Submitted:
21 December 2023
Posted:
22 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Chemistry
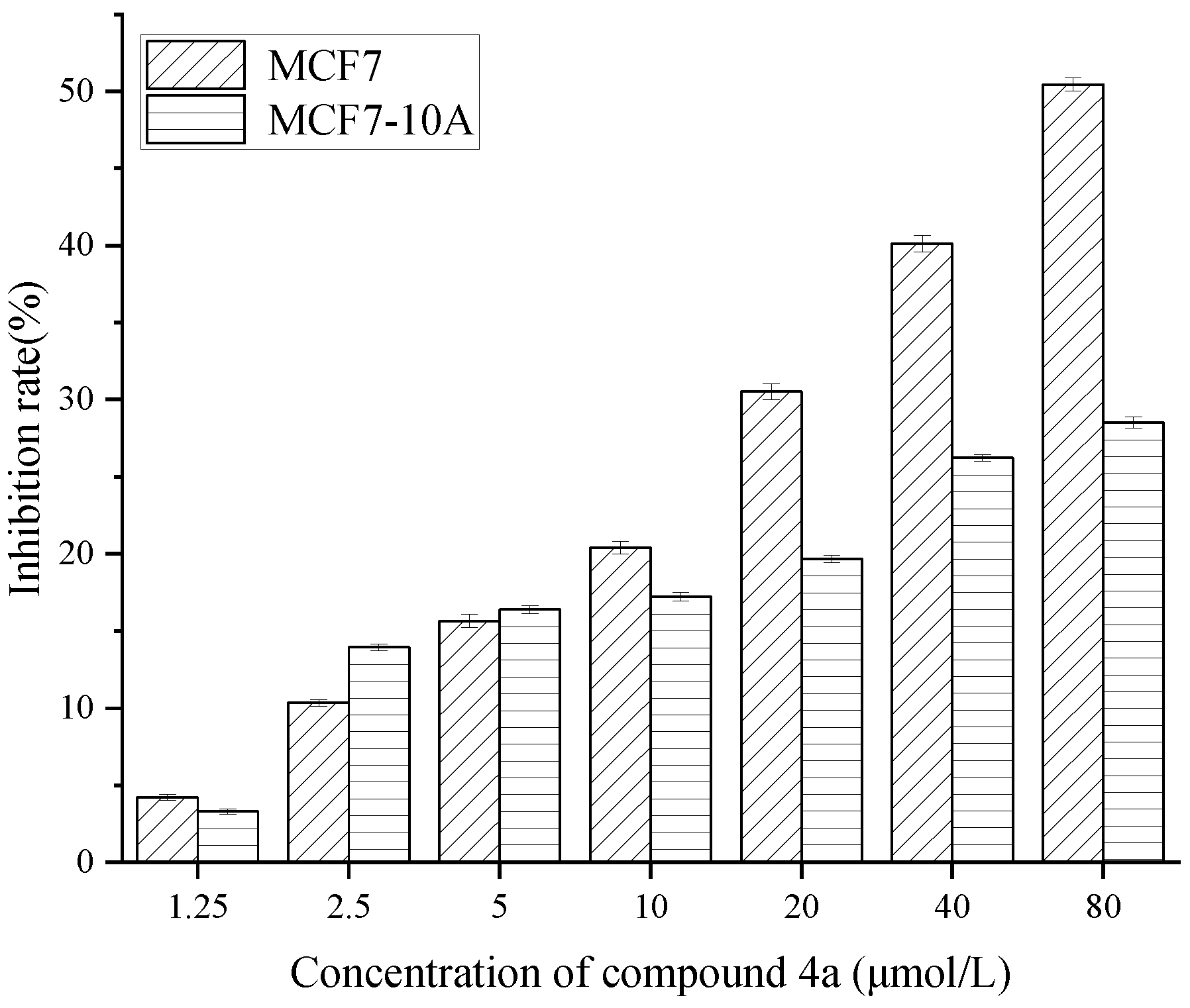
2.2. Effects of Cordycepin derivatives inhibits the proliferation on MCF7, HepG2, and SGC-7901
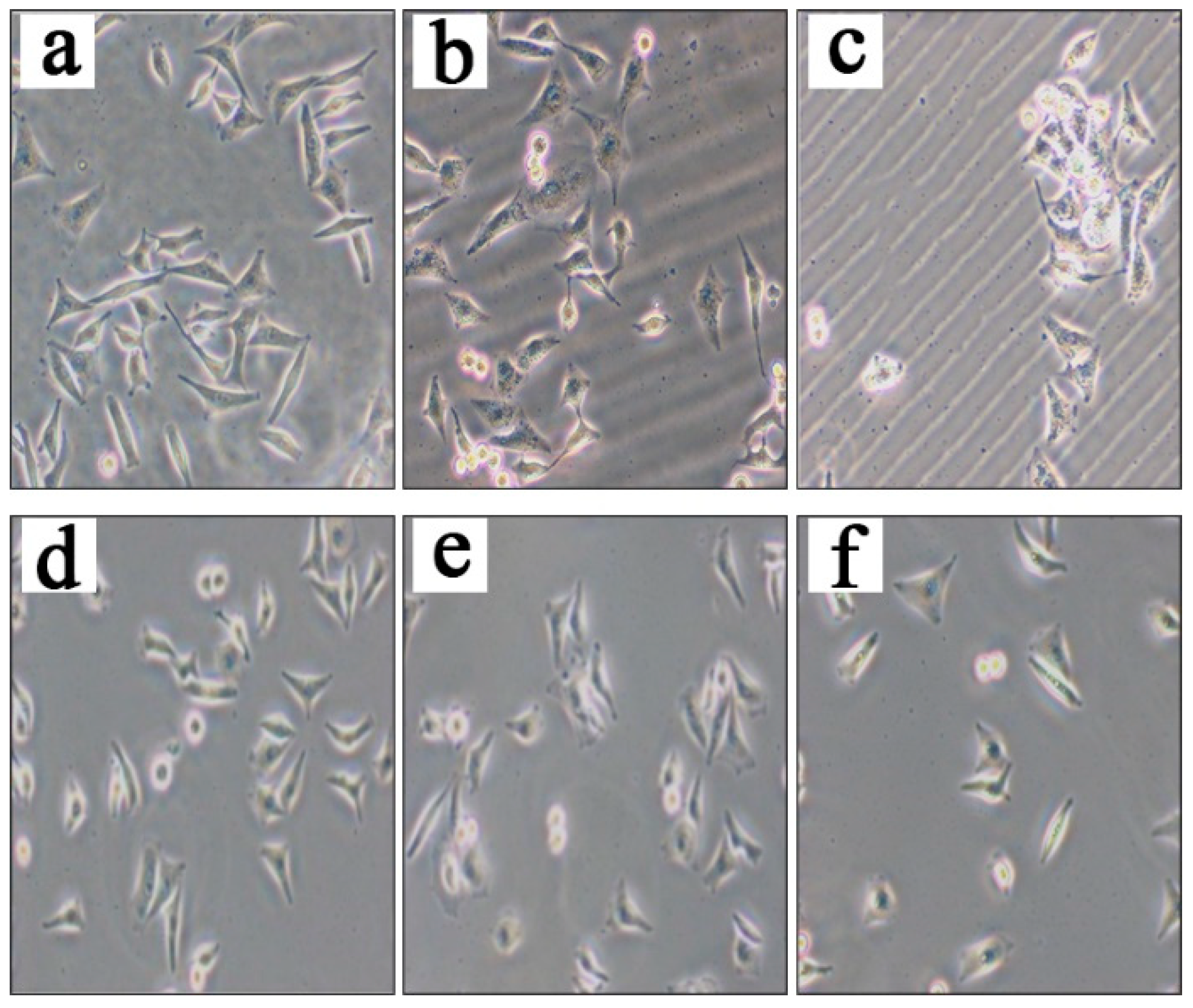
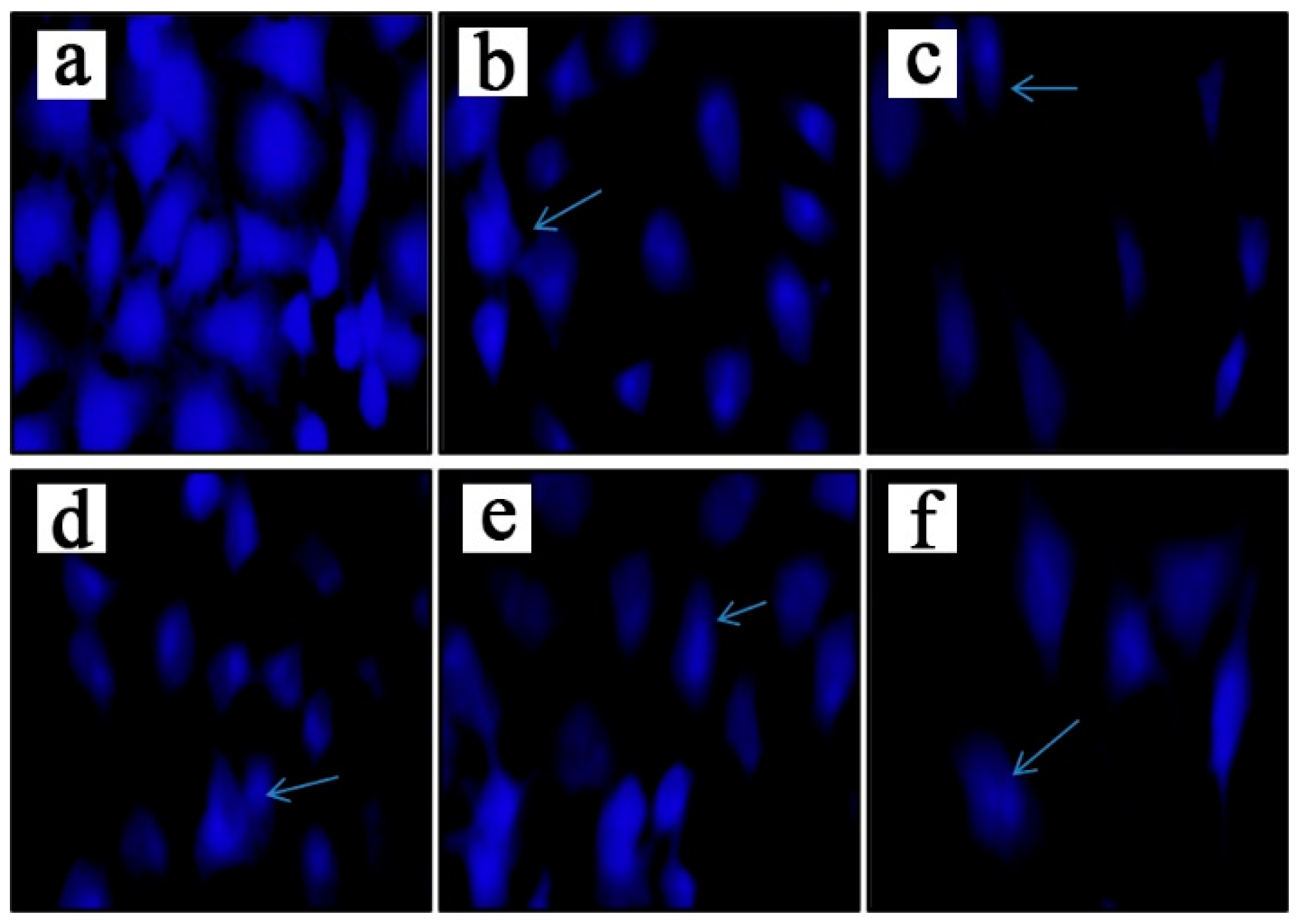
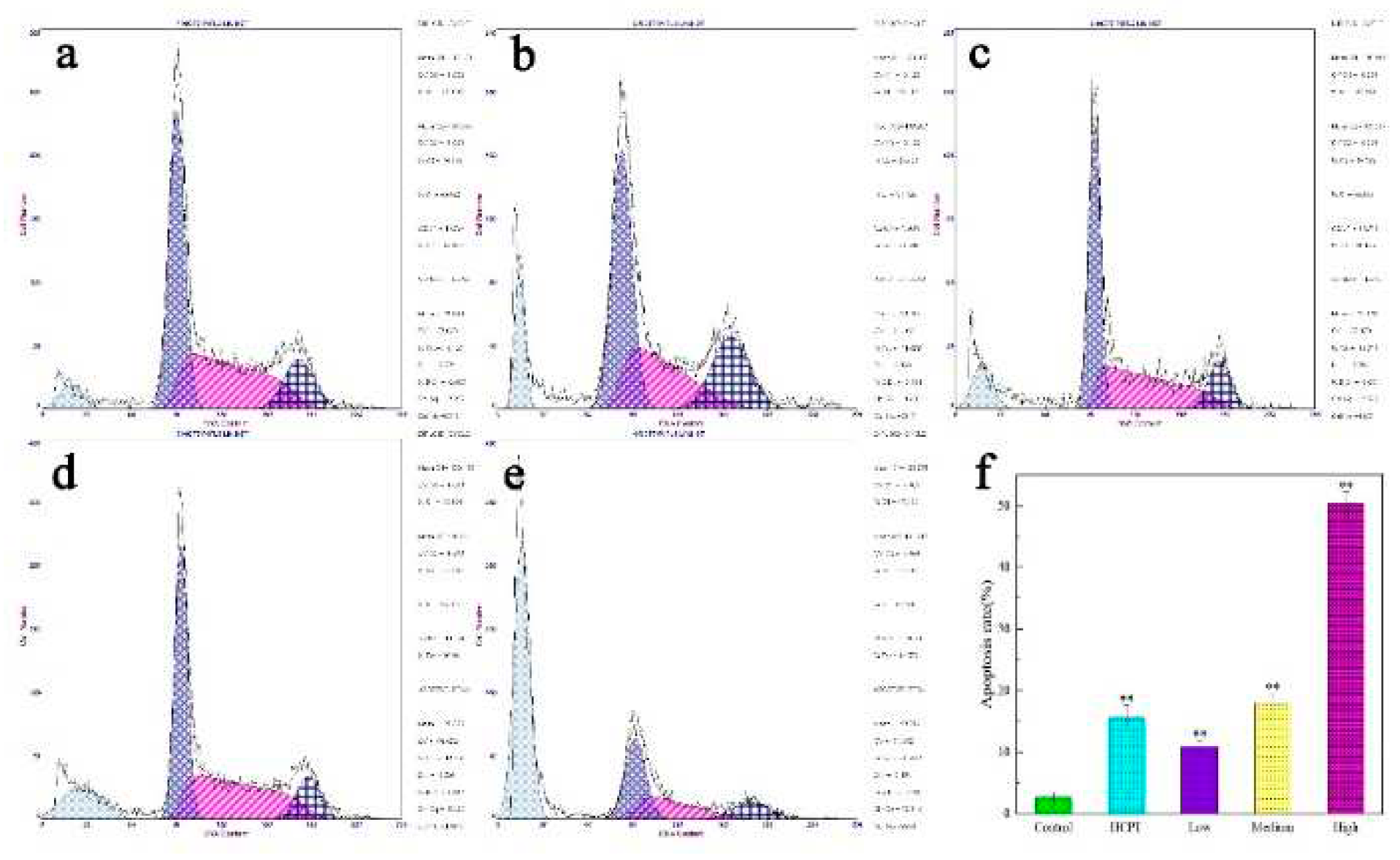
2.3. Compound 4a induces apoptosis in MCF 7 cells.
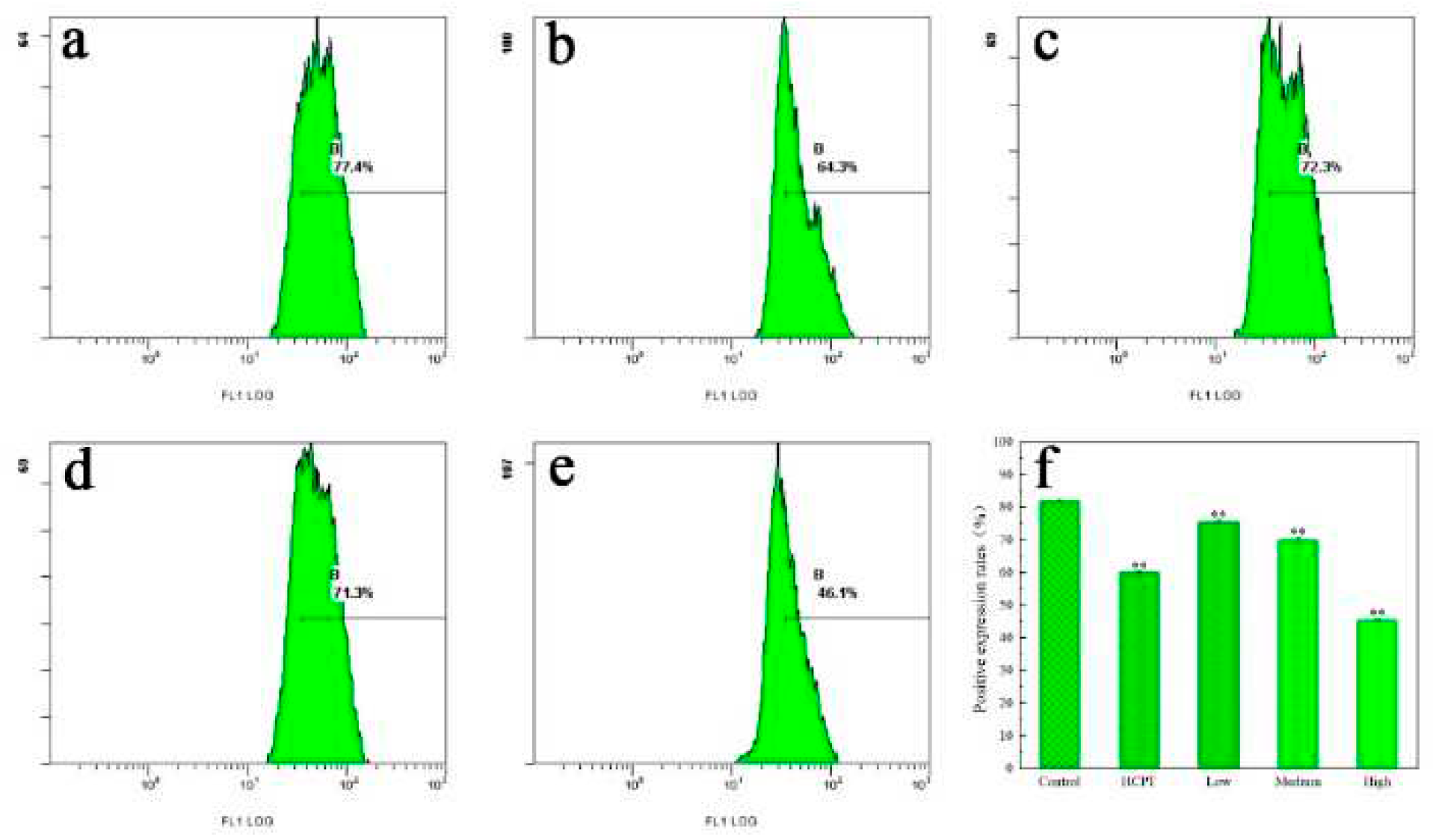
2.4. Results of western blot analysis
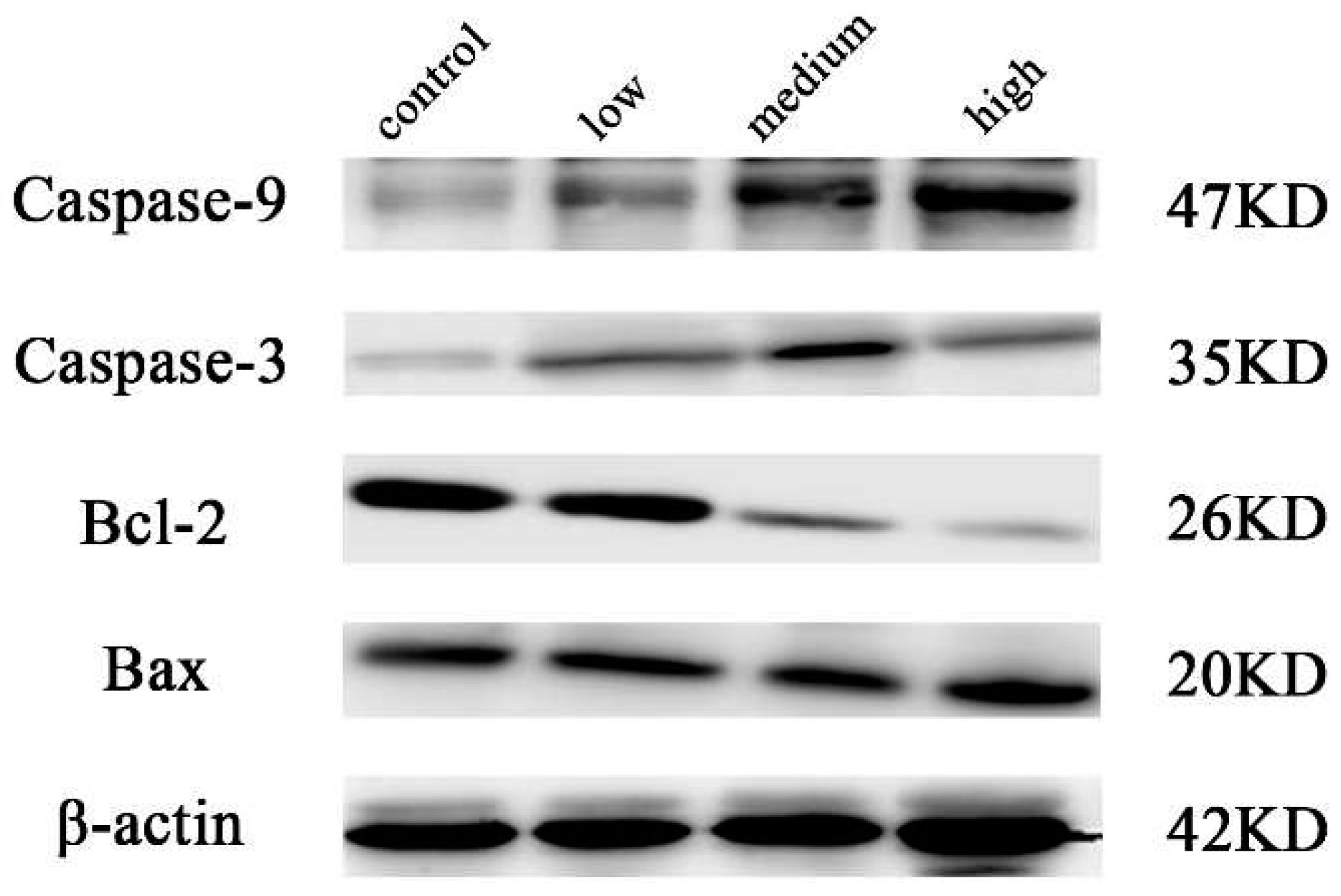
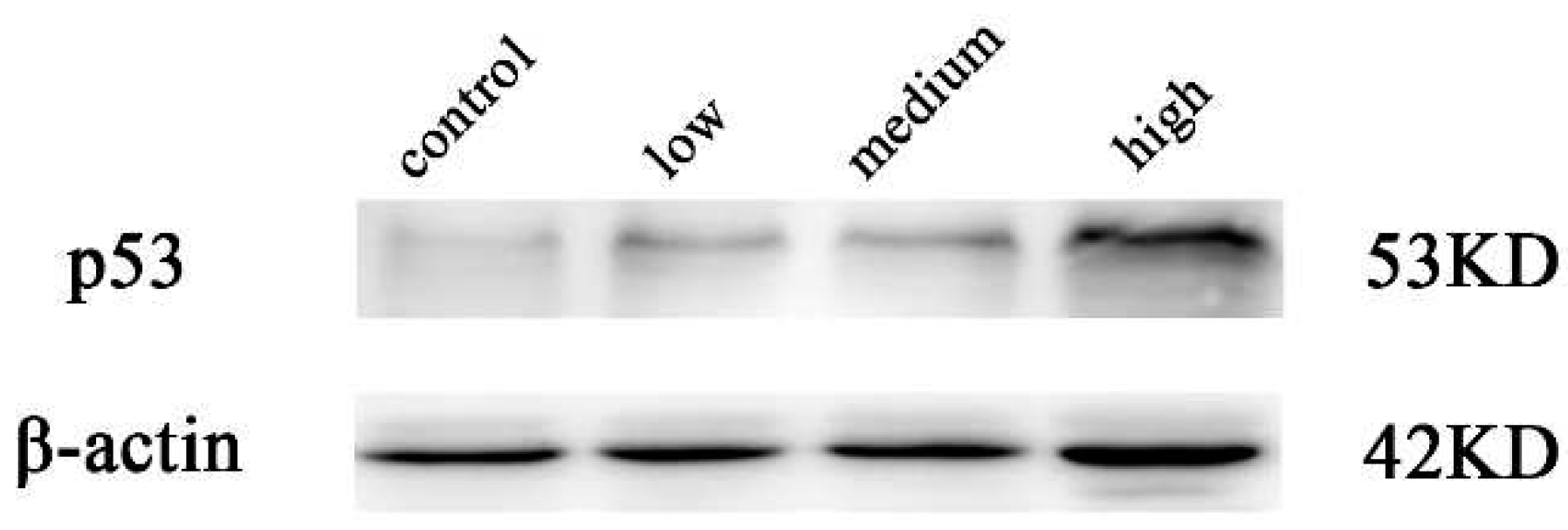
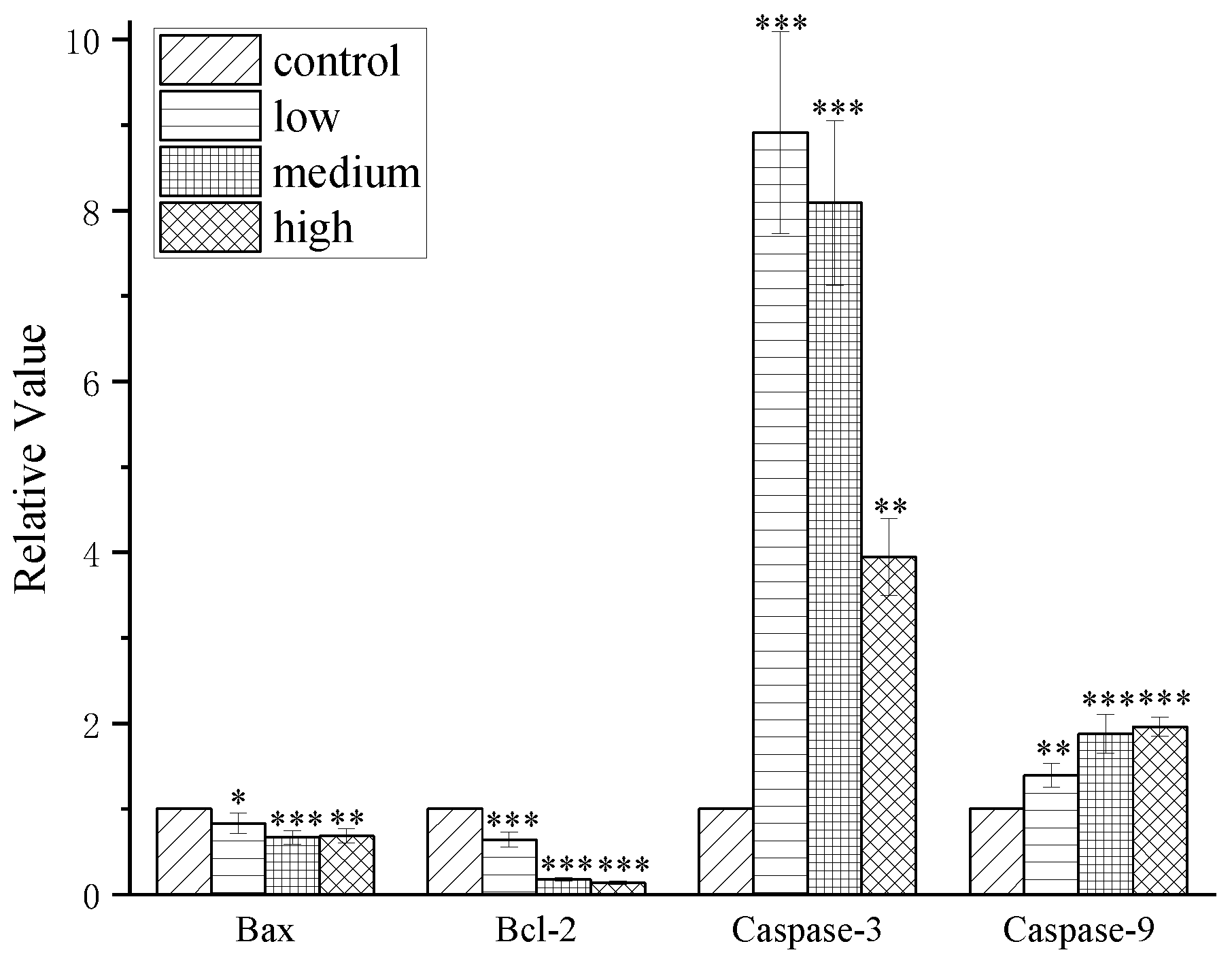
