Submitted:
22 December 2023
Posted:
25 December 2023
Read the latest preprint version here
Abstract
Keywords:
Introduction
Organization of the Retina
Neurotransmitters
Glutamate
γ-Aminobutyric Acid (GABA)
Dopamine
Endocannabinoid System
TRP Channels
TRP Channel in the Retinal Tissue
Location and Function of TRP Vanilloids in the Retina
Adenosine
Neuropeptides: PACAP
Nitric Oxide
Gliotransmitters
Nucleotide Receptors in the Retina
Nucleotides and Retinal Cell Proliferation
Nucleotide and Retinal Cell Migration
Nucleotides and the Induction of Cell Death in the Retina
P2X7 Glial Receptors and Retinal Development
Antioxidants
Glutathione
Vitamin C
Reciprocal Interactions between Retinal Transmitters
Dopamine and Adenosine
Glutamate and Adenosine
Glutamate and Vitamin C
Glutamate and GABA
Dopamine and Glutamate
Endocannabinoid and Dopamine
Dopamine, Glutamate and Vitamin C
Adenosine, Vitamin C and Nitric Oxide
The Diseased Retina
Glaucoma
Diabetic Retina
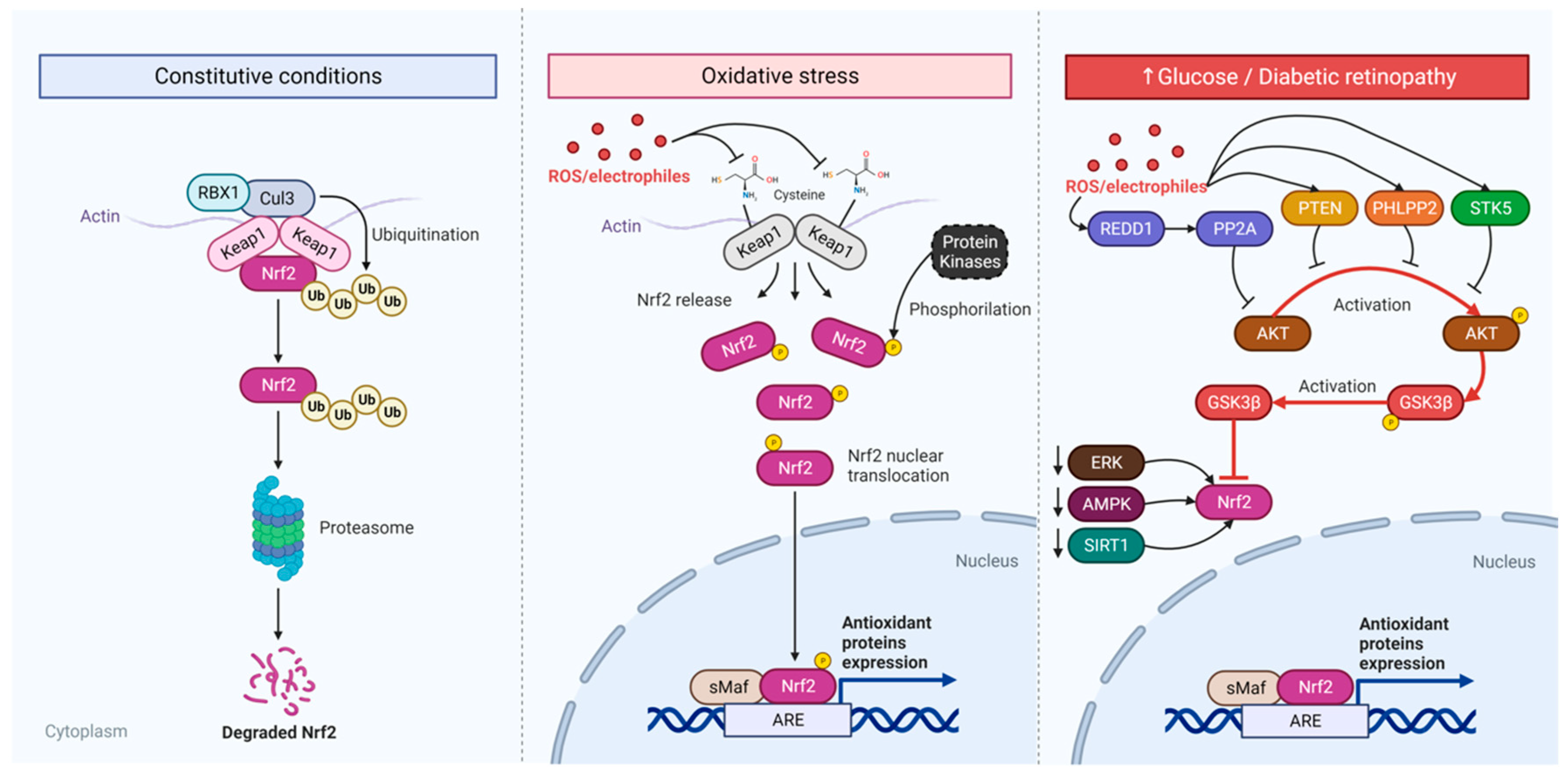
Investigation of Innovative Therapeutic Strategies
Neuroprotection
Gene Therapy and the Future of Vision Recovery
Cell Reprogramming
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vergara, M. N.; Canto-Soler, M. V., Rediscovering the chick embryo as a model to study retinal development. Neural development 2012, 7, 22. [CrossRef]
- L. Belecky-Adams, T.; Haynes, T.; M. Wilson, J.; Del Rio-Tsonis, K., Chapter 8 - The Chick as a Model for Retina Development and Regeneration. In Animal Models in Eye Research, Tsonis, P. A., Ed. Academic Press: London, 2008; pp 102-119. [CrossRef]
- Cebulla, C. M.; Zelinka, C. P.; Scott, M. A.; Lubow, M.; Bingham, A.; Rasiah, S.; Mahmoud, A. M.; Fischer, A. J., A chick model of retinal detachment: cone rich and novel. PLoS One 2012, 7, e44257. [CrossRef]
- Al Sabaani, N., Exendin-4 inhibits high glucose-induced oxidative stress in retinal pigment epithelial cells by modulating the expression and activation of p(66)Shc. Cutan Ocul Toxicol 2021, 40, 175-186. [CrossRef]
- Ventura, A. L. M.; De Mello, F. G.; De Melo Reis, R. A., Methods of dopamine research in retina cells. Methods in Molecular Biology 2013, 964, 25-42. [CrossRef]
- Tempone, M. H.; Freitas, H. R.; Schitine, C. S.; de Melo Reis, R. A., Visualizing Shifts on Neuron-Glia Circuit with the Calcium Imaging Technique. Journal of visualized experiments : JoVE 2022. [CrossRef]
- Arthur, P.; Muok, L.; Nathani, A.; Zeng, E. Z.; Sun, L.; Li, Y.; Singh, M., Bioengineering Human Pluripotent Stem Cell-Derived Retinal Organoids and Optic Vesicle-Containing Brain Organoids for Ocular Diseases. Cells 2022, 11. [CrossRef]
- Calaza, K. d. C.; Fluminense, U. F.; Gardino, P. F.; Janeiro, U. F. d. R. d., Neurochemical phenotype and birthdating of specific cell populations in the chick retina. Anais da Academia Brasileira de Ciencias 2010, 82, 595-608. [CrossRef]
- Li, M.; Key Laboratory of Animal Genetics, B. a. R. o. S. P., College of Animal Science and Technology, Northwest A&F University, Yangling 712100, China; Sun, C.; National Engineering Laboratory for Animal Breeding and Key Laboratory of Animal Genetics, B. a. R., Ministry of Agriculture and Rural Affairs, China Agricultural University, Beijing 100193, China; Xu, N.; Key Laboratory of Animal Genetics, B. a. R. o. S. P., College of Animal Science and Technology, Northwest A&F University, Yangling 712100, China; Bian, P.; Key Laboratory of Animal Genetics, B. a. R. o. S. P., College of Animal Science and Technology, Northwest A&F University, Yangling 712100, China; Tian, X.; Key Laboratory of Animal Genetics, B. a. R. o. S. P., College of Animal Science and Technology, Northwest A&F University, Yangling 712100, China; Wang, X.; Key Laboratory of Animal Genetics, B. a. R. o. S. P., College of Animal Science and Technology, Northwest A&F University, Yangling 712100, China; Wang, Y.; State Key Laboratory of Agrobiotechnology, C. o. B. S., China Agricultural University, Beijing 100193, China; National Research Facility for Phenotypic and Genotypic Analysis of Model Animals (Beijing), C. A. U., Beijing 100193, China; Jia, X.; Department of Animal Science, I. S. U., Ames, IA 50011, USA; School of Life Science and Engineering, F. U., Foshan 528225, China; Heller, R.; Section for Computational and RNA Biology, D. o. B., University of Copenhagen, Copenhagen N 2200, Denmark; Wang, M.; Howard Hughes Medical Institute, U. o. C. S. C., Santa Cruz, CA 95064, USA; Department of Ecology and Evolutionary Biology, U. o. C. S. C., Santa Cruz, CA 95064, USA; Wang, F.; Key Laboratory of Animal Genetics, B. a. R. o. S. P., College of Animal Science and Technology, Northwest A&F University, Yangling 712100, China; Dai, X.; Key Laboratory of Animal Genetics, B. a. R. o. S. P., College of Animal Science and Technology, Northwest A&F University, Yangling 712100, China; Luo, R.; Key Laboratory of Animal Genetics, B. a. R. o. S. P., College of Animal Science and Technology, Northwest A&F University, Yangling 712100, China; Guo, Y.; Key Laboratory of Animal Genetics, B. a. R. o. S. P., College of Animal Science and Technology, Northwest A&F University, Yangling 712100, China; Wang, X.; Key Laboratory of Animal Genetics, B. a. R. o. S. P., College of Animal Science and Technology, Northwest A&F University, Yangling 712100, China; Yang, P.; Key Laboratory of Animal Genetics, B. a. R. o. S. P., College of Animal Science and Technology, Northwest A&F University, Yangling 712100, China; Hu, D.; Key Laboratory of Animal Genetics, B. a. R. o. S. P., College of Animal Science and Technology, Northwest A&F University, Yangling 712100, China; Liu, Z.; Key Laboratory of Animal Genetics, B. a. R. o. S. P., College of Animal Science and Technology, Northwest A&F University, Yangling 712100, China; Fu, W.; Key Laboratory of Animal Genetics, B. a. R. o. S. P., College of Animal Science and Technology, Northwest A&F University, Yangling 712100, China; Zhang, S.; Key Laboratory of Animal Genetics, B. a. R. o. S. P., College of Animal Science and Technology, Northwest A&F University, Yangling 712100, China; Li, X.; National Engineering Laboratory for Animal Breeding and Key Laboratory of Animal Genetics, B. a. R., Ministry of Agriculture and Rural Affairs, China Agricultural University, Beijing 100193, China; Wen, C.; National Engineering Laboratory for Animal Breeding and Key Laboratory of Animal Genetics, B. a. R., Ministry of Agriculture and Rural Affairs, China Agricultural University, Beijing 100193, China; Lan, F.; National Engineering Laboratory for Animal Breeding and Key Laboratory of Animal Genetics, B. a. R., Ministry of Agriculture and Rural Affairs, China Agricultural University, Beijing 100193, China; Siddiki, A. Z.; Department of Pathology and Parasitology, F. o. V. M., Chittagong Veterinary and Animal Sciences University, Chittagong 4202, Bangladesh; Suwannapoom, C.; School of Agriculture and Natural Resources, U. o. P., Phayao, Thailand; Zhao, X.; Department of Animal Science, M. U., Montreal, QC, Canada; Nie, Q.; Department of Animal Genetics, B. a. R., College of Animal Science, South China Agricultural University, Guangzhou 510642, Guangdong, China; Hu, X.; State Key Laboratory of Agrobiotechnology, C. o. B. S., China Agricultural University, Beijing 100193, China; Jiang, Y.; Key Laboratory of Animal Genetics, B. a. R. o. S. P., College of Animal Science and Technology, Northwest A&F University, Yangling 712100, China; Center for Functional Genomics, I. o. F. A., Northwest A&F University, China; Yang, N.; National Engineering Laboratory for Animal Breeding and Key Laboratory of Animal Genetics, B. a. R., Ministry of Agriculture and Rural Affairs, China Agricultural University, Beijing 100193, China, De Novo Assembly of 20 Chicken Genomes Reveals the Undetectable Phenomenon for Thousands of Core Genes on Microchromosomes and Subtelomeric Regions. Molecular Biology and Evolution 2023, 39.
- Yamagata, M.; Yan, W.; Sanes, J. R., A cell atlas of the chick retina based on single-cell transcriptomics. eLife 2021, 10, 1-39. [CrossRef]
- Hoon, M.; Okawa, H.; Della Santina, L.; Wong, R. O. L., Functional architecture of the retina: Development and disease. In Progress in retinal and eye research, Elsevier Ltd: 2014; Vol. 42, pp 44-84. [CrossRef]
- Reichenbach, A.; Bringmann, A., New functions of Müller cells. Glia 2013, 61, 651-78. [CrossRef]
- Karl, M. O.; Reh, T. A., Regenerative medicine for retinal diseases: activating endogenous repair mechanisms. Trends Mol Med 2010, 16, 193-202. [CrossRef]
- Vecino, E.; Rodriguez, F. D.; Ruzafa, N.; Pereiro, X.; Sharma, S. C., Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res 2016, 51, 1-40. [CrossRef]
- Seifert, M.; Baden, T.; Osorio, D., The retinal basis of vision in chicken. In Seminars in Cell and Developmental Biology, Elsevier Ltd: 2020. [CrossRef]
- Baden, T.; Osorio, D., The Retinal Basis of Vertebrate Color Vision. Annu Rev Vis Sci 2019, 5, 177-200. [CrossRef]
- Barnstable, C. J., Glutamate and GABA in retinal circuitry. Current opinion in neurobiology 1993, 3, 520-525. [CrossRef]
- Münch, T. A.; da Silveira, R. A.; Siegert, S.; Viney, T. J.; Awatramani, G. B.; Roska, B., Approach sensitivity in the retina processed by a multifunctional neural circuit. Nature neuroscience 2009, 12, 1308-16. [CrossRef]
- Pourcho, R. G., Neurotransmitters in the retina. Curr Eye Res 1996, 15, 797-803. [CrossRef]
- Martins, R. A.; Pearson, R. A., Control of cell proliferation by neurotransmitters in the developing vertebrate retina. Brain Res 2008, 1192, 37-60. [CrossRef]
- Hoon, M.; Okawa, H.; Della Santina, L.; Wong, R. O., Functional architecture of the retina: development and disease. Prog Retin Eye Res 2014, 42, 44-84. [CrossRef]
- Ferreira, I. L.; Duarte, C. B.; Carvalho, A. P., Ca2+ influx through glutamate receptor-associated channels in retina cells correlates with neuronal cell death. European journal of pharmacology 1996, 302, 153-162. [CrossRef]
- Rodríguez Villanueva, J.; Martín Esteban, J.; Rodríguez Villanueva, L. J., Retinal Cell Protection in Ocular Excitotoxicity Diseases. Possible Alternatives Offered by Microparticulate Drug Delivery Systems and Future Prospects. Pharmaceutics 2020, 12, 94. [CrossRef]
- Carpi-Santos, R.; de Melo Reis, R. A.; Gomes, F. C. A.; Calaza, K. C., Contribution of Müller Cells in the Diabetic Retinopathy Development: Focus on Oxidative Stress and Inflammation. Antioxidants 2022, 11, 617. [CrossRef]
- Santos, A. E.; Carvalho, A. L.; Lopes, M. C.; Carvalho, A. P., Differential postreceptor signaling events triggered by excitotoxic stimulation of different ionotropic glutamate receptors in retinal neurons. J Neurosci Res 2001, 66, 643-55. [CrossRef]
- Lambuk, L.; Jafri, A. J. A.; Iezhitsa, I.; Agarwal, R.; Bakar, N. S.; Agarwal, P.; Abdullah, A.; Ismail, N. M., Dose-dependent effects of NMDA on retinal and optic nerve morphology in rats. International journal of ophthalmology 2019, 12, 746-753.
