Submitted:
11 December 2023
Posted:
12 December 2023
You are already at the latest version
Abstract
Keywords:
Introduction
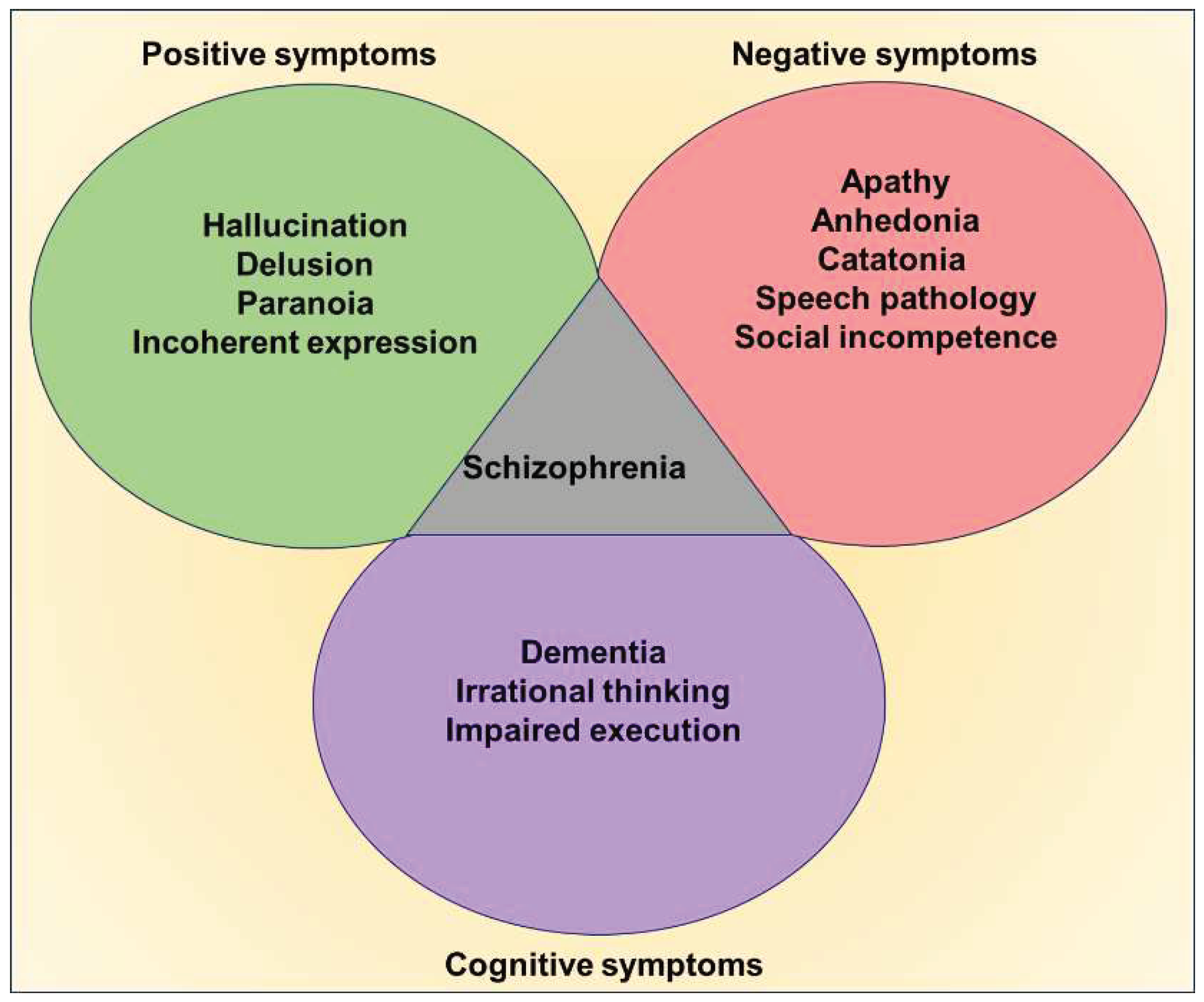
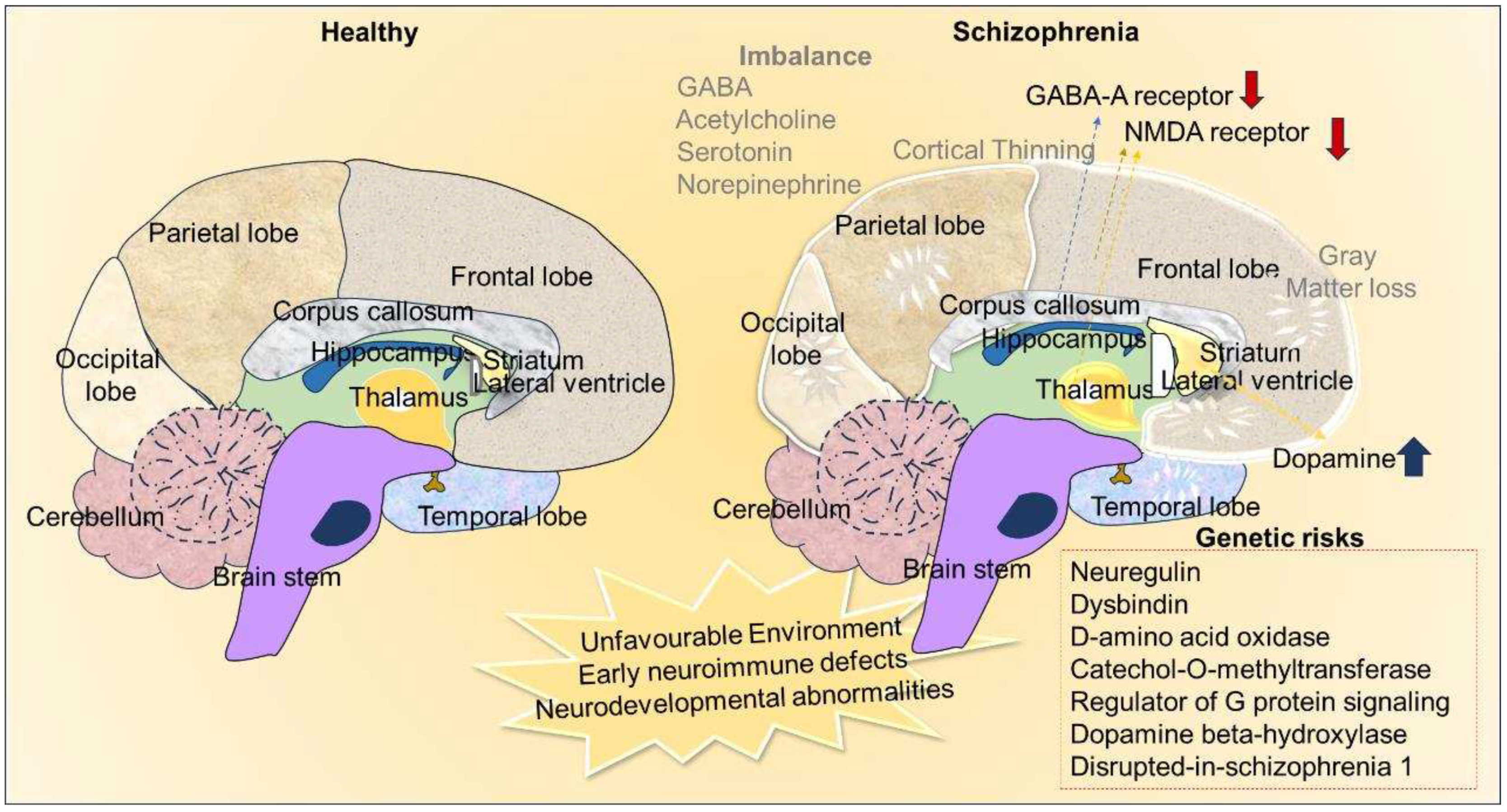
Risk factors and etiopathological relevance of schizophrenia
Neuro morphological and pathological sequelae of schizophrenia
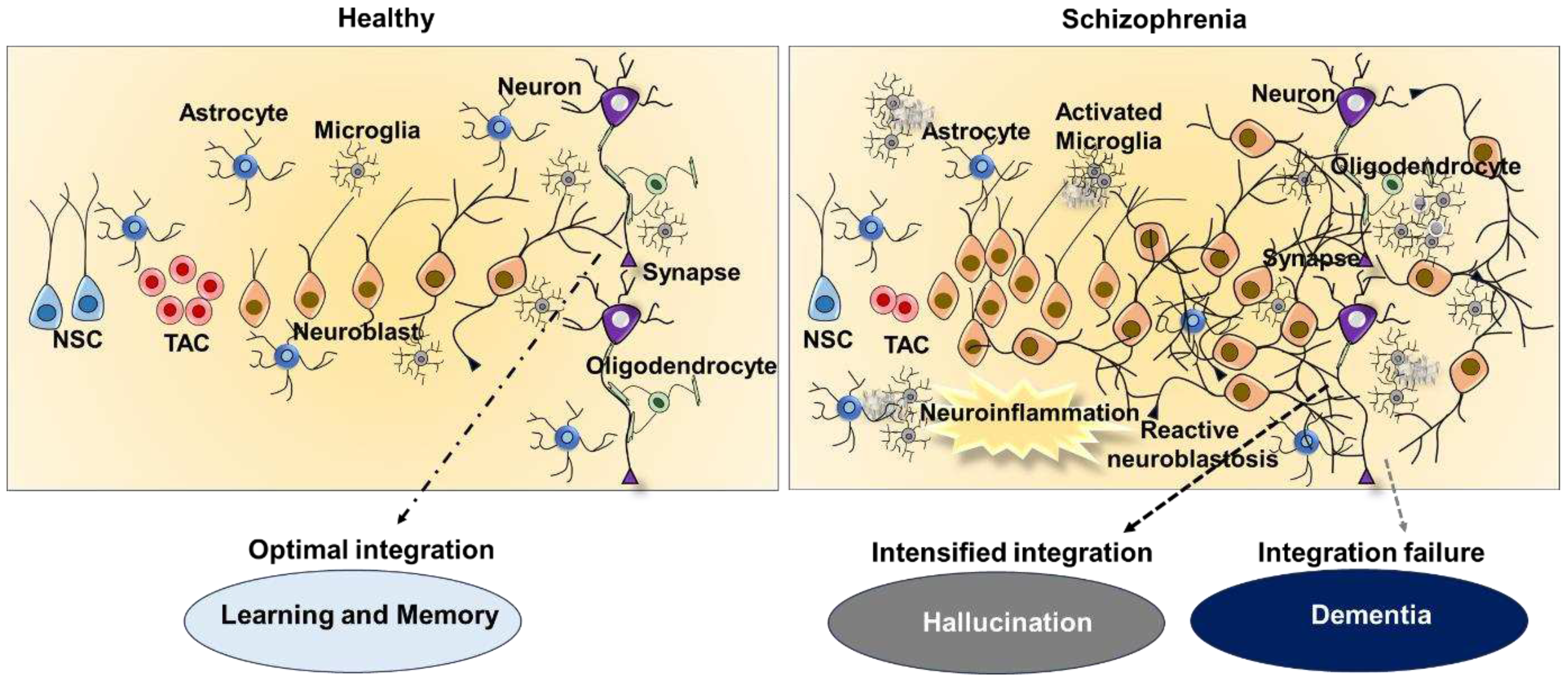
Reactive neuroblastosis as an underlying mechanism of hallucination in schizophrenia and other neurological disease
Conclusions
Author Contributions
Acknowledgment
References
- Patel, K.R.; Cherian, J.; Gohil, K.; Atkinson, D. Schizophrenia: Overview and Treatment Options. P T 2014, 39, 638–645. [Google Scholar]
- Hany, M.; Rehman, B.; Azhar, Y.; Chapman, J. Schizophrenia. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Zubin, J.; Spring, B. Vulnerability: A New View of Schizophrenia. Journal of Abnormal Psychology 1977, 86, 103–126. [Google Scholar] [CrossRef]