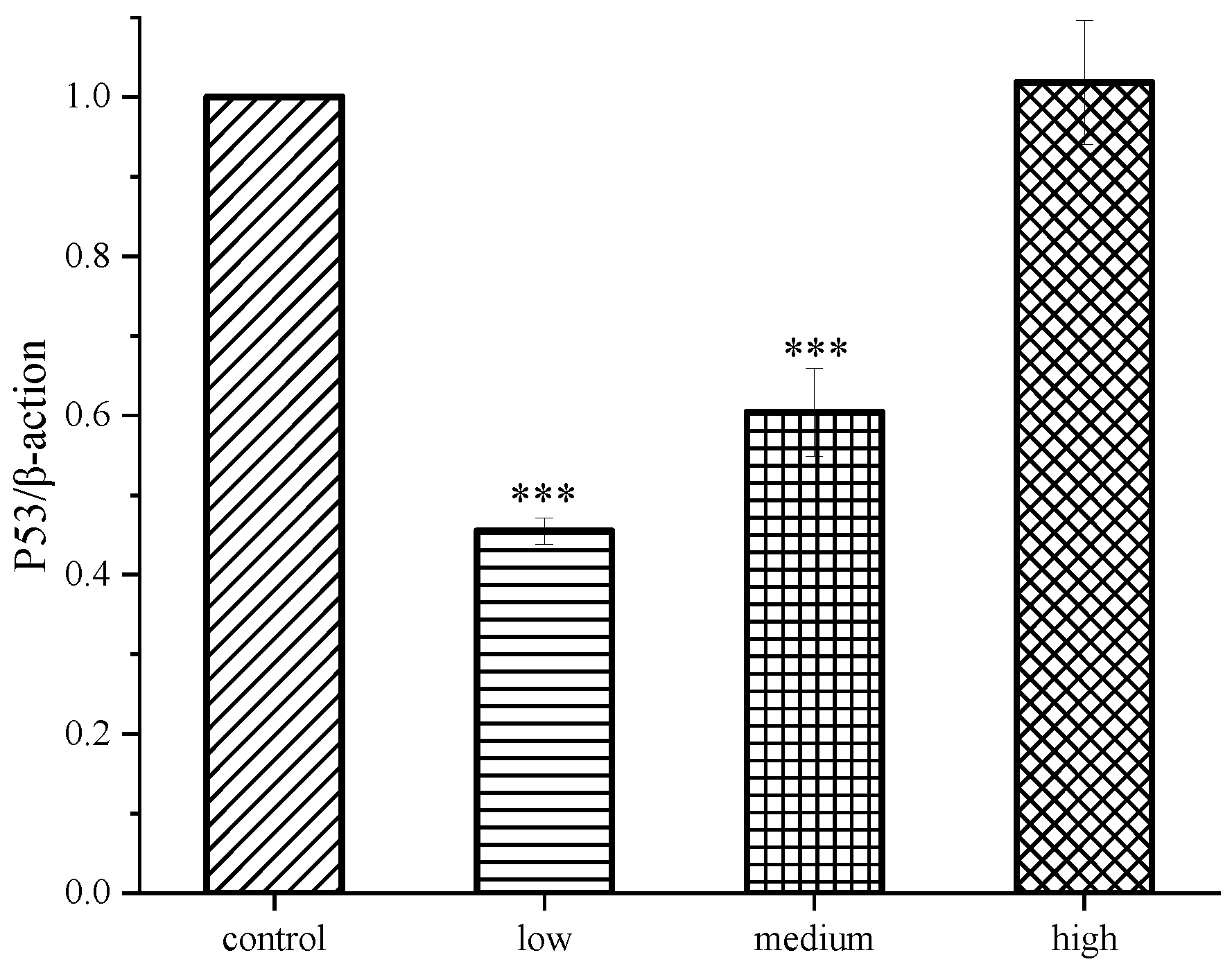
3. Conclusions
4. Experimental Section
4.1. Chemicals and Reagents
4.2. Synthesis
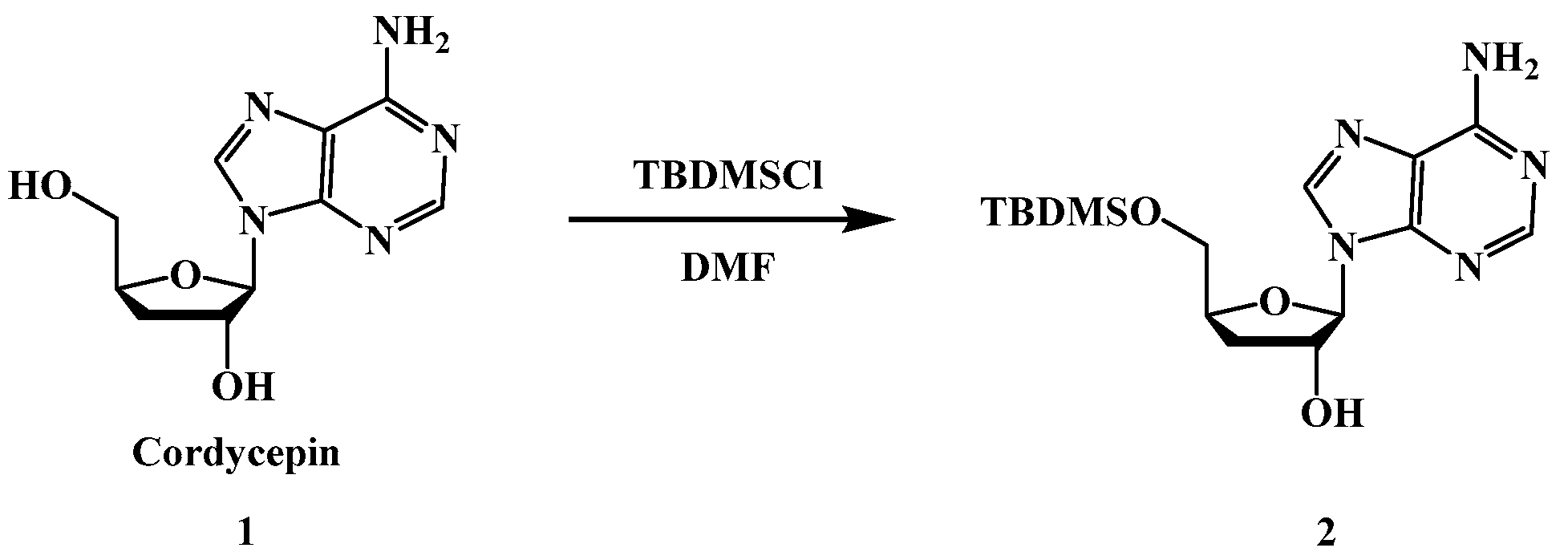
4.2.1. Synthesis of compound 2:
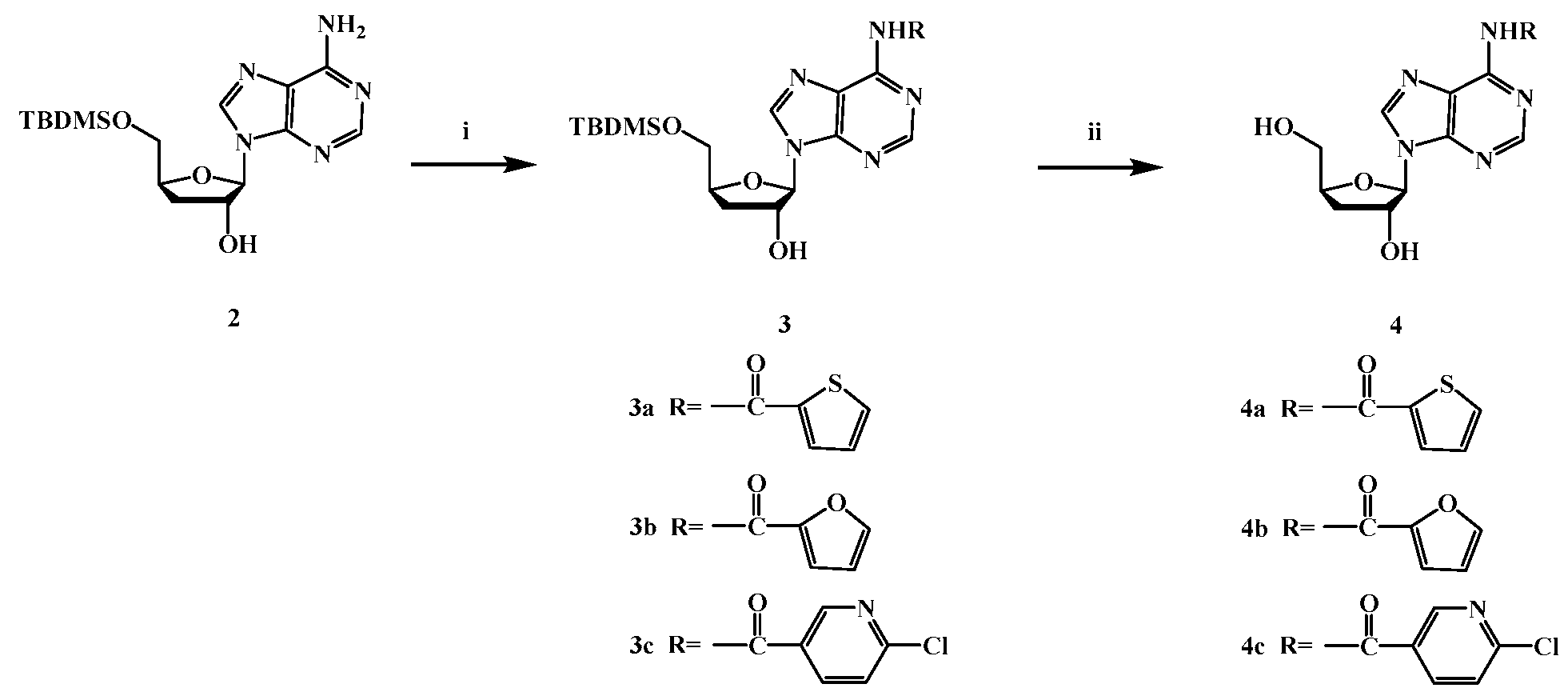
4.2.2. Synthesis of compounds 3a-3c(i)
4.2.3. Synthesis of compound 4a-4c (ii)
4.3. Characterization
4.4. Cell culture
4.5. Cell proliferation and cytotoxicity assays
4.6. Hoechst 33258 staining assay
4.7. Mitochondrial membrane potential assay
4.8. Cell cycle detection
4.9. Western blot assay
4.10. Statistical analysis
Author Contributions
Acknowledgments
References
