Submitted:
23 December 2023
Posted:
25 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Article Types
3. Materials and Methods
3.1. Ethics Approval
3.2. Preparation of Animals and Samples
3.3. Determination of Meat Quality
3.4. Lipidomics Analysis
3.5. Total RNA Extraction and qPCR
3.6. H&E staining, Oil Red Staining, and BODIPY Staining
3.7. DNA Extraction and 16SrRNA Gene Amplification and Sequencing
3.8. Untargeted Metabolomics Assays
3.9. Statistical Analysis
4. Results
4.1. Effect of Mulberry Leaves on Growth Performance and Meat Quality
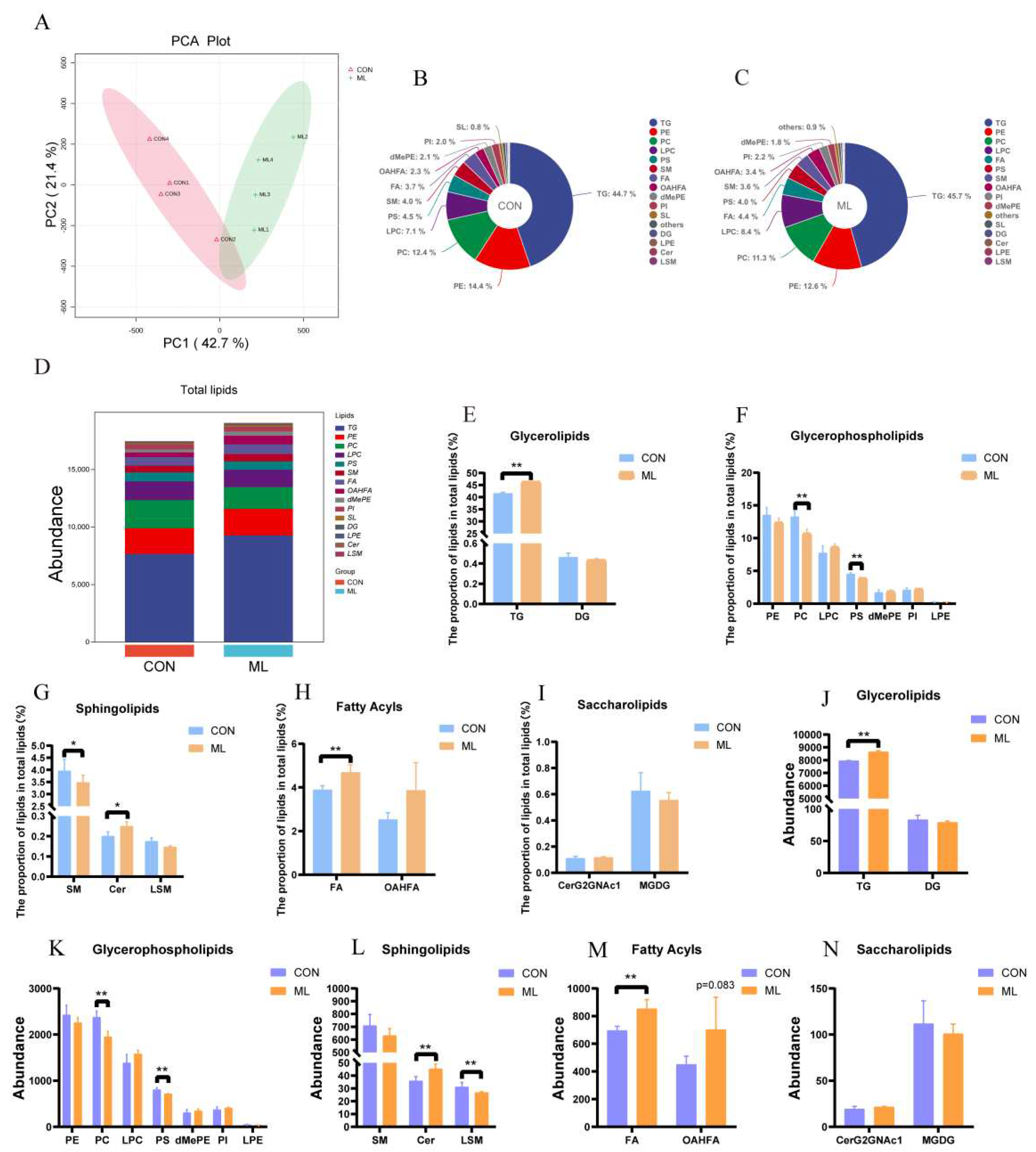
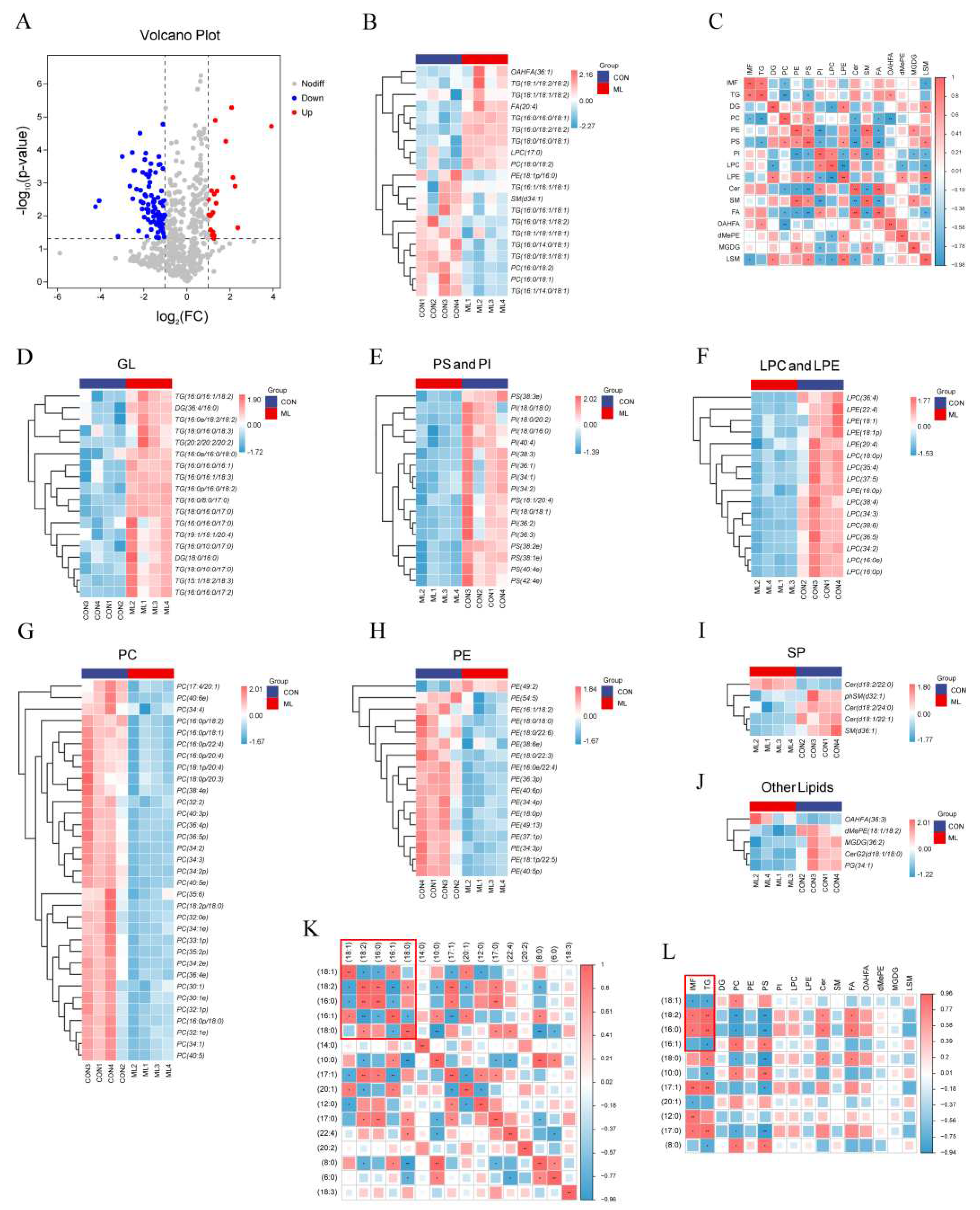
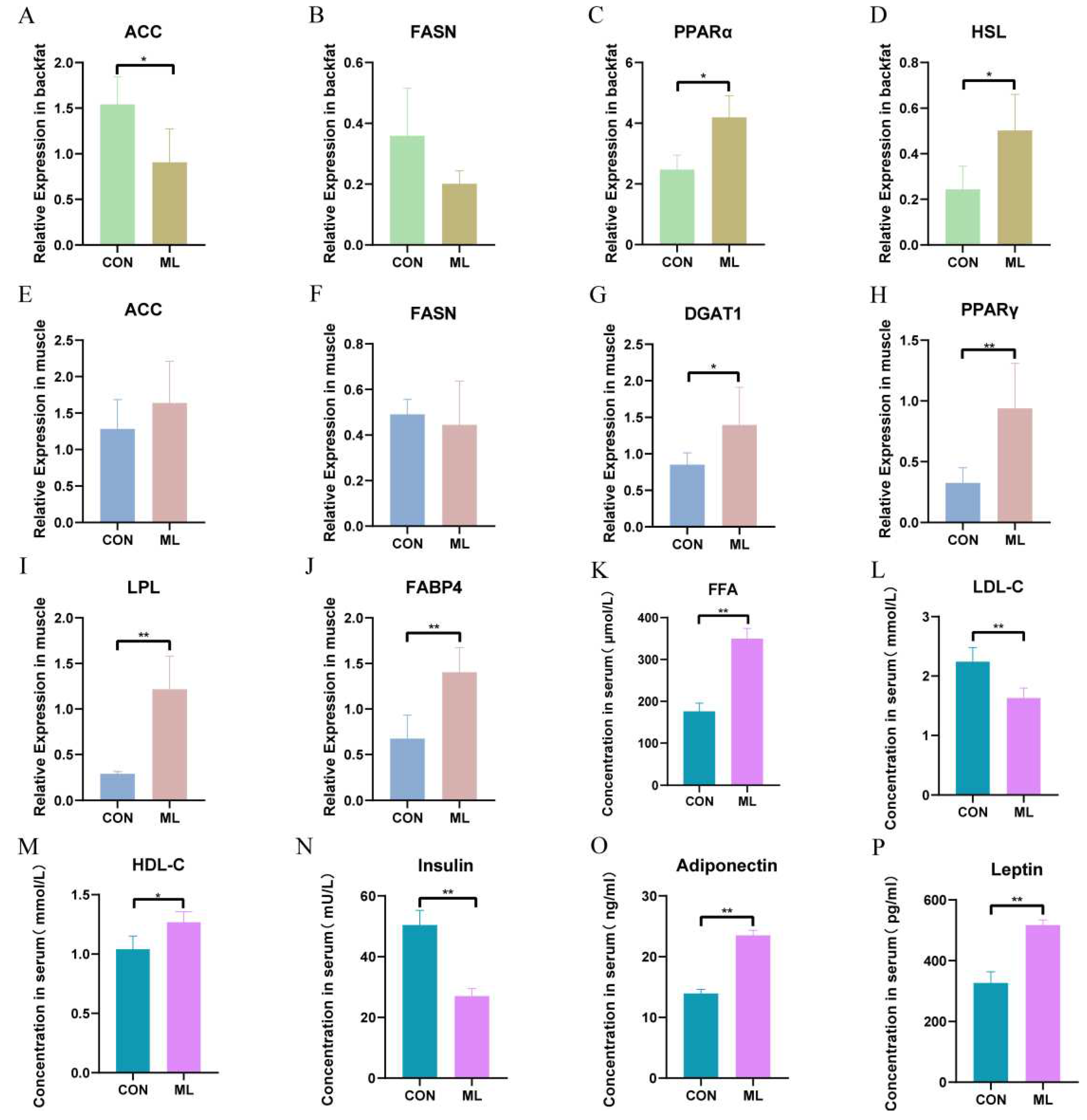
4.2. Distribution of Lipid Profiles of Muscle Tissue and Screening of Differential Lipid Molecules
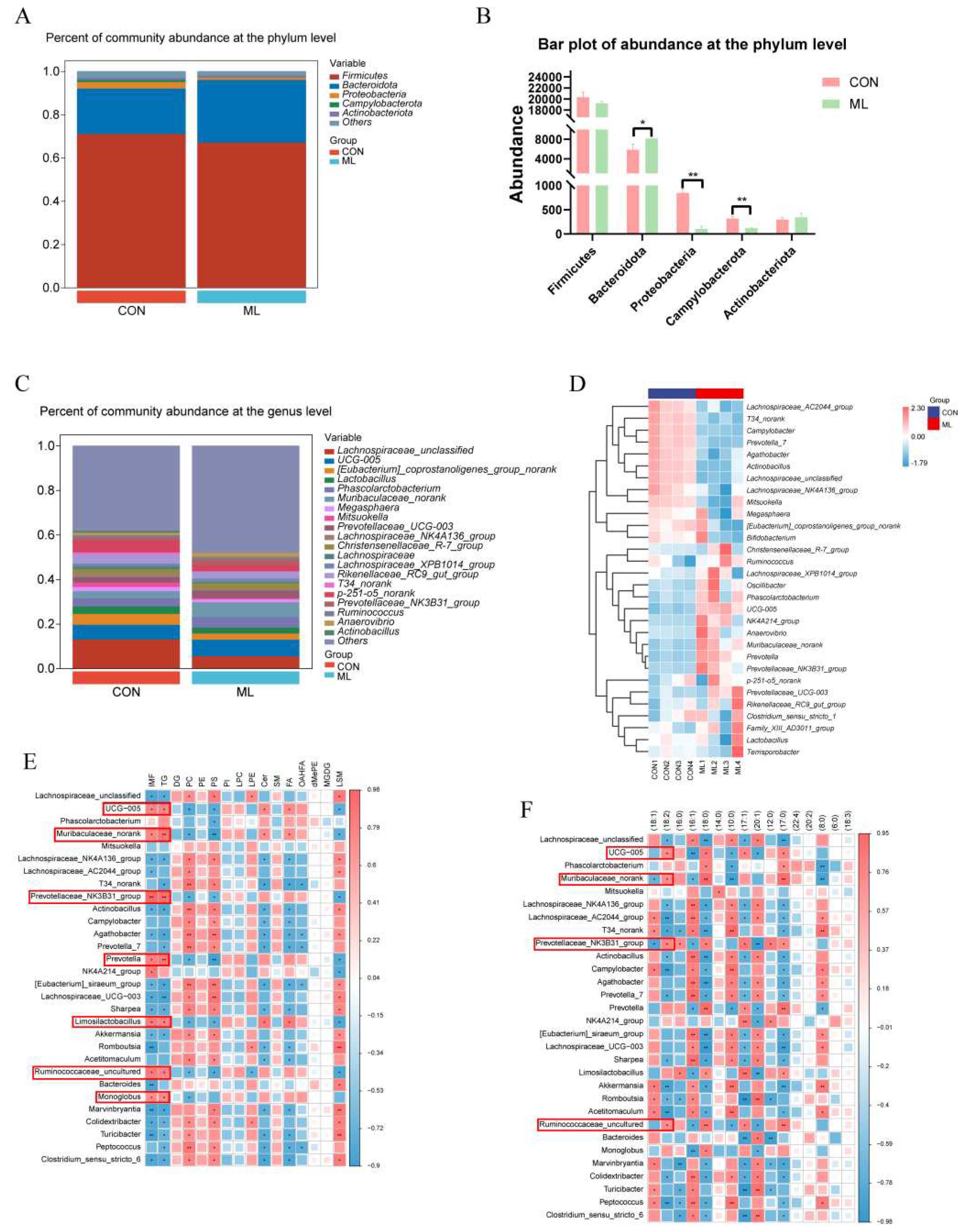
4.3. Effects of Mulberry Leaves on Gut Microbiota
4.4. Untargeted Metabolomics Analysis of Cecal Contents
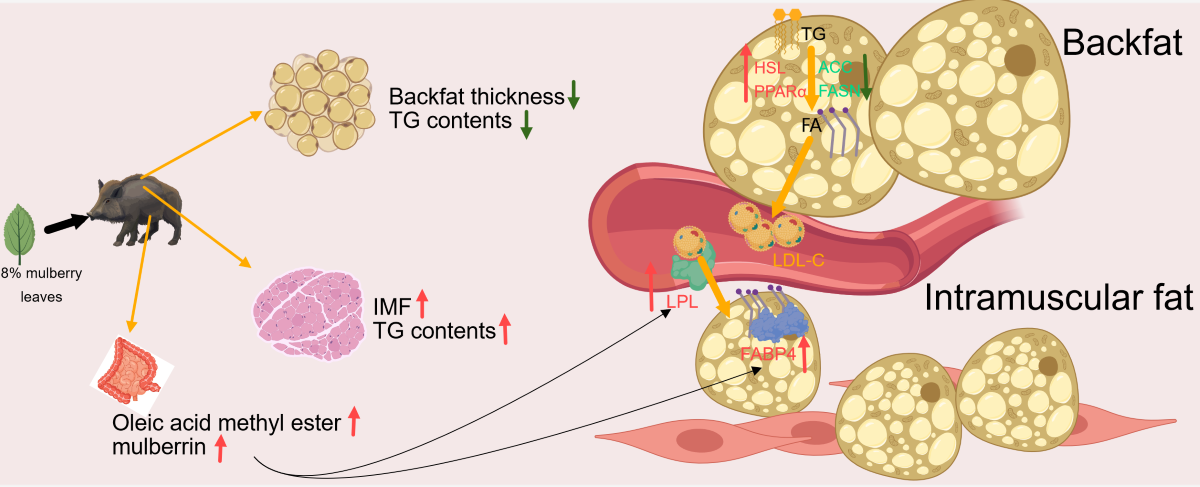
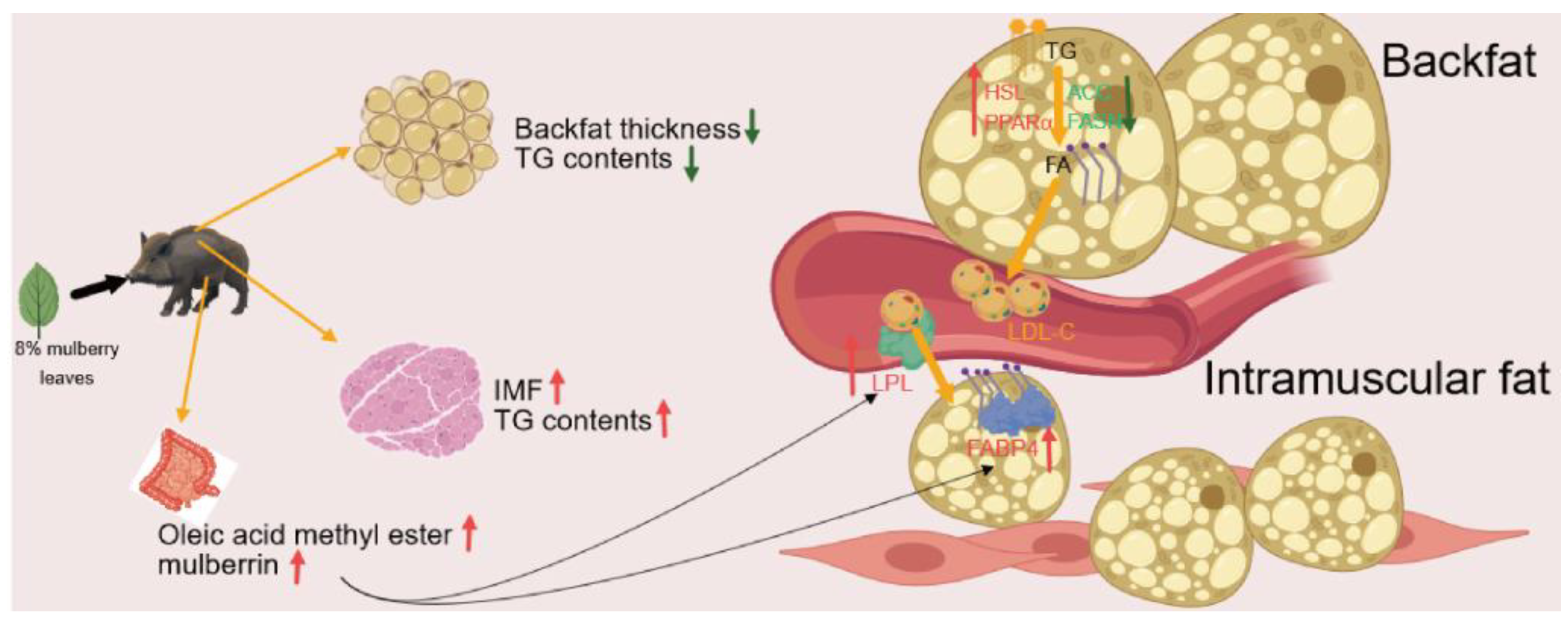
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-García, S.; Esteve-Llorens, X.; Moreira, M.T.; Feijoo, G. Carbon footprint and nutritional quality of different human dietary choices. Sci Total Environ 2018, 644, 77–94. [Google Scholar] [CrossRef]
- Reig, M.; Aristoy, M.C.; Toldrá, F. Variability in the contents of pork meat nutrients and how it may affect food composition databases. Food Chem 2013, 140, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.M.; Smith, L.M. Review: Smart agri-systems for the pig industry. Animal 2022, 16 Suppl 2, 100518. [Google Scholar] [CrossRef]
- Kong, C.; Yang, L.; Gong, H.; Wang, L.; Li, H.; Li, Y.; Wei, B.; Nima, C.; Deji, Y.; Zhao, S.; et al. Dietary and Food Consumption Patterns and Their Associated Factors in the Tibetan Plateau Population: Results from 73 Counties with Agriculture and Animal Husbandry in Tibet, China. Nutrients 