1. Introduction
Karst aquifers are among the richest in groundwater on Earth [
1,
2,
3,
4,
5], and globally provide drinking water to almost a quarter of the world population [
6,
7,
8,
9,
10,
11,
12,
13,
14]. They are used even more extensively in the Mediterranean area) [
15,
16,
17,
18,
19,
20], and in south-eastern Europe where some large cities, including Tirana, are supplied with water from karst sources [
21]. At the same time, carbonate aquifers are also large reservoirs often recharging important mineral and thermal springs in many countries of the world [
22,
23,
24], including the Balkan countries [
25].
Albania is characterised by wide presence of carbonate rocks [
26,
27]. They cover about 6490 km
2 and contain in total about 90 m
3/s exploitable groundwater resources [
28]. Likewise, Albania is rich also in thermal karst waters, which are mostly related to carbonate karst aquifers [
28,
29,
30,
31,
32]. The Makaresh karst massif, object of this article, although relatively small is the only carbonate massif in Albania rich in both cold and thermal karst waters. The main purpose of this study is therefore to highlight the hydrogeological functioning of Makaresh massif, in close relation to its geological and structural features, and the role of hypogene speleogenesis [
33,
34,
35] in the formation of its secondary porosity. Eventually, given the existing threats in the massif, some considerations about degradation of the Makaresh karst environment are also presented.
2. Study area
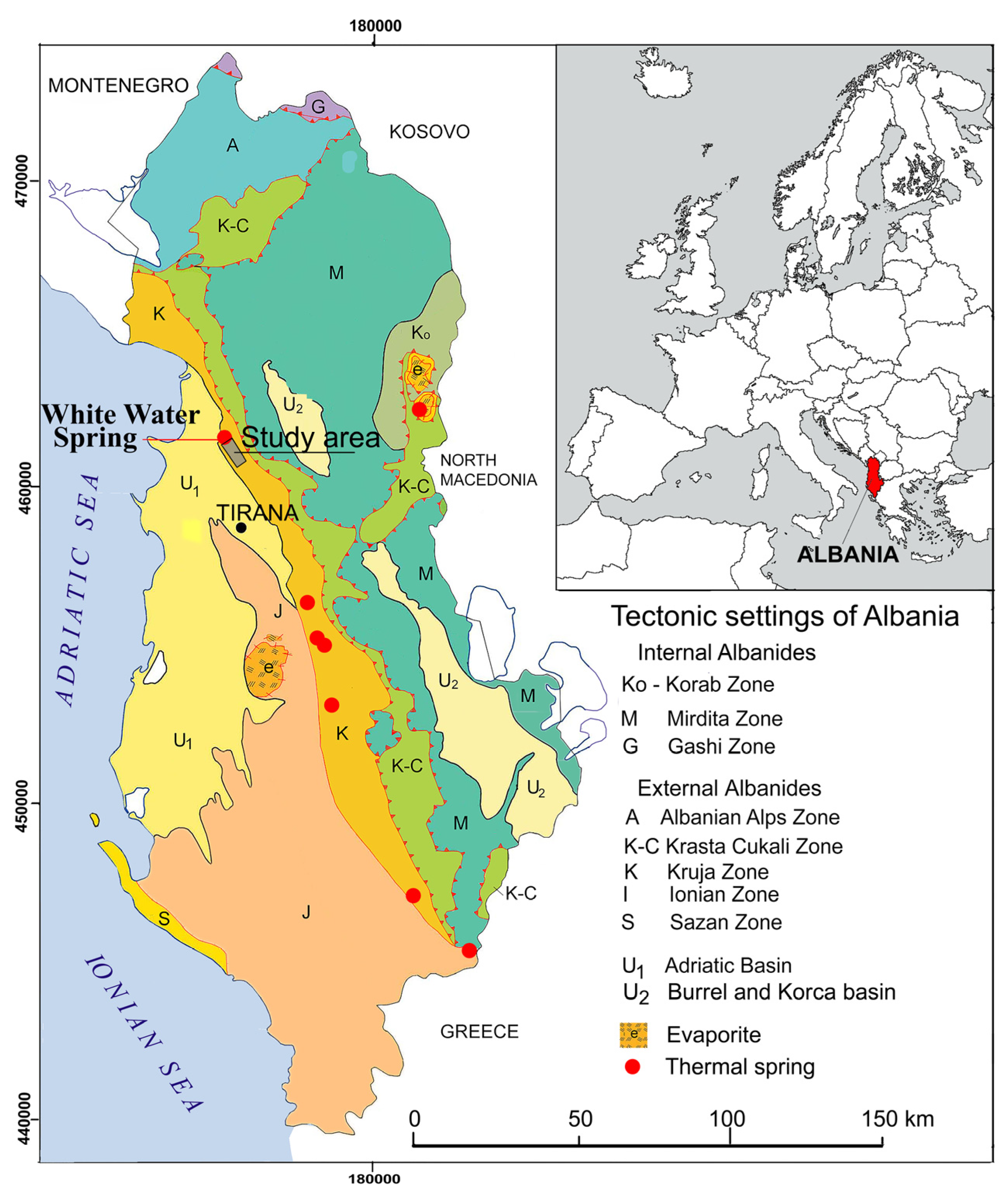
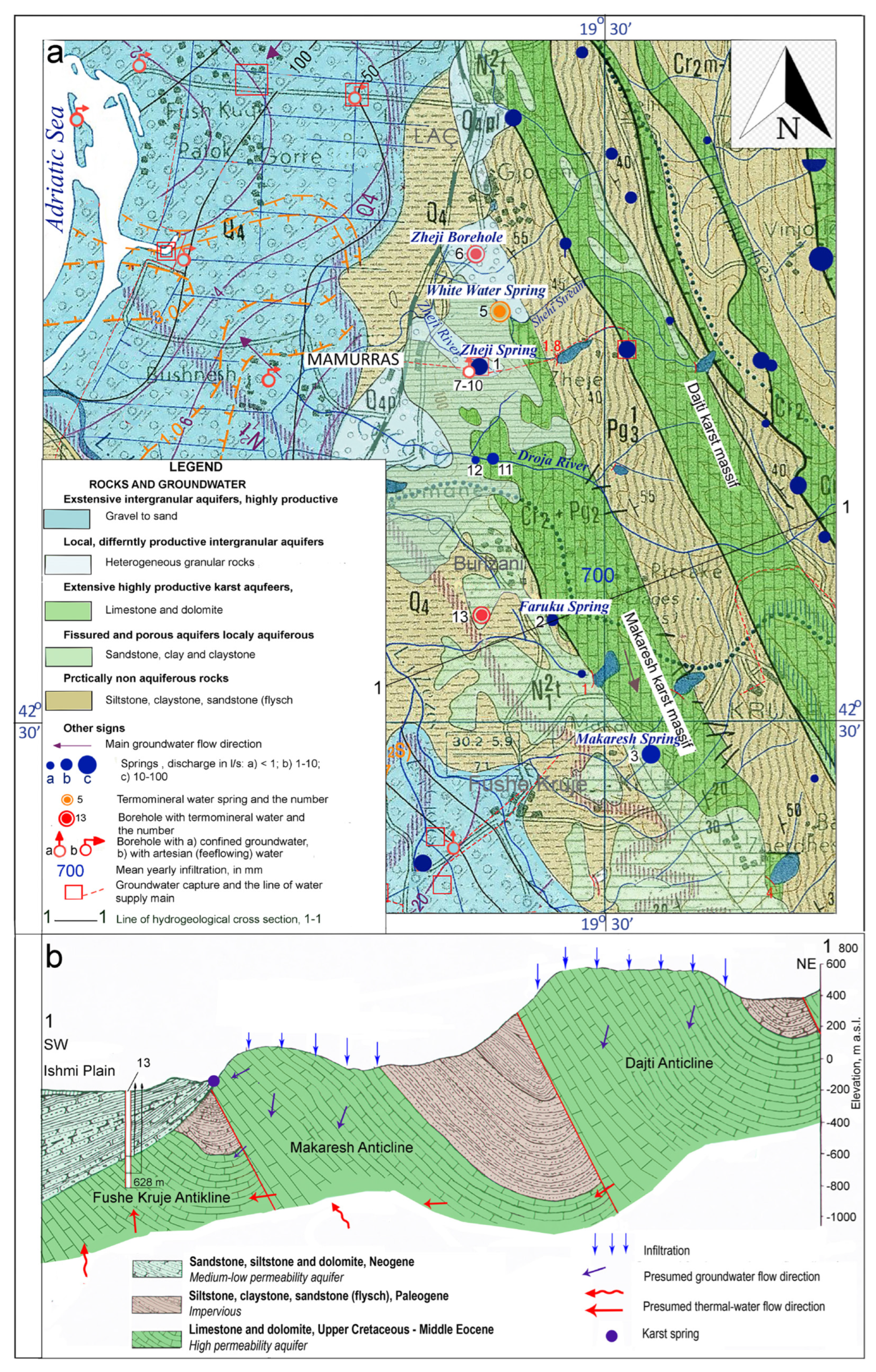
The karst massif of Makaresh is located about 30 km north-west of the city of Tirana (
Figure 1). Morphologically it represents a NW-SE oriented ridge where carbonate rocks crop out for about 22 km
2, while about 8 km
2, of the northern part are covered by Neogene formations. The highest peak of the massif (Picraga, 442 m above sea level), is in its central part, while the average altitude of the massif is about 300 m a.s.l. Climate of the area is a warm Mediterranean, characteristic for the coastal plain areas of the country [
36]: the mean yearly temperature is about 14° C, with 6.2 °C in January, and 23.0 °C in August. Mean yearly precipitation is about 1300 mm, with about 70% falling during the period October-April. Main hydrological elements of the study area are the Droja River, which canyon crosses the northern part of the karst massif in E-W direction, and the small stream of Zheji, south of the former river.