- K. Cunningham, W. Manson, F. Spring, S. Hutchinson, ‘Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.)Link’, Nature. 1950, 166, 949–949.
- H. S. Tuli, S. S. Sandhu, A. Sharma, ‘Pharmacological and therapeutic potential of Cordyceps with special reference to Cordycepin’, 3 Biotech. 2014, 4, 1–12.
- K. Yue, M. Ye, Z. Zhou, W. Sun, X. Lin, ‘The genus Cordyceps: a chemical and pharmacological review’, J. Pharm. Pharmacol. 2013, 65, 474–493.
- J. M. Park, J. S. Lee, K. R. Lee, S. J. Ha, E. K. Hong, ‘Cordyceps militaris extract protects human dermal fibroblasts against oxidative stress-induced apoptosis and premature senescence’, Nutrients. 2014, 6, 3711–3726.
- J. Yin, X. Xin, Y. Weng, Z. Gui, ‘Transcriptome-wide analysis reveals the progress of Cordyceps militaris subculture degeneration’, PLoS. One. 2017, 12, e0186279.
- W. Liu, L. Zhang, S. Sun, L. Tang, S. He, A. Chen, L. Yao, D. Ren, ‘Cordycepin inhibits inflammatory responses through suppression of ERK activation in zebrafish’, Dev. Comp. Immunol. 2021, 124, 104178.
- X. Zhang, W. Huang, P. Tang, Y. Sun, X. Zhang, L. Qiu, B. Yu, X. Zhang, Y. Hong, Y. He, ‘Anti-inflammatory and neuroprotective effects of natural cordycepin in rotenone-induced PD models through inhibiting Drp1-mediated mitochondrial fission’, Neurotoxicology. 2021, 84, 1–13.
