1. INTRODUCTION
An oral aqueous solution (Fintepla
®) of
d,l-fenfluramine HCl (referred to hereafter as fenfluramine) was approved in 2020 in the US and Europe for the treatment of seizures associated with Dravet syndrome and Lennox-Gastaut syndrome in patients aged 2 years and older.
1,2 Administration of fenfluramine results in four active chiral chemical entities being present in the systemic circulation, namely
d- and
l-fenfluramine and
d- and
l-norfenfluramine (
Figure 1s).
3 Although differences in the pharmacokinetics and pharmacodynamics of fenfluramine and its primary active metabolite
d,l-norfenfluramine HCl (referred to hereafter as norfenfluramine) have been known for decades,
4-10 it is remarkable that recent articles on fenfluramine’s clinical pharmacology, mechanisms of action and drug interactions consider the medication as if it were a single molecular entity.
11-15 Fenfluramine and its d-enantiomer had been marketed in the past as appetite suppressants, and both were withdrawn from the market in 1997 after being found to cause valvular heart disease and pulmonary hypertension.3 Although to date no cardiovascular toxicity has been reported in patients with epilepsy treated with fenfluramine, the drug is currently available in the US and Europe through a controlled access program requiring echocardiogram assessments before, during and after treatment.1,2
Available evidence indicates that appetite suppression and cardiovascular toxicity are mediated by different serotonergic mechanisms. In particular, appetite suppression can be ascribed mainly to the d-enantiomers of fenfluramine and norfenfluramine,5,9 whereas cardiovascular toxicity can be ascribed mainly to activation of 5-HT2B receptors by d-norfenfluramine.5,7,16-19 d-Norfenfluramine and l-norfenfluramine are also the major circulating metabolites of the fenfluramine analogue benfluorex ((±)-N-(2-benzoyloxyethy1)-norfenfluramine, Mediator®), which was approved in France as an adjuvant antidiabetic and withdrawn in 2009 due to its association with valvular heart disease and pulmonary hypertension. 3,18-20
With respect to antiseizure activity in preclinical models, fenfluramine has been found to protect against maximal electroshock seizures (MES) and audiogenic seizures in rodents.21-23 It has also been shown to inhibit seizure-related locomotor activity and brain epileptiform discharges in zebrafish models of Dravet syndrome.24 We recently reported the results of a comparative assessment of the antiseizure activity of the individual enantiomers of fenfluramine and norfenfluramine in after intraperitoneal (i.p.) administration in rats.25 Median effective doses (ED50) in the MES test were 8.4 mg/kg for d-fenfluramine, 13.4 mg/kg for l-fenfluramine and 10.2 mg/kg for l-norfenfluramine. A median effective dose for d-norfenfluramine could not be determined due to dose-limiting neurotoxicity. Because of the evidence linking d-fenfluramine and d-norfenfluramine to cardiovascular toxicity, these findings justify interest in developing l-fenfluramine or l-norfenfluramine as a potential, enantiomerically pure follow-up compound to the marketed fenfluramine.26,27
The objective of the present study was to assess the comparative anticonvulsant activity of fenfluramine, norfenfluramine and their individual enantiomers in the MES test in mice. Additional experiments were conducted to assess the antiseizure effects of fenfluramine, norfenfluramine and their individual l-enantiomers in the DBA/2 mouse model of audiogenic seizures, and their correlation with the concentration of the same compounds in plasma and brain. Together with the results of our previous pharmacodynamics/pharmacokinetic analysis in rats, 25,28 these data will assist in determining whether l-fenfluramine or l-norfenfluramine is a better candidate compound for further development as a follow-up to the marketed racemic-fenfluramine.
2. MATERIALS AND METHODS
2.1. Materials
Fenfluramine HCl (lot no. 10-JKL-74-1), d-fenfluramine HCl (lot no. 10-DHL-18-1) and l-fenfluramine HCl (lot no. 10-DHL-26-1) were obtained from Toronto Research Chemicals (North York, ON, Canada). Norfenfluramine HCl (lot no. CD-890-72) d-norfenfluramine HCl (lot no. MRA-879-77) and l-norfenfluramine HCl (lot no. TC-055-208 and TC-150-097) were obtained from BioVectra (Charlottetown, PE, Canada) and sodium valproate (lot n. MKCD3350) from Sigma Aldrich (Oakville, ON, Canada). Mice for the MES studies were purchased from Charles River (Kingston, NY, USA). DBA/2 mice were obtained from Charles River (Kingston, NY, USA) for studies in Canada and from Janvier Laboratories (Le Genest-Saint-Isle, France) for studies in France.
All doses of fenfluramine, norfenfluramine and their individual enantiomers are expressed as the HCl salt. All concentrations are expressed as free base.
2.2. Assessment of antiseizure activity in the mouse MES model
All experiments were conducted in male albino CF-1 / Charles River mice (18–30 g) as part of the NIH Epilepsy Therapy Screening Program (ETSP) at the ETSP contract site at the University of Utah (Salt Lake City, UT, USA).29-31 Investigators at the testing site were blinded to the source and identity of all compounds tested. All protocols involving the use of animals were approved by the Institutional Animal Care and Use Committee at the University of Utah.
