Submitted:
14 December 2023
Posted:
15 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
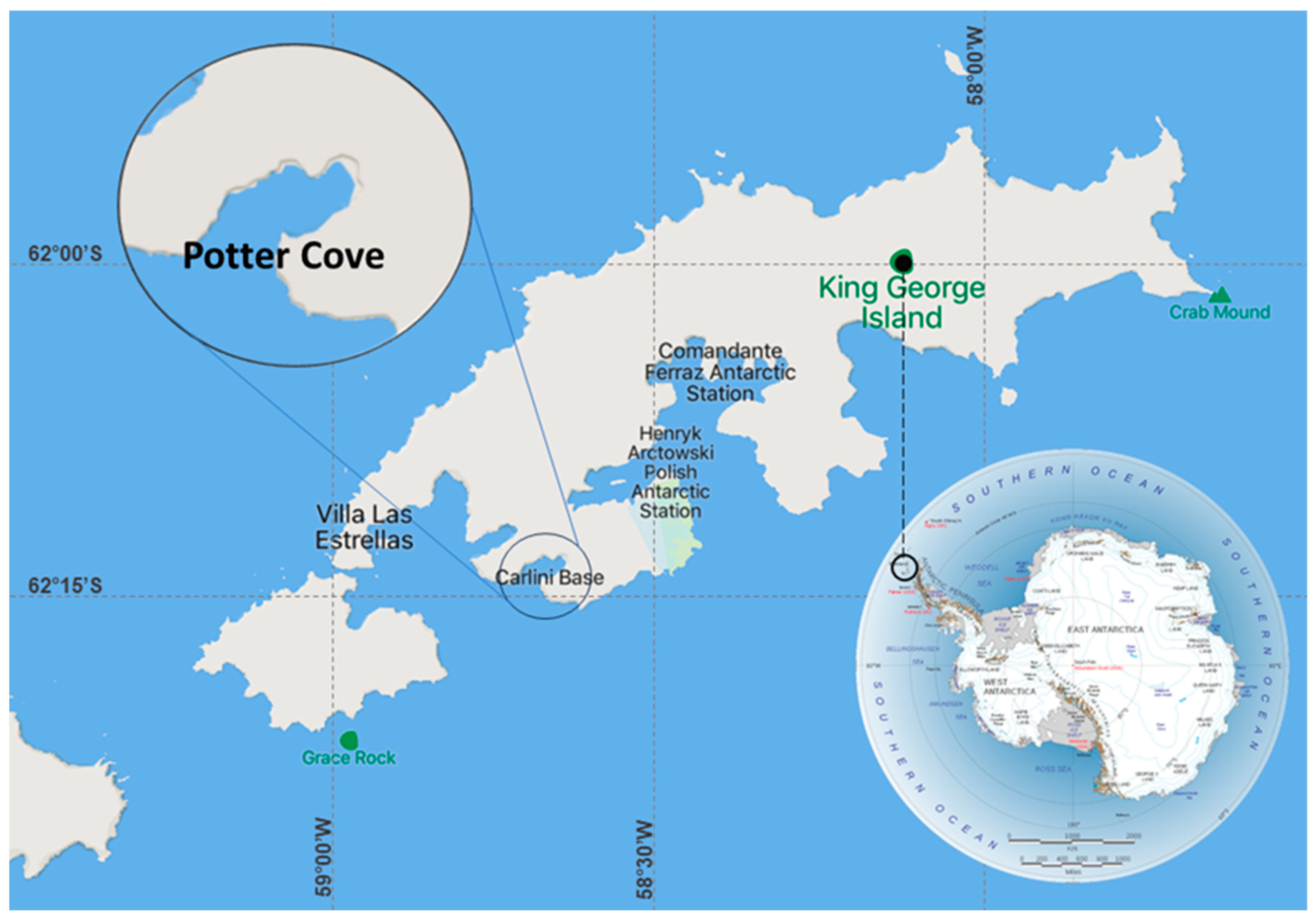
2.1. Study Site
2.2. Food web data set
2.3. Extinction simulations
2.4. Thresholds on secondary extinctions
2.5. Effect on food web properties
3. Results
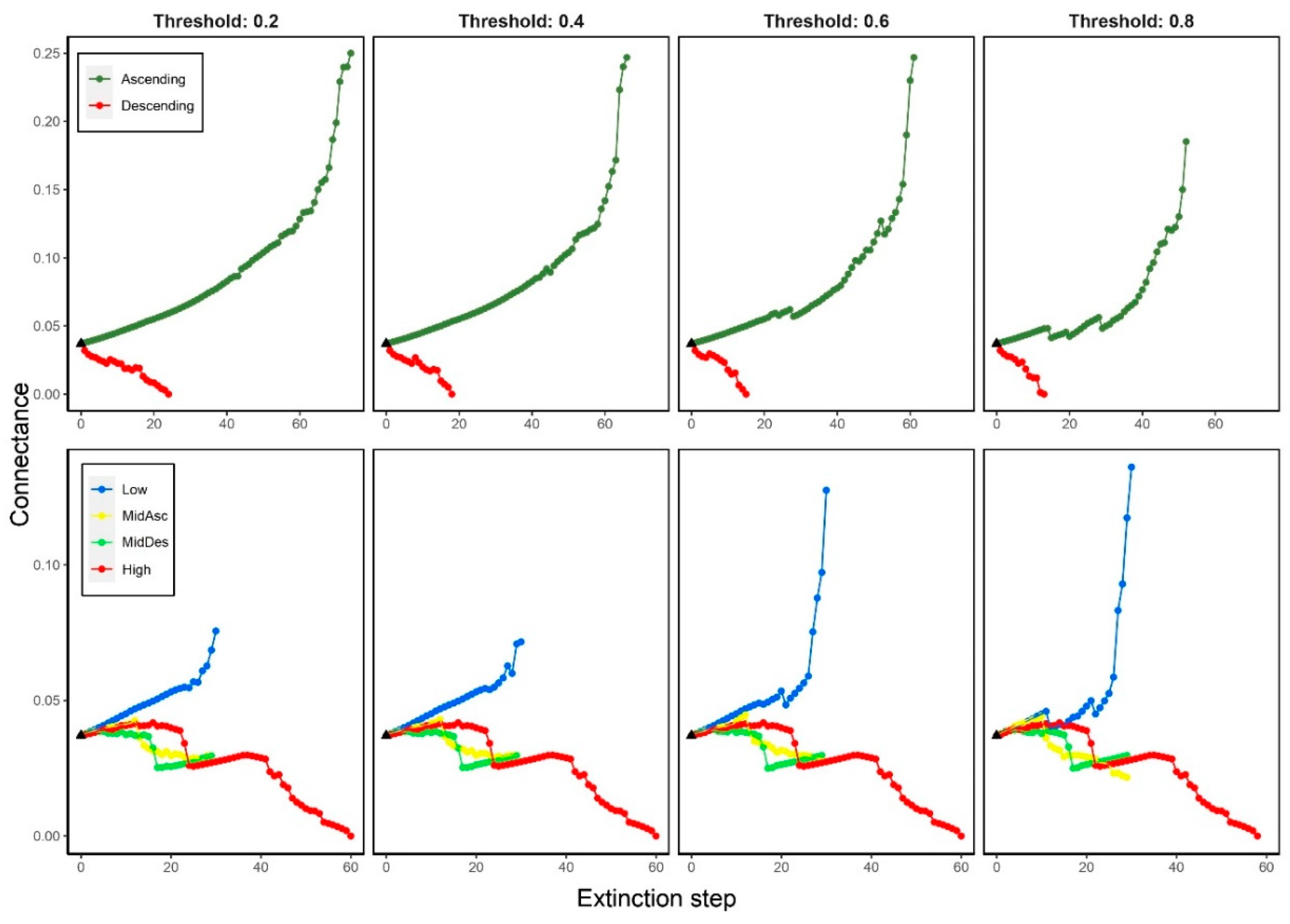
3.1. Effects on connectance (C)
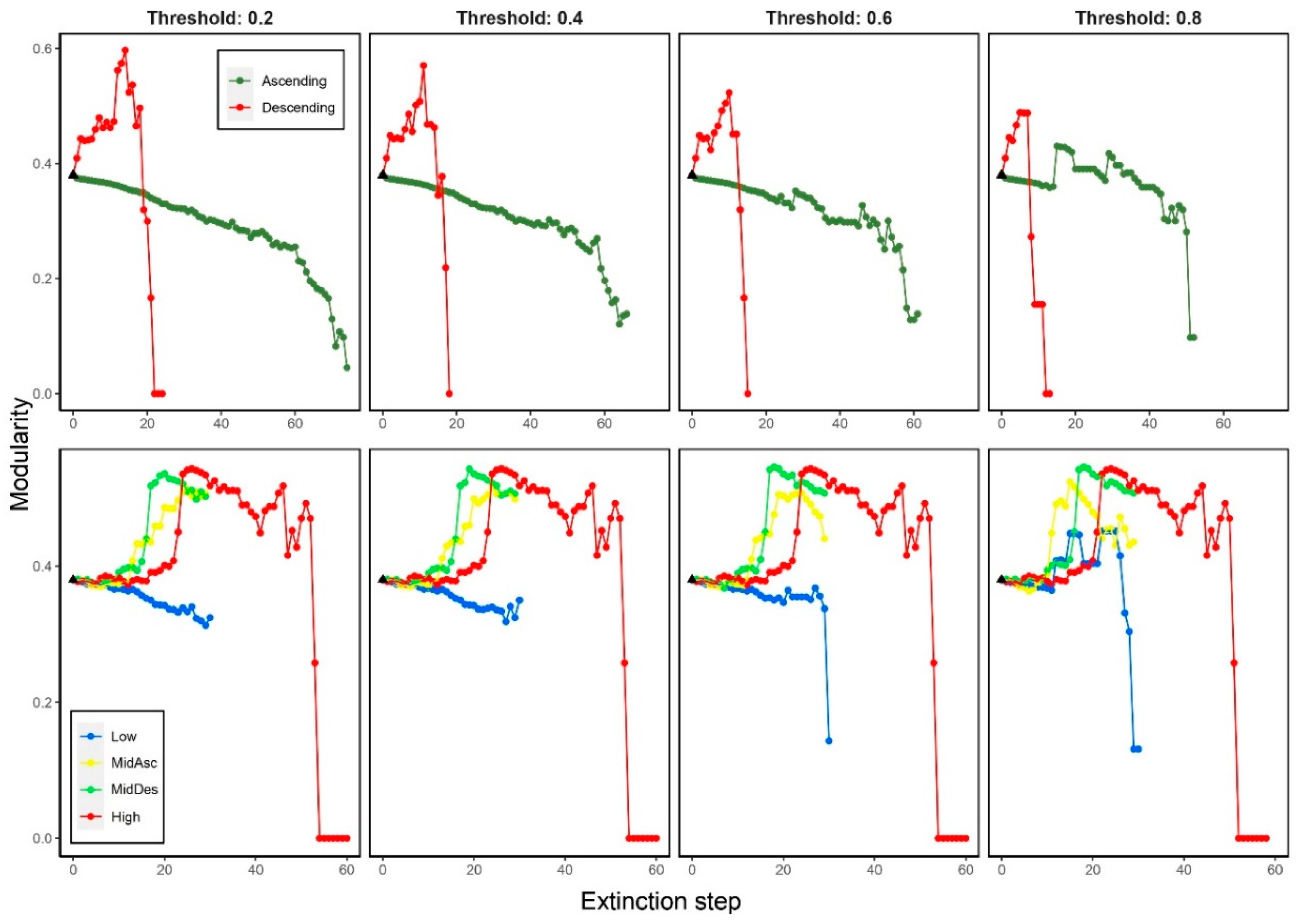
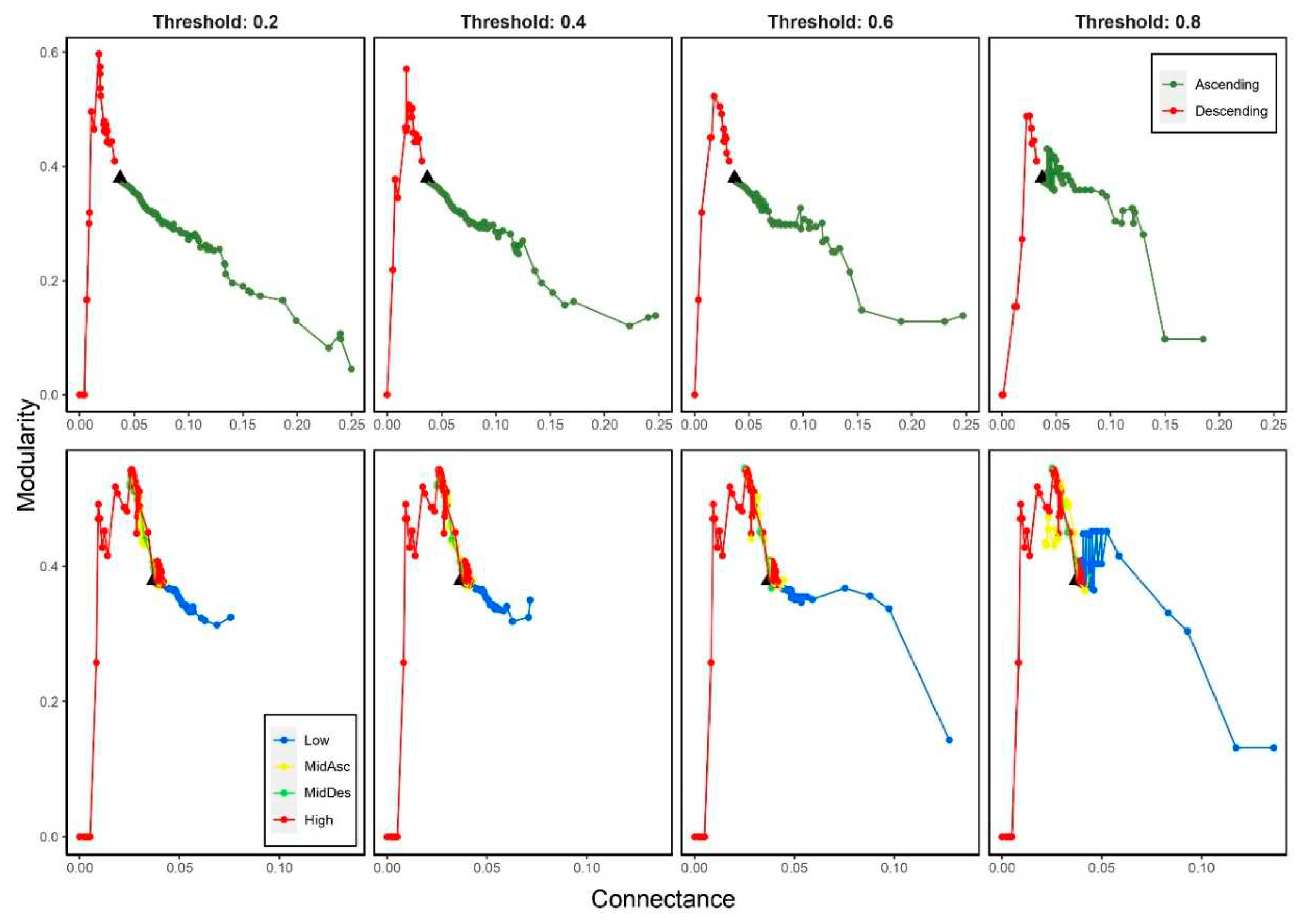
3.2. Effects on modularity (M)
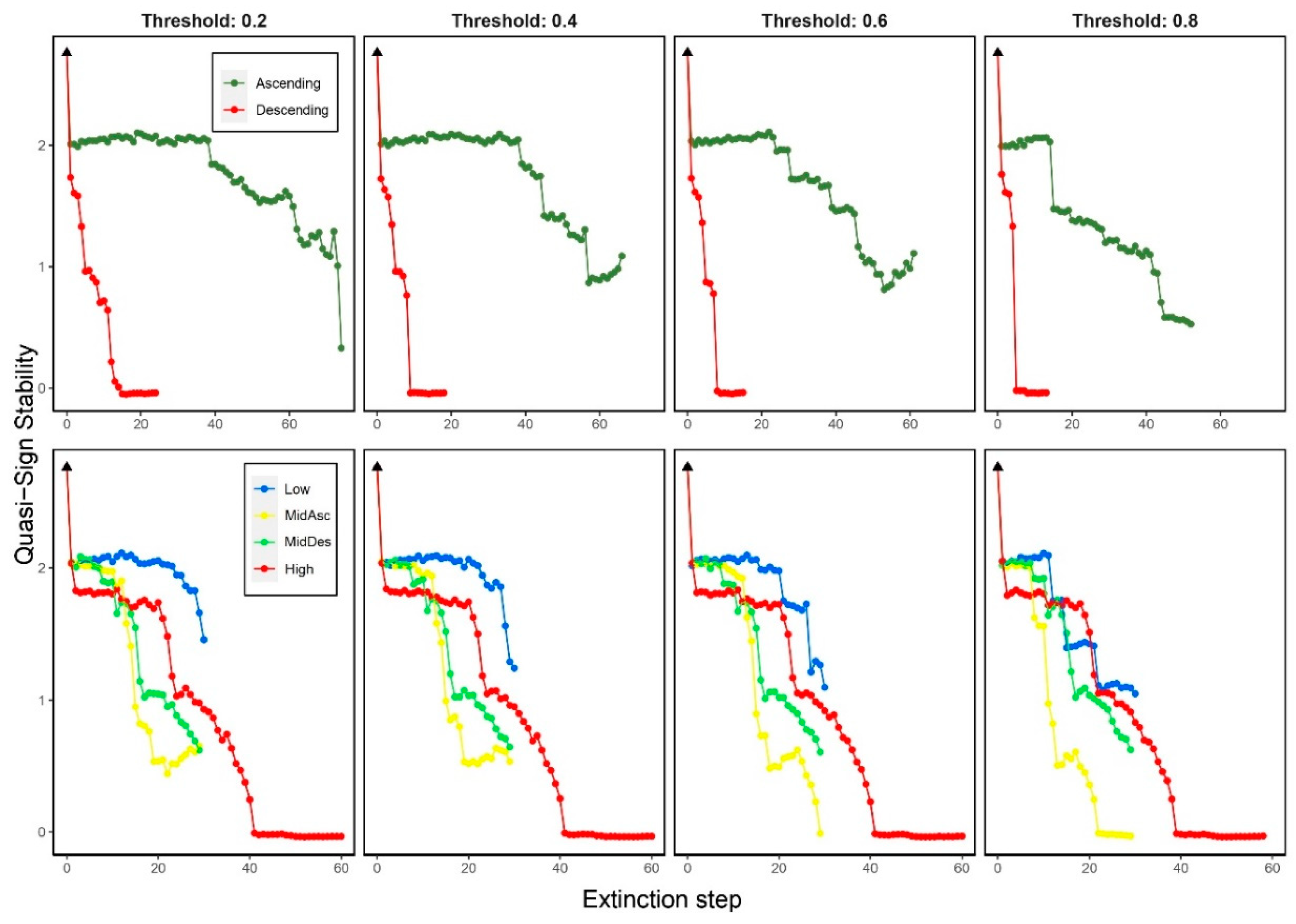
3.3. Effects on stability (QSS)
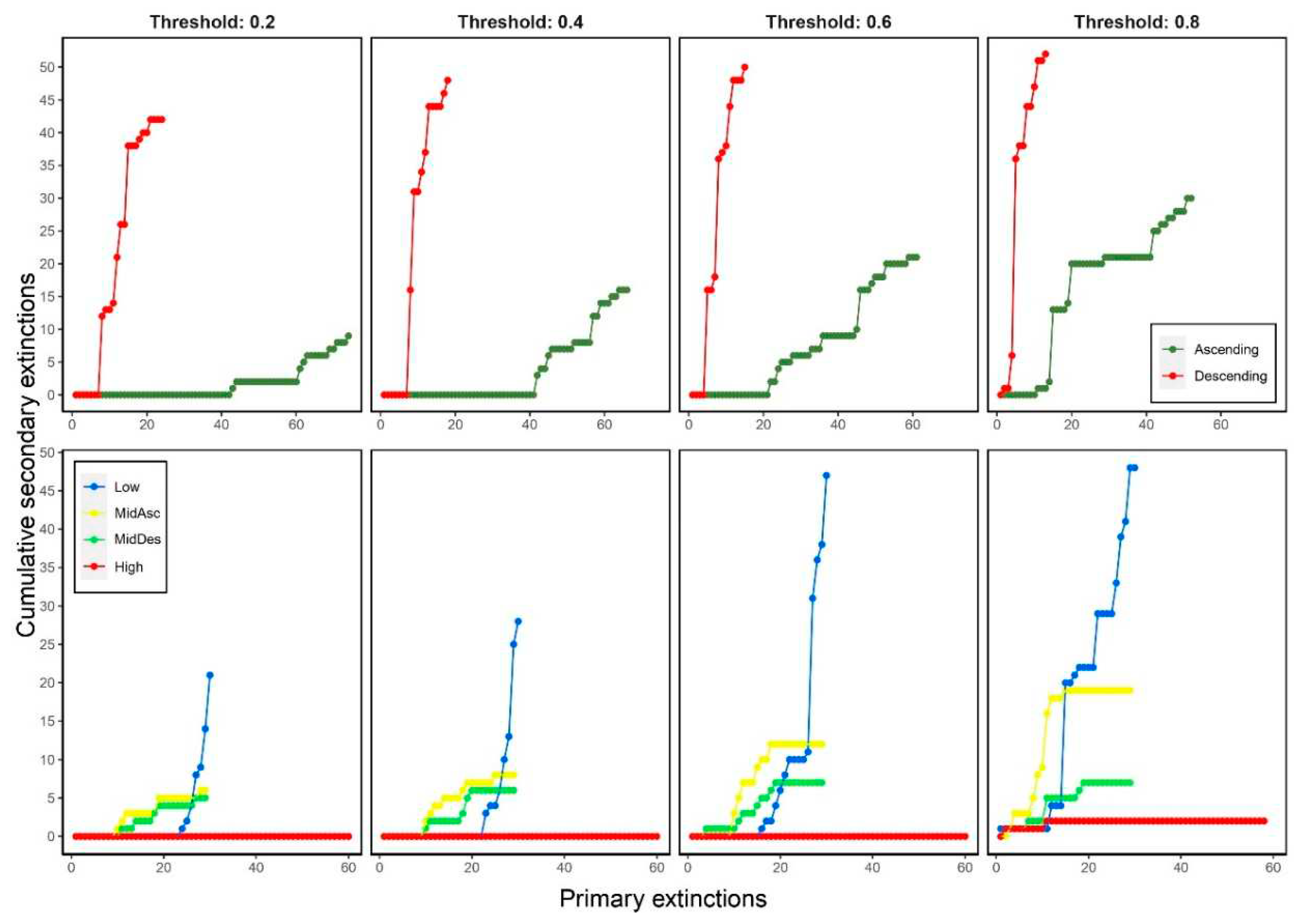
3.4. Cumulative secondary extinctions
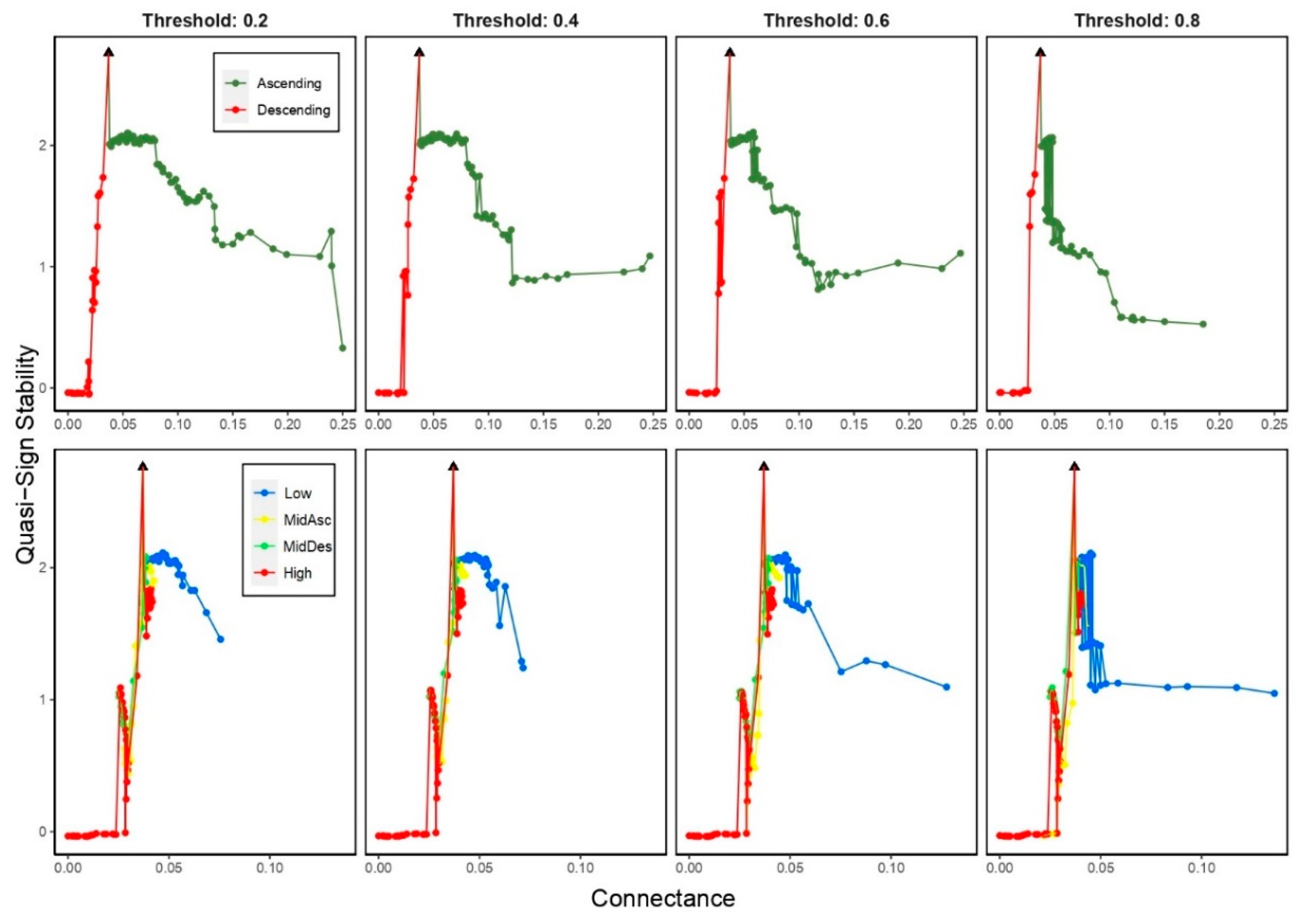
3.5. Properties dependency on food web connectance
3.5.1. Modularity
3.5.2. Quasi-Sign Stability
4. Discussion
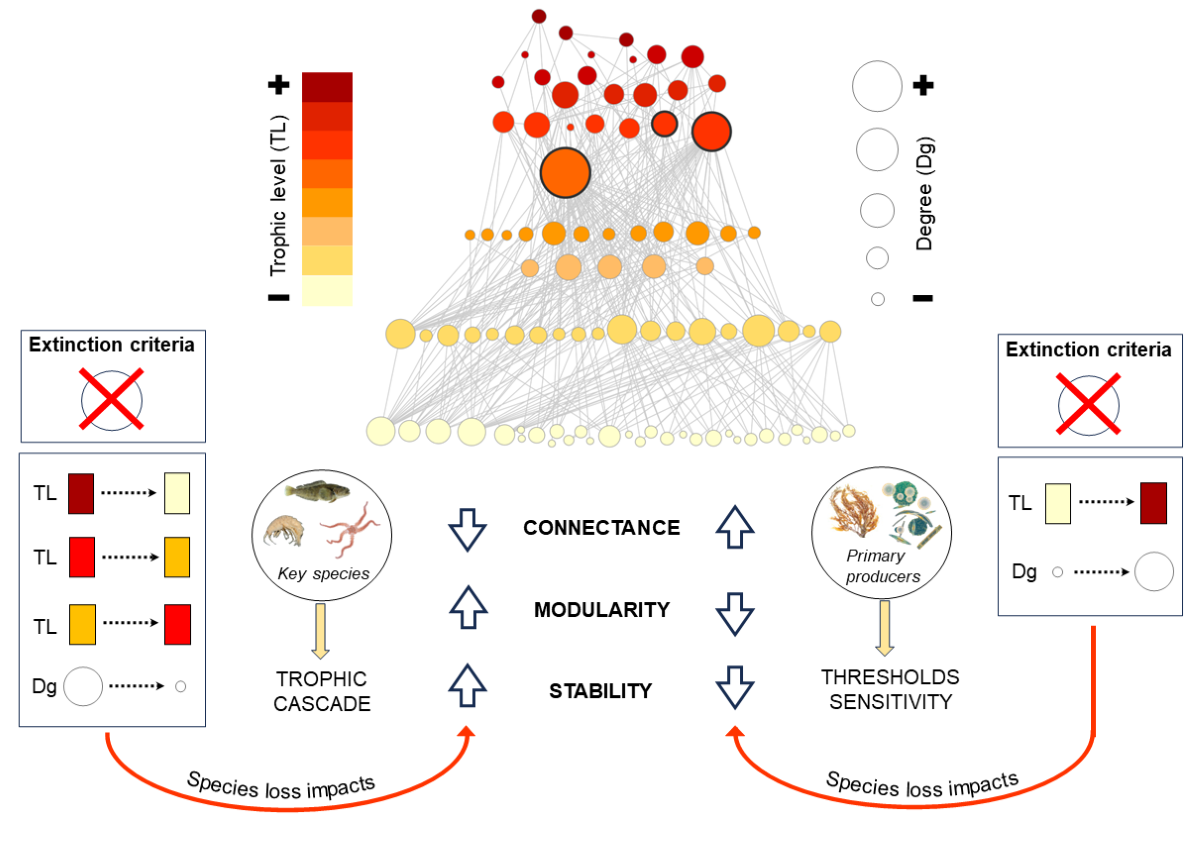
4.1. Topological role of species
4.2. Effects of thresholds on food web properties
4.3. Multidimensional stability criteria
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | TL | Degree |
|---|---|---|
| Urticinopsis antartica | 4.27 | 4 |
| Octopus | 4.13 | 4 |
| Chaenocephalus aceratus | 4.02 | 4 |
| Protomyctophum | 3.70 | 1 |
| Diplasterias brucei | 3.67 | 1 |
| Trematomus newnesi | 3.65 | 10 |
| Trematomus bernacchi | 3.59 | 7 |
| Parachaenichthys charcoti | 3.50 | 1 |
| Perknaster fuscus antarticus | 3.46 | 4 |
| Parborlasia corrugatus | 3.41 | 9 |
| Odontaster meridionalis | 3.35 | 7 |
| Hyperiids | 3.33 | 6 |
| Harpagifer antarcticus | 3.32 | 11 |
| Notothenia rossii | 3.25 | 8 |
| Margarella antarctica | 3.25 | 10 |
| Perknaster aurorae | 3.25 | 2 |
| Sterechinus neumayeri | 3.21 | 17 |
| Glyptonotus antarcticus | 3.13 | 8 |
| Lepidonotothen nudifrons | 3.07 | 7 |
| Austrodoris kerguelensis | 3.07 | 10 |
| Odontaster validus | 3.06 | 10 |
| Bovallia gigantea | 3.00 | 18 |
| Ophionotus victoriae | 2.97 | 33 |
| Notothenia coriiceps | 2.80 | 49 |
| Salps | 2.70 | 8 |
| Neobuccinum eatoni | 2.67 | 11 |
| Dacrydyum sp. | 2.50 | 3 |
| Euphausia superba | 2.50 | 11 |
| Copepods | 2.50 | 5 |
| Ascidians | 2.50 | 5 |
| Oligochaetes | 2.50 | 3 |
| Hydrozoans | 2.50 | 4 |
| Bryozoans | 2.50 | 5 |
| Priapulids | 2.50 | 2 |
| Mysids | 2.50 | 3 |
| Malacobelmnon daytoni | 2.50 | 2 |
| Laternulla elliptica | 2.33 | 6 |
| Haliclonidae sp. | 2.25 | 11 |
| Stylo-Myca | 2.25 | 13 |
| Rosella sp. | 2.25 | 11 |
| Dendrilla antarctica | 2.25 | 6 |
| Nereidae | 2.00 | 17 |
| Eatoniella sp. | 2.00 | 7 |
| Nacella concinna | 2.00 | 9 |
| Laevilacunaria antarctica | 2.00 | 9 |
| Paradexamine sp. | 2.00 | 7 |
| Eurymera monticulosa | 2.00 | 9 |
| Pontogeneiella sp. | 2.00 | 8 |
| Gondogeneia antarctica | 2.00 | 20 |
| Pariphimedia integricauda | 2.00 | 3 |
| Cheirimedon femoratus | 2.00 | 4 |
| Gitanopsis antarctica | 2.00 | 5 |
| Prostebbingia gracilis | 2.00 | 14 |
| Waldeckia obesa | 2.00 | 6 |
| Hippo-Orcho | 2.00 | 3 |
