Submitted:
24 December 2023
Posted:
26 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Progress in lead-based PeLEDs
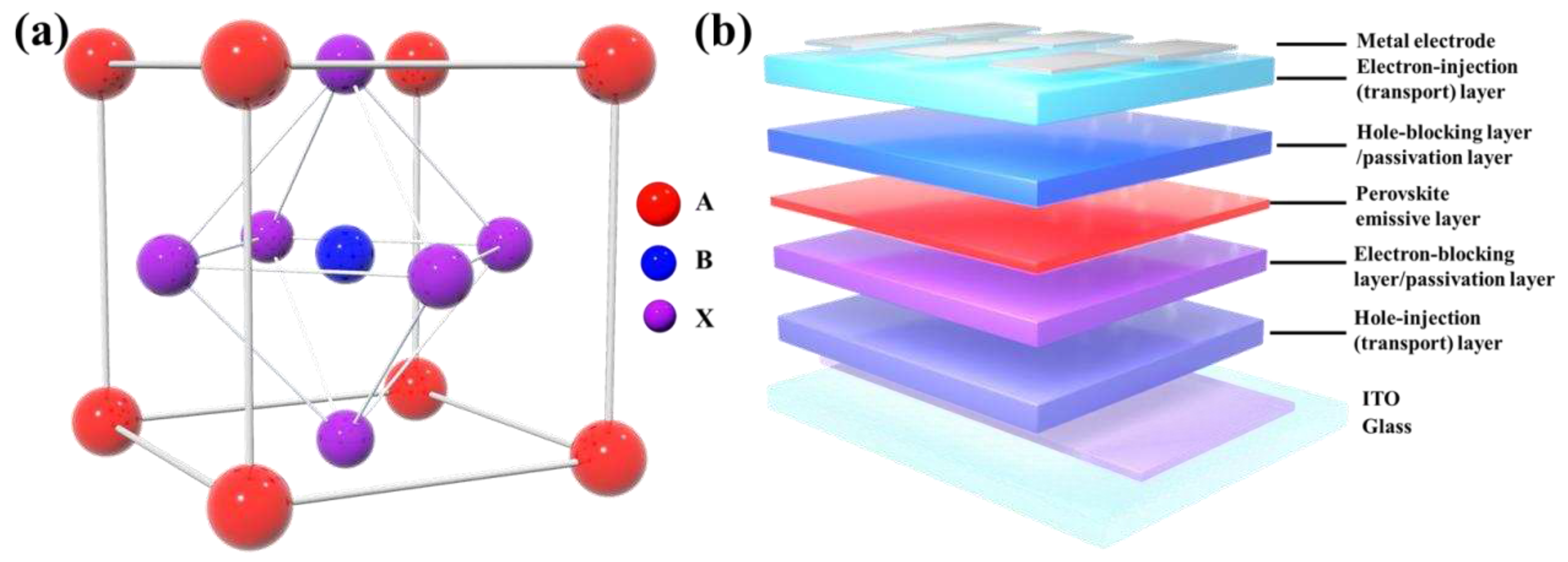
2.1. Progress in PeLEDs based on 3D lead-based perovskites
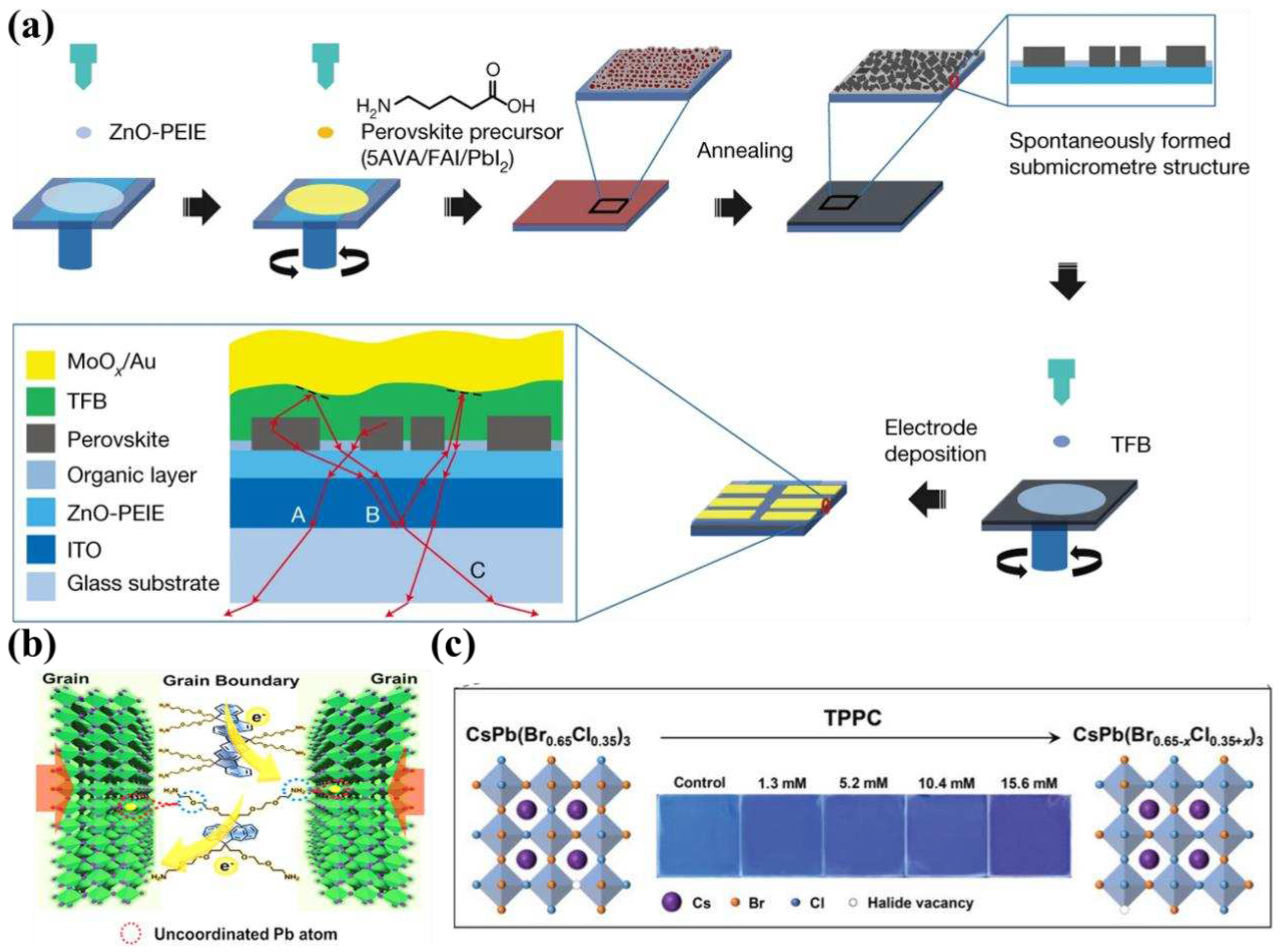
2.2. Progress in PeLEDs based on quasi-2D lead-based perovskites
2.3. Progress in PeLEDs based on 0D lead-based perovskites
3. Progress in lead-free PeLEDs
4. Challenges for PeLEDs
4.1. Poor devices operational stability
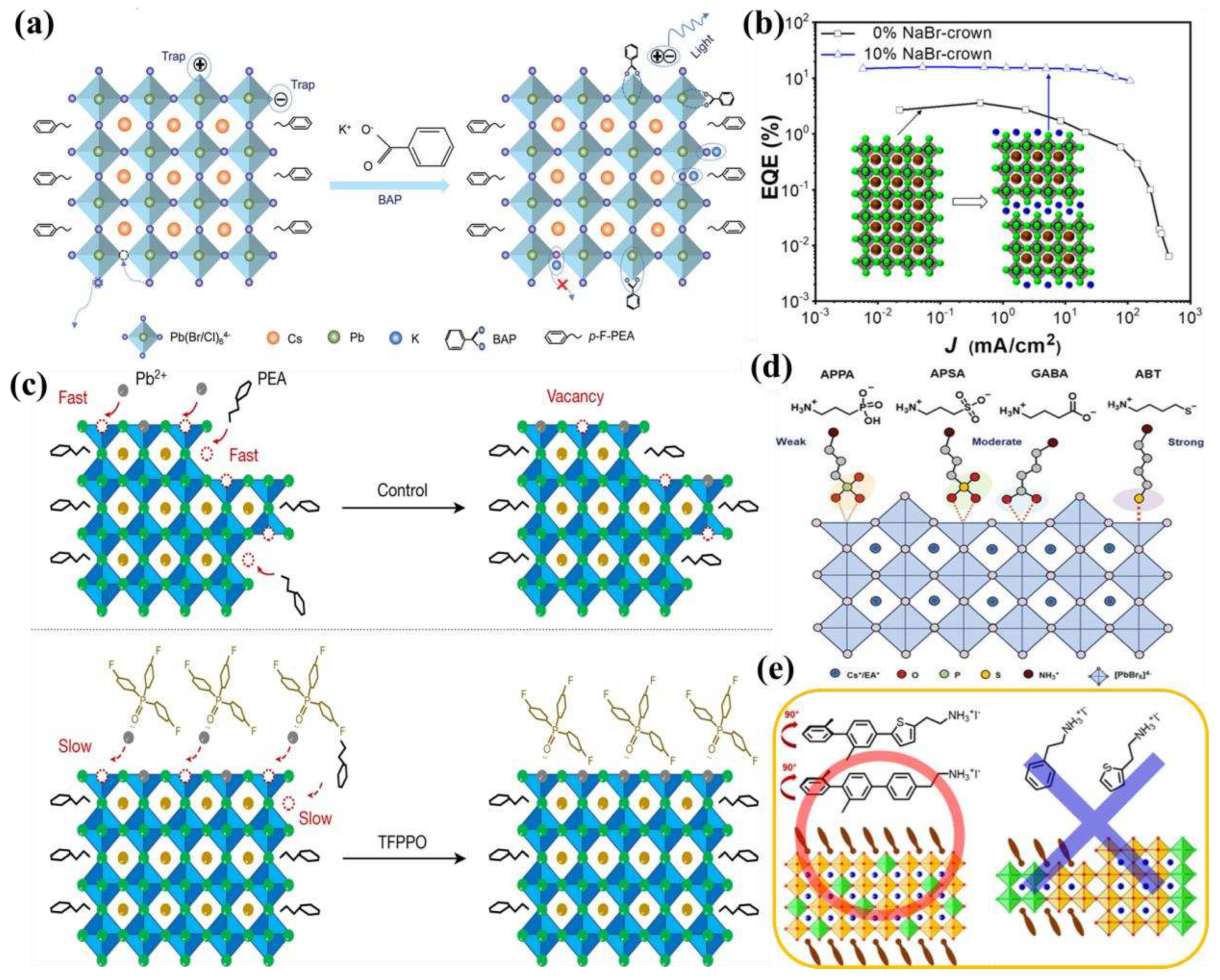
4.2. EQE roll-off
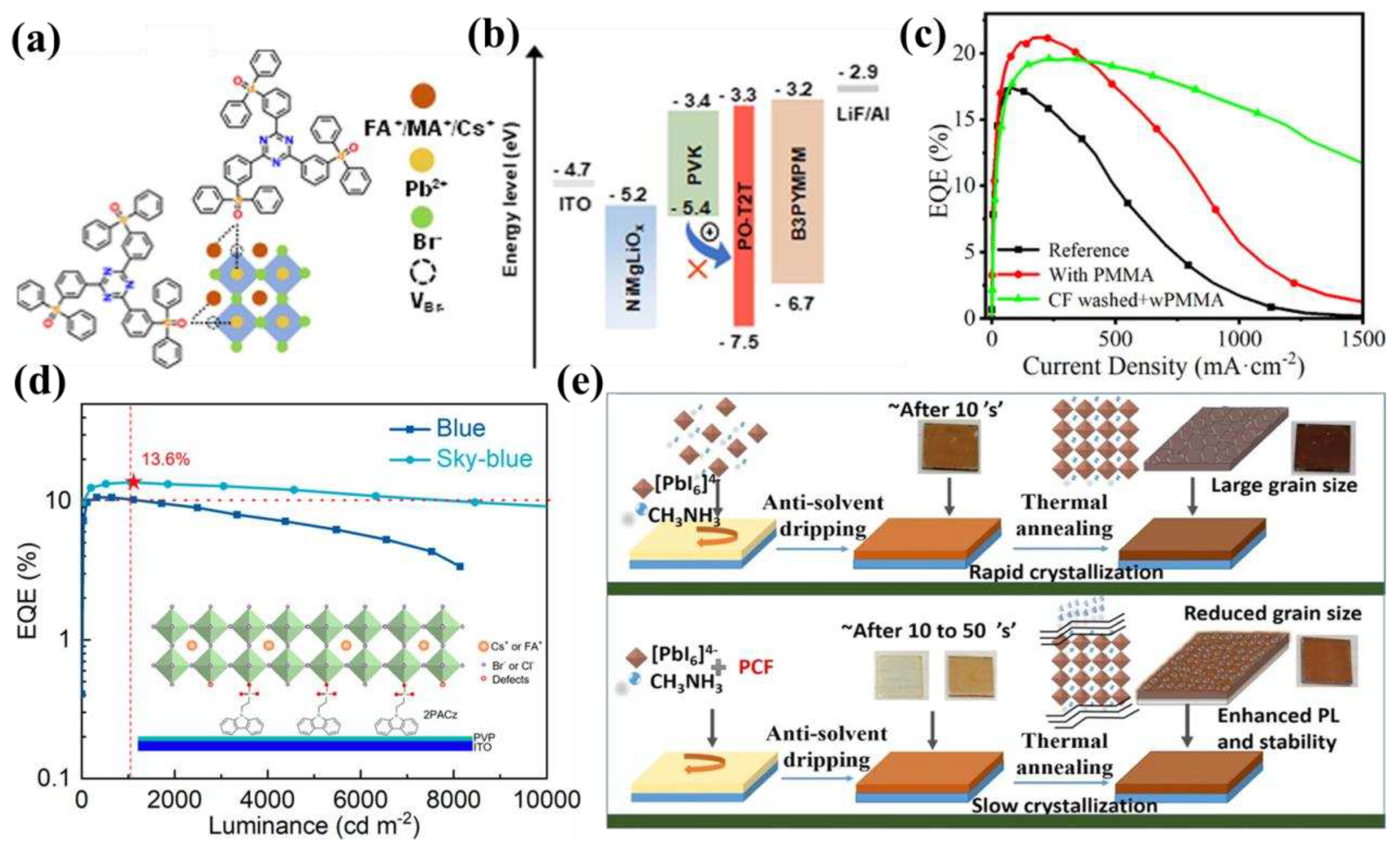
4.3. Low EQE for blue PeLEDs
5. Summary and outlook
Acknowledgments
References
- Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding Trihalide Perovskite Absorber. Science. 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Sutherland, B.R.; Sargent, E.H. Perovskite Photonic Sources. Nat. Photonics 2016, 10, 295–302. [Google Scholar] [CrossRef]
- Quan, L.N.; Rand, B.P.; Friend, R.H.; Mhaisalkar, S.G.; Lee, T.W.; Sargent, E.H. Perovskites for Next-Generation Optical Sources. Chem. Rev. 2019, 119, 7444–7477. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M. V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, Y.; Wang, S.; Guo, J.; Yu, W.W.; Rogach, A.L. Metal Halide Perovskite Light-Emitting Devices: Promising Technology for Next-Generation Displays. Adv. Funct. Mater. 2019, 29, 1902008. [Google Scholar] [CrossRef]
- Hassan, Y.; Park, J.H.; Crawford, M.L.; Sadhanala, A.; Lee, J.; Sadighian, J.C.; Mosconi, E.; Shivanna, R.; Radicchi, E.; Jeong, M.; et al. Ligand-Engineered Bandgap Stability in Mixed-Halide Perovskite LEDs. Nature 2021, 591, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; et al. Bright Light-Emitting Diodes Based on Organometal Halide Perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef]
- Zhou, W.; Shen, Y.; Cao, L.X.; Lu, Y.; Tang, Y.Y.; Zhang, K.; Ren, H.; Xie, F.M.; Li, Y.Q.; Tang, J.X. Manipulating Ionic Behavior with Bifunctional Additives for Efficient Sky-Blue Perovskite Light-Emitting Diodes. Adv. Funct. Mater. 2023, 33, 2301425. [Google Scholar] [CrossRef]
- Bai, W.; Xuan, T.; Zhao, H.; Dong, H.; Cheng, X.; Wang, L.; Xie, R.J. Perovskite Light-Emitting Diodes with an External Quantum Efficiency Exceeding 30%. Adv. Mater. 2023, 35, 2302283. [Google Scholar] [CrossRef]
- Jiang, J.; Chu, Z.; Yin, Z.; Li, J.; Yang, Y.; Chen, J.; Wu, J.; You, J.; Zhang, X. Red Perovskite Light-Emitting Diodes with Efficiency Exceeding 25% Realized by Co-Spacer Cations. Adv. Mater. 2022, 34, 2204460. [Google Scholar] [CrossRef]
- Sun, Y.; Ge, L.; Dai, L.; Cho, C.; Ferrer Orri, J.; Ji, K.; Zelewski, S.J.; Liu, Y.; Mirabelli, A.J.; Zhang, Y.; et al. Bright and Stable Perovskite Light-Emitting Diodes in the near-Infrared Range. Nature 2023, 615, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Fakharuddin, A.; Gangishetty, M.K.; Abdi-Jalebi, M.; Chin, S.H.; bin Mohd Yusoff, A.R.; Congreve, D.N.; Tress, W.; Deschler, F.; Vasilopoulou, M.; Bolink, H.J. Perovskite Light-Emitting Diodes. Nat. Electron. 2022, 5, 203–216. [Google Scholar] [CrossRef]
- Liu, X.K.; Xu, W.; Bai, S.; Jin, Y.; Wang, J.; Friend, R.H.; Gao, F. Metal Halide Perovskites for Light-Emitting Diodes. Nat. Mater. 2021, 20, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Kerner, R.A.; Zhao, L.; Tran, N.L.; Lee, K.M.; Koh, T.W.; Scholes, G.D.; Rand, B.P. Efficient Perovskite Light-Emitting Diodes Featuring Nanometre-Sized Crystallites. Nat. Photonics 2017, 11, 108–115. [Google Scholar] [CrossRef]
- Zhao, X.; Tan, Z.K. Large-Area near-Infrared Perovskite Light-Emitting Diodes. Nat. Photonics 2020, 14, 215–218. [Google Scholar] [CrossRef]
- Zhao, B.; Lian, Y.; Cui, L.; Divitini, G.; Kusch, G.; Ruggeri, E.; Auras, F.; Li, W.; Yang, D.; Zhu, B.; et al. Efficient Light-Emitting Diodes from Mixed-Dimensional Perovskites on a Fluoride Interface. Nat. Electron. 2020, 3, 704–710. [Google Scholar] [CrossRef]
- Zhao, B.; Bai, S.; Kim, V.; Lamboll, R.; Shivanna, R.; Auras, F.; Richter, J.M.; Yang, L.; Dai, L.; Alsari, M.; et al. High-Efficiency Perovskite–Polymer Bulk Heterostructure Light-Emitting Diodes. Nat. Photonics 2018, 12, 783–789. [Google Scholar] [CrossRef]
- Zhao, B.; Vasilopoulou, M.; Fakharuddin, A.; Gao, F.; Mohd Yusoff, A.R. bin; Friend, R.H.; Di, D. Light Management for Perovskite Light-Emitting Diodes. Nat. Nanotechnol. 2023, 18, 981–992. [Google Scholar] [CrossRef]
- Moore, D.T.; Sai, H.; Tan, K.W.; Smilgies, D.M.; Zhang, W.; Snaith, H.J.; Wiesner, U.; Estroff, L.A. Crystallization Kinetics of Organic-Inorganic Trihalide Perovskites and the Role of the Lead Anion in Crystal Growth. J. Am. Chem. Soc. 2015, 137, 2350–2358. [Google Scholar] [CrossRef]
- Chu, Z.; Yang, M.; Schulz, P.; Wu, D.; Ma, X.; Seifert, E.; Sun, L.; Li, X.; Zhu, K.; Lai, K. Impact of Grain Boundaries on Efficiency and Stability of Organic-Inorganic Trihalide Perovskites. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Nie, W.; Tsai, H.; Asadpour, R.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A.; Wang, H.; et al. High-Efficiency Solution-Processed Perovskite Solar Cells with Millimeter-Scale Grains. Science. 2015, 347, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-Halide Anion Engineering for α-FAPbI3 Perovskite Solar Cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Tang, P.; Luo, D.; Dar, M.I.; Eickemeyer, F.T.; Arora, N.; Hu, Q.; Luo, J.; Liu, Y.; Zakeeruddin, S.M.; et al. Enabling Full-Scale Grain Boundary Mitigation in Polycrystalline Perovskite Solids. Sci. Adv. 2022, 8, eabo3733. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, D.; Dar, M.I.; Dini, D.; Gontrani, L.; Caminiti, R.; Mattoni, A.; Graetzel, M.; Meloni, S. Dual Effect of Humidity on Cesium Lead Bromide: Enhancement and Degradation of Perovskite Films. J. Mater. Chem. A 2019, 7, 12292–12302. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Y.; Juarez-Perez, E.J.; Ono, L.K.; Qi, Y. Accelerated Degradation of Methylammonium Lead Iodide Perovskites Induced by Exposure to Iodine Vapour. Nat. Energy 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Ghosh, S.; Pal, S.K.; Karki, K.J.; Pullerits, T. Ion Migration Heals Trapping Centers in CH3NH3PbBr3 Perovskite. ACS Energy Lett. 2017, 2, 2133–2139. [Google Scholar] [CrossRef]