Test compounds were administered i.p.. Details of the experimental procedure have been described before.25 After an exploratory assessment of dose-response relationships and time course of activity, ED50 values with 95% confidence intervals (95%CI) were determined by testing 4 to 5 doses, each in 8 animals, at the time of peak effect. The protective index (PI) was calculated as the ratio between the median minimal neurotoxic dose (TD50) and the ED50 at the time of peak effect. Determination of the TD50 was based on assessment of minimal motor impairment (MMI) in the rotarod test as detailed in our previous report.25
2.3. Assessment of antiseizure activity in the DBA/2 mouse model of audiogenic seizures
The audiogenic seizure assay was conducted at 1 hour after dosing, except for sodium valproate (active control) which was assessed at 0.5 h after dosing. All compounds were administered i.p., dissolved in 0.9% sodium chloride in water. The volume injected was 10 mL/kg for all compounds and doses. The study was conducted at two sites, Xenon Pharmaceuticals Inc. (Vancouver, BC, Canada) and Porsolt (Le Genest-Saint-Isle, France). The two sites evaluated different treatments using virtually identical experimental protocols. Protocols involving the use of animals at Xenon Pharmaceuticals Inc. were approved by the Xenon Animal Care Committee and followed the guidelines of the Canadian Council on Animal Care (CCAC). Studies performed at Porsolt were done at an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited facility.
The Canadian site tested l-norfenfluramine (0.5-20 mg/kg), norfenfluramine (0.3-15 mg/kg) and fenfluramine (15 mg/kg). Male DBA/2 mice (7.6-18.5 g, 3-4 weeks old) were individually placed in a clear acrylic cubic box with lid (40.6 cm each side) and allowed to explore the box freely for 1 minute. After this period of habituation, an auditory stimulus was generated by an electrical bell at an intensity of about 105 dB for a maximum duration of 60 seconds or until mice exhibited a tonic seizure.
The French site tested l-fenfluramine (8.7-34.7 mg/kg), mg/kg), fenfluramine (17.4 and 34.7 mg/kg) and sodium valproate (180 mg/kg). Male DBA/2 mice (5-10 g, 3-4 weeks old) were placed in a Plexiglas jar (diameter 40 cm; height 35 cm) mounted with an electric bell (105-120 dB). The bell was activated for a maximum duration of 60 seconds unless death occurred earlier.
At both sites, seizure-related behavior after sound stimulation was evaluated for 10 min according to the method described by Dürmüller et al. 1993.32 Specifically, the score was 0 for no seizure, 1 for wild running, 2 for clonic convulsion, 3 for tonic extension, and 4 for death. Soon after completion of the experiment, samples of blood and brain were obtained for pharmacokinetic analysis (see below). Experimenters were blind to the treatment conditions, except for sodium valproate (dosed 30 min prior to seizure induction).
2.4. Assay of fenfluramine and norfenfluramine in biological samples
2.4.1. Sample collection and preparation
At the Canadian site, DBA/2 mice were anesthetized by isoflurane inhalation soon after the completion of the antiseizure activity testing. A 0.5 mL of blood was collected from the heart, deposited into a K2EDTA tube and stored on ice. Animals were then euthanized by cervical dislocation and their brains were removed, placed into pre-weighed vials, and snap frozen on dry ice. At the end of the sample collection, blood was centrifuged at 4000 rpm for 10 minutes at 4°C, and the plasma pipetted into a labeled tube. All samples were stored in a -80°C freezer until bioanalysis.
At the French site, animals were decapitated at the completion of each experiment. The brain was quickly removed and rinsed with physiological saline. The whole brain and individual hemispheres were weighed, placed in separated pre-labelled vials and snap frozen in liquid nitrogen (or equivalent). The vials were stored at -80°C until shipment on dry ice to Xenon Pharmaceuticals for the measurement of fenfluramine, l-fenfluramine, norfenfluramine and l-norfenfluramine concentrations.
2.4.2. Quantification of norfenfluramine and l-norfenfluramine in plasma
Extraction of plasma samples was carried out by protein precipitation using acetonitrile. Fifty microliters of plasma sample were mixed with 50 μL of internal standard solution in 1:1 acetonitrile: water (v/v) followed by the addition of 50 μL of 6% (v/v) phosphoric acid in water and 200 μL of acetonitrile. The mass of the internal standard and mass fragments used for detection as a transition were 203.96/159 m/z. Samples were vortexed for 30 seconds, then centrifuged at 13,000 rpm for 20 minutes. Fifty microliter aliquots of each supernatant were added into a 96-well plate followed by the addition of 1:1 acetonitrile: water (v/v) to bring the final volume to 250 μL in the well plate. After mixing, the plate was further centrifuged at 4000 rpm for 20 minutes. The samples were analyzed by ultra-high pressure liquid chromatography electrospray ionization tandem mass spectrometry (UHPLC-ESI-MS/MS) using a Sciex TQ-5500 triple quadrupole mass spectrometer equipped with a Shimadzu Nexera UHPLC pump and auto-sampler system using an ACE Excel C18-PFP, 50 x 2.1 mm, 2 μm particle size column and gradient elution consisting of solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in acetonitrile). The analytes (norfenfluramine and its l-enantiomer) were detected by electrospray in the positive ion mode.
K2EDTA blank mouse plasma purchased from Valley Biomedical, California, USA, was used to prepare standards and quality control (QC) samples for plasma quantitation and as surrogates for brain homogenate quantitation. Twelve points calibration samples were prepared by spiking the corresponding analytes into blank mouse plasma resulting in a concentration range from 2.34 ng/mL to 4800 ng/mL. QC samples were prepared at 14.0 ng/mL, 225 ng/mL, and 3600 ng/mL and analyzed in triplicate. Sample concentrations were determined using a linear calibration function, weighted 1/x or 1/x2, or a quadratic calibration function, weighted 1/x2, generated by the regression of the ratio of peak area of the analyte to the peak area of its corresponding internal standard against the concentration of the analytes in the standard samples.