- Kendler, K.S. Kraepelin’s Final Views on Dementia Praecox. Schizophr Bull 2020, 47, 635–643. [Google Scholar] [CrossRef]
- Ashok, A.H.; Baugh, J.; Yeragani, V.K. Paul Eugen Bleuler and the Origin of the Term Schizophrenia (SCHIZOPRENIEGRUPPE). Indian J Psychiatry 2012, 54, 95–96. [Google Scholar] [CrossRef]
- Schultz, S.H.; North, S.W.; Shields, C.G. Schizophrenia: A Review. Am Fam Physician 2007, 75, 1821–1829. [Google Scholar]
- George, M.; Maheshwari, S.; Chandran, S.; Manohar, J.S.; Sathyanarayana Rao, T.S. Understanding the Schizophrenia Prodrome. Indian J Psychiatry 2017, 59, 505–509. [Google Scholar] [CrossRef]
- Saha, S.; Chant, D.; Welham, J.; McGrath, J. A Systematic Review of the Prevalence of Schizophrenia. PLoS Med 2005, 2, e141. [Google Scholar] [CrossRef]
- Cederlöf, M.; Lichtenstein, P.; Larsson, H.; Boman, M.; Rück, C.; Landén, M.; Mataix-Cols, D. Obsessive-Compulsive Disorder, Psychosis, and Bipolarity: A Longitudinal Cohort and Multigenerational Family Study. Schizophr Bull 2015, 41, 1076–1083. [Google Scholar] [CrossRef]
