Submitted:
19 December 2023
Posted:
22 December 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Results and Discussion
2.1. ATR-FTIR analysis of biomolecular mechanisms of inhibitor binding to the proteolytic enzyme
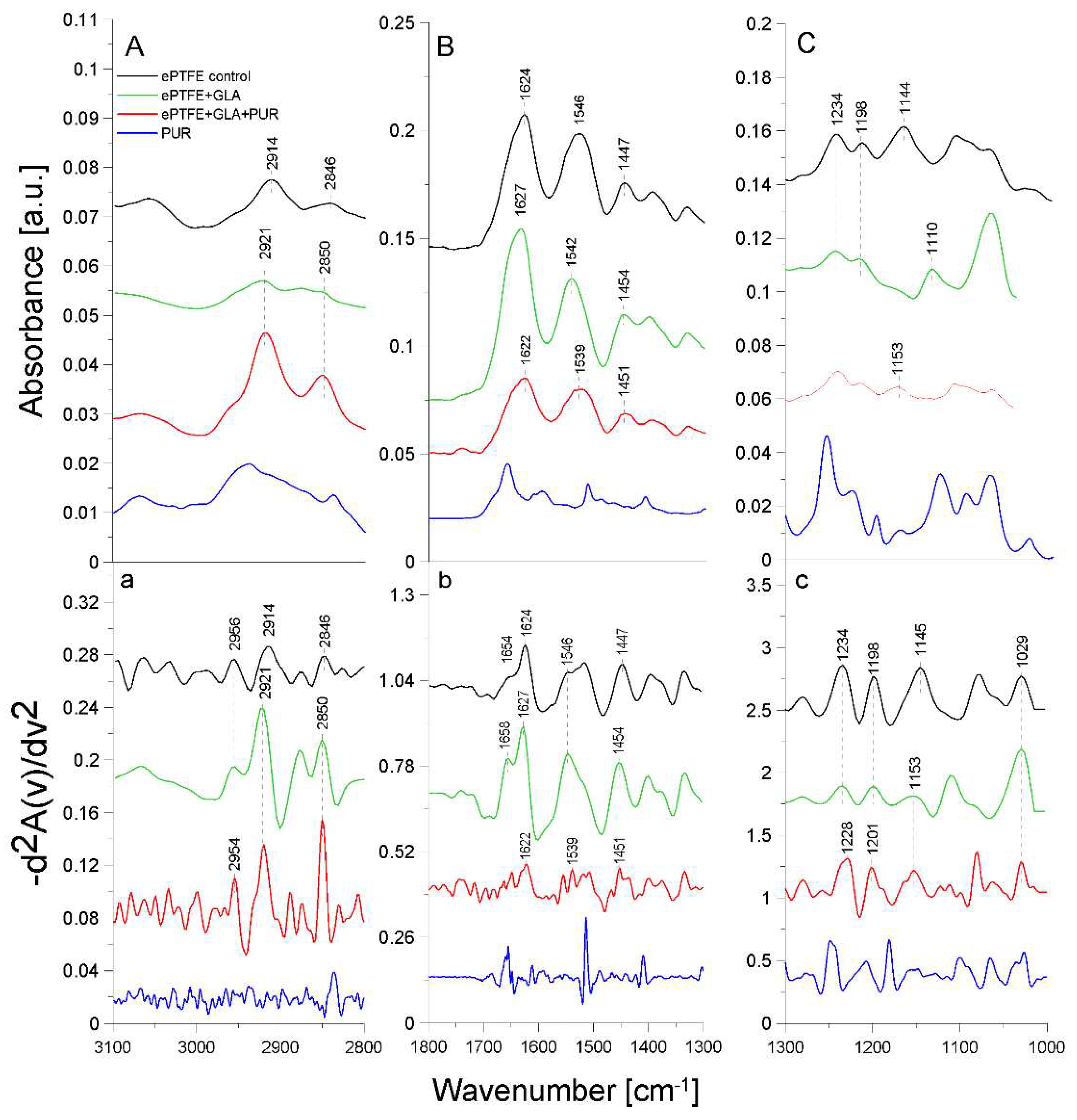
2.2. ATR-FTIR analysis of structural changes after immobilization of tested inhibitors on the ePTFE prosthesis
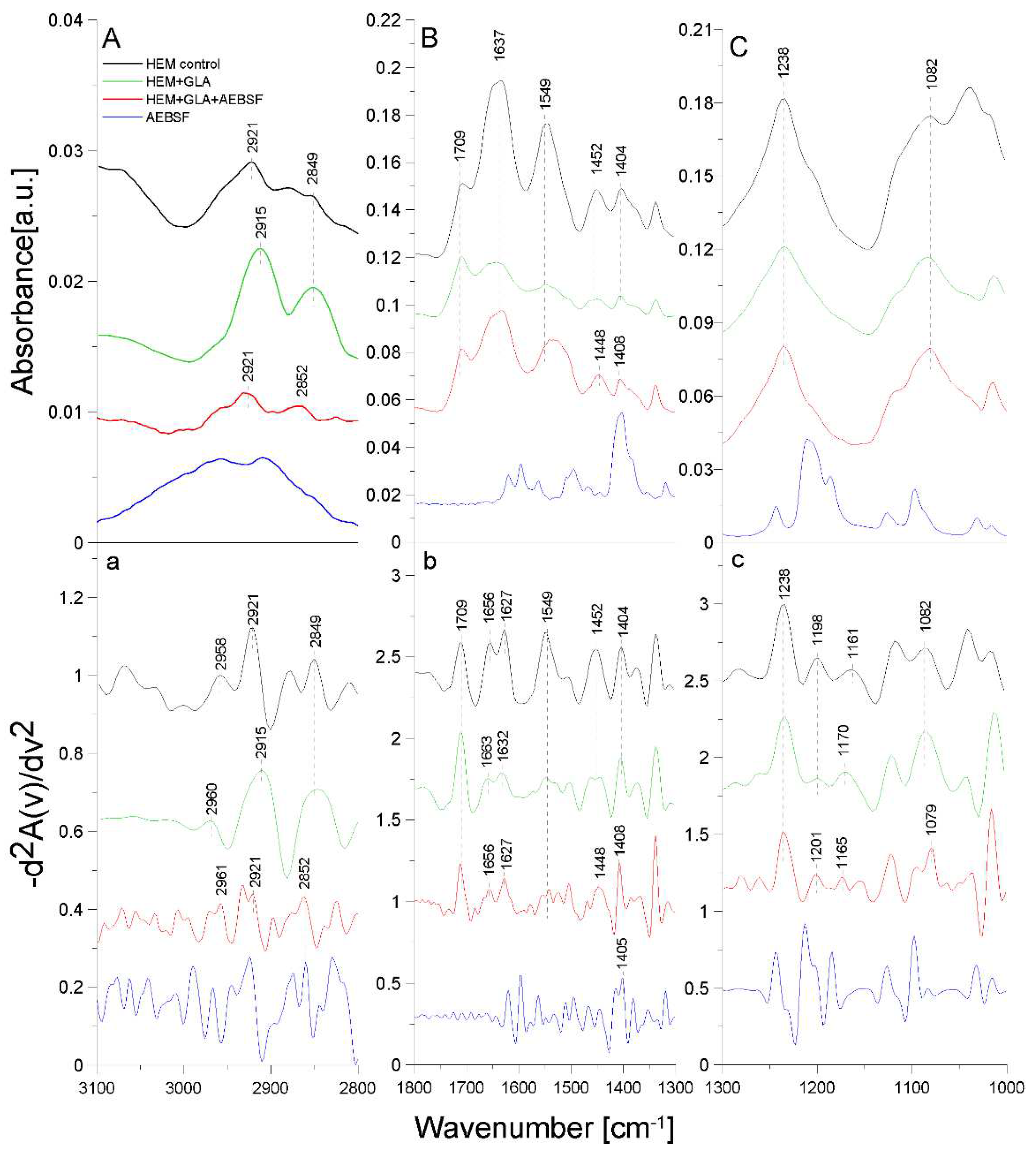
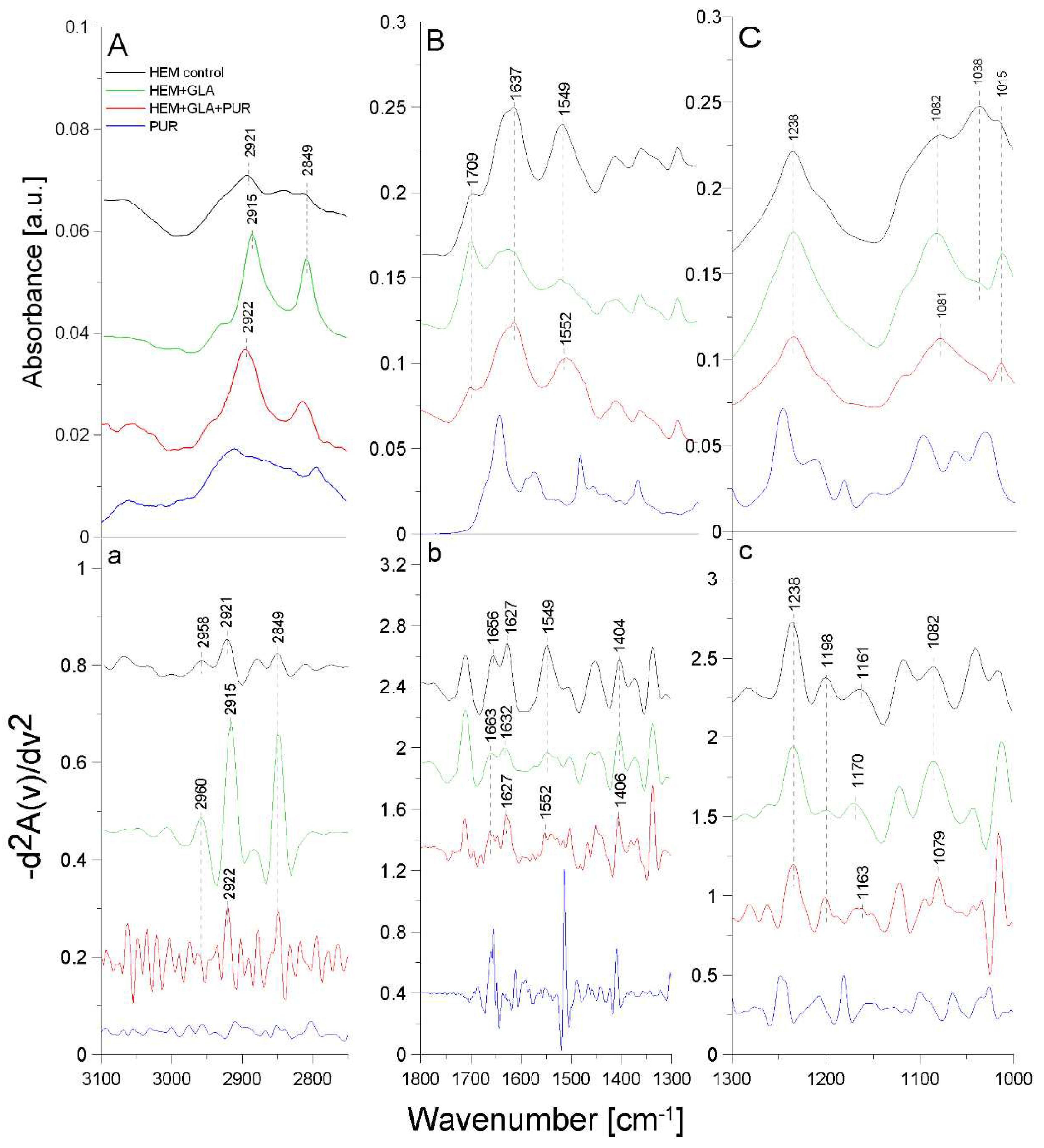
2.3. ATR-FTIR analysis of structural changes after immobilization of tested inhibitors on the HEM prosthesis
