Submitted:
23 December 2023
Posted:
27 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
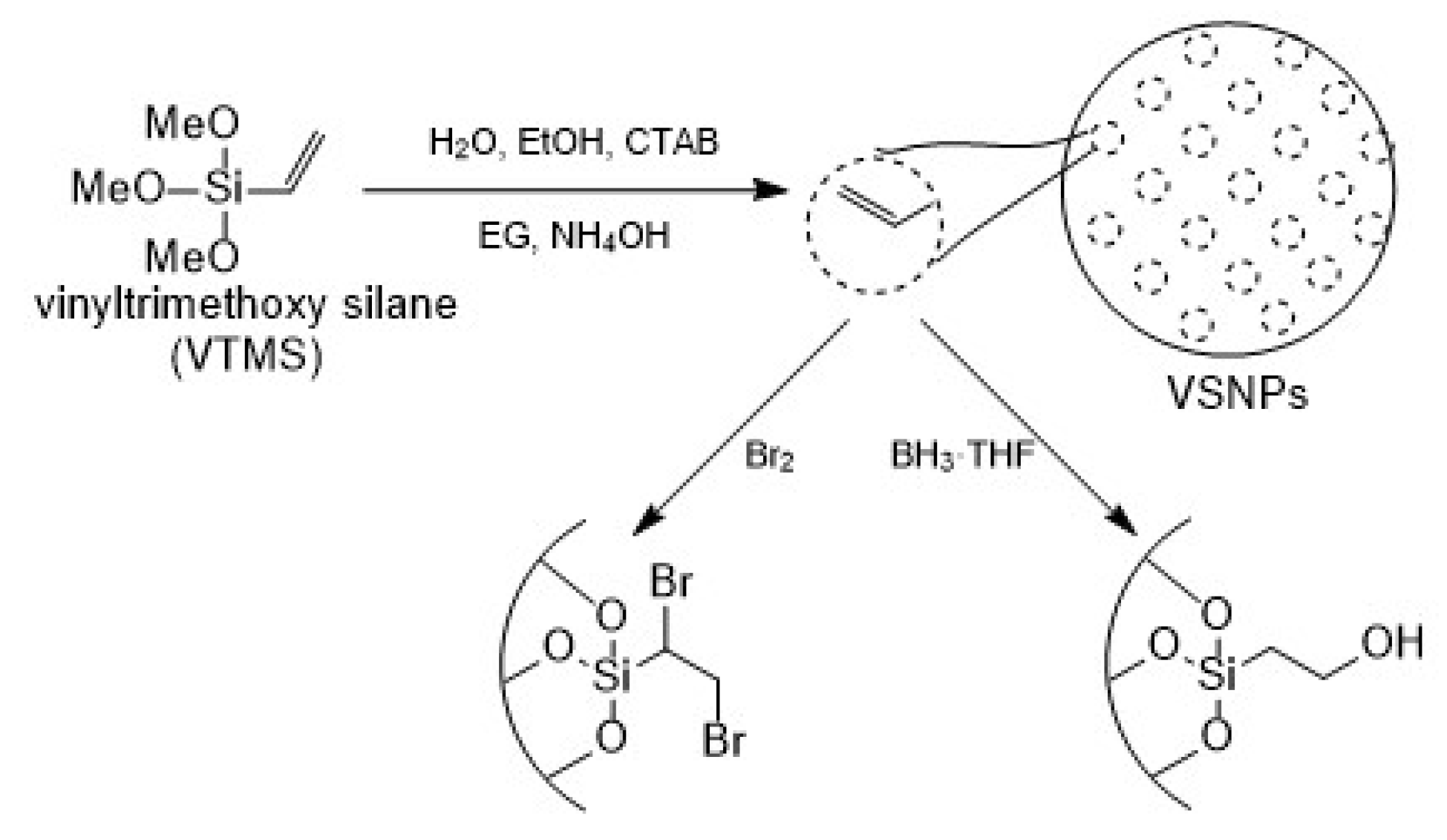
2. Materials and Methods


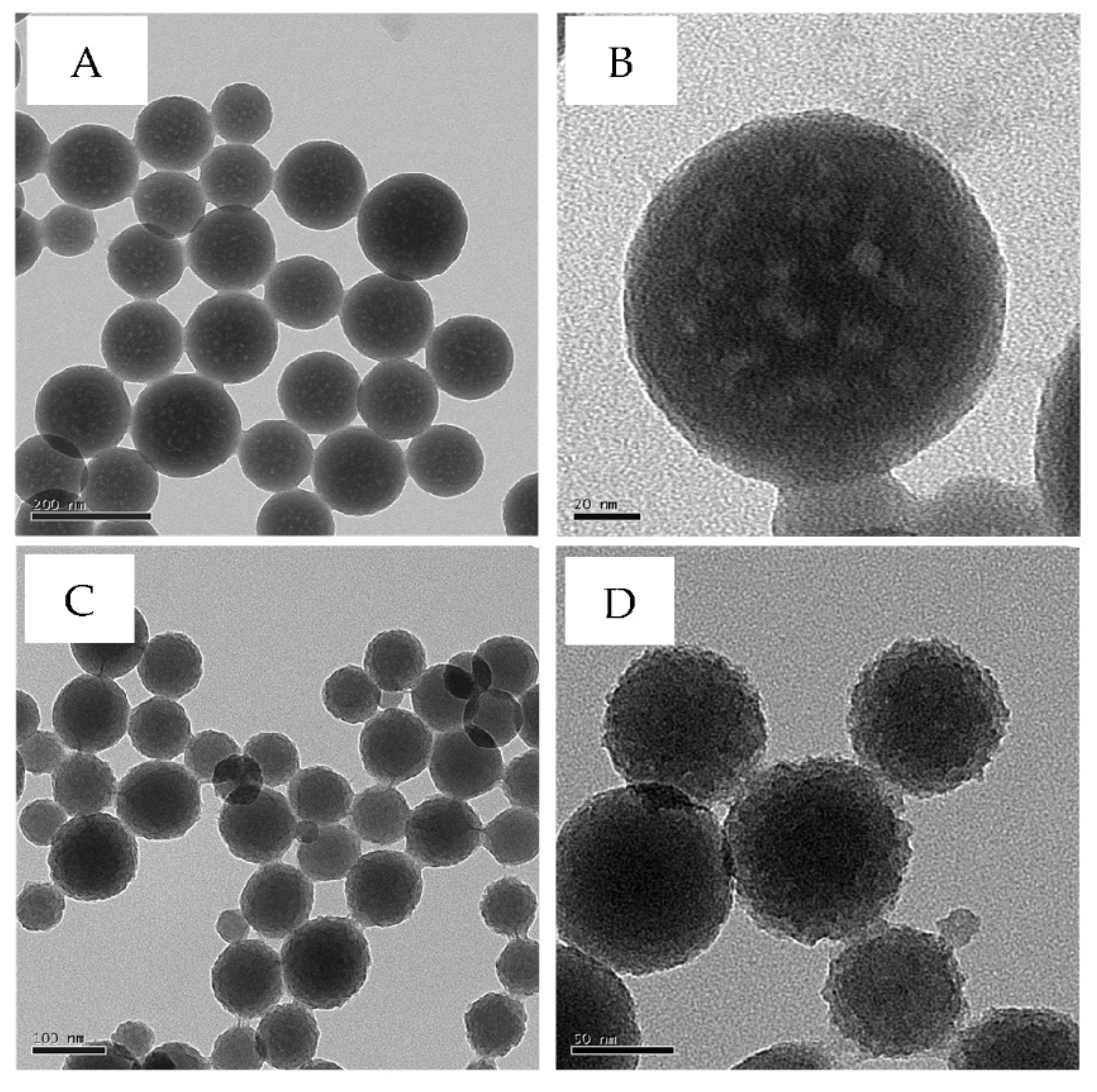
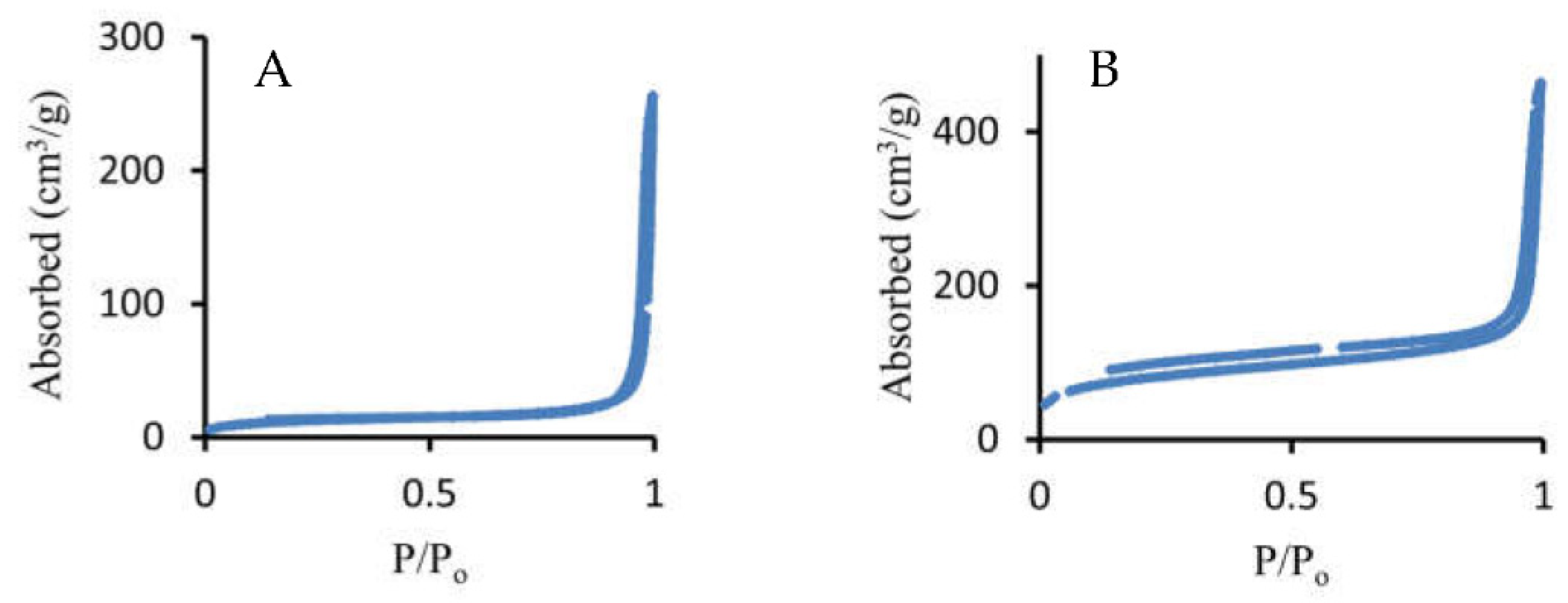
| Particles | Size, nm | Surface area, m2·g−1 | Calculated pore size, nm |
|---|---|---|---|
| mVSNPs | 137 ± 8 | 44.0 | 37-50 |
| rVSNPs | 97 ± 6 | 278.7 | 18* |
| sVSNPs | 592 ± 18 | 8.5 | 0 |
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted application of silica nanoparticles. A review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous silica and organosilica nanoparticles: physical chemistry, biosafety, delivery strategies, and biomedical applications. Adv. Healthcare Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Cui, Y.; Ren, Z.; Qiao, Z.A.; Wang, L.; Liu, Y.; Huo, Q. Highly Ordered Periodic Mesoporous Organosilica Nanoparticles With Controllable Pore Structures. Nanoscale 2012, 4, 6588–6596. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Li, W.; Tang, Y.; Elzatahry, A.; Lu, G.; Zhao, D. Mesoporous Organosilica Hollow Nanoparticles: Synthesis And Applications. Adv. Mater. 2019, 31, 1707612. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Ren, Y. Synthetic strategies for nonporous organosilica nanoparticles from organosilanes. Nanoscale 2023, 15, 10484–10497. [Google Scholar] [CrossRef]
- Jin, Y.; Li, A.; Hazelton, S.G.; Liang, S.; John, C.L.; Selid, P.D.; Pierce, D.T.; Zhao, J.X. Amorphous silica Nanohybrids: Synthesis, Properties and Applications. Coord. Chem. Rev. 2009, 253, 2998–3014. [Google Scholar] [CrossRef]
- Qiao, Z.A.; Zhang, L.; Guo, M.; Liu, Y.; Huo, Q. Synthesis Of Mesoporous Silica Nanoparticles Via Controlled Hydrolysis And Condensation Of Silicon Alkoxide. Chem. Mater. 2009, 21, 3823–3829. [Google Scholar] [CrossRef]