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Malgwi, I.H.; Halas, V.; Grünvald, P.; Schiavon, S.; Jócsák, I. Genes Related to Fat Metabolism in Pigs and Intramuscular Fat Content of Pork: A Focus on Nutrigenetics and Nutrigenomics. Animals (Basel) 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Fortin, A.; Robertson, W.M.; Tong, A.K. The eating quality of Canadian pork and its relationship with intramuscular fat. Meat Sci 2005, 69, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Y.; Wu, Z.; Xiong, X.; Zhang, J.; Ma, J.; Xiao, S.; Huang, L.; Yang, B. Subcutaneous and intramuscular fat transcriptomes show large differences in network organization and associations with adipose traits in pigs. Sci China Life Sci 2021, 64, 1732–1746. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, J.; Francisco, A.; Portugal, A.P.; Paulos, K.; Dentinho, M.T.; Almeida, J.M.; Regedor, L.; Fialho, L.; Cachucho, L.; Jerónimo, E.; et al. Effects of partial substitution of grain by agroindustrial byproducts and sunflower seed supplementation in beef haylage-based finisher diets on growth, in vitro methane production and carcass and meat quality. Meat Sci 2022, 188, 108782. [Google Scholar] [CrossRef] [PubMed]
- Fixen, P.E.; Johnston, A.M. World fertilizer nutrient reserves: a view to the future. J Sci Food Agric 2012, 92, 1001–1005. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Q.; Zhu, S.; Liu, B.; Liu, F.; Xu, Y. Mulberry leaf (Morus alba L.): A review of its potential influences in mechanisms of action on metabolic diseases. Pharmacol Res 2022, 175, 106029. [Google Scholar] [CrossRef]
- Ma, G.; Chai, X.; Hou, G.; Zhao, F.; Meng, Q. Phytochemistry, bioactivities and future prospects of mulberry leaves: A review. Food Chem 2022, 372, 131335. