Submitted:
13 December 2023
Posted:
14 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
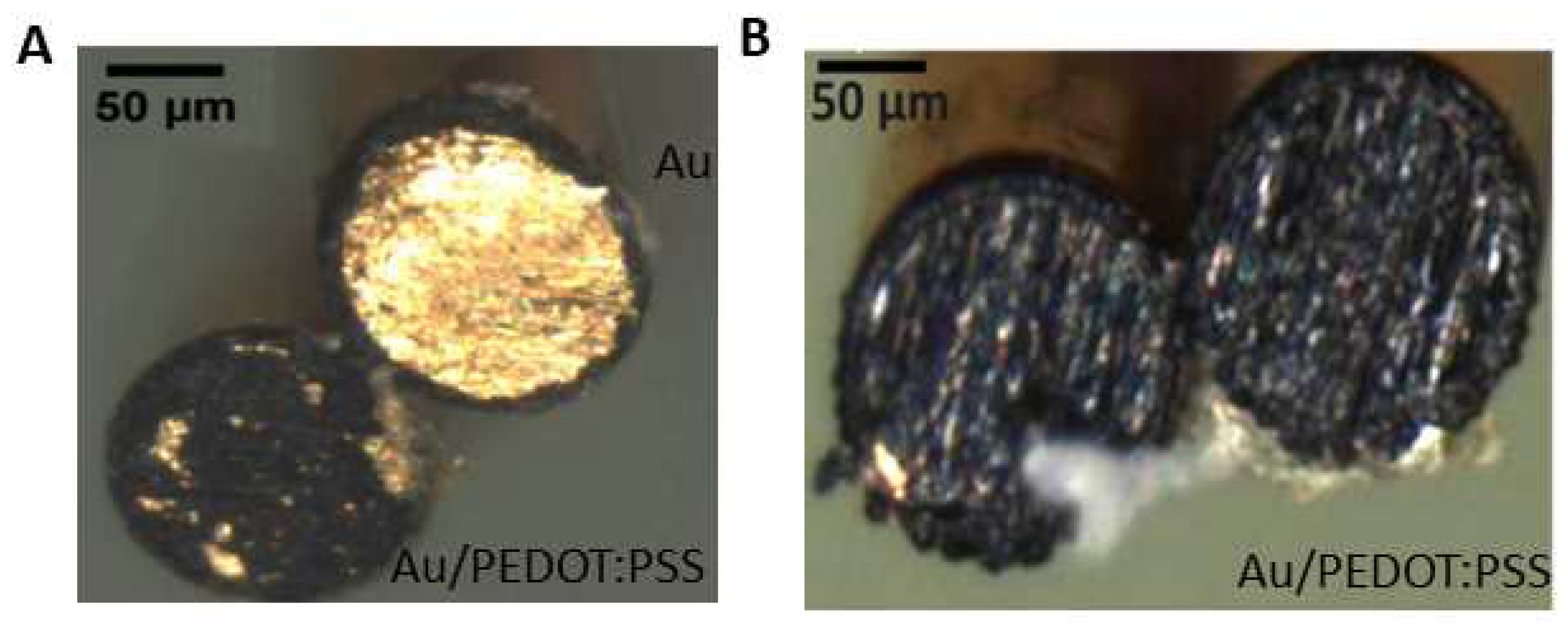
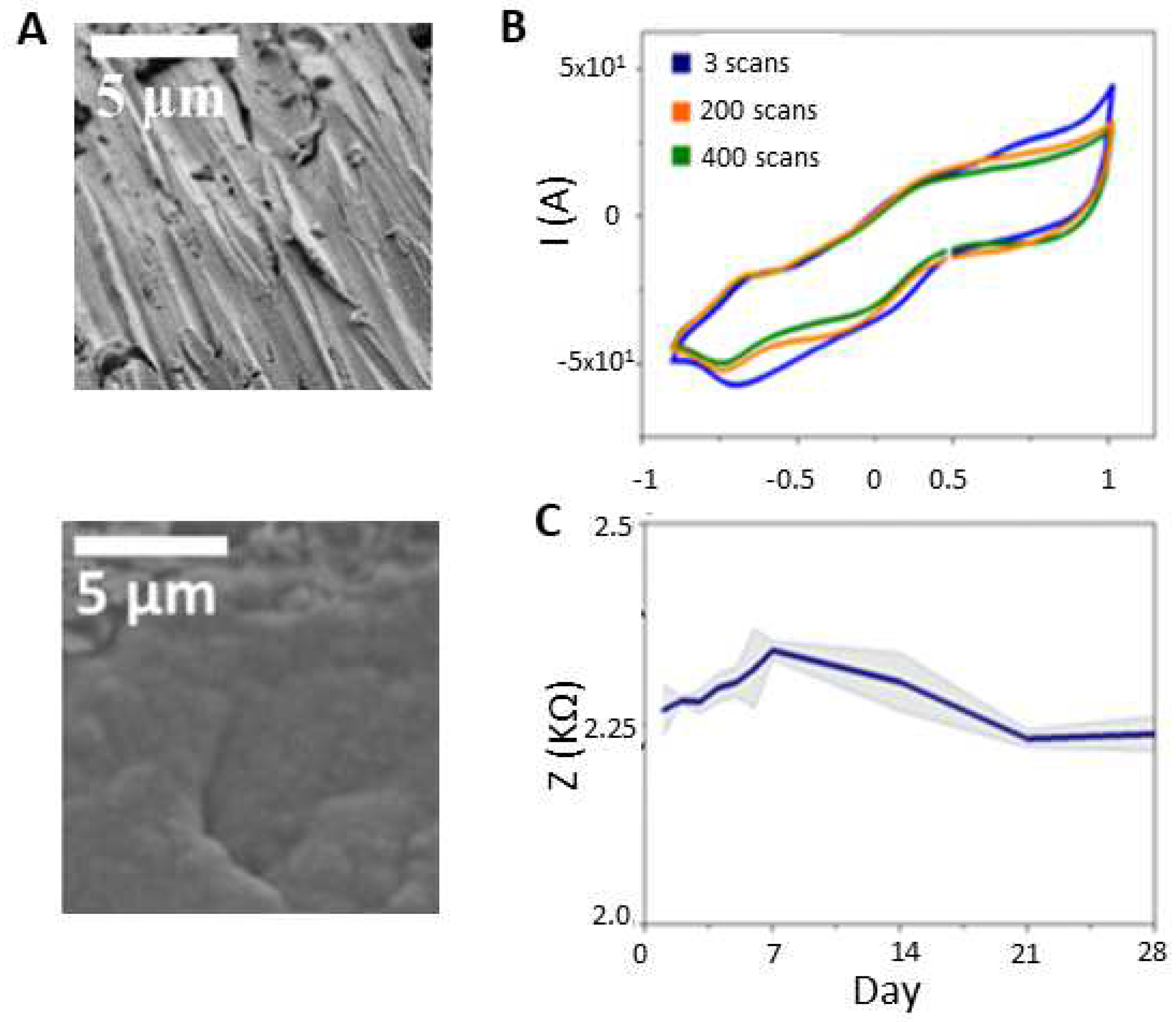
2.1. Electrode preparation
2.2. Electrodes modeling
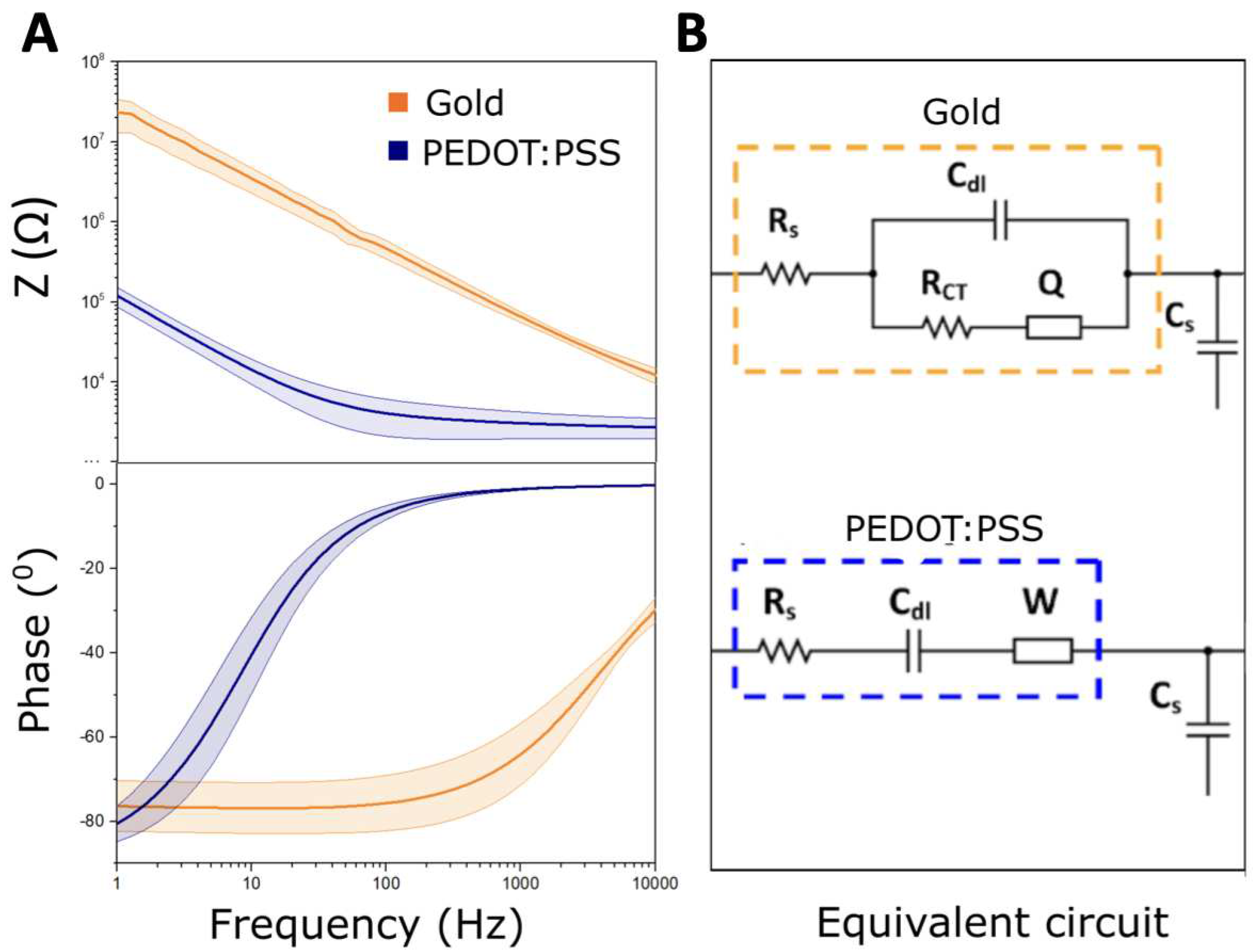
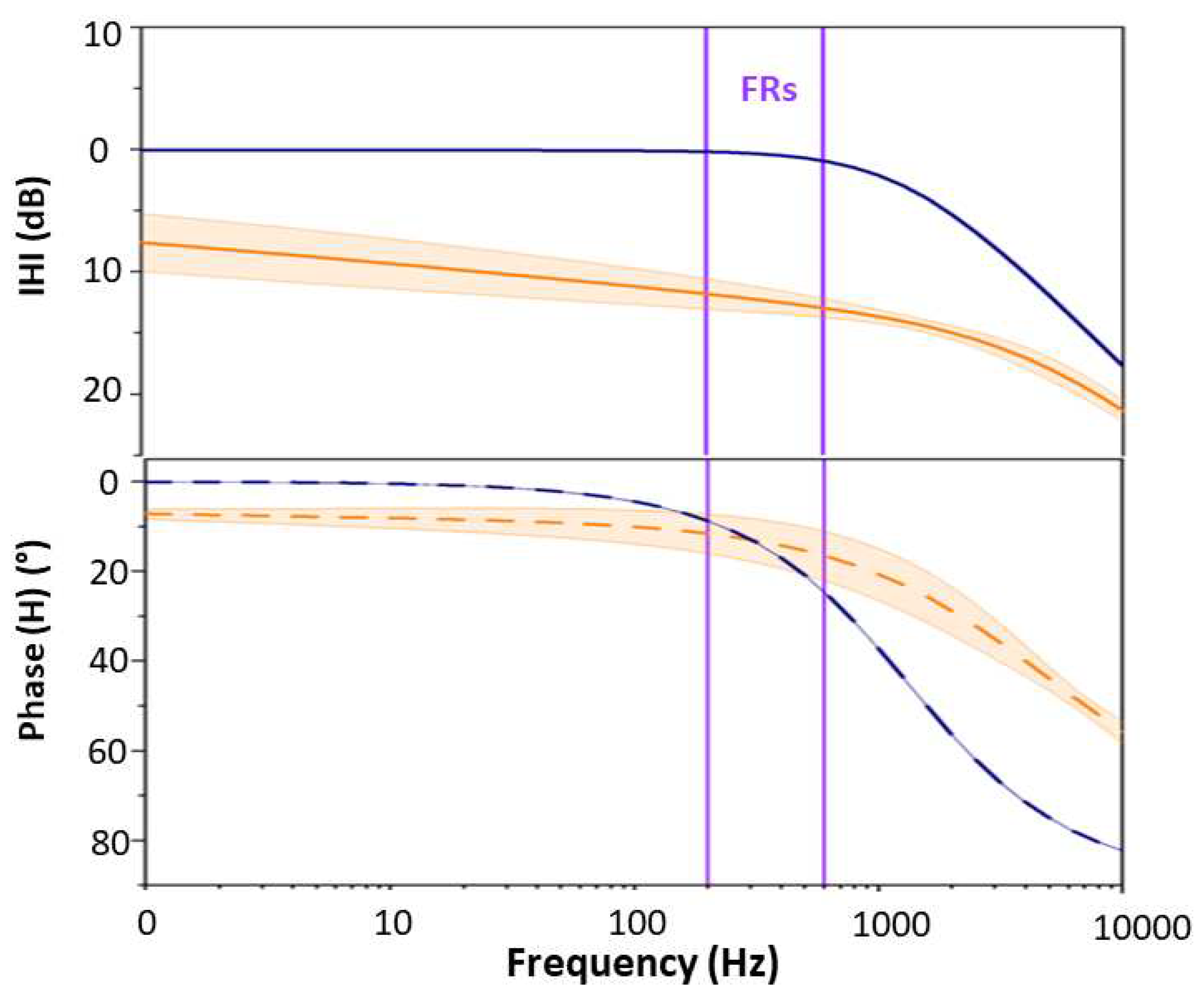
2.3. Transfer function definition
| Circuit | Au | Au/PEDOT:PSS |
|---|---|---|
| Elements | ||
| 3 | 3 | |
| 12.24 | — | |
| 1.036 | 1.73 | |
| 10.97 | — | |
| — | 10.24 |
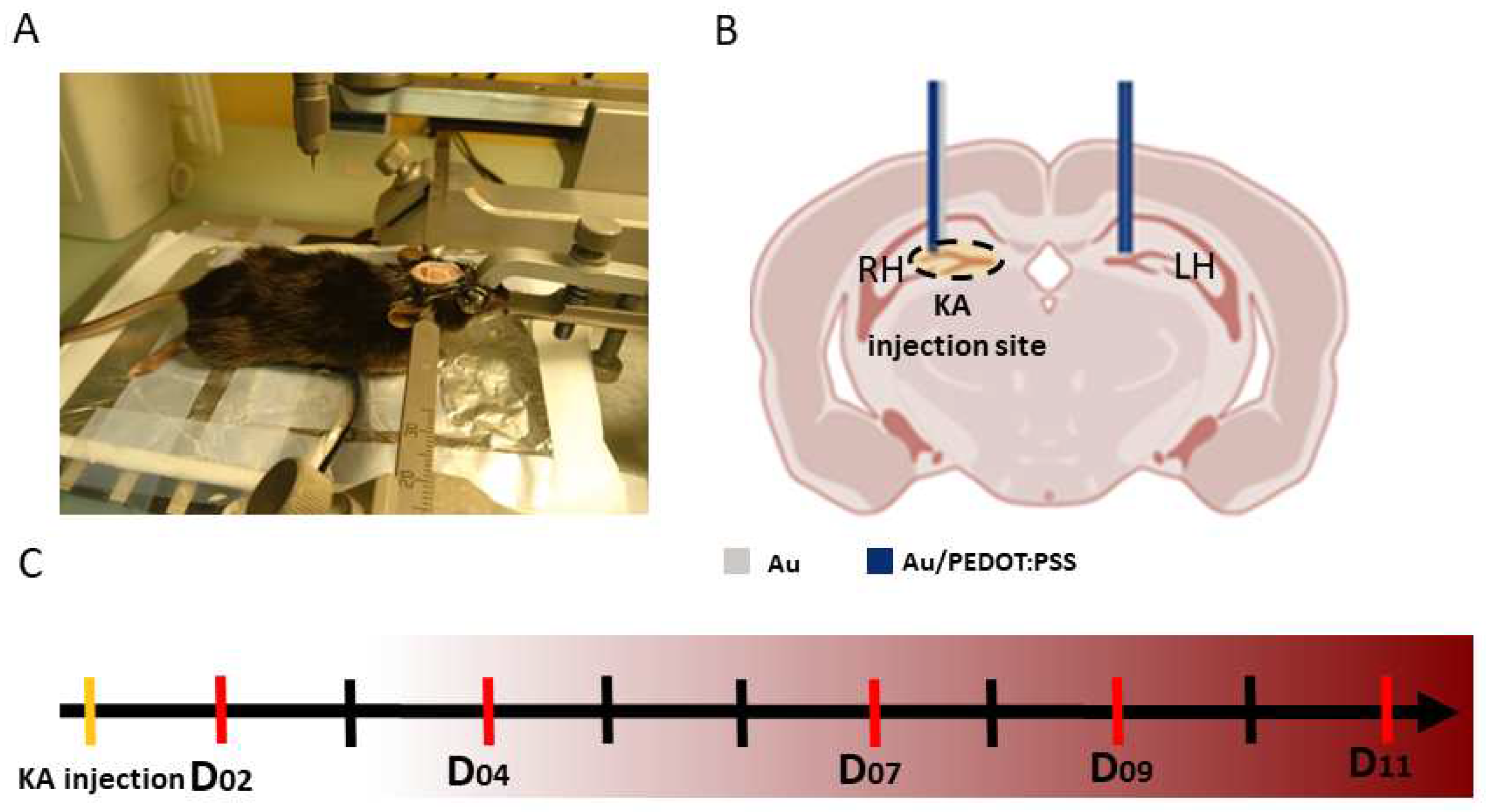
2.4. Experimental recordings
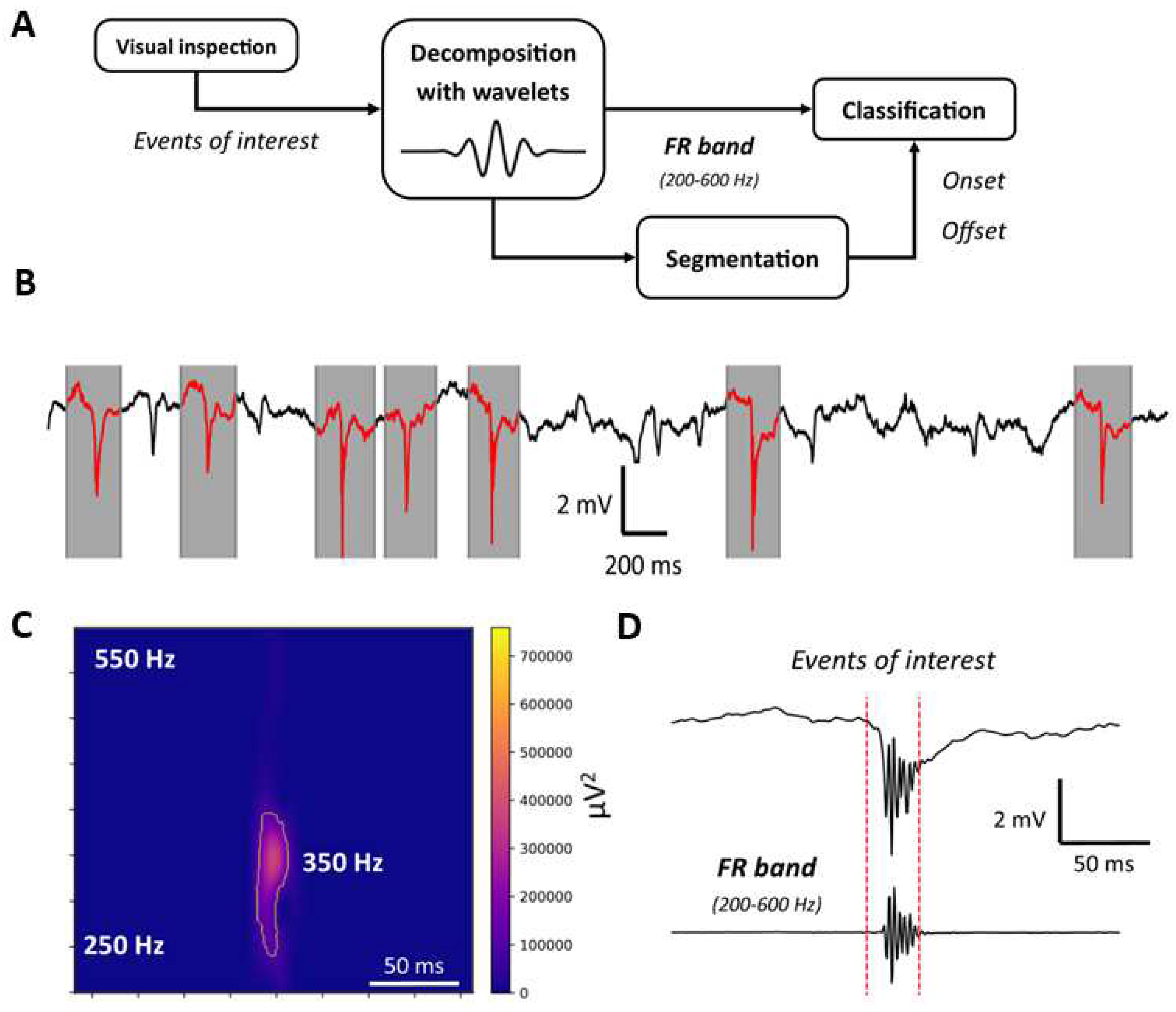
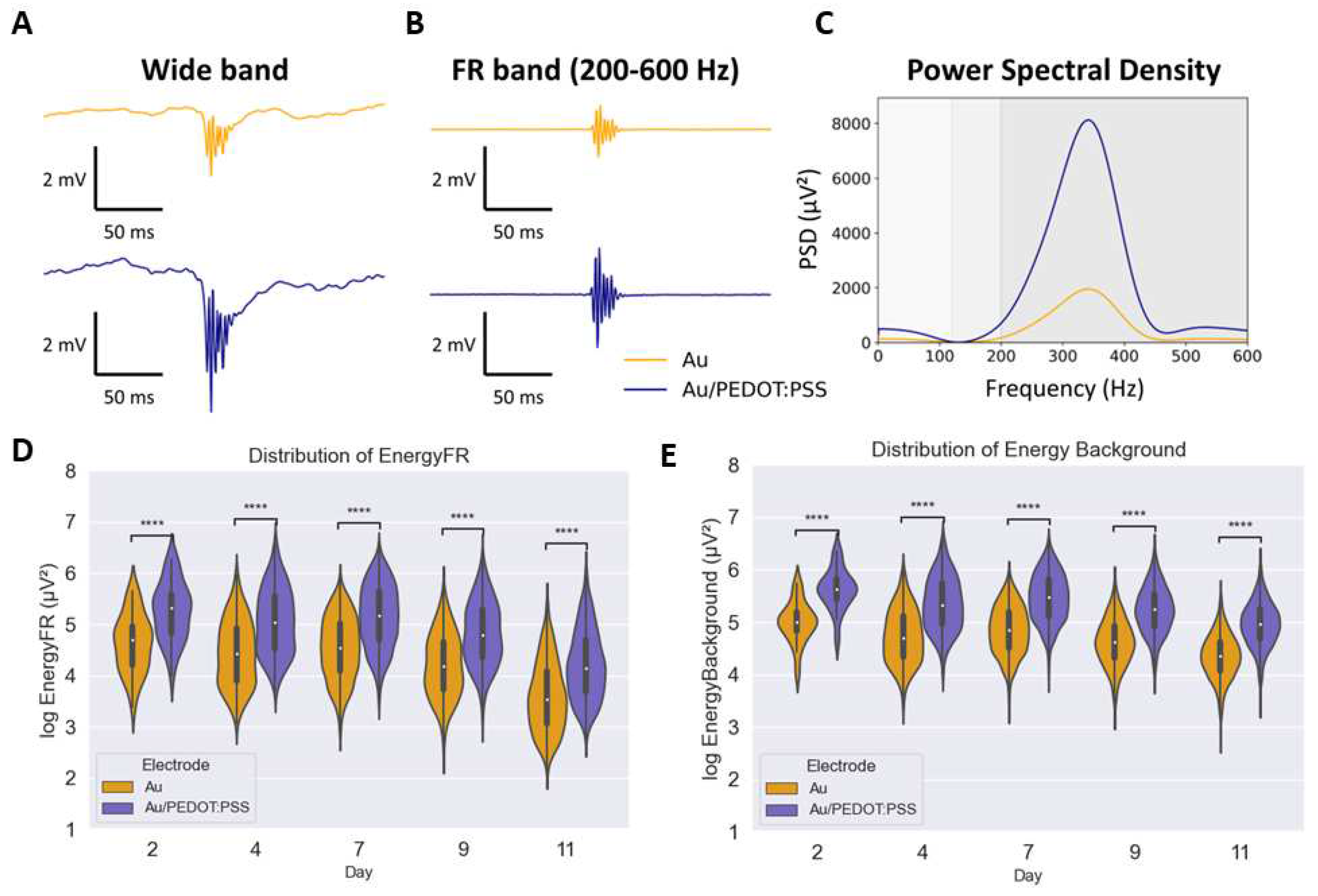
2.5. FRs identification and analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EZ | Epileptogenic Zone |
| FRs | Fast Ripples |
| Au | Gold |
| PEDOT:PSS | poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonate) |
| Au/PEDOT:PSS | PEDOT:PSS coated Au |
| LFPs | Local Field Potentials |
| SNR | Signal to Noise Ratio |
| CP | Conductive Polymers |
| EDOT | 3,4-ethylenedioxythiophene |
| ETI | Electrode Tissue Interface |
| LH | Left Hippocampus |
| LR | Right Hippocampus |
| DG | Dentate Gyrus |
| SS | Stainless Steel |
| R | Ripples |
| EIS | Electrochemical Impedance Spectroscopy |
| CV | Cyclic Voltammetry |
References