- Yano, K.; Fukushima, Y. Synthesis Of Mono-Dispersed Mesoporous Silica Spheres With Highly Ordered Hexagonal Regularity Using Conventional Alkyltrimethylammonium Halide As A Surfactant. J. Mater. Chem. 2004, 14, 1579–1584. [Google Scholar] [CrossRef]
- Yamada, H.; Urata, C.; Ujiie, H.; Yamauchi, Y.; Kuroda, K. Preparation Of Aqueous Colloidal Mesostructured And Mesoporous Silica Nanoparticles With Controlled Particle Size In A Very Wide Range From 20 nm to 700 nm. Nanoscale 2013, 5, 6145–6153. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef]
- Lin, H.P.; Tsai, C.P. Synthesis of Mesoporous Silica Nanoparticles from a Low-concentration CnTMAX-Sodium Silicate Components. Chem. Lett. 2003, 32, 1092–1093. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, D. On The Controllable Soft-Templating Approach To Mesoporous Silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef] [PubMed]
- Lelong, G.; Bhattacharyya, S.; Kline, S.; Cacciaguerra, T.; Gonzalez, M.A.; Saboungi, M.L. Effect Of Surfactant Concentration On The Morphology And Texture Of MCM-41 Materials. J. Phys. Chem. C 2008, 112, 10674–10680. [Google Scholar] [CrossRef]
- Chen, B.; Wang, Z.; Quan, G.; Peng, X.; Pan, X.; Wang, R.; Xu, Y.; Li, G.; Wu, C. In Vitro And In Vivo Evaluation Of Ordered Mesoporous Silica As A Novel Adsorbent In Liquisolid Formulation. Int. J. Nanomed. 2012, 7, 199–209. [Google Scholar] [CrossRef]
- Möller, K.; Bein, T. Talented Mesoporous Silica Nanoparticles. Chem. Mater. 2017, 29, 371–388. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Shi, B.; Chaikittisilp, W.; Li, M.; Wang, Y.; Liu, Y.; Gao, L.; Mao, L. A General Method To Synthesize A Family Of Mesoporous Silica Nanoparticles Less Than 100 nm And Their Applications In Anti-Reflective/Fogging Coating. J. Mater. Sci. 2016, 51, 6192–6206. [Google Scholar] [CrossRef]