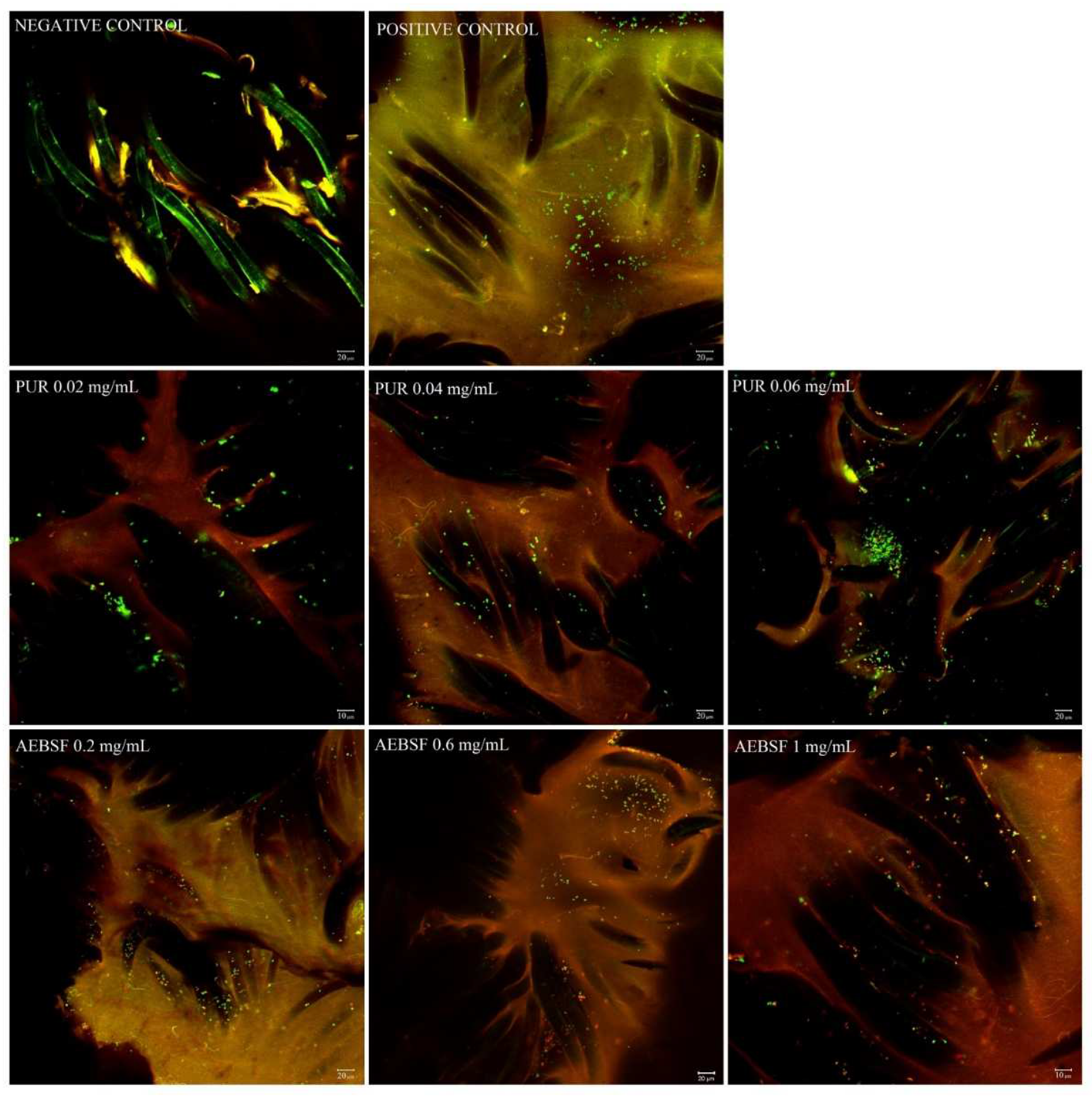
2.4. CLSM analysis of PUR and AEFBS antimicrobial activity (live/dead analysis)
3. Materials and Methods
3.1. Enzymes and inhibitors
- a)
- puromycin (PUR) - a natural aminonucleoside antibiotic (Figure 7a) with a molecular weight of 544.4 Da isolated from Streptomyces alboniger [41]. PUR inhibits protein synthesis by induction of premature chain termination acting as an analogue of the 3′-terminal ends of aminoacyl-tRNA. Additionally, PUR is a reversible inhibitor of dipeptidyl-peptidase II (serine peptidase) and cytosol alanyl aminopeptidase (metallopeptidase). PUR is active against gram-positive bacteria, less active against acid-fast bacilli, and more weakly active against gram-negative microorganisms [42].
- b)
- c)
- trypsin (TRYP) – a model serine protease cleaving peptides on the C-terminal side of lysine and arginine residues. TRYP is produced in pancreatic acinar cells in an inactive form (known as trypsinogen) and is activated only in the lumen of the small intestine to digest proteins [44].
3.3. Analysis of enzyme-inhibitor interactions using ATR-FTIR spectroscopy
3.4. Covalent immobilization of protease inhibitors on the vascular prosthesis
3.5. Analysis of biomaterial surface structure using ATR-FTIR spectroscopy
3.6. Biofilm formation on GLA-activated HEM prostheses
3.7. Confocal laser scanning microscopy analysis (CLSM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barua, E.; Deoghare, A.B.; Deb, P.; Lala, S.D. Naturally derived biomaterials for development of composite bone scaffold: A review. Materials Science and Engineering 2018, 377, 012013. [Google Scholar] [CrossRef]
- Hasirci, V.; Hasirci, N. Fundamentals of Biomaterials; Springer 2018.