- Rosenstein, R. E., New actors in optic neuritis pathogenesis: An Editorial for "Influence of retinal NMDA receptor activity during autoimmune optic neuritis" on page 693. Journal of neurochemistry 2020, 153, 671-673. [CrossRef]
- Ikonomidou, C.; Turski, L., Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol 2002, 1, 383-386. [CrossRef]
- Traynelis, S. F.; Wollmuth, L. P.; McBain, C. J.; Menniti, F. S.; Vance, K. M.; Ogden, K. K.; Hansen, K. B.; Yuan, H.; Myers, S. J.; Dingledine, R., Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 2010, 62, 405-96. [CrossRef]
- Pinto, M. C. X.; Kihara, A. H.; Goulart, V. A. M.; Tonelli, F. M. P.; Gomes, K. N.; Ulrich, H.; Resende, R. R., Calcium signaling and cell proliferation. Cellular signalling 2015, 27, 2139-2149. [CrossRef]
- de Melo Reis, R. A.; Freitas, H. R.; de Mello, F. G., Cell Calcium Imaging as a Reliable Method to Study Neuron-Glial Circuits. Frontiers in neuroscience 2020, 14, 569361. [CrossRef]
- Dawson, T. M.; Dawson, V. L., Chapter Four - Nitric Oxide Signaling in Neurodegeneration and Cell Death. In Advances in Pharmacology, Pasternak, G. W.; Coyle, J. T., Eds. Academic Press: 2018; Vol. 82, pp 57-83. [CrossRef]
- Marshall, J.; Wong, K. Y.; Rupasinghe, C. N.; Tiwari, R.; Zhao, X.; Berberoglu, E. D.; Sinkler, C.; Liu, J.; Lee, I.; Parang, K.; Spaller, M. R.; Hüttemann, M.; Goebel, D. J., Inhibition of N-Methyl-D-aspartate-induced Retinal Neuronal Death by Polyarginine Peptides Is Linked to the Attenuation of Stress-induced Hyperpolarization of the Inner Mitochondrial Membrane Potential. The Journal of biological chemistry 2015, 290, 22030-48. [CrossRef]
- Martel, M. A.; Ryan, T. J.; Bell, K. F.; Fowler, J. H.; McMahon, A.; Al-Mubarak, B.; Komiyama, N. H.; Horsburgh, K.; Kind, P. C.; Grant, S. G.; Wyllie, D. J.; Hardingham, G. E., The subtype of GluN2 C-terminal domain determines the response to excitotoxic insults. Neuron 2012, 74, 543-56. [CrossRef]
- Opere, C. A.; Heruye, S.; Njie-Mbye, Y. F.; Ohia, S. E.; Sharif, N. A., Regulation of Excitatory Amino Acid Transmission in the Retina: Studies on Neuroprotection. J Ocul Pharmacol Ther 2018, 34, (1-2), 107-118. [CrossRef]
- Park, Y. H.; Broyles, H. V.; He, S.; McGrady, N. R.; Li, L.; Yorio, T., Involvement of AMPA Receptor and Its Flip and Flop Isoforms in Retinal Ganglion Cell Death Following Oxygen/Glucose Deprivation. Invest Ophthalmol Vis Sci 2016, 57, 508-26. [CrossRef]
- Cossenza, M.; Cadilhe, D. V.; Coutinho, R. N.; Paes-de-Carvalho, R., Inhibition of protein synthesis by activation of NMDA receptors in cultured retinal cells: a new mechanism for the regulation of nitric oxide production. Journal of neurochemistry 2006, 97, 1481-93. [CrossRef]
- Gladulich, L. F. H.; Peixoto-Rodrigues, M. C.; Campello-Costa, P.; Paes-de-Carvalho, R.; Cossenza, M., NMDA-induced nitric oxide generation and CREB activation in central nervous system is dependent on eukaryotic elongation factor 2 kinase. Biochim Biophys Acta Mol Cell Res 2020, 1867, 118783. [CrossRef]
- Carlberg, U.; Nilsson, A.; Nygård, O., Functional properties of phosphorylated elongation factor 2. Eur J Biochem 1990, 191, 639-45. [CrossRef]
- Nairn, A. C.; Matsushita, M.; Nastiuk, K.; Horiuchi, A.; Mitsui, K.; Shimizu, Y.; Palfrey, H. C., Elongation factor-2 phosphorylation and the regulation of protein synthesis by calcium. Prog Mol Subcell Biol 2001, 27, 91-129. [CrossRef]
- Price, N. T.; Redpath, N. T.; Severinov, K. V.; Campbell, D. G.; Russell, J. M.; Proud, C. G., Identification of the phosphorylation sites in elongation factor-2 from rabbit reticulocytes. FEBS letters 1991, 282, 253-8. [CrossRef]
- Rodnina, M. V.; Savelsbergh, A.; Wintermeyer, W., Dynamics of translation on the ribosome: molecular mechanics of translocation. FEMS Microbiol Rev 1999, 23, 317-33. [CrossRef]
- Ryazanov, A. G.; Shestakova, E. A.; Natapov, P. G., Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature 1988, 334, 170-3. [CrossRef]
- Scheetz, A. J.; Nairn, A. C.; Constantine-Paton, M., N-methyl-D-aspartate receptor activation and visual activity induce elongation factor-2 phosphorylation in amphibian tecta: a role for N-methyl-D-aspartate receptors in controlling protein synthesis. Proceedings of the National Academy of Sciences of the United States of America 1997, 94, 14770-5. [CrossRef]
- Hsu, W. L.; Chung, H. W.; Wu, C. Y.; Wu, H. I.; Lee, Y. T.; Chen, E. C.; Fang, W.; Chang, Y. C., Glutamate Stimulates Local Protein Synthesis in the Axons of Rat Cortical Neurons by Activating α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptors and Metabotropic Glutamate Receptors. The Journal of biological chemistry 2015, 290, 20748-20760. [CrossRef]
- Dieterich, D. C.; Hodas, J. J.; Gouzer, G.; Shadrin, I. Y.; Ngo, J. T.; Triller, A.; Tirrell, D. A.; Schuman, E. M., In situ visualization and dynamics of newly synthesized proteins in rat hippocampal neurons. Nature neuroscience 2010, 13, 897-905. [CrossRef]
- Scheetz, A. J.; Nairn, A. C.; Constantine-Paton, M., NMDA receptor-mediated control of protein synthesis at developing synapses. Nature neuroscience 2000, 3, 211-6. [CrossRef]
- Verpelli, C.; Piccoli, G.; Zibetti, C.; Zanchi, A.; Gardoni, F.; Huang, K.; Brambilla, D.; Di Luca, M.; Battaglioli, E.; Sala, C., Synaptic activity controls dendritic spine morphology by modulating eEF2-dependent BDNF synthesis. The Journal of neuroscience : the official journal of the Society for Neuroscience 2010, 30, 5830-42. [CrossRef]
- Cossenza, M.; Socodato, R.; Mejía-García, T. A.; Domith, I.; Portugal, C. C.; Gladulich, L. F. H.; Duarte-Silva, A. T.; Khatri, L.; Antoine, S.; Hofmann, F.; Ziff, E. B.; Paes-de-Carvalho, R., Protein synthesis inhibition promotes nitric oxide generation and activation of CGKII-dependent downstream signaling pathways in the retina. Biochim Biophys Acta Mol Cell Res 2020, 1867, 118732. [CrossRef]
- Numakawa, T.; Suzuki, S.; Kumamaru, E.; Adachi, N.; Richards, M.; Kunugi, H., BDNF function and intracellular signaling in neurons. Histology and histopathology 2010, 25, 237-58.
- Schmid, R. S.; Graff, R. D.; Schaller, M. D.; Chen, S.; Schachner, M.; Hemperly, J. J.; Maness, P. F., NCAM stimulates the Ras-MAPK pathway and CREB phosphorylation in neuronal cells. Journal of neurobiology 1999, 38, 542-58. [CrossRef]
- Singh, L.; Bhatti, R., Signaling Pathways Involved in the Neuroprotective Effect of Osthole: Evidence and Mechanisms. Mol Neurobiol 2023. [CrossRef]
- Luhmann, H. J.; Kirischuk, S.; Sinning, A.; Kilb, W., Early GABAergic circuitry in the cerebral cortex. Current opinion in neurobiology 2014, 26, 72-8. [CrossRef]
- Mosinger, J. L.; Yazulla, S.; Studholme, K. M., GABA-like immunoreactivity in the vertebrate retina: a species comparison. Exp Eye Res 1986, 42, 631-44. [CrossRef]
- Wu, C.; Sun, D., GABA receptors in brain development, function, and injury. Metab Brain Dis 2015, 30, 367-79. [CrossRef]
- Siucinska, E., Γ-Aminobutyric acid in adult brain: an update. Behav Brain Res 2019, 376, 112224. [CrossRef]
- Nuss, P., Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr Dis Treat 2015, 11, 165-75. [CrossRef]
- Wässle, H., Parallel processing in the mammalian retina. Nature reviews. Neuroscience 2004, 5, 747-57. [CrossRef]
- Calaza Kda, C.; Gardino, P. F., Neurochemical phenotype and birthdating of specific cell populations in the chick retina. Anais da Academia Brasileira de Ciencias 2010, 82, 595-608. [CrossRef]
- De Sampaio Schitine, C.; Kubrusly, R. C.; De Melo Reis, R. A.; Yamasaki, E. N.; De Mello, M. C.; De Mello, F. G., GABA uptake by purified avian Müller glia cells in culture. Neurotox Res 2007, 12, 145-53. [CrossRef]
- Ferreira, D. D.; Stutz, B.; de Mello, F. G.; Reis, R. A.; Kubrusly, R. C., Caffeine potentiates the release of GABA mediated by NMDA receptor activation: Involvement of A1 adenosine receptors. Neuroscience 2014, 281, 208-15. [CrossRef]
- Frederick, J. M., The emergence of GABA-accumulating neurons during retinal histogenesis in the embryonic chick. Exp Eye Res 1987, 45, 933-45. [CrossRef]
- Hokoç, J. N.; Ventura, A. L.; Gardino, P. F.; De Mello, F. G., Developmental immunoreactivity for GABA and GAD in the avian retina: possible alternative pathway for GABA synthesis. Brain Res 1990, 532, (1-2), 197-202. [CrossRef]
- Sun, H.; Crossland, W. J., Quantitative assessment of localization and colocalization of glutamate, aspartate, glycine, and GABA immunoreactivity in the chick retina. The Anatomical record 2000, 260, 158-79. [CrossRef]
- Lee, S. E.; Lee, Y.; Lee, G. H., The regulation of glutamic acid decarboxylases in GABA neurotransmission in the brain. Arch Pharm Res 2019, 42, 1031-1039. [CrossRef]
- Soghomonian, J. J.; Martin, D. L., Two isoforms of glutamate decarboxylase: why? Trends in pharmacological sciences 1998, 19, 500-5. [CrossRef]
- Yamasaki, E. N.; Barbosa, V. D.; De Mello, F. G.; Hokoc, J. N., GABAergic system in the developing mammalian retina: dual sources of GABA at early stages of postnatal development. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 1999, 17, 201-13. [CrossRef]
- Madsen, K. K.; White, H. S.; Schousboe, A., Neuronal and non-neuronal GABA transporters as targets for antiepileptic drugs. Pharmacol Ther 2010, 125, 394-401. [CrossRef]
- Pinal, C. S.; Tobin, A. J., Uniqueness and redundancy in GABA production. Perspect Dev Neurobiol 1998, 5, (2-3), 109-18.
- Wu, Z.; Guo, Z.; Gearing, M.; Chen, G., Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer's [corrected] disease model. Nature communications 2014, 5, 4159. [CrossRef]
- Kwak, H.; Koh, W.; Kim, S.; Song, K.; Shin, J. I.; Lee, J. M.; Lee, E. H.; Bae, J. Y.; Ha, G. E.; Oh, J. E.; Park, Y. M.; Kim, S.; Feng, J.; Lee, S. E.; Choi, J. W.; Kim, K. H.; Kim, Y. S.; Woo, J.; Lee, D.; Son, T.; Kwon, S. W.; Park, K. D.; Yoon, B. E.; Lee, J.; Li, Y.; Lee, H.; Bae, Y. C.; Lee, C. J.; Cheong, E., Astrocytes Control Sensory Acuity via Tonic Inhibition in the Thalamus. Neuron 2020, 108, 691-706.e10. [CrossRef]
- Krantis, A., GABA in the Mammalian Enteric Nervous System. News Physiol Sci 2000, 15, 284-290. [CrossRef]
- De, A.; Dos, S.; Nora, H.; Yamasaki, E.; Gardino, P.; Mello, F., Regulation of glutamic acid decarboxylase of chick and rat retina cells by GABA and excitatory amino acids. Anais da Academia Brasileira de Ciencias 2000, 72. [CrossRef]
- Sequerra, E. B.; Gardino, P.; Hedin-Pereira, C.; de Mello, F. G., Putrescine as an important source of GABA in the postnatal rat subventricular zone. Neuroscience 2007, 146, 489-93. [CrossRef]
- Kim, J. I.; Ganesan, S.; Luo, S. X.; Wu, Y. W.; Park, E.; Huang, E. J.; Chen, L.; Ding, J. B., Aldehyde dehydrogenase 1a1 mediates a GABA synthesis pathway in midbrain dopaminergic neurons. Science 2015, 350, 102-6. [CrossRef]
- Magri, C.; Giacopuzzi, E.; La Via, L.; Bonini, D.; Ravasio, V.; Elhussiny, M. E. A.; Orizio, F.; Gangemi, F.; Valsecchi, P.; Bresciani, R.; Barbon, A.; Vita, A.; Gennarelli, M., A novel homozygous mutation in GAD1 gene described in a schizophrenic patient impairs activity and dimerization of GAD67 enzyme. Scientific reports 2018, 8, 15470. [CrossRef]
- Fletcher, E. L.; Phipps, J. A.; Ward, M. M.; Puthussery, T.; Wilkinson-Berka, J. L., Neuronal and glial cell abnormality as predictors of progression of diabetic retinopathy. Curr Pharm Des 2007, 13, 2699-712. [CrossRef]
- Malomouzh, A.; Ilyin, V.; Nikolsky, E., Components of the GABAergic signaling in the peripheral cholinergic synapses of vertebrates: a review. Amino acids 2019, 51, 1093-1102. [CrossRef]
- Eskandari, S.; Willford, S. L.; Anderson, C. M., Revised Ion/Substrate Coupling Stoichiometry of GABA Transporters. Adv Neurobiol 2017, 16, 85-116. [CrossRef]
- Gether, U.; Andersen, P. H.; Larsson, O. M.; Schousboe, A., Neurotransmitter transporters: molecular function of important drug targets. Trends in pharmacological sciences 2006, 27, 375-83. [CrossRef]
- Kubrusly, R. C.; da Cunha, M. C.; Reis, R. A.; Soares, H.; Ventura, A. L.; Kurtenbach, E.; de Mello, M. C.; de Mello, F. G., Expression of functional receptors and transmitter enzymes in cultured Muller cells. Brain Res 2005, 1038, 141-9. [CrossRef]
- Scimemi, A., Structure, function, and plasticity of GABA transporters. Frontiers in cellular neuroscience 2014, 8, 161. [CrossRef]
- Calaza, K. C.; Gardino, P. F.; de Mello, F. G., Transporter mediated GABA release in the retina: role of excitatory amino acids and dopamine. Neurochemistry international 2006, 49, 769-77. [CrossRef]
- Schwartz, E. A., Transport-mediated synapses in the retina. Physiological reviews 2002, 82, 875-91. [CrossRef]
- Leviel, V., Dopamine release mediated by the dopamine transporter, facts and consequences. Journal of neurochemistry 2011, 118, 475-89. [CrossRef]
- Nicholls, D.; Attwell, D., The release and uptake of excitatory amino acids. Trends Pharmacol Sci 1990, 11, 462-8. [CrossRef]
- Roux, M. J.; Supplisson, S., Neuronal and glial glycine transporters have different stoichiometries. Neuron 2000, 25, 373-83. [CrossRef]
- Calaza Kda, C.; de Mello, M. C.; de Mello, F. G.; Gardino, P. F., Local differences in GABA release induced by excitatory amino acids during retina development: selective activation of NMDA receptors by aspartate in the inner retina. Neurochem Res 2003, 28, 1475-85.
- Yazulla, S.; Kleinschmidt, J., Dopamine blocks carrier-mediated release of GABA from retinal horizontal cells. Brain Res 1982, 233, 211-5. [CrossRef]
- Do Nascimento, J. L.; Kubrusly, R. C.; Reis, R. A.; De Mello, M. C.; De Mello, F. G., Atypical effect of dopamine in modulating the functional inhibition of NMDA receptors of cultured retina cells. Eur J Pharmacol 1998, 343, 103-10. [CrossRef]
- Maggesissi, R. S.; Gardino, P. F.; Guimarães-Souza, E. M.; Paes-de-Carvalho, R.; Silva, R. B.; Calaza, K. C., Modulation of GABA release by nitric oxide in the chick retina: different effects of nitric oxide depending on the cell population. Vision research 2009, 49, 2494-502. [CrossRef]
- Ferreira, I. L.; Duarte, C. B.; Santos, P. F.; Carvalho, C. M.; Carvalho, A. P., Release of [3H]GABA evoked by glutamate receptor agonists in cultured chick retina cells: effect of Ca2+. Brain Res 1994, 664, (1-2), 252-6. [CrossRef]
- Melone, M.; Ciappelloni, S.; Conti, F., Plasma membrane transporters GAT-1 and GAT-3 contribute to heterogeneity of GABAergic synapses in neocortex. Front Neuroanat 2014, 8, 72. [CrossRef]
- do Nascimento, J. L.; Ventura, A. L.; Paes de Carvalho, R., Veratridine- and glutamate-induced release of [3H]-GABA from cultured chick retina cells: possible involvement of a GAT-1-like subtype of GABA transporter. Brain Res 1998, 798, (1-2), 217-22. [CrossRef]
- Borges-Martins, V. P. P.; Ferreira, D. D. P.; Souto, A. C.; Oliveira Neto, J. G.; Pereira-Figueiredo, D.; da Costa Calaza, K.; de Jesus Oliveira, K.; Manhaes, A. C.; de Melo Reis, R. A.; Kubrusly, R. C. C., Caffeine regulates GABA transport via A1R blockade and cAMP signaling. Neurochem Int 2019, 104550. [CrossRef]
- Tapia, R.; Arias, C., Selective stimulation of neurotransmitter release from chick retina by kainic and glutamic acids. Journal of neurochemistry 1982, 39, 1169-78. [CrossRef]
- Calaza, K. C.; de Mello, F. G.; Gardino, P. F., GABA release induced by aspartate-mediated activation of NMDA receptors is modulated by dopamine in a selective subpopulation of amacrine cells. J Neurocytol 2001, 30, 181-93.
- Pohl-Guimarães, F.; Calaza Kda, C.; Yamasaki, E. N.; Kubrusly, R. C.; Reis, R. A., Ethanol increases GABA release in the embryonic avian retina. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 2010, 28, 189-94. [CrossRef]
- Cristóvão-Ferreira, S.; Vaz, S. H.; Ribeiro, J. A.; Sebastião, A. M., Adenosine A2A receptors enhance GABA transport into nerve terminals by restraining PKC inhibition of GAT-1. Journal of neurochemistry 2009, 109, 336-47. [CrossRef]
- Ehinger, B.; Florén, I., Quantitation of the uptake of indoleamines and dopamine in the rabbit retina. Exp Eye Res 1978, 26, 1-11. [CrossRef]
- Feldkaemper, M.; Schaeffel, F., An updated view on the role of dopamine in myopia. Exp Eye Res 2013, 114, 106-19. [CrossRef]
- Reis, R. A.; Ventura, A. L.; Kubrusly, R. C.; de Mello, M. C.; de Mello, F. G., Dopaminergic signaling in the developing retina. Brain Res Rev 2007, 54, 181-8. [CrossRef]
- Lankford, K. L.; DeMello, F. G.; Klein, W. L., D1-type dopamine receptors inhibit growth cone motility in cultured retina neurons: evidence that neurotransmitters act as morphogenic growth regulators in the developing central nervous system. Proceedings of the National Academy of Sciences of the United States of America 1988, 85, 4567-71. [CrossRef]
- Gardino, P. F.; dos Santos, R. M.; Hokoç, J. N., Histogenesis and topographical distribution of tyrosine hydroxylase immunoreactive amacrine cells in the developing chick retina. Brain Res Dev Brain Res 1993, 72, 226-36. [CrossRef]
- Kubrusly, R. C.; Guimarães, M. Z.; Vieira, A. P.; Hokoç, J. N.; Casarini, D. E.; de Mello, M. C.; de Mello, F. G., L-DOPA supply to the neuro retina activates dopaminergic communication at the early stages of embryonic development. Journal of neurochemistry 2003, 86, 45-54. [CrossRef]
- Ming, M.; Li, X.; Fan, X.; Yang, D.; Li, L.; Chen, S.; Gu, Q.; Le, W., Retinal pigment epithelial cells secrete neurotrophic factors and synthesize dopamine: possible contribution to therapeutic effects of RPE cell transplantation in Parkinson's disease. J Transl Med 2009, 7, 53. [CrossRef]
- de Mello, M. C.; Ventura, A. L.; Paes de Carvalho, R.; Klein, W. L.; de Mello, F. G., Regulation of dopamine- and adenosine-dependent adenylate cyclase systems of chicken embryo retina cells in culture. Proceedings of the National Academy of Sciences of the United States of America 1982, 79, 5708-12. [CrossRef]
- Callier, S.; Snapyan, M.; Le Crom, S.; Prou, D.; Vincent, J. D.; Vernier, P., Evolution and cell biology of dopamine receptors in vertebrates. Biol Cell 2003, 95, 489-502. [CrossRef]
- Soares, H. C.; de Melo Reis, R. A.; De Mello, F. G.; Ventura, A. L.; Kurtenbach, E., Differential expression of D(1A) and D(1B) dopamine receptor mRNAs in the developing avian retina. Journal of neurochemistry 2000, 75, 1071-5. [CrossRef]
- de Mello, M. C.; Pinheiro, M. C.; de Mello, F. G., Transient expression of an atypical D1-like dopamine receptor system during avian retina differentiation. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica ... [et al.] 1996, 29, 1035-1044.