- Cai, Q.; Luo, Z.S.; Pang, W.Q.; Fan, Y.W.; Chen, X.H.; Cui, F.Z. Dilute Solution Routes to Various Controllable Morphologies of MCM-41 Silica with a Basic Medium. Chem. Mater. 2001, 13, 258–263. [Google Scholar] [CrossRef]
- Lai, C.Y.; Trewyn, B.G.; Jeftinija, D.M.; Jeftinija, K.; Xu, S.; Jeftinija, S.; Lin, V.S.Y. A Mesoporous Silica Nanosphere-Based Carrier System with Chemically Removable CdS Nanoparticle Caps for Stimuli-Responsive Controlled Release of Neurotransmitters and Drug Molecules. J. Am. Chem. Soc. 2003, 125, 4451–4459. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, C.; Li, F. Fluorescence Turn-On Chemodosimeter-Functionalized Mesoporous Silica Nanoparticles and Their Application in Cell Imaging. J. Mater. Chem. 2011, 21, 7175–7181. [Google Scholar] [CrossRef]
- Lu, F.; Wu, S.H.; Hung, Y.; Mou, C.Y. Size Effect on Cell Uptake in Well-Suspended, Uniform Mesoporous Silica Nanoparticles. Small 2009, 5, 1408–1413. [Google Scholar] [CrossRef]
- Lin, Y.S.; Tsai, C.P.; Huang, H.Y.; Kuo, C.T.; Hung, Y.; Huang, D.M.; Chen, Y.C.; Mou, C.Y. Well-Ordered Mesoporous Silica Nanoparticles as Cell Markers. Chem. Mater. 2005, 17, 4570–4573. [Google Scholar] [CrossRef]
- Yokoi, T.; Karouji, T.; Ohta, S.; Kondo, J.N.; Tatsumi, T. Synthesis of Mesoporous Silica Nanospheres Promoted by Basic Amino Acids and Their Catalytic Application. Chem. Mater. 2010, 22, 3900–3908. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, L.L.; Jiang, J.G.; Calin, N.; Lam, K.F.; Zhang, S.J.; Wu, H.H.; Wu, G.D.; Albela, B.; Bonneviot, L.; Wu, P. Facile Large-Scale Synthesis of Monodisperse Mesoporous Silica Nanospheres with Tunable Pore Structure. J. Am. Chem. Soc. 2013, 135, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.P.; Mou, C.Y. Structural and Morphological Control of