3. Materials and Methods
To characterize the hydrogeology of the study area, multiple geological and regional hydrogeological studies have been performed, and integrated data collected from different archives. Among the most important regional studies are geological maps [
27] and neotectonics map [
37], as well as the hydrogeological map of Albania [
26]. The first detailed and important hydrogeological study carried out on
Uji Bardhe (White Water) thermal spring, accompanied with detailed chemical analyses, was performed since 1957 [
29]. Detailed hydrogeological observation on Makaresh karst massif, including new chemical analyses of White Water have been carried out in the framework of the hydrogeological survey of the territory of Albania [
26]. This spring was also treated in a special edition dedicated to the thermal springs of Albania [
32]. Some archive materials of the Albanian Hydrogeological Service (AHS) reflect the results of detailed karst studies for the water supply of the city of Mamurras [
38,
39]. The water samples are analysed for the macro-components Mg
2+, Ca
2+, N
++K
+, Cl
-, SO
42-, HCO
3-, partially in the field, as well as in the AHS laboratory. The main physical parameters, such as ph, temperature and electrical conductivity (EC), have been measured in the field for 7 karst springs and 5 wells. Volumetric, spectro-photometric and colorimetric methods have been used for analysis. The Piper diagram, as well as other hydrochemical graphs created using the AquaChem program, have been used for the hydrochemistry characterization of the groundwater. The comparative assessment of the results of the chemical analyses of thermal springs performed at different times, as well as the comparison of the chemical composition between thermal and cold-water springs, represents an important element of this study. This enables to clarify the groundwater formation and circulation in the Makaresh massif, as a pre-condition for its rational use and protection.
4. Geology
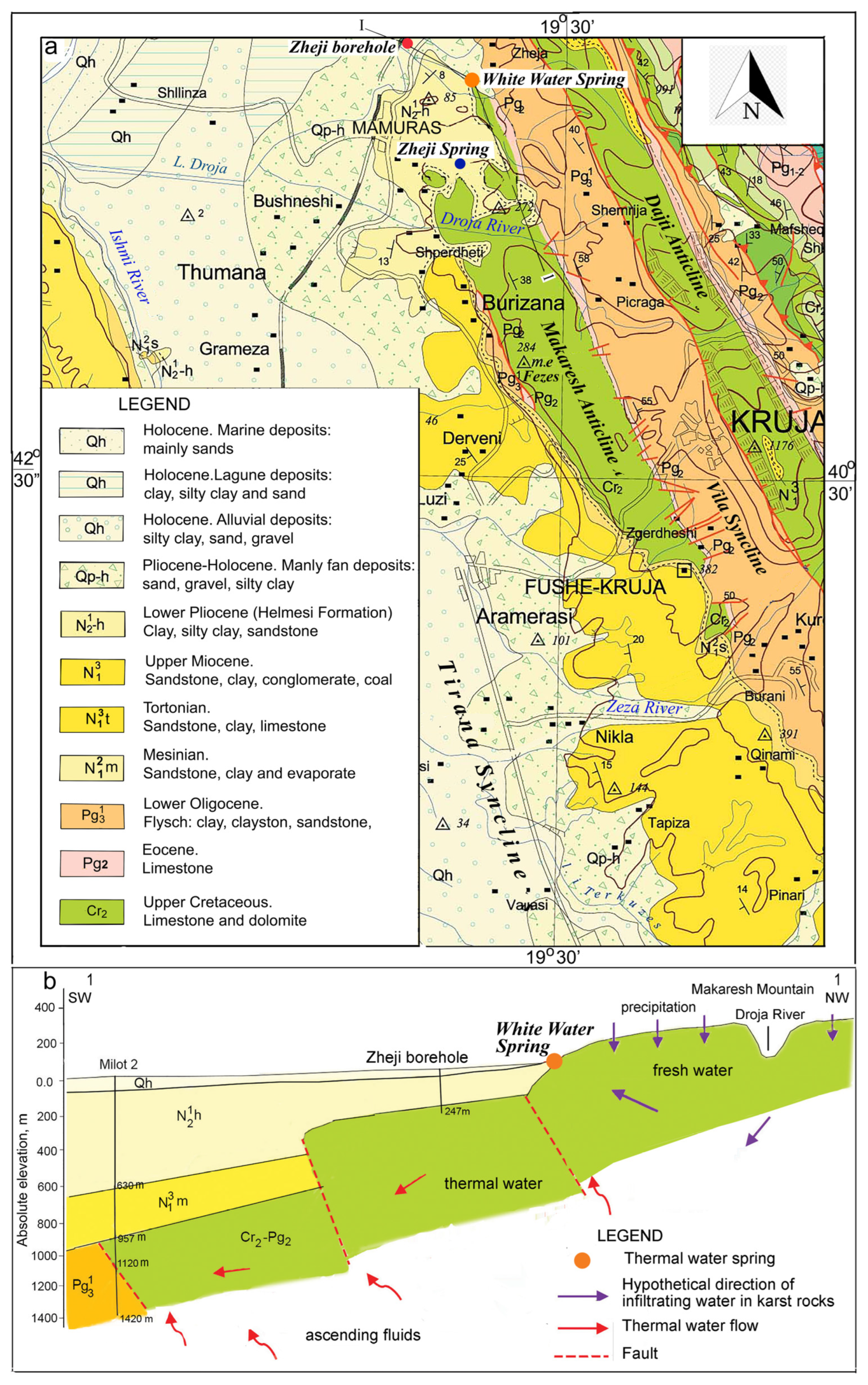
The geological structure of Albania (
Figure 1) comprises two major units, namely the Internal Albanides to the east and the External Albanides to the west [
27,
37,
40]. The Makaresh karst structure belongs to the Kruja zone, which is the easternmost sector of the External Albanides (
Figure 1), and is characterized by the presence of long NNW-SSE structures built up by sedimentary rocks. The Makaresh structure represents an anticline consisting of carbonate rocks (limestone, dolomitic limestone and dolomites) of Upper Cretaceous to Eocene age (
Figure 2). These deposits dip eastward with angles of about 35° and are covered by Oligocene clay-claystone flysch filling the Vila Syncline. In accordance with the tectonic style of the Kruja zone, the Makaresh structure was affected by westward longitudinal thrusting during Eocene and late Oligocene to Miocene times [
27,
40,
41].
Beside this, the Makaresh massif is broken by a series of deep transversal NE-SW faults, that fragmentize the buried carbonate structures [
42,
43]. This is testified also by deep boreholes located in the northern part of the Makaresh structure (
Figure 2a and 2b). West of it, the wide Tirana syncline, filled with Paleogene and Neogene molasses, is located, to cover two buried parallel carbonate structures (
Figure 3b) hosting thermal water tapped by deep oil wells [
32]. East of this structure, and parallel to it, the Dajti carbonate anticline is present (
Figure 2a). On the northern part of the Makaresh carbonate structure, it is covered by Lower Pliocene deposits of the Helmes suite, represented by intercalations of clays, claystones and sandstones.