- Li, G.; Lai, Z.; Shan, A. Advances of antimicrobial peptide-based biomaterials for the treatment of bacterial infections. Advanced Science 2023, 10, e2206602. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, X.; Savino, K.; Gabrys, P.; Gao, Y.; Chaimayo, W.; Miller, B.L.; Yates, M.Z. Antimicrobial silver-hydroxyapatite composite coatings through two-stage electrochemical synthesis. Surface and Coatings Technology 2016, 301, 13–19. [Google Scholar] [CrossRef]
- Hennessey, H.; Luckham, E.; Kayssi, A.; Wheatcroft, M.D.; Greco, E.; Al-Omran, M.; Harlock, J.; Qadura, M. Optimization of rifampin coating on covered Dacron endovascular stent grafts for infected aortic aneurysms. Journal of Vascular Surgery 2019, 69, 242–248. [Google Scholar] [CrossRef]
- Zawrotniak, M.; Bochenska, O.; Karkowska-Kuleta, J.; Seweryn-Ozog, K.; Aoki, W.; Ueda, M.; Kozik, A.; Rapala-Kozik, M. Aspartic proteases and major cell wall components in Candida albicans trigger the release of neutrophil extracellular traps. Frontiers in Cellular and Infection Microbiology 2017, 7, 414. [Google Scholar] [CrossRef]
- Backert, S.; Bernegger, S.; Skórko-Glonek, J.; Wessler, S. Extracellular HtrA serine proteases: an emerging new strategy in bacterial pathogenesis. Cellular Microbiology 2018, 20, e12845. [Google Scholar] [CrossRef]
- Murphy, J.; Ramezanpour, M.; Stach, N.; Dubin, G.; Psaltis, A.J.; Wormald, P.J.; Vreugde, S. Staphylococcus aureus V8 protease disrupts the integrity of the airway epithelial barrier and impairs IL-6 production in vitro. The Laryngoscope 2018, 128, 8–15. [Google Scholar] [CrossRef]
- Burchacka, E.; Pięta, P.; Łupicka-Słowik, A. Recent advances in fungal serine protease inhibitors. Biomedicine & Pharmacotherapy 2022, 146, 112523. [Google Scholar] [CrossRef]
- Szałapata, K.; Osińska-Jaroszuk, M.; Kapral-Piotrowska, J.; Pawlikowska-Pawlęga, B.; Łopucki, R.; Mroczka, R.; Jarosz-Wilkołazka, A. Serine protease inhibitors — new molecules for modification of polymeric biomaterials. Biomolecules 2020, 10, 82. [Google Scholar] [CrossRef]
- Gerbino, E.; Mobili, P.; Tymczyszyn, E.; Fausto, R.; Gómez-Zavaglia, A. FTIR spectroscopy structural analysis of the interaction between S-layers and metal ions. Journal of Molecular Structure 2011, 987, 186–192. [Google Scholar] [CrossRef]
- Jaiswal, P.; Jha, S.N.; Borah, A.; Gautam, A.; Grewal, M.K.; Jindal, G. Detection and quantification of soymilk in cow-buffalo milk using Attenuated Total Reflectance Fourier Transform Infrared spectroscopy (ATR-FTIR). Food Chemistry 2015, 168, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hebia, C.; Bekale, L.; Chanphai, P.; Agbebavi, J.; Tajmir-Riahi, H.A. Trypsin inhibitor complexes with human and bovine serum albumins: TEM and spectroscopic analysis. Journal of Photochemistry and Photobiology B: Biology 2014, 130, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.C.; Söderhäll, K.; Lind, M.I. Pefabloc–A sulfonyl fluoride serine protease inhibitor blocks induction of Diptericin in Drosophila l(2)mbn cells. Insect Science 2012, 19, 472–476. [Google Scholar] [CrossRef]