| Oradarea bidentata | 2.00 | 3 |
| Serolis sp. | 2.00 | 3 |
| Plakarthrium puncattissimum | 2.00 | 5 |
| Hemiarthrum setulosum | 2.00 | 3 |
| Zooplankton | 2.00 | 17 |
| Callophyllis atrosanguinea | 1.00 | 1 |
| Curdiea racovitzae | 1.00 | 3 |
| Georgiella confluens | 1.00 | 3 |
| Gigartina skottsbergii | 1.00 | 5 |
| Iridaea cordata | 1.00 | 5 |
| Myriogramme manginii | 1.00 | 2 |
| Neuroglossum delesseriae | 1.00 | 1 |
| Palmaria decipiens | 1.00 | 9 |
| Pantoneura plocamioides | 1.00 | 1 |
| Picconiella plumosa | 1.00 | 1 |
| Plocamium cartilagineum | 1.00 | 4 |
| Pyropia plocamiestris | 1.00 | 1 |
| Trematocarpus antarcticus | 1.00 | 1 |
| Adenocystis utricularis | 1.00 | 3 |
| Ascoseira mirabilis | 1.00 | 3 |
| Desmarestia anceps | 1.00 | 2 |
| Desmarestia antarctica | 1.00 | 3 |
| Desmarestia menziesii | 1.00 | 5 |
| Geminocarpus geminatus | 1.00 | 2 |
| Phaeurus antarcticus | 1.00 | 3 |
| Lambia antarctica | 1.00 | 1 |
| Monostroma hariotii | 1.00 | 3 |
| Urospora penicilliformis | 1.00 | 1 |
| Ulothrix sp. | 1.00 | 1 |
| Epiphytes diatoms | 1.00 | 8 |
| Benthic diatoms | 1.00 | 15 |
| Phytoplankton | 1.00 | 16 |
| Aged detritus | 1.00 | 5 |
| Squids | 1.00 | 3 |
| Fresh detritus | 1.00 | 12 |
| Necromass | 1.00 | 9 |
References
- Seddon, A. W.; Macias-Fauria, M.; Long, P. R.; Benz, D.; Willis, K. J. Sensitivity of global terrestrial ecosystems to climate variability. Nature 2016, 531, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.; Overpeck, J. T.; Allen, J. R.; Anderson, P. M.; Betancourt, J. L.; Binney, H. A. ; ... Jackson, S. T. Past and future global transformation of terrestrial ecosystems under climate change. Science 2018, 361, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Guldberg, O.; Bruno, J. F. The impact of climate change on the world’s marine ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Doney, S. C.; Ruckelshaus, M.; Emmett Duffy, J.; Barry, J. P.; Chan, F.; English, C. A. ; ... Talley, L. D. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Science 2012, 4, 11–37. [Google Scholar] [CrossRef] [PubMed]
- Heleno, R. H.; Ripple, W. J.; Traveset, A. Scientists' warning on endangered food webs. Web Ecol. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Cahill, A. E.; Aiello-Lammens, M. E.; Fisher-Reid, M. C.; Hua, X. , Karanewsky, C. J.; Yeong Ryu, H.; ... Wiens, J. J. How does climate change cause extinction? Proc. R. Soc. Ser. B Biol. Sci. 2013, 280, 20121890. [Google Scholar] [CrossRef] [PubMed]
- Mastrantonis, S.; Craig, M. D.; Renton, M.; Kirkby, T.; Hobbs, R. J. Climate change indirectly reduces breeding frequency of a mobile species through changes in food availability. Ecosphere 2019, 10, e02656. [Google Scholar] [CrossRef]
- Hirt, M. R.; Barnes, A. D.; Gentile, A. , Pollock, L. J., Rosenbaum, B.; Thuiller, W.; ... Brose, U. Environmental and anthropogenic constraints on animal space use drive extinction risk worldwide. Ecol. Lett. 2021, 24, 2576–2585. [Google Scholar] [CrossRef] [PubMed]