Cationic Surfactant-Templated Mesoporous Silica. Acc. Chem. Res. 2002, 35, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.D.; Yang, Y.X.; Zheng, J.L.; Cao, L.; Ding, H.J.; Liu, X.N. Synthesis of Mesoporous Silica by Cationic Surfactant Templating in Various Inorganic Acid Sources. Mater. Sci. Pol. 2010, 28, 709–730. [Google Scholar]
- Bagshaw, S.A. The Effect of Dilute Electrolytes on the Formation of Non-Ionically Templated [Si]-MSU-X Mesoporous Silica Molecular Sieves. J. Mater. Chem. 2001, 11, 831–840. [Google Scholar] [CrossRef]
- Lin, H.P.; Kao, C.P.; Mou, C.Y.; Liu, S.B. Counterion Effect in Acid Synthesis of Mesoporous Silica Materials. J. Phys. Chem. B 2000, 104, 7885–7894. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Huang, G.; Ma, X.; Gao, I. A Surprising Chaotropic Anion-Induced Supramolecular Self- Assembly of Ionic Polymeric Micelles. Angew. Chem. Int. Ed. 2014, 53, 8074–8078. [Google Scholar] [CrossRef]
- Yu, C.; Fan, J.; Tian, B.; Zhao, D.; Stucky, G.D. High-Yield Synthesis of Periodic Mesoporous. Adv. Mater. 2002, 14, 1742–1745. [Google Scholar] [CrossRef]
- Issa, A.A.; Luyt, A.S. Kinetics of Alkoxysilanes and Organoalkoxysilanes Polymerization: A Review. Polymers 2019, 11, 537. [Google Scholar] [CrossRef]
- Yu, C.; Tian, B.; Fan, J.; Stucky, G.D.; Zhao, D. Salt Effect in the Synthesis of Mesoporous Silica Templated by Non-Ionic Block Copolymers. Chem. Commun. 2001, 24, 2726–2727. [Google Scholar] [CrossRef]
- Yu, C.; Fan, J.; Tian, B.; Zhao, D. Morphology Development of Mesoporous Materials: A Colloidal Phase Separation Mechanism. Chem. Mater. 2004, 16, 889–898. [Google Scholar] [CrossRef]
- Trompette, J.L. Influence of Co-Ion Nature on the Gelation Kinetics of Colloidal Silica Suspensions. J. Phys. Chem. B 2017, 121, 5654–5659. [Google Scholar] [CrossRef] [PubMed]
