Submitted:
12 December 2023
Posted:
14 December 2023
You are already at the latest version
Abstract
Keywords:

1. Introduction
2. Type I Homodimeric Reaction Center-Photosystems of Anoxygenic Phototrophs
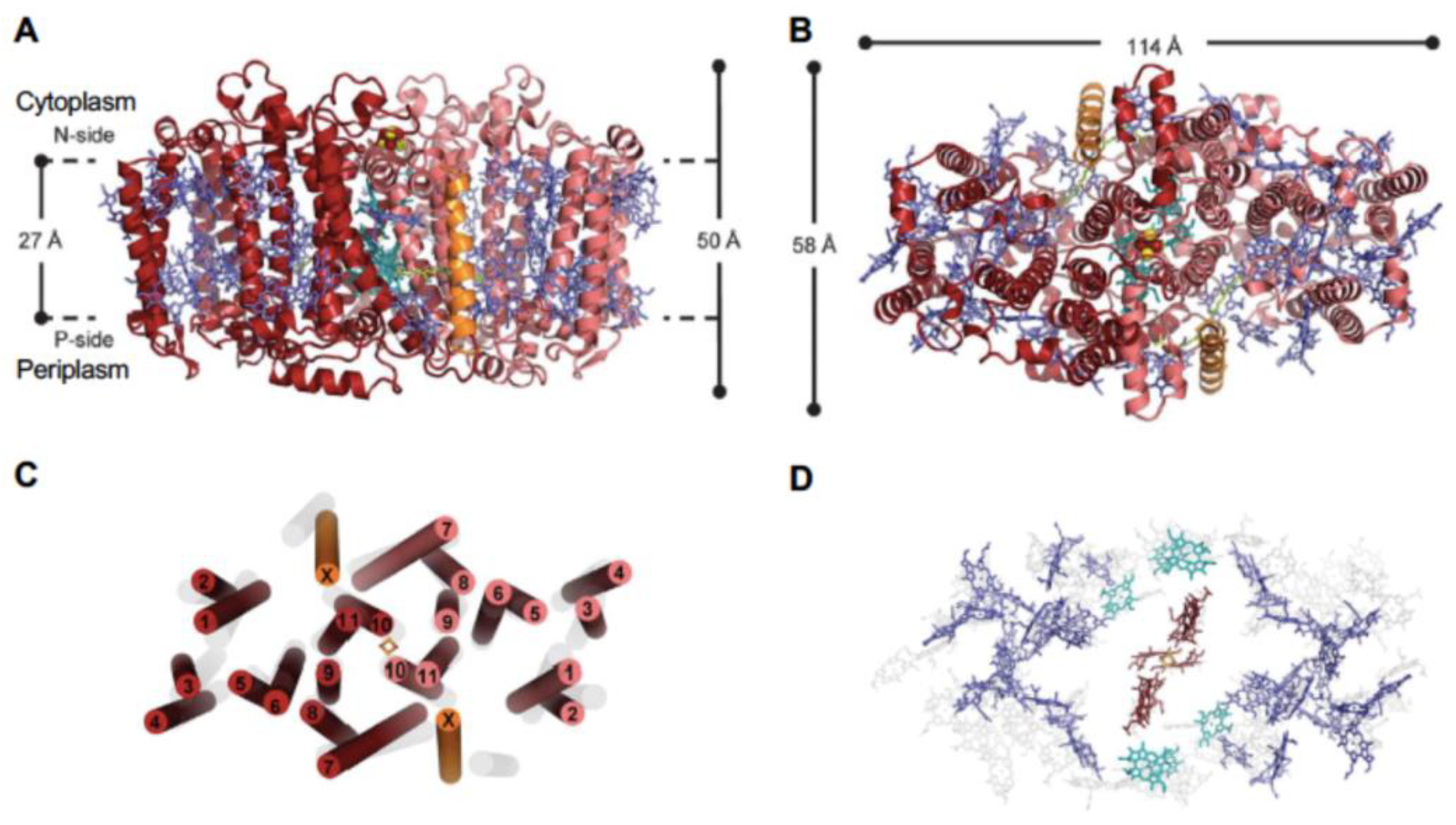
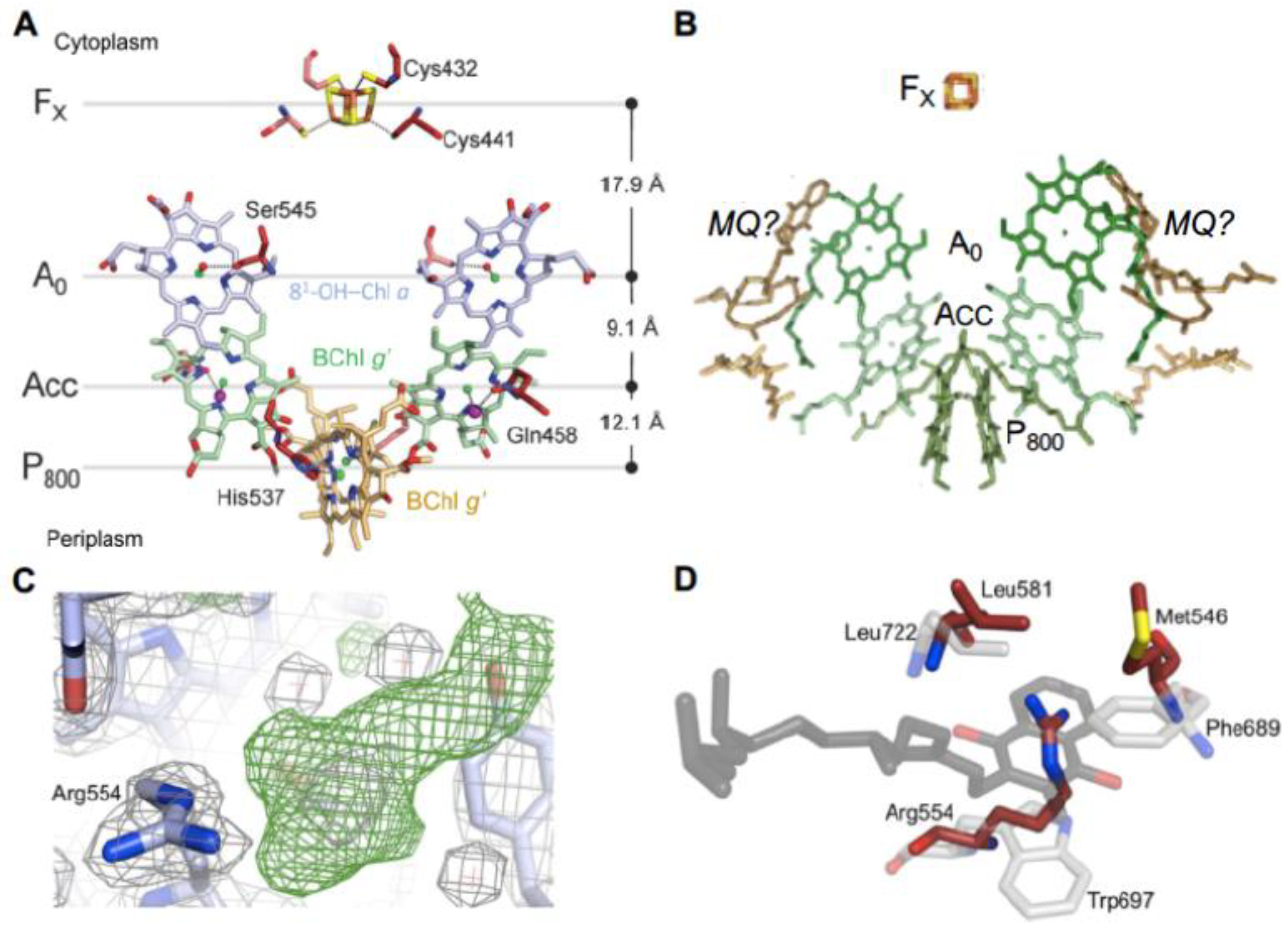
2.1. Structure of the Reaction Center-Photosystem of the Heliobacteria
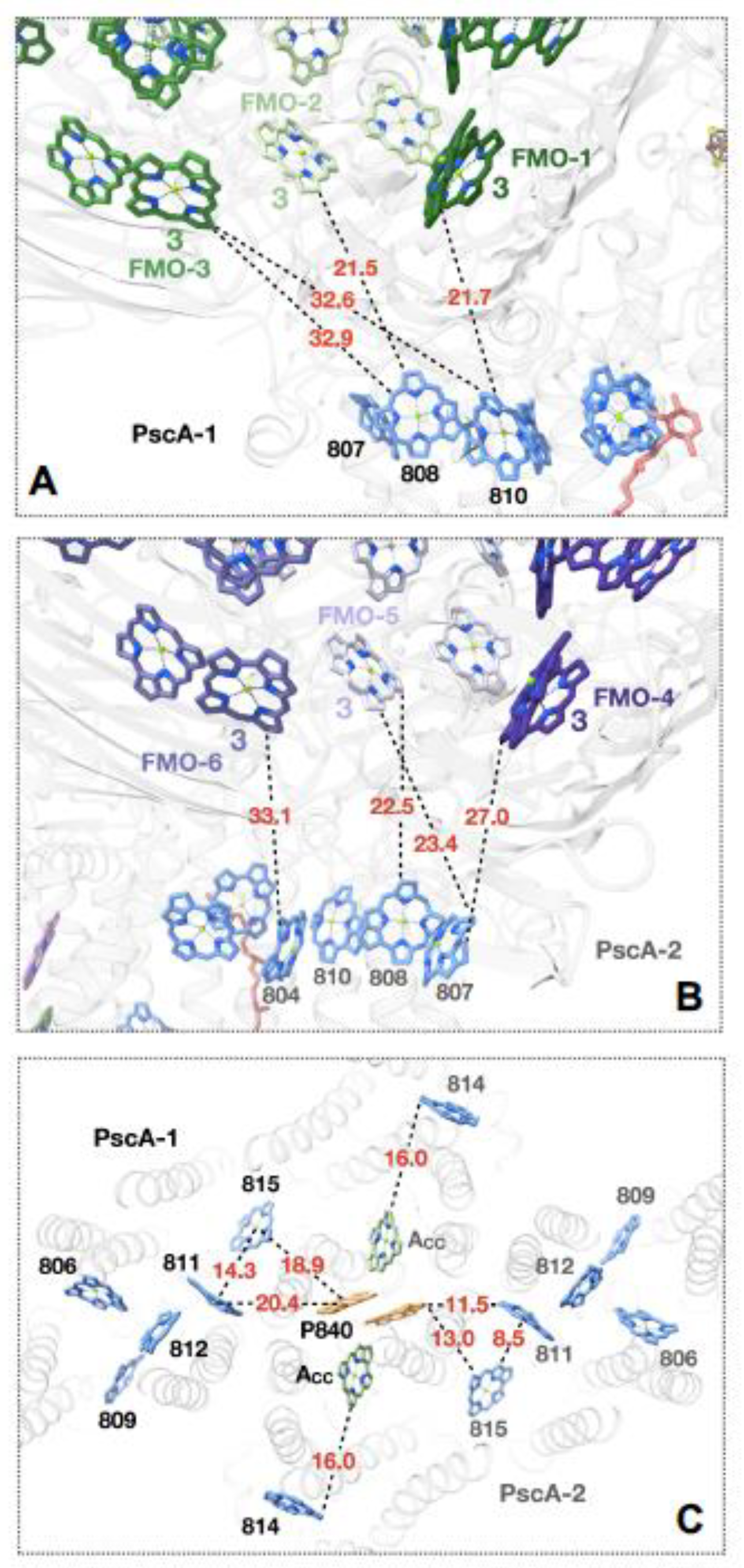
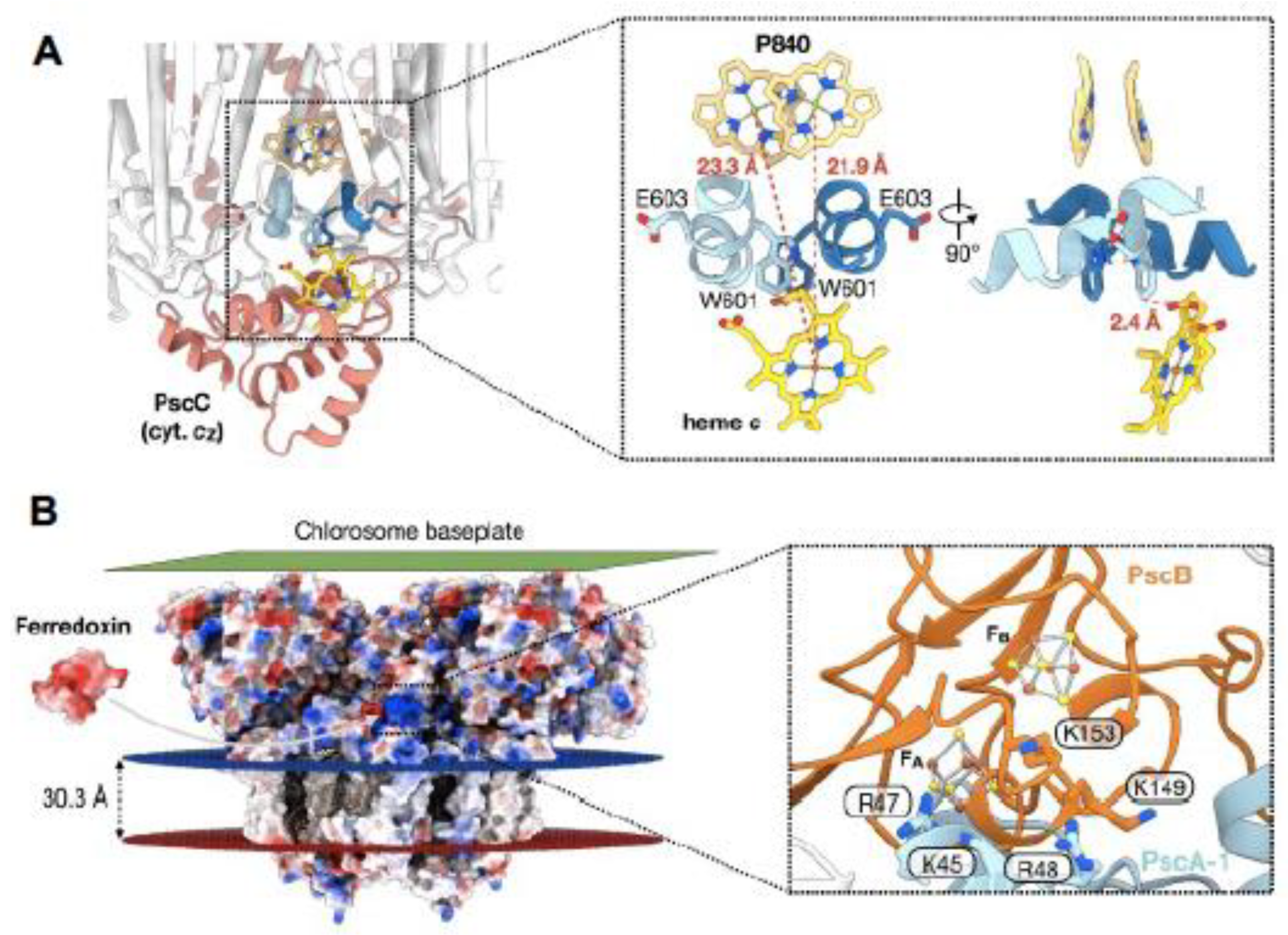
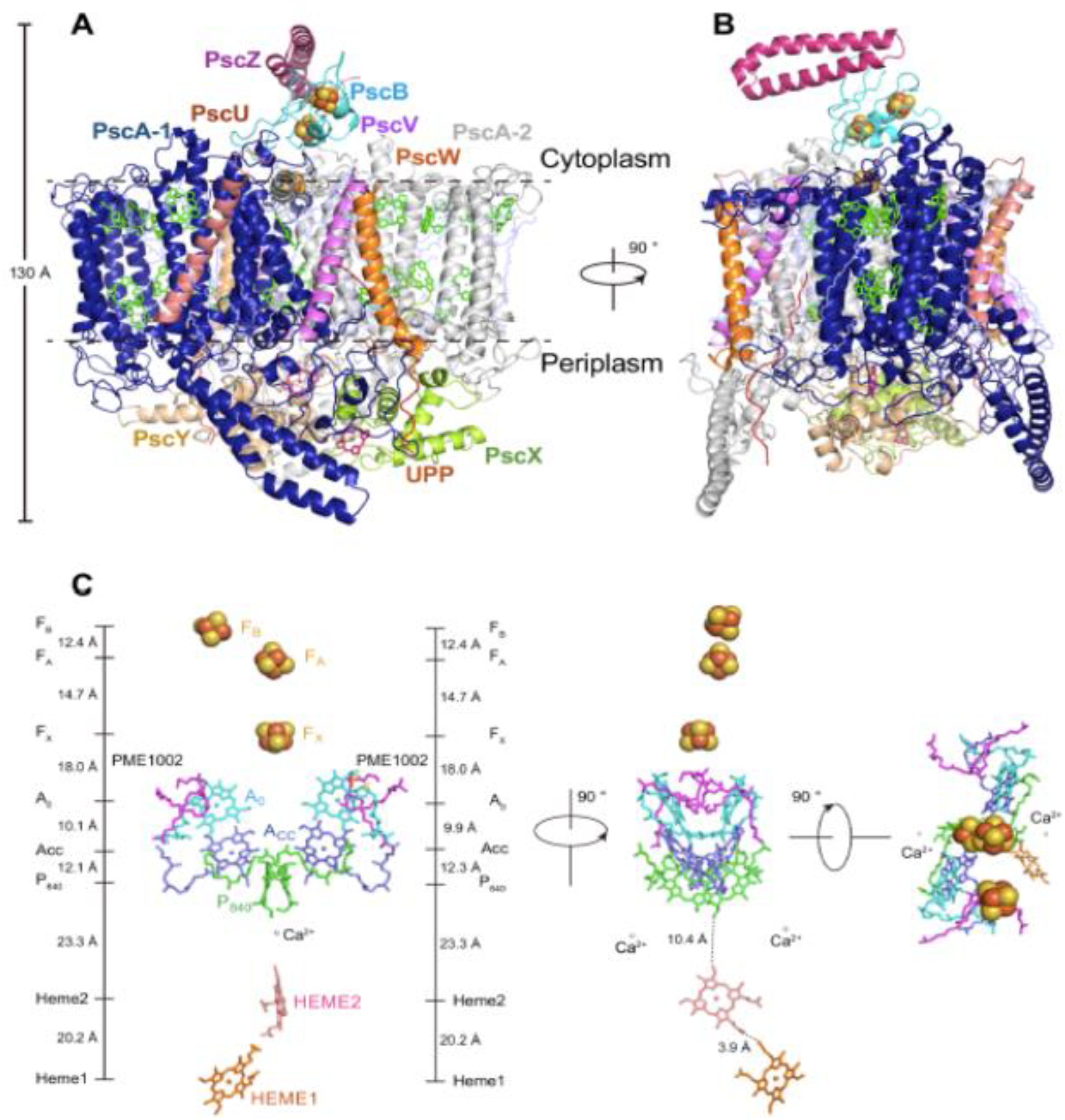
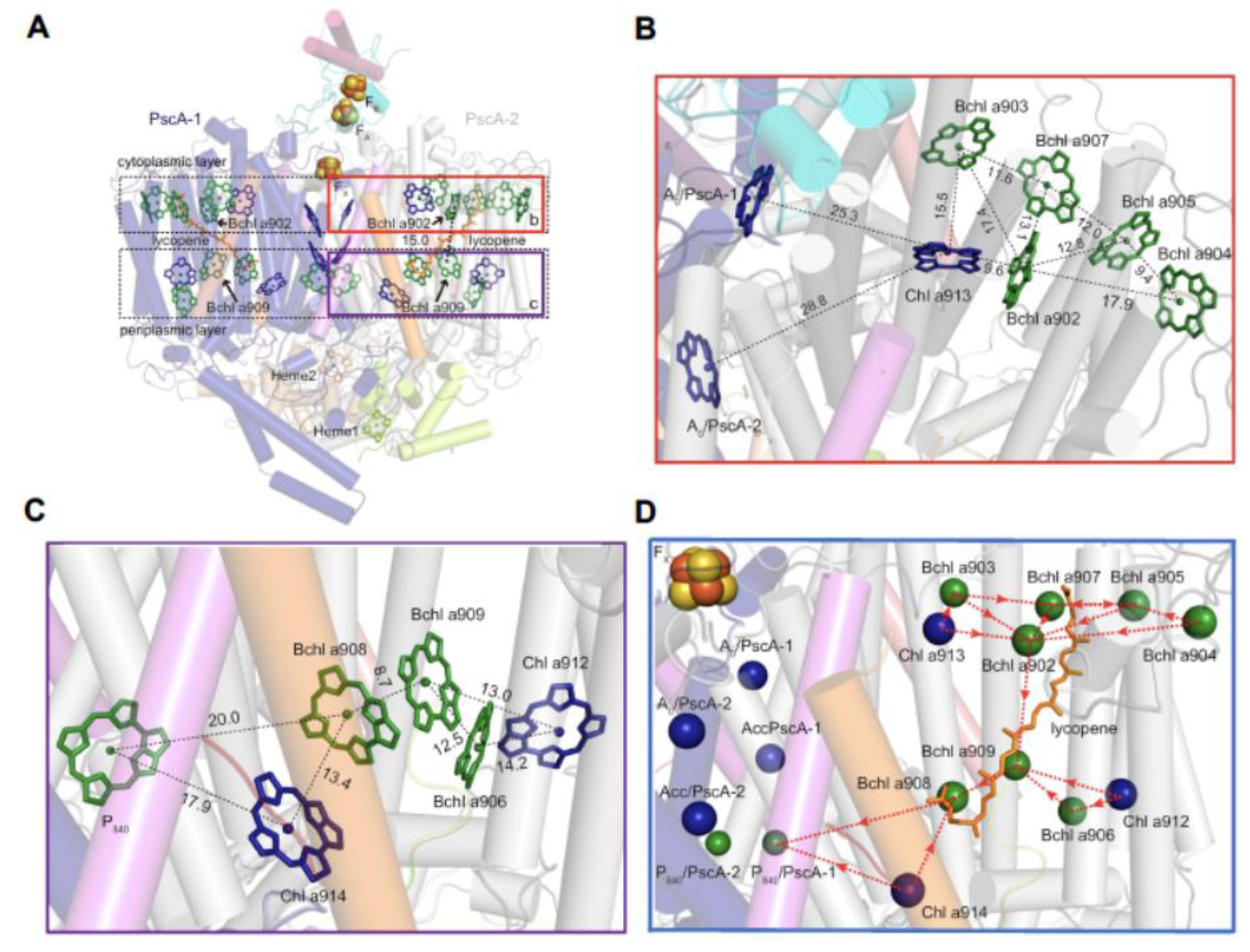
2.2. Structure of the Reaction Center-Photosystem of the Green Sulfur Bacteria
2.3. Structure of the Reaction Center-Photosystem of the Chloroacidobacteria
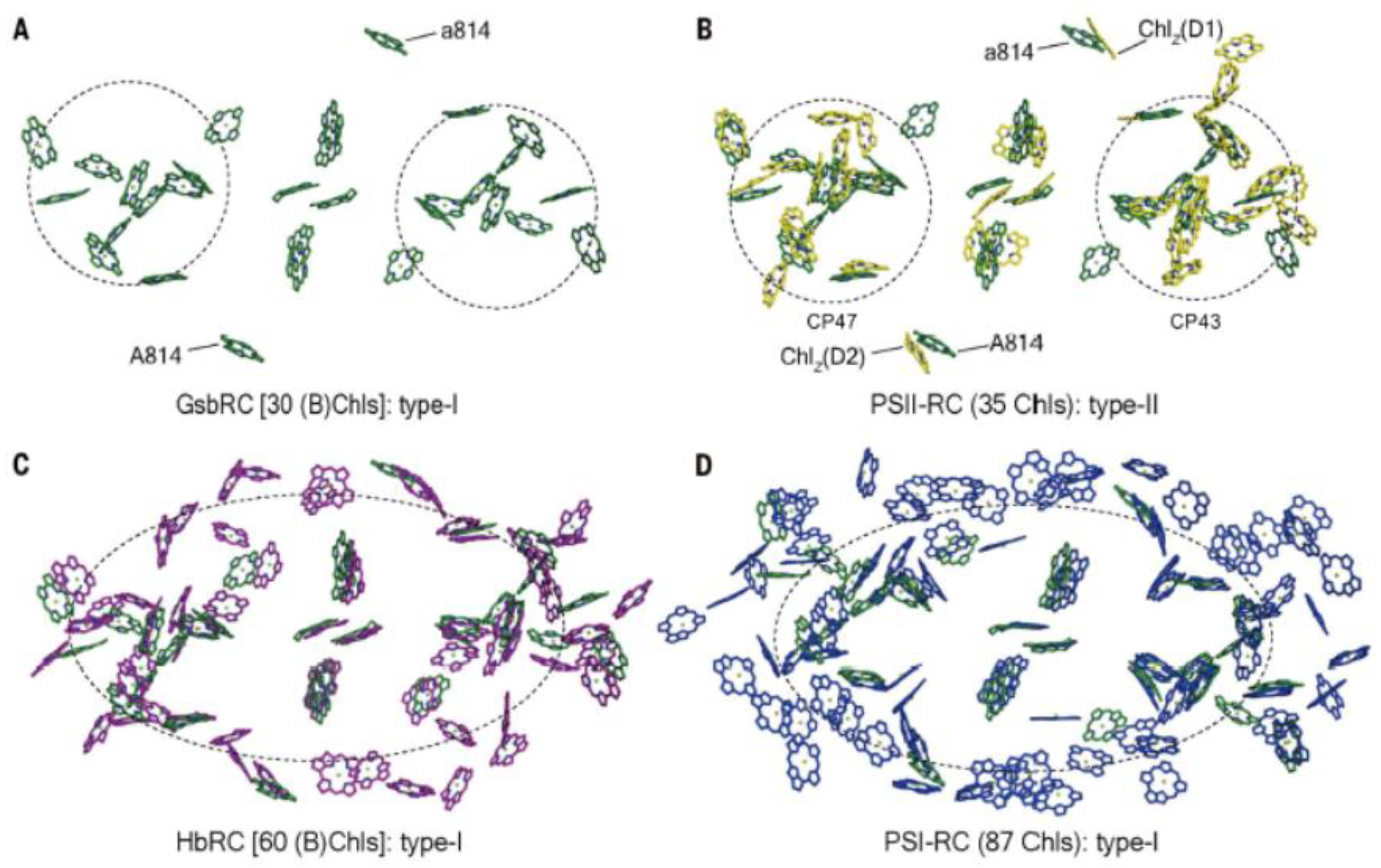
3. Further Insights into the Evolutionary Origins of Oxygenic Photosynthesis Provided by Structures of Homodimeric Type I Reaction Center-Photosystems
References
- Gisriel, C.; Sarrou, I.; Ferlez, B.; Golbeck, J.H.; Redding, K.E.; Fromme, R. Structure of a Symmetric Photosynthetic Reaction Center-Photosystem, Science 2017, 357, 1021–1025. [CrossRef]
- Xie, H.; Lyratzakis, A.; Khera, R.; Koutantou, M.; Welsch, S.; Michel, H.; Tsiotis, G. Cryo-EM Structure of the Whole Photosynthetic Reaction Center Apparatus from the Green Sulfur Bacterium Chlorobaculum tepidum. Proc. Natl. Acad. Sci. USA 2023, 120, e2216734120. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Wu, H.; Xu, C.; Liu, X.-C.; Huang, Z.; Chang, S.; Wang, W.; Han, G.; Kuang, T.; Shen, J.-R.; et al. Architecture of the Photosynthetic Complex from a Green Sulfur Bacterium. Science 2020, 370, eabb6350. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Huang, G.; Wang, C.; Wang, J.; Sui, S.-F.; Qin, X. Structure of the Acidobacteria Homodimeric Reaction Center Bound with Cytochrome c. Nat. Commun. 2022, 13, 7745. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis, 3rd ed.; John Wiley & Sons: Hoboken, NJ USA, 2021; p. 352p. ISBN 978-1-119-80001-9. [Google Scholar]
- Cardona, T.; Rutherford, A.W. Evolution of Photochemical Reaction Centres: More Twists. Trends Plant Sci. 2019, 24, 1008–1021. [Google Scholar] [CrossRef] [PubMed]