- Chichorro, F.; Juslén, A.; Cardoso, P. A review of the relation between species traits and extinction risk. Biol. Conserv. 2019, 237, 220–229. [Google Scholar] [CrossRef]
- Albert, R.; Jeong, H.; Barabasi, A. L. Error and attack tolerance of complex networks. Nature 2000, 409, 542–542. [Google Scholar] [CrossRef]
- Sole, R. V.; Montoya, M. Complexity and fragility in ecological networks. Proc. R. Soc. Ser. B Biol. Sci. 2001, 268, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Dunne, J. A.; Williams, R. J.; Martinez, N. D. Network Topology and Species Loss in Food Webs: Robustness Increases with Connectance. In SFI Working Papers; Santa Fe Institute: Santa Fe, NM 87501, USA, 2002. [Google Scholar]
- Dunne, J. A.; Williams, R. J.; Martinez, N. D. Network structure and robustness of marine food webs. Mar. Ecol. Prog. Ser. 2004, 273, 291–302. [Google Scholar] [CrossRef]
- Srinivasan, U. T.; Dunne, J. A.; Harte, J.; Martinez, N. D. Response of complex food webs to realistic extinction sequences. Ecology 2007, 88, 671–682. [Google Scholar] [CrossRef]
- Riede, J. O.; Binzer, A.; Brose, U.; de Castro, F.; Curtsdotter, A.; Rall, B. C.; Eklöf, A. Size-based food web characteristics govern the response to species extinctions. Basic Appl. Ecol. 2011, 12, 581–589. [Google Scholar] [CrossRef]
- Carscallen, W. M. A.; Romanuk, T. N. Structure and robustness to species loss in Arctic and Antarctic ice-shelf meta-ecosystem webs. Ecol. Modell. 2012, 245, 208–218. [Google Scholar] [CrossRef]
- Donohue, I.; Petchey, O. L.; Kéfi, S.; Génin, A.; Jackson, A. L.; Yang, Q.; O'Connor, N. E. Loss of predator species, not intermediate consumers, triggers rapid and dramatic extinction cascades. Global Change Biol. 2012, 23, 2962–2972. [Google Scholar] [CrossRef] [PubMed]
- Keyes, A. A.; McLaughlin, J. P.; Barner, A. K.; Dee, L. E. An ecological network approach to predict ecosystem service vulnerability to species losses. Nat. Commun. 2021, 12, 1586. [Google Scholar] [CrossRef]
- Bellingeri, M.; Bodini, A. Threshold extinction in food webs. Theor. Ecol. 2013, 6, 143–152. [Google Scholar] [CrossRef]
- Cordone, G.; Marina, T. I.; Salinas, V.; Doyle, S. R.; Saravia, L. A.; Momo, F. R. Effects of macroalgae loss in an Antarctic marine food web: applying extinction thresholds to food web studies. PeerJ 2018, 6, e5531. [Google Scholar] [CrossRef]
- Dunne, J. A.; Williams, R. J. Cascading extinctions and community collapse in model food webs. Philos. Trans. R. Soc. London, Ser. B 2009, 364, 1711–1723. [Google Scholar] [CrossRef]
- Montoya, J. M.; Pimm, S. L.; Solé, R. V. Ecological networks and their fragility. Nature 2006, 442, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Hastings, A.; McCann, K. S.; de Ruiter, P. C. Introduction to the special issue: theory of food webs. Theor. Ecol. 2016, 9, 1–2. [Google Scholar] [CrossRef]