- Kubrusly, R. C.; Ventura, A. L.; de Melo Reis, R. A.; Serra, G. C.; Yamasaki, E. N.; Gardino, P. F.; de Mello, M. C.; de Mello, F. G., Norepinephrine acts as D1-dopaminergic agonist in the embryonic avian retina: late expression of beta1-adrenergic receptor shifts norepinephrine specificity in the adult tissue. Neurochem Int 2007, 50, 211-8. [CrossRef]
- Paes de Carvalho, R.; de Mello, F. G., Expression of A1 adenosine receptors modulating dopamine-dependent cyclic AMP accumulation in the chick embryo retina. Journal of neurochemistry 1985, 44, 845-51. [CrossRef]
- Guimarães, M. Z.; Hokoç, J. N.; Duvoisin, R.; Reis, R. A.; De Mello, F. G., Dopaminergic retinal cell differentiation in culture: modulation by forskolin and dopamine. The European journal of neuroscience 2001, 13, 1931-7. [CrossRef]
- Borba, J. C.; Henze, I. P.; Silveira, M. S.; Kubrusly, R. C.; Gardino, P. F.; de Mello, M. C.; Hokoc, J. N.; de Mello, F. G., Pituitary adenylate cyclase-activating polypeptide (PACAP) can act as determinant of the tyrosine hydroxylase phenotype of dopaminergic cells during retina development. Brain Res Dev Brain Res 2005, 156, 193-201. [CrossRef]
- Katona, I.; Freund, T. F., Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nature medicine 2008, 14, 923-30. [CrossRef]
- Heifets, B. D.; Castillo, P. E., Endocannabinoid signaling and long-term synaptic plasticity. Annual review of physiology 2009, 71, 283-306. [CrossRef]
- Bockmann, E. C.; Brito, R.; Madeira, L. F.; da Silva Sampaio, L.; de Melo Reis, R. A.; França, G. R.; Calaza, K. D. C., The Role of Cannabinoids in CNS Development: Focus on Proliferation and Cell Death. Cell Mol Neurobiol 2022. [CrossRef]
- Miranzadeh Mahabadi, H.; Bhatti, H.; Laprairie, R. B.; Taghibiglou, C., Cannabinoid receptors distribution in mouse cortical plasma membrane compartments. Molecular brain 2021, 14, 89. [CrossRef]
- Fernández-Ruiz, J. J.; Berrendero, F.; Hernández, M. L.; Romero, J.; Ramos, J. A., Role of endocannabinoids in brain development. Life Sci 1999, 65, (6-7), 725-36. [CrossRef]
- da Silva Sampaio, L.; Kubrusly, R. C. C.; Colli, Y. P.; Trindade, P. P.; Ribeiro-Resende, V. T.; Einicker-Lamas, M.; Paes-de-Carvalho, R.; Gardino, P. F.; de Mello, F. G.; De Melo Reis, R. A., Cannabinoid Receptor Type 1 Expression in the Developing Avian Retina: Morphological and Functional Correlation With the Dopaminergic System. Frontiers in cellular neuroscience 2018, 12, 58. [CrossRef]
- Kubrusly, R. C. C.; Gunter, A.; Sampaio, L.; Martins, R. S.; Schitine, C. S.; Trindade, P.; Fernandes, A.; Borelli-Torres, R.; Miya-Coreixas, V. S.; Rego Costa, A. C.; Freitas, H. R.; Gardino, P. F.; de Mello, F. G.; Calaza, K. C.; Reis, R. A. M., Neuro-glial cannabinoid receptors modulate signaling in the embryonic avian retina. Neurochem Int 2018, 112, 27-37. [CrossRef]
- Jo, A. O.; Noel, J. M.; Lakk, M.; Yarishkin, O.; Ryskamp, D. A.; Shibasaki, K.; McCall, M. A.; Križaj, D., Mouse retinal ganglion cell signalling is dynamically modulated through parallel anterograde activation of cannabinoid and vanilloid pathways. The Journal of physiology 2017, 595, 6499-6516. [CrossRef]
- Straiker, A.; Sullivan, J. M., Cannabinoid receptor activation differentially modulates ion channels in photoreceptors of the tiger salamander. Journal of neurophysiology 2003, 89, 2647-54. [CrossRef]
- Gallo Afflitto, G.; Aiello, F.; Scuteri, D.; Bagetta, G.; Nucci, C., CB(1)R, CB(2)R and TRPV1 expression and modulation in in vivo, animal glaucoma models: A systematic review. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2022, 150, 112981. [CrossRef]
- Cairns, E. A.; Baldridge, W. H.; Kelly, M. E., The Endocannabinoid System as a Therapeutic Target in Glaucoma. Neural plasticity 2016, 2016, 9364091. [CrossRef]
- Nucci, C.; Gasperi, V.; Tartaglione, R.; Cerulli, A.; Terrinoni, A.; Bari, M.; De Simone, C.; Agrò, A. F.; Morrone, L. A.; Corasaniti, M. T.; Bagetta, G.; Maccarrone, M., Involvement of the endocannabinoid system in retinal damage after high intraocular pressure-induced ischemia in rats. Investigative ophthalmology & visual science 2007, 48, 2997-3004. [CrossRef]
- Rapino, C.; Tortolani, D.; Scipioni, L.; Maccarrone, M., Neuroprotection by (endo)Cannabinoids in Glaucoma and Retinal Neurodegenerative Diseases. Current neuropharmacology 2018, 16, 959-970. [CrossRef]
- Schlicker, E.; Timm, J.; Göthert, M., Cannabinoid receptor-mediated inhibition of dopamine release in the retina. Naunyn Schmiedebergs Arch Pharmacol 1996, 354, 791-5. [CrossRef]
- Buckley, N. E.; Hansson, S.; Harta, G.; Mezey, E., Expression of the CB1 and CB2 receptor messenger RNAs during embryonic development in the rat. Neuroscience 1998, 82, 1131-49. [CrossRef]
- Diacou, R.; Nandigrami, P.; Fiser, A.; Liu, W.; Ashery-Padan, R.; Cvekl, A., Cell fate decisions, transcription factors and signaling during early retinal development. Progress in retinal and eye research 2022, 101093. [CrossRef]
- Buckley, N. E.; Hansson, S.; Harta, G.; Mezey, É., Expression of the CB1 and CB2 receptor messenger RNAs during embryonic development in the rat. Neuroscience 1998, 82, 1131-1149. [CrossRef]
- Schwitzer, T.; Schwan, R.; Angioi-Duprez, K.; Giersch, A.; Laprevote, V., The Endocannabinoid System in the Retina: From Physiology to Practical and Therapeutic Applications. Neural Plast 2016, 2016, 2916732. [CrossRef]
- Straiker, A.; Stella, N.; Piomelli, D.; Mackie, K.; Karten, H. J.; Maguire, G., Cannabinoid CB1 receptors and ligands in vertebrate retina: localization and function of an endogenous signaling system. Proceedings of the National Academy of Sciences of the United States of America 1999, 96, 14565-70. [CrossRef]
- Matsuda, S.; Kanemitsu, N.; Nakamura, A.; Mimura, Y.; Ueda, N.; Kurahashi, Y.; Yamamoto, S., Metabolism of Anandamide, an Endogenous Cannabinoid Receptor Ligand, in Porcine Ocular Tissues. Experimental Eye Research 1997, 64, 707-711. [CrossRef]
- Freitas, H. R.; Isaac, A. R.; Silva, T. M.; Diniz, G. O. F.; Dos Santos Dabdab, Y.; Bockmann, E. C.; Guimaraes, M. Z. P.; da Costa Calaza, K.; de Mello, F. G.; Ventura, A. L. M.; de Melo Reis, R. A.; Franca, G. R., Cannabinoids Induce Cell Death and Promote P2X7 Receptor Signaling in Retinal Glial Progenitors in Culture. Molecular neurobiology 2019, 56, 6472-6486. [CrossRef]
- Yates, C. F.; Huang, J. Y.; Protti, D. A., Tonic Endocannabinoid Levels Modulate Retinal Signaling. Int J Environ Res Public Health 2022, 19. [CrossRef]
- Matsuda, S.; Kanemitsu, N.; Nakamura, A.; Mimura, Y.; Ueda, N.; Kurahashi, Y.; Yamamoto, S., Metabolism of anandamide, an endogenous cannabinoid receptor ligand, in porcine ocular tissues. Exp Eye Res 1997, 64, 707-11. [CrossRef]
- Begbie, J.; Doherty, P.; Graham, A., Cannabinoid receptor, CB1, expression follows neuronal differentiation in the early chick embryo. Journal of Anatomy 2004, 205, 213-218. [CrossRef]
- Leonelli, M.; Britto, L. R. G.; Chaves, G. P.; Torrão, A. S., Developmental expression of cannabinoid receptors in the chick retinotectal system. Developmental Brain Research 2005, 156, 176-182. [CrossRef]
- Hu, S. S.; Arnold, A.; Hutchens, J. M.; Radicke, J.; Cravatt, B. F.; Wager-Miller, J.; Mackie, K.; Straiker, A., Architecture of cannabinoid signaling in mouse retina. The Journal of comparative neurology 2010, 518, 3848-66. [CrossRef]
- Felder, C. C.; Glass, M., Cannabinoid receptors and their endogenous agonists. Annual review of pharmacology and toxicology 1998, 38, 179-200. [CrossRef]
- Warrier, A.; Wilson, M., Endocannabinoid signaling regulates spontaneous transmitter release from embryonic retinal amacrine cells. Visual neuroscience 2007, 24, 25-35. [CrossRef]
- Chaves, G. P.; Nogueira, T. C. A.; Britto, L. R. G.; Bordin, S.; Torrão, A. S., Retinal removal up-regulates cannabinoid CB1 receptors in the chick optic tectum. Journal of Neuroscience Research 2008, 86, 1626-1634. [CrossRef]
- Araújo, D. S. M.; Miya-Coreixas, V. S.; Pandolfo, P.; Calaza, K. C., Cannabinoid receptors and TRPA1 on neuroprotection in a model of retinal ischemia. Experimental Eye Research 2017, 154, 116-125. [CrossRef]
- Faria, R. X.; Freitas, H. R.; Reis, R. A. M., P2X7 receptor large pore signaling in avian Müller glial cells. J Bioenerg Biomembr 2017, 49, 215-229. [CrossRef]
- Faria, R. X.; Reis, R. A.; Ferreira, L. G.; Cezar-de-Mello, P. F.; Moraes, M. O., P2X7R large pore is partially blocked by pore forming proteins antagonists in astrocytes. J Bioenerg Biomembr 2016, 48, 309-24. [CrossRef]
- Zhao, Y.-F.; Tang, Y.; Illes, P., Astrocytic and Oligodendrocytic P2X7 Receptors Determine Neuronal Functions in the CNS. Frontiers in Molecular Neuroscience 2021, 14. [CrossRef]
- Freitas, H. R.; Reis, R. A. M.; Ventura, A. L. M.; Franca, G. R., Interaction between cannabinoid and nucleotide systems as a new mechanism of signaling in retinal cell death. Neural Regen Res 2019, 14, 2093-2094. [CrossRef]
- De Melo Reis, R. A.; Schitine, C. S.; Köfalvi, A.; Grade, S.; Cortes, L.; Gardino, P. F.; Malva, J. O.; de Mello, F. G., Functional identification of cell phenotypes differentiating from mice retinal neurospheres using single cell calcium imaging. Cell Mol Neurobiol 2011, 31, 835-46. [CrossRef]
- Campbell, W. A.; Blum, S.; Reske, A.; Hoang, T.; Blackshaw, S.; Fischer, A. J., Cannabinoid signaling promotes the de-differentiation and proliferation of Müller glia-derived progenitor cells. Glia 2021, 69, 2503-2521. [CrossRef]
- Cosens, D. J.; Manning, A., Abnormal electroretinogram from a Drosophila mutant. Nature 1969, 224, 285-7. [CrossRef]
- Gees, M.; Owsianik, G.; Nilius, B.; Voets, T., TRP channels. Compr Physiol 2012, 2, 563-608. [CrossRef]
- Zhao, Y.; McVeigh, B. M.; Moiseenkova-Bell, V. Y., Structural Pharmacology of TRP Channels. J Mol Biol 2021, 433, 166914. [CrossRef]
- Bisogno, T.; Delton-Vandenbroucke, I.; Milone, A.; Lagarde, M.; Di Marzo, V., Biosynthesis and inactivation of N-arachidonoylethanolamine (anandamide) and N-docosahexaenoylethanolamine in bovine retina. Arch Biochem Biophys 1999, 370, 300-7. [CrossRef]
- Bazan, N. G., Metabolism of arachidonic acid in the retina and retinal pigment epithelium: biological effects of oxygenated metabolites of arachidonic acid. Prog Clin Biol Res 1989, 312, 15-37.