- Jordan, P.; Fromme, P.; Witt, H.T.; Klukas, O.; Saenger, W.; Krauss, N. Three-Dimensional Structure of Cyanobacterial Photosystem I at 2.5 Å Resolution. Nature 2001, 411, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, A.T.; Nguyen-Phan, T.C.; Cogdell, R.J. A Comparative Look at Structural Variation Among RC-LH1 'Core' Complexes Present in Anoxygenic Phototrophic Bacteria. Photosynth. Res. 2020, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Niederman, R.A. The Structural Diversity of Bacterial Reaction Center-Light Harvesting 1 Complexes and Their Role in Developing Biohybrid Photoelectrochemical Cells. In Photosynthesis: From Plants to Nanomaterials, Hou, H.J.M.; Allakhverdiev, S.I. Eds, Ed.; .Elsevier: Amsterdam, NL, 2023; Chapter 12; pp. 239–291. [Google Scholar] [CrossRef]
- Swainsbury, D.J.K.; Qian, P.; Hitchcock, A.; Hunter, C.N. The Structure and Assembly of Reaction Centre-Light-Harvesting 1 Complexes in Photosynthetic Bacteria. Biosci. Rep. 2023, 43, BSR20220089. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bracun, L.; Li, M. Structural Diversity and Modularity of Photosynthetic RC-LH1 Complexes. Trends Microbiol. 2023; 26, S0966-842X(23)00173-7. [Google Scholar] [CrossRef]
- Orf, G.S.; Gisriel, C.J.; Redding, K.E. Evolution of Photosynthetic Reaction Centers: Insights from the Structure of the Heliobacterial Reaction Center. Photosynth. Res. 2018, 138, 11–37. [Google Scholar] [CrossRef]
- Cardona, T. Early Archean Origin of Heterodimeric Photosystem I. Heliyon 2018, 4, e00548. [Google Scholar] [CrossRef]
- Deisenhofer, J.; Epp, O.; Miki, K.; Huber, R.; Michel, H. Structure of the Protein Subunits in the Photosynthetic Reaction Centre of Rhodopseudomonas viridis at 3Å Resolution. Nature 1985, 318, 618–624. [Google Scholar] [CrossRef]
- Allen, J.P.; Feher, G.; Yeates, T.O.; Komiya, H.; Rees, D.C. Structure of the Reaction Center from Rhodobacter sphaeroides R-26: The Protein Subunits. Proc. Natl. Acad. Sci. USA 1987, 84, 6162–6166. [Google Scholar] [CrossRef] [PubMed]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal Structure of Oxygen-Evolving Photosystem II at a Resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Kimble, L.K.; Mandelco, L.; Woese, C.R.; Madigan, M.T. Heliobacterium modesticaldum, sp. nov., a Thermophilic Heliobacterium of Hot Springs and Volcanic Soils. Arch. Microbiol. 1983, 136, 17–19. [Google Scholar] [CrossRef]
- Brockmann, H.; Lipinski, A. Bacteriochlorophyll g. A New Bacteriochlorophyll from Heliobacterium chlorum. Arch. Microbiol. 1983, 136, 17–19. [Google Scholar] [CrossRef]