2.4.3. Quantification of fenfluramine, norfenfluramine and their l-enantiomers in brain
Bead mill tubes containing brain tissues were thawed at room temperature and 2 mL of homogenization solvent (water: acetonitrile (1:1, v/v) was added. The tubes were placed in the Omni Bead Mill Homogenizer (Bead Ruptor Elite Model) and were shaken at a velocity of 3.70 m/s for a single cycle lasting 30 seconds, with a second cycle if the homogenate was not uniform. The homogenized tubes were centrifuged at 4000 rpm for 20 minutes, the supernatants transferred to 1.5 mL Eppendorf tubes and stored frozen at -80°C until analysis. The supernatants were assayed as described for supernatants obtained from plasma (section 2.3.3) except that fenfluramine and its l-enantiomer were added to the calibrators and assayed together with metabolically formed norfenfluramine and l-norfenfluramine.
2.5. Assessment of concentration-response relationships and statistical analysis
Concentration response curves were generated using the following Hill-Langmuir equation33:
E= B + (T - B) Cn / (IC50n + Cn),
where:
E=response
B = bottom of the maximal effect (Emax) set as 0.
T = top of the maximal effect (Emax) set as 4.
C=concentration
n= the Hill coefficient or steepness factor, constrained to less than zero.
IC50 = concentration that reduces the response by half.
All data are expressed as mean ± SD. Between-group differences were analyzed using one-way ANOVA with Dunnett’s post-hoc test. Statistical significance was set at p<0.05. All statistics were calculated using Microsoft Excel and GraphPad Prism version 9.
3. RESULTS
3.1. Antiseizure activity and protective index in the MES model in mice
Fenfluramine, norfenfluramine and their individual enantiomers were active in protecting mice against electrically-induced seizures at doses ranging from 4 to 30 mg/kg. Median effective doses (ED50) were assessed at time of peak seizure protection, which occurred at 6 h after dosing for all compounds with the exception of l-fenfluramine and l-norfenfluramine, for which the time to peak seizure protection was 4 h and 8 h, respectively. By contrast, peak toxicity (MMI scores) in the rotarod test developed early after dosing and median toxic doses (TD50) were assessed at 0.25 h after dosing for all compounds.
As shown in
Table 1, fenfluramine,
d-fenfluramine and
l-fenfluramine were equipotent in terms of seizure protection, but the
l-enantiomer was better tolerated and consequently had a higher PI. With respect to norfenfluramine, the
d-enantiomer was more potent than the
l-enantiomer, but also more toxic and the PI tended to favor
l-norfenfluramine.
For comparison purposes,
Table 1 also shows results in the MES test for the same compounds in rats, based on a previous publication.
25 In rats, all enantiomers had similar ED
50 values, except for
d-norfenfluramine which was more potent but also more toxic.
3.2. Studies in the DBA/2 mouse model of audiogenic seizures
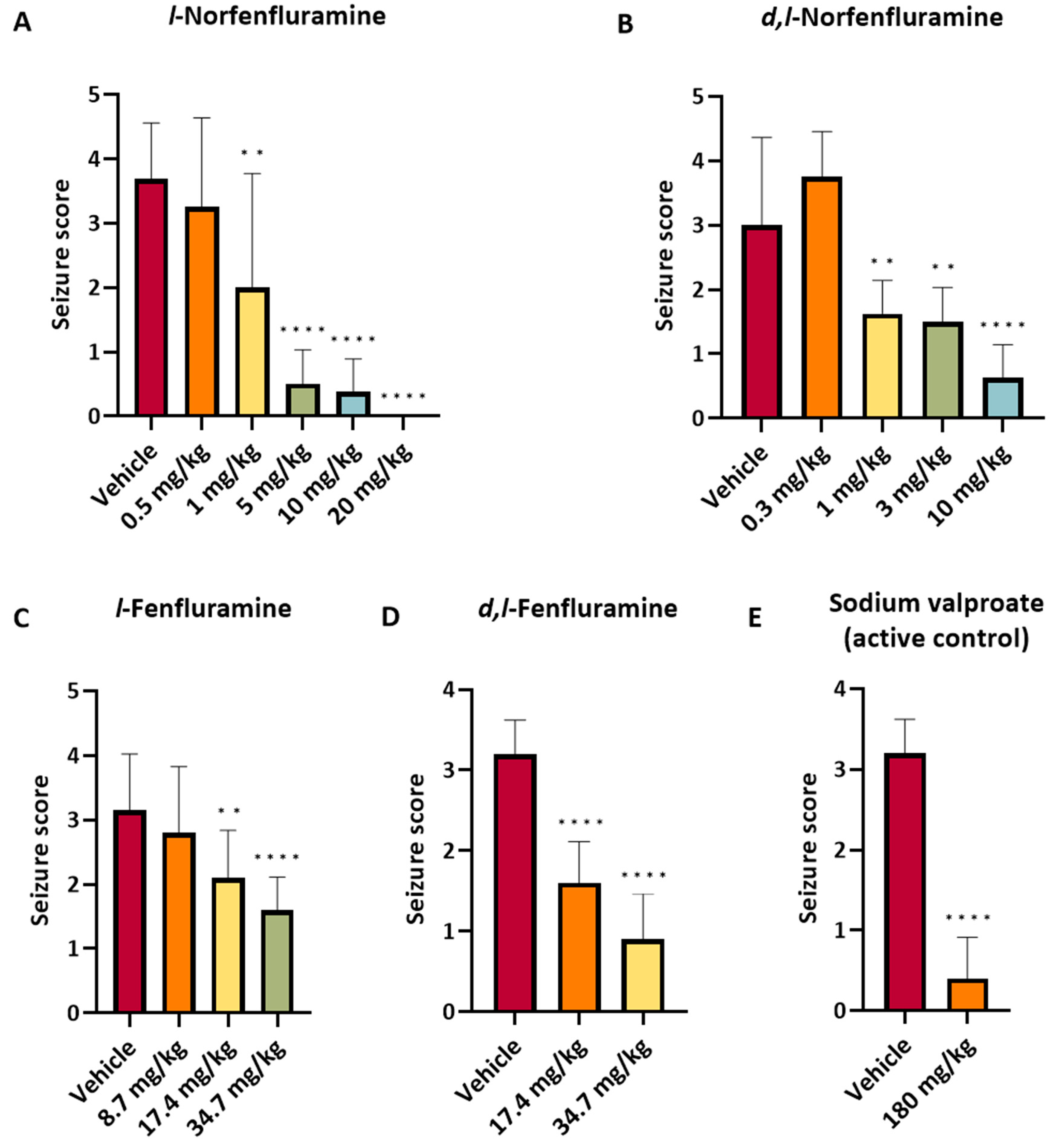
All compounds tested caused a dose-dependent protection against audiogenic seizures (
Table 2 and
Figure 1). ED
50 values of norfenfluramine and
l-norfenfluramine were almost identical (1.3 and 1.2 mg/kg, respectively) and greater than the ED
50 values of fenfluramine and
l-fenfluramine respectively (10.2 and 17.7 mg/kg, respectively) (
Table 3). Of note,
l-norfenfluramine at the dose of 20 mg/kg was the only compound that fully suppressed seizures in all 8 animals tested. The active control sodium valproate (180 mg/kg) exerted a degree of seizure protection similar to that observed with 0.5 and 10 mg/kg
l-norfenfluramine, and 10 mg/kg norfenfluramine (
Figure 1).