- Van Der Linden, M.; Conchúir, B.O.; Spigone, E.; Niranjan, A.; Zaccone, A.; Cicuta, P. Microscopic Origin of the Hofmeister Effect in Gelation Kinetics of Colloidal Silica. J. Phys. Chem. Lett. 2015, 6, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.F.; Lyklema, J. Adsorption of Potential-Determining Ions at the Silica-Aqueous Electrolyte Interface and the Role of Some Cations. J. Electroanal. Chem. Interfacial Electrochem. 1968, 17, 267–275. [Google Scholar] [CrossRef]
- Matsoukas, T.; Gulari, E. Dynamics of Growth of Silica Particles from Ammonia-Catalyzed Hydrolysis of Tetra-Ethyl-Orthosilicate. J. Colloid Interface Sci. 1988, 124, 252–261. [Google Scholar] [CrossRef]
- Zainal, N.A.; Shukor, S.R.A.; Wab, H.A.A.; Razak, K.A. Study on the Effect of Synthesis Parameters of Silica Nanoparticles Entrapped with Rifampicin. Chem. Eng. Trans. 2013, 32, 2245–2250. [Google Scholar] [CrossRef]
- Issa, A.A.; El-Azazy, M.; Luyt, A.S. Kinetics of alkoxysilanes hydrolysis: An empirical approach. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Yamada, Y.; Yano, K. Synthesis of Monodispersed Super-Microporous/Mesoporous Silica Spheres with Diameters in the Low Submicron Range. Micropor. Mesopor. Mater. 2006, 93, 190–198. [Google Scholar] [CrossRef]
- Gu, J.; Huang, K.; Zhu, X.; Li, Y.; Wei, J.; Zhao, W.; Liu, C.; Shi, J. Sub-150 nm Mesoporous Silica Nanoparticles with Tunable Pore Sizes and Well-Ordered Mesostructure for Protein Encapsulation. J. Colloid Interface Sci. 2013, 407, 236–242. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Mesoporous silica/organosilica nanoparticles: Synthesis, biological effect and biomedical application. Mater. Sci. Engineering R 2019, 137, 66–105. [Google Scholar] [CrossRef]