- Guo, C.; Guo, X.; Chu, W.; Jiang, N.; Li, H. FTIR-ATR study for adsorption of trypsin in aqueous environment on bare and TiO2 coated ZnSe surfaces. Chinese Chemical Letters 2020, 31, 150–154. [Google Scholar] [CrossRef]
- Glorieux, S.; Favoreel, H.W.; Steukers, L.; Vandekerckhove, A.P.; Nauwynck, H.J. A trypsin-like serine protease is involved in pseudorabies virus invasion through the basement membrane barrier of porcine nasal respiratory mucosa. Veterinary Research 2011, 42, 58. [Google Scholar] [CrossRef] [PubMed]
- Mihály, J.; Sterkel, S.; Ortner, H.M.; Kocsis, L.; Hajba, L.; Furdyga, É.; Mink, J. FTIR and FT-Raman spectroscopic study on polymer based high pressure digestion vessels. Croatica Chemica Acta 2006, 79, 497–501. [Google Scholar]
- Chandy, T.; Das, G.S.; Wilson, R.F.; Rao, G.H. Use of plasma glow for surface-engineering biomolecules to enhance bloodcompatibility of Dacron and PTFE vascular prosthesis. Biomaterials 2000, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman characteristic group frequencies: tables and charts; John Wiley & Sons: United Kingdom, 2004. [Google Scholar]
- Piwowarczyk, J.; Jędrzejewski, R.; Moszyński, D.; Kwiatkowski, K.; Niemczyk, A.; Baranowska, J. XPS and FTIR studies of polytetrafluoroethylene thin films obtained by physical methods. Polymers 2019, 11, 1629. [Google Scholar] [CrossRef]
- Lech, A.; Butruk-Raszeja, B.A.; Ciach, T.; Lawniczak-Jablonska, K.; Kuzmiuk, P.; Bartnik, A.; Wachulak, P.; Fiedorowicz, H. Surface modification of PLLA, PTFE and PVDF with extreme ultraviolet (EUV) to enhance cell adhesion. International Journal of Molecular Sciences 2020, 21, 9679. [Google Scholar] [CrossRef]
- Reiter, T.; Panick, T.; Schuhladen, K.; Roether, J.A.; Hum, J.; Boccaccini, A.R. Bioactive glass based scaffolds coated with gelatin for the sustained release of icariin. Bioactive Materials 2019, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Karaszewska, A.; Bucheńska, J. Polyester vascular prostheses — antibacterial and athrombogenic biomaterials. Part II. Effect of two-stage modification of polyester vascular prostheses on the selected physicochemical, mechanical and microbiological properties. Polimery 2013, 58, 33–38. [Google Scholar] [CrossRef]
- Sendrea, C.; Carsote, C.; Badea, E.; Adams, A.; Niculescu, M.; Iovu, H. Non-invasive characterisation of collagen-based materials by NMR-mouse and ATR-FTIR. UPB Science Bulletin 2016, 78, 27–38. [Google Scholar]
- Mazumder, M.A.J.; Sheardown, H.; Al-Ahmed, A. Functional Polymers, 1st ed.; Springer Cham: Switzerland, 2019. [Google Scholar]
- Walker, J.N.; Horswill, A.R. A coverslip-based technique for evaluating Staphylococcus aureus biofilm formation on human plasma. Frontiers in Cellular and Infection Microbiology 2012, 2, 39. [Google Scholar] [CrossRef] [PubMed]
- Shanks, R.M.; Donegan, N.P.; Graber, M.L.; Buckingham, S.E.; Zegans, M.E.; Cheung, A.L.; O'Toole, G.A. Heparin stimulates Staphylococcus aureus biofilm formation. Infection and Immunity 2005, 73, 4596–4606. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Xiao, S.; Yin, M.; Su, M.; Gao, C.; Tang, R. The self-adaptive nanosystem for implant-related infections theranostics via phase-change driven anti-biofilm and the enhancement of immune memory. Advanced Functional Materials 2023, 2302322. [Google Scholar] [CrossRef]