- Aristidou, N.; Eames, C.; Sanchez-Molina, I.; Bu, X.; Kosco, J.; Saiful Islam, M.; Haque, S.A. Fast Oxygen Diffusion and Iodide Defects Mediate Oxygen-Induced Degradation of Perovskite Solar Cells. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Han, T.H.; Lee, J.W.; Choi, Y.J.; Choi, C.; Tan, S.; Lee, S.J.; Zhao, Y.; Huang, Y.; Kim, D.; Yang, Y. Surface-2D/Bulk-3D Heterophased Perovskite Nanograins for Long-Term-Stable Light-Emitting Diodes. Adv. Mater. 2020, 32, 1905674. [Google Scholar] [CrossRef]
- Vashishtha, P.; Halpert, J.E. Field-Driven Ion Migration and Color Instability in Red-Emitting Mixed Halide Perovskite Nanocrystal Light-Emitting Diodes. Chem. Mater. 2017, 29, 5965–5973. [Google Scholar] [CrossRef]
- Fan, Z.; Xiao, H.; Wang, Y.; Zhao, Z.; Lin, Z.; Cheng, H.C.; Lee, S.J.; Wang, G.; Feng, Z.; Goddard, W.A.; et al. Layer-by-Layer Degradation of Methylammonium Lead Tri-Iodide Perovskite Microplates. Joule 2017, 1, 548–562. [Google Scholar] [CrossRef]
- Eperon, G.E.; Habisreutinger, S.N.; Leijtens, T.; Bruijnaers, B.J.; Van Franeker, J.J.; Dequilettes, D.W.; Pathak, S.; Sutton, R.J.; Grancini, G.; Ginger, D.S.; et al. The Importance of Moisture in Hybrid Lead Halide Perovskite Thin Film Fabrication. ACS Nano 2015, 9, 9380–9393. [Google Scholar] [CrossRef] [PubMed]
- Rehman, W.; McMeekin, D.P.; Patel, J.B.; Milot, R.L.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. Photovoltaic Mixed-Cation Lead Mixed-Halide Perovskites: Links between Crystallinity, Photo-Stability and Electronic Properties. Energy Environ. Sci. 2017, 10, 361–369. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Jiang, Q.; Wang, P.; Yin, Z.; Zhang, X.; Tan, H.; Yang, Y.M.; Wei, M.; Sutherland, B.R.; et al. Ultra-Bright and Highly Efficient Inorganic Based Perovskite Light-Emitting Diodes. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Qin, C.; Cui, M.; He, T.; Liu, K.; Huang, Y.; Luo, M.; Zhang, L.; Xu, H.; Li, S.; et al. Spectra Stable Blue Perovskite Light-Emitting Diodes. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Tao, S.X.; Bobbert, P.A.; Wong, C.P.; Zhao, N. Interstitial Occupancy by Extrinsic Alkali Cations in Perovskites and Its Impact on Ion Migration. Adv. Mater. 2018, 30, 1707350. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Li, J.; Wang, L.; Sun, L.; Wang, X.; Xu, X.; Yang, L.; Pang, J.; Liang, W.; Luo, J.; et al. Efficient and Large-Area All Vacuum-Deposited Perovskite Light-Emitting Diodes via Spatial Confinement. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kim, Y.H.; Wolf, C.; Lee, H.D.; Lee, T.W. Improving the Stability of Metal Halide Perovskite Materials and Light-Emitting Diodes. Adv. Mater. 2018, 30, 1704587. [Google Scholar] [CrossRef]
- Liu, M.; Matuhina, A.; Zhang, H.; Vivo, P. Advances in the Stability of Halide Perovskite Nanocrystals. Materials. 2019, 12. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.K.; Yuan, F.; Johnston, A.; Liu, Y.; Ma, D.; Choi, M.J.; Chen, B.; Chekini, M.; Baek, S.W.; et al. Bipolar-Shell Resurfacing for Blue LEDs Based on Strongly Confined Perovskite Quantum Dots. Nat. Nanotechnol. 2020, 15, 668–674. [Google Scholar] [CrossRef]
- Mei, A.; Li, X.; Liu, L.; Ku, Z.; Liu, T.; Rong, Y.; Xu, M.; Hu, M.; Chen, J.; Yang, Y.; et al. A Hole-Conductor-Free, Fully Printable Mesoscopic Perovskite Solar Cell with High Stability. Science. 2014, 345, 295–298. [Google Scholar] [CrossRef]
- Han, G.S.; Kim, J.; Bae, S.; Han, S.; Kim, Y.J.; Gong, O.Y.; Lee, P.; Ko, M.J.; Jung, H.S. Spin-Coating Process for 10 Cm × 10 Cm Perovskite Solar Modules Enabled by Self-Assembly of SnO2 Nanocolloids. ACS Energy Lett. 2019, 4, 1845–1851. [Google Scholar] [CrossRef]
- Jeong, D.N.; Lee, D.K.; Seo, S.; Lim, S.Y.; Zhang, Y.; Shin, H.; Cheong, H.; Park, N.G. Perovskite Cluster-Containing Solution for Scalable D-Bar Coating toward High-Throughput Perovskite Solar Cells. ACS Energy Lett. 2019, 4, 1189–1195. [Google Scholar] [CrossRef]
- Xu, M.; Ji, W.; Sheng, Y.; Wu, Y.; Cheng, H.; Meng, J.; Yan, Z.; Xu, J.; Mei, A.; Hu, Y.; et al. Efficient Triple-Mesoscopic Perovskite Solar Mini-Modules Fabricated with Slot-Die Coating. Nano Energy 2020, 74, 1–8. [Google Scholar] [CrossRef]
- Zeng, L.; Chen, S.; Forberich, K.; Brabec, C.J.; Mai, Y.; Guo, F. Controlling the Crystallization Dynamics of Photovoltaic Perovskite Layers on Larger-Area Coatings. Energy Environ. Sci. 2020, 13, 4666–4690. [Google Scholar] [CrossRef]
- Ball, J.M.; Petrozza, A. Defects in Perovskite-Halides and Their Effects in Solar Cells. Nat. Energy 2016, 1. [Google Scholar] [CrossRef]
- Jeong, J.E.; Park, J.H.; Jang, C.H.; Song, M.H.; Woo, H.Y. Multifunctional Charge Transporting Materials for Perovskite Light-Emitting Diodes. Adv. Mater. 2020, 32, 2002176. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, N.; Tian, H.; Guo, J.; Wei, Y.; Chen, H.; Miao, Y.; Zou, W.; Pan, K.; He, Y.; et al. Perovskite Light-Emitting Diodes Based on Spontaneously Formed Submicrometre-Scale Structures. Nature 2018, 562, 249–253. [Google Scholar] [CrossRef]
- Cao, J.; Yin, J.; Yuan, S.; Zhao, Y.; Li, J.; Zheng, N. Thiols as Interfacial Modifiers to Enhance the Performance and Stability of Perovskite Solar Cells. Nanoscale 2015, 7, 9443–9447. [Google Scholar] [CrossRef]