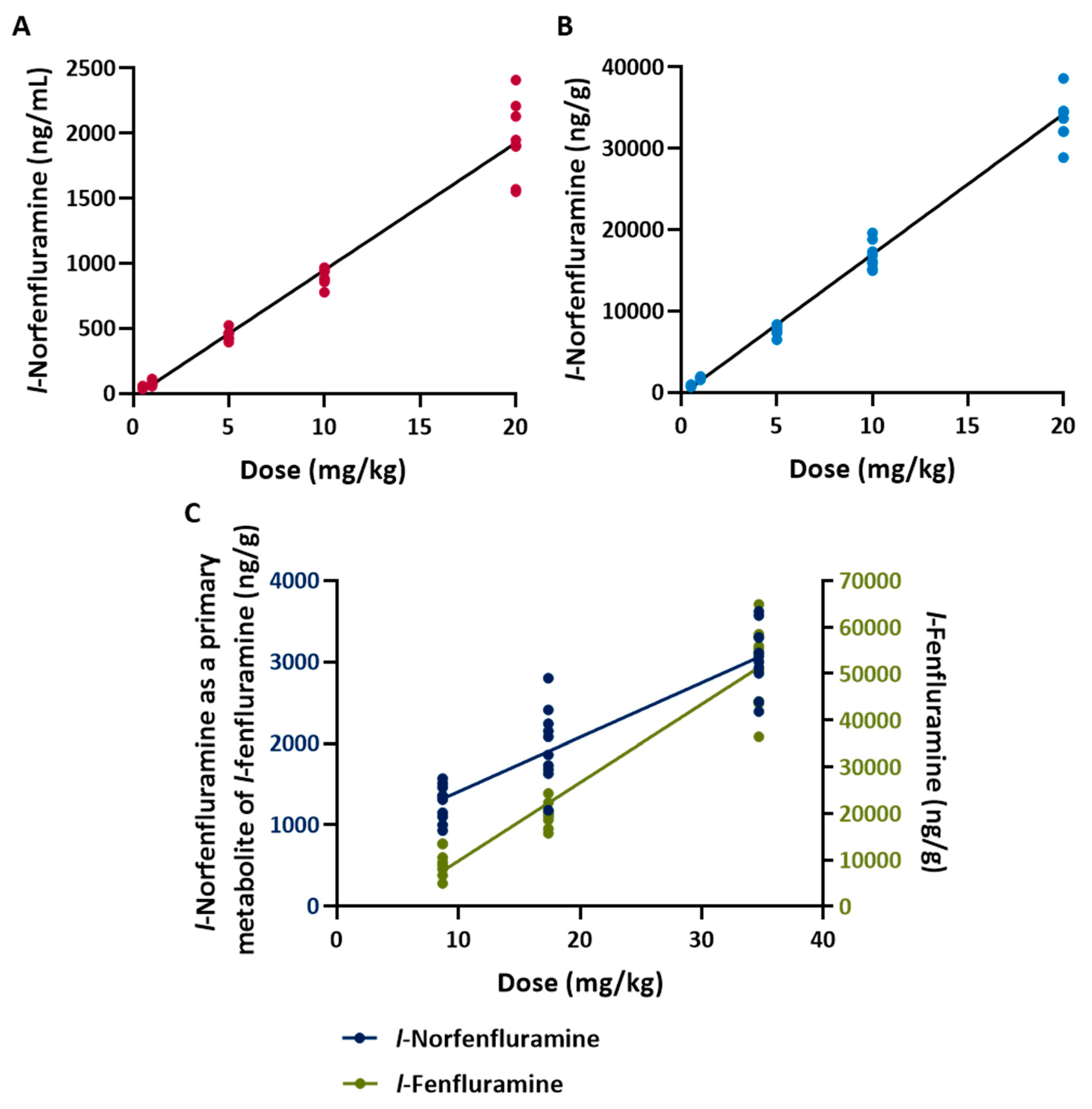
The concentrations of each of the compounds tested (including the concentrations of metabolically formed norfenfluramine and
l-norfenfluramine in animals treated with fenfluramine and its
l-enantiomer, respectively) measured in brain samples collected soon after completion of audiogenic seizure testing are reported in
Table 2. Irrespective of the dose administered, fenfluramine, norfenfluramine and
l-norfenfluramine were found in brain tissue at concentrations about 20-fold higher than in plasma (
Table 2). After administration of fenfluramine and
l-fenfluramine, the concentration of metabolically formed norfenfluramine and
l-norfenfluramine in brain was about 20-fold higher than the brain concentration of the parent compound (
Table 2). For all compounds tested, brain concentrations appeared to be linearly related to dose (
Table 2 and
Figure 2).