- Sawamura, S.; Shirakawa, H.; Nakagawa, T.; Mori, Y.; Kaneko, S., Frontiers in Neuroscience TRP Channels in the Brain: What Are They There For? In Neurobiology of TRP Channels, Emir, T. L. R., Ed. CRC Press/Taylor & Francis © 2018 by Taylor & Francis Group, LLC.: Boca Raton (FL), 2017; pp 295-322. [CrossRef]
- Thébault, S., Minireview: Insights into the role of TRP channels in the retinal circulation and function. Neuroscience letters 2021, 765, 136285. [CrossRef]
- Gilliam, J. C.; Wensel, T. G., TRP channel gene expression in the mouse retina. Vision Res 2011, 51, (23-24), 2440-52. [CrossRef]
- Rychkov, G.; Barritt, G. J., TRPC1 Ca(2+)-permeable channels in animal cells. Handb Exp Pharmacol 2007, 23-52. [CrossRef]
- Lakk, M.; Young, D.; Baumann, J. M.; Jo, A. O.; Hu, H.; Križaj, D., Polymodal TRPV1 and TRPV4 Sensors Colocalize but Do Not Functionally Interact in a Subpopulation of Mouse Retinal Ganglion Cells. Frontiers in cellular neuroscience 2018, 12, 353. [CrossRef]
- Molnar, T.; Barabas, P.; Birnbaumer, L.; Punzo, C.; Kefalov, V.; Križaj, D., Store-operated channels regulate intracellular calcium in mammalian rods. The Journal of physiology 2012, 590, 3465-81. [CrossRef]
- Tóth, A.; Czikora, A.; Pásztor, E. T.; Dienes, B.; Bai, P.; Csernoch, L.; Rutkai, I.; Csató, V.; Mányiné, I. S.; Pórszász, R.; Edes, I.; Papp, Z.; Boczán, J., Vanilloid receptor-1 (TRPV1) expression and function in the vasculature of the rat. J Histochem Cytochem 2014, 62, 129-44. [CrossRef]
- Crousillac, S.; LeRouge, M.; Rankin, M.; Gleason, E., Immunolocalization of TRPC channel subunits 1 and 4 in the chicken retina. Vis Neurosci 2003, 20, 453-63. [CrossRef]
- Da Silva, N.; Herron, C. E.; Stevens, K.; Jollimore, C. A.; Barnes, S.; Kelly, M. E., Metabotropic receptor-activated calcium increases and store-operated calcium influx in mouse Müller cells. Invest Ophthalmol Vis Sci 2008, 49, 3065-73. [CrossRef]
- Witkovsky, P.; Gábriel, R.; Krizaj, D., Anatomical and neurochemical characterization of dopaminergic interplexiform processes in mouse and rat retinas. The Journal of comparative neurology 2008, 510, 158-74. [CrossRef]
- Maddox, J. W.; Khorsandi, N.; Gleason, E., TRPC5 is required for the NO-dependent increase in dendritic Ca(2+) and GABA release from chick retinal amacrine cells. Journal of neurophysiology 2018, 119, 262-273. [CrossRef]
- Morgans, C. W.; Zhang, J.; Jeffrey, B. G.; Nelson, S. M.; Burke, N. S.; Duvoisin, R. M.; Brown, R. L., TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proceedings of the National Academy of Sciences of the United States of America 2009, 106, 19174-8. [CrossRef]
- Hasan, N.; Pangeni, G.; Cobb, C. A.; Ray, T. A.; Nettesheim, E. R.; Ertel, K. J.; Lipinski, D. M.; McCall, M. A.; Gregg, R. G., Presynaptic Expression of LRIT3 Transsynaptically Organizes the Postsynaptic Glutamate Signaling Complex Containing TRPM1. Cell reports 2019, 27, 3107-3116.e3. [CrossRef]
- Anastassov, I. A.; Wang, W.; Dunn, F. A., Synaptogenesis and synaptic protein localization in the postnatal development of rod bipolar cell dendrites in mouse retina. The Journal of comparative neurology 2019, 527, 52-66. [CrossRef]
- Kozuka, T.; Chaya, T.; Tamalu, F.; Shimada, M.; Fujimaki-Aoba, K.; Kuwahara, R.; Watanabe, S. I.; Furukawa, T., The TRPM1 Channel Is Required for Development of the Rod ON Bipolar Cell-AII Amacrine Cell Pathway in the Retinal Circuit. The Journal of neuroscience : the official journal of the Society for Neuroscience 2017, 37, 9889-9900. [CrossRef]
- Takeuchi, H.; Horie, S.; Moritoh, S.; Matsushima, H.; Hori, T.; Kimori, Y.; Kitano, K.; Tsubo, Y.; Tachibana, M.; Koike, C., Different Activity Patterns in Retinal Ganglion Cells of TRPM1 and mGluR6 Knockout Mice. BioMed research international 2018, 2018, 2963232. [CrossRef]
- Meléndez García, R.; Arredondo Zamarripa, D.; Arnold, E.; Ruiz-Herrera, X.; Noguez Imm, R.; Baeza Cruz, G.; Adán, N.; Binart, N.; Riesgo-Escovar, J.; Goffin, V.; Ordaz, B.; Peña-Ortega, F.; Martínez-Torres, A.; Clapp, C.; Thebault, S., Prolactin protects retinal pigment epithelium by inhibiting sirtuin 2-dependent cell death. EBioMedicine 2016, 7, 35-49. [CrossRef]
- Malko, P.; Syed Mortadza, S. A.; McWilliam, J.; Jiang, L. H., TRPM2 Channel in Microglia as a New Player in Neuroinflammation Associated With a Spectrum of Central Nervous System Pathologies. Frontiers in pharmacology 2019, 10, 239. [CrossRef]
- Webster, C. M.; Tworig, J.; Caval-Holme, F.; Morgans, C. W.; Feller, M. B., The Impact of Steroid Activation of TRPM3 on Spontaneous Activity in the Developing Retina. eNeuro 2020, 7. [CrossRef]
- McGahon, M. K.; Fernández, J. A.; Dash, D. P.; McKee, J.; Simpson, D. A.; Zholos, A. V.; McGeown, J. G.; Curtis, T. M., TRPV2 Channels Contribute to Stretch-Activated Cation Currents and Myogenic Constriction in Retinal Arterioles. Investigative ophthalmology & visual science 2016, 57, 5637-5647. [CrossRef]
- Souza Monteiro de Araújo, D.; De Logu, F.; Adembri, C.; Rizzo, S.; Janal, M. N.; Landini, L.; Magi, A.; Mattei, G.; Cini, N.; Pandolfo, P.; Geppetti, P.; Nassini, R.; Calaza, K. D. C., TRPA1 mediates damage of the retina induced by ischemia and reperfusion in mice. Cell Death Dis 2020, 11, 633. [CrossRef]
- Davis, J. B.; Gray, J.; Gunthorpe, M. J.; Hatcher, J. P.; Davey, P. T.; Overend, P.; Harries, M. H.; Latcham, J.; Clapham, C.; Atkinson, K.; Hughes, S. A.; Rance, K.; Grau, E.; Harper, A. J.; Pugh, P. L.; Rogers, D. C.; Bingham, S.; Randall, A.; Sheardown, S. A., Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000, 405, 183-7. [CrossRef]
- Dhaka, A.; Uzzell, V.; Dubin, A. E.; Mathur, J.; Petrus, M.; Bandell, M.; Patapoutian, A., TRPV1 is activated by both acidic and basic pH. The Journal of neuroscience : the official journal of the Society for Neuroscience 2009, 29, 153-158. [CrossRef]
- Clapham, D. E., TRP channels as cellular sensors. Nature 2003, 426, 517-524. [CrossRef]
- Di Marzo, V., Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol 2008, 160, 1-24. [CrossRef]
- Benítez-Angeles, M.; Morales-Lázaro, S. L.; Juárez-González, E.; Rosenbaum, T., TRPV1: Structure, Endogenous Agonists, and Mechanisms. International journal of molecular sciences 2020, 21. [CrossRef]
- Jo, A. O.; Ryskamp, D. A.; Phuong, T. T.; Verkman, A. S.; Yarishkin, O.; MacAulay, N.; Križaj, D., TRPV4 and AQP4 Channels Synergistically Regulate Cell Volume and Calcium Homeostasis in Retinal Müller Glia. The Journal of neuroscience : the official journal of the Society for Neuroscience 2015, 35, 13525-13537. [CrossRef]
- Ryskamp, D. A.; Redmon, S.; Jo, A. O.; Križaj, D., TRPV1 and Endocannabinoids: Emerging Molecular Signals that Modulate Mammalian Vision. Cells 2014, 3, 914-938. [CrossRef]
- Sappington, R. M.; Calkins, D. J., Contribution of TRPV1 to microglia-derived IL-6 and NFkappaB translocation with elevated hydrostatic pressure. Invest Ophthalmol Vis Sci 2008, 49, 3004-3017. [CrossRef]
- Sappington, R. M.; Sidorova, T.; Long, D. J.; Calkins, D. J., TRPV1: contribution to retinal ganglion cell apoptosis and increased intracellular Ca2+ with exposure to hydrostatic pressure. Invest Ophthalmol Vis Sci 2009, 50, 717-728. [CrossRef]
- Yazulla, S., Endocannabinoids in the retina: from marijuana to neuroprotection. Prog Retin Eye Res 2008, 27, 501-526. [CrossRef]
- Leonelli, M.; Martins, D. O.; Kihara, A. H.; Britto, L. R., Ontogenetic expression of the vanilloid receptors TRPV1 and TRPV2 in the rat retina. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 2009, 27, 709-718. [CrossRef]
- Shen, Y.; Heimel, J. A.; Kamermans, M.; Peachey, N. S.; Gregg, R. G.; Nawy, S., A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. The Journal of neuroscience : the official journal of the Society for Neuroscience 2009, 29, 6088-6093. [CrossRef]
- Glaser, S. T.; Deutsch, D. G.; Studholme, K. M.; Zimov, S.; Yazulla, S., Endocannabinoids in the intact retina: 3 H-anandamide uptake, fatty acid amide hydrolase immunoreactivity and hydrolysis of anandamide. Visual neuroscience 2005, 22, 693-705. [CrossRef]
- Bisogno, T.; Hanus, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D. E.; Brandi, I.; Moriello, A. S.; Davis, J. B.; Mechoulam, R.; Di Marzo, V., Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 2001, 134, 845-852. [CrossRef]
- Anand, U.; Jones, B.; Korchev, Y.; Bloom, S. R.; Pacchetti, B.; Anand, P.; Sodergren, M. H., CBD Effects on TRPV1 Signaling Pathways in Cultured DRG Neurons. J Pain Res 2020, 13, 2269-2278. [CrossRef]
- de Almeida, D. L.; Devi, L. A., Diversity of molecular targets and signaling pathways for CBD. Pharmacol Res Perspect 2020, 8, e00682. [CrossRef]
- Yazulla, S.; Studholme, K. M., Vanilloid receptor like 1 (VRL1) immunoreactivity in mammalian retina: colocalization with somatostatin and purinergic P2X1 receptors. The Journal of comparative neurology 2004, 474, 407-18. [CrossRef]
- Thermos, K., Functional mapping of somatostatin receptors in the retina: a review. Vision Res 2003, 43, 1805-15. [CrossRef]
- Snyder, S. H., Adenosine as a neuromodulator. Annual review of neuroscience 1985, 8, 103-24. [CrossRef]
- Fredholm, B. B.; Chen, J. F.; Cunha, R. A.; Svenningsson, P.; Vaugeois, J. M., Adenosine and brain function. Int Rev Neurobiol 2005, 63, 191-270. [CrossRef]
- Blazynski, C.; Perez, M. T., Adenosine in vertebrate retina: localization, receptor characterization, and function. Cell Mol Neurobiol 1991, 11, 463-484. [CrossRef]
- Shewan, D.; Dwivedy, A.; Anderson, R.; Holt, C. E., Age-related changes underlie switch in netrin-1 responsiveness as growth cones advance along visual pathway. Nature neuroscience 2002, 5, 955-962. [CrossRef]
- Zhang, M.; Budak, M. T.; Lu, W.; Khurana, T. S.; Zhang, X.; Laties, A. M.; Mitchell, C. H., Identification of the A3 adenosine receptor in rat retinal ganglion cells. Mol Vis 2006, 12, 937-948.
- Portugal, C. C.; da Encarnação, T. G.; Sagrillo, M. A.; Pereira, M. R.; Relvas, J. B.; Socodato, R.; Paes-de-Carvalho, R., Activation of adenosine A3 receptors regulates vitamin C transport and redox balance in neurons. Free Radic Biol Med 2021, 163, 43-55. [CrossRef]
- Duarte-Silva, A. T.; Ximenes, L. G. R.; Guimarães-Souza, M.; Domith, I.; Paes-de-Carvalho, R., Chemical signaling in the developing avian retina: Focus on cyclic AMP and AKT-dependent pathways. Front Cell Dev Biol 2022, 10, 1058925. [CrossRef]
- Paes de Carvalho, R., Development of A1 adenosine receptors in the chick embryo retina. J Neurosci Res 1990, 25, 236-42. [CrossRef]
- Paes de Carvalho, R.; de Mello, F. G., Adenosine-elicited accumulation of adenosine 3', 5'-cyclic monophosphate in the chick embryo retina. Journal of neurochemistry 1982, 38, 493-500. [CrossRef]
- de Carvalho, R. P.; Braas, K. M.; Adler, R.; Snyder, S. H., Developmental regulation of adenosine A1 receptors, uptake sites and endogenous adenosine in the chick retina. Brain Res Dev Brain Res 1992, 70, 87-95. [CrossRef]
- dos Santos-Rodrigues, A.; Ferreira, J. M.; Paes-de-Carvalho, R., Differential adenosine uptake in mixed neuronal/glial or purified glial cultures of avian retinal cells: modulation by adenosine metabolism and the ERK cascade. Biochem Biophys Res Commun 2011, 414, 175-80. [CrossRef]
- Pereira, M. R.; Hang, V. R.; Vardiero, E.; de Mello, F. G.; Paes-de-Carvalho, R., Modulation of A1 adenosine receptor expression by cell aggregation and long-term activation of A2a receptors in cultures of avian retinal cells: involvement of the cyclic AMP/PKA pathway. Journal of neurochemistry 2010, 113, 661-73. [CrossRef]
- Paes-de-Carvalho, R.; Maia, G. A.; Ferreira, J. M., Adenosine regulates the survival of avian retinal neurons and photoreceptors in culture. Neurochem Res 2003, 28, 1583-90. [CrossRef]
- Ferreira, J. M.; Paes-de-Carvalho, R., Long-term activation of adenosine A(2a) receptors blocks glutamate excitotoxicity in cultures of avian retinal neurons. Brain Res 2001, 900, 169-176. [CrossRef]
- Socodato, R.; Brito, R.; Calaza, K. C.; Paes-de-Carvalho, R., Developmental regulation of neuronal survival by adenosine in the in vitro and in vivo avian retina depends on a shift of signaling pathways leading to CREB phosphorylation or dephosphorylation. Journal of neurochemistry 2011, 116, 227-239. [CrossRef]
- Paes de Carvalho, R.; Braas, K. M.; Snyder, S. H.; Adler, R., Analysis of adenosine immunoreactivity, uptake, and release in purified cultures of developing chick embryo retinal neurons and photoreceptors. Journal of neurochemistry 1990, 55, 1603-1611. [CrossRef]
- Paes-de-Carvalho, R.; Dias, B. V.; Martins, R. A.; Pereira, M. R.; Portugal, C. C.; Lanfredi, C., Activation of glutamate receptors promotes a calcium-dependent and transporter-mediated release of purines in cultured avian retinal cells: possible involvement of calcium/calmodulin-dependent protein kinase II. Neurochemistry international 2005, 46, 441-451. [CrossRef]
- Langer, I.; Jeandriens, J.; Couvineau, A.; Sanmukh, S.; Latek, D., Signal Transduction by VIP and PACAP Receptors. Biomedicines 2022, 10. [CrossRef]
- Hirabayashi, T.; Nakamachi, T.; Shioda, S., Discovery of PACAP and its receptors in the brain. J Headache Pain 2018, 19, 28. [CrossRef]
- May, V.; Parsons, R. L., G Protein-Coupled Receptor Endosomal Signaling and Regulation of Neuronal Excitability and Stress Responses: Signaling Options and Lessons From the PAC1 Receptor. J Cell Physiol 2017, 232, 698-706. [CrossRef]
- Onali, P.; Olianas, M. C., PACAP is a potent and highly effective stimulator of adenylyl cyclase activity in the retinas of different mammalian species. Brain Res 1994, 641, 132-134. [CrossRef]
- Denes, V.; Geck, P.; Mester, A.; Gabriel, R., Pituitary Adenylate Cyclase-Activating Polypeptide: 30 Years in Research Spotlight and 600 Million Years in Service. J Clin Med 2019, 8. [CrossRef]
- Shioda, S.; Takenoya, F.; Wada, N.; Hirabayashi, T.; Seki, T.; Nakamachi, T., Pleiotropic and retinoprotective functions of PACAP. Anat Sci Int 2016, 91, 313-324. [CrossRef]
- Njaine, B.; Martins, R. A.; Santiago, M. F.; Linden, R.; Silveira, M. S., Pituitary adenylyl cyclase-activating polypeptide controls the proliferation of retinal progenitor cells through downregulation of cyclin D1. The European journal of neuroscience 2010, 32, 311-321. [CrossRef]
- Njaine, B.; Rocha-Martins, M.; Vieira-Vieira, C. H.; De-Melo, L. D.; Linden, R.; Braas, K.; May, V.; Martins, R. A.; Silveira, M. S., Pleiotropic functions of pituitary adenylyl cyclase-activating polypeptide on retinal ontogenesis: involvement of KLF4 in the control of progenitor cell proliferation. J Mol Neurosci 2014, 54, 430-442. [CrossRef]
- Fleming, R. L.; Silveira, M. S.; Santos, L. E.; Henze, I. P.; Gardino, P. F.; de Mello, M. C.; de Mello, F. G., Pituitary adenylyl cyclase-activating polypeptide receptor re-sensitization induces plastic changes in the dopaminergic phenotype in the mature avian retina. Journal of neurochemistry 2013, 124, 621-631. [CrossRef]
- Silveira, M. S.; Costa, M. R.; Bozza, M.; Linden, R., Pituitary adenylyl cyclase-activating polypeptide prevents induced cell death in retinal tissue through activation of cyclic AMP-dependent protein kinase. The Journal of biological chemistry 2002, 277, 16075-16080. [CrossRef]
- Denes, V.; Hideg, O.; Nyisztor, Z.; Lakk, M.; Godri, Z.; Berta, G.; Geck, P.; Gabriel, R., The Neuroprotective Peptide PACAP1-38 Contributes to Horizontal Cell Development in Postnatal Rat Retina. Invest Ophthalmol Vis Sci 2019, 60, 770-778. [CrossRef]
- Seki, T.; Itoh, H.; Nakamachi, T.; Endo, K.; Wada, Y.; Nakamura, K.; Shioda, S., Suppression of rat retinal ganglion cell death by PACAP following transient ischemia induced by high intraocular pressure. J Mol Neurosci 2011, 43, 30-34. [CrossRef]
- Danyadi, B.; Szabadfi, K.; Reglodi, D.; Mihalik, A.; Danyadi, T.; Kovacs, Z.; Batai, I.; Tamas, A.; Kiss, P.; Toth, G.; Gabriel, R., PACAP application improves functional outcome of chronic retinal ischemic injury in rats-evidence from electroretinographic measurements. J Mol Neurosci 2014, 54, 293-299. [CrossRef]
- Kvarik, T.; Mammel, B.; Reglodi, D.; Kovacs, K.; Werling, D.; Bede, B.; Vaczy, A.; Fabian, E.; Toth, G.; Kiss, P.; Tamas, A.; Ertl, T.; Gyarmati, J.; Atlasz, T., PACAP Is Protective in a Rat Model of Retinopathy of Prematurity. J Mol Neurosci 2016, 60, 179-185. [CrossRef]
- Kvarik, T.; Reglodi, D.; Werling, D.; Vaczy, A.; Kovari, P.; Szabo, E.; Kovacs, K.; Hashimoto, H.; Ertl, T.; Gyarmati, J.; Atlasz, T., The Protective Effects of Endogenous PACAP in Oxygen-Induced Retinopathy. J Mol Neurosci 2021, 71, 2546-2557. [CrossRef]
- Patko, E.; Szabo, E.; Vaczy, A.; Molitor, D.; Tari, E.; Li, L.; Csutak, A.; Toth, G.; Reglodi, D.; Atlasz, T., Protective Effects of Pituitary Adenylate-Cyclase-Activating Polypeptide on Retinal Vasculature and Molecular Responses in a Rat Model of Moderate Glaucoma. International journal of molecular sciences 2023, 24. [CrossRef]
