1. Introduction
Rethinking packaging, for recyclability, circularity and sustainability is required to overcome the current packaging challenges as fossil raw material dependency or waste management. The use of an edible coating is one of the strategies, to improve the sustainability of food packages, which combines the need to use renewable natural biomaterials and reduces packaging waste by being edible or biodegradable. A coating is a thin homogeneous layer formed from a natural film forming material, as carbohydrates, proteins or lipids, on the product surface [
1] and acts as a packaging by preventing or controlling deteriorative reactions of the product. Potential renewable natural film forming polysaccharides, sourced from maritime environment are for example, chitosan and alginate. Advantages of using maritime polymeric materials as edible coatings for seafood products are their availability in the production area of the seafood products, and generation of a new market for processing waste products.
Chitosan is a derivative of chitin the second most abundant natural polymer in nature and is mainly produced from shrimp and crab shells, a waste product of the seafood industry with a global waste accumulation of 6 to 8 million tonnes [
2,
3]. Chitosan is a suitable material for edible coatings, as it is nontoxic, biodegradable, biocompatible and general recognized as safe by US Food and Drug Administration [
4]. Chitosan coatings showed an excellent coating behaviour by forming a transparent, invisible and adhesive layer around food products, and was shown to control microbial growth [
5,
6] and reduce fat oxidation [
7]. Variations in chitosan coatings composition and processing factors were reported, e.g., chitosan with different degrees of acetylation from 70 to 95%, chitosan concentrations from 0.5 to 3%, different solvents with concentrations from 1 to 2%, stirring temperature from 23 to 40°C, or addition of glycerol from 0.5 to 75% of w/w chitosan [
5,
6,
8,
9,
10,
11,
12,
13]. The potential of chitosan coatings is well studied for different fresh fish products [
5,
8,
11,
14], but limited studies for Ready-to-Eat (RTE) food products, and particularly RTE seafood products [
6,
7,
9] could be found.
Alginate can be found in cell-walls and intercellular regions of most brown algae species which are abundant in many coastlines. Alginates are seen as valuable and sustainable resource, avoiding competition for land use with food production and are a low cost product [
15]. Alginate is biodegradable, biocompatibility, nontoxic, has excellent film forming properties and is widely studied as carrier coating material for natural antimicrobial or antioxidant agents for fresh fish products [
16,
17,
18,
19,
20,
21,
22]. Alginate coating was reported to control food product degradations processes, as lipid oxidation [
17,
18,
19,
20,
21,
22] and microbiological growth [
17,
18,
19]. Studies with alginate coatings applied to RTE seafood products are rare [
7] and the effect can depend on the coating composition and development process.
RTE seafood products are in high demand considering the current consumer trends of convenience, healthy, nutritious, mildly preserved foods and products with an enhanced shelf-life and controlled product quality [
23]. Additionally, considering an upward trend on the growth of elderly population in every country in the world [
24], seafood products can provide an important food source with high nutritional value [
25]. RTE food is defined as food intended for direct human consumption without the need for cooking or other processing preparation steps to eliminate or reduce microorganisms of concern [
26]. The shelf life of chilled RTE products is limited, and suitable handling until consumption is crucial to maintain the quality and provide a safe product. The use of hurdle technology, as for example pre-preservation steps, cooled storage and packaging is a common strategy applied to provide a product with an extended shelf life. The application of coatings for RTE seafood products is still limited beside several advantages as enhancing the product safety and quality and being an alternative edible packaging option.
The objective of this study was to develop an edible coating for controlling degradation processes of a RTE baked fish fillet. As the protection functions of a coating are affected by material properties, coating composition and development process, for this study two maritime based coatings, based on chitosan and alginate were developed and optimised. Additionally, the performance of the selected coatings as well as a bi-layer coating, to combine the advantages of both coatings, were compared under optimal and abused storage conditions. The shelf life of the baked fish product with and without coatings were estimated using a desirability analysis to compare the overall performance of the studied coatings.
2. Materials and Methods
2.1. Chemicals
Acetic acid, calcium chloride anhydrous, chitosan (medium molecular weight), glycerol, peptone buffer, plate count agar, sodium alginate, 1,1,3,3-tetraethoxypropane, thiobarbituric acid and trichloroacetic acid were purchased from Sigma-Aldrich.
2.2. Experimental Design & Set Up
An overview of the performed experimental designs are illustrated in
Table 1. A 3^2 full factorial design was used to study the effect of chitosan concentration (1, 2 and 3%), and glycerol concentration (0, 15 and 30% w/w chitosan) on the coating performance to control degradation processes of a baked fish sample stored at 4°C for 14 days. The effect of the composition of an alginate coating was studying with a 2^3 full factorial design. The studied factors were alginate concentration (1 or 2% (w/v)), glycerol concentration (0 or 1.5% (w/w) alginate) and crosslinking the alginate coating with CaCl
2 to form calcium alginate (yes/no). Additionally, the performance of the selected chitosan coating, selected alginate coating, and samples coated with a bilayer coating consisting of a chitosan and alginate layer at optimal storage conditions (4°C) for 21 days and abused storage conditions (14°C) for 7 days were investigated. All samples were placed into a storage container (KIS C, XS) with a lid to control airflow and stored in an incubator (Sanyo) at defined temperature. The functionality of the coatings were assessed by measuring the lipid oxidation, water loss and the microbial total aerobic growth at day 0 and defined storage times (2, 4, 7 days or 7, 14, 21 days for samples stored at 14 and 4°C, respectively). An uncoated product was used as reference. All measurements were at least conducted in triplicates and values presented in this study are average values.
2.3. Product Preparation
Frozen white fish fillet was purchased from local supermarket. The nutritional composition of the product was 0.8% fat, 17% protein, 0.25% salt, and 0.5% fibre. The frozen fillets were placed on a baking tray and prepared with a mild heating treatment in an oven at 80°C for 70 min. The baked fillets were allowed to cool down to room temperature in a sterile storage box to avoid any cross contamination, before the fillets were cut into pieces of 20 to 25 g. The samples were examined and pieces with a similar thickness was selected for this study. The average water content of the RTE fish product after baking was 75%. Quality characteristics and microbiological contamination of the products were analysed at day 0, which was taken as reference point to analyse the deterioration process during storage.
2.4. Coating Preparation & Application
Chitosan film forming solutions (FFS) were prepared by either dissolving 1, 2 or 3% (w/v) chitosan in a 1% (v/v) acidic acid solution. The mixture was stirred with a head stirrer (IKA, Staufen) for 2 h at room temperature. Afterwards, when required 15 or 30% glycerol (w/w chitosan powder) was added and the mixture was stirred for additional 10 min. Alginate FFSs were prepared by dissolving sodium alginate (1 or 2% w/v) in distilled water by stirring for 30 min at 80°C. Afterwards, when required glycerol was added and the mixture was stirred for additional 10 min. The solutions were cooled down to room temperature. Both FFSs were allowed to rest for at least 12 h at 4°C before use.