- Zuberi, S.M.; Wirrell, E.; Yozawitz, E.; Wilmshurst, J.M.; Specchio, N.; Riney, K.; Pressler, R.; Auvin, S.; Samia, P.; Hirsch, E.; Galicchio, S.; Triki, C.; Snead, O.C.; Wiebe, S.; Cross, J.H.; Tinuper, P.; Scheffer, I.E.; Perucca, E.; Moshé, S.L.; Nabbout, R. ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia 2022, 63, 1349–1397. [Google Scholar] [CrossRef] [PubMed]
- Bragin, A.; Engel, J.; Staba, R.J. High-frequency oscillations in epileptic brain. Current Opinion in Neurology 2010, 23, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Burnos et al., S. The morphology of high frequency oscillations (HFO) does not improve delineating the epileptogenic zone. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology 2016, 127, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.; Pitkanen, A.; Loeb, J.A.; Dudek, F.E.; Bertram, E.H.; Cole, A.J.; Moshé, S.L.; Wiebe, S.; Jensen, F.E.; Mody, I.; Nehlig, A.; Vezzani, A. Epilepsy biomarkers. Epilepsia 2013, 54, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zijlmans, M.; Jiruska, P.; Zelmann, R.; Leijten, F.S.; Jefferys, J.G.; Gotman, J. High-frequency oscillations as a new biomarker in epilepsy. Annals of Neurology 2012, 71, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Bragin, A.; Engel, J.; Wilson, C.L.; Fried, I.; Buzsáki, G. High-frequency oscillations in human brain. Hippocampus 1999, 9, 137–142. [Google Scholar] [CrossRef]
- Staba, R.J.; Stead, M.; Worrell, G.A. Electrophysiological Biomarkers of Epilepsy. Neurotherapeutics 2014, 11, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Urrestarazu, E.; Chander, R.; Dubeau, F.; Gotman, J. Interictal high-frequency oscillations (10-500 Hz) in the intracerebral EEG of epileptic patients. Brain 2007, 130, 2354–2366. [Google Scholar] [CrossRef]