5. Hydrogeology
The carbonate rocks forming the Makaresh structure are intensively fractured, at least with three main discontinuity systems, the most developed of which is represented by the bedding, dipping in the same direction of the axis of the structure itself. Fissured carbonate rocks often have bitumen content, related to weathering processes from the early pre-Neogene time when the Makaresh structure was an oil-bearing structure. Broken rock surfaces often smell of hydrogen sulphide (H
2S), as observed in the neighbouring Dajti anticline structure [
44]. On top of this structure, a karst plateau hosting a great variety of karst landforms, with innumerable sinkholes [
45,
46,
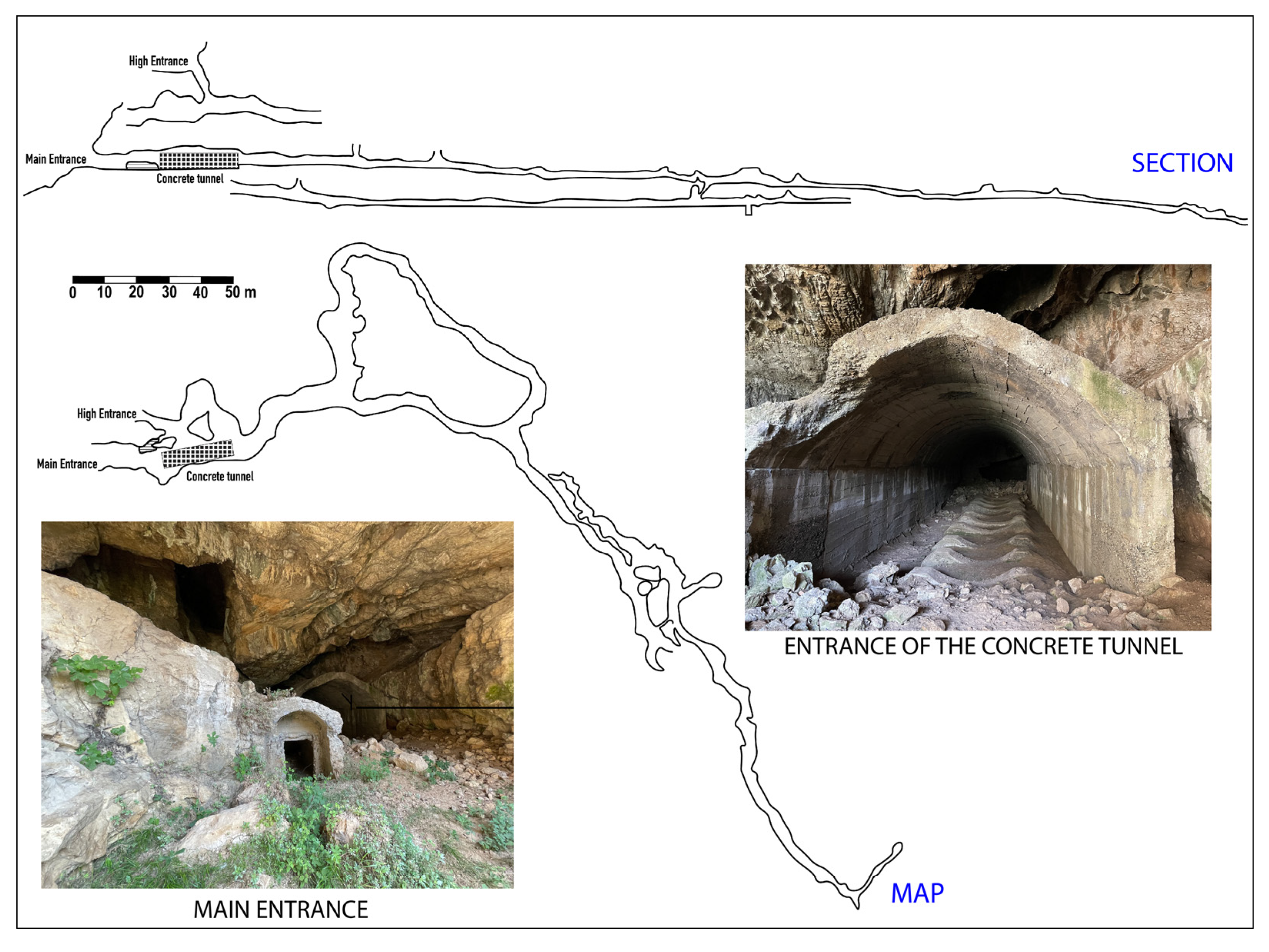
47] and vertical cave opening and fractures, is developed. One of the most interesting karst phenomena is Shpella Sallas, a cave located in the south-western part of the carbonate massif, in the immediate proximity of Makaresh spring—No. 3 (
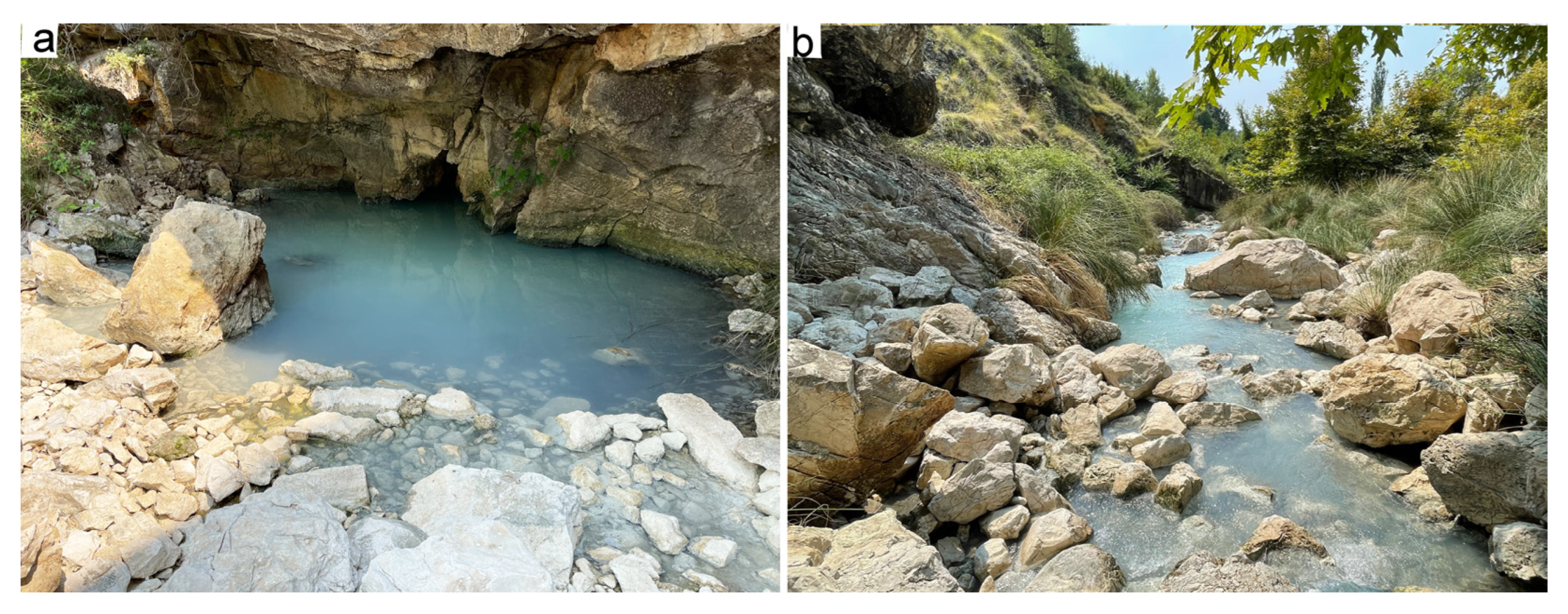
Figure 3).
The entrance of the cave, after the initial part with a concrete tunnel built during the Communist time, is about 8-10 m wide, with maximum height of about 6 m. It then continues like a tunnel for about 25 m, 4-4.5 m wide and about 3.0 m high (
Figure 3). The cave walls are coated with calcite and sulphur pigments. It is not excluded that in a such environment the sulfuric acid formed by the oxidation of pyrite pigments of the limestone plane fissures [
44], as many authors describe [
19,
33,
35,
48] mixes with the ascending thermal fluids and produces morphologies typical of speleogenesis by sulphuric acid. Shpella Sallas has an overall length of over 700 meters. About 6-7 below the described cave there is another small cavity where the largest karst spring of the massif, known as the Makaresh spring, issues (
Figure 3).
Figure 3.
Shpella Sallas section and map with details of the main cave entrance and the concrete tunnel structure. This latter is a man-made tunnel built along the initial part of the natural cave; it has been used during communist time for defending purposes (redrawn after [
49]).
Figure 3.
Shpella Sallas section and map with details of the main cave entrance and the concrete tunnel structure. This latter is a man-made tunnel built along the initial part of the natural cave; it has been used during communist time for defending purposes (redrawn after [
49]).
The intensive karstification of the Makaresh massif facilitates infiltration of the abundant rainfall. Based on climatic data [
36], the extrapolated annual rainfall for the average elevation of this structure is about 1300 mm, while the average annual temperature is 14.0 °C. The evapotranspiration calculated with the formula by Turc [
50] is 600 mm and the infiltration results 700 mm/year. The fast discharge during short periods following the heavy rains is estimated at about 10%, or 130 mm/year, while the efficient infiltration of the precipitation is equal to 570 mm/year. For the entire outcrop area of carbonate rocks at the Makaresh massif (22 km
2), the renewable groundwater resources consist approximately in 12.54*10
6 m
3/year, or 400 l/s corresponding to an underground flow module of the massif of about 18 l/s/km
2. The calculated values match with the estimated values for low-elevation karst massifs in Central Albania [
51].
The springs emerging from the Makaresh massif essentially differ regarding their physical and hydro-chemical characteristics, and consist of two well different groups; cold and thermal (
Figure 4). Temperature of the cold springs is about the yearly average temperature of the area (15 °C), while the thermal springs show temperature higher than 5 °C [
22,
52].
5.1. Cold springs
The few cold springs are located in the western periphery of the Makaresh karst structure, where the limestone formations crop out at lower elevation, or at the base level of the structure (
Figure 4a). Among them there is Zheji spring (no. 1) discharging about 5 to over 50 l/s. Several small sources emerge in the village Burizan, the largest of which is the Farruku spring (no. 2), flowing about 2 l/s. In the narrow gorge of river Droja, crossing the karst massif, there are several minor springs with total flow of about 20 l/s; among them, springs nos. 11 and 12 discharge about 6.5 and 0.5 l/s, respectively. It is believed that the groundwater seepage from the limestone massive to the Droja River along the canyon could be in average some tenths of litres per second.