- Orf, G.S.; Gisriel, C.J.; Granstrom, J.; Baker, P.L.; Redding, K.E. The PshX Subunit of the Photochemical Reaction Center from Heliobacterium modesticaldum Acts as a Low-Energy Antenna. Photosynth. Res. 2022, 151, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Gorka, M.; Charles, P.; Kalendra, V.; Baldansuren, A.; Lakshmi, K.V.; Golbeck, J.H. A Dimeric Chlorophyll Electron Acceptor Differentiates Type I from Type II Photosynthetic Reaction Centers. iScience 2021, 24, 102719. [Google Scholar] [CrossRef]
- Kashey, T.S.; Luu, D.D.; Cowgill, J.C.; Baker, P.L.; Redding, K.E. Light-Driven Quinone Reduction in Heliobacterial Membranes. Photosynth. Res. 2018, 138, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sarrou, I.; Khan, Z.; Cowgill, J.; Lin, S.; Brune, D.; Romberger, S.; Golbeck, J.H.; Redding, K.E. Purification of the Photosynthetic Reaction Center from Heliobacterium modesticaldum. Photosynth. Res. 2012, 111, 291–302. [Google Scholar] [CrossRef]
- Romberger, S.P.; Golbeck, J.H. The FX Iron–Sulfur Cluster Serves as the Terminal Bound Electron Acceptor in Heliobacterial Reaction Centers. Photosynth. Res. 2012, 111, 285–290. [Google Scholar] [CrossRef]
- Khadka, B.; Adeolu, M.; Blankenship, R.E.; Gupta, R.S. Novel Insights into the Origin and Diversification of Photosynthesis Based on Analyses of Conserved Indels in the Core Reaction Center Proteins. Photosynth. Res. 2017, 131, 159–171. [Google Scholar] [CrossRef]
- Sattley, W.M.; Madigan, M.T.; Swingley, W.D.; Cheung, P.C.; Clocksin, K.M.; Conrad, A.L.; Dejesa, L.C.; Honchak, B.M.; Jung, D.O.; Karbach, L.E.; et al. The Genome of Heliobacterium modesticaldum, a Phototrophic Representative of the Firmicutes Containing the Simplest Photosynthetic Apparatus. J. Bacteriol. 2008, 190, 4687–4696. [Google Scholar] [CrossRef]
- Psencík, J.; Ikonen, T.P.; Laurinmäki, P.; Merckel, M., C.; Butcher, S.J.; Serimaa, R., E.; Tuma, R. Lamellar Organization of Pigments in Chlorosomes, the Light Harvesting Complexes of Green Photosynthetic Bacteria. Biophys. J. 2004, 87, 1165–1172. [Google Scholar] [CrossRef]