- Barnes, A. D.; Jochum, M.; Lefcheck, J. S.; Eisenhauer, N.; Scherber, C.; O’Connor, M. I. ; ... Brose, U. Energy flux: the link between multitrophic biodiversity and ecosystem functioning. Trends Ecol. Evol. 2018, 33, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Momo, F. R.; Cordone, G.; Marina, T. I.; Salinas, V.; Campana, G. L.; Valli, M. A. ; ... Saravia, L. A. Seaweeds in the Antarctic Marine Coastal Food Web. In Antarctic Seaweeds: Diversity, Adaptation and Ecosystem Services, 1st ed.; Gómez, I., Huovinen, P., Eds.; Springer Cham: Springer Nature, Switzerland AG, 2020; Volume 1, pp. 293–307. ISBN 978-3-030-39447-9. [Google Scholar]
- Allesina, S.; Bodini, A. Who dominates whom in the ecosystem? Energy flow bottlenecks and cascading extinctions. J. Theor. Biol. 2004, 230, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Bascompte, J. Disentangling the web of life. Science 2009, 325, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Eayrs, C.; Li, X.; Raphael, M. N.; Holland, D. M. Rapid decline in Antarctic Sea ice in recent years hints at future change. Nat. Geosci. 2012, 14, 460–464. [Google Scholar] [CrossRef]
- Rantanen, M.; Karpechko, A. Y.; Lipponen, A.; Nordling, K.; Hyvärinen, O.; Ruosteenoja, K. ; ... Laaksonen, A. The Arctic has warmed nearly four times faster than the globe since 1979. Commun. Earth Environ. 2022, 3, 168. [Google Scholar] [CrossRef]
- Constable, A. J.; Harper, S.; Dawson, J.; Holsman, K.; Mustonen, T.; Piepenburg, D.; ... Van Dam, B. Cross-chapter paper 6: polar regions. In Climate Change 2022: Impacts, adaptation and vulnerability; Contribution of the WGII to the 6th assessment report of the intergovernmental panel on climate change, IPCC AR WGII; Cambridge University Press, 2022. [Google Scholar]
- Ives, A. R.; Cardinale, B. J. Food-web interactions govern the resistance of communities after non-random extinctions. Nature 2004, 429, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Thébault, E.; Huber, V.; Loreau, M. Cascading extinctions and ecosystem functioning: contrasting effects of diversity depending on food web structure. Oikos 2007, 116, 163–173. [Google Scholar] [CrossRef]
- Sanders, D.; Thébault, E.; Kehoe, R.; Frank van Veen, F. J. Trophic redundancy reduces vulnerability to extinction cascades. PNAS 2018, 115, 2419–2424. [Google Scholar] [CrossRef]
- Kehoe, R.; Frago, E.; Sanders, D. Cascading extinctions as a hidden driver of insect decline. Ecol. Entomol. 2021, 46, 743–756. [Google Scholar] [CrossRef]
- Pimm, S. L. The complexity and stability of ecosystems. Nature 1984, 307, 321–326. [Google Scholar] [CrossRef]
- Allesina, S.; Bodini, A.; Bondavalli, C. Secondary extinctions in ecological networks: bottlenecks unveiled. Ecol. Modell. 2006, 194, 150–161. [Google Scholar] [CrossRef]