- Frauscher, B.; Bartolomei, F.; Kobayashi, K.; Cimbalnik, J.; van ‘t Klooster, M.A.; Rampp, S.; Otsubo, H.; Höller, Y.; Wu, J.Y.; Asano, E.; Engel, J.; Kahane, P.; Jacobs, J.; Gotman, J. High-frequency oscillations: The state of clinical research. Epilepsia 2017, 58, 1316–1329. [Google Scholar] [CrossRef]
- Ibarz, J.M.; Foffani, G.; Cid, E.; Inostroza, M.; De La Prida, L.M. Emergent dynamics of fast ripples in the epileptic hippocampus. Journal of Neuroscience 2010, 30, 16249–16261. [Google Scholar] [CrossRef]
- Li, L.; Bragin, A.; Staba, R.; Engel, J. Unit firing and oscillations at seizure onset in epileptic rodents, 2019. [CrossRef]
- Al Harrach, M.; Benquet, P.; Wendling, F. Long term evolution of fast ripples during epileptogenesis. Journal of Neural Engineering 2021, 18, 046027. [Google Scholar] [CrossRef] [PubMed]
- Demont-Guignard, S.; Benquet, P.; Gerber, U.; Biraben, A.; Martin, B.; Wendling, F. Distinct hyperexcitability mechanisms underlie fast ripples and epileptic spikes. Annals of Neurology 2012, 71, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Luo, J.; Chen, X.; Zhang, Y.; Yu, S.; Shu, H. Association Between Removal of High-Frequency Oscillations and The Effect of Epilepsy Surgery: A Meta-Analysis. Journal of Neurological surgery. Part A, Central European Neurosurgery. [CrossRef]