One of the most interesting springs in the study area is the Makaresh Spring (no. 3 in
Figure 6), emerging inside the homonymous cave. The spring has ascending character, and according to non-systematic observation its discharge varies from about 100 l/s to more than 500 l/s, while the water temperature is 16.2° C. The spring water has a weak smell of sulphurous gas and a light white colour (
Figure 5).
5.2. Thermal springs
These springs are represented by a group called White Water springs, as well as by the Zheji borehole free-flowing thermal water (
Figure 4). The spring emerges at the contact between Pleistocene deposits (local name “Helmasi Series”) consisting of sandstone, conglomerates and clay, and the underlying Cretaceous-Eocene carbonate rocks. White Water is a group of springs issuing closely within an area of about 120x70 m (
Figure 6). Discharge of the different springs varies from about 1 to more than 20 l/s, the largest one being no. 5, located near some travertine deposits (
Figure 6). According to non-systematic measurements, the total discharge of White Water Spring varies from 20 to about 100 l/s, with average discharge of about 40-50 l/s, and water temperature ranging from 20 to 22.8 °C.
Zheji borehole (no. 6) is located about 1.4 km northwest of White Water springs, at the northern periclinal of the submerged Makaresh anticline (
Figure 4a) and was drilled during 1958 for bauxite investigations [
53]. After passing Holocene and Pliocene deposits, the borehole at depth 178.0 m tapped Upper Cretaceous dolomite limestones and limestones, down until depth of 241. 6 m. The borehole tapped artesian free-flowing groundwater, which initial discharge in 1959 was 4 l/s, but today it has a constant discharge of about 1.8 l/s (
Figure 6b). The water is warm with a temperature ranging during the year from 22.0 to 22.8 °C and has a strong sulphur gas smell.
6. Hydrogeochemical characteristics
Groundwater circulation can be understood in the framework of hierarchical flow systems, consisting of local, intermediate, and regional flow system [
54,
55,
56], or shallow groundwater, and deep groundwater reservoirs [
57]. Water circulation in thermal karst systems is generally gravity-driven, caused by topographic gradients [
56], however temperature-induced density gradients and reduced viscosities facilitate the upward flow of hot water toward the springs [
22]. The simplest approach to delineate flow components in a karst aquifer is to use thermal data [
57].
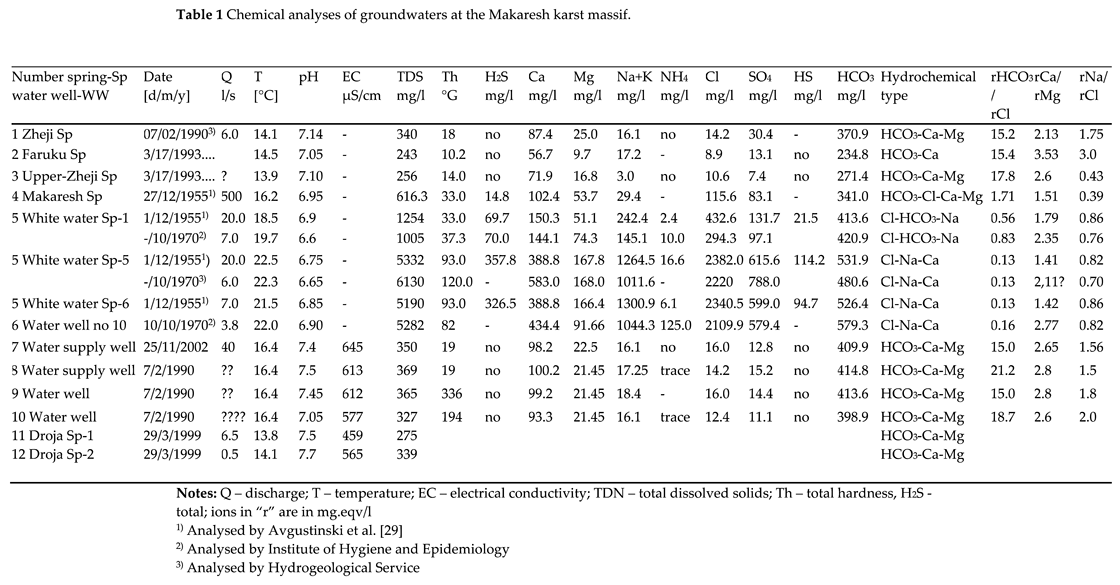
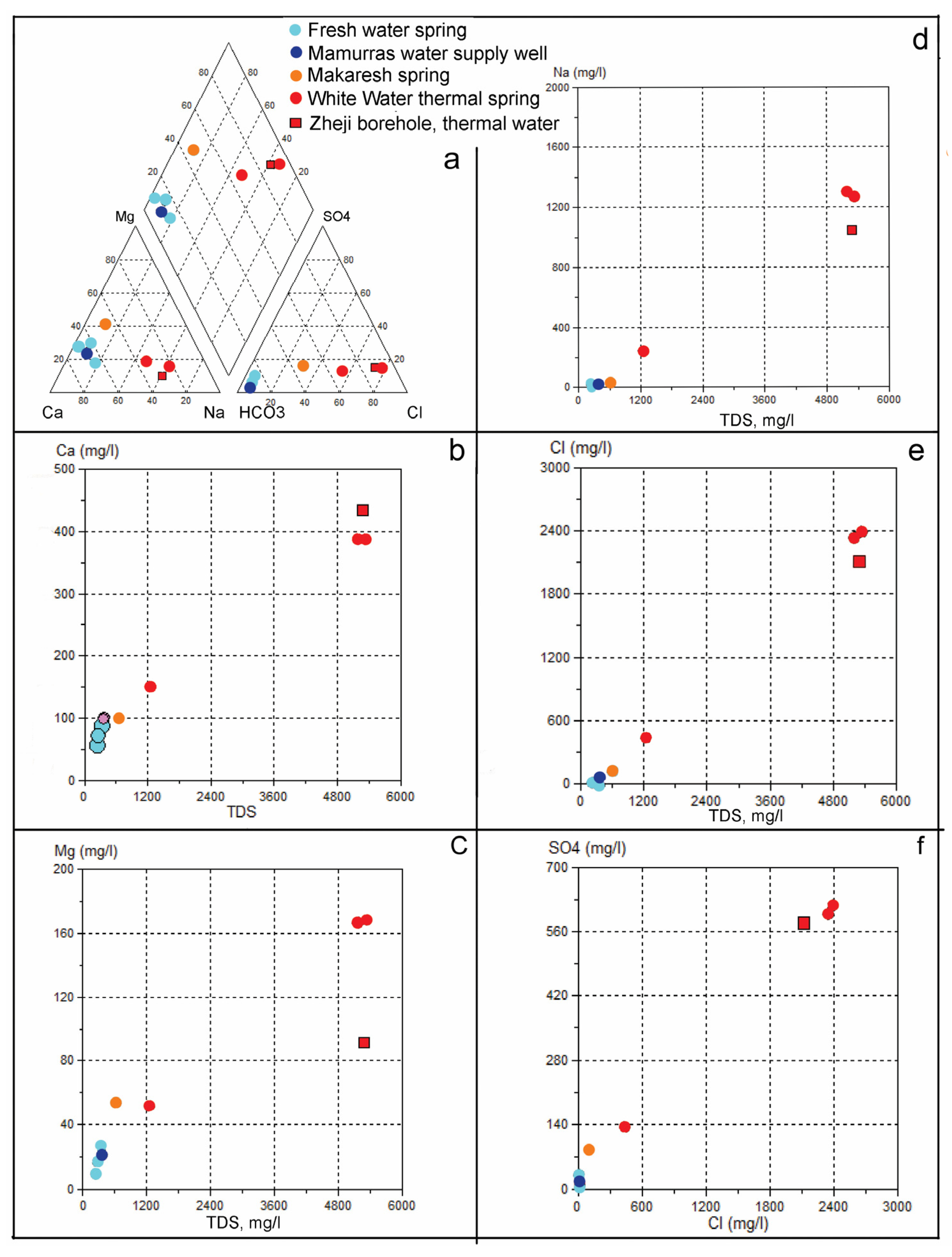
The results of the physic-chemical analysis of the springs at the Makaresh carbonate structure (Table 1) and the following Piper diagram, as well as other hydrochemical graphs created using the AquaChem program, have been used for the hydrochemistry characterization of the groundwater of the studied area (
Figure 7 and
Figure 8). The hydrochemical assessment includes the springs, as well as the groundwater of wells no. 1, 2, 3 and 4, located near the Zheji spring (no. 1). The chemical parameters like TDS, Mg
2+, Ca
2+, Cl
-, SO
42-, as well as the groundwater temperature (
Figure 7 and
Figure 8) evidence the differences between the two groundwater groups of cold and thermal waters, likely related to presence of two groundwater circulation systems, one shallow and one deep.