- Brauer, F.; Gläsel, H.-J.; Decker, U.; Ernst, H.; Freyer, A.; Hartmann, E.; Sauerland, V.; Mehnert, R. Trialkoxysilane grafting onto nanoparticles for the preparation of clear coat polyacrylate systems with excellent scratch performance. Prog. Org. Coatings 2003, 47, 147–153. [Google Scholar] [CrossRef]
- Wang, L.; Tan, W. Multicolor FRET silica nanoparticles by single wavelength excitation. Nano Lett. 2006, 6, 84–88. [Google Scholar] [CrossRef]
- Zhang, W.H.; Hu, X.X.; Zhang, X.B. Dye-doped fluorescent silica nanoparticles for live cell and in vivo bioimaging. Nanomater. 2016, 6, 81. [Google Scholar] [CrossRef]
- Wang, C.; Yan, J.; Cui, X.; Wang, H. Synthesis of raspberry-like monodisperse magnetic hollow hybrid nanospheres by coating polystyrene template with Fe3O4@ SiO2 particles. J. Colloid Interface Sci. 2011, 354, 94–99. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, Q.; Wei, W.; Lu, Y.; Wang, S.; Chen, H.; Zhou, Y. Roughness surface of raspberry-shaped silica nanoparticles effect on shear thickening colloidal suspensions. Appl. Surf. Sci. 2022, 606, 154917. [Google Scholar] [CrossRef]
- Du, X.; He, J. A self-templated etching route to surface-rough silica nanoparticles for superhydrophobic coatings. ACS Appl. Mater. Interfaces 2011, 3, 1269–1276. [Google Scholar] [CrossRef]
- Li, X.; He, J. In situ assembly of raspberry-and mulberry-like silica nanospheres toward antireflective and antifogging coatings. ACS Appl. Mater. Interfaces 2012, 4, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.B.; Zhang, D.M.; Meng, Y.F.; Tai, L.; Jiang, Y. One-pot fabrication of fluoride-silica@ silica raspberry-like nanoparticles for superhydrophobic coating. Ceramics International 2016, 42, 14601–14608. [Google Scholar] [CrossRef]
- Semeykina, V.S.; Zharov, I. Medium Controlled Aggregative Growth as a Key Step in Mesoporous Silica Nanoparticle Formation. J. Colloid Interface Sci. 2022, 615, 236–247. [Google Scholar] [CrossRef]
