Submitted:
13 December 2023
Posted:
14 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Overview of Anticoagulation
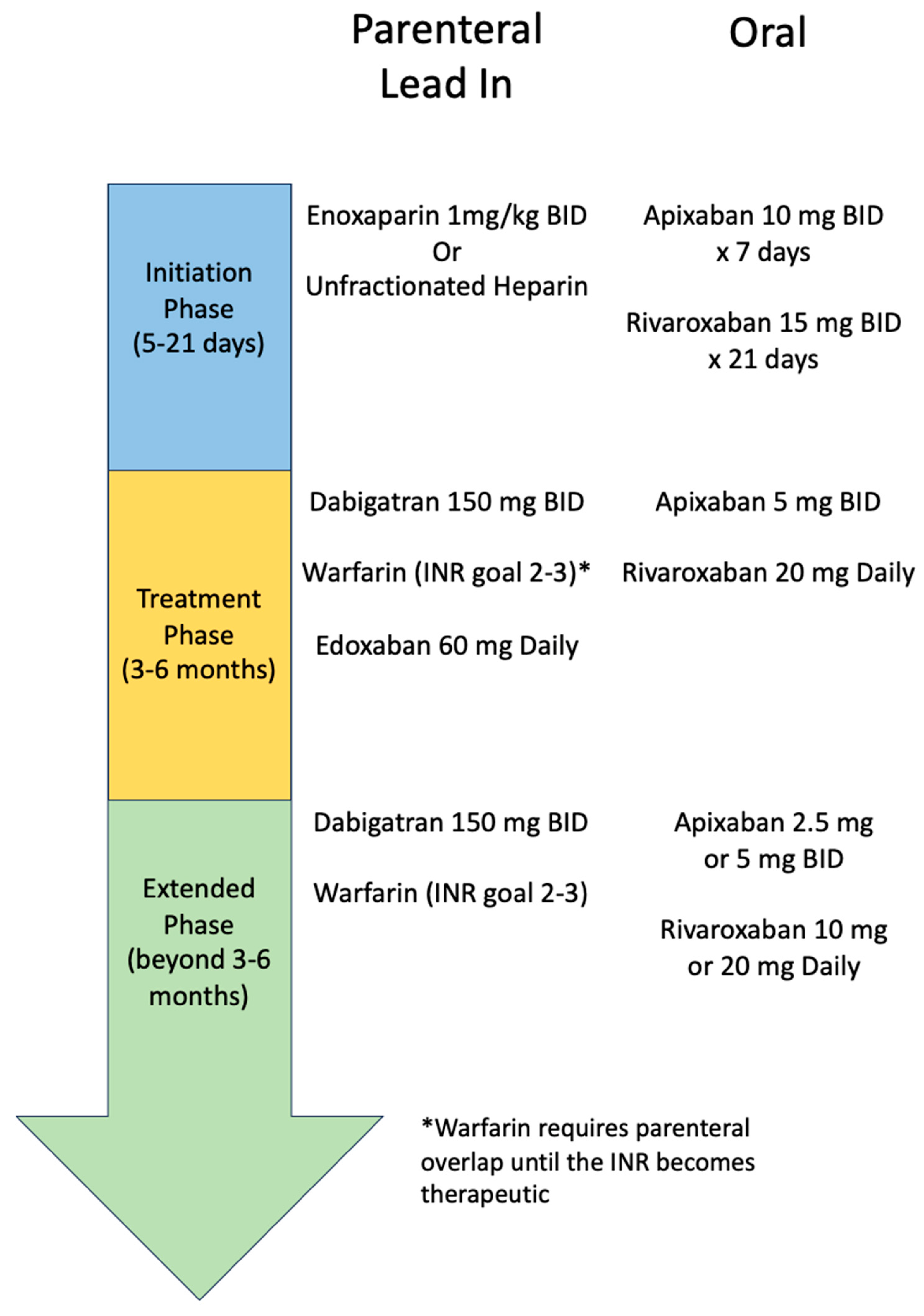
3. Phases of Management of VTE
3.1. Initiation Phase
3.2. Treatment Phase
3.2. Extended Phase
4. Special Considerations
4.1. Cancer-associated Thrombosis Treatment
4.2. Thrombotic Antiphospholipid Antibody Syndrome Treatment
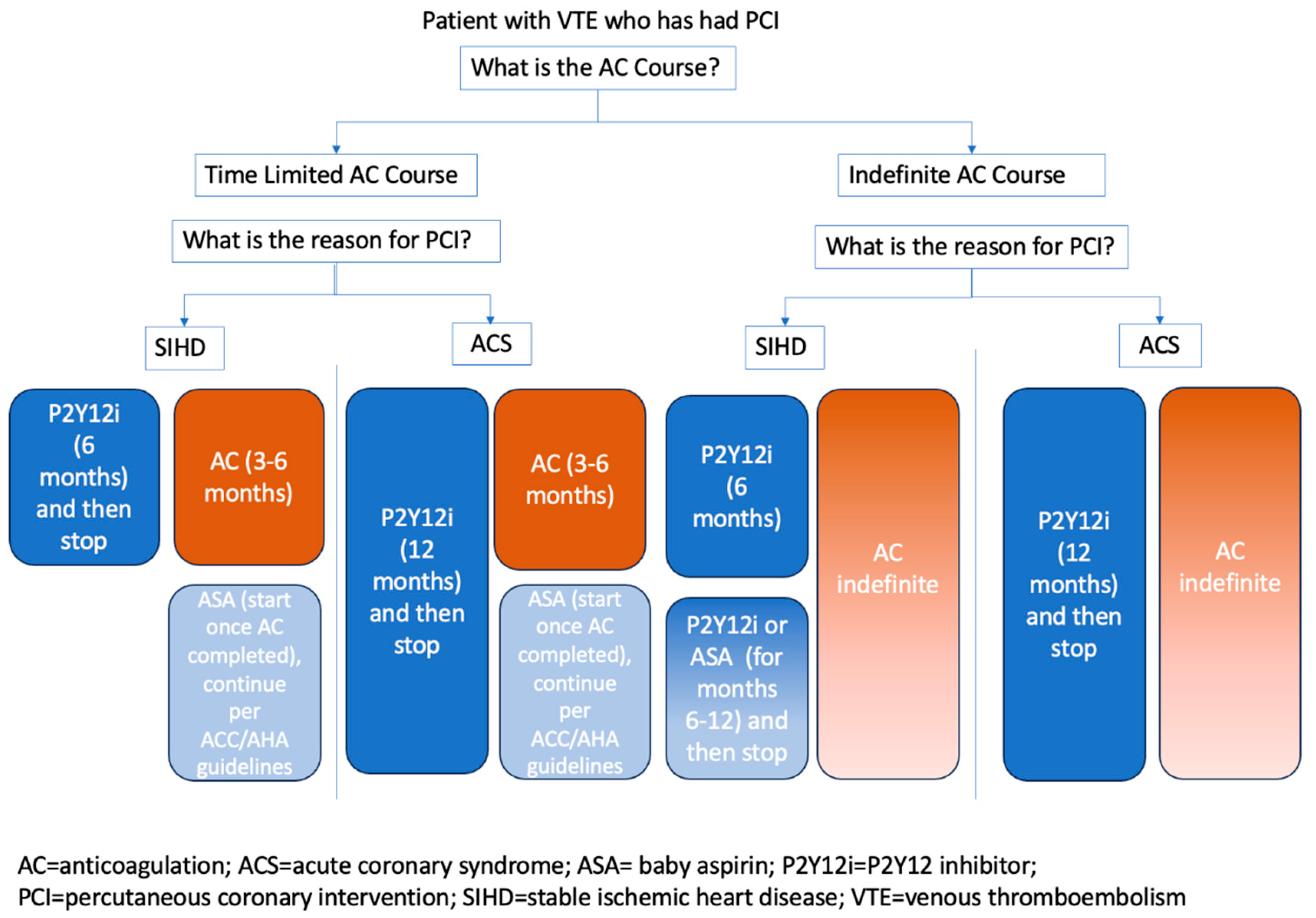
4.3. Concurrent Coronary Artery Disease and Venous Thromboembolism
4.4. COVID-19 Infection
5. Future Anticoagulation Options
6. Final Thoughts
Author Contributions
Funding
Conflicts of Interest
References
- Beckman, M.G; Hooper, W.C.; Critchley, S.E.; Ortel, T.L. Venous thromboembolism: a public health concern. Am J Prev Med 2010, 38 (Suppl. 4), S495–501. [Google Scholar] [CrossRef] [PubMed]
- CDC. Data and Statistics on Venous Thromboembolism. Available online: https://www.cdc.gov/ncbddd/dvt/data.html (accessed on 11/6/2023).