- Wu, J.; Sankar, R.; Lerner, J.; Matsumoto, J.; Vinters, H.; Mathern, G. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology 2010, 75, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Zijlmans, M.; Zelmann, R.; Chatillon, C.É.; Hall, J.; Olivier, A.; Dubeau, F.; Gotman, J. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 2010, 67, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Al Harrach, M.; Dauly, G.; Seyedeh-Mousavi, H.; Dieuset, G.; Benquet, P.; Ismailova, E.; Wendling, F. Improving Fast Ripples Recording with Model-Guided Design of Microelectrodes. IEEE Transactions on Biomedical Engineering 2023. [Google Scholar] [CrossRef]
- Amiri, M.; Lina, J.M.; Pizzo, F.; Gotman, J. High Frequency Oscillations and spikes: Separating real HFOs from false oscillations. Clinical Neurophysiology 2016, 127, 187–196. [Google Scholar] [CrossRef]
- Roehri, N.; Pizzo, F.; Bartolomei, F.; Wendling, F.; Bénar, C.G. What are the assets and weaknesses of HFO detectors? A benchmark framework based on realistic simulations. PLoS ONE 2017, 12, 1–20. [Google Scholar] [CrossRef]
- Buzsáki, G.; Anastassiou, C.A.; Koch, C. The origin of extracellular fields and currents-EEG, ECoG, LFP and spikes. Nature Reviews Neuroscience 2012, 13, 407–420. [Google Scholar] [CrossRef]
- Boehler, C.; Carli, S.; Fadiga, L.; Stieglitz, T.; Asplund, M. Tutorial: guidelines for standardized performance tests for electrodes intended for neural interfaces and bioelectronics. Nature Protocols 2020, 15, 3557–3578. [Google Scholar] [CrossRef]