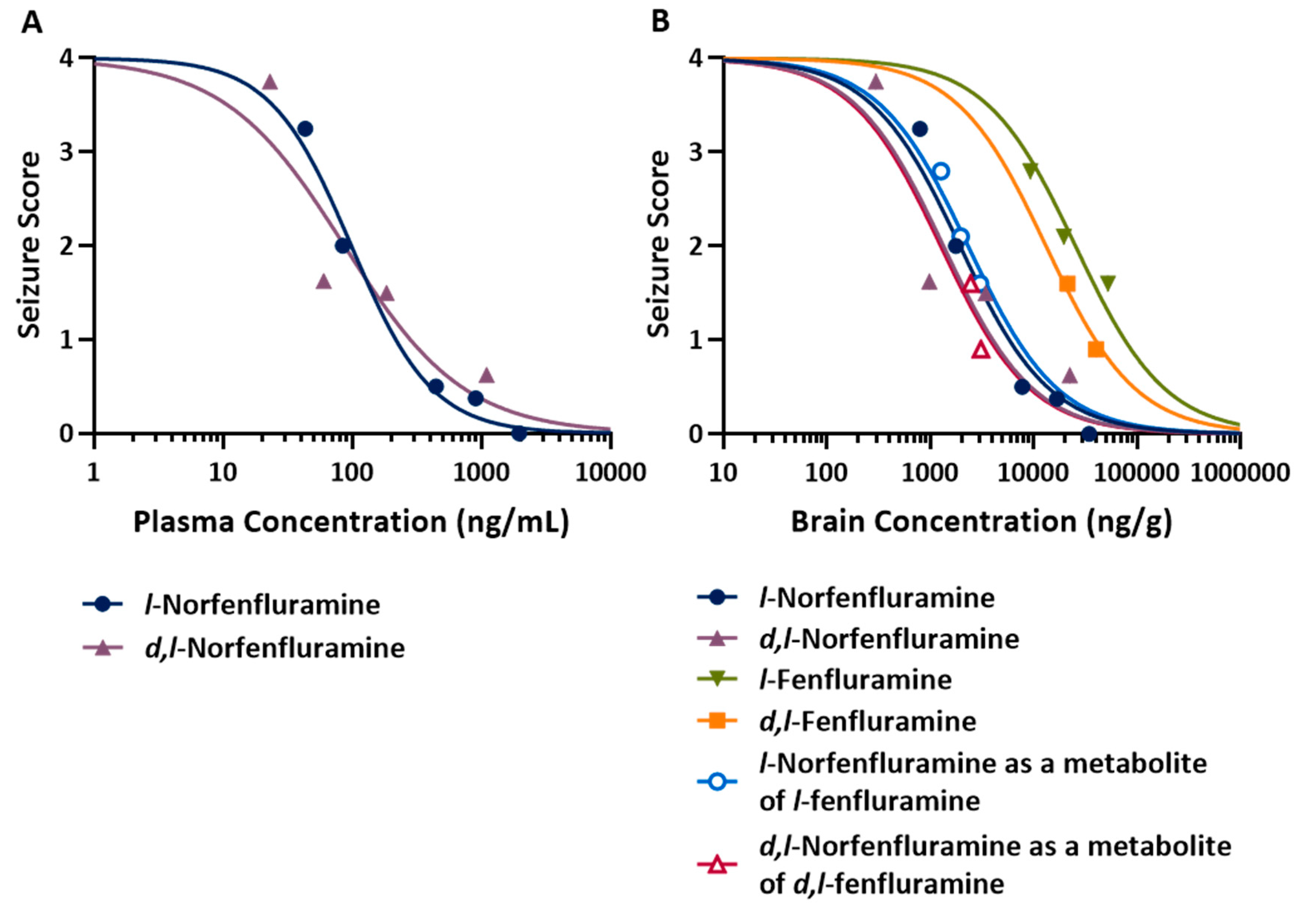
Assessment of the concentration-response relationship (
Figure 3) allowed calculation of median effective concentrations (EC
50) in both plasma and brain tissue. Because plasma samples were not collected in dose-response experiments with fenfluramine and
l-norfenfluramine, plasma EC
50 values could be determined only for norfenfluramine and its
l-enantiomer, and were found to be similar for both compounds (81 and 101 ng/mL respectively), and much lower than their EC
50 in brain (1340 and 2330 ng/mg respectively). The antiseizure potencies of norfenfluramine and
l-norfenfluramine as estimated by EC
50 values in brain were 10- and 13-fold higher than those of fenfluramine and
l-fenfluramine, respectively (
Table 3). Of note, brain EC
50 values for metabolically formed norfenfluramine and
l-norfenfluramine were very similar to brain EC
50 values of the same compounds administered as such (
Table 3).
4. DISCUSSION
Clarification of potential differences in antiseizure activity and safety profile of the individual enantiomers of fenfluramine and norfenfluramine has important implications, because it could provide the rationale for developing a single enantiomer as a novel molecular entity with a better therapeutic index than the marketed racemic-fenfluramine. The evidence linking d-fenfluramine and d-norfenfluramine to cardiovascular adverse effects (and the withdrawal of d-fenfluramine from the market due to these complications) 5,7,16-18may justify a chiral switch approach 26,27,34 aimed at developing l-fenfluramine or l-norfenfluramine as safer alternatives to fenfluramine. Assessing the comparative antiseizure activity of fenfluramine, norfenfluramine and their l-enantiomers is a key step in determining the viability of this approach.