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Peng, Y.; He, J.; Xiao, D.; Chen, C.; Li, F.; Huang, R.; Yin, Y. Dietary mulberry leaf powder affects growth performance, carcass traits and meat quality in finishing pigs. J Anim Physiol Anim Nutr (Berl) 2019, 103, 1934–1945. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Xiao, Y.; Peng, Y.; He, J.; Chen, C.; Xiao, D.; Yin, Y.; Li, F. Mulberry leaf powder regulates antioxidative capacity and lipid metabolism in finishing pigs. Anim Nutr 2021, 7, 421–429. [Google Scholar] [CrossRef]
- Parida, I.S.; Takasu, S.; Nakagawa, K. A comprehensive review on the production, pharmacokinetics and health benefits of mulberry leaf iminosugars: Main focus on 1-deoxynojirimycin, d-fagomine, and 2-O-ɑ-d-galactopyranosyl-DNJ. Crit Rev Food Sci Nutr 2023, 63, 3468–3496. [Google Scholar] [CrossRef]
- Cui, W.; Luo, K.; Xiao, Q.; Sun, Z.; Wang, Y.; Cui, C.; Chen, F.; Xu, B.; Shen, W.; Wan, F.; et al. Effect of mulberry leaf or mulberry leaf extract on glycemic traits: a systematic review and meta-analysis. Food Funct 2023, 14, 1277–1289. [Google Scholar] [CrossRef]
- Fan, L.; Peng, Y.; Wu, D.; Hu, J.; Shi, X.; Yang, G.; Li, X. Dietary supplementation of Morus nigra L. leaves decrease fat mass partially through elevating leptin-stimulated lipolysis in pig model. J Ethnopharmacol 2020, 249, 112416. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol Mol Biol Rev 2017, 81. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.R. Lipid Biogeochemistry and Modern Lipidomic Techniques. Ann Rev Mar Sci 2023, 15, 485–508. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, W.; Li, Z.; Xia, Y.; Ouyang, Z. Enabling High Structural Specificity to Lipidomics by Coupling Photochemical Derivatization with Tandem Mass Spectrometry. Acc Chem Res 2021, 54, 3873–3882. [Google Scholar] [CrossRef]
- Hillesheim, E.; Brennan, L. Distinct patterns of personalised dietary advice delivered by a metabotype framework similarly improve dietary quality and metabolic health parameters: secondary analysis of a randomised controlled trial. Front Nutr 2023, 10, 1282741. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath 2013, 3, 71–85. [Google Scholar] [PubMed]