- Harris, K.D.; Quiroga, R.Q.; Freeman, J.; Smith, S.L. Improving data quality in neuronal population recordings. Nature Neuroscience 2016, 19, 1165–1174. [Google Scholar] [CrossRef]
- Liu, X.; Demosthenous, A.; Donaldson, N. Platinum electrode noise in the ENG spectrum. Medical and Biological Engineering and Computing 2008, 46, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Stacey et al., W. C. Signal distortion from microelectrodes in clinical EEG acquisition systems. Journal of Neural Engineering 2012, 9, 056007. [Google Scholar] [CrossRef]
- Rivnay, J.; Wang, H.; Fenno, L.; Deisseroth, K.; Malliaras, G.G. Next-generation probes, particles, and proteins for neural interfacing. Science Advances 2017, 3. [Google Scholar] [CrossRef]
- Wang et al., K. High-performance graphene-fiber-based neural recording microelectrodes. Advanced materials 2019, 31, 1805867. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; del Agua, I.; Malliaras, G.G.; Mecerreyes, D. Conductive Poly(3,4-Ethylenedioxythiophene) (PEDOT)-Based Polymers and Their Applications in Bioelectronics, second edi ed.; Elsevier Ltd., 2019; pp. 191–218. [CrossRef]
- Donahue, M.J.; Sanchez-Sanchez, A.; Inal, S.; Qu, J.; Owens, R.M.; Mecerreyes, D.; Malliaras, G.G.; Martin, D.C. Tailoring PEDOT properties for applications in bioelectronics. Materials Science and Engineering R: Reports 2020, 140, 100546. [Google Scholar] [CrossRef]