- Francke, C.; Otte, S.C.M.; Miller, M.; Amesz, J.; Olson, J.M. Energy Transfer from Carotenoid and FMO-protein in Subcellular Preparations from Green Sulfur Bacteria. Spectroscopic Characterization of an FMO-Reaction Center Core Complex at Low Temperature. Photosynth. Res. 1996, 50, 71–77. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Niedzwiedzki, D.M.; Orf, G.S.; Zhang, H.; Blankenship, R.E. Dynamics of Energy and Electron Transfer in the FMO-Reaction Center Core Complex from the Phototrophic Green Sulfur Bacterium Chlorobaculum tepidum. J. Phys. Chem. B. 2015, 119, 8321–8329. [Google Scholar] [CrossRef] [PubMed]
- Magdaong, N.C.M.; Niedzwiedzki, D.M.; Saer, R.G.; Goodson, C.; Blankenship, R.E. Excitation Energy Transfer Kinetics and Efficiency in Phototrophic Green Sulfur Bacteria. Biochim. Biophys. Acta 2018, 1859, 1180–1190. [Google Scholar] [CrossRef]
- Dostál, J.; Pšenčík, J.; Zigmantas, D. In Situ Mapping of the Energy Flow Through the Entire Photosynthetic Apparatus. Nat. Chem. 2016, 8, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, M.; Itoh, S.; Kamei, S.; Matsubara, H.; Oh-oka, H. Time-Resolved Spectroscopy of Chlorophyll-a like Electron Acceptor in the Reaction Center Complex of the Green Sulfur Bacterium Chlorobium tepidum. Plant Cell Physiol., 1999, 40, 1021–1028. [CrossRef]
- Kjaer, B.; Frigaard, N.-U.; Yang, F.; Zybailov, B.; Miller, M.; Golbeck, J.H.; Scheller, H.V. Menaquinone-7 in the Reaction Center Complex of the Green Sulfur Bacterium Chlorobium vibrioforme Functions as the Electron Acceptor A1. Biochemistry 1998, 37, 3237–3242. [Google Scholar] [CrossRef]
- Van der Est, A.; Scheller, H.; Hager-Braun, C.; Hauska, G.; Stehlik, D. Forward Electron Transfer in Chlorobium Reaction Centres Studied by Transient EPR. In Photosynthesis: Mechanisms and Effects, Garab, G. Ed.; Springer: Berlin, DE, 1998; pp. 547–550. [Google Scholar]
- Fixen, K.R.; Oda, Y.; Harwood, C.S. Clades of Photosynthetic Bacteria Belonging to the Genus Rhodopseudomonas Show Marked Diversity in Light-Harvesting Antenna Complex Gene Composition and Expression. mSystems 2015, 1, e00006-15. [Google Scholar] [CrossRef]
- Southall, J.; Henry, S.L.; Gardiner, A.T.; Roszak, A.W.; Mullen, W.; Carey, A.M.; Kelly, S.M.; de Percin Northumberland, C.O.; Cogdell, R.J. Characterisation of a pucBA Deletion Mutant from Rhodopseudomonas palustris Lacking All But the pucBAd Genes. Photosynth. Res. 2018, 135, 9–21. [Google Scholar] [CrossRef]
- Ben-Shem, A.; Frolow, F.; Nelson, N. Crystal Structure of Plant Photosystem I. Nature 2003, 426, 630–635. [Google Scholar] [CrossRef]