To date, few studies have investigated the antiseizure effects of l-fenfluramine and l-norfenfluramine in seizure models. In the MES test in rats, all enantiomers of fenfluramine and norfenfluramine have been found to be active in protecting against seizures, with a potency comparable to that of the corresponding racemates. 25 Our results show that in the MES test in mice, ED50 values of individual enantiomers are similar to those obtained previously in rats, including a trend for d-norfenfluramine to be more potent but also more toxic. Both in rats and in mice, ED50 values were assessed at the time of peak seizure protection, which differed between species. In rats, peak antiseizure effects occurred at 15 to 30 min after dosing (except for d-fenfluramine with a peak effect at 2 h), 25 whereas in mice the peak effect was observed at 4-8 h after dosing. In contrast, peak effect for neurotoxic manifestations (MMI) was shorter and occurred between 15-30 min in both species.
A limitation of the MES studies summarized above is that no measurements were made of plasma and brain concentrations of the test compounds at the time at which response was assessed. Our subsequent experiments conducted to compare l-fenfluramine and l-norfenfluramine with the corresponding racemic compounds in the DBA/2 mouse model of audiogenic seizures assessed not only dose-response relationships, but also concentration-response relationships. ED50 values for l-norfenfluramine and norfenfluramine in this model were almost identical (1.2 and 1.3 mg/kg). Based on ED50, l-norfenfluramine and norfenfluramine were 15 and 8 times more potent, respectively, than l-fenfluramine and fenfluramine. This finding is interesting, because in the MES model those compounds showed similar anticonvulsant potency. A brain-to-plasma concentration ratio close to 20 for fenfluramine, norfenfluramine and their l-enantiomers is similar to the brain-to-plasma exposure (AUC) ratio previously reported for the same compounds in rats25, indicating rapid and extensive brain penetration in both species. Based on brain EC50 values, norfenfluramine is 10 times more potent than fenfluramine, and l-norfenfluramine is 13 times more potent than l-fenfluramine, in protecting against audiogenic seizures. The fact that the brain EC50 of l-norfenfluramine administered as such (1,940 ng/g) was similar to that of metabolically formed l-norfenfluramine after administration of l-fenfluramine (2,330 ng/g), strongly suggests that the antiseizure activity of l-fenfluramine in this model can be mainly ascribed to its primary metabolite. Likewise, the metabolite norfenfluramine appears to be the main determinant of the antiseizure effect observed after administration of fenfluramine. Of note, the plasma EC50 of d,l-norfenfluramine in the DBA/2 audiogenic seizure model (81 ng/mL) is well within the plasma concentration range (2.6-149.6 ng/mL) reported in patients with Dravet syndrome and other developmental and epileptic encephalopathies treated with fenfluramine in routine clinical practice.11
Overall, the findings in the MES model in rats and mice and the audiogenic seizure model in DBA/2 mice provide clear evidence that both l-fenfluramine and l-norfenfluramine possess antiseizure activity, and therefore support the rationale for their clinical development as enantiomerically pure ASMs. While the MES studies did not permit direct assessment of the relative contribution of metabolically formed norfenfluramine and l-norfenfluramine to the activity of fenfluramine and l-fenfluramine, the findings in DBA/2 mice provide strong evidence that the metabolite was responsible for the seizure protection observed after administration of the parent compound. The results in DBA/2 mice support the development of l-norfenfluramine preferably to l-fenfluramine, although it is unclear to what extent the same findings can be extrapolated to other models or to the clinical setting. While the MES test is generally considered predictive of activity against generalized tonic-clonic seizures and focal-to-bilateral tonic-clonic seizures, 35, the specificity of the audiogenic seizure model in predicting spectrum of activity against different seizure types is less clearly defined.36 Both enantiomers of fenfluramine and norfenfluramine have been found to be inactive in the 6 Hz seizure model in mice. 25 Only one study evaluated fenfluramine and norfenfluramine enantiomers in a model designed to mimic Dravet syndrome. In the zebrafish scn1Lab-/- mutant model of Dravet syndrome, all fenfluramine and norfenfluramine enantiomers were found to be similarly effective in inhibiting behavioural (locomotor) epileptic activity, but at the concentrations tested (up to 100 μM) l-norfenfluramine differed from the other enantiomers in failing to reduce the frequency and duration of epileptiform events in local field potential recordings.24 In view of these findings, studies on the comparative activity of l-fenfluramine and l-norfenfluramine in other seizure models would be desirable.
There is sound evidence linking the cardiovascular adverse effects of fenfluramine to stimulation of 5-HT2B receptors.5,7,16-18 While fenfluramine per se does not bind significantly to 5-HT2B receptors, d-norfenfluramine activates these receptors with at least 10-fold greater potency compared with l-norfenfluramine.16,37-39 In addition to minimizing the risk of cardiovascular adverse effects, l-norfenfluramine (or l-fenfluramine) as a safer follow-up compound to fenfluramine could have additional advantages. In particular, the appetite suppressant effects of fenfluramine are known to be mediated at least in part by activation of 5-HT2c receptors by the metabolite norfenfluramine.3,40 Because l-norfenfluramine is at least 3-times less potent than d-norfenfluramine in activating this receptor,16 it would be expected to have a lower potential to induce anorexia and weight loss.