- Bellingeri, M.; Vincenzi, S. Robustness of empirical food webs with varying consumer's sensitivities to loss of resources. J. Theor. Biol. 2013, 333, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.; Pimenov, A.; Palmer, C.; Emmerson, M.; Jonsson, T. Ecological communities are vulnerable to realistic extinction sequences. Oikos 2015, 124, 486–496. [Google Scholar] [CrossRef]
- Ávila-Thieme, M. I.; Corcoran, D.; Pérez-Matus, A.; Wieters, E. A.; Navarrete, S. A.; Marquet, P. A.; Valdovinos, F. S. Alteration of coastal productivity and artisanal fisheries interact to affect a marine food web. Sci. Rep. 2021, 11, 1765. [Google Scholar] [CrossRef] [PubMed]
- Calizza, E.; Costantini, M. L.; Rossi, L. Effect of multiple disturbances on food web vulnerability to biodiversity loss in detritus-based systems. Ecosphere 2015, 6, 1–20. [Google Scholar] [CrossRef]
- Mendonça, V.; Madeira, C.; Dias, M.; Flores, A. A.; Vinagre, C. Robustness of temperate versus tropical food webs: comparing species trait-based sequential deletions. Mar. Ecol. Prog. Ser. 2022, 691, 19–28. [Google Scholar] [CrossRef]
- Mestre, F.; Rozenfeld, A.; Araújo, M. B. Human disturbances affect the topology of food webs. Ecol. Lett. 2022, 25, 2476–2488. [Google Scholar] [CrossRef]
- Mérillet, L.; Robert, M.; Hernvann, P. Y.; Pecuchet, L.; Pavoine, S.; Mouchet, M. ; ... Kopp, D. Effects of life-history traits and network topological characteristics on the robustness of marine food webs. Global Ecol. Conserv. 2022, 34, e02048. [Google Scholar] [CrossRef]
- Bers, A. V.; Momo, F.; Schloss, I. R.; Abele, D. Analysis of trends and sudden changes in long-term environmental data from King George Island (Antarctica): relationships between global climatic oscillations and local system response. Clim. Change 2013, 116, 789–803. [Google Scholar] [CrossRef]
- Lagger, C.; Nime, M.; Torre, L.; Servetto, N.; Tatián, M.; Sahade, R. Climate change, glacier retreat and a new ice-free island offer new insights on Antarctic benthic responses. Ecography 2018, 41, 579–591. [Google Scholar] [CrossRef]
- Barlett, E. R.; Sierra, M. E.; Costa, A. J.; Tosonotto, G. V. Interannual variability of hydrographic properties in Potter Cove during summers between 2010 and 2017. Antarct. Sci. 2021, 33, 281–300. [Google Scholar] [CrossRef]
- Pasotti, F.; Manini, E.; Giovannelli, D.; Wölfl, A. C.; Monien, D.; Verleyen, E. ; ... Vanreusel, A. Antarctic shallow water benthos in an area of recent rapid glacier retreat. Mar. Ecol. 2015, 36, 716–733. [Google Scholar] [CrossRef]
- Sahade, R.; Lagger, C.; Torre, L.; Momo, F.; Monien, P.; Schloss, I. ; ... Abele, D. Climate change and glacier retreat drive shifts in an Antarctic benthic ecosystem. Sci. Adv. 2015, 1, e1500050. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M. D.; Severini, M. D. F.; Spetter, C.; Abbate, M. C. L.; Tartara, M. N.; Nahuelhual, E. G. ; ... Hoffmeyer, M. S. Effects of glacier melting on the planktonic communities of two Antarctic coastal areas (Potter Cove and Hope Bay) in summer. Reg. Stud. Mar. Sci. 2019, 30, 100731. [Google Scholar] [CrossRef]