Samples were coated by immersing the samples into FFS for 30s, followed by a draining step for 5 min. The process was repeated to ensure a complete coating formation on the sample surface. For the calcium-alginate coating the sodium alginate coated samples were immersed into a 0.2 M CaCl2 solution. A double coating was applied by first immersing the sample into chitosan FFS, drained for 5 min and afterwards immersed into the alginate FFS. Samples were allowed to air dry during storage.
2.5. Characterisation of Coating
Apparent viscosity of the FFS was measured using a Haake Rotor viscometer equipped with a coaxial cylinder geometry at 30°C ± 0.1. The shear rate was increased from 0.1 to 1000 s- 1 over 4 min, held at 1000 s-1 for 3 min, after which it was decreased from 100 to 0.1 s- 1 over 4 min.
The wet coating load (mg liquid coating/cm2 sample) defined as the coating adhered to the sample after the coating process was calculated by weighting the samples before and after the coating process. Additionally, the surface area of the samples was manually measured with the aid of a ruler and calculated by assuming a rectangular shape of the fish samples.
2.6. Characterisation of Product Quality
Thiobarbituric acid reactive substance assay (TBARS) was used to determine the lipid oxidation of samples [
27]. Briefly, 4 g sample was mixed with 10 mL of 10% trichloroacetic acid (w/v). Then, 5 mL of control solution, containing an aliquot of 30 μL of a 10
-3 M 1,1,3,3-tetraethoxypropane (TEP) or 5 mL of distilled water was added and vortex for 5 min. Freshly prepared 0.001 M thiobarbituric acid solution was added (5 mL) and mixed for 1 min. The reaction mixture was centrifugated at 3500 rpm for 5 min (Universal 16R, Hettich, Tuttlingen, Germany) and the supernatant was filter through a Whatman No. 1 filter. Afterwards the mixture was heated in a water bath (WB14, Memmert, Schwabach, Germany) at 95°C for 35 min and placed for 10 min into a cooled water bath for cooling. The supernatant absorbance was recorded against a blank, prepared as described above without the addition of sample, at 532 nm using a spectrophotometer (Libra S22, Biochrom, Cambridge, England). Standard solutions were prepared using 1,1,3,3-tetraethoxypropane to quantify the malondialdehyde (MDA) content, and results were expressed as mg MDA equivalent/Kg sample.
The weight of RTE fish samples was measured using a precision balance (Sartorius, Germany). The weight loss (WL) in % was calculated based on the initial (W
0) and final weight (W
f) of the product, as follows:
For coated samples the initial weight was set to the product weight after the coating was applied.
The total aerobic count (TAC) of the product was analysed by aseptically transferring around 10 - 15 g of sample to a sterile stomacher bag (Sewards LTD, UK) and adding peptone buffer to achieve a 10-fold dilution. The sample was mashed and hand shacked for 2 min. Appropriate dilutions were prepared of the resulting suspension with serially diluting in peptone buffer. For the total microbial count, the pour plate method with a plate count agar (PCA, Sigma Aldrich) was applied. The agar plates were incubated at 30°C for 72 h [
5]. Results were expressed as log10 colony forming units per gram sample (log CFU/g).
2.7. Statistical Analysis
Analysis of Variance (ANOVA) and post-hoc analysis using Tukey’s test at 95% confidence level were used to assess significant differences between the tested coating formulations. Full factorial designs were analysed with a significance level of 5%. Additionally, the desirability was calculated [
28], as follows:
Where, D is the total desirability, d is the desirability score of each property studied and w is the weight conferred to each property. Each property was weighted equally. The desirability score is determined using a linear transformation to the values from the Pareto Front, scaling the objectives between 0 and 1 from worst to best quality. The optimal combination of the tested coatings was validated qualitatively using characteristic quality and safety properties describing a lower level of product degradation, namely low lipid oxidation, water loss and TAC during storage. In order to avoid the elimination of some results that could be important to explain some observed phenomena, results with 0.05
< p < 0.1 were considered marginally significant, and also considered to the analysis [
29]. The statistical analysis was performed by using Statistica software for Windows v. 7.1 (Tulsa, USA).
3. Results
The coating material, coating formulation, and addition of additives as plasticisers can influence physical and protection properties of edible coatings. Therefore, the first step was to identify a suitable coating formulation for a RTE seafood product based on chitosan and alginate. The coating performance was measured by studying critical quality parameters of RTE baked fish fillet stored over 14 days at 4°C. Lipid oxidation is one of the main factors limiting the shelf-life of muscle foods due to present of higher amounts unsaturated fatty acids, leading to off-flavour, colour and odour development and contribute to texture deterioration [
4,
30]. Therefore, lipid oxidation measured by the malondialdehyde content developed during storage indicating the degree of secondary lipid oxidation [
31], was used as the first critical quality parameter. Controlling weight loss during storage was an additional important factor for quantifying the shelf life. Weight loss during storage can alter the product appearance and reduces consumer acceptance. The third critical quality parameter was the determination of the total aerobic count to assess the overall safety and quality of the RTE fish product. RTE food products which received only a mild heat treatment and are distributed at cold temperatures are likely to spoil due to microbial action [
32]. As they are intended for direct consumption without any heat treatment prior to consumption, the safety of the product needs to be assured.
3.1. Development of a Chitosan Coating
The overall appearance of the coating formulations and coating properties were investigated visually. The chitosan FFS had a clear, slightly yellowish appearance. The viscosity was highly depended on the used chitosan concentration with higher viscosity with higher concentrations. When applied to the RTE fish samples, using the coating dipping method, all tested chitosan coatings adhered to the sample surface and coated samples had a wet, shiny appearance. After air drying during storage, no differences between coated and uncoated samples could be identified, indicating that all tested coating formulations are invisible. Samples coated with 1% chitosan solution with a viscosity of 86 mPas, had a wet coating load of around 18.6 mg liquid coating/cm
2. It could be observed that a higher viscosity increased the amount of coating adhering to the sample. A relation between the coating thickness and FFS properties as viscosity, density and draining time was also reported in literature [
33]. The used acidic acid solvent for dissolving the chitosan had a pH value of around 3.1, and the pH of the final chitosan FFSs were around 4.4. Previous studies showed that the low pH of the solvent alone did not affect the product quality [
4].