Cold water springs derive from the shallow circulating system, characterized by temperatures around 14-16.4 °C and by a weakly alkaline pH (7.05-7.45). These waters present low mineralization (TDS 240-370 mg/l; TDS 580-650 μS/cm) and low hardness Th=10-20 °G. Major ion concentrations fall within the following ranges (in mg/l): Ca2+ 57-100; Mg2+ 10-25; Na- 3-18; HCO3- 270-415; SO42- 7-30; Cl- 9-16. Taking into consideration the ions with concentration > 25 % mg/eqv/l, the hydrochemical type of cold waters is mainly of the HCO3-Ca-Mg type. To this typology springs such as Faruku (no. 2), and Zheji (no. 1) belong, as well as four shallow water supply wells located near the latter (Table 1).
In the cold waters group, the Makaresh spring is slightly different, being characterized by temperature of 16.2 °C, pH 6.95, TDS 616 mg/l, and a total hardness Th = 26.7 °G. The other chemical parameters generally show increased concentrations (mg/l): Ca
2+ 102.4; Mg
2+ 53.7, Na
- 29; HCO
32- 341; SO
42- 83; Cl
- 115.6. The hydrochemical type is in this case HCO
3-Cl-Ca-Mg (Table 1), due to the significant increase in the concentration of Cl
- and Mg
2+ ions. However, the main characteristics of the Makaresh spring is the presence of H
2S gas (about 14.8 mg/l) classifying it as week sulphide mineral spring [
58].
Thermal springs are represented by White Water Spring and the Zheji borehole. The eight issues of White Water have very similar physical and chemical characteristics, excluding the issue no. 1, where the water temperature and the concentration of the chemical parameters is lower than at the other springs (Table 1). The groundwater temperature varies from about 19 °C in issue no. 1 to 21.5-22.3 °C in issues nos. 5 and 6, and have weakly acidic reaction, with pH 6.6-6.9.
The concentration of hydrochemical parameters of thermal water (issues nos. 5 and 6) are distinctly higher than for the cold waters, with increased mineralization (TDS 4100-7800 mg/l) and hardness (Th = 33-93 °G). The major ion concentrations fall within the following ranges (mg/l): Ca2+ 388-583; Mg2+ about 168; Na+ 1100-1300; HCO3- 480-530; SO42- 600-788; Cl- 2200-2380. Taking into consideration the ions with concentration > 25 % mg/eqv/l, the hydrochemical typology of the thermal springs is mainly of the Cl-Na-Ca type.
The most important hydrochemical characteristics of the White-Water Spring is the high concentration of H
2S, about 325-360 mg/l. Comparing with other thermal springs of Albania, only in that of Llixha Elbasan, the concentration of this gas is higher, about 410 mg/l [
32]. In
Table 2 the concentrations of some micro-components and gases in White Water Spring, are provided: among the gases it could be noticed the increased presence of hydrogens sulphide HS (114.2 mg/l) and free carbonic gas CO
2 (141.7 mg/l).
Among the gases freely released from water, nitrogen (N) dominates with 71.5% of the volume, followed by carbon dioxide (CO
2 15.41%), and further methane and sulphur gas representing the sum of S
2O
32+ and SO
32+. As for dissolved gases, H
2S (155 mg/l) and CO
2 (71.7 mg/l) prevail. The only analysed micro-components bromide and iodide have low concentrations, respectively 1.2 and 0.4 mg/l. Based on the classification of thermal waters [
58], White Water Spring can be classified as:
“Very strong hydrogen sulphide (H2S) gas warm water with medium salinity”.
7. Groundwater circulation
Cold water springs are fed by the shallow circulation system and moves into a predominantly dolomite-limestone environment. The main process conditioning the formation of the chemical composition of shallow-circulating groundwater is solution of the carbonate rocks by infiltrating waters, enriched in carbon dioxide in the vegetal cover of the karst massif, a process strongly dependent by the contact time between water and rock [
59,
60]. Although the groundwater moves into the dolomite environment the concentration of Ca
2+ in water is higher than that of Mg
2+ due to higher solubility of the limestones [
44,
61,
62]. According to many researchers the rCa
2+/rMg
2+ ratio (r indicating the concentration in mg/eqv/l) is a sensitive indicator of the composition of carbonate rocks. The ratio rCa
2+/rMg
2+ in dolomite groundwater fluctuates in the range 1.5 to 2.2, while in limestone waters it is usually over 2.5, but can reach values up to 10 [
63,
64,
65]. This ratio varies from 1.6 to 2.1 in the groundwater of the dolomite massif of Dajti Mountain [
44], while in the pure limestone massif of Mali me Gropa it varies from 7.2 to 13.8 [
66]. Data of the cold waters of Makaresh karst massif support the above-mentioned conclusions, since the rCa
2+/rMg
2+ value generally varies between 2.1 and 2.8, with the lower value (1.5) at the Makaresh spring.
Thermal waters are recharged by the deep circulation system, characterised by high water temperature, high concentration of chemical parameters and the presence of gases dissolved in water. Based on the hydrogeological regionalization of the thermal waters of Albania, the Makaresh carbonate aquifer is part of the Kruja tectonic zone [
32] and has a great potential for hypogenic karstification in the Upper Cretaceous to Eocene carbonate formations [
67].
Karst reservoirs are characterized by very low matrix porosity and by high porosity related to fissures and karst, developed more intensively at the crests of anticlinal structures [
42,
67]. Transverse trend (NW-SE) breakdowns in the external tectonic areas of Albania are predominant as fluid movement routes, as well as these are favoured by presence of open gaps, calcium fillings and bitumen [
41]. In conditions of difficult groundwater circulation in deep structures, their enrichment with different chemical components is mainly related to two processes.
The first relates to the presence of evaporitic rocks below the Mesozoic carbonate structures [
68,
69], which facilitates the formation and movement of sulphatic fluids rich in salts and gases such as H
2S and CO
2 and micro-components, that transfer warm water to sources [
22,
23,
70]. Fluids may also be rich in Na and Cl ions, as related to the halite deficiency usually found in evaporite deposits [
32,
71]. The second process is the enrichment of thermal waters with sulphate by oxidation of pyrite, a phenomenon occurring in oxidation environment [
72], and that is likely to be present in the Makaresh massif, as well as in the neighbouring Dajti [
44]. This could explain the mainly Cl-Na-Ca composition with high concentration of H
2S and CO
2 gases at the White Water thermal spring.
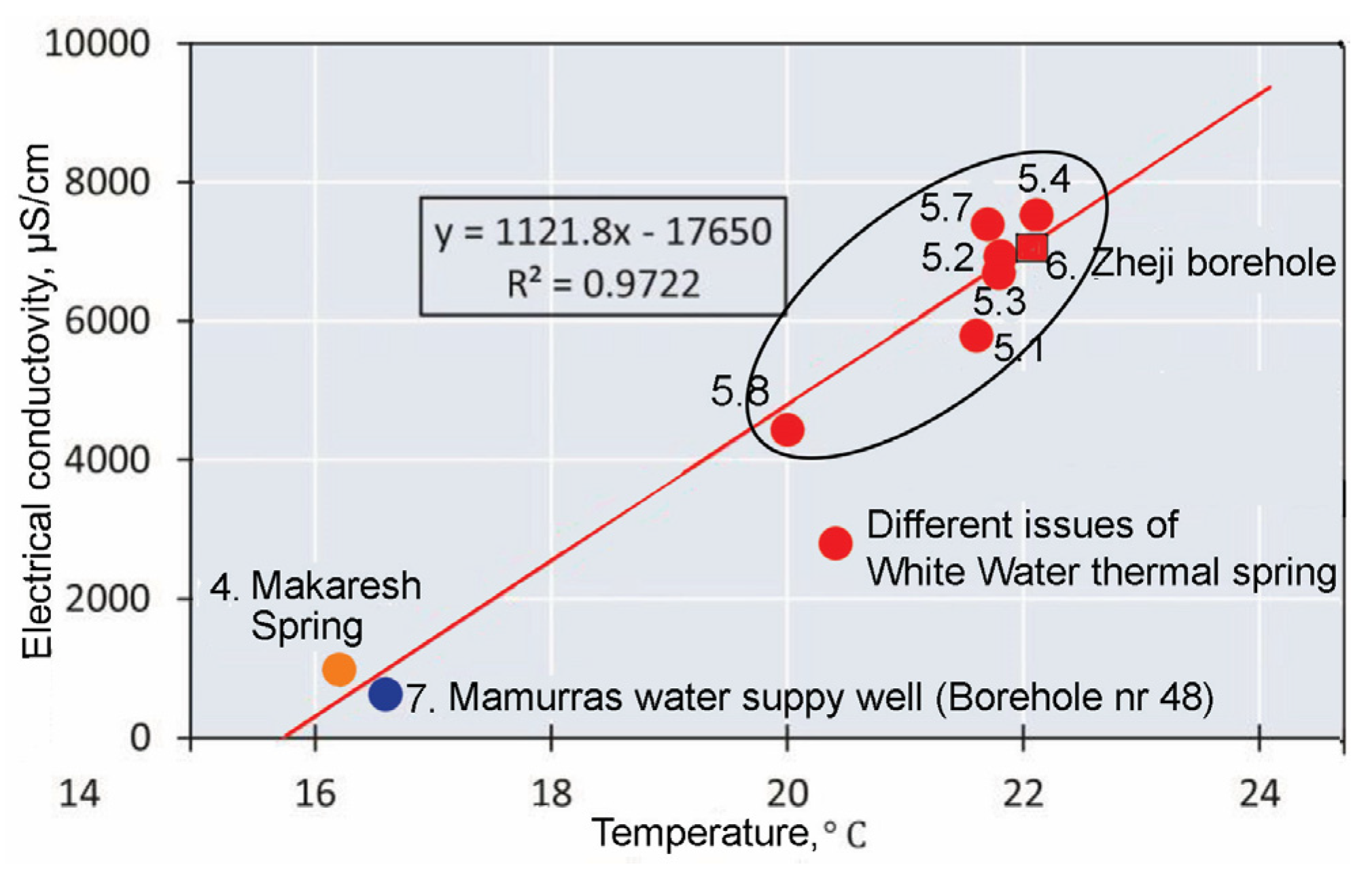
Correlating electrical conductivity and water temperature for the different issues of White Water spring (
Figure 8), it appears that issue no. 8, and to a lesser extent issue no. 1, have the highest contribution of cold water. Accepting as the mixing end-members the well no. 48 and the issue no. 4, we can calculate the rate of mixing of water points located between them: issue no. 8 consists of a mixing of approximately the same amount of cold and thermal waters, while issue no. 1 derives from a mixing of about 75% thermal water with 25% cold water. In the remaining springs (nos. 2, 3, 4, 6 and 7) the mixing of thermal and cold water is not significant.