- Hoffmann, R.; Al-Handal, A. Y.; Wulff, A.; Deregibus, D.; Zacher, K.; Quartino, M. L. ; ... Braeckman, U.Implications of glacial melt-related processes on the potential primary production of a microphytobenthic community in Potter Cove (Antarctica). Front. Mar. Sci. 2019, 6, 655. [Google Scholar] [CrossRef]
- Marina, T. I.; Salinas, V.; Cordone, G.; Campana, G.; Moreira, E.; Deregibus, D. ; ... Momo, F. R. The food web of Potter Cove (Antarctica): complexity, structure and function. Estuarine Coastal Shelf Sci. 2018, 200, 141–151. [Google Scholar] [CrossRef]
- Briand, F.; Cohen, J. E. Community food webs have scale-invariant structure. Nature 1984, 307, 264–267. [Google Scholar] [CrossRef]
- Martinez, N. D. Artifacts or attributes? Effects of resolution on the Little Rock Lake food web. Ecol. Monogr. 1991, 61, 367–392. [Google Scholar] [CrossRef]
- Eklöf, A.; Tang, S.; Allesina, S. Secondary extinctions in food webs: a Bayesian network approach. Methods Ecol. Evol. 2013, 4, 760–770. [Google Scholar] [CrossRef]
- Martinez, N. D. Constant connectance in community food webs. Am. Nat. 1992, 139, 1208–1218. [Google Scholar] [CrossRef]
- Vermaat, J. E.; Dunne, J. A.; Gilbert, A. J. Major dimensions in food-web structure properties. Ecology 2009, 90, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Poisot, T.; Gravel, D. When is a network complex? Connectance drives degree distribution and emerging network properties. PeerJ Preprints, 2013, 1, e50v1. [Google Scholar] [CrossRef]
- Newman, M. E.; Girvan, M. Finding and evaluating community structure in networks. Phys. Rev. E 2004, 69, 026113. [Google Scholar] [CrossRef]
- Fortuna, M. A.; Stouffer, D. B.; Olesen, J. M.; Jordano, P.; Mouillot, D.; Krasnov, B. R. ; ... Bascompte, J. Nestedness versus modularity in ecological networks: two sides of the same coin? J. Anim. Ecol. 2010, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Grilli, J.; Rogers, T.; Allesina, S. Modularity and stability in ecological communities. Nat. Commun. 2016, 7, 12031. [Google Scholar] [CrossRef]
- Allesina, S.; Pascual, M. Network structure, predator–prey modules, and stability in large food webs. Theor. Ecol. 2008, 1, 55–64. [Google Scholar] [CrossRef]
- Pimm, S. L.; Lawton, J. H. Number of trophic levels in ecological communities. Nature 1977, 268, 329–331. [Google Scholar] [CrossRef]
- Pimm, S. L. Food webs. In Food Webs. Population and Community Biology; Springer: Dordrecht, The Netherlands, 1982; pp. 1–11. ISBN 978-94-009-5927-9. [Google Scholar]
- Moore, J. C.; McCann, K.; de Ruiter, P. C. Modeling trophic pathways, nutrient cycling, and dynamic stability in soils. Pedobiologia 2005, 49, 499–510. [Google Scholar] [CrossRef]