- X. Liao, Y. Gao, H. Zhao, M. Zhou, D. Chen, L. Tao, W. Guo, L. Sun, C. Gu, H. Chen, ‘Cordycepin reverses cisplatin resistance in non-small cell lung cancer by activating AMPK and inhibiting AKT signaling pathway’, Front. Cell. Dev. Biol. 2021, 8, 609285.
- N. X. D. Mai, U.-C. N. Le, L. H. T. Nguyen, H. T. K. Ta, N. H. Van, T. M. Le, T. B. Phan, L. T. T. Nguyen, F. Tamanoi, T. L. H. Doan, ‘Facile synthesis of biodegradable mesoporous functionalized-organosilica nanoparticles for enhancing the anti-cancer efficiency of cordycepin’, Microporous. Mesoporous. Mater. 2021, 315, 110913.
- Z. Wen, X. Du, N. Meng, Y. Li, R. Mi, X. Li, Y. Sun, S. Ma, S. Li, ‘Tussah silkmoth pupae improve anti-tumor properties of Cordyceps militaris (L.) Link by increasing the levels of major metabolite cordycepin’, RSC. Adv. 2019, 9, 5480–5491.
- C. Lee, K. Huang, J. Shaw, J. Chen, W. Kuo, G. Shen, A. M. Grumezescu, A. M. Holban, Y. Wang, J. Wang, ‘Trends in the immunomodulatory effects of Cordyceps militaris: total extracts, polysaccharides and cordycepin’, Front. Pharmacol. 2020, 1824, 10–3389. [CrossRef]
- X. Fei, X. Zhang, G. Zhang, W. Bao, Y. Zhang, M. Zhang, X. Zhou, ‘Cordycepin inhibits airway remodeling in a rat model of chronic asthma’, Biomed. Pharmacother. 2017, 88, 335–341.
- F. Han, M. Dou, Y. Wang, C. Xu, Y. Li, X. Ding, W. Xue, J. Zheng, P. Tian, C. Ding, ‘Cordycepin protects renal ischemia/reperfusion injury through regulating inflammation, apoptosis, and oxidative stress’, Acta. Biochim. Biophys. Sin. 2020, 52, 125–132.
- Y. Jia, H. Li, H. Bao, D. Zhang, L. Feng, Y. Xiao, K. Zhu, Y. Hou, S. Luo, Y. Zhang, ‘Cordycepin (3′-deoxyadenosine) promotes remyelination via suppression of neuroinflammation in a cuprizone-induced mouse model of demyelination’, Int. Immunopharmacol. 2019, 75, 105777.