- Kearon, C.; Ageno, W.; Cannegieter, S.C.; Cosmi, B.; Geersing, G.J.; Kyrle, P.A. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. Journal of Thrombosis and Haemostasis 2016, 14, 1483. [Google Scholar] [CrossRef] [PubMed]
- Renner, E.; Barnes, G.D. Antithrombotic Management of Venous Thromboembolism: JACC Focus Seminar. J Am Coll Cardiol 2020, 76, 2142–2154. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.M.; Woller, S.C.; Baumann Kreuziger, L.; et al. Executive Summary: Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report. Chest 2021, 160, 2247–2259. [Google Scholar] [CrossRef] [PubMed]
- Vinson, D.R.; Ballard, D.W.; Huang, J.; et al. MAPLE Investigators of the KP CREST Network. Outpatient Management of Emergency Department Patients With Acute Pulmonary Embolism: Variation, Patient Characteristics, and Outcomes. Ann Emerg Med 2018, 72, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Ortel, T.L.; Neuman, I.; et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv 2020, 4, 4693–4738. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). European Heart Journal 2020, 41, 41–543. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, S.; Heinze, G.; Jandeck, L.M.; Kyrle, P.A. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010, 121, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Rodger, M.A.; Kahn, S.R.; Wells, P.S.; et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008, 179, 417–426. [Google Scholar] [CrossRef]
- Tosetto, A.; Iorio, A.; Marcucci, M.; et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012, 10, 1019–1025. [Google Scholar] [CrossRef]
- Bauersachs, R.; Berkowitz, S.D.; Brenner, B.; et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010, 363, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kearon, C.; Kakkar, AK.; et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013, 368, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Buller, H.R.; Cohen, A.; et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013, 368, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I.; Lensing, A.W.A.; Prins, M.H.; et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med 2017, 376, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Young, A.M.; Marshall, A.; Thirlwall, J.; et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol 2018, 36, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- McBane, R. 2nd.; Wysokinski, W.E.; Le-Rademacher, J.G.; et al. Apixaban and dalteparin inactive malignancy-associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost 2020, 18, 411–421. [CrossRef] [PubMed]
- Agnelli, G.; Becattini, C.; Meyer, G. , et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med 2020, 382, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Carrier, M.; Ay, C.; et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv 2021, 5, 927–974. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Garcia, D.A.; Lyman, G.H.; Carrier, M. ; Direct oral anticoagulant (DOAC) versus low-molecular weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): a systematic review and meta-analysis. Thromb Res 2019, 173, 158–163. [Google Scholar] [CrossRef]
- Mosarla, R.C.; Vaduganathan, M.; Qamar, A.; et al. Anticoagulation Strategies in Patients With Cancer: JACC Review Topic of the Week. J Am Coll Cardiol, 2019, 73, 1336–1349. [Google Scholar] [CrossRef]
- Khairani, C.D.; Bejjani, A.; Piazza, G.; et al. Direct Oral Anticoagulants vs Vitamin K Antagonists in Patients With Antiphospholipid Syndromes: Meta-Analysis of Randomized Trials. Journal of the American College of Cardiology 2023, 81, 16–30. [Google Scholar] [CrossRef]
- Cohen, H.; Hunt, B.J.; Efthymiou, M.; et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol 2016, 3, e426–e436. [Google Scholar] [CrossRef]
- Pengo, V.; Denas, G.; Zoppellaro, G.; et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018, 132, 1365–1371. [Google Scholar] [CrossRef]
- Ordi-Ros, J.; Saez-Comet, L.; Perez-Conesa, M.; et al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: a randomized noninferiority trial. Ann Intern Med 2019, 171, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Woller, S.C.; Stevens, S.M.; Kaplan, D.; et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: a randomized trial. Blood Adv 2022, 6, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.L.; Sorensen, R.; Clausen, M.T.; et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Archives of Internal Medicine 2010, 170, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Kumbhani, D.J.; Cannon, C.P.; Beavers, C.J.; et al. 2020 ACC Expert Consensus Decision Pathway for Anticoagulant and Antiplatelet Therapy in Patients With Atrial Fibrillation or Venous Thromboembolism Undergoing Percutaneous Coronary Intervention or With Atherosclerotic Cardiovascular Disease: A Report of the American College of Cardiology Solution Set Oversight Committee. Journal of the American College of Cardiology 2021, 77, 629–658. [Google Scholar] [PubMed]
- NIH. Antithrombotic Therapy in Patients With COVID-19. Available online: https://www.covid19treatmentguidelines.nih.gov/therapies/antithrombotic-therapy (accessed on 11/26/2023).