- Wang, R.; Xue, J.; Meng, L.; Lee, J.W.; Zhao, Z.; Sun, P.; Cai, L.; Huang, T.; Wang, Z.; Wang, Z.K.; et al. Caffeine Improves the Performance and Thermal Stability of Perovskite Solar Cells. Joule 2019, 3, 1464–1477. [Google Scholar] [CrossRef]
- Huang, G.; Wang, C.; Zhang, H.; Xu, S.; Xu, Q.; Cui, Y. Post-Healing of Defects: An Alternative Way for Passivation of Carbon-Based Mesoscopic Perovskite Solar Cells: Via Hydrophobic Ligand Coordination. J. Mater. Chem. A 2018, 6, 2449–2455. [Google Scholar] [CrossRef]
- Du, K.; Wang, A.; Li, Y.; Xu, Y.; Li, L.; Yuan, N.; Ding, J. The Synergistic Effect of Phosphonic and Carboxyl Acid Groups for Efficient and Stable Perovskite Solar Cells. Materials. 2023, 16, 7306. [Google Scholar] [CrossRef] [PubMed]
- Wang, K. li; Li, M.; Lou, Y.H.; Chen, J.; Shi, Y.R.; Chen, C.H.; Zhou, Y.H.; Wang, Z.K.; Liao, L.S. Aniline Sulfonic Acid Induced Uniform Perovskite Film for Large-Scale Photovoltaics. Adv. Energy Mater. 2023, 13, 2203471. [Google Scholar] [CrossRef]
- Zhang, H.; Pfeifer, L.; Zakeeruddin, S.M.; Chu, J.; Grätzel, M. Tailoring Passivators for Highly Efficient and Stable Perovskite Solar Cells. Nat. Rev. Chem. 2023, 7, 632–652. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Tong, J.; Xian, Y.; Kerner, R.A.; Dunfield, S.P.; Xiao, C.; Scheidt, R.A.; Kuciauskas, D.; Wang, X.; Hautzinger, M.P.; et al. Surface Reaction for Efficient and Stable Inverted Perovskite Solar Cells. Nature 2022, 611, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tan, Z.K.; Di, D.; Lai, M.L.; Jiang, L.; Lim, J.H.W.; Friend, R.H.; Greenham, N.C. Efficient Light-Emitting Diodes Based on Nanocrystalline Perovskite in a Dielectric Polymer Matrix. Nano Lett. 2015, 15, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Peng, X.; Wang, Q.; Ren, J.; Xiong, Z.; Yang, X. On the Performance of Polymer:Organometal Halide Perovskite Composite Light Emitting Devices: The Effects of Polymer Additives. Org. Electron. 2018, 52, 350–355. [Google Scholar] [CrossRef]
- Jang, C.H.; Kim, Y.I.; Harit, A.K.; Ha, J.M.; Park, S.; Noh, Y.W.; Lee, A. young; Kim, K.S.; Jung, J.W.; Woo, H.Y.; et al. Multifunctional Conjugated Molecular Additives for Highly Efficient Perovskite Light-Emitting Diodes. Adv. Mater. 2023, 35, 2210511. [Google Scholar] [CrossRef]
- Kumawat, N.K.; Dey, A.; Kumar, A.; Gopinathan, S.P.; Narasimhan, K.L.; Kabra, D. Band Gap Tuning of CH3NH3Pb(Br1-XClx)3 Hybrid Perovskite for Blue Electroluminescence. ACS Appl. Mater. Interfaces 2015, 7, 13119–13124. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, C.; Jiang, X.F.; Liu, M.; Xia, R.; Shi, T.; Chen, D.; Xue, Q.; Zhao, Y.J.; Su, S.; et al. High-Performance Color-Tunable Perovskite Light Emitting Devices through Structural Modulation from Bulk to Layered Film. Adv. Mater. 2017, 29, 1603157. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Shin, Y.S.; Jang, H.; Son, J.G.; Kim, J.W.; Park, C.B.; Yuk, D.; Seo, J.; Kim, G.H.; Kim, J.Y. Highly Stable Bulk Perovskite for Blue LEDs with Anion-Exchange Method. Nano Lett. 2021, 21, 3473–3479. [Google Scholar] [CrossRef]
- Park, C.B.; Shin, Y.S.; Yoon, Y.J.; Jang, H.; Son, J.G.; Kim, S.; An, N.G.; Kim, J.W.; Jun, Y.C.; Kim, G.H.; et al. Suppression of Halide Migration and Immobile Ionic Surface Passivation for Blue Perovskite Light-Emitting Diodes. J. Mater. Chem. C 2022, 10, 2060–2066. [Google Scholar] [CrossRef]
- Cheng, L.; Yi, C.; Tong, Y.; Zhu, L.; Kusch, G.; Wang, X.; Wang, X.; Jiang, T.; Zhang, H.; Zhang, J.; et al. Halide Homogenization for High-Performance Blue Perovskite Electroluminescence. Research 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Bi, X.; Xu, S.; Min, H.; Cheng, L.; Kuang, Z.; Yuan, L.; Zhou, F.; Chu, Y.; Xu, L.; et al. In Situ Halide Exchange of Cesium Lead Halide Perovskites for Blue Light-Emitting Diodes. Adv. Mater. 2023, 35, 2207111. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, Y.; Chen, Y.; Wang, C.; Zhao, G.; Du, X.; Wang, C.; Xiao, L.; Lu, Z.; Wang, J.; et al. Surface Defect Suppression for High Color Purity Light-Emitting Diode of Free-Standing Single-Crystal Perovskite Film. Adv. Funct. Mater. 2023, 33, 2301205. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Yan, C.; Zeng, X.; Fu, X.; Pan, L.; Cao, J.; Yang, S.; Li, W.; Chen, X.; et al. Molecularly Designing a Passivation ETL to Suppress EQE Roll-off of PeLEDs. ACS Energy Lett. 2023, 8, 3710–3719. [Google Scholar] [CrossRef]
- Wei, J.; Li, J.; Duan, C.; Yuan, L.; Zou, S.; Pang, Q.; Yan, K. High Efficiency Near-Infrared Perovskite Light Emitting Diodes With Reduced Rolling-Off by Surface Post-Treatment. Small 2023, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite Light-Emitting Diodes with External Quantum Efficiency Exceeding 20 per Cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, W.; Li, M.; Lu, J.; Qin, X.; Lin, K.; Luo, J.; Zhang, W.H.; Lim, E.L.; Wei, Z. Efficient Perovskite Light-Emitting Diodes with Chemically Bonded Contact and Regulated Charge Behavior. Nano Lett. 2023, 23, 8560–8567. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, H.; Zhou, Y.; Li, N.; Guo, Y.; Xie, F.; Qin, Z.; Lu, X.; Zhao, N. Excess Ion-Induced Efficiency Roll-Off in High-Efficiency Perovskite Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2021, 13, 28546–28554. [Google Scholar] [CrossRef]