Overall, lipid oxidation increased during storage for all samples, but even results after 14 days of storage with an average MDA value of 1.5 mg MDA eq./Kg sample were low. They can be classified into the category of ‘perfect quality material’ based on threshold criteria [
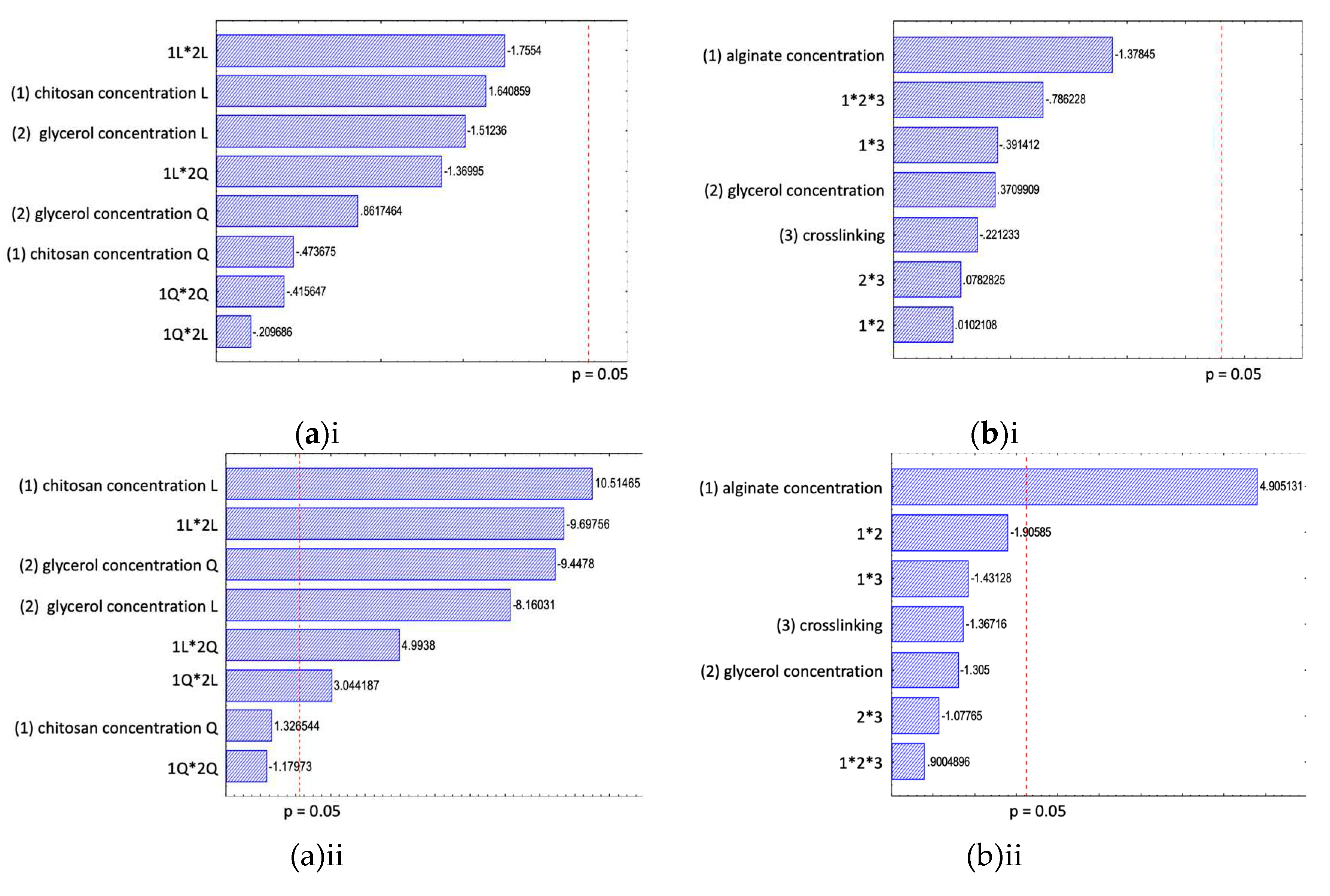
34], which were values < 3 mg MDA eq./Kg for perfect quality material, 3 ≤ 5 mg MDA eq./Kg for good quality material, and 5 < 8 mg MDA eq./Kg suitable for human consumption. The statistical analysis showed that there was no significant differences between the tested coating formulations, as well as no significant differences between 7 and 14 days of storage in regards to lipid oxidation (
Figure 1 – ai). Variations in the selected design variables were in a similar range of magnitude as an identified noise factor, possible trace back to natural product variations, and therefore it was not possible to distinguish between these two. In conclusion, no effect of the tested chitosan coating composition on controlling lipid oxidation of RTE baked fish product could be observed within this experimental set up. These findings are in disagreement with previous findings [
7,
35]. Both observed an effect of the tested chitosan coating on retarding oxidation when applied to a precooked beef patty or smoked sea bass fillet, respectively. A possible explanation for the different results, could be that the sample in this study are less prolonged to lipid oxidation or the effect was not visible under the selected experimental conditions.
All samples lost water during storage, as the driving force for the water gradient was in the direction of the surrounding air with a relative humidity of around 75%. Significant differences in the water loss between the tested coatings could be observed. The results of the full factorial design after 14 days of storage showed that the variation in the data set can be explained by the factors chitosan concentration, glycerol concentration and an interactive effect between these two factors (
Figure 1 - aii). The lowest water loss could be seen when a coating with a low chitosan concentration in combination with a medium glycerol concentration was applied. When the chitosan concentration is increasing the water loss is increased, whereby this effect can be reduced with an increase of glycerol up to 15% (w/w chitosan) in the coating. With higher glycerol concentrations (30% w/w chitosan), the combination with a higher chitosan concentration reduces the water loss the most. Nevertheless, the lowest obtained value with 30% glycerol concentration was still higher than values with lower glycerol and chitosan concentrations. A similar effect of a chitosan coating, with a 2% (w/v) chitosan coating with 25% (w/w chitosan) glycerol, on the water loss of a precooked beef patty was reported [
35].