- Qin, X.; Suga, M.; Kuang, T.; Shen, J.-R. Photosynthesis. Structural Basis for Energy Transfer Pathways in the Plant PSI-LHCI Supercomplex. Science 2015, 348, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Tsukatani, Y.; Azai, C.; Kondo, T.; Itoh, S.; Oh-Oka, H. Parallel Electron Donation Pathways to Cytochrome cZ in The Type I Homodimeric Photosynthetic Reaction Center Complex of Chlorobium tepidum. Biochim. Biophys. Acta 2008, 1777, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Oh-oka, H.; Kamei, S.; Matsubara, H.; Iwaki, M.; Itoh, S. Viscosity Dependence of the Electron Transfer Rate from Bound Cytochrome c to P840 in the Photosynthetic Reaction Center of the Green Sulfur Bacterium Chlorobium tepidum. Biochemistry 1997, 36, 9267–9272. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Higuchi, M.; Azai, C.; Oh-Oka, H.; Miki, K.; Wang, Z.-Y. Crystal Structure of the Electron Carrier Domain of the Reaction Center Cytochrome cZ Subunit from Green Photosynthetic Bacterium Chlorobium tepidum. J. Mol. Biol. 2010, 397, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Kölsch, A.; Madhi, H.; Stieger, K.R.; Feifel, S.C.; Kern, J.F.; Müh, F.; Lisdat, F.; Lokstein, H.; Zouni, A. Insights into the Binding Behavior of Native and Non-Native Cytochromes to Photosystem I from Thermosynechococcus elongatus. J. Biol. Chem. 2018, 293, 9090–9100. [Google Scholar] [CrossRef]
- Axelrod, H., L.; Abresch, E.C.; Okamura, M.Y.; Yeh, A.P.; Rees, D.C.; Feher, G. X-ray Structure Determination of the Cytochrome c2: Reaction Center Electron Transfer Complex from Rhodobacter sphaeroides. J. Mol. Biol. 2002, 319, 501–515. [Google Scholar] [CrossRef]
- Kubota-Kawai, H.; Mutoh, R.; Shinmura, K.; Sétif, P.; Nowaczyk, M.M.; Rögner, M.; Ikegami, T.; Tanaka, H.; Kurisu, G. X-ray Structure of an Asymmetrical Trimeric Ferredoxin-Photosystem I Complex. Nat. Plants 2018, 4, 218–224. [Google Scholar] [CrossRef]
- Tsukatani, Y.; Miyamoto, R.; Itoh, S.; Oh-Oka, H. Function of a PscD Subunit in a Homodimeric Reaction Center Complex of the Photosynthetic Green Sulfur Bacterium Chlorobium tepidum Studied by Insertional Gene Inactivation. Regulation of Energy Transfer and Ferredoxin-Mediated NADP+ Reduction on the Cytoplasmic Side. J. Biol. Chem. 2004, 279, 51122–51130. [Google Scholar] [CrossRef]