- Ciacotich, N.; Kragh, K.N.; Lichtenberg, M.; Tesdorpf, J.E.; Bjarnsholt, T.; Gram, L. In situ monitoring of the antibacterial activity of a copper–silver alloy using confocal laser scanning microscopy and pH microsensors. Global Challenges 2019, 3, 1900044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, X.; Tian, L.; Wang, N.; Li, Y.; Kushmaro, A.; Marks, R.; Sun, Q. Antibiofilm activity of 3, 3'-diindolylmethane on Staphylococcus aureus and its disinfection on common food-contact surfaces. Food Science and Human Wellness 2022, 11, 1222–1232. [Google Scholar] [CrossRef]
- Mootz, J.M.; Malone, C.L.; Shaw, L.N.; Horswill, A.R. Staphopains modulate Staphylococcus aureus biofilm integrity. Infection and Immunity 2013, 81, 3227–3238. [Google Scholar] [CrossRef]
- Anjum, A.; Chung, P.-Y.; Ng, S.-F. PLGA/xylitol nanoparticles enhance antibiofilm activity via penetration into biofilm extracellular polymeric substances. RSC Advances 2019, 9, 14198–14208. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Lee, J. Inhibition of Staphylococcus aureus biofilm formation and virulence factor production by petroselinic acid and other unsaturated C18 fatty acids. Microbiology Spectrum 2022, 10, e01330–01322. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.; Miranda-Hernandez, S.; Rush, C.; Warner, J.; Eisen, D.P. Effect of savirin in the prevention of biofilm-related Staphylococcus aureus prosthetic joint infection. Frontiers in Pharmacology 2022, 13, 989417. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.A.; Souza, R.R.; Bonelli, R.R.; Américo, M.A.; Fracalanzza, S.E.L.; Figueiredo, A.M.S. Comparison of in vitro and in vivo systems to study ica-independent Staphylococcus aureus biofilms. Journal of Microbiological Methods 2012, 88, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Kleine, D.; Chodorski, J.; Mitra, S.; Schlegel, C.; Huttenlochner, K.; Müller-Renno, C.; Mukherjee, J.; Ziegler, C.; Ulber, R. Monitoring of biofilms grown on differentially structured metallic surfaces using confocal laser scanning microscopy. Engineering in Life Sciences 2019, 19, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Reichhardt, C.; Parsek, M.R. Confocal laser scanning microscopy for analysis of Pseudomonas aeruginosa biofilm architecture and matrix localization. Frontiers in Microbiology 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Madec, J.-Y.; Bousquet-Mélou, A.; Haenni, M.; Ferran, A.A. Destruction of Staphylococcus aureus biofilms by combining an antibiotic with subtilisin A or calcium gluconate. Scientific Reports 2021, 11, 6225. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-Q.; Li, T.-T.; Li, H.-Q. Biofilm formation monitored by confocal laser scanning microscopy during startup of MBBR operated under different intermittent aeration modes. Process Biochemistry 2018, 74, 132–140. [Google Scholar] [CrossRef]