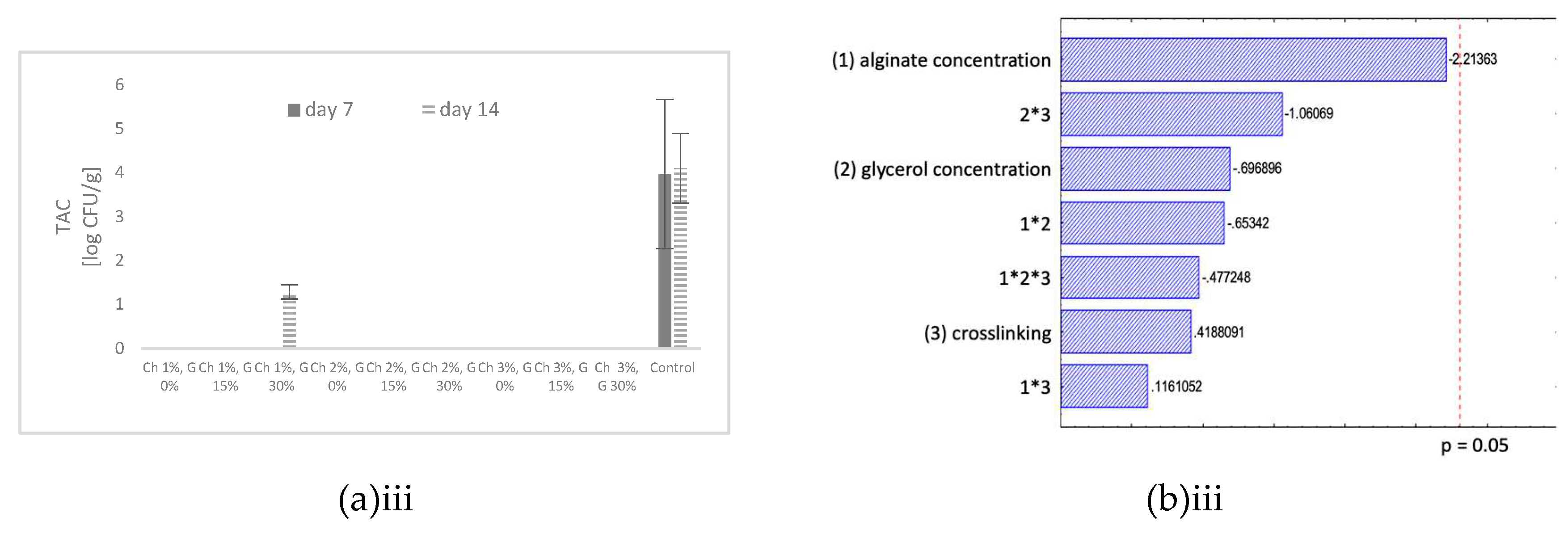
All tested chitosan coatings showed a significant effect on the microbiological growth. The TAC of the coated samples were below the detection limit after 14 days of storage at 4°C, with one exception of samples coated with 1% chitosan solution with 30% (w/w chitosan) glycerol addition with a TAC below 2 log CFU/g after 14 days (
Figure 1 – aiii). The control samples showed a TAC of around 4 log CFU/g after 14 days stored at 4°C. All tested chitosan coating formulations showed a similar effect on controlling the TAC of the RTE fish samples. Therefore, it can be concluded that the presence of glycerol in the solutions does not influence the antimicrobial effect of the chitosan and a 1% chitosan concentration showed the same effect as the highest tested chitosan concentration of 3%. The reported antimicrobial effect in literature of chitosan coatings can be confirmed with the observed results. The effect of a chitosan coating on ready-to-cook meat products packed in LDPE pouch showed that a chitosan coating can lead to retention of the good quality characteristics, improvement of microbiological safety and extension of shelf life during storage [
4]. The application of a chitosan coating with and without the incorporation of essential oils applied to RTE peeled shrimp tails at refrigerated storage packed under MAP conditions was studied [
9], showing that chitosan coatings could be used as a promising emerging technology in order to maintain the microbiological quality. Martínez et al. [
7] studied the effect of a chitosan coating in combination with a vacuum package, inter alia, as a conservative method for smoked sea bass fillets and found that the coating inhibited microbiological growth. An increasing microbial stability and improve colour stability in RTE maki sushi when coated with chitosan and packed under low-CO
2 MAP conditions, was reported by Sørbø und Lerfall (2022) [
6].
Summarizing, based on the findings of all three quality parameters, a FFS with 1% chitosan with 15% (w/w chitosan) glycerol showed the best performance in controlling the tested degradation processes during cold storage, mainly based on the effect of the coating to reduce water loss and control microbiological growth.
Figure 1.
Results of the screening experiment of (a) chitosan and (b) alginate coatings on the quality parameters (i) lipid oxidation, (ii) water loss and (iii) total aerobic microbiological count stored at 4 °C under controlled air circulation over 14 days.
Figure 1.
Results of the screening experiment of (a) chitosan and (b) alginate coatings on the quality parameters (i) lipid oxidation, (ii) water loss and (iii) total aerobic microbiological count stored at 4 °C under controlled air circulation over 14 days.
3.2. Development of a Chitosan Coating
The overall appearance of the coating formulations and coating properties were investigated. The alginate FFSs had a clear appearance and a similar viscosity of around 9.43 mPas for all tested compositions. When applied to the RTE fish samples, using the coating dipping method, FFSs adhered to the sample surface. When the alginate coating was cross-linked with calcium chloride a gel was formed at the product surface. Coated samples had a wet, shiny appearance. After drying during storage, no differences between coated and uncoated samples could be identified, indicating that the coatings were invisible. The wet coating load of the alginate coatings was determined at around 10 mg liquid coating/cm
2. Confirming that a lower viscosity decreases the coating adhering to the product, when compared to the higher viscosity of the FFS of the chitosan coatings (Kanatt et al. 2013). The results of the statistical analysis of the effect of the alginate concentration, glycerol concentration and crosslinking the alginate with CaCl
2 on the lipid oxidation, water loss and TAC of the baked fish sample are shown in
Figure 1 – b.
An increase in MDA value was observed, indicating fat oxidation of all tested alginate coated samples, with the highest value of around 2 mg MDA eq./Kg for samples stored for 14 days. Under the assessed conditions, no tested factor on the alginate coating showed that the lipid oxidation was significant (
Figure 1 – bi) and no significant differences to control samples were found. A higher standard deviation was found within each coating formulation, which could be related to natural variations within the tested baked fish samples. The tested alginate concentrations, glycerol addition or crosslinking with CaCl
2 did not have an effect on the performance of the alginate coating, in providing an oxygen barrier to reduce lipid oxidation. In several studies, alginate coated fish products showed a reduction in lipid oxidation during cold storage. Martínez et al. [
7] studied the effect of an alginate coating in combination with a vacuum package, inter alia, as a conservative method for smoked sea bass fillets, and concluded that an alginate coating protected the fillets against oxidation. Other studies with fresh fish fillet showed that with longer storage time the use of an alginate coating can reduce lipid oxidation [
17,
20]. Nevertheless in these studies the lipid oxidation of the tested fish products was of a higher level, than in the present study. Therefore, the minimal detected effect can be potentially related to the low fat content of the RTE product, overall low lipid oxidation of the product during the tested storage conditions or were not yet visible under the selected storage conditions of 4°C up to 14 days. It could potentially be recommended to investigate the effect of the alginate coating on the lipid oxidation at higher storage temperature to increase the lipid oxidation reaction rate or for longer storage times. In general, the product degradation regarding the lipid oxidation could be of higher importance for high fat content RTE food products.
The statistical results of the effect of the tested alginate coatings on the moisture loss are presented in
Figure 1 – bii. The moisture loss increased with longer storage times, whereby the moisture loss rate depends on the coating formulation. The results of the full factorial design showed that the factor alginate concentration was significant after 14 days of storage, emphasizing the importance of a lower alginate concentration to decrease the water loss. For shorter storage times also the interactive effect between alginate concentration and glycerol concentration was significant. For storage times up to 14 days the interactive effect was only marginally significant. When the alginate concentration was increased, the glycerol concentration needed to be increased for a better control of the water loss during cold storage. The third tested factor crosslinking of the alginate coating with CaCl
2 was not significant. Based on the effect of the coatings on the water loss a 1% alginate concentration, with no glycerol and not crosslinking was favoured for a RTE seafood product.