- Liu, W.; Chen, L.; Miao, K.; You, Y.; Li, J.; Lu, J.; Zhang, Y. Identification and validation of diagnostic biomarkers for intrahepatic cholestasis of pregnancy based on untargeted and targeted metabolomics analyses of urine metabolite profiles. BMC Pregnancy Childbirth 2023, 23, 828. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Meng, L.; Mi, L. Effects of Leymus chinensis hay and alfalfa hay on growth performance, rumen microbiota, and untargeted metabolomics of meat in lambs. Front Vet Sci 2023, 10, 1256903. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, H.; Billon, Y.; Brossard, L.; Faure, J.; Gatellier, P.; Gondret, F.; Labussière, E.; Lebret, B.; Lefaucheur, L.; Le Floch, N.; et al. Review: divergent selection for residual feed intake in the growing pig. Animal 2017, 11, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiong, X.; Wu, X.; Liu, G.; Zhou, K.; Yin, Y. Effects of stocking density on growth performance, blood parameters and immunity of growing pigs. Anim Nutr 2020, 6, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liao, S.; Pang, D.; Li, E.; Liu, T.; Liu, F.; Zou, Y. The transported active mulberry leaf phenolics inhibited adipogenesis through PPAR-γ and Leptin signaling pathway. J Food Biochem 2022, 46, e14270. [Google Scholar] [CrossRef]
- He, L.; Xing, Y.; Ren, X.; Zheng, M.; Yu, S.; Wang, Y.; Xiu, Z.; Dong, Y. Mulberry Leaf Extract Improves Metabolic Syndrome by Alleviating Lipid Accumulation In Vitro and In Vivo. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Huang, T.; Jing, R.; Wen, J.; Cao, M. Mulberry leaf extract reduces abdominal fat deposition via adenosine-activated protein kinase/sterol regulatory element binding protein-1c/acetyl-CoA carboxylase signaling pathway in female Arbor Acre broilers. Poult Sci 2023, 102, 102638. [Google Scholar] [CrossRef]