- Li, Z.; Sun, X.; Zheng, X.; Li, B.; Gao, D.; Zhang, S.; Wu, X.; Li, S.; Gong, J.; Luther, J.M.; et al. Stabilized Hole-Selective Layer for High-Performance Inverted p-i-n Perovskite Solar Cells. Science. 2023, 382, 284–289. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, F.; Wang, X.; Chen, R.; Zhang, H.; Zhan, L.; Jiang, X.; Li, Y.; Ji, X.; Liu, S.; et al. Minimizing Buried Interfacial Defects for Efficient Inverted Perovskite Solar Cells. Science. 2023, 380, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zou, C.; Tang, W.; Xing, S.; Wang, Z.; Zhao, B.; Di, D. Efficient and Bright Blue Perovskite LEDs Enabled by a Carbazole-Phosphonic Acid Interface. ACS Energy Lett. 2023, 8, 2897–2903. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chu, C.Y.; Huang, Y.C.; Huang, C.W.; Chang, S.Y.; Chen, C.A.; Chao, C.Y.; Su, W.F. Tuning Perovskite Morphology by Polymer Additive for High Efficiency Solar Cell. ACS Appl. Mater. Interfaces 2015, 7, 4955–4961. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Huang, F.; Wang, S.; Zhou, L.; Jin, P.; Xin, Y.; Cai, Z.; Yin, Z.; Pang, Q.; Zhang, J.Z. Highly Stable Hybrid Perovskite Solar Cells Modified with Polyethylenimine via Ionic Bonding. ChemNanoMat 2018, 4, 649–655. [Google Scholar] [CrossRef]
- Kim, M.; Motti, S.G.; Sorrentino, R.; Petrozza, A. Enhanced Solar Cell Stability by Hygroscopic Polymer Passivation of Metal Halide Perovskite Thin Film. Energy Environ. Sci. 2018, 11, 2609–2619. [Google Scholar] [CrossRef]
- Shivarudraiah, S.B.; Chan, C.C.S.; Chen, D.; Zhou, Z.; Ng, M.; Tewari, N.; Tao, C.K.; Wong, K.S.; Halpert, J.E. In Situ MAPbI3 Perovskite Nanostructures Formed by a Poly [(Phenylglycidyl Ether)-Co-Formaldehyde] Epoxide for Enhanced Stability and Photoluminescence. ACS Appl. Nano Mater. 2023, 6, 12240–12247. [Google Scholar] [CrossRef]
- Calabrese, J.; Jones, N.L.; Harlow, R.L.; Herron, N.D.; Thorn, L.; Wang, Y. Preparation and Characterization of Layered Rare Earth Compound. J. Am. Chem. Soc. 1991, 113, 2328–2330. [Google Scholar] [CrossRef]
- Hanmandlu, C.; Singh, A.; Chen, L.; Wang, H.; Fang, C.; Luo, H. Two / Quasi-Two-Dimensional Perovskite-Based Heterostructures : Construction, Properties And. Int. J. Extrem. Manuf. 2023, 5, 012004. [Google Scholar]
- Stoumpos, C.C.; Cao, D.H.; Clark, D.J.; Young, J.; Rondinelli, J.M.; Jang, J.I.; Hupp, J.T.; Kanatzidis, M.G. Ruddlesden-Popper Hybrid Lead Iodide Perovskite 2D Homologous Semiconductors. Chem. Mater. 2016, 28, 2852–2867. [Google Scholar] [CrossRef]
- Lee, J.-W.; Park, N.-G. Quasi-Two-Dimensional Perovskite Light Emitting Diodes for Bright Future. Light Sci. Appl. 2021, 10, 10–11. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Peng, J.; Tang, J.; Zheng, K.; Liang, Z. 2D Ruddlesden–Popper Perovskites for Optoelectronics. Adv. Mater. 2018, 30, 1703487. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Quan, L.N.; Comin, R.; Walters, G.; Sabatini, R.; Voznyy, O.; Hoogland, S.; Zhao, Y.; Beauregard, E.M.; Kanjanaboos, P.; et al. Perovskite Energy Funnels for Efficient Light-Emitting Diodes. Nat. Nanotechnol. 2016, 11, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.; Nie, W.; Blancon, J.C.; Stoumpos, C.C.; Asadpour, R.; Harutyunyan, B.; Neukirch, A.J.; Verduzco, R.; Crochet, J.J.; Tretiak, S.; et al. High-Efficiency Two-Dimensional Ruddlesden-Popper Perovskite Solar Cells. Nature 2016, 536, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wu, T.; Yang, Y.; McLeod, J.A.; Wang, Y.; Zou, Y.; Zhai, T.; Li, J.; Ban, M.; Song, T.; et al. Alternative Type Two-Dimensional-Three-Dimensional Lead Halide Perovskite with Inorganic Sodium Ions as a Spacer for High-Performance Light-Emitting Diodes. ACS Nano 2019, 13, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Jiang, T.; Cao, Y.; Yi, C.; Wang, N.; Huang, W.; Wang, J. Multiple-Quantum-Well Perovskites for High-Performance Light-Emitting Diodes. Adv. Mater. 2020, 32, 201904163. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.N.; Zhao, Y.; García De Arquer, F.P.; Sabatini, R.; Walters, G.; Voznyy, O.; Comin, R.; Li, Y.; Fan, J.Z.; Tan, H.; et al. Tailoring the Energy Landscape in Quasi-2D Halide Perovskites Enables Efficient Green-Light Emission. Nano Lett. 2017, 17, 3701–3709. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, X.; Li, Y.; Wang, H.; Jiang, Y.; Wang, S.; You, M.; Zhang, C.; Zhang, T.; Kershaw, S. V.; et al. Smoothing the Energy Transfer Pathway in Quasi-2D Perovskite Films Using Methanesulfonate Leads to Highly Efficient Light-Emitting Devices. Nat. Commun. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Jiang, Y.; Cui, M.; Li, S.; Sun, C.; Huang, Y.; Wei, J.; Zhang, L.; Lv, M.; Qin, C.; Liu, Y.; et al. Reducing the Impact of Auger Recombination in Quasi-2D Perovskite Light-Emitting Diodes. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Ma, D.; Lin, K.; Dong, Y.; Choubisa, H.; Proppe, A.H.; Wu, D.; Wang, Y.K.; Chen, B.; Li, P.; Fan, J.Z.; et al. Distribution Control Enables Efficient Reduced-Dimensional Perovskite LEDs. Nature 2021, 599, 594–598. [Google Scholar] [CrossRef]
- Liu, S.; Guo, Z.; Wu, X.; Liu, X.; Huang, Z.; Li, L.; Zhang, J.; Zhou, H.; Sun, L.D.; Yan, C.H. Zwitterions Narrow Distribution of Perovskite Quantum Wells for Blue Light-Emitting Diodes with Efficiency Exceeding 15%. Adv. Mater. 2023, 35, 2208078. [Google Scholar] [CrossRef]
- Yang, S.J.; Wang, K.; Luo, Y.; Park, J.Y.; Yang, H.; Coffey, A.H.; Ma, K.; Sun, J.; Wieghold, S.; Zhu, C.; et al. Two-Factor Phase Separations in Mixed-Halide Quasi-2D Perovskite LEDs: Dimensionality and Halide Segregations. ACS Energy Lett. 2023, 8, 3693–3701. [Google Scholar] [CrossRef]
- Chu, Z.; Zhang, W.; Jiang, J.; Qu, Z.; Ma, F.; Zhao, Y.; Chu, X.; Shen, Y.; Li, Y.; Yin, Z.; et al. Blue Light-Emitting Diodes Based on Quasi-Two-Dimensional Perovskite with Efficient Charge Injection and Optimized Phase Distribution via an Alkali Metal Salt. Nat. Electron. 2023, 6, 360–369. [Google Scholar] [CrossRef]
- Lin, Y.K.; Chen, C.H.; Wang, Y.Y.; Yu, M.H.; Yang, J.W.; Ni, I.C.; Lin, B.H.; Zhidkov, I.S.; Kurmaev, E.Z.; Lu, Y.J.; et al. Realizing High Brightness Quasi-2D Perovskite Light-Emitting Diodes with Reduced Efficiency Roll-Off via Multifunctional Interface Engineering. Adv. Sci. 2023, 10, 2302232. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.X.; Wu, W.Q.; Liao, J.F.; Feng, W.; Jiang, Y.; Wang, L.; Kuang, D. Bin The Rise of Textured Perovskite Morphology: Revolutionizing the Pathway toward High-Performance Optoelectronic Devices. Adv. Energy Mater. 2020, 10, 1902256. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Chen, Z.; Xia, R.; Zou, G.; Chu, L.; Su, S.J.; Peng, J.; Yip, H.L.; Cao, Y. Utilization of Trapped Optical Modes for White Perovskite Light-Emitting Diodes with Efficiency over 12%. Joule 2021, 5, 456–466. [Google Scholar] [CrossRef]