- Aviner, R. The science of puromycin: from studies of ribosome function to applications in biotechnology. Computational and Structural Biotechnology Journal 2020, 18, 1074–1083. [Google Scholar] [CrossRef]
- Lawana, V.; Korrapati, M.; Mehendale, H.; Wexler, P. Encyclopedia of Toxicology, 3rd ed.; Academic Press: London, England, 2014. [Google Scholar]
- Rose, N.L.; Palcic, M.M.; Helms, L.M.; Lakey, J.R. Evaluation of Pefabloc as a serine protease inhibitor during human-islet isolation. Transplantation 2003, 75, 462–466. [Google Scholar] [CrossRef]
- Liu, K.; Liu, J.; Zou, B.; Li, C.; Zeh, H.J.; Kang, R.; Kroemer, G.; Huang, J.; Tang, D. Trypsin-mediated sensitization to ferroptosis increases the severity of pancreatitis in mice. Cellular and Molecular Gastroenterology and Hepatology 2022, 13, 483–500. [Google Scholar] [CrossRef]
- Dobrzelecka, A.; Mazurkiewicz, A. Przegląd materiałów stosowanych do produkcji sztucznych ścięgien i więzadeł (The overview of materials used for artificial tendons and ligaments production). Aktualne Problemy Biomechaniki 2015, 35–40. [Google Scholar]
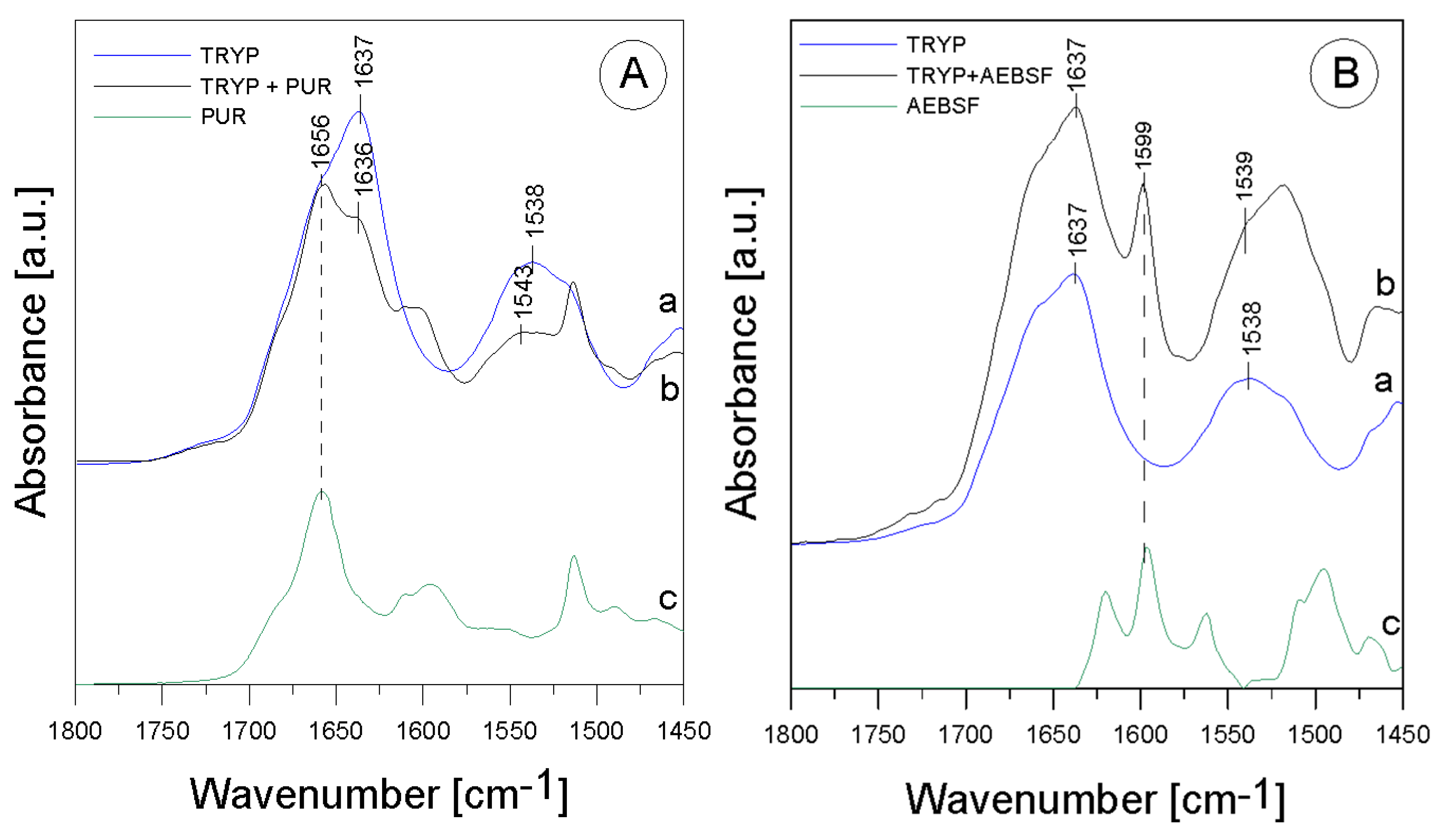


| Band [cm-1] | Origin | Characteristic |
|---|---|---|
| 1637; 1636 |
amide I group C=O (70-85%) C-N (10-20%) (β-helix) |
protein region |
| 1543; 1538; 1539 |
amide II group N-H bond bend. C-N bond str. |
protein region |
| 1656 | aromatic ring vibrations |
from PUR |
| 1599 | -C=C group str. | from AEBSF |
| Band [cm-1] | Origin | Characteristic |
|---|---|---|
| 2959; 2956; 2954 | -CH group str. |
polyethylene level |
| 2921; 2914 | -CH2 group sym. |
polytetrafluoroethylene region |
| 2850; 2846 | -CH2CH3 group str. |
polytetrafluoroethylene region |
| 1654; 1658 1627; 1624; 1622; 1629 |
amide I group C=O bond str.(70-85%) C-N bond bend. (10-20%) N-H bond bend. (α-helix and β-helix) |
protein (gelatin) region |
| 1546; 1549; 1539 |
amide II group N-H bond bend. C-N bond str. |
protein (gelatin) region |
| 1447; 1454; 1450; 1451 | -CH2 | polytetrafluoroethylene region |
| 1403; 1397 | -CH3 | carboxylate portion of polyethylene |
| 1234; 1230; 1228 | -CF2 group str. |
polytetrafluoroethylene region |
| 1200; 1201; 1198 | -CF2 group asym. str. |
polytetrafluoroethylene region |
| 1145; 1153; 1157 | -CF2 group sym. str. |
polytetrafluoroethylene region |
| 1029 | -C-O and C-C str. | carbohydrate structure of gelatin |
| Band [cm-1] | Origin | Characteristic |
|---|---|---|
| 2960; 2961; 2958 | -CH group str. |
from methylene group |
| 2921; 2915; 2922 | -CH2 group sym. |
from polyester |
| 2849; 2852 | -CH2 group str. |
from polyester |
| 1709 | -C=O group sym. |
carbonyl group from polyester |
| 1627; 1632; 1656; 1663 |
amide I group C=O bond str. (70-85%) N-H bond bend. (β-helix) |
protein (gelatin) region |
| 1549; 1552 |
amide II group N-H bond bend. C-N bond str. |
protein (gelatin) region |
| 1452; 1448 | -CH group def. |
from polyester |
| 1404; 1405; 1406; 1408 | -OH group |
carboxylate portion of polyester fiber |
| 1238; 1201; 1198; 1170; 1165; 1161; 1163; 1082; 1079 | -CO group |
carbonyl group in polyester chains |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