- Atlasz, T.; Szabadfi, K.; Kiss, P.; Marton, Z.; Griecs, M.; Hamza, L.; Gaal, V.; Biro, Z.; Tamas, A.; Hild, G.; Nyitrai, M.; Toth, G.; Reglodi, D.; Gabriel, R., Effects of PACAP in UV-A radiation-induced retinal degeneration models in rats. J Mol Neurosci 2011, 43, 51-7. [CrossRef]
- Gábriel, R.; Pöstyéni, E.; Dénes, V., Neuroprotective Potential of Pituitary Adenylate Cyclase Activating Polypeptide in Retinal Degenerations of Metabolic Origin. Frontiers in neuroscience 2019, 13, 1031. [CrossRef]
- Wang, T.; Li, Y.; Guo, M.; Dong, X.; Liao, M.; Du, M.; Wang, X.; Yin, H.; Yan, H., Exosome-Mediated Delivery of the Neuroprotective Peptide PACAP38 Promotes Retinal Ganglion Cell Survival and Axon Regeneration in Rats With Traumatic Optic Neuropathy. Front Cell Dev Biol 2021, 9, 659783. [CrossRef]
- Van, C.; Condro, M. C.; Ko, H. H.; Hoang, A. Q.; Zhu, R.; Lov, K.; Ricaflanca, P. T.; Diep, A. L.; Nguyen, N. N. M.; Lipshutz, G. S.; MacKenzie-Graham, A.; Waschek, J. A., Targeted deletion of PAC1 receptors in retinal neurons enhances neuron loss and axonopathy in a model of multiple sclerosis and optic neuritis. Neurobiology of disease 2021, 160, 105524. [CrossRef]
- Goldstein, I. M.; Ostwald, P.; Roth, S., Nitric oxide: a review of its role in retinal function and disease. Vision Res 1996, 36, 2979-2994. [CrossRef]
- Toda, N.; Nakanishi-Toda, M., Nitric oxide: ocular blood flow, glaucoma, and diabetic retinopathy. Prog Retin Eye Res 2007, 26, 205-38. [CrossRef]
- Cossenza, M.; Socodato, R.; Portugal, C. C.; Domith, I. C.; Gladulich, L. F.; Encarnação, T. G.; Calaza, K. C.; Mendonça, H. R.; Campello-Costa, P.; Paes-de-Carvalho, R., Nitric oxide in the nervous system: biochemical, developmental, and neurobiological aspects. Vitamins and hormones 2014, 96, 79-125. [CrossRef]
- Cossenza, M.; Paes de Carvalho, R., L-arginine uptake and release by cultured avian retinal cells: differential cellular localization in relation to nitric oxide synthase. Journal of neurochemistry 2000, 74, 1885-1894. [CrossRef]
- Do, K. Q.; Grima, G.; Benz, B.; Salt, T. E., Glial-neuronal transfer of arginine and S-nitrosothiols in nitric oxide transmission. Ann N Y Acad Sci 2002, 962, 81-92. [CrossRef]
- Grima, G.; Benz, B.; Do, K. Q., Glutamate-induced release of the nitric oxide precursor, arginine, from glial cells. The European journal of neuroscience 1997, 9, 2248-2258. [CrossRef]
- Grima, G.; Benz, B.; Do, K. Q., Glial-derived arginine, the nitric oxide precursor, protects neurons from NMDA-induced excitotoxicity. The European journal of neuroscience 2001, 14, 1762-1770. [CrossRef]
- Bredt, D. S.; Ferris, C. D.; Snyder, S. H., Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. The Journal of biological chemistry 1992, 267, 10976-10981. [CrossRef]
- Lamas, S.; Marsden, P. A.; Li, G. K.; Tempst, P.; Michel, T., Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proceedings of the National Academy of Sciences of the United States of America 1992, 89, 6348-6352. [CrossRef]
- Cho, H. J.; Xie, Q. W.; Calaycay, J.; Mumford, R. A.; Swiderek, K. M.; Lee, T. D.; Nathan, C., Calmodulin is a subunit of nitric oxide synthase from macrophages. The Journal of experimental medicine 1992, 176, 599-604. [CrossRef]
- Garthwaite, J., Nitric oxide signalling in the nervous system. Seminars in Neuroscience 1993, 5, 171-180. [CrossRef]
- Garthwaite, J.; Boulton, C. L., Nitric oxide signaling in the central nervous system. Annual review of physiology 1995, 57, 683-706. [CrossRef]
- Garthwaite, J.; Charles, S. L.; Chess-Williams, R., Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 1988, 336, 385-388. [CrossRef]
- Garthwaite, J.; Garthwaite, G., Cellular origins of cyclic GMP responses to excitatory amino acid receptor agonists in rat cerebellum in vitro. Journal of neurochemistry 1987, 48, 29-39. [CrossRef]
- Brenman, J. E.; Bredt, D. S., Synaptic signaling by nitric oxide. Current opinion in neurobiology 1997, 7, 374-378. [CrossRef]
- Brenman, J. E.; Chao, D. S.; Gee, S. H.; McGee, A. W.; Craven, S. E.; Santillano, D. R.; Wu, Z.; Huang, F.; Xia, H.; Peters, M. F.; Froehner, S. C.; Bredt, D. S., Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 1996, 84, 757-767. [CrossRef]
- Dawson, T. M.; Bredt, D. S.; Fotuhi, M.; Hwang, P. M.; Snyder, S. H., Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proceedings of the National Academy of Sciences of the United States of America 1991, 88, 7797-7801. [CrossRef]
- Hope, B. T.; Michael, G. J.; Knigge, K. M.; Vincent, S. R., Neuronal NADPH Diaphorase is a Nitric Oxide Synthase. Proceedings of the National Academy of Sciences of the United States of America 1991, 88, 2811-2814. [CrossRef]
- Kurenny, D. E.; Moroz, L. L.; Turner, R. W.; Sharkey, K. A.; Barnes, S., Modulation of ion channels in rod photoreceptors by nitric oxide. Neuron 1994, 13, 315-324. [CrossRef]
- Yamamoto, R.; Bredt, D. S.; Snyder, S. H.; Stone, R. A., The localization of nitric oxide synthase in the rat eye and related cranial ganglia. Neuroscience 1993, 54, 189-200. [CrossRef]
- Vielma, A. H.; Retamal, M. A.; Schmachtenberg, O., Nitric oxide signaling in the retina: what have we learned in two decades? Brain Res 2012, 1430, 112-125. [CrossRef]
- Andrade da Costa, B. L.; Hokoç, J. N., Coexistence of GAD-65 and GAD-67 with tyrosine hydroxylase and nitric oxide synthase in amacrine and interplexiform cells of the primate, Cebus apella. Visual neuroscience 2003, 20, 153-163. [CrossRef]
- Vardi, N.; Auerbach, P., Specific cell types in cat retina express different forms of glutamic acid decarboxylase. The Journal of comparative neurology 1995, 351, 374-384. [CrossRef]
- Socodato, R.; Brito, R.; Portugal, C. C.; de Oliveira, N. A.; Calaza, K. C.; Paes-de-Carvalho, R., The nitric oxide-cGKII system relays death and survival signals during embryonic retinal development via AKT-induced CREB1 activation. Cell death and differentiation 2014, 21, 915-928. [CrossRef]
- Pang, J. J.; Gao, F.; Wu, S. M., Light responses and morphology of bNOS-immunoreactive neurons in the mouse retina. The Journal of comparative neurology 2010, 518, 2456-2474. [CrossRef]
- Blom, J.; Giove, T.; Deshpande, M.; Eldred, W. D., Characterization of nitric oxide signaling pathways in the mouse retina. The Journal of comparative neurology 2012, 520, 4204-4217. [CrossRef]
- Tekmen-Clark, M.; Gleason, E., Nitric oxide production and the expression of two nitric oxide synthases in the avian retina. Visual neuroscience 2013, 30, 91-103. [CrossRef]
- Djamgoz, M. B.; Sekaran, S.; Angotzi, A. R.; Haamedi, S.; Vallerga, S.; Hirano, J.; Yamada, M., Light-adaptive role of nitric oxide in the outer retina of lower vertebrates: a brief review. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 2000, 355, 1199-1203. [CrossRef]
- Giove, T. J.; Deshpande, M. M.; Eldred, W. D., Identification of alternate transcripts of neuronal nitric oxide synthase in the mouse retina. J Neurosci Res 2009, 87, 3134-3142. [CrossRef]
- Shi, Q.; Teves, M. M.; Lillywhite, A.; Pagtalunan, E. B.; Stell, W. K., Light adaptation in the chick retina: Dopamine, nitric oxide, and gap-junction coupling modulate spatiotemporal contrast sensitivity. Exp Eye Res 2020, 195, 108026. [CrossRef]
- Sato, M.; Ohtsuka, T.; Stell, W. K., Endogenous nitric oxide enhances the light-response of cones during light-adaptation in the rat retina. Vision Res 2011, 51, 131-137. [CrossRef]
- DeVries, S. H.; Schwartz, E. A., Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. The Journal of physiology 1989, 414, 351-375. [CrossRef]
- Mills, S. L.; Massey, S. C., Differential properties of two gap junctional pathways made by AII amacrine cells. Nature 1995, 377, 734-737. [CrossRef]
- Ding, J. D.; Weinberg, R. J., Distribution of soluble guanylyl cyclase in rat retina. The Journal of comparative neurology 2007, 500, 734-745. [CrossRef]
- Hirooka, K.; Kourennyi, D. E.; Barnes, S., Calcium channel activation facilitated by nitric oxide in retinal ganglion cells. Journal of neurophysiology 2000, 83, 198-206. [CrossRef]
- Wexler, E. M.; Stanton, P. K.; Nawy, S., Nitric oxide depresses GABAA receptor function via coactivation of cGMP-dependent kinase and phosphodiesterase. The Journal of neuroscience : the official journal of the Society for Neuroscience 1998, 18, 2342-9. [CrossRef]
- McMahon, D. G.; Ponomareva, L. V., Nitric oxide and cGMP modulate retinal glutamate receptors. Journal of neurophysiology 1996, 76, 2307-2315. [CrossRef]
- McMahon, D. G.; Schmidt, K. F., Horizontal cell glutamate receptor modulation by NO: mechanisms and functional implications for the first visual synapse. Visual neuroscience 1999, 16, 425-433. [CrossRef]
- Ientile, R.; Pedale, S.; Picciurro, V.; Macaione, V.; Fabiano, C.; Macaione, S., Nitric oxide mediates NMDA-evoked [3H]GABA release from chick retina cells. FEBS letters 1997, 417, 345-348. [CrossRef]
- Ientile, R.; Picciurro, V.; Pedale, S.; Nucci, C.; Malecka, B.; Nisticò, G.; Macaione, S., Nitric oxide enhances amino acid release from immature chick embryo retina. Neuroscience letters 1996, 219, 79-82. [CrossRef]
- Yu, D.; Eldred, W. D., Nitric oxide stimulates gamma-aminobutyric acid release and inhibits glycine release in retina. The Journal of comparative neurology 2005, 483, 278-291. [CrossRef]
- Tsukaguchi, H.; Tokui, T.; Mackenzie, B.; Berger, U. V.; Chen, X. Z.; Wang, Y.; Brubaker, R. F.; Hediger, M. A., A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 1999, 399, 70-75. [CrossRef]
- Portugal, C. C.; da Encarnação, T. G.; Socodato, R.; Moreira, S. R.; Brudzewsky, D.; Ambrósio, A. F.; Paes-de-Carvalho, R., Nitric oxide modulates sodium vitamin C transporter 2 (SVCT-2) protein expression via protein kinase G (PKG) and nuclear factor-κB (NF-κB). The Journal of biological chemistry 2012, 287, 3860-3872. [CrossRef]
- Portugal, C. C.; Miya, V. S.; Calaza Kda, C.; Santos, R. A.; Paes-de-Carvalho, R., Glutamate receptors modulate sodium-dependent and calcium-independent vitamin C bidirectional transport in cultured avian retinal cells. Journal of neurochemistry 2009, 108, 507-520. [CrossRef]
- Socodato, R. E.; Magalhaes, C. R.; Paes-de-Carvalho, R., Glutamate and nitric oxide modulate ERK and CREB phosphorylation in the avian retina: evidence for direct signaling from neurons to Muller glial cells. Journal of neurochemistry 2009, 108, 417-429. [CrossRef]
- Moriyama, S.; Hiasa, M., Expression of Vesicular Nucleotide Transporter in the Mouse Retina. Biol Pharm Bull 2016, 39, 564-569. [CrossRef]
- Xia, J.; Lim, J. C.; Lu, W.; Beckel, J. M.; Macarak, E. J.; Laties, A. M.; Mitchell, C. H., Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7 receptors. The Journal of physiology 2012, 590, 2285-304. [CrossRef]
- Mitchell, C. H., Release of ATP by a human retinal pigment epithelial cell line: potential for autocrine stimulation through subretinal space. The Journal of physiology 2001, 534, (Pt 1), 193-202. [CrossRef]
- Santos, P. F.; Caramelo, O. L.; Carvalho, A. P.; Duarte, C. B., Characterization of ATP release from cultures enriched in cholinergic amacrine-like neurons. Journal of neurobiology 1999, 41, 340-348. [CrossRef]
- Newman, E. A., Calcium increases in retinal glial cells evoked by light-induced neuronal activity. The Journal of neuroscience : the official journal of the Society for Neuroscience 2005, 25, 5502-5510. [CrossRef]
- Uckermann, O.; Wolf, A.; Kutzera, F.; Kalisch, F.; Beck-Sickinger, A. G.; Wiedemann, P.; Reichenbach, A.; Bringmann, A., Glutamate release by neurons evokes a purinergic inhibitory mechanism of osmotic glial cell swelling in the rat retina: activation by neuropeptide Y. J Neurosci Res 2006, 83, 538-550. [CrossRef]
- Loiola, E. C.; Ventura, A. L., Release of ATP from avian Müller glia cells in culture. Neurochemistry international 2011, 58, 414-422. [CrossRef]
- Ventura, A. L. M.; Dos Santos-Rodrigues, A.; Mitchell, C. H.; Faillace, M. P., Purinergic signaling in the retina: From development to disease. Brain Res Bull 2019, 151, 92-108. [CrossRef]
- de Almeida-Pereira, L.; Magalhães, C. F.; Repossi, M. G.; Thorstenberg, M. L. P.; Sholl-Franco, A.; Coutinho-Silva, R.; Ventura, A. L. M.; Fragel-Madeira, L., Adenine Nucleotides Control Proliferation In Vivo of Rat Retinal Progenitors by P2Y(1) Receptor. Molecular neurobiology 2017, 54, 5142-5155. [CrossRef]
- Jacques, F. J.; Silva, T. M.; da Silva, F. E.; Ornelas, I. M.; Ventura, A. L. M., Nucleotide P2Y13-stimulated phosphorylation of CREB is required for ADP-induced proliferation of late developing retinal glial progenitors in culture. Cell Signal 2017, 35, 95-106. [CrossRef]
- Sugioka, M.; Fukuda, Y.; Yamashita, M., Ca2+ responses to ATP via purinoceptors in the early embryonic chick retina. The Journal of physiology 1996, 493 ( Pt 3), (Pt 3), 855-63. [CrossRef]
- Pearson, R.; Catsicas, M.; Becker, D.; Mobbs, P., Purinergic and muscarinic modulation of the cell cycle and calcium signaling in the chick retinal ventricular zone. The Journal of neuroscience : the official journal of the Society for Neuroscience 2002, 22, 7569-7579. [CrossRef]
- Pearson, R. A.; Catsicas, M.; Becker, D. L.; Bayley, P.; Lüneborg, N. L.; Mobbs, P., Ca(2+) signalling and gap junction coupling within and between pigment epithelium and neural retina in the developing chick. The European journal of neuroscience 2004, 19, 2435-2445. [CrossRef]
- Sanches, G.; de Alencar, L. S.; Ventura, A. L., ATP induces proliferation of retinal cells in culture via activation of PKC and extracellular signal-regulated kinase cascade. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 2002, 20, 21-27. [CrossRef]
- França, G. R.; Freitas, R. C.; Ventura, A. L., ATP-induced proliferation of developing retinal cells: regulation by factors released from postmitotic cells in culture. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 2007, 25, 283-291. [CrossRef]
- Sholl-Franco, A.; Fragel-Madeira, L.; Macama Ada, C.; Linden, R.; Ventura, A. L., ATP controls cell cycle and induces proliferation in the mouse developing retina. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 2010, 28, 63-73. [CrossRef]
- Nunes, P. H.; Calaza Kda, C.; Albuquerque, L. M.; Fragel-Madeira, L.; Sholl-Franco, A.; Ventura, A. L., Signal transduction pathways associated with ATP-induced proliferation of cell progenitors in the intact embryonic retina. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 2007, 25, 499-508. [CrossRef]
- Sugioka, M.; Zhou, W. L.; Hofmann, H. D.; Yamashita, M., Ca2+ mobilization and capacitative Ca2+ entry regulate DNA synthesis in cultured chick retinal neuroepithelial cells. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 1999, 17, 163-172. [CrossRef]
- Sugioka, M.; Zhou, W. L.; Hofmann, H. D.; Yamashita, M., Involvement of P2 purinoceptors in the regulation of DNA synthesis in the neural retina of chick embryo. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 1999, 17, 135-144. [CrossRef]
- Yamashita, M., From neuroepithelial cells to neurons: changes in the physiological properties of neuroepithelial stem cells. Arch Biochem Biophys 2013, 534, (1-2), 64-70. [CrossRef]
- Ornelas, I. M.; Ventura, A. L., Involvement of the PI3K/AKT pathway in ATP-induced proliferation of developing retinal cells in culture. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 2010, 28, 503-511. [CrossRef]
- Moll, V.; Weick, M.; Milenkovic, I.; Kodal, H.; Reichenbach, A.; Bringmann, A., P2Y receptor-mediated stimulation of Müller glial DNA synthesis. Investigative ophthalmology & visual science 2002, 43, 766-773.
- Milenkovic, I.; Weick, M.; Wiedemann, P.; Reichenbach, A.; Bringmann, A., P2Y receptor-mediated stimulation of Müller glial cell DNA synthesis: dependence on EGF and PDGF receptor transactivation. Investigative ophthalmology & visual science 2003, 44, 1211-1220. [CrossRef]
- Ornelas, I. M.; Silva, T. M.; Fragel-Madeira, L.; Ventura, A. L., Inhibition of PI3K/Akt pathway impairs G2/M transition of cell cycle in late developing progenitors of the avian embryo retina. PLoS One 2013, 8, e53517. [CrossRef]
- Massé, K.; Bhamra, S.; Eason, R.; Dale, N.; Jones, E. A., Purine-mediated signalling triggers eye development. Nature 2007, 449, 1058-1062. [CrossRef]
- Gampe, K.; Haverkamp, S.; Robson, S. C.; Gachet, C.; Hüser, L.; Acker-Palmer, A.; Zimmermann, H., NTPDase2 and the P2Y1 receptor are not required for mammalian eye formation. Purinergic signalling 2015, 11, 155-60. [CrossRef]
- Lewis, G. P.; Chapin, E. A.; Luna, G.; Linberg, K. A.; Fisher, S. K., The fate of Müller's glia following experimental retinal detachment: nuclear migration, cell division, and subretinal glial scar formation. Mol Vis 2010, 16, 1361-1372.