- Rivnay, J.; Owens, R.M.; Malliaras, G.G. The rise of organic bioelectronics. Chemistry of Materials 2014, 26, 679–685. [Google Scholar] [CrossRef]
- Bianchi, M.; Salvo, A.D.; Asplund, M.; Carli, S.; Lauro, M.D.; Schulze-bonhage, A.; Stieglitz, T.; Fadiga, L.; Biscarini, F. Poly ( 3 , 4-ethylenedioxythiophene ) -Based Neural Interfaces for Recording and Stimulation : Fundamental Aspects and In Vivo Applications. 2022, 210470, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Abidian, M.R.; Martin, D.C. Multifunctional nanobiomaterials for neural interfaces. Advanced Functional Materials 2009, 19, 573–585. [Google Scholar] [CrossRef]
- Cui, X.; Martin, D.C. Electrochemical deposition and characterization of poly(3,4-ethylenedioxythiophene) on neural microelectrode arrays. Sensors and Actuators, B: Chemical 2003, 89, 92–102. [Google Scholar] [CrossRef]
- Robinson, D.A. The Electrical Properties of Metal Microelectrodes. Proceedings of the IEEE 1968, 56, 1065–1071. [Google Scholar] [CrossRef]
- Karki, J. Effect of parasitic capacitance in op amp circuits. Application Report sloa013a, 2000; 1–26. [Google Scholar]
- Suzuki, F.; Junier, M.P.; Guilhem, D.; Sørensen, J.C.; Onteniente, B. Morphogenetic effect of kainate on adult hippocampal neurons associated with a prolonged expression of brain-derived neurotrophic factor. Neuroscience 1995, 64, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, M.; Avoli, M. High-frequency oscillations and focal seizures in epileptic rodents. Neurobiology of Disease 2019, 124, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B. Paxinos and Franklin’s the mouse brain in stereotaxic coordinates; Academic press, 2019.
- Bénar, C.G.; Chauvière, L.; Bartolomei, F.; Wendling, F. Pitfalls of high-pass filtering for detecting epileptic oscillations: A technical note on "false" ripples. Clinical Neurophysiology 2010, 121, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Jrad, N.; Kachenoura, A.; Merlet, I.; Bartolomei, F.; Nica, A.; Biraben, A.; Wendling, F. Automatic Detection and Classification of High-Frequency Oscillations in Depth-EEG Signals. IEEE Transactions on Biomedical Engineering 2017, 64, 2230–2240. [Google Scholar] [CrossRef]
- Harrach, M.A.; Benquet, P.; Wendling, F. Long term evolution of fast ripples during epileptogenesis. Journal of Neural Engineering 2021, 18. [Google Scholar] [CrossRef] [PubMed]
- Mark, E. Orazem, B.T. Electrochemical Impedance Espectroscopy.
- Liang, Y.; Offenhäusser, A.; Ingebrandt, S.; Mayer, D. PEDOT: PSS-based bioelectronic devices for recording and modulation of electrophysiological and biochemical cell signals. Advanced Healthcare Materials 2021, 10, 2100061. [Google Scholar] [CrossRef]
- Rathore, P.; Schiffman, J.D. Effect of pH Value on the Electrical Properties of PEDOT: PSS-Based Fiber Mats. ACS Engineering Au 2023. [Google Scholar] [CrossRef]