- Cui, J.; Liu, Y.; Deng, Y.; Lin, C.; Fang, Z.; Xiang, C.; Bai, P.; Du, K.; Zuo, X.; Wen, K.; et al. Efficient Light-Emitting Diodes Based on Oriented Perovskite Nanoplatelets. Sci. Adv. 2021, 7, eabg8458. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Yadav, A. Recent Progress in Inverted Perovskite Solar Cells Employing Nickel Oxide (NiOx) as a Hole Transport Materials. Mater. Today Proc. 2021, 46, 5827–5832. [Google Scholar] [CrossRef]
- Kazes, M.; Udayabhaskararao, T.; Dey, S.; Oron, D. Effect of Surface Ligands in Perovskite Nanocrystals: Extending in and Reaching Out. Acc. Chem. Res. 2021, 54, 1409–1418. [Google Scholar] [CrossRef]
- Kovalenko, M. V.; Protesescu, L.; Bodnarchuk, M.I. Properties and Potential Optoelectronic Applications of Lead Halide Perovskite Nanocrystals. Science. 2017, 358, 745–750. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M. V.; Manna, L. Genesis, Challenges and Opportunities for Colloidal Lead Halide Perovskite Nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef]
- Shamsi, J.; Rainò, G.; Kovalenko, M. V.; Stranks, S.D. To Nano or Not to Nano for Bright Halide Perovskite Emitters. Nat. Nanotechnol. 2021, 16, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.C.; Pertegás, A.; Gonzalez-carrero, S.; Malinkiewicz, O.; Agouram, S.; Espallargas, G.M.; Bolink, H.J.; Galian, R.E.; Pérez-prieto, J.; González-carrero, S. Non-Template Synthesis of CH3NH3PbBr3 Perovskite Nanoparticles Non-Template Synthesis of CH 3 NH 3 PbBr 3 Perovskite Nanopar- Ticles. J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, S.; Kakekhani, A.; Park, J.; Park, J.; Lee, Y.H.; Xu, H.; Nagane, S.; Wexler, R.B.; Kim, D.H.; et al. Comprehensive Defect Suppression in Perovskite Nanocrystals for High-Efficiency Light-Emitting Diodes. Nat. Photonics 2021, 15, 148–155. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, J.; Kim, S.; Kim, J.S.; Xu, H.; Jeong, S.H.; Hu, B.; Lee, T.W. Exploiting the Full Advantages of Colloidal Perovskite Nanocrystals for Large-Area Efficient Light-Emitting Diodes. Nat. Nanotechnol. 2022, 17, 590–597. [Google Scholar] [CrossRef]
- Yun, Q.; Ge, Y.; Huang, B.; Wa, Q.; Zhang, H. Ligand-Assisted Phase Engineering of Nanomaterials. Acc. Chem. Res. 2023, 56, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, T.W. Engineering Colloidal Perovskite Nanocrystals and Devices for Efficient and Large-Area Light-Emitting Diodes. Accounts Mater. Res. 2023, 4, 655–667. [Google Scholar] [CrossRef]
- Yassitepe, E.; Yang, Z.; Voznyy, O.; Kim, Y.; Walters, G.; Castañeda, J.A.; Kanjanaboos, P.; Yuan, M.; Gong, X.; Fan, F.; et al. Amine-Free Synthesis of Cesium Lead Halide Perovskite Quantum Dots for Efficient Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 8757–8763. [Google Scholar] [CrossRef]
- Shynkarenko, Y.; Bodnarchuk, M.I.; Bernasconi, C.; Berezovska, Y.; Verteletskyi, V.; Ochsenbein, S.T.; Kovalenko, M. V. Direct Synthesis of Quaternary Alkylammonium-Capped Perovskite Nanocrystals for Efficient Blue and Green Light-Emitting Diodes. ACS Energy Lett. 2019, 4, 2703–2711. [Google Scholar] [CrossRef]
- Yang, D.; Li, X.; Zhou, W.; Zhang, S.; Meng, C.; Wu, Y.; Wang, Y.; Zeng, H. CsPbBr3 Quantum Dots 2.0: Benzenesulfonic Acid Equivalent Ligand Awakens Complete Purification. Adv. Mater. 2019, 31, 1900767. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, C.; Yu, Y.; Xue, W.; Liu, X.; Yuan, F.; Dai, J.; Wang, S.; Jiao, B.; Wu, Z. Multifunctional Short-Chain 2-Thiophenealkylammonium Bromide Ligand-Assisted Perovskite Quantum Dots for Efficient Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2023, 15, 40080–40087. [Google Scholar] [CrossRef]
- Guo, J.; Lu, M.; Zhang, X.; Sun, S.; Han, C.; Zhang, Y.; Yang, X.; Kershaw, S. V.; Zheng, W.; Rogach, A.L. Highly Stable and Efficient Light-Emitting Diodes Based on Orthorhombic γ-CsPbI3Nanocrystals. ACS Nano 2023, 17, 9290–9301. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Quan, L.N.; Zhao, Y.; Peng, W.; Murali, B.; Sarmah, S.P.; Yuan, M.; Sinatra, L.; Alyami, N.M.; Liu, J.; et al. Highly Efficient Perovskite-Quantum-Dot Light-Emitting Diodes by Surface Engineering. Adv. Mater. 2016, 28, 8718–8725. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Wang, J.; Zhang, X.; Zhuang, R.; Hua, Y.; He, K.; Zheng, W.; Zhang, X. In Situ Ligand Compensation of Perovskite Quantum Dots for Efficient Light-Emitting Diodes. ACS Energy Lett. 2023, 8, 4386–4396. [Google Scholar] [CrossRef]
- Boles, M.A.; Ling, D.; Hyeon, T.; Talapin, D. V. The Surface Science of Nanocrystals. Nat. Mater. 2016, 15, 141–153. [Google Scholar] [CrossRef]
- Yin, J.; Yang, H.; Gutiérrez-Arzaluz, L.; Zhou, Y.; Brédas, J.L.; Bakr, O.M.; Mohammed, O.F. Luminescence and Stability Enhancement of Inorganic Perovskite Nanocrystals via Selective Surface Ligand Binding. ACS Nano 2021, 15, 17998–18005. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ko, P.K.; Li, C.H.A.; Zou, B.; Geng, P.; Guo, L.; Halpert, J.E. Amino Acid-Passivated Pure Red CsPbI3 Quantum Dot LEDs. ACS Energy Lett. 2023, 8, 410–416. [Google Scholar] [CrossRef]
- Fiuza-Maneiro, N.; Sun, K.; López-Fernández, I.; Gómez-Graña, S.; Müller-Buschbaum, P.; Polavarapu, L. Ligand Chemistry of Inorganic Lead Halide Perovskite Nanocrystals. ACS Energy Lett. 2023, 8, 1152–1191. [Google Scholar] [CrossRef]