The statistical results of the TAC are shown in
Figure 1 – biii. The microbiological growth increased during storage, after 7 days the average TAC was around 4 log CFU/g sample and after 14 days around 7 log CFU/g sample. The results are within the same range as the control sample without any coating. Statistical analysis of the results showed no significant differences between the tested alginate coating formulations after 7 and 14 days stored at 4°C. After 14 days of storage only a marginal effect of the alginate concentration was observed. Indicating that an alginate concentration of 2% was marginally significant for reducing the TAC. Different findings of the effect of alginate coating on microbiological growth were presented in literature. Some studies reported a reduction of microbiological growth for samples coated with sodium alginate due to the ability of the coating to act as a barrier against oxygen transfer [
19], and the effect could be increased by the used of additives [
17,
18]. Nevertheless, other researchers reported no reduction in microbial growth [
7,
20,
36].
In summary, considering the findings for all the tested quality parameters, a coating formulation with 1% alginate, without glycerol and no crosslinking was recommended to enhance the product quality. Nevertheless, the alginate coating showed good film forming properties but the effect of the alginate coating on the lipid oxidation, water loss and TAC on RTE baked fish product is limited and could be possible enhanced by the inclusion of natural additives as antioxidants or antimicrobials.
3.3. Comparing the Performance of a Chitosan, Alginate and Bilayer Coating under Optimal and Abused Storage Conditions
A follow up experiment was conducted to further investigate and compare the properties of the selected chitosan and alginate coatings. A 1% chitosan solution with 15% glycerol (w/w chitosan) and 1% alginate coating were previously identified to be the most suitable for the RTE fish product under study. Additionally, the potential of combining the individual coating properties of the chitosan and alginate coating was studied by applying a bilayer coating to the RTE baked fish fillet. A full factorial experimental design was used to study the effect of the coatings at optimal storage conditions at 4°C and abusive conditions of 14°C, over 21 days and 7 days, respectively (
Table 2). The effect of the storage temperature was investigated after 7 days of storage.
All tested samples, including the chitosan coated, alginate coated, bi-layer coated samples and an untreated sample as control, showed a significant increase in MDA value over the tested storage time, indicating lipid oxidation. Though, the highest value measured over the tested storage conditions, with 4.4 mg MDA eq./Kg for uncoated and alginate coated samples stored at 4°C for 21 days, was still in the range of a good quality material based on the threshold criteria suggested [
34]. Results showed that even for longer storage times, up to 21 days for samples stored at 4°C and up to 7 days for samples stored at 14°C, the lipid oxidation was not a critical quality parameter for the product under study. The statistical analysis showed that there was no significant differences between the control, chitosan, alginate or a double coating at each sampling day regarding lipid oxidation (
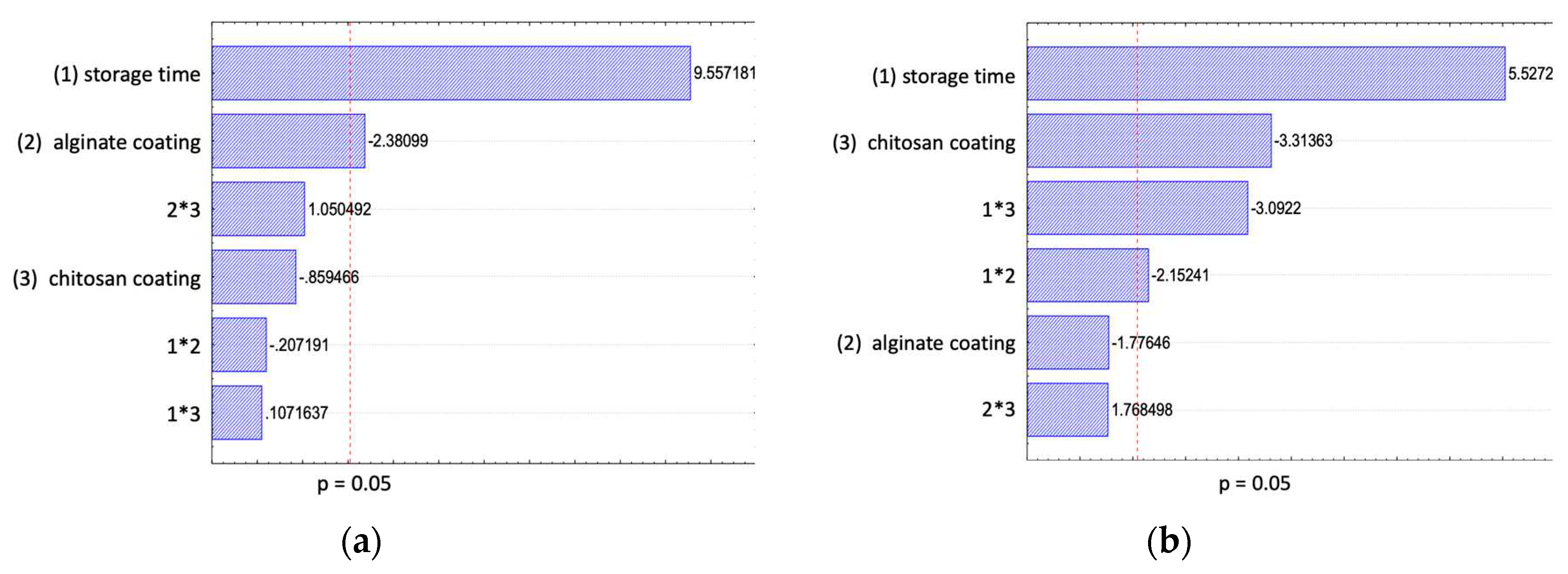
Table 2). When studying the effect of the storage temperature, a significant interactive effect between the storage temperature and chitosan coating could be observed (
Figure 2). In general, as expected a slightly higher increase of lipid oxidation for samples stored at 14°C compared to samples stored at 4°C were seen. At higher storage temperatures, samples coated with chitosan showed a lower lipid oxidation. These findings are in accordance with other studies reporting no effect for short storage times and a slight effect for longer storage times of chitosan coatings on the lipid oxidation of RTE meat products. Wu et al. [
35] reported that chitosan coating on precooked beef patties stored for 3 days at 4°C, showed no significant effect of the chitosan coating reducing TBARS, when compared to an uncoated, unpacked sample. Kanatt et al. [
4] reported that chitosan coated ready-to-cook meat products displayed a longer shelf life compared to uncoated samples as the tested chitosan coating slightly retarded lipid oxidation in all the meat products during storage. No effect of the alginate coating regarding the storage temperature could be identified. Nevertheless, a bi-layer coating showed a reduced lipid oxidation at higher storage temperature. Most likely, due to the positive effect of the chitosan layer of the bi-layer coating.