- ATTACC, ACTIV-4a, and REMAP-CAP Investigators. Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19. N Engl J Med 2021, 385, 790–802. [Google Scholar] [CrossRef]
- Sholzberg, M.; Tang, G.H.; Rahhal, H.; et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with COVID-19 admitted to hospital: RAPID randomised clinical trial. BMJ 2021, 375, n2400. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Goldin, M.; Giannis, D.; et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med 2021, 181, 1612–1620. [Google Scholar] [CrossRef]
- Stone, G.W.; Farkouh, M.E.; Lala, A.; et al. Randomized trial of anticoagulation strategies for noncritically ill patients hospitalized with COVID-19. J Am Coll Cardiol 2023, 81, 1762. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; de Barros, E.S.P.G.M.; Furtado, R.H.M.; et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet 2021, 397, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Ramacciotti, E.; Agati, L.B.; Calderaro, D.; et al. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. The Lancet 2022, 399, 50–59. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2022, 399, 143–151. [Google Scholar]
- A Study Comparing Abelacimab to Apixaban in the Treatment of Cancer-associated VTE (ASTER) Available online:. Available online: https://www.clinicaltrials.gov/study/NCT05171049 (accessed on 11/26/2023).
- A Study Comparing Abelacimab to Dalteparin in the Treatment of Gastrointestinal/Genitourinary Cancer and Associated VTE (MAGNOLIA) Available online:. Available online: https://www.clinicaltrials.gov/study/NCT05171075 (accessed on 11/26/2023).
- Xisomab 3G3 for the Prevention of Catheter-Associated Thrombosis in Patients With Cancer Receiving Chemotherapy Available online:. Available online: https://clinicaltrials.gov/study/NCT04465760 (accessed on 11/26/2023).
| Major Transient Risk Factor | Minor Transient Risk Factor | Persistent Risk Factor |
| -Cesarean section -Confined to hospital bed for 3 days -Surgery with general anesthesia for >30 minutes |
-Confined to bed out of hospital for 3 days -Hospitalization < 3 days -Leg Injury -Pregnancy -Estrogen therapy -Acute infectious illness (e.g., COVID-19) without hospitalization |
-Active cancer -Inflammatory bowel disease -Obesity -Chronic inflammatory condition -Advanced age -Previous venous thromboembolism -Genetic/Acquired thrombophilia (APLS, protein C&S deficiency, etc) |
| Generic Name | Mechanism of Action | Dose and Regimen | Consideration of Renal Function | Consideration of Drug Interactions | Other Considerations |
| Apixaban | Factor Xa Inhibitor | 10 mg BID x7days, followed by 5 mg BID | Not studied in patients with SCr ≥ 2.5 mg/dl or CrCl <25 ml/min | Reducing dose by 50% in patients taking strong dual inhibitors of p-glycoprotein and CYP 3A4. Avoiding in patients taking dual inducers of CYP 34A and p-glycoprotein. | N/a |
| Dabigatran | Direct Thrombin Inhibitor | 150 mg BID after 5-10 days of parenteral anticoagulation lead in | Avoid in CrCl ≤ 30 ml/min | If CrCl ≤ 50 ml/min, patients taking p-glycoprotein inhibitors should avoid dabigatran. Patients taking p-glycoprotein inducers should avoid dabigatran. | N/a |
| Edoxaban | Factor Xa Inhibitor | 60 mg daily after 5-10 days of parenteral anticoagulation lead in | Renally dose to 30 mg daily for CrCl 15-50 ml/min. Avoid in CrCl <15 ml/min | Reduce dose to 30 mg daily for patients taking p-glycoprotein inhibitors. Avoid using with p-glycoprotein inducers. | Reduce dose to 30 mg daily for body weight ≤ 60 kg. |