- Bryant, D.A.; Costas, A.; Maresca, J.; Chew, A.; Klatt, C.G.; Bateson, M.M.; Tallon, L.J.; Hostetler, J.; Nelson, W.C.; Heidelberg, J.F. et al.; et al. Candidatus Chloracidobacterium thermophilum: an Aerobic Phototrophic Acidobacterium. Science 2007, 317, 523–526. [Google Scholar] [CrossRef]
- Tsukatani, Y.; Romberger, S.P.; Golbeck, J.H.; Bryant, D.A. Isolation and Characterization of Homodimeric Type-I Reaction Center Complex from Candidatus Chloracidobacterium thermophilum, an Aerobic Chlorophototroph. J. Biol. Chem. 2012, 287, 5720–5732. [Google Scholar] [CrossRef]
- He, Z.; Ferlez, B.; Kurashov, V.; Tank, M.; Golbeck, J.H.; Bryant, D.A. Reaction Centers of the Thermophilic Microaerophile, Chloracidobacterium thermophilum (Acidobacteria) I: Biochemical and Biophysical Characterization. Photosynth. Res. 2019, 142, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Garcia Costas, A.M.; Tsukatani, Y.; Rijpstra, W.I.; Schouten, S.; Welander, P.V.; Summons, R.E.; Bryant, D.A. Identification of the Bacteriochlorophylls, Carotenoids, Quinones, Lipids, and Hopanoids of "Candidatus Chloracidobacterium thermophilum." J. Bacteriol. 2012 194,1158-1168. 194. [CrossRef]
- Tsukatani, Y.; Wen, J.; Blankenship, R.E.; Bryant, D.A. Characterization of the FMO Protein from the Aerobic Chlorophototroph, Candidatus Chloracidobacterium thermophilum. Photosynth. Res. 2010, 104, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Weyer, K.A.; Schaefer, W.; Lottspeich, F.; Michel, H. Cytochrome Subunit of the Photosynthetic Reaction Center from Rhodopseudomonas viridis is a Lipoprotein, Biochemistry 1987, 26, 2909–2914. 26. [CrossRef]
- Qian, P.; Siebert, C.A.; Wang, P.; Canniffe, D.P.; Hunter, C.N. Cryo-EM Structure of the Blastochloris viridis LH1-RC Complex at 2.9 Å. Nature 2018, 556, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.J.; Suga, M.; Wang-Otomo, Z.Y.; Shen, J.R. Structure of Photosynthetic LH1-RC Supercomplex At 1.9 A Resolution. Nature 2018, 556, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Tani, K.; Kanno, R.; Kurosawa, K.; Takaichi, S.; Nagashima, K.V.P.; Hall, M.; Yu, L.J.; Kimura, Y.; Madigan, M.T.; Mizoguchi, A.; et al. An LH1-RC Photocomplex from an Extremophilic Phototroph Provides Insight into Origins of Two Photosynthesis Proteins. Commun. Biol. 2022, 5, 1197. [Google Scholar] [CrossRef] [PubMed]