- Li, R.; Xue, Z.; Li, S.; Zhou, J.; Liu, J.; Zhang, M.; Panichayupakaranant, P.; Chen, H. Mulberry leaf polysaccharides ameliorate obesity through activation of brown adipose tissue and modulation of the gut microbiota in high-fat diet fed mice. Food Funct 2022, 13, 561–573. [Google Scholar] [CrossRef]
- Li, R.; Xue, Z.; Jia, Y.; Wang, Y.; Li, S.; Zhou, J.; Liu, J.; Zhang, M.; He, C.; Chen, H. Polysaccharides from mulberry (Morus alba L.) leaf prevents obesity by inhibiting pancreatic lipase in high-fat diet induced mice. Int J Biol Macromol 2021, 192, 452–460. [Google Scholar] [CrossRef]
- Cesar, A.S.; Regitano, L.C.; Koltes, J.E.; Fritz-Waters, E.R.; Lanna, D.P.; Gasparin, G.; Mourão, G.B.; Oliveira, P.S.; Reecy, J.M.; Coutinho, L.L. Putative regulatory factors associated with intramuscular fat content. PLoS One 2015, 10, e0128350. [Google Scholar] [CrossRef] [PubMed]
- Moisá, S.J.; Shike, D.W.; Faulkner, D.B.; Meteer, W.T.; Keisler, D.; Loor, J.J. Central Role of the PPARγ Gene Network in Coordinating Beef Cattle Intramuscular Adipogenesis in Response to Weaning Age and Nutrition. Gene Regul Syst Bio 2014, 8, 17–32. [Google Scholar] [CrossRef]
- Naowaboot, J.; Chung, C.H.; Pannangpetch, P.; Choi, R.; Kim, B.H.; Lee, M.Y.; Kukongviriyapan, U. Mulberry leaf extract increases adiponectin in murine 3T3-L1 adipocytes. Nutr Res 2012, 32, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yuan, W.; Peng, X.; Wang, M.; Xiao, J.; Wu, C.; Luo, L. PPAR γ/Nnat/NF-κB Axis Involved in Promoting Effects of Adiponectin on Preadipocyte Differentiation. Mediators Inflamm 2019, 2019, 5618023. [Google Scholar] [CrossRef] [PubMed]