- Yoon, S.M.; Min, H.; Kim, J.B.; Kim, G.; Lee, K.S.; Seok, S. Il Surface Engineering of Ambient-Air-Processed Cesium Lead Triiodide Layers for Efficient Solar Cells. Joule 2021, 5, 183–196. [Google Scholar] [CrossRef]
- Wang, Y.K.; Yuan, F.; Dong, Y.; Li, J.Y.; Johnston, A.; Chen, B.; Saidaminov, M.I.; Zhou, C.; Zheng, X.; Hou, Y.; et al. All-Inorganic Quantum-Dot LEDs Based on a Phase-Stabilized α-CsPbI3 Perovskite. Angew. Chemie - Int. Ed. 2021, 60, 16164–16170. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Dong, Z.; Wei, X.; Li, W.; Zhu, L.; Zhu, C.; Bai, Y.; Chen, H. Dimethyl Sulfoxide: A Promising Solvent for Inorganic CsPbI3 Perovskite. Sci. Bull. 2023, 68, 192–202. [Google Scholar] [CrossRef]
- Zhao, F.; Duan, H.W.; Li, S.N.; Pan, J.L.; Shen, W.S.; Li, S.M.; Zhang, Q.; Wang, Y.K.; Liao, L.S. Iodotrimethylsilane as a Reactive Ligand for Surface Etching and Passivation of Perovskite Nanocrystals toward Efficient Pure-Red to Deep-Red LEDs. Angew. Chemie - Int. Ed. 2023, 62, eabg8458. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Yu, C.; Je, H.; Park, J.Y.; Kim, T.; Baik, H.; Tomboc, G.M.; Kim, Y.; Ha, J.M.; Joo, J.; et al. Perovskite Nanocrystals Protected by Hermetically Sealing for Highly Bright and Stable Deep-Blue Light-Emitting Diodes. Adv. Sci. 2023, 10, 2302906. [Google Scholar] [CrossRef] [PubMed]
- Koscher, B.A.; Swabeck, J.K.; Bronstein, N.D.; Alivisatos, A.P. Essentially Trap-Free CsPbBr3 Colloidal Nanocrystals by Postsynthetic Thiocyanate Surface Treatment. J. Am. Chem. Soc. 2017, 139, 6566–6569. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, J.S.; Lee, T.W. Strategies to Improve Luminescence Efficiency of Metal-Halide Perovskites and Light-Emitting Diodes. Adv. Mater. 2019, 31, 1804595. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Zheng, W.; Zou, C.; Carulli, F.; Zhang, C.; Song, H.; Liu, M.; Zhang, Q.; Lin, L.Y.; Kong, L.; et al. Ultrathin Light-Emitting Diodes with External Efficiency over 26% Based on Resurfaced Perovskite Nanocrystals. ACS Energy Lett. 2023, 8, 927–934. [Google Scholar] [CrossRef]
- Yang, F.; Chen, H.; Zhang, R.; Liu, X.; Zhang, W.; Zhang, J. Bin; Gao, F.; Wang, L. Efficient and Spectrally Stable Blue Perovskite Light-Emitting Diodes Based on Potassium Passivated Nanocrystals. Adv. Funct. Mater. 2020, 30, 1908760. [Google Scholar] [CrossRef]
- Song, J.; Fang, T.; Li, J.; Xu, L.; Zhang, F.; Han, B.; Shan, Q.; Zeng, H. Organic–Inorganic Hybrid Passivation Enables Perovskite QLEDs with an EQE of 16.48%. Adv. Mater. 2018, 30, 1805409. [Google Scholar] [CrossRef]
- Xu, J.; Yan, H.; Ali, M.U.; Wu, L.; Miao, J.; Cai, J.; Ning, J.; Yang, B.; Liu, M.; Shen, C.K.F.; et al. Efficient NIR Perovskite Light-Emitting Diodes Enabled by Incorporating an Anthracene Derivative as a Bifunctional Electron Transport Layer. ACS Appl. Electron. Mater. 2022, 4, 1669–1677. [Google Scholar] [CrossRef]
- Chiba, T.; Hayashi, Y.; Ebe, H.; Hoshi, K.; Sato, J.; Sato, S.; Pu, Y.J.; Ohisa, S.; Kido, J. Anion-Exchange Red Perovskite Quantum Dots with Ammonium Iodine Salts for Highly Efficient Light-Emitting Devices. Nat. Photonics 2018, 12, 681–687. [Google Scholar] [CrossRef]
- Yuan, S.; Fang, T.; Huang, J.; Li, X.; Wei, C.; Zhou, Y.; Li, Y.; Zheng, X.; Huang, J.; Su, J.; et al. Balancing Charge Injection via a Tailor-Made Electron-Transporting Material for High Performance Blue Perovskite QLEDs. ACS Energy Lett. 2023, 8, 818–826. [Google Scholar] [CrossRef]
- Jiang, Y.; Wei, K.; Sun, C.; Feng, Y.; Zhang, L.; Cui, M.; Li, S.; Li, W. Di; Kim, J.T.; Qin, C.; et al. Unraveling Size-Dependent Ion-Migration for Stable Mixed-Halide Perovskite Light-Emitting Diodes. Adv. Mater. 2023, 35, 2304094. [Google Scholar] [CrossRef]
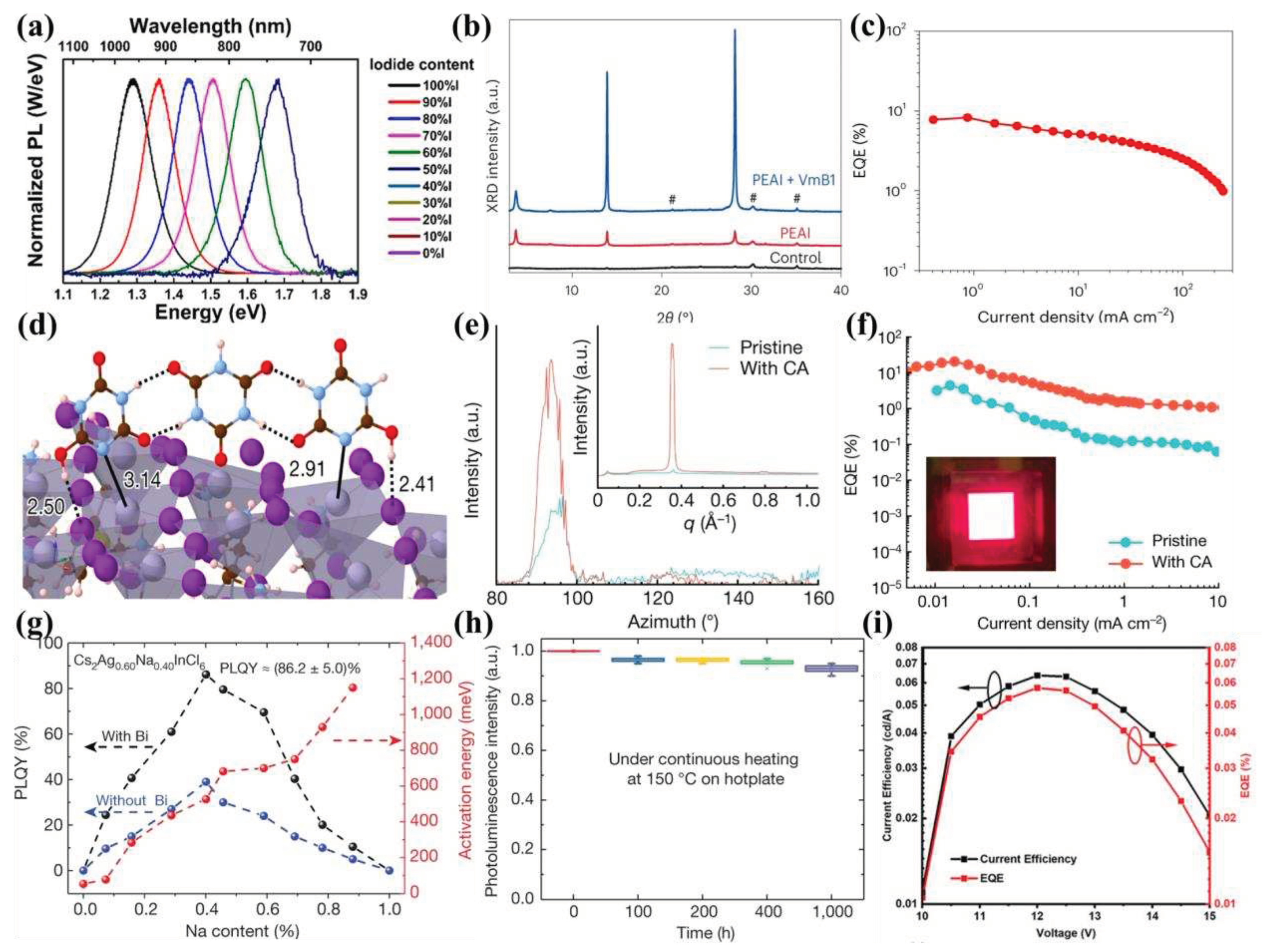
- Kim, J.S.; Heo, J.M.; Park, G.S.; Woo, S.J.; Cho, C.; Yun, H.J.; Kim, D.H.; Park, J.; Lee, S.C.; Park, S.H.; et al. Ultra-Bright, Efficient and Stable Perovskite Light-Emitting Diodes. Nature 2022, 611, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, W.; Wei, Q.; Peng, S.; Shang, Y.; Jiang, X.; Yu, D.; Wang, K.; Pu, R.; Zhao, C.; et al. In-Situ Growth of Low-Dimensional Perovskite-Based Insular Nanocrystals for Highly Efficient Light Emitting Diodes. Light Sci. Appl. 2023, 12, 62. [Google Scholar] [CrossRef]
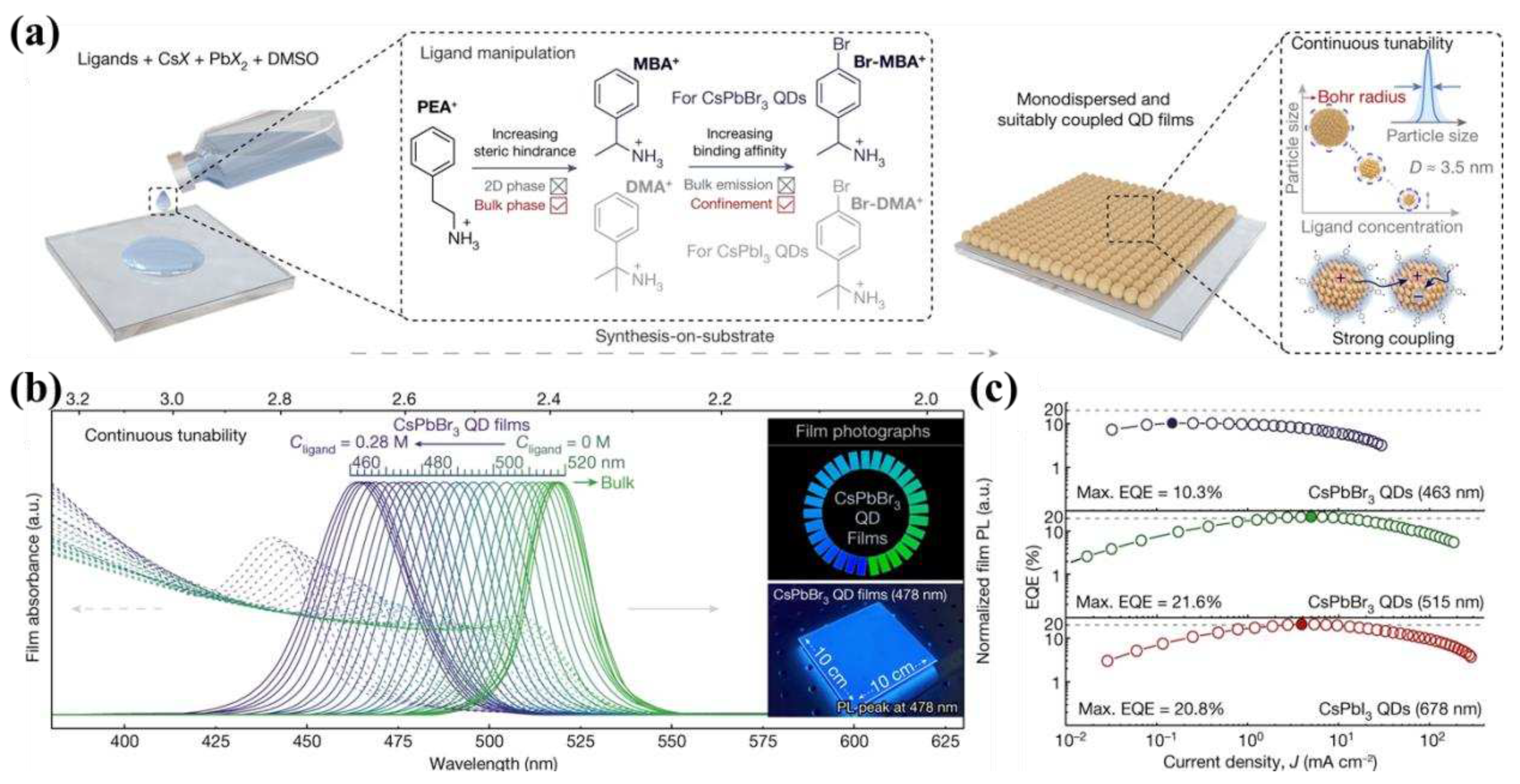
- Jiang, Y.; Sun, C.; Xu, J.; Li, S.; Cui, M.; Fu, X.; Liu, Y.; Liu, Y.; Wan, H.; Wei, K.; et al. Synthesis-on-Substrate of Quantum Dot Solids. Nature 2022, 612, 679–684. [Google Scholar] [CrossRef]
- López-Fernández, I.; Valli, D.; Wang, C.Y.; Samanta, S.; Okamoto, T.; Huang, Y.T.; Sun, K.; Liu, Y.; Chirvony, V.S.; Patra, A.; et al. Lead-Free Halide Perovskite Materials and Optoelectronic Devices: Progress and Prospective. Adv. Funct. Mater. 2023. [Google Scholar] [CrossRef]
- Toshniwal, A.; Kheraj, V. Development of Organic-Inorganic Tin Halide Perovskites: A Review. Sol. Energy 2017, 149, 54–59. [Google Scholar] [CrossRef]
- Kim, D.Y.; Jung, J.G.; Lee, Y.J.; Park, M.H. Lead-Free Halide Perovskite Nanocrystals for Light-Emitting Diodes. Materials. 2023, 16, 6317. [Google Scholar] [CrossRef]
- Lai, M.L.; Tay, T.Y.S.; Sadhanala, A.; Dutton, S.E.; Li, G.; Friend, R.H.; Tan, Z.K. Tunable Near-Infrared Luminescence in Tin Halide Perovskite Devices. J. Phys. Chem. Lett. 2016, 7, 2653–2658. [Google Scholar] [CrossRef]
- Yuan, F.; Zheng, X.; Johnston, A.; Wang, Y.; Zhou, C.; Dong, Y.; Chen, B.; Chen, H.; Fan, J.Z.; Sharma, G.; et al. Color-Pure Red Light-Emitting Diodes Based on Two-Dimensional Lead-Free Perovskites. Sci. Adv. 2020, 6, eabb0253. [Google Scholar] [CrossRef]
- Dong, H.; Ran, C.; Gao, W.; Sun, N.; Liu, X.; Xia, Y.; Chen, Y.; Huang, W. Crystallization Dynamics of Sn-Based Perovskite Thin Films: Toward Efficient and Stable Photovoltaic Devices. Adv. Energy Mater. 2022, 12, 2102213. [Google Scholar] [CrossRef]
