| Ultraviolet light | Enzymatic reaction | E. coli | UV-Vis full-wavelength detection system to monitor E. coli growth at 280 nm and thiouric acid assay at 311 nm to identify strain expression ergothionease activity | (17) |
| Visible light | Direct measurement of absorbance | Yeast | Integrated droplet-digital microfluidic system to measure the growth of mutant and wild-type yeasts in ionic liquids | (39) |
| Visible light | Enzymatic reaction | HL-60 Cell population | Differential detection photothermal interferometry combined with droplet microfluidics, relying on electronic media and mitochondrial succinate-tetrazolium reductase reaction to generate absorbance, high-throughput analysis of HL-60 cell population metabolic activity | (40) |
| Visible light | Enzyme-coupled reaction | Glucose oxidase | Using the principle of enzyme colorimetry, glucose hydrolysis intermediate H2O2, 4-aminoantipyrine and phenol generate red quinone imine, thereby continuously determining glucose oxidase activity with high throughput | (38) |
| Fluorescence | Direct measurement of target metabolite | Lactic acid bacteria | Riboflavin has a natural fluorescent signal, and high-yield mutant strains are screened for use in milk fermentation, and riboflavin reaches 2.81 mg/L | (89) |
| Fluorescence | Addition of metabolite dyes | Microalgae strain | The fluorescent stain BODIPY was added to stain the lipids, and microalgae strains with a 2.75-fold increase in lipid yield were screened | (90) |
| Fluorescence | Embedding metal chelating agents | - | Add EtBr to bind to RNA to determine ribonuclease activity, the higher the activity, the lower the fluorescence signal | (44) |
| Fluorescence | Enzymatic reaction | Environmental microorganisms | Fluorescein dibutyrate was introduced as a fluorescent substrate, and 11 lipase-producing strains were screened from environmental microorganisms by FADS | (91) |
| Fluorescence | Enzymatic reaction | E. coli | A method was developed to rapidly form a large-scale droplet array using microcage array chips, which improved the operability of droplets and introduced fluorescent substrates to screen strains expressing esterase AFEST from mixed bacteria | (92) |
| Fluorescence | Enzymatic reaction | Viable bacteria in wastewater | The fluorescent substrate diphenyl dibenzoate can be degraded by PETase to produce a fluorescent signal, which can screen for strains that express PETase efficiently | (93) |
| Fluorescence | Enzyme-coupled reaction | Bacillus licheniformus | According to the modified 3,5-dinitrosalicylic acid (DNS) method, starch substrates are decomposed into glucose, DNS and glucose undergo redox reactions to produce fluorescent substances, and strains with α-amylase expression increased by 67% were screened | (94) |
| Fluorescence | Enzyme-coupled reaction | Yeast | The glucose in the microdroplets was decomposed by oxidase to produce H2O2, H2O2 reacted with vanadium bromoperoxidase to produce BrO-, and BrO- reacted with aminophenoxyfluorescein to produce strong fluorescence, and an enzyme coupling strategy was established to screen out glucose oxidase mutants 2.1 times higher than that of wild-type Kcat | (95) |
| Fluorescence | Protein-based biosensors | Candida glabrum | A biosensor expressing pH-sensitive fluorescent protein was constructed, and the ankyrin gene was introduced for expression, so that the fluorescent protein was anchored on the surface of Candida glabricis, so as to show the accumulation of pyruvate, and the strain with a 73.6% increase in pyruvate yield, reaching 48.6 g/L, was screened | (96) |
| Fluorescence | Transcription factor-based biosensors | E. coli | A biosensor in response to 3-dehydroshikimic acid (3-DHS) was constructed, and 3-DHS was positively regulated, expressing fluorescent protein, and screening mutant strains with high yield of 3-DHS, with a yield of 2.46 g/L at 24 h | (62) |
| Fluorescence | Nucleic acid-based biosensors(RNA Spinach) | Saccharomyces cerevisiae | Innovated on the basis of RNA Spinach, a universal sensing technology that provides small molecule metabolites by altering RNA sequences, and screened mutant strains with 28-fold and 3-fold higher yields of tyrosine and recombinant protein streptomycin, respectively | (25) |
| Non-spectral | Coupled mass spectrometry analysis | Transaminase | Combined with droplet microfluidics, a high-throughput and stable droplet analysis system was constructed to detect the reaction products of intradroplet transaminases, quantify enzyme activity, and evaluate enzyme mutants | (97) |
| Non-spectral | Coupled mass spectrometry analysis | E. coli | Transaminases can convert methyl 4-methyl-3-oxovalerate into the corresponding amine, and the amine is then spontaneously hydrolyzed to β-leucine, which is detected by the microfluidic-mass spectrometry analysis system and screened out a variety of mutants of transaminases | (98) |
| Raman spectroscopy | Coupled surface-enhanced Raman scattering | Prostate cancer cell population | Surface-enhanced Raman scattering combined with microfluidic system improved the sensitivity of the detection system, and the glycan expression and cell variability of prostate cancer cells were analyzed at high throughput by wheat germ lectin-modified nanometal particles | (83) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