8. Groundwater exploitation
8.1. Cold waters
Groundwater of the Makaresh karst massif is used for water supply of some important urban, rural and industrial centres such as the city of Mamurras, the villages of Burizan and Zgërdhesh, and the Titan Cement Factory. The study of the water supply of the city of Mamurras through drilling pointed out to some important features of the karstification, and highlighted the abundance of groundwater in the karst massif [
39]. To meet the water requirements of the growing city, in 2002 a water supply capacity of 60 l/s was required. Taking into account the capacity of natural springs in the Makaresh massif, drilling water wells remains the only possibility to solve the problem. In the area around the existing water tapping there are two aquifers: (a) one in intergranular rocks, and (b) a carbonate rock aquifer. Analysing the hydrogeological conditions of the Zheji stream area it was concluded that the gravelly aquifer has no capacity to provide the required amount of 60 l/s.
As concerns groundwater flow in the karst rocks, it is usually concentrated in conduits, which location is difficult to determine, since in short distances they alternate with impervious rocks [
3,
8,
11,
48,
73]. After the detailed observations of the hydrogeological situation in the Zheji stream area, it was thus decided to drill two water wells, 50m-deep, in the immediate vicinity of Mamurras spring (no. 1,
Figure 4), and to isolate the gravel aquifer. During the implementation of the wells, the EC and groundwater temperature were continuously measured in order to record any possible change of groundwater quality with depth. Well no. 7 flowed 40 l/s, while the pumped capacity was recommended to be 60 l/s. According to the chemical analyses the water quality respects the Albanian Drinking Water Standard (
Table 2); TDS was measured in 350 mg/l, the temperature is 16.4 °C and the water chemical type is HCO
3-Ca.
8.2. Thermal waters
White Water thermal spring is not used for balneological purposes, due to its temperature, not sufficiently high, although the content of hydrogen sulphide (H
2S) in water shows significant values, even though not reaching that at the well-known Peshkopi thermal springs [
32]. A restricted number of the inhabitants of local villages use them for curative baths using primitively-made ponds. However, if special pools were built, they would be sufficiently useable in the winter season when the spring water temperature of 22° is perceived as warm, while during the summer the water temperature in the ponds would increase because the summer air temperature in this area usually exceeds 30 °C. Since the White Water thermal spring water is not used, it is not protected, too.
Boreholes with depth about 1000 m could provide thermal waters with temperatures of about 30° C with high balneological qualities (high salinity of about 5 gr/l and significantly higher content of H2S gas of about 400 mg/l) in the area of White Water spring. The geological-hydrogeological data here presented support such an assumption. Further, it must be stressed the significant environmental value of this thermal area, due to its privileged geographical location, at a distance of only 35 km from the capital city of Tirana.
8.3. Groundwater protection
Two cement factories have been built in the Makaresh massif, respectively at its southern suburbs and in the central part of the massif (
Figure 9). To provide the factories with limestones, as well as for construction purposes in general, several quarries exercise their activity in the massif, too. Location of such factories and quarries on the Makaresh karst plateau (
Figure 9) represents a typical negative example of human activities degrading the beautiful mountain karst landscape that borders the eastern side of the Tirana depression. It is well known and documented that quarrying activity has many negative impacts on the karst environment: first, it destroys the epikarst [
74], the most surficial part of the karst that acta as the main recharge zone for karst aquifer; then, it impacts through clearing of the vegetation cover, in turn causing an increase in surface runoff, and in erosion as well, even on low-gradient slopes [
75].
Advancement of the quarrying face results in destruction of karst caves, and of the natural resources therein, often without any possibility to ascertain whether these might be scientifically important or not [
76,
77,
78,
79,
80]. Further, these activities eventually result in pollution of karst waters, as already documented in several sites of the Albanian karst [
81,
82,
83]. An example worth to be mentioned is the pollution of Bogovo spring used for water supply of the cities of Berat, Kuçova and Urra Vajgurore, by the quarrying activity at Mount Tomori [
84]. The high vulnerability of the karst environment by activity of the cement factories, the likely produced damage, or the possibility of future environmental problems, should represent issues of further analysis, as a pressing task for the future, also by using some of the dedicated indices defined for karst environment [
85,
86,
87,
88,
89], aimed at ascertaining and qualitatively assess the damage produced by such anthropogenic activities.
9. Conclusions
The Kruja tectonic zone is characterized by presence of SE-NW oriented structures of Upper Cretaceous to Eocene carbonate formations, locally exposed below the overlying Oligocene flysch deposits. In this zone there are over 80% of the thermal springs of Albania. The Makaresh karst massif, with an area of 22 km2, is one of the most interesting karst structures hosting, within relatively short distances, both fresh and thermal waters. Geological and hydrogeological investigations, combined with physico-chemical analyses, allowed to identify the presence of two groundwater circulation systems in the Makaresh karst massif.
The shallow circulating waters are cold, fresh and belong to a HCO3-Ca or HCO3-Ca-Mg hydrochemical facies, with EC varying around 580-650 μS/cm, and water temperature ranging from about 13.9 to 16.6 °C. The deeper circulating waters are mineralized, with lower acidic pH and higher total hardness; they are mainly of the Cl-Na-Ca type, whilst EC varies in the range 7200-7800 μS/cm, and the water temperature is about 18.5-22.5° C. Thermal waters can be also distinguished for the high content of the total sulphide gas H2S (about 350 mg/l), a concentration higher than for most of the thermal waters in Albania.
The main factors responsible for the qualitative formation of shallow circulating groundwater is the solution of the carbonate rocks and of the metallic elements they contain, like pyrite and marcasite. Deep ascending fluids moving upward along transversal faults are the main recharge source for the thermal springs. Their mixing in different proportion with cold water is not very significant.
The renewable groundwater resources of Makaresh massif are estimated at about 400 l/s. Cold water is used for the water supply of the city of Mamurras for about 60 l/s. The White Water thermal spring is not used for curative purposes, due to its insufficient temperature, notwithstanding the high content of hydrogen sulphide (H2S). In the area of White Water (Uji Bardhe) thermal spring, through deep boreholes about 1000 m deep, it could be possible to provide thermal waters with temperatures of about 30 °C, with high balneological qualities.
In the Makaresh massif, presence of anthropic activities (namely, a cement factory situated on the karst plateau in its central part, and several quarries providing limestones for cement production and for construction purposes) is definitely a negative aspect for the preservation of the pristine landscape, at the origin of degradation of the natural karst in this area bordering the eastern side of the Tirana depression.
Author Contributions
Conceptualization, Methodology, Writing—Original Draft, R.E.; Conceptualization, Methodology, Writing—Review and Editing, Supervision, M.P.; Conceptualization, Methodology, Writing-Review and Editing, I.S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Readers can contact authors for availability of data and materials.
Conflicts of Interest
No conflict of interest is declared for this article.
References
- Stringfield, V.T.; Legrand, H.E. Hydrology of carbonate rock terrenes. A review with special reference to the United States. J Hydrology 1969, 8, 349–417. [Google Scholar] [CrossRef]
- Ford, D.C.; Williams, P. Karst hydrogeology and geomorphology; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Kresić N. Hydrogeology. Introduction to Groundwater Science and Engineering. Blue Ridge Press. ISBN 970-8-2-218-06984-1, 2023.
- Stevanović, Z. Global distribution and use of water from karst aquifers. In Advances in Karst Research: Theory, Fieldwork and Applications; Parise, M.; Gabrovsek, F.; Kaufmann, G.; Ravbar, N., Eds.; Geological Society, London, UK, 2018; Volume 466, pp. 217–236.