- .Vivero-Escoto, J.L.; Slowing, I.I.; Trewyn, B.G.; Lin, V.S-Y. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small 2010, 6, 1952–1967. [Google Scholar] [CrossRef] [PubMed]
- .Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles for drug delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Feldmann, V.; Engelmann, J.; Gottschalk, S.; Mayer, H.A. Synthesis, characterization and examination of Gd [DO3A-hexylamine]-functionalized silica nanoparticles as contrast agent for MRI-applications. J. Colloid Interface Sci. 2012, 366, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Cha, B.G.; Kim, J. Functional mesoporous silica nanoparticles for bio-imaging applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1515. [Google Scholar] [CrossRef]
- Niculescu, V.C. Mesoporous silica nanoparticles for bio-applications. Front. Mater. 2020, 7, 36. [Google Scholar] [CrossRef]
- Brozek, E.M.; Zharov, I. Internal Functionalization and Surface Modification of Vinylsilsesquioxane Nanoparticles. Chem. Mater. 2009, 21, 1451–1456. [Google Scholar] [CrossRef]
- Tripathi, V.S.; Kandimalla, V.B.; Ju, H. Preparation of Ormosil and its Applications in the Immobilizing Biomolecules. Sens. Actuators B 2006, 114, 1071–1082. [Google Scholar] [CrossRef]
- Gonçalves, M.C. Sol-gel silica nanoparticles in medicine: A natural choice. Design, synthesis and products. Molecules 2018, 23, 2021. [Google Scholar] [CrossRef]
- Soloway, A.H.; Tjarks, W.; Barnum, B.A.; Rong, F.-G.; Barth, R.F.; Codogni, I.M.; Wilson, J.G. The Chemistry of Neutron Capture Therapy. Chem. Rev. 1998, 98, 1515–1562. [Google Scholar] [CrossRef]
- Gao, Z.; Moghaddam, S.P.H.; Ghandehari, H.; Zharov, I. Synthesis of Water-Degradable Silica Nanoparticles from Carbamate-Containing Bridged Silsesquioxane Precursor. RSC Adv. 2018, 8, 4914–4920. [Google Scholar] [CrossRef]