- Reichenbach, A.; Bringmann, A., Role of Purines in Müller Glia. J Ocul Pharmacol Ther 2016, 32, 518-533. [CrossRef]
- Silva, T. M.; França, G. R.; Ornelas, I. M.; Loiola, E. C.; Ulrich, H.; Ventura, A. L., Involvement of nucleotides in glial growth following scratch injury in avian retinal cell monolayer cultures. Purinergic Signal 2015, 11, 183-201. [CrossRef]
- Resta, V.; Novelli, E.; Vozzi, G.; Scarpa, C.; Caleo, M.; Ahluwalia, A.; Solini, A.; Santini, E.; Parisi, V.; Di Virgilio, F.; Galli-Resta, L., Acute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATP. The European journal of neuroscience 2007, 25, 2741-2754. [CrossRef]
- Anccasi, R. M.; Ornelas, I. M.; Cossenza, M.; Persechini, P. M.; Ventura, A. L., ATP induces the death of developing avian retinal neurons in culture via activation of P2X7 and glutamate receptors. Purinergic Signal 2013, 9, 15-29. [CrossRef]
- Zhang, X.; Zhang, M.; Laties, A. M.; Mitchell, C. H., Stimulation of P2X7 receptors elevates Ca2+ and kills retinal ganglion cells. Invest Ophthalmol Vis Sci 2005, 46, 2183-2191. [CrossRef]
- Hu, H.; Lu, W.; Zhang, M.; Zhang, X.; Argall, A. J.; Patel, S.; Lee, G. E.; Kim, Y. C.; Jacobson, K. A.; Laties, A. M.; Mitchell, C. H., Stimulation of the P2X7 receptor kills rat retinal ganglion cells in vivo. Exp Eye Res 2010, 91, 425-432. [CrossRef]
- Sugiyama, T.; Oku, H.; Shibata, M.; Fukuhara, M.; Yoshida, H.; Ikeda, T., Involvement of P2X7 receptors in the hypoxia-induced death of rat retinal neurons. Invest Ophthalmol Vis Sci 2010, 51, 3236-3243. [CrossRef]
- Niyadurupola, N.; Sidaway, P.; Ma, N.; Rhodes, J. D.; Broadway, D. C.; Sanderson, J., P2X7 receptor activation mediates retinal ganglion cell death in a human retina model of ischemic neurodegeneration. Investigative ophthalmology & visual science 2013, 54, 2163-2170. [CrossRef]
- Campagno, K. E.; Lu, W.; Jassim, A. H.; Albalawi, F.; Cenaj, A.; Tso, H. Y.; Clark, S. P.; Sripinun, P.; Gómez, N. M.; Mitchell, C. H., Rapid morphologic changes to microglial cells and upregulation of mixed microglial activation state markers induced by P2X7 receptor stimulation and increased intraocular pressure. Journal of neuroinflammation 2021, 18, 217. [CrossRef]
- Hu, X.; Zhao, G. L.; Xu, M. X.; Zhou, H.; Li, F.; Miao, Y.; Lei, B.; Yang, X. L.; Wang, Z., Interplay between Müller cells and microglia aggravates retinal inflammatory response in experimental glaucoma. Journal of neuroinflammation 2021, 18, 303. [CrossRef]
- Kakurai, K.; Sugiyama, T.; Kurimoto, T.; Oku, H.; Ikeda, T., Involvement of P2X(7) receptors in retinal ganglion cell death after optic nerve crush injury in rats. Neuroscience letters 2013, 534, 237-241. [CrossRef]
- Xue, B.; Xie, Y.; Xue, Y.; Hu, N.; Zhang, G.; Guan, H.; Ji, M., Involvement of P2X(7) receptors in retinal ganglion cell apoptosis induced by activated Müller cells. Exp Eye Res 2016, 153, 42-50. [CrossRef]
- Franke, H.; Klimke, K.; Brinckmann, U.; Grosche, J.; Francke, M.; Sperlagh, B.; Reichenbach, A.; Liebert, U. G.; Illes, P., P2X(7) receptor-mRNA and -protein in the mouse retina; changes during retinal degeneration in BALBCrds mice. Neurochem Int 2005, 47, 235-242. [CrossRef]
- Puthussery, T.; Fletcher, E., Extracellular ATP induces retinal photoreceptor apoptosis through activation of purinoceptors in rodents. The Journal of comparative neurology 2009, 513, 430-440. [CrossRef]
- Notomi, S.; Hisatomi, T.; Kanemaru, T.; Takeda, A.; Ikeda, Y.; Enaida, H.; Kroemer, G.; Ishibashi, T., Critical involvement of extracellular ATP acting on P2RX7 purinergic receptors in photoreceptor cell death. The American journal of pathology 2011, 179, 2798-2809. [CrossRef]
- Cao, M.; Huang, X.; Zou, J.; Peng, Y.; Wang, Y.; Zheng, X.; Tang, L.; Zhang, L., Attenuation of Microglial Activation and Pyroptosis by Inhibition of P2X7 Pathway Promotes Photoreceptor Survival in Experimental Retinal Detachment. Invest Ophthalmol Vis Sci 2023, 64, 34. [CrossRef]
- Rice, M. E.; Russo-Menna, I., Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience 1998, 82, 1213-1223. [CrossRef]
- Raj Rai, S.; Bhattacharyya, C.; Sarkar, A.; Chakraborty, S.; Sircar, E.; Dutta, S.; Sengupta, R., Glutathione: Role in Oxidative/Nitrosative Stress, Antioxidant Defense, and Treatments. ChemistrySelect 2021, 6, 4566-4590. [CrossRef]
- Gu, F.; Chauhan, V.; Chauhan, A., Glutathione redox imbalance in brain disorders. Current Opinion in Clinical Nutrition & Metabolic Care 2015, 18. [CrossRef]
- Bjørklund, G.; Tinkov, A. A.; Hosnedlová, B.; Kizek, R.; Ajsuvakova, O. P.; Chirumbolo, S.; Skalnaya, M. G.; Peana, M.; Dadar, M.; El-Ansary, A.; Qasem, H.; Adams, J. B.; Aaseth, J.; Skalny, A. V., The role of glutathione redox imbalance in autism spectrum disorder: A review. Free Radical Biology and Medicine 2020, 160, 149-162. [CrossRef]
- Freitas, H. R.; Reis, R. A., Glutathione induces GABA release through P2X7R activation on Muller glia. Neurogenesis (Austin, Tex.) 2017, 4, e1283188. [CrossRef]
- Freitas, H. R.; Ferraz, G.; Ferreira, G. C.; Ribeiro-Resende, V. T.; Chiarini, L. B.; do Nascimento, J. L.; Matos Oliveira, K. R.; Pereira Tde, L.; Ferreira, L. G.; Kubrusly, R. C.; Faria, R. X.; Herculano, A. M.; Reis, R. A., Glutathione-Induced Calcium Shifts in Chick Retinal Glial Cells. PloS one 2016, 11, e0153677. [CrossRef]
- Pow, D. V.; Crook, D. K., Immunocytochemical evidence for the presence of high levels of reduced glutathione in radial glial cells and horizontal cells in the rabbit retina. Neuroscience letters 1995, 193, 25-28. [CrossRef]
- Schütte, M.; Werner, P., Redistribution of glutathione in the ischemic rat retina. Neuroscience letters 1998, 246, 53-56. [CrossRef]
- Castagné, V.; Clarke, P. G. H., Inhibition of glutathione synthesis can enhance cycloheximide-induced protection of developing neurons against axotomy. Developmental Brain Research 1997, 102, 285-290. [CrossRef]
- Castagné, V.; Clarke, P. G. H., Cooperation between glutathione depletion and protein synthesis inhibition against naturally occurring neuronal death. Neuroscience 1998, 86, 895-902. [CrossRef]
- Corpe, C. P.; Tu, H.; Eck, P.; Wang, J.; Faulhaber-Walter, R.; Schnermann, J.; Margolis, S.; Padayatty, S.; Sun, H.; Wang, Y.; Nussbaum, R. L.; Espey, M. G.; Levine, M., Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. The Journal of clinical investigation 2010, 120, 1069-1083. [CrossRef]
- Ferrada, L.; Magdalena, R.; Barahona, M. J.; Ramírez, E.; Sanzana, C.; Gutiérrez, J.; Nualart, F., Two Distinct Faces of Vitamin C: AA vs. DHA. Antioxidants (Basel) 2021, 10. [CrossRef]
- Padayatty, S. J.; Levine, M., Vitamin C: the known and the unknown and Goldilocks. Oral Dis 2016, 22, 463-493. [CrossRef]
- Diliberto, E. J., Jr.; Allen, P. L., Semidehydroascorbate as a product of the enzymic conversion of dopamine to norepinephrine. Coupling of semidehydroascorbate reductase to dopamine-beta-hydroxylase. Molecular pharmacology 1980, 17, 421-426.
- Qiu, S.; Li, L.; Weeber, E. J.; May, J. M., Ascorbate transport by primary cultured neurons and its role in neuronal function and protection against excitotoxicity. J Neurosci Res 2007, 85, 1046-1056. [CrossRef]
- Eldridge, C. F.; Bunge, M. B.; Bunge, R. P.; Wood, P. M., Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. The Journal of cell biology 1987, 105, 1023-1034. [CrossRef]
- Covarrubias-Pinto, A.; Acuña, A. I.; Beltrán, F. A.; Torres-Díaz, L.; Castro, M. A., Old Things New View: Ascorbic Acid Protects the Brain in Neurodegenerative Disorders. Int J Mol Sci 2015, 16, 28194-28217. [CrossRef]
- Kocot, J.; Luchowska-Kocot, D.; Kiełczykowska, M.; Musik, I.; Kurzepa, J., Does Vitamin C Influence Neurodegenerative Diseases and Psychiatric Disorders? Nutrients 2017, 9. [CrossRef]
- Moretti, M.; Fraga, D. B.; Rodrigues, A. L. S., Ascorbic Acid to Manage Psychiatric Disorders. CNS Drugs 2017, 31, 571-583. [CrossRef]
- Renner, O.; Burkard, M.; Michels, H.; Vollbracht, C.; Sinnberg, T.; Venturelli, S., Parenteral high-dose ascorbate - A possible approach for the treatment of glioblastoma (Review). Int J Oncol 2021, 58. [CrossRef]
- De Mello, F. G., The ontogeny of dopamine-dependent increase of adenosine 3',5'-cyclic monophosphate in the chick retina. Journal of neurochemistry 1978, 31, 1049-1053. [CrossRef]
- Majumdar, S., Role of glutamate in the development of visual pathways. Frontiers in Ophthalmology 2023, 3. [CrossRef]
- Domith, I.; Socodato, R.; Portugal, C. C.; Munis, A. F.; Duarte-Silva, A. T.; Paes-de-Carvalho, R., Vitamin C modulates glutamate transport and NMDA receptor function in the retina. Journal of neurochemistry 2018, 144, 408-420. [CrossRef]
- Telegina, D. V.; Antonenko, A. K.; Fursova, A. Z.; Kolosova, N. G., The glutamate/GABA system in the retina of male rats: effects of aging, neurodegeneration, and supplementation with melatonin and antioxidant SkQ1. Biogerontology 2022, 23, 571-585. [CrossRef]
- do Nascimento, J. L.; de Mello, F. G., Induced release of gamma-aminobutyric acid by a carrier-mediated, high-affinity uptake of L-glutamate in cultured chick retina cells. Journal of neurochemistry 1985, 45, 1820-1827. [CrossRef]
- Schitine, C. S.; de Mello, F. G.; Reis, R. A., Neurochemical plasticity of Müller cells after retinal injury: overexpression of GAT-3 may potentiate excitotoxicity. Neural Regen Res 2015, 10, 1376-1378. [CrossRef]
- de Almeida, O. M.; Gardino, P. F.; Loureiro dos Santos, N. E.; Yamasaki, E. N.; de Mello, M. C.; Hokoç, J. N.; de Mello, F. G., Opposite roles of GABA and excitatory amino acids on the control of GAD expression in cultured retina cells. Brain Res 2002, 925, 89-99. [CrossRef]
- Socodato, R.; Santiago, F. N.; Portugal, C. C.; Domith, I.; Encarnação, T. G.; Loiola, E. C.; Ventura, A. L.; Cossenza, M.; Relvas, J. B.; Castro, N. G.; Paes-de-Carvalho, R., Dopamine promotes NMDA receptor hypofunction in the retina through D(1) receptor-mediated Csk activation, Src inhibition and decrease of GluN2B phosphorylation. Scientific reports 2017, 7, 40912. [CrossRef]
- Lowry, W. E.; Huang, J.; Ma, Y. C.; Ali, S.; Wang, D.; Williams, D. M.; Okada, M.; Cole, P. A.; Huang, X. Y., Csk, a critical link of g protein signals to actin cytoskeletal reorganization. Dev Cell 2002, 2, 733-744. [CrossRef]
- Salter, M. W.; Kalia, L. V., Src kinases: a hub for NMDA receptor regulation. Nature reviews. Neuroscience 2004, 5, 317-328. [CrossRef]
- Batty, N. J.; Fenrich, K. K.; Fouad, K., The role of cAMP and its downstream targets in neurite growth in the adult nervous system. Neuroscience letters 2017, 652, 56-63. [CrossRef]
- Lankford, K.; De Mello, F. G.; Klein, W. L., A transient embryonic dopamine receptor inhibits growth cone motility and neurite outgrowth in a subset of avian retina neurons. Neuroscience letters 1987, 75, 169-174. [CrossRef]
- da Encarnação, T. G.; Portugal, C. C.; Nogueira, C. E.; Santiago, F. N.; Socodato, R.; Paes-de-Carvalho, R., Dopamine Promotes Ascorbate Release from Retinal Neurons: Role of D(1) Receptors and the Exchange Protein Directly Activated by cAMP type 2 (EPAC2). Mol Neurobiol 2018, 55, 7858-7871. [CrossRef]
- Portugal, C. C.; da Encarnacao, T. G.; Domith, I.; Dos Santos Rodrigues, A.; de Oliveira, N. A.; Socodato, R.; Paes-de-Carvalho, R., Dopamine-Induced Ascorbate Release From Retinal Neurons Involves Glutamate Release, Activation of AMPA/Kainate Receptors and Downstream Signaling Pathways. Frontiers in neuroscience 2019, 13, 453. [CrossRef]
- Paes-De-Carvalho, R., Adenosine as a signaling molecule in the retina: biochemical and developmental aspects. Anais da Academia Brasileira de Ciencias 2002, 74, 437-451. [CrossRef]
- Garthwaite, J., Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends in neurosciences 1991, 14, 60-67. [CrossRef]
- Sohocki, M. M.; Daiger, S. P.; Bowne, S. J.; Rodriquez, J. A.; Northrup, H.; Heckenlively, J. R.; Birch, D. G.; Mintz-Hittner, H.; Ruiz, R. S.; Lewis, R. A.; Saperstein, D. A.; Sullivan, L. S., Prevalence of mutations causing retinitis pigmentosa and other inherited retinopathies. Hum Mutat 2001, 17, 42-51. [CrossRef]
- Kumaran, N.; Michaelides, M.; Smith, A. J.; Ali, R. R.; Bainbridge, J. W. B., Retinal gene therapy. Br Med Bull 2018, 126, 13-25. [CrossRef]
- Carss, K. J.; Arno, G.; Erwood, M.; Stephens, J.; Sanchis-Juan, A.; Hull, S.; Megy, K.; Grozeva, D.; Dewhurst, E.; Malka, S.; Plagnol, V.; Penkett, C.; Stirrups, K.; Rizzo, R.; Wright, G.; Josifova, D.; Bitner-Glindzicz, M.; Scott, R. H.; Clement, E.; Allen, L.; Armstrong, R.; Brady, A. F.; Carmichael, J.; Chitre, M.; Henderson, R. H. H.; Hurst, J.; MacLaren, R. E.; Murphy, E.; Paterson, J.; Rosser, E.; Thompson, D. A.; Wakeling, E.; Ouwehand, W. H.; Michaelides, M.; Moore, A. T.; Webster, A. R.; Raymond, F. L., Comprehensive Rare Variant Analysis via Whole-Genome Sequencing to Determine the Molecular Pathology of Inherited Retinal Disease. American journal of human genetics 2017, 100, 75-90.