As an indication of the interest in developing individual enantiomers as follow-up compounds to fenfluramine, at least two companies have filed patent applications for the use of the enantiomers of fenfluramine and norfenfluramine as antiseizure agents. 3 On June 28, 2023 the European Patent Office issued a Notice of Intent to grant a patent on the use of l-norfenfluramine in epilepsy.41 The comparative antiseizure activity of l-norfenfluramine and l-fenfluramine has been discussed above. In addition to the data in the audiogenic seizure model, arguments favoring the preferential development of l-norfenfluramine over l-fenfluramine include the advantage of a single active molecular entity, a longer half-life consistent with once-daily dosing, 6 and a lower susceptibility to enzyme inhibition- or induction-based drug-drug interactions.12 Compared to l-fenfluramine, l-norfenfluramine would be expected to have a lower pharmacokinetic variability, due to minimal cytochrome P450 (CYP)-mediated metabolism and avoidance of the CYP2D6 genetic polymorphism affecting fenfluramine’s disposition.42
Acknowledgments
This work is abstracted from the Ph.D. thesis of Natalia Erenburg in partial fulfillment of the Ph.D. degree requirements for The Hebrew University of Jerusalem. We thank Brian Klein and Raol Yogendra at the NIH-NINDS-Epilepsy Therapy Screening Program (ETSP) for the seizure and minimal motor impairment (MMI) testing of the compounds in the mice-MES model. We further thank Girish Bankar, Kate Montgomery and Luis Bettio from Xenon Pharmaceuticals, Inc., for their contributions to data collection and analysis of the audiogenic seizure assay, and Luis Sojo and team for bioanalytical quantification of compounds in brain and plasma samples. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. There was no financial support for this research.
Conflicts of Interest
NE has nothing to disclose. JB, CD, NW and RS are employees of Xenon Pharmaceuticals Inc.. EP received speaker’s or consultancy fees from Eisai, Janssen, PMI Life Sciences, Sanofi group of companies, Shackelford Pharma, Sintetica, SKL Life Science, Takeda, UCB Pharma, Xenon Pharmaceuticals, and royalties from Wiley, Elsevier, and Wolters Kluwers. MB received consultancy fees from Clexio Therapeutics, Meditec (Sam-On), Pharma2B, Selene Therapeutics, Shackelford Pharma, US WorldMeds and Xenon Pharmaceuticals.
References
- Fintepla (fenfluramine) oral solution. Prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/212102s003lbl.pdf (accessed October 26, 2023).
- Fintepla (2.2 mg/ml oral solution) (2022). Summary of Product Characteristics (last updated 26 July 2022). Available at: https://www.ema.europa.eu/en/documents/product-information/fintepla-epar-product-information_en.pdf (accessed October, 26, 2023).
- Odi R, Invernizzi RW, Gallily T, Bialer M, Perucca E. Fenfluramine repurposing from weight loss to epilepsy: What we do and do not know. Pharmacol Ther 2021, 226, 107866. [Google Scholar] [CrossRef] [PubMed]
- Caccia S, Ballabio M, De Ponte P. Pharmacokinetics of fenfluramine enantiomers in man. Eur J Drug Metab Pharmacokinet 1979, 4, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Garattini S, Caccia S, Mennini T, Samanin R, Consolo S, Ladinski H. Biochemical pharmacology of the anorectic drug fenfluramine; a review. Curr Med Res Opin 1979, 1 suppl 6, 15–27.
- Caccia S, Conforti I, Duchier J, Garattini S. Pharmacokinetics of fenfluramine and norfenfluramine in volunteers given D-and DL-fenfluramine for 15 days. Eur J Clin Pharmacol. 1985, 9, 221–224. [Google Scholar]
- Mennini T, Garattini S, Caccia S. Anorectic effect of fenfluramine isomers and metabolites: Relationship between brain levels and in vitro potencies on serotonergic mechanisms. Psychopharmacology 1985, 85, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi R, Berettera C, Garattini S, Samanin R. D- and L-isomers of fenfluramine differ markedly in their interaction with brain serotonin and catecholamines in the rat. Eur J Pharmacol. 1986, 120, 9–15.
- Hirsch JA, Goldberg S, Wurtman RJ. Effect of (+)- or (-)-enantiomers of fenfluramine or norfenfluramine on nutrient selection by rats. J Pharm Pharmacol. 1982, 34, 18–21. [Google Scholar]
- Spinelli R, Fracasso C, Guiso G, Garattini S, Caccia S. Disposition of (-)-fenfluramine and its active metabolite, (-)-norfenfluramine in rat: a single dose-proportionality study. Xenobiotica. 1988, 18, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Schoonjans A-S, Roosens L, Dewals W, Paelinck BP, Ceulemans B. Therapeutic drug monitoring of fenfluramine in clinical practice: Pharmacokinetic variability and impact of concomitant antiseizure medications. Epilepsia 2022, 63, 686–696. [Google Scholar] [CrossRef]
- Martin P, Czerwiński M, Limaye PB, Muranjan S, Ogilvie BW, Smith S, Boyd B. In vitro evaluation of fenfluramine and norfenfluramine as victims of drug interactions. Pharmacol Res Perspect 2022, 10, e00958. [CrossRef]
- Martin P, Czerwiński M, Limaye PB, Ogilvie BW, Smith S, Boyd B. In vitro evaluation suggests fenfluramine and norfenfluramine are unlikely to act as perpetrators of drug interactions. Pharmacol Res Perspect 2022, 10, e00959. [CrossRef] [PubMed]
- Frampton, JE. Fenfluramine: A review in Dravet and Lennox-Gastaut Syndromes. Drugs 2023, 83, 923–934. [Google Scholar] [CrossRef]
- Sourbron J, Lagae L. Fenfluramine: A plethora of mechanisms. Front Pharmacol 2023. [CrossRef]
- Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, Sun JH, Link JR, Abbaszade I, Hollis JM, Largent BL, Hartig PR, Hollis GF, Meunier PC, Robichaud AJ, Robertson DW. Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol. 2000, 57, 75–81. [Google Scholar]
- Hutcheson JD, Setola V, Roth BL, Merryman WD. Serotonin receptors and heart valve disease--it was meant 2B. Pharmacol Ther. 2011, 132, 146–157.
- Szymanski C, Andrejak M, Peltier M, Marchaux S, Tribouilloy C. Adverse effects of benfluorex on heart valves and pulmonary circulation. Pharmacoepidem Drug Safety, 2014, 23, 679–686.