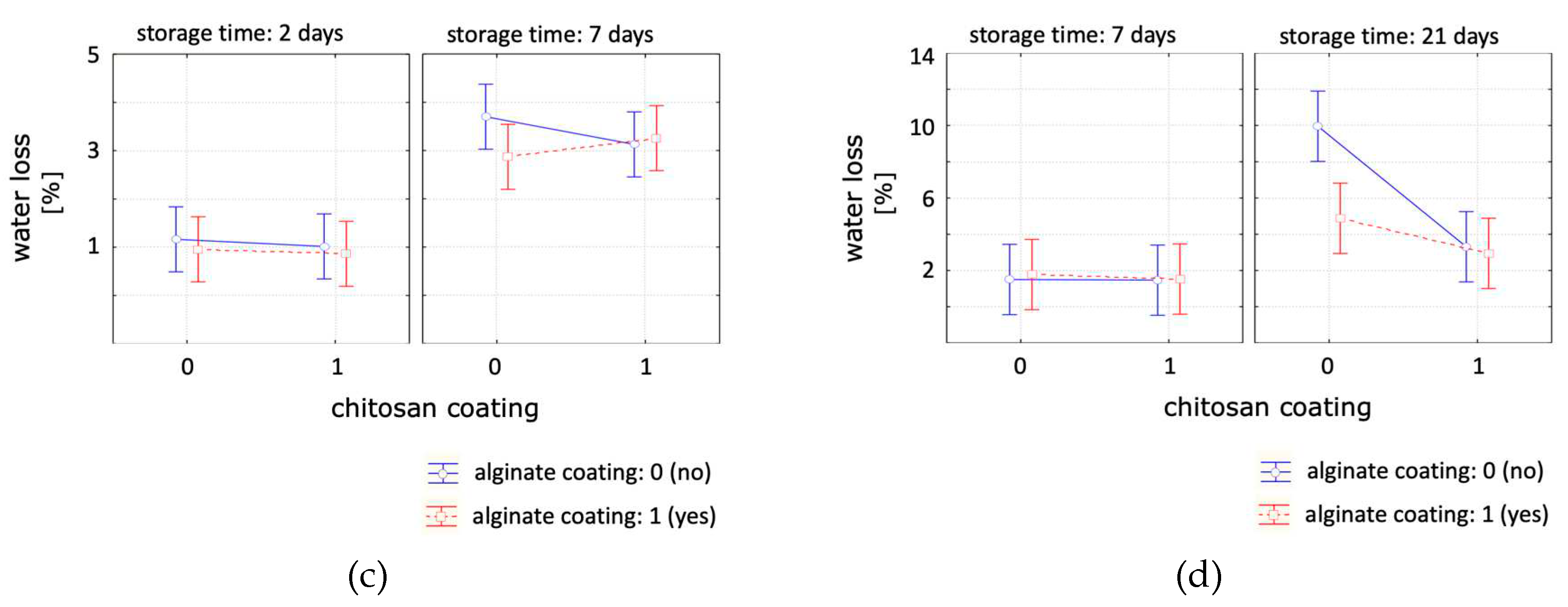
The effect of chitosan, alginate and a bilayer coating, compared to an uncoated control sample, on the product water loss under optimal storage temperature of 4°C and abusive storage temperature of 14°C were studied and the results are presented in
Table 2. Overall, for all the studied scenarios a decrease in water loss was observed. The effect of the coating treatments in regard to the weight loss was studied at 14°C (
Figure 3- i) and 4°C (
Figure 3-ii). For samples stored at 14°C only the alginate coating was significant with a slight reduction in water loss over time. The chitosan coating, the interactive effect between factors ‘storage time and chitosan coating’ and the interactive effect between the factors ‘alginate coating and storage time’ was significant for samples stored under cooled storage. For longer storage times, the applied coating reduced the water loss. When studying the storage temperature effect after 7 days, the storage temperature was the only significant parameter with a high effect on weight loss. The average moisture loss after 7 days stored at 14°C was 3.2% and at 4°C was 1.6%. In conclusion, the tested coating formulations were effective on reducing weight loss of the samples during storage. Samples, either coated with a single chitosan coating or a double coating had a weight loss of around 3.3 and 3% after 21 days of storage, compared to the control sample with a 10% weight loss. With the double coating it was not possible to combine the effect of the chitosan and the single alginate coating.
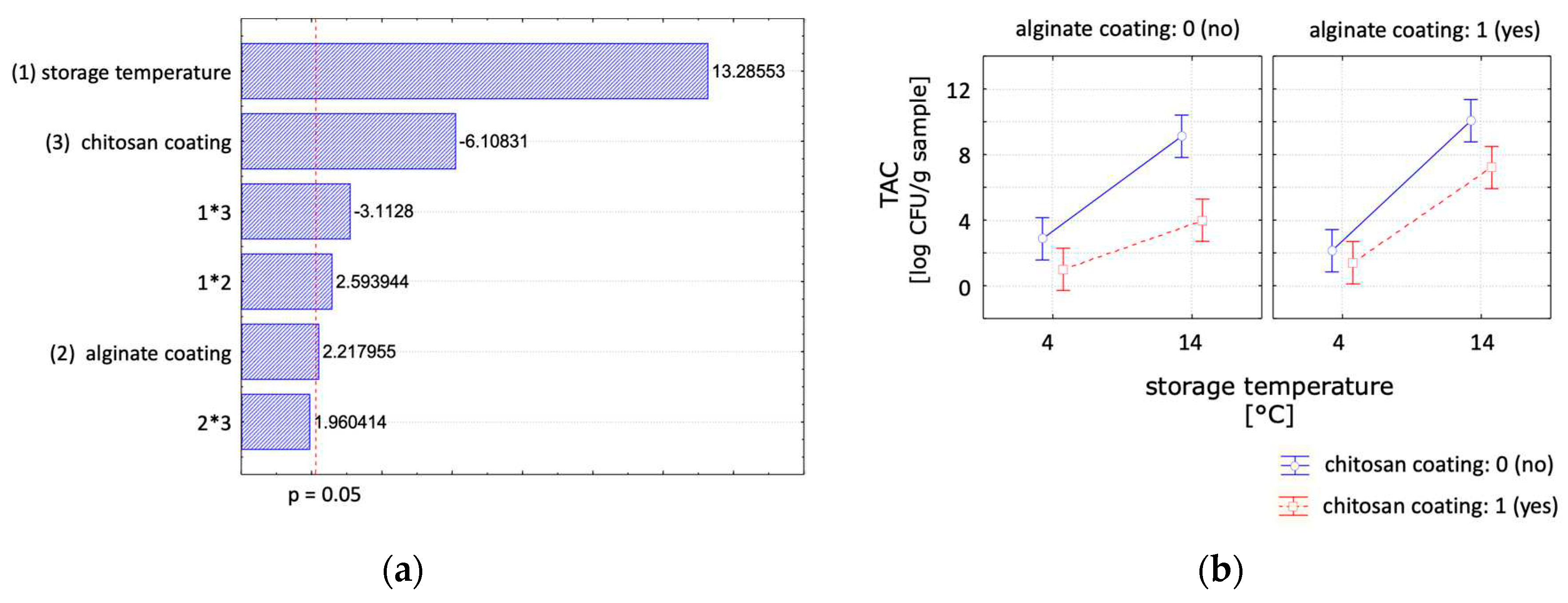
In this study the TAC was used to assess the overall safety and quality of the RTE fish product. The observed results of the TAC of the RTE fish samples are presented in
Table 2. The initial TAC of freshly baked uncoated RTE fish product was in the range of 1 log CFU/g sample. In general, the TAC increases with storage time, though the growth rate can be significantly influenced by the storage temperature and by applying a coating to the product. The microbiological growth rate was reduced with decreasing temperature, shown by reaching the maximal allowed microbiological load of 7 log CFU/g sample at 14°C after 4 days and at 4°C after 14 days for uncoated control samples. After the storage temperature, the chitosan coating was the second significant parameter (
Figure 4). The results showed that the microbial growth during storage can be reduced when a chitosan coating was applied. The chitosan coated samples were below the critical value of 7 log CFU/g during the tested storage times of 7 days at 14°C and 21 days at 4°C. The alginate coating showed a negative effect on the microbial growth. With longer storage times, samples coated with alginate resulted in a higher CFU values compared to the control samples. The samples coated with chitosan and alginate showed a reduced microbial growth when compared to the uncoated samples, nevertheless the former showed a higher CFU count than using a single chitosan coating. These findings are in accordance with other chitosan coating studies, reporting an antimicrobial effect of chitosan [
5,
7,
8,
10]. The effect of chitosan on microbial stability in RTE maki sushi under abused storage conditions was also reported [
6]. Emphasizing, an increased safety of RTE products during optimal cooled chain, but also when exposed to abused conditions. Therefore, it is recommended to further test the ability of a chitosan coating under simulated real shelf life conditions.
3.4. Effect of the Edible Coatings on the Product Shelf Life
A multi-response analysis was used to determine the shelf life of the uncoated and coated products. The cut of points for the shelf life estimation were 7 log CFU/g for TAC (Food Safety Authority of Ireland 2020), 10% water loss and lipid oxidation value of below 8 mg MD eq./Kg sample [
34]. The results of the total desirability score of the RTE fish products, weighting each quality parameter equally without the ability to comprise for another quality parameter, are presented in
Table 3. Overall, the highest score at each storage time and temperature was reached with a chitosan coating providing an acceptable quality score until 21 and 7 days stored at 4 and 14°C, respectively. The second best coating was a bi-layer coating, though with a reduced quality and consequently a lower maximal shelf life. The used alginate coating only provides marginal advantage for prolonging the quality. The most critical drawback of the alginate coating under the tested three quality factors was the negative effect of the coating on the aerobic microbial growth.
4. Discussion