- Wong, W. L.; Su, X.; Li, X.; Cheung, C. M.; Klein, R.; Cheng, C. Y.; Wong, T. Y., Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014, 2, e106-e116. [CrossRef]
- Fleckenstein, M.; Keenan, T. D. L.; Guymer, R. H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C. C.; Wong, W. T.; Chew, E. Y., Age-related macular degeneration. Nat Rev Dis Primers 2021, 7, 31. [CrossRef]
- Saaddine, J. B.; Honeycutt, A. A.; Narayan, K. M.; Zhang, X.; Klein, R.; Boyle, J. P., Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005-2050. Arch Ophthalmol 2008, 126, 1740-1747. [CrossRef]
- Lucchesi, M.; Marracci, S.; Amato, R.; Filippi, L.; Cammalleri, M.; Dal Monte, M., Neurosensory Alterations in Retinopathy of Prematurity: A Window to Neurological Impairments Associated to Preterm Birth. Biomedicines 2022, 10. [CrossRef]
- Quigley, H. A., Understanding Glaucomatous Optic Neuropathy: The Synergy Between Clinical Observation and Investigation. Annu Rev Vis Sci 2016, 2, 235-254. [CrossRef]
- Amerasinghe, N.; Zhang, J.; Thalamuthu, A.; He, M.; Vithana, E. N.; Viswanathan, A.; Wong, T. Y.; Foster, P. J.; Aung, T., The heritability and sibling risk of angle closure in Asians. Ophthalmology 2011, 118, 480-485. [CrossRef]
- Nickells, R. W., Apoptosis of retinal ganglion cells in glaucoma: an update of the molecular pathways involved in cell death. Surv Ophthalmol 1999, 43 Suppl 1, S151-61. [CrossRef]
- Tham, Y. C.; Li, X.; Wong, T. Y.; Quigley, H. A.; Aung, T.; Cheng, C. Y., Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014, 121, 2081-2090. [CrossRef]
- Bravo Filho, V. T.; Ventura, R. U.; Brandt, C. T.; Sarteschi, C.; Ventura, M. C., [Visual impairment impact on the quality of life of the elderly population that uses the public health care system from the western countryside of Pernambuco State, Brazil]. Arq Bras Oftalmol 2012, 75, 161-165. [CrossRef]
- Allison, K.; Patel, D.; Alabi, O., Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [CrossRef]
- Lusthaus, J.; Goldberg, I., Current management of glaucoma. Med J Aust 2019, 210, 180-187. [CrossRef]
- Lo, J.; Mehta, K.; Dhillon, A.; Huang, Y. K.; Luo, Z.; Nam, M. H.; Al Diri, I.; Chang, K. C., Therapeutic strategies for glaucoma and optic neuropathies. Molecular aspects of medicine 2023, 94, 101219. [CrossRef]
- Killer, H. E.; Pircher, A., Normal tension glaucoma: review of current understanding and mechanisms of the pathogenesis. Eye 2018, 32, 924-930. [CrossRef]
- Souza Monteiro de Araújo, D.; De Logu, F.; Adembri, C.; Rizzo, S.; Janal, M. N.; Landini, L.; Magi, A.; Mattei, G.; Cini, N.; Pandolfo, P.; Geppetti, P.; Nassini, R.; Calaza, K. d. C., TRPA1 mediates damage of the retina induced by ischemia and reperfusion in mice. Cell Death & Disease 2020, 11, 633. [CrossRef]
- Heng, L. Z.; Comyn, O.; Peto, T.; Tadros, C.; Ng, E.; Sivaprasad, S.; Hykin, P. G., Diabetic retinopathy: pathogenesis, clinical grading, management and future developments. Diabetic medicine : a journal of the British Diabetic Association 2013, 30, 640-650. [CrossRef]
- Lechner, J.; O'Leary, O. E.; Stitt, A. W., The pathology associated with diabetic retinopathy. Vision Res 2017, 139, 7-14. [CrossRef]
- Wang, W.; Lo, A. C. Y., Diabetic Retinopathy: Pathophysiology and Treatments. International journal of molecular sciences 2018, 19. [CrossRef]
- Zheng, Y.; He, M.; Congdon, N., The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol 2012, 60, 428-31. [CrossRef]
- Ting, D. S.; Cheung, G. C.; Wong, T. Y., Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol 2016, 44, 260-277. [CrossRef]
- Leasher, J. L.; Bourne, R. R.; Flaxman, S. R.; Jonas, J. B.; Keeffe, J.; Naidoo, K.; Pesudovs, K.; Price, H.; White, R. A.; Wong, T. Y.; Resnikoff, S.; Taylor, H. R., Global Estimates on the Number of People Blind or Visually Impaired by Diabetic Retinopathy: A Meta-analysis From 1990 to 2010. Diabetes Care 2016, 39, 1643-9. [CrossRef]
- Barber, A. J.; Baccouche, B., Neurodegeneration in diabetic retinopathy: Potential for novel therapies. Vision research 2017, 139, 82-92. [CrossRef]
- Seki, M.; Tanaka, T.; Nawa, H.; Usui, T.; Fukuchi, T.; Ikeda, K.; Abe, H.; Takei, N., Involvement of brain-derived neurotrophic factor in early retinal neuropathy of streptozotocin-induced diabetes in rats: therapeutic potential of brain-derived neurotrophic factor for dopaminergic amacrine cells. Diabetes 2004, 53, 2412-2419. [CrossRef]
- Gastinger, M. J.; Singh, R. S.; Barber, A. J., Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Investigative ophthalmology & visual science 2006, 47, 3143-3150. [CrossRef]
- Miya-Coreixas, V. S.; Maggesissi Santos, R.; Carpi Santos, R.; Gardino, P. F.; Calaza, K., Regulation of GABA content by glucose in the chick retina. Exp Eye Res 2013, 115, 206-215. [CrossRef]
- Carpi-Santos, R.; Ferreira, M. J.; Pereira Netto, A. D.; Giestal-de-Araujo, E.; Ventura, A. L. M.; Cossenza, M.; Calaza, K. C., Early changes in system [Formula: see text] and glutathione in the retina of diabetic rats. Exp Eye Res 2016, 146, 35-42. [CrossRef]
- Carpi-Santos, R.; Calaza, K. C., Alterations in System x(c)(-) Expression in the Retina of Type 1 Diabetic Rats and the Role of Nrf2. Molecular neurobiology 2018, 55, 7941-7948. [CrossRef]
- Wong, T. Y.; Cheung, C. M.; Larsen, M.; Sharma, S.; Simó, R., Diabetic retinopathy. Nat Rev Dis Primers 2016, 2, 16012. [CrossRef]
- Barber, A. J.; Lieth, E.; Khin, S. A.; Antonetti, D. A.; Buchanan, A. G.; Gardner, T. W., Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest 1998, 102, 783-791. [CrossRef]
- Mendonca, H. R.; Carpi-Santos, R.; da Costa Calaza, K.; Blanco Martinez, A. M., Neuroinflammation and oxidative stress act in concert to promote neurodegeneration in the diabetic retina and optic nerve: galectin-3 participation. Neural Regen Res 2020, 15, 625-635. [CrossRef]
- Carpineto, P.; Toto, L.; Aloia, R.; Ciciarelli, V.; Borrelli, E.; Vitacolonna, E.; Di Nicola, M.; Di Antonio, L.; Mastropasqua, R., Neuroretinal alterations in the early stages of diabetic retinopathy in patients with type 2 diabetes mellitus. Eye (Lond) 2016, 30, 673-9. [CrossRef]
- Zhao, X.; Wang, J.; Li, P.; Tang, L.; Bai, Y., Casein Kinase 2-Interacting Protein-1 Alleviates High Glucose-Reduced Autophagy, Oxidative Stress, and Apoptosis in Retinal Pigment Epithelial Cells via Activating the p62/KEAP1/NRF2 Signaling Pathway. J Ophthalmol 2021, 2021, 6694050. [CrossRef]
- Lopes de Faria, J. M.; Duarte, D. A.; Simó, R.; García-Ramirez, M.; Dátilo, M. N.; Pasqualetto, F. C.; Lopes de Faria, J. B., δ Opioid Receptor Agonism Preserves the Retinal Pigmented Epithelial Cell Tight Junctions and Ameliorates the Retinopathy in Experimental Diabetes. Invest Ophthalmol Vis Sci 2019, 60, 3842-3853. [CrossRef]
- Feng, L.; Liang, L.; Zhang, S.; Yang, J.; Yue, Y.; Zhang, X., HMGB1 downregulation in retinal pigment epithelial cells protects against diabetic retinopathy through the autophagy-lysosome pathway. Autophagy 2022, 18, 320-339. [CrossRef]
- Janani, R.; Anitha, R. E.; Perumal, M. K.; Divya, P.; Baskaran, V., Astaxanthin mediated regulation of VEGF through HIF1α and XBP1 signaling pathway: An insight from ARPE-19 cell and streptozotocin mediated diabetic rat model. Exp Eye Res 2021, 206, 108555. [CrossRef]
- Gao, L. M.; Fu, S.; Liu, F.; Wu, H. B.; Li, W. J., Astragalus Polysaccharide Regulates miR-182/Bcl-2 Axis to Relieve Metabolic Memory through Suppressing Mitochondrial Damage-Mediated Apoptosis in Retinal Pigment Epithelial Cells. Pharmacology 2021, 106, (9-10), 520-533. [CrossRef]
- Giacco, F.; Brownlee, M., Oxidative stress and diabetic complications. Circulation research 2010, 107, 1058-1070. [CrossRef]
- Kowluru, R. A.; Mishra, M., Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochimica et biophysica acta 2015, 1852, 2474-83. [CrossRef]
- Rodríguez, M. L.; Pérez, S.; Mena-Mollá, S.; Desco, M. C.; Ortega Á, L., Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxidative medicine and cellular longevity 2019, 2019, 4940825. [CrossRef]
- Sun, F.; Sun, Y.; Zhu, J.; Wang, X.; Ji, C.; Zhang, J.; Chen, S.; Yu, Y.; Xu, W.; Qian, H., Mesenchymal stem cells-derived small extracellular vesicles alleviate diabetic retinopathy by delivering NEDD4. Stem cell research & therapy 2022, 13, 293. [CrossRef]
- Tang, X.; Li, X.; Zhang, D.; Han, W., Astragaloside-IV alleviates high glucose-induced ferroptosis in retinal pigment epithelial cells by disrupting the expression of miR-138-5p/Sirt1/Nrf2. Bioengineered 2022, 13, 8240-8254. [CrossRef]
- Li, R.; Ye, Z.; Yang, W.; Xu, Y. J.; Tan, C. P.; Liu, Y., Blueberry Anthocyanins from Commercial Products: Structure Identification and Potential for Diabetic Retinopathy Amelioration. Molecules 2022, 27. [CrossRef]
- D'Agata, V.; D'Amico, A. G.; Maugeri, G.; Bucolo, C.; Rossi, S.; Giunta, S., Carnosol attenuates high glucose damage in human retinal endothelial cells through regulation of ERK/Nrf2/HO-1 pathway. J Asian Nat Prod Res 2023, 25, 783-795. [CrossRef]
- Albert-Garay, J. S.; Riesgo-Escovar, J. R.; Salceda, R., High glucose concentrations induce oxidative stress by inhibiting Nrf2 expression in rat Müller retinal cells in vitro. Scientific reports 2022, 12, 1261. [CrossRef]
- Liu, X.; Liu, Y.; Chen, L.; Zhang, Z.; Cui, L.; Wei, T., Loss of pleckstrin homology domain and leucine-rich repeat protein phosphatase 2 has protective effects on high glucose-injured retinal ganglion cells via the effect on the Akt-GSK-3β-Nrf2 pathway. Inflamm Res 2023, 72, 373-385. [CrossRef]
- Fang, J.; Bai, W.; Yang, L., Astaxanthin inhibits oxidative stress and apoptosis in diabetic retinopathy. Acta Histochem 2023, 125, 152069. [CrossRef]
- Yang, X.; Li, D., Tricin attenuates diabetic retinopathy by inhibiting oxidative stress and angiogenesis through regulating Sestrin2/Nrf2 signaling. Hum Exp Toxicol 2023, 42, 9603271231171642. [CrossRef]
- Bannai, S.; Tateishi, N., Role of membrane transport in metabolism and function of glutathione in mammals. The Journal of membrane biology 1986, 89, 1-8. [CrossRef]
- Zhou, Z.; Li, H.; Bai, S.; Xu, Z.; Jiao, Y., Loss of serine/threonine protein kinase 25 in retinal ganglion cells ameliorates high glucose-elicited damage through regulation of the AKT-GSK-3β/Nrf2 pathway. Biochem Biophys Res Commun 2022, 600, 87-93. [CrossRef]
- Hayes, J. D.; Chowdhry, S.; Dinkova-Kostova, A. T.; Sutherland, C., Dual regulation of transcription factor Nrf2 by Keap1 and by the combined actions of β-TrCP and GSK-3. Biochemical Society transactions 2015, 43, 611-20. [CrossRef]
- Rojo, A. I.; Sagarra, M. R.; Cuadrado, A., GSK-3beta down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. Journal of neurochemistry 2008, 105, 192-202. [CrossRef]
- Rojo, A. I.; Rada, P.; Egea, J.; Rosa, A. O.; López, M. G.; Cuadrado, A., Functional interference between glycogen synthase kinase-3 beta and the transcription factor Nrf2 in protection against kainate-induced hippocampal cell death. Molecular and cellular neurosciences 2008, 39, 125-132. [CrossRef]
- Giacco, F.; Du, X.; Carratú, A.; Gerfen, G. J.; D'Apolito, M.; Giardino, I.; Rasola, A.; Marin, O.; Divakaruni, A. S.; Murphy, A. N.; Shah, M. S.; Brownlee, M., GLP-1 Cleavage Product Reverses Persistent ROS Generation After Transient Hyperglycemia by Disrupting an ROS-Generating Feedback Loop. Diabetes 2015, 64, 3273-3284. [CrossRef]
- Miller, W. P.; Toro, A. L.; Barber, A. J.; Dennis, M. D., REDD1 Activates a ROS-Generating Feedback Loop in the Retina of Diabetic Mice. Investigative ophthalmology & visual science 2019, 60, 2369-2379. [CrossRef]
- Miller, W. P.; Sunilkumar, S.; Giordano, J. F.; Toro, A. L.; Barber, A. J.; Dennis, M. D., The stress response protein REDD1 promotes diabetes-induced oxidative stress in the retina by Keap1-independent Nrf2 degradation. The Journal of biological chemistry 2020, 295, 7350-7361. [CrossRef]
- Schrufer, T. L.; Antonetti, D. A.; Sonenberg, N.; Kimball, S. R.; Gardner, T. W.; Jefferson, L. S., Ablation of 4E-BP1/2 prevents hyperglycemia-mediated induction of VEGF expression in the rodent retina and in Muller cells in culture. Diabetes 2010, 59, 2107-2116. [CrossRef]
- Dennis, M. D.; Kimball, S. R.; Fort, P. E.; Jefferson, L. S., Regulated in development and DNA damage 1 is necessary for hyperglycemia-induced vascular endothelial growth factor expression in the retina of diabetic rodents. The Journal of biological chemistry 2015, 290, 3865-74. [CrossRef]
- Hao, Y.; Gao, X., Diosgenin protects retinal pigment epithelial cells from inflammatory damage and oxidative stress induced by high glucose by activating AMPK/Nrf2/HO-1 pathway. Immun Inflamm Dis 2022, 10, e698. [CrossRef]
- Barouch, F. C.; Miyamoto, K.; Allport, J. R.; Fujita, K.; Bursell, S. E.; Aiello, L. P.; Luscinskas, F. W.; Adamis, A. P., Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Invest Ophthalmol Vis Sci 2000, 41, 1153-1158.
- Joussen, A. M.; Poulaki, V.; Le, M. L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B.; Kern, T. S.; Adamis, A. P., A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2004, 18, 1450-1452. [CrossRef]
- Kasza, M.; Meleg, J.; Vardai, J.; Nagy, B., Jr.; Szalai, E.; Damjanovich, J.; Csutak, A.; Ujhelyi, B.; Nagy, V., Plasma E-selectin levels can play a role in the development of diabetic retinopathy. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 2017, 255, 25-30. [CrossRef]
- Miyamoto, K.; Khosrof, S.; Bursell, S. E.; Rohan, R.; Murata, T.; Clermont, A. C.; Aiello, L. P.; Ogura, Y.; Adamis, A. P., Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proceedings of the National Academy of Sciences of the United States of America 1999, 96, 10836-10841. [CrossRef]
- Schröder, S.; Palinski, W.; Schmid-Schönbein, G. W., Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. The American journal of pathology 1991, 139, 81-100.
- Boss, J. D.; Singh, P. K.; Pandya, H. K.; Tosi, J.; Kim, C.; Tewari, A.; Juzych, M. S.; Abrams, G. W.; Kumar, A., Assessment of Neurotrophins and Inflammatory Mediators in Vitreous of Patients With Diabetic Retinopathy. Invest Ophthalmol Vis Sci 2017, 58, 5594-5603. [CrossRef]
- Koleva-Georgieva, D. N.; Sivkova, N. P.; Terzieva, D., Serum inflammatory cytokines IL-1beta, IL-6, TNF-alpha and VEGF have influence on the development of diabetic retinopathy. Folia Med (Plovdiv) 2011, 53, 44-50. [CrossRef]
- Rangasamy, S.; McGuire, P. G.; Franco Nitta, C.; Monickaraj, F.; Oruganti, S. R.; Das, A., Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS One 2014, 9, e108508. [CrossRef]
- Suzuki, Y.; Nakazawa, M.; Suzuki, K.; Yamazaki, H.; Miyagawa, Y., Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Jpn J Ophthalmol 2011, 55, 256-263. [CrossRef]
- Abcouwer, S. F., Müller Cell-Microglia Cross Talk Drives Neuroinflammation in Diabetic Retinopathy. Diabetes 2017, 66, 261-263. [CrossRef]
- Sorrentino, F. S.; Allkabes, M.; Salsini, G.; Bonifazzi, C.; Perri, P., The importance of glial cells in the homeostasis of the retinal microenvironment and their pivotal role in the course of diabetic retinopathy. Life Sci 2016, 162, 54-59. [CrossRef]
- Xu, Z.; Li, S.; Li, K.; Wang, X.; Li, X.; An, M.; Yu, X.; Long, X.; Zhong, R.; Liu, Q.; Wang, X.; Yang, Y.; Tian, N., Urolithin A ameliorates diabetic retinopathy via activation of the Nrf2/HO-1 pathway. Endocr J 2022, 69, 971-982. [CrossRef]
- Mansour, S. E.; Browning, D. J.; Wong, K.; Flynn, H. W., Jr.; Bhavsar, A. R., The Evolving Treatment of Diabetic Retinopathy. Clin Ophthalmol 2020, 14, 653-678. [CrossRef]