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. InterJournal, complex systems 2006, 1695, 1–9. [Google Scholar]
- Saravia, L. A.; Marina, T. I.; Kristensen, N. P.; De Troch, M.; Momo, F. R. Ecological network assembly: how the regional metaweb influences local food webs. J. Anim. Ecol. 2022, 91, 630–642. [Google Scholar] [CrossRef]
- Borrvall, C.; Ebenman, B.; Tomas Jonsson, T. J. Biodiversity lessens the risk of cascading extinction in model food webs. Ecol. Lett. 2000, 3, 131–136. [Google Scholar] [CrossRef]
- Myers, R. A.; Baum, J. K.; Shepherd, T. D.; Powers, S. P.; Peterson, C. H. Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 2007, 315, 1846–1850. [Google Scholar] [CrossRef]
- Murphy, G. E.; Romanuk, T. N.; Worm, B. Cascading effects of climate change on plankton community structure. Ecol. Evol. 2020, 10, 2170–2181. [Google Scholar] [CrossRef] [PubMed]
- Paine, R. T. Food web complexity and species diversity. Am. Nat. 1966, 100, 65–75. [Google Scholar] [CrossRef]
- Paine, R. T. A note on trophic complexity and community stability. Am. Nat. 1969, 103, 91–93. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Sui, H.; Xu, B.; Zhang, C.; Ren, Y.; Xue, Y. Using network analysis to identify keystone species in the food web of Haizhou Bay, China. Mar. Freshwater Res. 2019, 71, 469–481. [Google Scholar] [CrossRef]
- Gouveia, C.; Móréh, Á.; Jordán, F. Combining centrality indices: maximizing the predictability of keystone species in food webs. Ecol. Indic. 2021, 126, 107617. [Google Scholar] [CrossRef]
- Delmas, E.; Besson, M.; Brice, M. H.; Burkle, L. A.; Dalla Riva, G. V.; Fortin, M. J. ; ... Poisot, T. Analysing ecological networks of species interactions. Biol. Rev. 2019, 94, 16–36. [Google Scholar] [CrossRef]
- Cirtwill, A. R.; Dalla Riva, G. V.; Gaiarsa, M. P.; Bimler, M. D.; Cagua, E. F.; Coux, C.; Dehling, D. M. A review of species role concepts in food webs. Food Webs 2018, 16, e00093. [Google Scholar] [CrossRef]
- Baum, J. K.; Worm, B. Cascading top-down effects of changing oceanic predator abundances. J. Anim. Ecol. 2009, 78, 699–714. [Google Scholar] [CrossRef]
- Lynam, C. P.; Llope, M.; Möllmann, C.; Helaouët, P.; Bayliss-Brown, G. A.; Stenseth, N. C. Interaction between top-down and bottom-up control in marine food webs. PNAS 2017, 114, 1952–1957. [Google Scholar] [CrossRef]
- Rodriguez, I. D.; Marina, T. I.; Schloss, I. R.; Saravia, L. A. Marine food webs are more complex but less stable in sub-Antarctic (Beagle channel, Argentina) than in Antarctic (Potter cove, Antarctic peninsula) regions. Mar. Environ. Res. 2022, 174, 105561. [Google Scholar] [CrossRef]
- Harrison, W. G.; Cota, G. F. Primary production in polar waters: relation to nutrient availability. Polar Res. 1991, 10, 87–104. [Google Scholar] [CrossRef]