- Boss, M.; Kemmerer, M.; Brüne, B.; Namgaladze, D. FABP4 inhibition suppresses PPARγ activity and VLDL-induced foam cell formation in IL-4-polarized human macrophages. Atherosclerosis 2015, 240, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.Z.; Fang, J.C.; Zhang, J.S.; Zhang, L.M.; Xu, C.; Xu, H.Y.; Shao, J.; Xia, G.J. Correlations between single nucleotide polymorphisms in FABP4 and meat quality and lipid metabolism gene expression in Yanbian yellow cattle. PLoS One 2020, 15, e0234328. [Google Scholar] [CrossRef]
- Opgenorth, J.; Sordillo, L.M.; VandeHaar, M.J. Colostrum supplementation with n-3 fatty acids and α-tocopherol alters plasma polyunsaturated fatty acid profile and decreases an indicator of oxidative stress in newborn calves. J Dairy Sci 2020, 103, 3545–3553. [Google Scholar] [CrossRef]
- Yagoubi, Y.; Joy, M.; Ripoll, G.; Mahouachi, M.; Bertolín, J.R.; Atti, N. Rosemary distillation residues reduce lipid oxidation, increase alpha-tocopherol content and improve fatty acid profile of lamb meat. Meat Sci 2018, 136, 23–29. [Google Scholar] [CrossRef]
- Fu, Y.; Cao, S.; Yang, L.; Li, Z. Flavor formation based on lipid in meat and meat products: A review. J Food Biochem 2022, 46, e14439. [Google Scholar] [CrossRef]
- Mapiye, C.; Aldai, N.; Turner, T.D.; Aalhus, J.L.; Rolland, D.C.; Kramer, J.K.; Dugan, M.E. The labile lipid fraction of meat: from perceived disease and waste to health and opportunity. Meat Sci 2012, 92, 210–220. [Google Scholar] [CrossRef]
- Mariutti, L.R.; Bragagnolo, N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Res Int 2017, 94, 90–100. [Google Scholar] [CrossRef]
- Bloukas, I.; Honikel, K.O. The influence of mincing and temperature of storage on the oxidation of pork back fat and its effect on water- and fat-binding in finely comminuted batters. Meat Sci 1992, 32, 215–227. [Google Scholar] [CrossRef]