- Barz, W.P.; Vermeglio, A.; Francia, F.; Venturoli, G.; Melandri, B.A.; Oesterhelt, D. Role of the PufX Protein in Photosynthetic Growth of Rhodobacter sphaeroides. 2. PufX is Required for Efficient Ubiquinone/Ubiquinol Exchange Between the Reaction Center QB Site and the Cytochrome bc1 Complex. Biochemistry 1995, 34, 15248–15258. [Google Scholar] [CrossRef]
- Hucke, O.; Schiltz, E.; Drews, G.; Labahn, A. Sequence Analysis Reveals New Membrane Anchor of Reaction Center-Bound Cytochromes Possibly Related to PufX. FEBS Lett, 2003; 535, 166–170. [Google Scholar] [CrossRef]
- Hanada, S.; Takaichi, S.; Matsuura, K.; Nakamura, K. Roseiflexus castenholzii gen. nov., sp. nov., a Thermophilic, Filamentous, Photosynthetic Bacterium that Lacks Chlorosomes. Int. J. Syst. Evol. Microbiol. 2002, 52, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Shi, Y.; Niu, T.; Wang, Q.; Niu, W.; Huang, X.; Ding, W.; Yang, L.; Blankenship, R.E.; Xu, X. Cryo-EM Structure of the RC-LH Core Complex from an Early Branching Photosynthetic Prokaryote. Nature Commun. 2018, 9, 1568. [Google Scholar] [CrossRef]
- Qi, C.H.; Wang, G.L.; Wang, F.F.; Xin, Y.; Zou, M.J.; Madigan, M.T.; Wang-Otomo, Z.Y.; Ma, F.; Yu, L.J. New Insights on the Photocomplex of Roseiflexus castenholzii Revealed from Comparisons of Native and Carotenoid-Depleted Complexes. J. Biol. Chem. 2023, 299, 105057. [Google Scholar] [CrossRef]
- Woese, C.R. Bacterial Evolution. Microbiol. Rev. 1987, 51, 221–271. [Google Scholar] [CrossRef] [PubMed]
- Garcia Costas, A.M.; Liu, Z.; Tomsho, L.P.; Schuster, S.C.; Ward, D.M.; Bryant, D.A. Complete Genome of Candidatus Chloracidobacterium thermophilum, a Chlorophyll-Based Photoheterotroph Belonging to the Phylum Acidobacteria. Environ. Microbiol. 2012, 14, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Gisriel, C.J.; Azai, C.; Cardona, T. Recent Advances in the Structural Diversity of Reaction Centers. Photosynth. Res. 2021, 149, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Cardona, T. Photosystem II is a Chimera of Reaction Centers. J. Mol. Evol. 2017, 84, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Azai, C.; Harada, J.; Fujimoto, S.; Masuda, S.; Kosumi, D. Anaerobic Energy Dissipation by Glycosylated Carotenoids in the Green Sulfur Bacterium Chlorobaculum tepidum. J. Photochem. Photobiol. A: Chemistry 2020, 403, 112828. [Google Scholar] [CrossRef]