- Parise, M.; Ravbar, N.; Živanović, V.; Mikszewski, A.; Kresic, N.; Mádl-Szόnyi, J.; Kukurić, N. Hazards in Karst and Managing Water Resources Quality. In Karst Aquifers—Characterization and Engineering. Stevanović, Z., Ed.; Professional Practice in Earth Sciences; Springer: Heidelberg, Germany, 2015; pp. 601–687. 601–687. [CrossRef]
- Custodio, E. Coastal aquifers of Europe: an overview. Hydrogeol J 2010, 10, 340–345. [Google Scholar] [CrossRef]
- Hartmann, A.; Goldscheider, N.; Wagner, T.; Lange, J.; Weiler, M. Karst water resources in a changing world: Review of hydrogeological modelling approaches. Rev Geophys 2014, 52. [Google Scholar] [CrossRef]
- Bakalowicz, M. Karst and karst groundwater resources in the Mediterranean. Environ Earth Sci 2015, 74, 5–14. [Google Scholar] [CrossRef]
- Chen, Z.; Auler, A.S.; Bakalowicz, M.; Drew, D.; Griger, F.; Hartmann, J.; Jiang, G.; Moosdorf, N.; Richts, A.; Stevanović, Z.; Veni, G.; Goldscheider, N. The World Karst Aquifer Mapping project: concept, mapping procedure and map of Europe. Hydrogeol J 2017, 25, 771–785. [Google Scholar] [CrossRef]
- Chen, Z.; Goldscheider, N.; Auler, A.S.; Bakalowicz, M.; Broda, S.; Drew, D.; Hartmann, J.; Jiang, G.; Moosdorf, N.; Richts, A.; Stevanović, Z.; Veni, G.; Dumont, A.; Aureli, A.; Clos, P.; Krombholz, M. World Karst Aquifer Map. IAH-UNESCO, Paris, France, 2017. [CrossRef]
- Stevanović, Z., Ed. Karst aquifers—Characterization and engineering. Professional practice in earth sciences Springer: Heidelberg, Germany, 2015. ISBN 978-3-319-12849-8.
- Stevanović, Z. Kast water in potable water supply: a global scale overview. Environ Earth Sc 2019, 78, 662. [Google Scholar] [CrossRef]
- Parise, M.; Gabrovsek, F.; Kaufmann, G.; Ravbar, N. Recent advances in karst research: from theory to fieldwork and applications. In Advances in Karst Research: Theory, Fieldwork and Applications; Parise, M.; Gabrovsek, F.; Kaufmann, G.; Ravbar, N., Eds.; Geological Society, London, UK, 2018; Volume 466, pp. 1–24. [CrossRef]
- Goldscheider, N.; Chen, Z.; Auler, A.S.; Bakalowicz, M.; Broda, S.; Drew, D.; Hartmann, J.; Jiang, G.; Moosdorf, N.; Stevanović, Z.; Veni, G. Global distribution of carbonate rocks and karst water resources. Hydrogeol J 2020, 28, 1661–1667. [Google Scholar] [CrossRef]
- Margat, J. Les eaux souterraines dans le basin méditerranén. Ressources et utilisations. Documents BRGM 1998, 282, BRGM, Orléans, France.
- Bakalowicz, M. Coastal Karst groundwater in the Mediterranean: a resource to be preferably exploited onshore, not from karst submarine springs. Geosciences 2018, 8, 258. [Google Scholar] [CrossRef]
- Günay, G.; Güner, N.; Törk, N. Turkish karst aquifers. Environ Earth Sci 2015, 74, 217–226. [Google Scholar] [CrossRef]
- Kalioras, A.; Marinos, P. Water resources assessment and management of karst aquifer systems in Greece. Environ Earth Sci 2015, 74, 83–100. [Google Scholar] [CrossRef]
- Liso, I.S.; Parise, M. Apulian karst springs: a review. J Environ Sci Engng Technology 2020, 8, 63–83. [Google Scholar] [CrossRef]
- Olarinoye, T.; Gleeson, T.; Marx, V.; Seeger, S.; Adinehvand, R.; Allocca, V.; Andreo, B.; Apaéstegui, J.; Apolit, C.; Arfib, B.; et al. Global karst springs hydrograph dataset for research and management of the world’s fastes flowing groundwater. Scientific Data 2020, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, Z.; Eftimi, R. Karstic sources of water supply for large consumers in south-eastern Europe—sustainability, disputes and advantages. In Proceedings International Interdisciplinary Scientific Conference “Sustainability of the karst environment. Dinaric karst and other karst regions”, Plitvice Lakes, Croatia, 23–26 September, 2009; Bonacci, O., Ed.; Series on Groundwater; IHP-UNESCO: Paris, France, 2010; no. 2, pp. 181–185.
- Goldscheider, N.; Mádl-Szönyi, J.; Eröss, A.; Schill, E. Thermal water resources in carbonate rock aquifers. Hydrogeol J 2010, 18, 1303–1318. [Google Scholar] [CrossRef]
- Mádl-Szönyi, J.; Tóth, A. Basin-scale conceptual groundwater flow model for an unconfined and confined thick carbonate region. Hydrogeol J 2015, 23, 1359–1380. [Google Scholar] [CrossRef]
- Judit, M.S.; Tóth, A. Basin-scale conceptual groundwater flow model for an unconfined and confined thick carbonate region. Hydrogeol J 2015, 23, 1359–1380. [Google Scholar] [CrossRef]
- Papić, P., Ed. Mineral and thermal waters of Southeastern Europe. Springer, 2015.
- Eftimi, R.; Bisha, G.; Tafilaj, I.; Habilaj, L. Hydrogeological map of Albania, scale 1: 200,000. Hamid Shijaku: Tirana, Albania, 1985.
- Xhomo, A.; Kodra, A.; Xhafa, Z.; Shallo, M. Gjeologjia e Shqipërisë. Shërbimi Gjeologjik, Shqiptar: Tirana, Albania, 2002; 410 pp.
- Eftimi, R. Hydrogeological characteristics of Albania. AQUAmundi 2010, 1012, 079–092. [Google Scholar] [CrossRef]
- Avgustinski, V.L.; Astashkina, A.A.; Shukevich, Ll. Mineral water resources of Albania. Health Ministry. Central Archive, Albanian Geological Survey: Tirana, Albania, 1957.
- Eftimi, R. Karst and karst water resources of Albania and their management. Carbonates and Evaporites 2020, 35, 1–14. [Google Scholar] [CrossRef]
- Frashëri, A., Ed. Geothermal Atlas of Albania. Academy of Science of Albania, Faculty of Geology and Mining, Polytechnical University of Tirana. Tirana 2013.
- Eftimi, R.; Frashëri, A. Thermal and mineral waters of Albania. PRINT-AL: Tirana, Albania, 2016; 214 pp.
- Klimchouk, A. Hypogene Speleogenesis: Hydrogeological and Morphogenetic Perspective. Special paper no 1, National Cave and Karst Research Institute, Carlsbad, NM, 2007.
- Klimchouk, A. Morphogenesis of hypogenic caves. Geomorphology 2009, 106, 100–117. [Google Scholar] [CrossRef]
- Klimchouk, A. Types and settings of hypogene karst. In: Klimchouk, A.B.; Palmer, A.N.; De Waele, J.; Audra, P.; Eds. Hypogene karst regions and caves of the world. Springer 2017; pp 1-39. [CrossRef]
- Jaho, S., Ed. Climate of Albania. Institute of Hydrometeorology, Tirana, 1985.
- Aliaj, Sh.; Melo, V.; Hyseni, A.; Skrami, J.; Mëhillka, Ll.; Muço, B.; Profiti, K.; Prillo, S. The technical structure of Albania. Seismological Institute: Tirana, Albania, 1996.
- Tartari, M. Hydrogeological investigation for the water supply of the town of Mamurras. Archive of AGS, 1990.
- Eftimi, R. Hydrogeological investigation: The result of the water supply wells of the town of Mamurras (in Albanian). Archive of ITA Consult, Tirana, 2002.
- Meço, N.; Aliaj, Sh. Geology of Albania. Gebruder Bornatrager: Berlin, Germany, 2000; 246 pp.
- Wall, B.R.G.; Girbacea, R.; Mesonjesi, A.; Aydin, A. Evolution of fracture and fault-controlled fluid pathways in Carbonates of the Albanides fold-thrust belt. AAPG Bulletin 2006, 90, 1227–1249. [Google Scholar] [CrossRef]
- Van Geet, J.; Swennen, R.; Durmishi, C.; Rour, F.; Muchez, P.H. Paragenesis of Cretaceous to Eocene carbonate reservoirs in the Ionian fold and thrust belt (Albania): relation between tectonism and fluid flow. Sedimentology 2002, 49, 697–718. [Google Scholar] [CrossRef]
- Aliaj, S. Seismotectonic of the Albanides collision zone: Geometry of the under-thrusting Adria microplate beneath the Albanides. JNTS 2020, 51, 3–40. [Google Scholar]
- Eftimi, R. Some data about the hydrochemistry of groundwater of Krujë-Dajt mountain chain (in Albanian). Stud Gjeograf 1998, 11, 60–65. [Google Scholar]
- Gutierrez, F.; Parise, M.; De Waele, J.; Jourde, H. A review on natural and human-induced geohazards and impacts in karst. Earth Science Reviews 2014, 138, 61–88. [Google Scholar] [CrossRef]
- Parise, M. Sinkholes. In Encyclopaedia of Caves, 3rd ed.; White, W.B.; Culver, D.C.; Pipan, T.; Eds.; Academic Press, Elsevier: Amsterdam, The Netherlands, 2019; pp. 934–942. ISBN 978-0-12-814124-3.