- Zheng, B.; Li, X.; Hao, J.; Xu, D. Meat systems produced with Monascus pigment water-in-oil-in-water multiple emulsion as pork fat replacers. Food Chem 2023, 402, 134080. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gross, R.W. The foundations and development of lipidomics. J Lipid Res 2022, 63, 100164. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.K.; Catanese, S.; Emond, P.; Corcia, P.; Blasco, H.; Pisella, P.J. Metabolomics and lipidomics approaches in human tears: A systematic review. Surv Ophthalmol 2022, 67, 1229–1243. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, B.; Lam, S.M.; Shui, G. Integration of lipidomics and metabolomics for in-depth understanding of cellular mechanism and disease progression. J Genet Genomics 2020, 47, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; You, R.; Lv, Y.; Liu, H.; Yang, G. Conjugated linoleic acid regulates adipocyte fatty acid binding protein expression via peroxisome proliferator-activated receptor α signaling pathway and increases intramuscular fat content. Front Nutr 2022, 9, 1029864. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mai, K.; Ai, Q. Palmitic acid activates NLRP3 inflammasome through NF-κB and AMPK-mitophagy-ROS pathways to induce IL-1β production in large yellow croaker (Larimichthys crocea). Biochim Biophys Acta Mol Cell Biol Lipids 2023, 159428. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.R.; Teng, F.Y.; Fan, W.; Xu, B.T.; Li, X.Y.; Tan, X.Z.; Guo, M.; Gao, C.L.; Zhang, C.X.; Jiang, Z.Z.; et al. BDH1-mediated βOHB metabolism ameliorates diabetic kidney disease by activation of NRF2-mediated antioxidative pathway. Aging (Albany NY) 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Liu, X.; Xu, J.; Li, F.; Wang, J.; Zhang, X.; Yang, X.; Wang, L.; Ma, S.; Li, D.; et al. Effects of Silage Diet on Meat Quality through Shaping Gut Microbiota in Finishing Pigs. Microbiol Spectr 2023, 11, e0241622. [Google Scholar] [CrossRef]