- Júnior, C.; Spinelli, B.; Damasceno, I.; Fiuza, F.; Morya, E.; others. All-Polymeric Electrode Based on PEDOT: PSS for In Vivo Neural Recording. Biosensors 2022, 12, 853. [Google Scholar] [CrossRef] [PubMed]
- Boehler, C.; Kleber, C.; Martini, N.; Xie, Y.; Dryg, I.; Stieglitz, T.; Hofmann, U.G.; Asplund, M. Actively controlled release of Dexamethasone from neural microelectrodes in a chronic in vivo study. Biomaterials 2017, 129, 176–187. [Google Scholar] [CrossRef]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. Journal of Neuroscience Methods 2005, 148, 1–18. [Google Scholar] [CrossRef]
- Kozai, T.D.; Vazquez, A.L.; Weaver, C.L.; Kim, S.G.; Cui, X.T. In vivo two-photon microscopy reveals immediate microglial reaction to implantation of microelectrode through extension of processes. Journal of Neural Engineering 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Otto, K.J.; Kipke, D.R. Repeated voltage biasing improves unit recordings by reducing resistive tissue impedances. IEEE Transactions on Neural Systems and Rehabilitation Engineering 2005, 13, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Charkhkar, H.; Knaack, G.L.; Mchail, D.G.; Mandal, H.S.; Peixoto, N.; Rubinson, J.F.; Dumas, T.C.; Pancrazio, J.J. Chronic intracortical neural recordings using microelectrode arrays coated with PEDOT-TFB. Acta Biomaterialia 2016, 32, 57–67. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