Edible coatings are alternative eco packaging systems based on biobased materials and can provide packaging functions by controlling degradation processes of food products, resulting in advantages in terms of food safety and quality. In this study coatings based on chitosan and alginate were tested for controlling fat oxidation, water loss and microbial growth of a RTE baked fish product. First, the effect of changes in the coating composition was studied and secondly the effect of the developed coatings on the product shelf life was analysed under optimal and abused storage conditions. The results showed that the selected coating composition can influence the coating performance. A chitosan coating composition with 1% (w/v) chitosan, 15% (w/w chitosan) glycerol, and a 1% (w/v) sodium alginate coating was the most effective coating solutions for the RTE seafood product of this study. Both chitosan and alginate coating applied as a single or bi-layer coating formed invisible adherent layers around the product. The tested alginate coatings only showed a limited effect on the tested quality parameters of the RTE seafood product. Whereas, the chitosan coating presented a significant reduction in microbial growth during storage at optimal and abused temperatures, as well as reduction in weight loss. A shelf life extension of chitosan coated samples compared to uncoated control sample stored at optimal temperature of 4°C could be achieved from 7 days to 21 days, and under abused conditions from 2 to 7 days. An effect of the tested coatings both at optimal and abused storage conditions could be seen, and it is therefore recommended using an edible coating as a safety measure against temperature abused during the food supply chain. These results suggest that a chitosan coating is an effective packaging strategy to improve the safety and quality of a RTE seafood product during cold storage as well as under abused storage conditions.
Author Contributions
I.B. conceived the work, conducted the literature review, data extraction and drafted the manuscript. M.S.G. edited, developed and approved the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by INTERREG Atlantic Area funding program, grant number EAPA_758/2018.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Vicente, F.A.; Ventura, S.P.; Passos, H.; Dias, A.C.; Torres-Acosta, M.A.; Novak, U.; Likozar, B. Crustacean waste biorefinery as a sustainable cost-effective business model. Chem. Eng. J. 2022, 442, 135937. [Google Scholar] [CrossRef]
- Muñoz, I.; Rodríguez, C.; Gillet, D.; M. Moerschbacher, B. Life cycle assessment of chitosan production in India and Europe. Int. J. Life Cycle Assess. 2018, 23, 1151–1160. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Effects of chitosan coating on shelf-life of ready-to-cook meat products during chilled storage. LWT Food Sci. Technol. 2013, 321–326. [Google Scholar] [CrossRef]
- Zarandona, I.; López-Caballero, M.E.; Montero, M.P.; Guerrero, P.; La Caba, K. de; Gómez-Guillén, M.C. Horse mackerel (Trachurus trachurus) fillets biopreservation by using gallic acid and chitosan coatings. Food Control 2021, 120, 107511. [Google Scholar] [CrossRef]
- Sørbø, S.; Lerfall, J. Effect of edible coating and modified atmosphere packaging on the microbiological and physicochemical stability of retail maki sushi. J. Food Sci. 2022, 87, 1211–1229. [Google Scholar] [CrossRef]
- Martínez, O.; Salmerón, J.; Epelde, L.; Vicente, M.; Vega, C. de. Quality enhancement of smoked sea bass (Dicentrarchus labrax) fillets by adding resveratrol and coating with chitosan and alginate edible films. Food Control 2018, 85, 168–176. [Google Scholar] [CrossRef]
- Bonilla, F.; Chouljenko, A.; Lin, A.; Young, B.M.; Goribidanur, T.S.; Blake, J.C.; Bechtel, P.J.; Sathivel, S. Chitosan and water-soluble chitosan effects on refrigerated catfish fillet quality. Food Biosci. 2019, 31, 100426. [Google Scholar] [CrossRef]
- Carrión-Granda, X.; Fernández-Pan, I.; Jaime, I.; Rovira, J.; Maté, J.I. Improvement of the microbiological quality of ready-to-eat peeled shrimps (Penaeus vannamei) by the use of chitosan coatings. Int. J. Food Microbiol. 2016, 232, 144–149. [Google Scholar] [CrossRef]
- Ebadi, Z.; Khodanazary, A.; Hosseini, S.M.; Zanguee, N. The shelf life extension of refrigerated Nemipterus japonicus fillets by chitosan coating incorporated with propolis extract. Int. J. Biol. Macromol. 2019, 139, 94–102. [Google Scholar] [CrossRef]
- Li, T.; Hu, W.; Li, J.; Zhang, X.; Zhu, J.; Li, X. Coating effects of tea polyphenol and rosemary extract combined with chitosan on the storage quality of large yellow croaker (Pseudosciaena crocea). Food Control 2012, 101–106. [Google Scholar] [CrossRef]
- Arancibia, M.Y.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Chitosan coatings enriched with active shrimp waste for shrimp preservation. Food Control 2015, 54, 259–266. [Google Scholar] [CrossRef]
- Yu, D.; Xu, Y.; Regenstein, J.M.; Xia, W.; Yang, F.; Jiang, Q.; Wang, B. The effects of edible chitosan-based coatings on flavor quality of raw grass carp (Ctenopharyngodon idellus) fillets during refrigerated storage. Food Chem. 2018, 242, 412–420. [Google Scholar] [CrossRef]