- Wunderling, K.; Zurkovic, J.; Zink, F.; Kuerschner, L.; Thiele, C. Triglyceride cycling enables modification of stored fatty acids. Nat Metab 2023, 5, 699–709. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Wang, R.; Ji, G.; Elmassry, M.M.; Zabet-Moghaddam, M.; Vellers, H.; Hamood, A.N.; Gong, X.; Mirzaei, P.; Sang, S.; et al. Dietary supplementation of gingerols- and shogaols-enriched ginger root extract attenuate pain-associated behaviors while modulating gut microbiota and metabolites in rats with spinal nerve ligation. J Nutr Biochem 2022, 100, 108904. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cao, M.; Wei, Z.; Mei, J.; Zhang, Y.; Li, M.; Li, M.; Zhang, Y.; Wang, Z. The protective effect of Buzhong Yiqi decoction on ischemic stroke mice and the mechanism of gut microbiota. Front Neurosci 2022, 16, 956620. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, S.; Zhang, X.; Du, P.; Zhu, Y.; Huang, Y.; Michiels, J.; Zeng, Q.; Chen, W. Dietary resistant starch alleviates Escherichia coli-induced bone loss in meat ducks by promoting short-chain fatty acid production and inhibiting Malt1/NF-κB inflammasome activation. J Anim Sci Biotechnol 2022, 13, 92. [Google Scholar] [CrossRef]
- Krishnamurthy, H.K.; Pereira, M.; Bosco, J.; George, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K.; Rajasekaran, J.J. Gut commensals and their metabolites in health and disease. Front Microbiol 2023, 14, 1244293. [Google Scholar] [CrossRef]
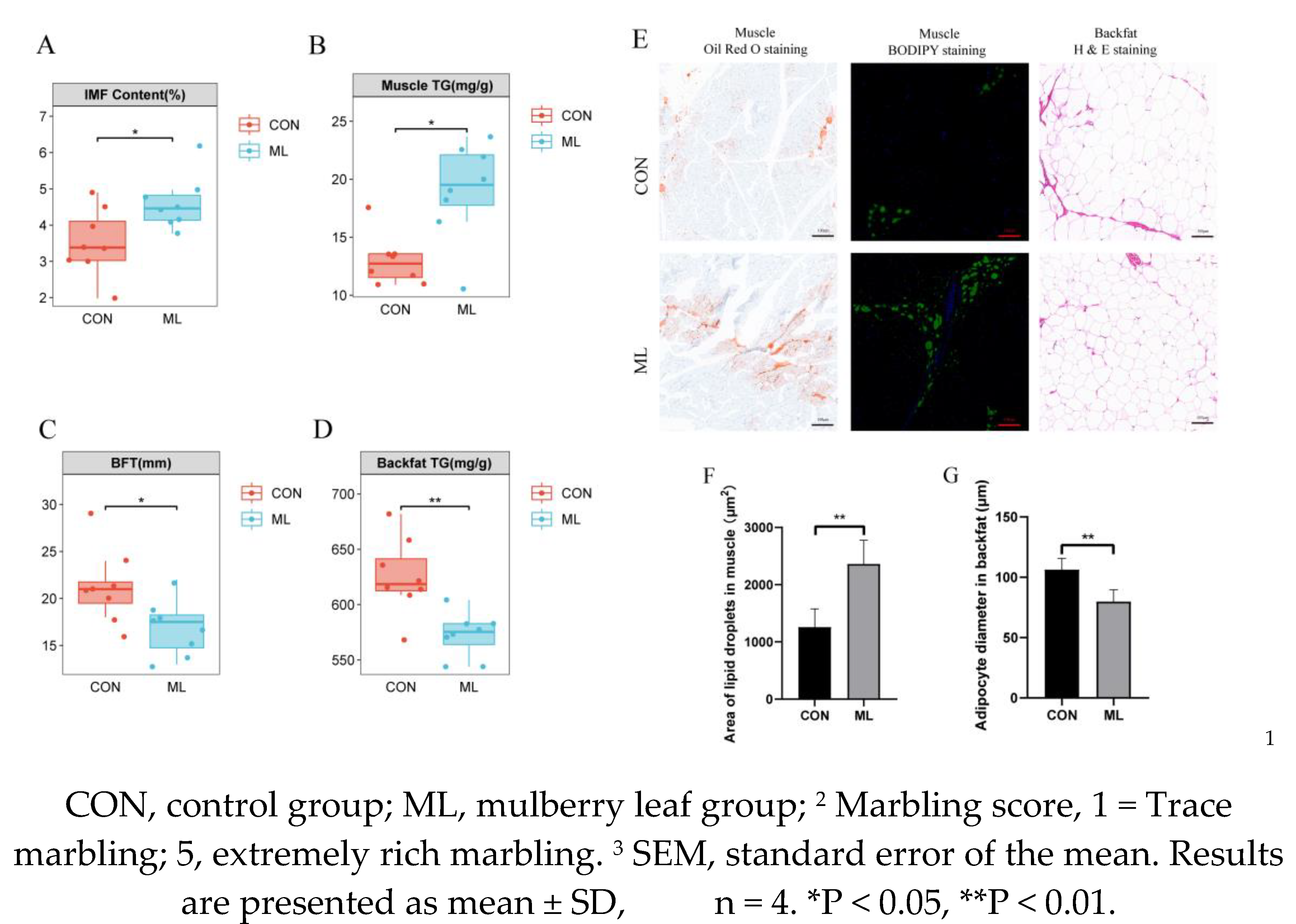

| Meat quality traits | CON | ML | SEM3 | P-Value |
| Lightness(L*) | 50.182 ± 1.595 | 52.652 ± 0.929* | 0.923 | 0.036 |
| Redness(a*) | 13.770 ± 1.435 | 13.210 ± 1.686 | 1.107 | 0.633 |
| Yellowness(b*) | 6.175 ± 1.786 | 5.255 ± 1.638 | 1.212 | 0.476 |
| Marbling score2 | 3.275 ± 0.320 | 4.075 ± 0.435* | 0.270 | 0.025 |
| pH | 6.260 ± 0.232 | 6.118 ± 0.284 | 0.183 | 0.467 |
| Shear force | 19.73 ± 2.275 | 21.28 ± 1.359 | 1.325 | 0.287 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