- Savale L, Chamais MC, Cottin V, Bergot E, Frachon I, Prevot, G. , et al. Pulmonary hypertension associated with benfluorex exposure. Eur Respir J 2012, 40, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Goldner V, Karst U. Benfluorex metabolism complemented by electrochemistry-mass spectrometry. J Pharm Biomed Anal 2023, 235, 115626. [Google Scholar]
- Martin P, White HS, Barker-Haliski ML. Evaluation of the acute anticonvulsant efficacy of fenfluramine in mouse models of acute and chronic seizures. Poster presented at the 73rd Annual Meeting of the American Epilepsy Society, Baltimore, MD, December 6-10, 2019. Available at https://zogenix.com/wp-content/uploads/2019/12/08.-FINAL-52352-AES-Martin-Mouse-Poster-2019-12-03v5.pdf (accessed December 21, 2022). 6 December.
- Silenieks LB, Carroll NK, Van Niekerk A, Van Niekerk E, Taylor C, Upton N, et al. Evaluation of selective 5-HT2C agonists in acute seizure models. ACS Chem Neurosci 2019, 10, 3284–3295. [Google Scholar] [CrossRef]
- Tupal S, Faingold CL. Fenfluramine, a serotonin-releasing drug, prevents seizure-induced respiratory arrest and is anticonvulsant in the DBA/1 mouse model of SUDEP. Epilepsia 2019, 60, 485–494. [Google Scholar] [CrossRef]
- Li J, Nelis M, Sourbron J, Copmans D, Lagae L Cabooter D, de Witte PAM. Efficacy of fenfluramine and norfenfluramine enantiomers and various antiepileptic drugs in a zebrafish model of Dravet syndrome. Neurochem Res 2021, 46, 2249–2261. [Google Scholar] [CrossRef]
- Erenburg N, Hamed R, Shaul C, Perucca E, Bialer M. Comparative activity of the enantiomers of fenfluramine and norfenfluramine in rodent seizure models, and relationship with their concentrations in plasma and brain. Epilepsia 2023, 64, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Tucker, GT. Chiral switches. Lancet 2000, 355, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Agranat I, Caner H, Caldwell J. Putting chirality to work: The strategy of chiral switches. Nature Rev Drug Discov 2002, 1, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Erenburg N, Hamed R, Shaul C, Barasch D, Perucca E, Bialer M. Pharmacokinetics of d- and l-norfenfluramine following their administration as individual enantiomers in rats. Epilepsia, in press.
- Barker-Haliski ML, Johnson K, Billingsley P, Huff J, Handy LJ, Khaleel R, et al. Validation of a Preclinical Drug Screening Platform for Pharmacoresistant Epilepsy. Neurochem Res. 2017, 42, 1904–1918. [Google Scholar] [CrossRef] [PubMed]
- Kehne JH, Klein BD, Raeissi S, Sharma S. The National Institute of Neurological Disorders and Stroke (NINDS) epilepsy therapy screening program (ETSP). Neurochem Res. 2017, 42, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Wilcox KS, West PJ, Metcalf CS. The current approach of the Epilepsy Therapy Screening Program contract site for identifying improved therapies for the treatment of pharmacoresistant seizures in epilepsy. Neuropharmacology. 2020, 166, 107811. [Google Scholar] [CrossRef] [PubMed]
- Dürmüller N, Smith SE, Meldrum BS. Proconvulsant and anticonvulsant effects of Evans blue dye in rodents. Neuroreport. 1993, 4, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Derendorf H, Schmidt S. Rowland and Tozer’s Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications. Fifth edition. Philadelphia: Wolters Kluwer; 2020; pp. 34–43.
- D’Acquarica I, Agranat I. The quest for secondary pharmaceuticals: Drug repurposing/chiral –switches combination strategy. ACS Pharmacol Trans Sci 2023, 6, 201–219. [Google Scholar]
- Castel-Branco MM, Alves GL, Figueiredo IV, Falcão AC, Caramona MM. The maximal electroshock seizure (MES) model in the preclinical assessment of potential new antiepileptic drugs. Methods Find Exp Clin Pharmacol. 2009, 31, 101–106.
- De Sarro G, Russo E, Citraro R, Meldrum BS. Genetically epilepsy-prone rats (GEPRs) and DBA/2 mice: Two animal models of audiogenic reflex epilepsy for the evaluation of new generation AEDs. Epilepsy Behav. 2017, 71 Pt B, 165–173. [CrossRef]
- Setola, V., Dukat, M., & Glennon, R.A.& Roth B.L. Molecular determinants for the interaction of the valvulopathic anorexigen norfenfluramine with the 5-HT2B receptor. MolPharmacol 2005, 68, 20–33.
- Rothman RB, Baumann MH. Serotonin releasing agents. Neurochemical, therapeutic and adverse effects. Pharmacol Biochem Behav 2002, 71, 825–836. [Google Scholar] [CrossRef]
- Kelly CR, Sharif NA. Pharmacological evidence for a functional serotonin-2B receptor in a human uterine smooth muscle cell line. J Pharmacol Exp Ther. 2006, 317, 1254–1261. [Google Scholar] [CrossRef]
- Bialer M, Perucca E. Lorcaserin for Dravet Syndrome: A Potential Advance Over Fenfluramine? CNS Drugs. 2022, 36, 113–122. [CrossRef]
- European Patent Office. Communication under Rule 71(3) EPC Application No 19750214.9-1112, ref N421012EP.
- Gross AS, Philips AC, Rieutord A, Shenfield GM. The influence of sparteine/debrisoquine genetic polymorphism on the disposition of dexfenfluramine. Brit J Clin Pharmacol 1996, 41, 311–7. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).