- M. M. Chang, S. Y. Hong, S. H. Yang, C. C. Wu, C. Y. Wang, B. M. Huang, ‘Anti-cancer effect of cordycepin on FGF9-induced testicular tumorigenesis’, Int. J. Mol. Sci. 2020, 21, 8336.
- M. Radhi, S. Ashraf, S. Lawrence, A. A. Tranholm, P. A. D. Wellham, A. Hafeez, A. S. Khamis, R. Thomas, D. McWilliams, C. H. DeMoor, ‘A systematic review of the biological effects of cordycepin’, Molecules. 2021, 26, 5886.
- S. Y. Yoon, S. J. Park, Y. J. Park, ‘The anticancer properties of cordycepin and their underlying mechanisms’, Int. J. Mol. Sci. 2018, 19, 3027.
- C. Liu, M. Qi, L. Li, Y. Yuan, X. Wu, J. Fu, ‘Natural cordycepin induces apoptosis and suppresses metastasis in breast cancer cells by inhibiting the Hedgehog pathway’, Food. Funct. 2020, 11, 2107–2116.
- H. J. Lee, P. Burger, M. Vogel, K. Friese, A. Brüning, ‘The nucleoside antagonist cordycepin causes DNA double strand breaks in breast cancer cells’, Invest. New. Drugs. 2012, 30, 1917–1925.
- E. M. Noh, S. H. Jung, J. H. Han, E. Y. Chung, J. Y. Jung, B. S. Kim, S. H. Lee, Y. R. Lee, J. S. Kim, ‘Cordycepin inhibits TPA-induced matrix metalloproteinase-9 expression by suppressing the MAPK/AP-1 pathway in MCF-7 human breast cancer cells’, Int. J. Mol. Med. 2010, 25, 255–260.
- H. Thomadaki, C. M. Tsiapalis, A. Scorilas, ‘Polyadenylate polymerase modulations in human epithelioid cervix and breast cancer cell lines, treated with etoposide or cordycepin, follow cell cycle rather than apoptosis induction’. 2005. [CrossRef]
- S. Choi, M. H. Lim, K. M. Kim, B. H. Jeon, W. O. Song, T. W. Kim, ‘Cordycepin-induced apoptosis and autophagy in breast cancer cells are independent of the estrogen receptor’, Toxicol. Appl. Pharmacol. 2011, 257, 165–173.
- Y. Bi, H. Li, D. Yi, Y. Sun, Y. Bai, S. Zhong, Y. Song, G. Zhao, Y. Chen, ‘Cordycepin augments the chemosensitivity of human glioma cells to temozolomide by activating AMPK and inhibiting the AKT signaling pathway’, Mol. Pharmaceutics. 2018, 15, 4912–4925.
- Y. Zhou, Z. Guo, Q. Meng, J. Lu, N. Wang, H. Liu, Q. Liang, Y. Quan, D. Wang, J. Xie, ‘Cordycepin affects multiple apoptotic pathways to mediate hepatocellular carcinoma cell death’, Anti-Cancer Agents Med. Chem. 2017, 17, 143–149.
- J. Dong, Y. Li, H. Xiao, D. Luo, S. Zhang, C. Zhu, M. Jiang, M. Cui, L. Lu, S. Fan, ‘Cordycepin sensitizes breast cancer cells toward irradiation through elevating ROS production involving Nrf2’, Toxicol. Appl. Pharmacol. 2019, 364, 12–21.
- S. R. Alberts, L. D. Wagman, ‘Chemotherapy for colorectal cancer liver metastases’, Oncologist. 2008, 13, 1063–1073.
- J. Song, Y. Wang, M. Teng, S. Zhang, M. Yin, J. Lu, Y. Liu, R. J. Lee, D. Wang, L. Teng, ‘Cordyceps militaris induces tumor cell death via the caspase-dependent mitochondrial pathway in HepG2 and MCF-7 cells’, Mol. Med. Rep. 2016, 13, 5132–5140.
- Z. Guo, W. Chen, G. Dai, Y. Huang, ‘Cordycepin suppresses the migration and invasion of human liver cancer cells by downregulating the expression of CXCR4’, Int. J. Mol. Med. 2020, 45, 141–150.
- H. Lu, X. Li, J. Zhang, H. Shi, X. Zhu, H. He, ‘Effects of cordycepin on HepG2 and EA. hy926 cells: Potential antiproliferative, antimetastatic and anti-angiogenic effects on hepatocellular carcinoma’, Oncol. Lett. 2014, 7, 1556–1562.
- M. I. Nasser, M. Masood, W. Wei, X. Li, Y. Zhou, B. Liu, J. Li, X. Li, ‘Cordycepin induces apoptosis in SGC-7901 cells through mitochondrial extrinsic phosphorylation of PI3K/Akt by generating ROS’, Int. J. Oncol. 2017, 50, 911–919.
- C. Wei, M. A. Khan, J. Du, J. Cheng, M. Tania, E. L. H. Leung, J. Fu, ‘Cordycepin inhibits triple-negative breast cancer cell migration and invasion by regulating EMT-TFs SLUG, TWIST1, SNAIL1, and ZEB1’, Front. Oncol. 2022, 12, 10–3389.
| Compounds | R1 | R2 | IC50[a](μM) | ||
| MCF7 | HepG2 | SGC-7901 | |||
| HCPT [b] | 7.561±0.05*** | 6.564±0.45** | 7.964±0.65*** | ||
| Cordycepin | -OH | -H | 46.852±1.62* | 51.836±1.36** | 51.271±3.77** |
| 3a | -OTBDMS | 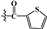 |
114.083±2.03** | 133.746±5.12** | 102.034±1.08* |
| 3b | -OTBDMS | 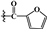 |
98.033±0.04** | 60.433±1.15** | 41.741±2.36** |
| 3c | -OTBDMS | 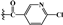 |
45.792±1.01** | 87.010±0.04** | 82.614±1.48** |
| 4a | -OH | 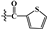 |
27.566±0.52*** | 68.793±3.34** | 38.929±0.06* |
| 4b | -OH |  |
40.938±1.67** | 33.365±0.08** | 86.307±2.35** |
| 4c | -OH | 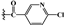 |
45.263±0.58** | 48.392±0.79** | 80.390±1.05** |
| [a] All compounds were examined in a set of experiments repeated three times; IC50 values of compounds represent the concentration that caused 50 % enzyme activity loss. [b] HCPT is positive control. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