| Rivaroxaban | Factor Xa Inhibitor | 15 mg twice a day for 21 days, then 20 mg daily | Avoid in CrCl ≤ 15 ml/min | In patients taking moderate dual inhibitors of CYP 3A4 and p-glycoprotein with CrCl ≤ 80 ml/min, use cautiously. Avoid use in patients taking strong dual inhibitors or inducers of CYP 3A4 and p-glycoprotein. | Administer with food. |
| Warfarin | Vitamin K Antagonist | Adjusted to target INR 2-3Require parenteral anticoagulation overlap at initiation | None | Consider reducing starting dose to 2.5 mg for patients with drug-drug interactions expected to increase exposure to warfarin. | Consider reducing starting dose to 2.5 mg for patients with multiple comorbidities, advanced age, and advanced end-organ dysfunction. |
| Clinical Trial [Ref. #] | Included Patients | N | Trial Design | Length of Follow-Up | Treatment Groups | Primary Efficacy Outcomes | Efficacy Outcomes | Major Bleeding Outcomes |
| RAPS [23] | Patients with APS who were taking warfarin for previous VTE | 116 | Open label RCT | 210 days | Continue warfarin vs rivaroxaban 20 mg daily | Percentage change in endogenous thrombin potential at day 42, with non-inferiority set at less than 20% difference from warfarin | ETP (nmol/L per min): Rivaroxaban 1086 vs warfarin 548 Treatment effect (ratio): 2.0 (1.7-2.4) |
Rivaroxaban: 0 Warfarin: 0 |
|
TRAPS [24] |
Patients with APS (triple positivity) with history of thrombus | 120 | Open label RCT | 569 days (mean) | Rivaroxaban 20 mg or 15 mg daily (dependent on creatine clearance) vs warfarin | Cumulative incidence of thromboembolic events, major bleeding, and vascular death | Rivaroxaban: 19% Warfarin: 3% HR: 6.7 (1.5-30.5) |
Rivaroxaban: 7% Warfarin: 3% HR: 2.5 (0.5-13.6) |
| Ordi-Ros et al [25] | Patients with APS (positive result on aPL testing on 2 occasions at least 3 months apart) with history of thrombus | 190 | Open label RCT | 36 months | Rivaroxaban 20 mg or 15 mg daily (dependent on creatine clearance) vs warfarin | Proportion of patients with new thrombotic event | Rivaroxaban: 11.6% Warfarin: 6.3% HR: 1.94 (0.72-5.24) |
Rivaroxaban: 6.3% Warfarin: 7.4% HR: 0.88 (0.3-2.63) |
| ASTRO-APS [26] | Patients with thrombotic antiphospholipid syndrome on anticoagulation for secondary prevention | 48 | Open label RCT | 12 months | Apixaban 2.5 mg BID then increased to 5 mg BID (after 25 patient was randomized) vs warfarin | Thrombosis and vascular death | Apixaban: 6 thrombotic events Warfarin: no thrombotic events |
Apixaban: 0 Warfarin: 1 event |
| Clinical Trial Reference (Status) | Drug | Mechanism of Action | N | Clinical Trial Summary | Results |
| ASTER NCT05171049 (Ongoing) [37] | Abelacimab | Binds and inhibits Factor XI and Factor XIa | 1655 | Phase III trial comparing the effect of abelacimab relative to apixaban on VTE recurrence and bleeding in patients with CAT | No results currently |
| MAGNOLIA NCT05171075 (Ongoing) [38] | Abelacimab | Binds and inhibits Factor XI and Factor XIa | 1020 | Phase III trial comparing the effect of abelacimab vs. dalteparin on VTE recurrence and bleeding in patients with gastrointestinal or genitourinary CAT | No results currently |
| NCT04465760 (Recruiting) [39] | Xisomab | Binds Factor XI and blocks activation by Factor XIIa | 50 | Phase II trial examining the efficacy of xisomab as measured by incidence of catheter associated thrombosis in individuals with a central venous catheter | No results currently |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