- Tremblay, JÉ. ; Robert, D.; Varela, D.E. et al. Current state and trends in Canadian Arctic marine ecosystems: I. Primary production. Clim. Change 2012, 115, 161–178. [Google Scholar] [CrossRef]
- Pinkerton, M. H.; Boyd, P. W.; Deppeler, S.; Hayward, A.; Höfer, J.; Moreau, S. Evidence for the impact of climate change on primary producers in the Southern Ocean. Front. Ecol. Evol. 2021, 9, 592027. [Google Scholar] [CrossRef]
- Amsler, C. D.; Rowley, R. J.; Laur, D. R.; Quetin, L. B.; Ross, R. M. Vertical distribution of Antarctic peninsular macroalgae: cover, biomass and species composition. Phycologia 1995, 34, 424–430. [Google Scholar] [CrossRef]
- Wiencke, C.; Clayton, M. N.; Gómez, I.; Iken, K.; Lüder, U. H.; Amsler, C. D. ; ... Dunton, K. Life strategy, ecophysiology and ecology of seaweeds in polar waters. Rev. Environ. Sci. Bio/Technol. 2007, 6, 95–126. [Google Scholar] [CrossRef]
- Wiencke, C.; Amsler, C. D. Seaweeds and their communities in polar regions. In Seaweed biology. Ecological Studies; Wiencke, C., Bischof, K., Eds.; Springer Berlin Heidelberg: Berlin, Germany, 2012; Volume 219, pp. 265–291. ISBN 978-3-642-28451-9. [Google Scholar]
- Quartino, M. L.; Saravia, L. A.; Campana, G. L.; Deregibus, D.; Matula, C. V.; Boraso, A. L.; Momo, F. R. Production and biomass of seaweeds in newly ice-free areas: implications for coastal processes in a changing Antarctic environment. In Antarctic Seaweeds: Diversity, Adaptation and Ecosystem Services, 1st ed.; Gómez, I., Huovinen, P., Eds.; Springer Cham: Springer Nature, Switzerland AG, 2020; Volume 1, pp. 155–171. ISBN 978-3-030-39447-9. [Google Scholar]
- Cordone, G.; Salinas, V.; Marina, T. I.; Doyle, S. R.; Pasotti, F.; Saravia, L. A.; Momo, F. R. Green vs brown food web: Effects of habitat type on multidimensional stability proxies for a highly-resolved Antarctic food web. Food Webs 2020, 25, e00166. [Google Scholar] [CrossRef]
- Salinas, V.; Marina, T. I.; Cordone, G.; Momo, F. R. Ecological networks of an Antarctic ecosystem: a full description of non-trophic interactions. Mar. Biol. 2023, 170, 9. [Google Scholar] [CrossRef]
- Petchey, O. L.; Beckerman, A. P.; Riede, J. O.; Warren, P. H. Size, foraging, and food web structure. PNAS 2008, 105, 4191–4196. [Google Scholar] [CrossRef] [PubMed]
- Yonatan, Y.; Amit, G.; Friedman, J.; Bashan, A. Complexity–stability trade-off in empirical microbial ecosystems. Nat. Ecol. Evol. 2022, 6, 693–700. [Google Scholar] [CrossRef] [PubMed]
- van Altena, C.; Hemerik, L.; de Ruiter, P. C. Food web stability and weighted connectance: the complexity-stability debate revisited. Theor. Ecol. 2016, 9, 49–58. [Google Scholar] [CrossRef]
- Eskuche-Keith, P.; Hill, S. L.; Hollyman, P.; Taylor, M. L.; O’Gorman, E. J. Trophic structuring of modularity alters energy flow through marine food webs. Front. Mar. Sci. 2023, 9, 1046150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