- Azaza, Y.B.; Hamdi, M.; Charmette, C.; Jridi, M.; Li, S.; Nasri, M.; Nasri, R. Development and characterization of active packaging films based on chitosan and sardinella protein isolate: Effects on the quality and the shelf life of shrimps. Food Packag. Shelf LIfe 2022, 31, 100796. [Google Scholar] [CrossRef]
- Beata Łabowska, M.; Michalak, I.; Detyna, J. Methods of extraction, physicochemical properties of alginates and their applications in biomedical field – a review. Open Chem. 2019, 17, 738–762. [Google Scholar] [CrossRef]
- Bazargani-Gilani, B.; Pajohi-Alamoti, M. The effects of incorporated resveratrol in edible coating based on sodium alginate on the refrigerated trout (Oncorhynchus mykiss) fillets' sensorial and physicochemical features. Food Sci. Biotechnol. 2020, 207–216. [Google Scholar] [CrossRef]
- Bazargani-Gilani, B. Activating sodium alginate-based edible coating using a dietary supplement for increasing the shelf life of rainbow trout fillet during refrigerated storage (4 ±1°C). J. Food Saf. 2017, 1–9. [Google Scholar] [CrossRef]
- Heydari, R.; Bavandi, S.; Javadian, S.R. Effect of sodium alginate coating enriched with horsemint (Mentha longifolia) essential oil on the quality of bighead carp fillets during storage at 4°C. Food Sci. Nutr. 2015, 3, 188–194. [Google Scholar] [CrossRef]
- Nie, X.; Wang, L.; Wang, Q.; Lei, J.; Hong, W.; Huang, B.; Zhang, C. Effect of a Sodium Alginate Coating Infused with Tea Polyphenols on the Quality of Fresh Japanese Sea Bass (Lateolabrax japonicas) Fillets. J. Food Sci. 2018, 83, 1695–1700. [Google Scholar] [CrossRef]
- Sáez, M.I.; Suárez, M.D.; Martínez, T.F. Effects of alginate coating enriched with tannins on shelf life of cultured rainbow trout (Oncorhynchus mykiss) fillets. LWT Food Sci. Technol. 2020, 108767. [Google Scholar] [CrossRef]
- Song, Y.; Liu, L.; Shen, H.; You, J.; Luo, Y. Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala). Food Control 2011, 608–615. [Google Scholar] [CrossRef]
- Vital, A.C.P.; Guerrero, A.; Ornaghi, M.G.; Kempinski, E.M.B.C.; Sary, C.; Monteschio, J.d.O.; Matumoto-Pintro, P.T.; Ribeiro, R.P.; do Prado, I.N. Quality and sensory acceptability of fish fillet (Oreochromis niloticus) with alginate-based coating containing essential oils. J. Food Sci. Technol. 2018, 55, 4945–4955. [Google Scholar] [CrossRef]
- Han, J.-W.; Ruiz-Garcia, L.; Qian, J.-P.; Yang, X.-T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef]
- United Nations. World Population Ageing 2019: Highlights, New York, 2019. Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf, /.
- Liu, C.; Ralston, N.V. Chapter Seven - Seafood and health: What you need to know? 2021, 97, 275–318. [CrossRef]
- The Commission of the European Communities. Commission Regulation (EC) No 2073/2005 of on microbiological criteria for foodstuffs 2005. 15 November.
- Rosmini, M.R.; Perlo, F.; Pérez-Alvarez, J.A.; Pagán-Moreno, M.J.; Gago-Gago, A.; López-Santoveña, F.; Aranda-Catalá, V. TBA test by an extractive method applied to ‘paté’. Meat Sci. 1996, 103–110. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response surface methodology: Process and product optimization using designed experiments, Fourth edition; Wiley: Hoboken, New Jersey, 2016; ISBN 978-1-118-91601-8. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons, 2013, ISBN 1118146921.
- Mendes, R.; Pestana, C.; Goncalves, A. The effect of soluble gas stabilisation on the quality of packed sardine fillets (Sarddina pilchardus) stored in air, VP and MAP. International Journal of Food Science and Technology 2008, 43, 2000–2009. [Google Scholar] [CrossRef]
- Anacleto, P.; Teixeira, B.; Marques, P.; Pedro, S.; Nunes, M.L.; Marques, A. Shelf-life of cooked edible crab (Cancer pagurus) stored under refrigerated conditions. LWT Food Sci. Technol. 2011, 44, 1376–1382. [Google Scholar] [CrossRef]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Krochta, J.M. Dependence of Coating Thickness on Viscosity of Coating Solution Applied to Fruits and Vegetables by Dipping Method. J. Food Science 2003, 68, 503–510. [Google Scholar] [CrossRef]
- Socaciu, M.-I.; Semeniuc, C.; Vodnar, D. Edible Films and Coatings for Fresh Fish Packaging: Focus on Quality Changes and Shelf-life Extension. Coatings 2018, 8, 366. [Google Scholar] [CrossRef]
- Wu, Y.; Rhim, J.W.; Weller, C.L.; Hamouz, F.; Cuppett, S.; Schnepf, M. Moisture Loss and Lipid Oxidation for Precooked Beef Patties Stored in Edible Coatings and Films. J. Food Science 2000, 65, 300–304. [Google Scholar] [CrossRef]
- Shankar, S.; Danneels, F.; Lacroix, M. Coating with alginate containing a mixture of essential oils and citrus extract in combination with ozonation or gamma irradiation increased the shelf life of Merluccius sp. fillets. Food Packag. Shelf LIfe 2019, 100434. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).