- Min, H.; Chang, J.; Tong, Y.; Wang, J.; Zhang, F.; Feng, Z.; Bi, X.; Chen, N.; Kuang, Z.; Wang, S.; et al. Additive Treatment Yields High-Performance Lead-Free Perovskite Light-Emitting Diodes. Nat. Photonics 2023, 17, 755–760. [Google Scholar] [CrossRef]
- Han, D.; Wang, J.; Agosta, L.; Zang, Z.; Zhao, B.; Kong, L.; Lu, H.; Mosquera-Lois, I.; Carnevali, V.; Dong, J.; et al. Tautomeric Mixture Coordination Enables Efficient Lead-Free Perovskite LEDs. Nature 2023, 622, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.; Mutukwa, D.; Zingwe, N.; Taziwa, R. Lead-Free Halide Double Perovskites: A Review of the Structural, Optical, and Stability Properties as Well as Their Viability to Replace Lead Halide Perovskites. Metals. 2018, 8, 667. [Google Scholar] [CrossRef]
- Gourji, F.H. ; Dhayalan Velauthapillai A Review on Cs-Based Pb-Free Double Halide Perovskites : From Theoretical and Experimental Studies to Doping and Applications. Molecules 2021, 26, 2010. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, Y.; Cai, T.; Yang, H.; Zhao, J.X.; Yin, T.; Hao, C.; Chen, M.; Shi, W.; Li, X.; et al. Two-Dimensional Cs2AgInxBi1-XCl6 Alloyed Double Perovskite Nanoplatelets for Solution-Processed Light-Emitting Diodes. Adv. Mater. 2023, 35, 2211235. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wu, Z.; Lu, M.; Gao, Y.; Li, X.; Bai, X.; Ji, Y.; Zhang, Y. Broadband Emission Origin in Metal Halide Perovskites: Are Self-Trapped Excitons or Ions? Adv. Mater. 2023, 2211088. [Google Scholar] [CrossRef] [PubMed]
- Arfin, H.; Kshirsagar, A.S.; Kaur, J.; Mondal, B.; Xia, Z.; Chakraborty, S.; Nag, A. Ns2Electron (Bi3+and Sb3+) Doping in Lead-Free Metal Halide Perovskite Derivatives. Chem. Mater. 2020, 32, 10255–10267. [Google Scholar] [CrossRef]
- Li, S.; Luo, J.; Liu, J.; Tang, J. Self-Trapped Excitons in All-Inorganic Halide Perovskites: Fundamentals, Status, and Potential Applications. J. Phys. Chem. Lett. 2019, 10, 1999–2007. [Google Scholar] [CrossRef]
- Luo, J.; Wang, X.; Li, S.; Liu, J.; Guo, Y.; Niu, G.; Yao, L.; Fu, Y.; Gao, L.; Dong, Q.; et al. Efficient and Stable Emission of Warm-White Light from Lead-Free Halide Double Perovskites. Nature 2018, 563, 541–545. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Yu, W.; He, Y.; Chen, Z.; Xiao, L.; Shi, J. jie; Guo, X.; Wang, S.; Qu, B. Lead-Free Double Perovskite Cs2AgIn0.9Bi0.1Cl6 Quantum Dots for White Light-Emitting Diodes. Adv. Sci. 2022, 9, 2102895. [Google Scholar] [CrossRef]
- Cao, L.; Klimes, K.; Ji, Y.; Fleetham, T.; Li, J. Efficient and Stable Organic Light-Emitting Devices Employing Phosphorescent Molecular Aggregates. Nat. Photonics 2021, 15, 230–237. [Google Scholar] [CrossRef]
- Lee, T.; Kim, B.J.; Lee, H.; Hahm, D.; Bae, W.K.; Lim, J.; Kwak, J. Bright and Stable Quantum Dot Light-Emitting Diodes. Adv. Mater. 2022, 34, 2106276. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, Y.; Zhu, T.; Ma, D.; Proppe, A.; Chen, B.; Zheng, C.; Hou, Y.; Lee, S.; Sun, B.; et al. Bright and Stable Light-Emitting Diodes Based on Perovskite Quantum Dots in Perovskite Matrix. J. Am. Chem. Soc. 2021, 143, 15606–15615. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, X.; Zhang, C.; Wang, L.; Wang, S.; Cao, F.; Zhao, D.; Rogach, A.L.; Yang, X. Stability of Perovskite Light-Emitting Diodes: Existing Issues and Mitigation Strategies Related to Both Material and Device Aspects. Adv. Mater. 2022, 34, 2205217. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.C.; Li, Y.Q.; Cai, X.Y.; Zhou, W.; Shen, Y.; Shen, K.C.; Wang, J.K.; Gao, X.; Zhidkov, I.S.; Tang, J.X. Minimizing Optical Energy Losses for Long-Lifetime Perovskite Light-Emitting Diodes. Adv. Funct. Mater. 2021, 31, 2105813. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Li, Y.; Cai, L.; Tan, Y.; Zhang, G.; Wang, Y.; Li, R.; Liang, D.; Song, T.; et al. Prominent Heat Dissipation in Perovskite Light-Emitting Diodes with Reduced Efficiency Droop for Silicon-Based Display. J. Phys. Chem. Lett. 2020, 11, 3689–3698. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Perez, E.J.; Ono, L.K.; Qi, Y. Thermal Degradation of Formamidinium Based Lead Halide Perovskites into: Sym -Triazine and Hydrogen Cyanide Observed by Coupled Thermogravimetry-Mass Spectrometry Analysis. J. Mater. Chem. A 2019, 7, 16912–16919. [Google Scholar] [CrossRef]
- Zhu, Z.; Hadjiev, V.G.; Rong, Y.; Guo, R.; Cao, B.; Tang, Z.; Qin, F.; Li, Y.; Wang, Y.; Hao, F.; et al. Interaction of Organic Cation with Water Molecule in Perovskite MAPbI3: From Dynamic Orientational Disorder to Hydrogen Bonding. Chem. Mater. 2016, 28, 7385–7393. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Hu, Y.; Campoy-Quiles, M.; Alonso, M.I.; Weber, O.J.; Azarhoosh, P.; Van Schilfgaarde, M.; Weller, M.T.; Bein, T.; Nelson, J.; et al. Reversible Hydration of CH3NH3PbI3 in Films, Single Crystals, and Solar Cells. Chem. Mater. 2015, 27, 3397–3407. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, X.; Tang, Y.; Wang, H.; Zhang, C.; Yu, W.W.; Wang, X.; Zhang, Y.; Xiao, M. Phase Segregation Due to Ion Migration in All-Inorganic Mixed-Halide Perovskite Nanocrystals. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Xiao, Z.; Yuan, Y.; Shao, Y.; Wang, Q.; Dong, Q.; Bi, C.; Sharma, P.; Gruverman, A.; Huang, J. Giant Switchable Photovoltaic Effect in Organometal Trihalide Perovskite Devices. Nat. Mater. 2015, 14, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, J.H.; Lee, B.R.; Jung, E.D.; Yu, J.C.; Di Nuzzo, D.; Friend, R.H.; Song, M.H. Amine-Based Passivating Materials for Enhanced Optical Properties and Performance of Organic-Inorganic Perovskites in Light-Emitting Diodes. J. Phys. Chem. Lett. 2017, 8, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Tiepelt, J.O.; Christians, J.A.; Levine, I.; Edri, E.; Sanehira, E.M.; Hodes, G.; Cahen, D.; Kahn, A. High-Work-Function Molybdenum Oxide Hole Extraction Contacts in Hybrid Organic-Inorganic Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 31491–31499. [Google Scholar] [CrossRef]