- Cardona, T. A Fresh Look at the Evolution and Diversification of Photochemical Reaction Centers. Photosynth. Res. 2015, 126, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.F.; Bryant, D.A.; Beatty, JT. A Physiological Perspective on the Origin and Evolution of Photosynthesis. FEMS Microbiol. Rev. 2018, 42, 205–231. [Google Scholar] [CrossRef] [PubMed]
- Cardona, T. Thinking Twice About the Evolution of Photosynthesis. Open Biol. 2019, 9, 180246. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Bauer, C.E. Complex Evolution of Photosynthesis. Annu. Rev. Plant Biol. 2002, 53, 503–5321. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.M.; Blankenship, R.E. Thinking About the Evolution of Photosynthesis. Photosynth. Res. 2004;80, 373-386. [CrossRef]
- Cardona, T. Reconstructing the Origin of Oxygenic Photosynthesis: Do Assembly and Photoactivation Recapitulate Evolution? Front. Plant Sci. 2016, 7, 257. [Google Scholar] [CrossRef]
- Cardona, T.; Sánchez-Baracaldo, P.; Rutherford, A.W.; Larkum, A.W. Early Archean Origin of Photosystem II. Geobiol. 2019, 17, 127–150. [Google Scholar] [CrossRef] [PubMed]
- Oliver, T.; Kim, T.D.; Trinugroho, J.P.; Cordón-Preciado, V.; Wijayatilake, N.; Bhatia, A.; Rutherford, A.W.; Cardona, T. The Evolution and Evolvability of Photosystem II. Annu. Rev. Plant Biol. 2023, 74, 225–257. [Google Scholar] [CrossRef] [PubMed]
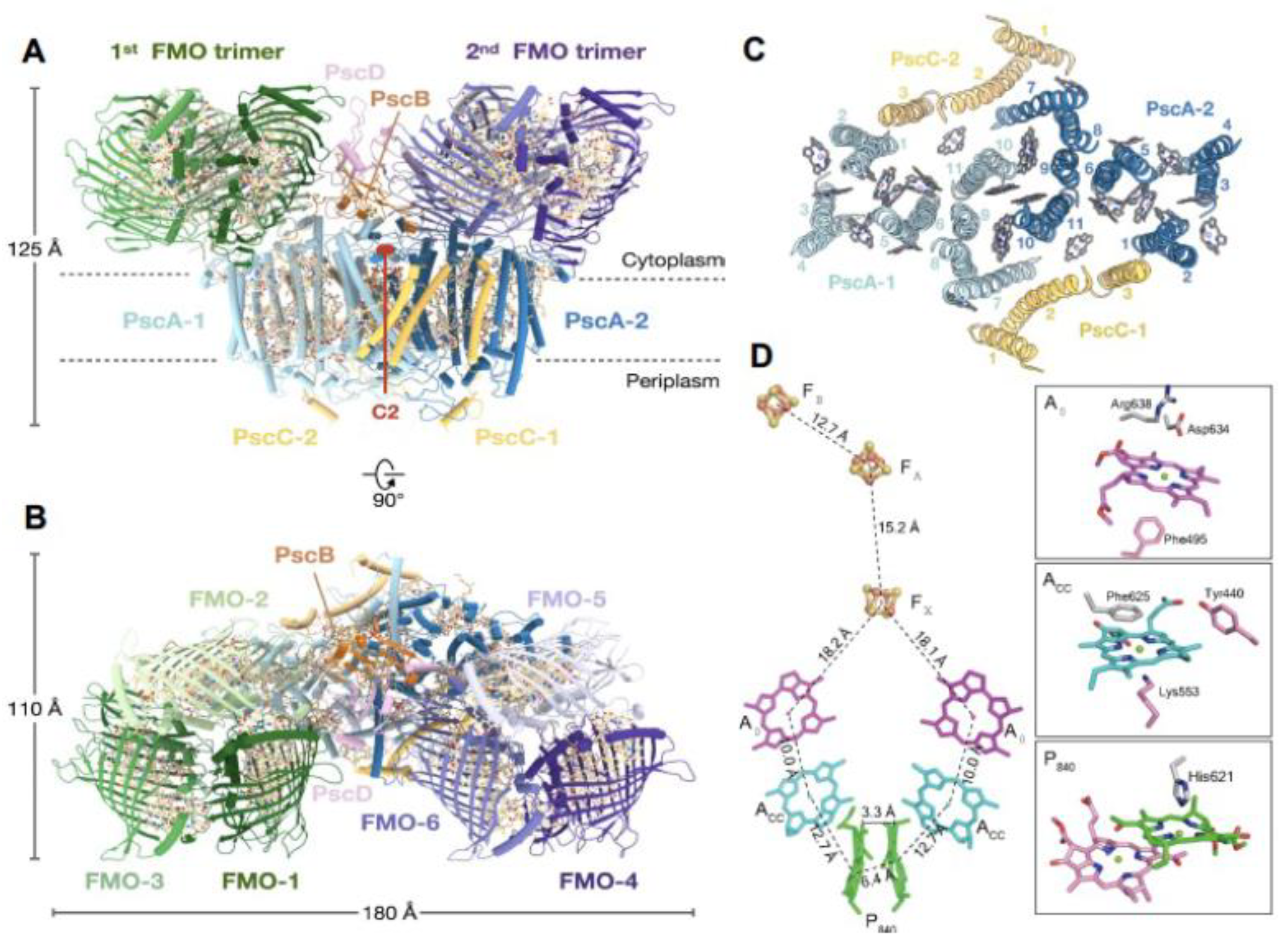
| Amino | |||
| Designation Acids | TMHs[2] | Functional assignment | |
| PscA-1 | 865 | 11 | Homodimeric RC-PS core, LH and RC charge separation roles |
| PscA-2 | 865 | 11 | Homodimeric RC-PS core, LH and RC charge separation roles |
| PscB | 179 | None | Houses terminal RC [4Fe-4S] electron acceptors FA and FB |
| PscU | 35 | 1 | Newly identified, unknown function |
| PscV | 36 | 1 | Newly identified, unknown function |
| PscW | 45 | 1 | Newly identified, unknown function |
| PscX | 189 | None | Cytochrome c electron donor 1 to RC, forms heterodimer with PscY |
| PscY | 221 | None | Cytochrome c electron donor 2 to RC, forms heterodimer with PscX |
| PscZ | 70 | None | Putative PscB stabilizer, may play PsaD role in enhancing PSI e- transfer |
| UPP | 19 | 1 | Unidentified polypeptide, non-helical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