- Gao, Z.; Zharov, I. Large Pore Mesoporous Silica Nanoparticles by Templating with a Nonsurfactant Molecule, Tannic Acid. Chem. Mater. 2014, 26, 2030–2037. [Google Scholar] [CrossRef]
- Brozek, E.M.; Washton, N.M.; Mueller, K.T.; Zharov, I. Silsesquioxane Particles with Internal Functional Groups. J. Nanopart. Res. 2017, 19, 85–97. [Google Scholar] [CrossRef]
- Walton, N.I.; Gao, Z.; Zharov, I. Theranostic Anticancer Agents Based on Internally Functionalized ORMOSIL Nanoparticles. MRS Proceedings 2014, 1526, 215–221. [Google Scholar] [CrossRef]
- Walton, N.I.; Naha, P.; Cormode, D.P.; Zharov, I. Mesoporous Organosilica Nanoparticles with Internal Metal-Chelating Groups: Transition Metal Uptake and Gd3+ Relaxivity. ChemistrySelec 2023, 8, e202301454. [Google Scholar] [CrossRef]
- Ottenbrite, R.M.; Wall, J.S.; Siddiqui, J.A. Self-Catalyzed Synthesis of Organo-Silica Nanoparticles. J. Am. Ceram. Soc. 2000, 83, 3214–3215. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E.J. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Gu, J.; Fan, W.; Shimojima, A.; Okubo, T. Organic–inorganic mesoporous nanocarriers integrated with biogenic ligands. Small 2007, 3, 1740–1744. [Google Scholar] [CrossRef]
- Hartono, S.B.; Gu, W.; Kleitz, F.; Liu, J.; He, L.; Middelberg, A.P.J.; Yu, C.; Lu, G.Q.; Qiao, S.Z. Poly-L-lysine functionalized large pore cubic mesostructured silica nanoparticles as biocompatible carriers for gene delivery. ACS Nano 2012, 6, 2104–2117. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure & Appl. Chem. 1985, 57, 603–619. [Google Scholar]
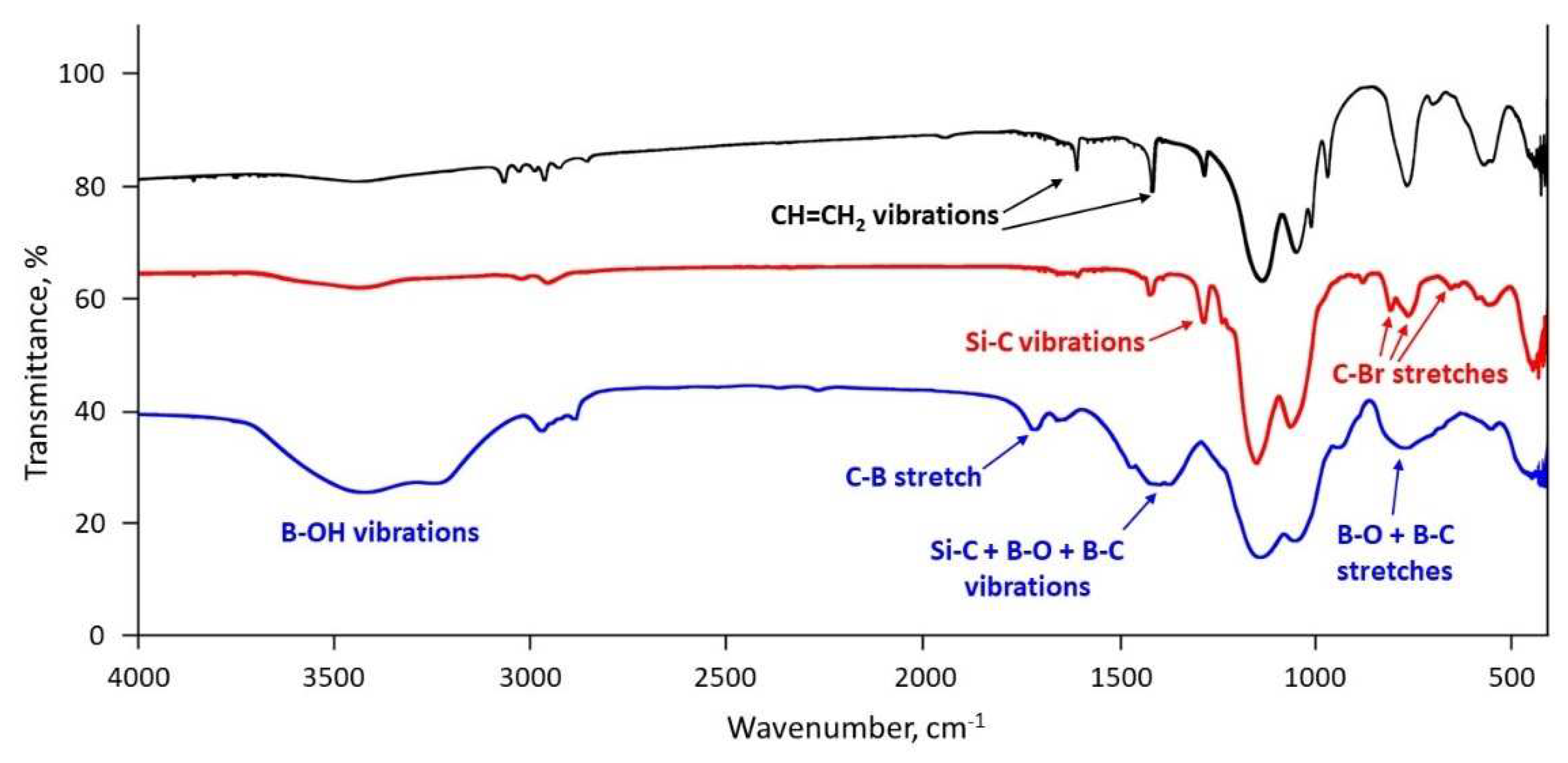
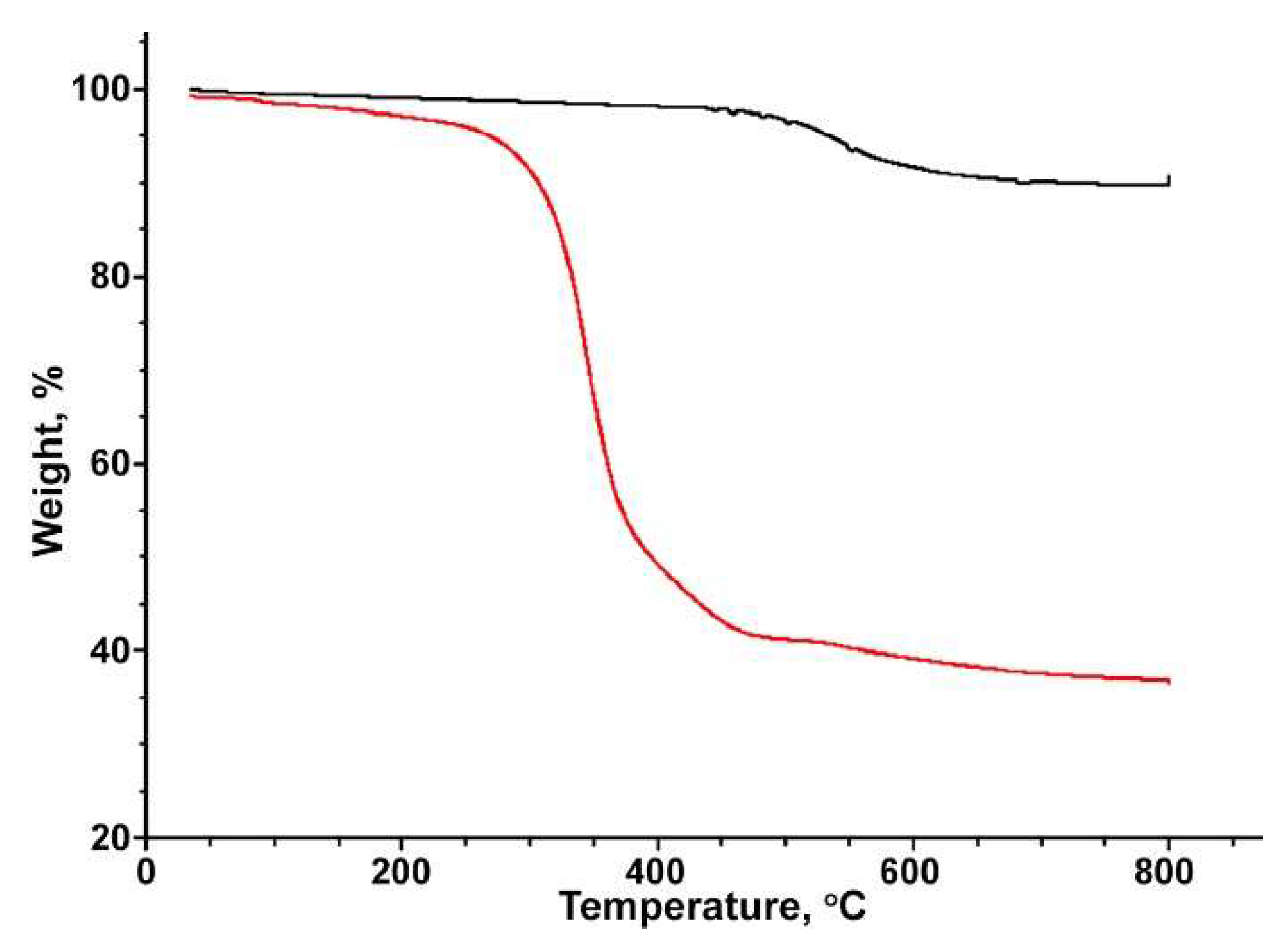
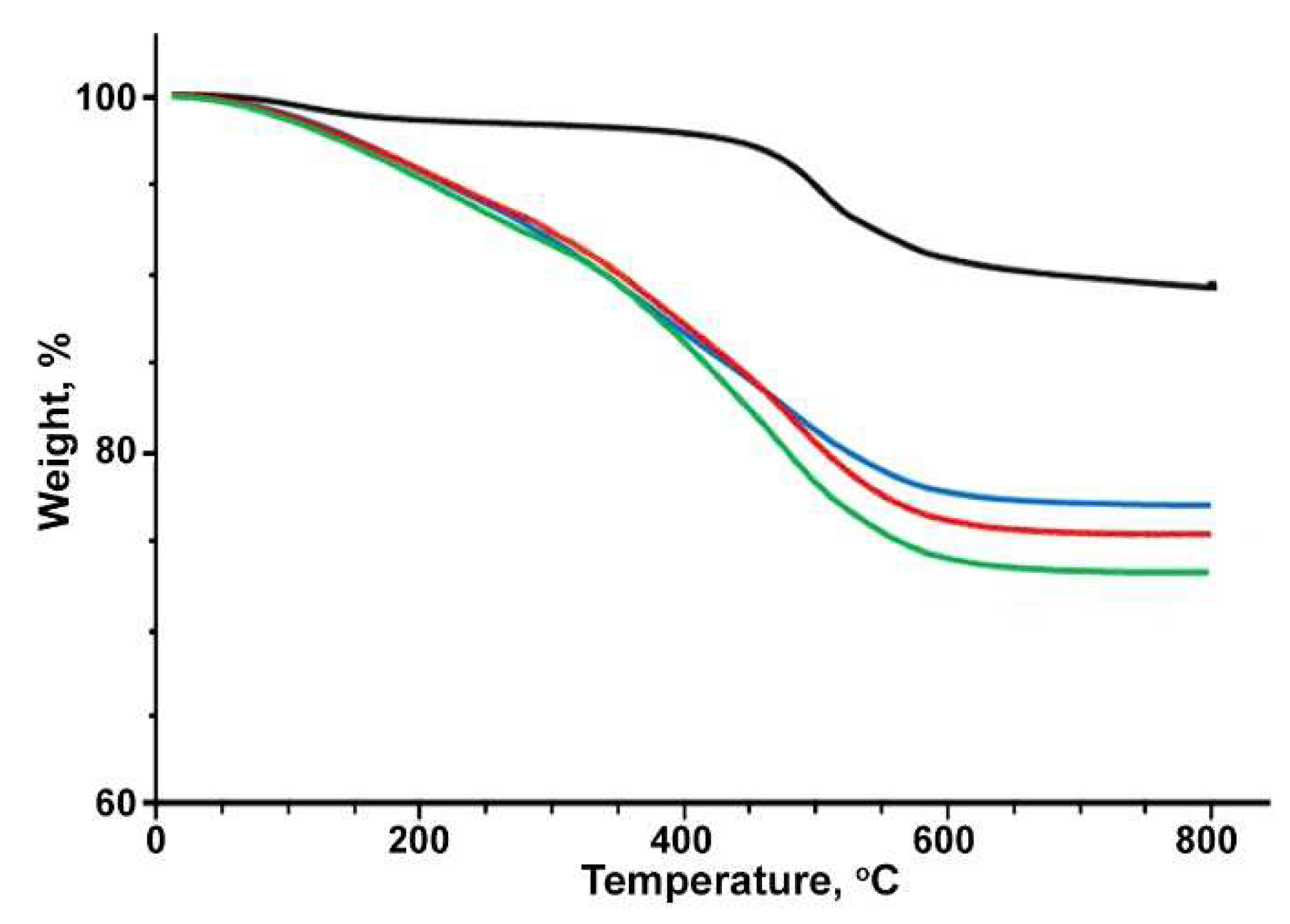
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