- Wang, Y.; Teng, Y.; Lu, P.; Shen, X.; Jia, P.; Lu, M.; Shi, Z.; Dong, B.; Yu, W.W.; Zhang, Y. Low Roll-Off Perovskite Quantum Dot Light-Emitting Diodes Achieved by Augmenting Hole Mobility. Adv. Funct. Mater. 2020, 30, 1910140. [Google Scholar] [CrossRef]
- Kim, H.; Zhao, L.; Price, J.S.; Grede, A.J.; Roh, K.; Brigeman, A.N.; Lopez, M.; Rand, B.P.; Giebink, N.C. Hybrid Perovskite Light Emitting Diodes under Intense Electrical Excitation. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Fakharuddin, A.; Qiu, W.; Croes, G.; Devižis, A.; Gegevičius, R.; Vakhnin, A.; Rolin, C.; Genoe, J.; Gehlhaar, R.; Kadashchuk, A.; et al. Reduced Efficiency Roll-Off and Improved Stability of Mixed 2D/3D Perovskite Light Emitting Diodes by Balancing Charge Injection. Adv. Funct. Mater. 2019, 29, 1904101. [Google Scholar] [CrossRef]
- Zou, W.; Li, R.; Zhang, S.; Liu, Y.; Wang, N.; Cao, Y.; Miao, Y.; Xu, M.; Guo, Q.; Di, D.; et al. Minimising Efficiency Roll-off in High-Brightness Perovskite Light-Emitting Diodes. Nat. Commun. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Klimov, V.I.; Mikhailovsky, A.A.; McBranch, D.W.; Leatherdale, C.A.; Bawendi, M.G. Quantization of Multiparticle Auger Rates in Semiconductor Quantum Dots. Science. 2000, 287, 1011–1014. [Google Scholar] [CrossRef]
- Bi, C.; Yao, Z.; Hu, J.; Wang, X.; Zhang, M.; Tian, S.; Liu, A.; Lu, Y.; de Leeuw, N.H.; Sui, M.; et al. Suppressing Auger Recombination of Perovskite Quantum Dots for Efficient Pure-Blue-Light-Emitting Diodes. ACS Energy Lett. 2023, 8, 731–739. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Y.; Zhang, D.; Jiang, X.; Cai, P.; Si, J.; Hu, Q.; Fang, Z.; Dai, X.; Song, J.; et al. Bilayer Phosphine Oxide Modification toward Efficient and Large-Area Pure-Blue Perovskite Quantum Dot Light-Emitting Diodes. Sci. Bull. 2023, 68, 2354–2361. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Shen, Y.; Yu, Y.; Zhang, K.; Wang, B.F.; Tang, J.X.; Li, Y.Q. Comprehensive Crystal Regulation Reduces Interfacial Energy Loss for Efficient Blue Perovskite Light-Emitting Diodes. Small 2023, 2309309. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Wang, C.; Yuan, S.; Zou, C.; Su, Z.; Wang, K.L.; Xia, Y.; Wang, B.; Di, D.; Wang, Z.K.; et al. Stabilized Low-Dimensional Species for Deep-Blue Perovskite Light-Emitting Diodes with EQE Approaching 3.4%. J. Am. Chem. Soc. 2022, 144, 18470–18478. [Google Scholar] [CrossRef] [PubMed]
- Boehme, S.C.; Bodnarchuk, M.I.; Burian, M.; Bertolotti, F.; Cherniukh, I.; Bernasconi, C.; Zhu, C.; Erni, R.; Amenitsch, H.; Naumenko, D.; et al. Strongly Confined CsPbBr3 Quantum Dots as Quantum Emitters and Building Blocks for Rhombic Superlattices. ACS Nano 2023, 17, 2089–2100. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, F.; Li, X.; Teale, S.; Xia, P.; Liu, Y.; Chan, P.T.; Wan, H.; Hassan, Y.; Imran, M.; et al. Self-Assembled Monolayer – Based Blue Perovskite LEDs. Sci. Adv. 2023, 9, eadh2140. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Cui, L.S.; Dai, L.; Liu, Y.; Liu, Q.W.; Sun, Y.Q.; Auras, F.; Anaya, M.; Zheng, X.; Ruggeri, E.; et al. Efficient and Spectrally Stable Blue Perovskite Light-Emitting Diodes Employing a Cationic π-Conjugated Polymer. Adv. Mater. 2021, 33, 2103640. [Google Scholar] [CrossRef]
- Feng, W.; Zhao, Y.; Lin, K.; Lu, J.; Liang, Y.; Liu, K.; Xie, L.; Tian, C.; Lyu, T.; Wei, Z. Polymer-Assisted Crystal Growth Regulation and Defect Passivation for Efficient Perovskite Light-Emitting Diodes. Adv. Funct. Mater. 2022, 32, 2203371. [Google Scholar] [CrossRef]
- Li, M.; Sun, R.; Chang, J.; Dong, J.; Tian, Q.; Wang, H.; Li, Z.; Yang, P.; Shi, H.; Yang, C.; et al. Orientated Crystallization of FA-Based Perovskite via Hydrogen-Bonded Polymer Network for Efficient and Stable Solar Cells. Nat. Commun. 2023, 14, 573. [Google Scholar] [CrossRef]
- Huang, Z.; Li, L.; Wu, T.; Xue, T.; Sun, W.; Pan, Q.; Wang, H.; Xie, H.; Chi, J.; Han, T.; et al. Wearable Perovskite Solar Cells by Aligned Liquid Crystal Elastomers. Nat. Commun. 2023, 14, 1–19. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, G.; Li, J.; Lin, X.; Yang, F.; Yang, H.; Chen, W.; Wu, Y.; Wu, X.; Cheng, Q.; et al. Functional Ionic Liquid Polymer Stabilizer for High-Performance Perovskite Photovoltaics. Angew. Chemie - Int. Ed. 2023, 62, e202300690. [Google Scholar] [CrossRef]
- Yang, J.N.; Song, Y.; Yao, J.S.; Wang, K.H.; Wang, J.J.; Zhu, B.S.; Yao, M.M.; Rahman, S.U.; Lan, Y.F.; Fan, F.J.; et al. Potassium Bromide Surface Passivation on CsPbI3-XBrx Nanocrystals for Efficient and Stable Pure Red Perovskite Light-Emitting Diodes. J. Am. Chem. Soc. 2020, 142, 2956–2967. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, B.; Zhou, X.; Yuan, F.; Yin, C.; Wang, H.; Chen, H.; Ji, X.; Liang, X.; Shen, C.; et al. Ligand-Induced Cation–π Interactions Enable High-Efficiency, Bright, and Spectrally Stable Rec. 2020 Pure-Red Perovskite Light-Emitting Diodes. Adv. Mater. 2023, 35, 2303938. [Google Scholar] [CrossRef]
- Ren, Z.; Xiao, X.; Ma, R.; Lin, H.; Wang, K.; Sun, X.W.; Choy, W.C.H. Hole Transport Bilayer Structure for Quasi-2D Perovskite Based Blue Light-Emitting Diodes with High Brightness and Good Spectral Stability. Adv. Funct. Mater. 2019, 29, 1905339. [Google Scholar] [CrossRef]
- Quan, L.N.; García de Arquer, F.P.; Sabatini, R.P.; Sargent, E.H. Perovskites for Light Emission. Adv. Mater. 2018, 30, 1801996. [Google Scholar] [CrossRef]
- Yang, X.; Ma, L.; Yu, M.; Chen, H.; Ji, Y.; Hu, A.; Zhong, Q.; Jia, X.; Wang, Y.; Zhang, Y.; et al. Focus on Perovskite Emitters in Blue Light-Emitting Diodes. Light Sci. Appl. 2023, 12, 177. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