- Jakus, V.; Rietbrock, N., Advanced glycation end-products and the progress of diabetic vascular complications. Physiological research 2004, 53, 131-42. [CrossRef]
- Haritoglou, C.; Gerss, J.; Sauerland, C.; Kampik, A.; Ulbig, M. W., Effect of calcium dobesilate on occurrence of diabetic macular oedema (CALDIRET study): randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2009, 373, 1364-71. [CrossRef]
- Mayer-Davis, E. J.; Bell, R. A.; Reboussin, B. A.; Rushing, J.; Marshall, J. A.; Hamman, R. F., Antioxidant nutrient intake and diabetic retinopathy: the San Luis Valley Diabetes Study. Ophthalmology 1998, 105, 2264-70. [CrossRef]
- Millen, A. E.; Klein, R.; Folsom, A. R.; Stevens, J.; Palta, M.; Mares, J. A., Relation between intake of vitamins C and E and risk of diabetic retinopathy in the Atherosclerosis Risk in Communities Study. The American journal of clinical nutrition 2004, 79, 865-73. [CrossRef]
- Yang, J.; Hua, Z.; Zheng, Z.; Ma, X.; Zhu, L.; Li, Y., Acteoside inhibits high glucose-induced oxidative stress injury in RPE cells and the outer retina through the Keap1/Nrf2/ARE pathway. Exp Eye Res 2023, 232, 109496. [CrossRef]
- Li, S.; Lu, S.; Wang, L.; Liu, S.; Zhang, L.; Du, J.; Wu, Z.; Huang, X., Effects of amygdalin on ferroptosis and oxidative stress in diabetic retinopathy progression via the NRF2/ARE signaling pathway. Exp Eye Res 2023, 234, 109569. [CrossRef]
- Blaustein, M. P.; Hamlyn, J. M., Ouabain, endogenous ouabain and ouabain-like factors: The Na(+) pump/ouabain receptor, its linkage to NCX, and its myriad functions. Cell Calcium 2020, 86, 102159. [CrossRef]
- Blanco, G., Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin Nephrol 2005, 25, 292-303. [CrossRef]
- Wetzel, R. K.; Arystarkhova, E.; Sweadner, K. J., Cellular and subcellular specification of Na,K-ATPase alpha and beta isoforms in the postnatal development of mouse retina. The Journal of neuroscience : the official journal of the Society for Neuroscience 1999, 19, 9878-89. [CrossRef]
- Maturana-Teixeira, S.; Braga, L. E.; Carpi Santos, R.; Calaza Kda, C.; Giestal-de-Araujo, E.; Leão-Ferreira, L. R., The (Na(+)/K (+))-ATPase activity in the developing rat retina: the role of insulin-like growth factor-I (IGF-I). Cell Mol Neurobiol 2015, 35, 243-54. [CrossRef]
- Demontis, G. C.; Ratto, G. M.; Bisti, S.; Cervetto, L., Effect of blocking the Na+/K+ ATPase on Ca2+ extrusion and light adaptation in mammalian retinal rods. Biophys J 1995, 69, 439-450. [CrossRef]
- Namekata, K.; Harada, C.; Kohyama, K.; Matsumoto, Y.; Harada, T., Interleukin-1 stimulates glutamate uptake in glial cells by accelerating membrane trafficking of Na+/K+-ATPase via actin depolymerization. Molecular and cellular biology 2008, 28, 3273-80. [CrossRef]
- Country, M. W., Retinal metabolism: A comparative look at energetics in the retina. Brain Res 2017, 1672, 50-57. [CrossRef]
- Nagaoka, K.; Kurauchi, Y.; Asano, D.; Morita, A.; Sakamoto, K.; Nakahara, T., Pharmacological inhibition of Na(+)/K(+)-ATPase induces neurovascular degeneration and glial cell alteration in the rat retina. Exp Eye Res 2022, 220, 109107. [CrossRef]
- McGinn, T. E.; Galicia, C. A.; Leoni, D. C.; Partington, N.; Mitchell, D. M.; Stenkamp, D. L., Rewiring the Regenerated Zebrafish Retina: Reemergence of Bipolar Neurons and Cone-Bipolar Circuitry Following an Inner Retinal Lesion. Front Cell Dev Biol 2019, 7, 95. [CrossRef]
- Barrett, L. M.; Mitchell, D. M.; Meighan, P. C.; Varnum, M. D.; Stenkamp, D. L., Dynamic functional and structural remodeling during retinal regeneration in zebrafish. Front Mol Neurosci 2022, 15, 1070509. [CrossRef]
- Corrêa Gde, R.; Cunha, K. C.; dos Santos, A. A.; de Araujo, E. G., The trophic effect of ouabain on retinal ganglion cell is mediated by EGF receptor and PKC delta activation. Neurochem Res 2010, 35, 1343-1352. [CrossRef]
- Sarkies, N., Traumatic optic neuropathy. Eye 2004, 18, 1122-5. [CrossRef]
- Almasieh, M.; Wilson, A. M.; Morquette, B.; Cueva Vargas, J. L.; Di Polo, A., The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res 2012, 31, 152-181. [CrossRef]
- Tribble, J. R.; Hui, F.; Quintero, H.; El Hajji, S.; Bell, K.; Di Polo, A.; Williams, P. A., Neuroprotection in glaucoma: Mechanisms beyond intraocular pressure lowering. Molecular aspects of medicine 2023, 92, 101193. [CrossRef]
- Fry, L. E.; Fahy, E.; Chrysostomou, V.; Hui, F.; Tang, J.; van Wijngaarden, P.; Petrou, S.; Crowston, J. G., The coma in glaucoma: Retinal ganglion cell dysfunction and recovery. Prog Retin Eye Res 2018, 65, 77-92. [CrossRef]
- Berkelaar, M.; Clarke, D. B.; Wang, Y. C.; Bray, G. M.; Aguayo, A. J., Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 1994, 14, 4368-74. [CrossRef]
- de Araujo, E. G.; Linden, R., Trophic factors produced by retinal cells increase the survival of retinal ganglion cells in vitro. The European journal of neuroscience 1993, 5, 1181-1188. [CrossRef]
- Yin, Y.; Benowitz, L. I., In Vitro and In Vivo Methods for Studying Retinal Ganglion Cell Survival and Optic Nerve Regeneration. Methods Mol Biol 2018, 1695, 187-205. [CrossRef]
- Kügler, S.; Straten, G.; Kreppel, F.; Isenmann, S.; Liston, P.; Bähr, M., The X-linked inhibitor of apoptosis (XIAP) prevents cell death in axotomized CNS neurons in vivo. Cell death and differentiation 2000, 7, 815-824. [CrossRef]
- Isenmann, S.; Kretz, A.; Cellerino, A., Molecular determinants of retinal ganglion cell development, survival, and regeneration. Prog Retin Eye Res 2003, 22, 483-543. [CrossRef]
- Kroeger, H.; Chiang, W. C.; Felden, J.; Nguyen, A.; Lin, J. H., ER stress and unfolded protein response in ocular health and disease. Febs j 2019, 286, 399-412. [CrossRef]
- Fudalej, E.; Justyniarska, M.; Kasarełło, K.; Dziedziak, J.; Szaflik, J. P.; Cudnoch-Jędrzejewska, A., Neuroprotective Factors of the Retina and Their Role in Promoting Survival of Retinal Ganglion Cells: A Review. Ophthalmic Res 2021, 64, 345-355. [CrossRef]
- de Rezende Corrêa, G.; Araujo dos Santos, A.; Frederico Leite Fontes, C.; Giestal de Araujo, E., Ouabain induces an increase of retinal ganglion cell survival in vitro: the involvement of protein kinase C. Brain Res 2005, 1049, 89-94. [CrossRef]
- Mázala-de-Oliveira, T.; de Figueiredo, C. S.; de Rezende Corrêa, G.; da Silva, M. S.; Miranda, R. L.; de Azevedo, M. A.; Cossenza, M.; Dos Santos, A. A.; Giestal-de-Araujo, E., Ouabain-Na(+)/K(+)-ATPase Signaling Regulates Retinal Neuroinflammation and ROS Production Preventing Neuronal Death by an Autophagy-Dependent Mechanism Following Optic Nerve Axotomy In Vitro. Neurochem Res 2022, 47, 723-738. [CrossRef]
- Salles von-Held-Ventura, J.; Mázala-de-Oliveira, T.; Cândida da Rocha Oliveira, A.; Granja, M. G.; Gonçalves-de-Albuquerque, C. F.; Castro-Faria-Neto, H. C.; Giestal-de-Araujo, E., The trophic effect of ouabain on retinal ganglion cells is mediated by IL-1β and TNF-α. Biochemical and biophysical research communications 2016, 478, 378-384. [CrossRef]
- Ail, D.; Ren, D.; Brazhnikova, E.; Nouvel-Jaillard, C.; Bertin, S.; Mirashrafi, S. B.; Fisson, S.; Dalkara, D., Systemic and local immune responses to intraocular AAV vector administration in non-human primates. Mol Ther Methods Clin Dev 2022, 24, 306-316. [CrossRef]
- Bennett, J.; Maguire, A. M., Lessons Learned from the Development of the First FDA-Approved Gene Therapy Drug, Voretigene Neparvovec-rzyl. Cold Spring Harbor perspectives in medicine 2023, 13. [CrossRef]
- Haider, N. B.; Ikeda, A.; Naggert, J. K.; Nishina, P. M., Genetic modifiers of vision and hearing. Human molecular genetics 2002, 11, 1195-206. [CrossRef]
- Dipple, K. M.; McCabe, E. R., Modifier genes convert "simple" Mendelian disorders to complex traits. Mol Genet Metab 2000, 71, (1-2), 43-50. [CrossRef]
- Toms, M.; Ward, N.; Moosajee, M., Nuclear Receptor Subfamily 2 Group E Member 3 (NR2E3): Role in Retinal Development and Disease. Genes 2023, 14. [CrossRef]
- Maeder, M. L.; Stefanidakis, M.; Wilson, C. J.; Baral, R.; Barrera, L. A.; Bounoutas, G. S.; Bumcrot, D.; Chao, H.; Ciulla, D. M.; DaSilva, J. A.; Dass, A.; Dhanapal, V.; Fennell, T. J.; Friedland, A. E.; Giannoukos, G.; Gloskowski, S. W.; Glucksmann, A.; Gotta, G. M.; Jayaram, H.; Haskett, S. J.; Hopkins, B.; Horng, J. E.; Joshi, S.; Marco, E.; Mepani, R.; Reyon, D.; Ta, T.; Tabbaa, D. G.; Samuelsson, S. J.; Shen, S.; Skor, M. N.; Stetkiewicz, P.; Wang, T.; Yudkoff, C.; Myer, V. E.; Albright, C. F.; Jiang, H., Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nature medicine 2019, 25, 229-233. [CrossRef]
- Nirenberg, S.; Pandarinath, C., Retinal prosthetic strategy with the capacity to restore normal vision. Proceedings of the National Academy of Sciences of the United States of America 2012, 109, 15012-15017. [CrossRef]
- Batabyal, S.; Gajjeraman, S.; Pradhan, S.; Bhattacharya, S.; Wright, W.; Mohanty, S., Sensitization of ON-bipolar cells with ambient light activatable multi-characteristic opsin rescues vision in mice. Gene Ther 2021, 28, (3-4), 162-176. [CrossRef]
- Sahel, J. A.; Boulanger-Scemama, E.; Pagot, C.; Arleo, A.; Galluppi, F.; Martel, J. N.; Esposti, S. D.; Delaux, A.; de Saint Aubert, J. B.; de Montleau, C.; Gutman, E.; Audo, I.; Duebel, J.; Picaud, S.; Dalkara, D.; Blouin, L.; Taiel, M.; Roska, B., Partial recovery of visual function in a blind patient after optogenetic therapy. Nature medicine 2021, 27, 1223-1229. [CrossRef]
- Miltner, A. M.; La Torre, A., Retinal Ganglion Cell Replacement: Current Status and Challenges Ahead. Developmental dynamics : an official publication of the American Association of Anatomists 2019, 248, 118-128. [CrossRef]
- Lahne, M.; Nagashima, M.; Hyde, D. R.; Hitchcock, P. F., Reprogramming Müller Glia to Regenerate Retinal Neurons. Annu Rev Vis Sci 2020, 6, 171-193. [CrossRef]
- Goldman, D., Muller glial cell reprogramming and retina regeneration. Nat Rev Neurosci 2014, 15, 431-42. [CrossRef]
- Blackshaw, S., Why Has the Ability to Regenerate Following CNS Injury Been Repeatedly Lost Over the Course of Evolution? Frontiers in neuroscience 2022, 16, 831062. [CrossRef]
- Hoang, T.; Wang, J.; Boyd, P.; Wang, F.; Santiago, C.; Jiang, L.; Yoo, S.; Lahne, M.; Todd, L. J.; Jia, M.; Saez, C.; Keuthan, C.; Palazzo, I.; Squires, N.; Campbell, W. A.; Rajaii, F.; Parayil, T.; Trinh, V.; Kim, D. W.; Wang, G.; Campbell, L. J.; Ash, J.; Fischer, A. J.; Hyde, D. R.; Qian, J.; Blackshaw, S., Gene regulatory networks controlling vertebrate retinal regeneration. Science 2020, 370. [CrossRef]
- Pollak, J.; Wilken, M. S.; Ueki, Y.; Cox, K. E.; Sullivan, J. M.; Taylor, R. J.; Levine, E. M.; Reh, T. A., ASCL1 reprograms mouse Muller glia into neurogenic retinal progenitors. Development 2013, 140, 2619-31. [CrossRef]
- Ueki, Y.; Wilken, M. S.; Cox, K. E.; Chipman, L.; Jorstad, N.; Sternhagen, K.; Simic, M.; Ullom, K.; Nakafuku, M.; Reh, T. A., Transgenic expression of the proneural transcription factor Ascl1 in Muller glia stimulates retinal regeneration in young mice. Proceedings of the National Academy of Sciences of the United States of America 2015, 112, 13717-13722. [CrossRef]
- Jorstad, N. L.; Wilken, M. S.; Grimes, W. N.; Wohl, S. G.; VandenBosch, L. S.; Yoshimatsu, T.; Wong, R. O.; Rieke, F.; Reh, T. A., Stimulation of functional neuronal regeneration from Muller glia in adult mice. Nature 2017, 548, 103-107. [CrossRef]
- Todd, L.; Hooper, M. J.; Haugan, A. K.; Finkbeiner, C.; Jorstad, N.; Radulovich, N.; Wong, C. K.; Donaldson, P. C.; Jenkins, W.; Chen, Q.; Rieke, F.; Reh, T. A., Efficient stimulation of retinal regeneration from Muller glia in adult mice using combinations of proneural bHLH transcription factors. Cell Rep 2021, 37, 109857. [CrossRef]
- Todd, L.; Jenkins, W.; Finkbeiner, C.; Hooper, M. J.; Donaldson, P. C.; Pavlou, M.; Wohlschlegel, J.; Ingram, N.; Rieke, F.; Reh, T. A.; Mu, X., Reprogramming Müller glia to regenerate ganglion-like cells in adult mouse retina with developmental transcription factors. Sci Adv 2022, 8, eabq7219. [CrossRef]
- Xiao, D.; Jin, K.; Qiu, S.; Lei, Q.; Huang, W.; Chen, H.; Su, J.; Xu, Q.; Xu, Z.; Gou, B.; Tie, X.; Liu, F.; Liu, S.; Liu, Y.; Xiang, M., In vivo Regeneration of Ganglion Cells for Vision Restoration in Mammalian Retinas. Front Cell Dev Biol 2021, 9, 755544. [CrossRef]
- Zhou, H.; Su, J.; Hu, X.; Zhou, C.; Li, H.; Chen, Z.; Xiao, Q.; Wang, B.; Wu, W.; Sun, Y.; Zhou, Y.; Tang, C.; Liu, F.; Wang, L.; Feng, C.; Liu, M.; Li, S.; Zhang, Y.; Xu, H.; Yao, H.; Shi, L.; Yang, H., Glia-to-Neuron Conversion by CRISPR-CasRx Alleviates Symptoms of Neurological Disease in Mice. Cell 2020, 181, 590-603 e16. [CrossRef]
- Yao, K.; Qiu, S.; Wang, Y. V.; Park, S. J. H.; Mohns, E. J.; Mehta, B.; Liu, X.; Chang, B.; Zenisek, D.; Crair, M. C.; Demb, J. B.; Chen, B., Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Nature 2018, 560, 484-488. [CrossRef]
- Le, N.; Appel, H.; Pannullo, N.; Hoang, T.; Blackshaw, S., Ectopic insert-dependent neuronal expression of GFAP promoter-driven AAV constructs in adult mouse retina. Front Cell Dev Biol 2022, 10, 914386. [CrossRef]
- Wang, L. L.; Zhang, C. L., Therapeutic Potential of PTBP1 Inhibition, If Any, Is Not Attributed to Glia-to-Neuron Conversion. Annual review of neuroscience 2023, 46, 1-15. [CrossRef]
- Xie, Y.; Zhou, J.; Chen, B., Critical examination of Ptbp1-mediated glia-to-neuron conversion in the mouse retina. Cell reports 2022, 39, 110960. [CrossRef]
- Blackshaw, S.; Sanes, J. R., Turning lead into gold: reprogramming retinal cells to cure blindness. The Journal of clinical investigation 2021, 131. [CrossRef]
- Ling, J. P.; Bygrave, A. M.; Santiago, C. P.; Carmen-Orozco, R. P.; Trinh, V. T.; Yu, M.; Li, Y.; Liu, Y.; Bowden, K. D.; Duncan, L. H.; Han, J.; Taneja, K.; Dongmo, R.; Babola, T. A.; Parker, P.; Jiang, L.; Leavey, P. J.; Smith, J. J.; Vistein, R.; Gimmen, M. Y.; Dubner, B.; Helmenstine, E.; Teodorescu, P.; Karantanos, T.; Ghiaur, G.; Kanold, P. O.; Bergles, D.; Langmead, B.; Sun, S.; Nielsen, K. J.; Peachey, N.; Singh, M. S.; Dalton, W. B.; Rajaii, F.; Huganir, R. L.; Blackshaw, S., Cell-specific regulation of gene expression using splicing-dependent frameshifting. Nature communications 2022, 13, 5773. [CrossRef]
- Gao, Y.; Fang, K.; Yan, Z.; Zhang, H.; Geng, G.; Wu, W.; Xu, D.; Zhang, H.; Zhong, N.; Wang, Q.; Cai, M.; Zuo, E.; Yang, H., Develop an efficient and specific AAV-based labeling system for Muller glia in mice. Scientific reports 2022, 12, 22410. [CrossRef]
- Tresenrider, A.; Hooper, M.; Todd, L.; Kierney, F.; Blasdel, N.; Trapnell, C.; Reh, T. A., A multiplexed, single-cell sequencing screen identifies compounds that increase neurogenic reprogramming of murine Muller glia. bioRxiv 2023. [CrossRef]
- Oliveira-Valenca, V. M.; Bosco, A.; Vetter, M. L.; Silveira, M. S., On the Generation and Regeneration of Retinal Ganglion Cells. Frontiers in cell and developmental biology 2020, 8, 581136. [CrossRef]
- Soucy, J. R.; Aguzzi, E. A.; Cho, J.; Gilhooley, M. J.; Keuthan, C.; Luo, Z.; Monavarfeshani, A.; Saleem, M. A.; Wang, X. W.; Wohlschlegel, J.; Baranov, P.; Di Polo, A.; Fortune, B.; Gokoffski, K. K.; Goldberg, J. L.; Guido, W.; Kolodkin, A. L.; Mason, C. A.; Ou, Y.; Reh, T. A.; Ross, A. G.; Samuels, B. C.; Welsbie, D.; Zack, D. J.; Johnson, T. V., Retinal ganglion cell repopulation for vision restoration in optic neuropathy: a roadmap from the RReSTORe Consortium. Molecular neurodegeneration 2023, 18, 64.
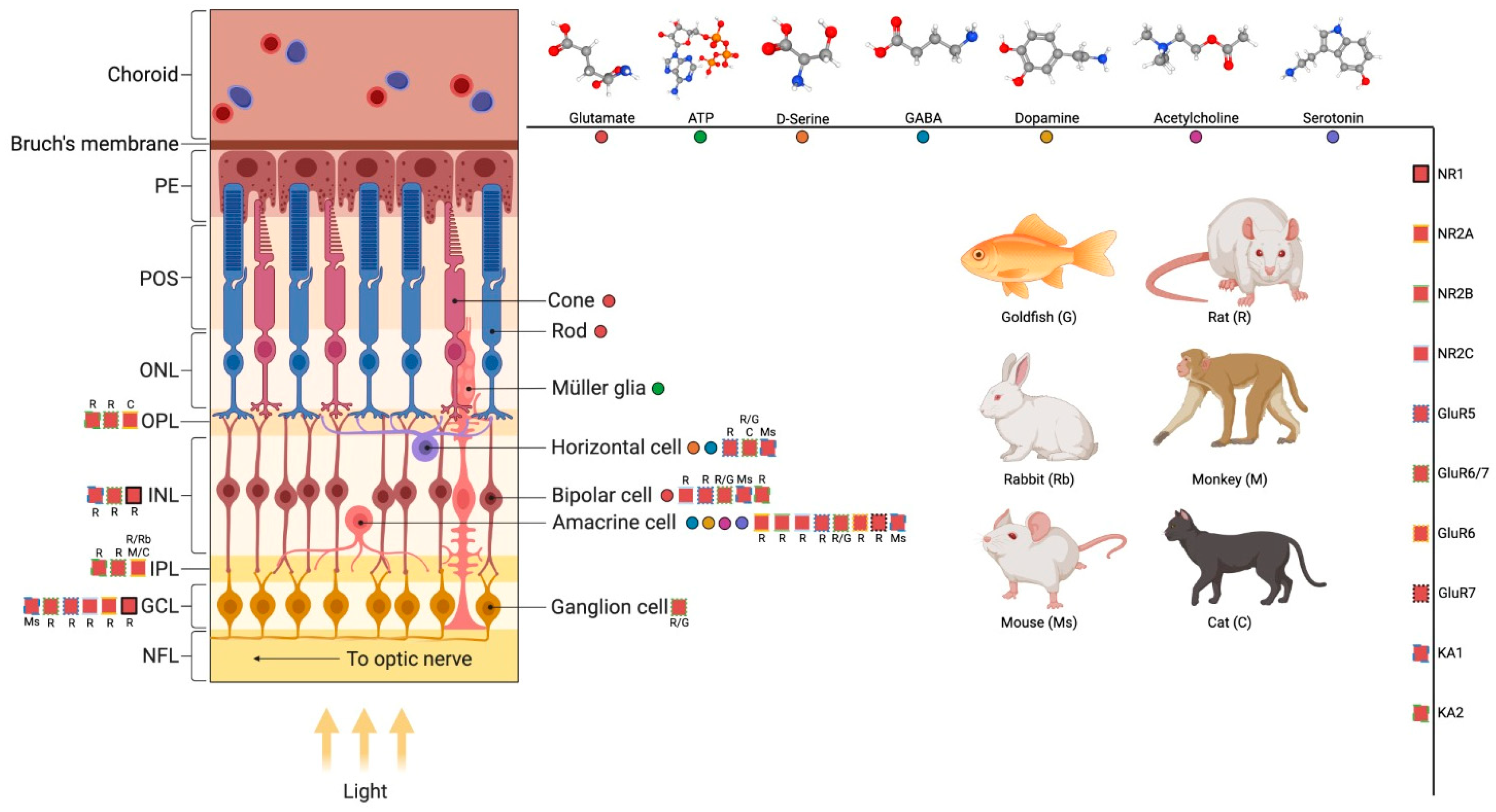
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