- Parise, M. Sinkholes, Subsidence and Related Mass Movements. In Treatise on Geomorphology, vol. 5, 2nd ed.; Shroder, J.J.F., Ed.; Elsevier, Academic Press: Amsterdam, The Netherlands, 2022; pp. 200–220. ISBN 9780128182345. [Google Scholar] [CrossRef]
- Stevanović, Z.; Gunn, J.; Goldscheider, N.; Ravbar, N. Karst Environment and Management of Aquifers. The Groundwater Project, Guelph, Ontario, Canada 2023. [CrossRef]
- Fabbri, I. La prima volta. Ipogea 2021, 56–59. [Google Scholar]
- Turc, L. Le bilan d’eau des sols. Relations entre les precipitation, l’évaporation et l’écoulement. Ann Agron 1954, 5, 491–595. [Google Scholar]
- Eftimi, R.; Malik, P. Assessment of regional flow type and groundwater sensitivity to pollution using hydrograph analyses and hydrochemical data of the Selita and Blue Eye karst springs, Albania. Hydrogeol J 2019, 27, 2045–2059. [Google Scholar] [CrossRef]
- White, W.B. Geomorphology and hydrology of karst terrains. Oxford University Press: Oxford, UK, 1988.
- Karoly, G. Investigation of bauxites in Albania during 1958. Archive of AGS, Tirana, 1959.
- Tóth, J. A theoretical analyses of groundwater flow in small drainage basins. J Geophys Res 1963, 68, 4795–4812. [Google Scholar] [CrossRef]
- Tóth, J. Groundwater as a geologic agent: an overview of the cause, processes, and manifestations. Hydrogeol J 1999, 7, 1–14. [Google Scholar] [CrossRef]
- Tóth, J. Springs seen and interpreted in the context of groundwater flow-systems. GSA Annual Meeting 2009, Portland, OR, 18-21 October 2009.
- Doucette, R.; Peterson, E.W. Identifying water sources in a karst aquifer using thermal signatures. Environ Earth Sci 2014. [CrossRef]
- Ivanov, V.V.; Nevrev, A.G. Klasifikacija podzemnih mineralni vod (in Russian), Classification of underground mineral waters. Nedra, Moskva, 1964.
- Hem, D.H. Study and interpretation of the chemical characteristics of natural water. U.S. Government printing Office, Washington, D.C. pp 360, 1970.
- Reiman, C.; Birke, M. Geochemistry of European Bottled water. Borntraeger Science Publishers, Stuttgart, 2010.
- Thraikill, J. Relative solubility of limestone and dolomite. Karst Hydrology AIH Mem1 1977, 12, 491–500. [Google Scholar]
- Appelo, C.A.J.; Postma, D. Geochemistry, groundwater and pollution. Balkema, Rotterdam, 1996.
- Longmuir, D. The geochemistry of some carbonate waters of Central Pennsylvania. Geochem Cosmochim Acta 1971, 35, 1023–1045. [Google Scholar] [CrossRef]
- Shuster, E.T.; White, W.B. Seasonal fluctuations in the chemistry of limestone springs: a possible mean for characterizing carbonate aquifers. J Hydrology 1971, 14, 93–128. [Google Scholar] [CrossRef]
- Zötl, J.G. Karst hydrogeology. Springer Verlag, Berlin 291, 1974.
- Eftimi, R. Hydrochemical characteristics of some lithologically different karst massifs of Albania. In Water Resources & Environmental Problems in Karst, Proceeding International Conference and Field Seminar, Belgrade, Serbia, 14–19 September 2005; Stevanović, Z.; Milanovic, P.; Eds.; Geological Faculty of Belgrade University, Belgrade, 2005, pp. 499–504.
- Klimchouk, A.; Eftimi, R.; Andreychouk, V. Hypogene karst in the External Albanides and its pronounced geomorphological effect. Proceedings 18th International Congress of Speleology, Savoie Mont Blanc 2022, Karstologia Mémoires 24 (4).
- Velaj, T. The effect of the evaporate tectonic in the structural model of the Berati belt of Albanides. Balkan Geophysical Society 1999, Istanbul, Turkey.
- Velaj, T. Evaporites in Albania and their impact on thrusting processes. J Balkan Geophys Soc 2001, 4, 9–18. [Google Scholar] [CrossRef]
- Tóth, J. Groundwater flow systems: analyses, characterization and agency in karst genesis. International Symposium on Hierarchical Flow Systems in Karst Regions, September 2013, Supp Note, pp. 1–14, 2013. http://www.karstflow2013.org/?nic=training-course.
- Andreychouk, V.; Eftimi, R.; Nita, J.; Klimchouk, A. Geomorphology and hydrogeology of an exposed evaporite dome: the Dumre karst area, Central Albania. Geological Quarterly 2021, 65, 55. [Google Scholar] [CrossRef]
- Guglielmi, Y.; Bertrand, C.; Compagnon, F.; Follaci, I.P.; Mudry, I. Acquisition of water chemistry in a mobile fissured basement massif: its role in the hydrogeology knowledge of the Clipière landslide (Mercauntour massif, southern Alps, France. J Hydrol 2000, 229, 138–146. [Google Scholar] [CrossRef]
- Bakalowicz, M. Karst groundwater: a challenge for new resources. Hydrogeology J 2005, 13, 148–160. [Google Scholar] [CrossRef]
- Williams, P.W. The role of the epikarst in karst and cave hydrogeology: A review. Int J Speleol 2008, 37, 1–10. [Google Scholar] [CrossRef]
- Hotzl, H. Industrial and urban produced impacts: In: Drew, D., Hotzl, H. (eds) Karst Hydrogeology and Human Activities, A.A. Balkema/Rotterdam 1999, pp 81–123.
- Gunn, J. The geomorphological impacts of limestone quarrying. Catena 1993, 25, 187–198. [Google Scholar]
- Gunn, J. Quarrying of limestones. In: Gunn, J. (Ed.) Encyclopedia of cave and karst science. Routledge, London 2004, pp. 608−611.
- Formicola, W.; Gueguen, E.; Martimucci, V.; Parise, M.; Ragone, G. Caves below quarries and quarries above caves: problems, hazard and research. A case study from southern Italy. Geological Society of America Abstracts with Program 2010, 42. [Google Scholar]
- Parise, M. Hazards in karst. In Proceedings International Interdisciplinary Scientific Conference “Sustainability of the karst environment. Dinaric karst and other karst regions”, Plitvice Lakes, Croatia, 23–26 September, 2009; Bonacci, O., Ed.; Series on Groundwater; IHP-UNESCO: Paris, France, 2010; no. 2, pp. 155–162.
- Parise, M. Modern resource use and its impact in karst areas—mining and quarrying. Zeitschrift fur Geomorphologie 2016, 60, 199–216. [Google Scholar] [CrossRef]
- Parise, M.; Qiriazi, P.; Sala, S. Natural and anthropogenic hazards in karst areas of Albania. Natural Hazards and Earth System Sciences 2004, 4, 569–581. [Google Scholar] [CrossRef]
- Parise, M.; Qiriazi, P.; Sala, S. Evaporite karst of Albania: main features and cases of environmental degradation. Environ Geol 2008, 53, 967–974. [Google Scholar] [CrossRef]
- Qiriazi, P.; Parise, M.; Sala, S. Il carsismo nei gessi del territorio albanese. Mem Ist Italian Speleol, ser. II, 2004, 16, 53–60. [Google Scholar]
- Eftimi, R.; Zojer, H. Human impacts on karst aquifers of Albania. Environ Earth Sci 2015, 74, 57–70. [Google Scholar] [CrossRef]
- van Beynen, P.E.; Townsend, K.M. A disturbance index for karst environments. Environmental Management 2005, 36, 101–116. [Google Scholar] [CrossRef]
- North, L.A.; van Beynen, P.E.; Parise, M. Interregional comparison of karst disturbance: West-central Florida and southeast Italy. J Environ Management 2009, 90, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Calò, F.; Parise, M. Evaluating the human disturbance to karst environments in southern Italy. Acta Carsologica 2006, 35, 47–56. [Google Scholar] [CrossRef]
- van Beynen, P.E.; Brinkmann, R.; van Beynen, K. A sustainability index for karst environments. J Cave and Karst Studies 2012, 74, 221–234. [Google Scholar] [CrossRef]
- Mazzei, M.; Parise, M. 2018, On the implementation of environmental indices in karst. In Karst Groundwater Contamination and Public Health. White, W.B.; Herman, J.S.; Herman, E.K.; Rutigliano, M., Eds.; Springer, Advances in Karst Science, 2018, ISBN 978-3-319-51069-9, pp. 245–247.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).