- Etchecopar-Etchart, D.; Korchia, T.; Loundou, A.; Llorca, P.-M.; Auquier, P.; Lançon, C.; Boyer, L.; Fond, G. Comorbid Major Depressive Disorder in Schizophrenia: A Systematic Review and Meta-Analysis. Schizophr Bull 2020, 47, 298–308. [Google Scholar] [CrossRef]
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23, 2087. [Google Scholar] [CrossRef]
- Stroup, T.S.; Gray, N. Management of Common Adverse Effects of Antipsychotic Medications. World Psychiatry 2018, 17, 341–356. [Google Scholar] [CrossRef]
- Kim, R.; Healey, K.L.; Sepulveda-Orengo, M.T.; Reissner, K.J. Astroglial Correlates of Neuropsychiatric Disease: From Astrocytopathy to Astrogliosis. Prog Neuropsychopharmacol Biol Psychiatry 2018, 87, 126–146. [Google Scholar] [CrossRef]
- Abazyan, S.; Yang, E.J.; Abazyan, B.; Xia, M.; Yang, C.; Rojas, C.; Slusher, B.; Sattler, R.; Pletnikov, M. Mutant Disrupted-In-Schizophrenia 1 in Astrocytes: Focus on Glutamate Metabolism. J Neurosci Res 2014, 92, 1659–1668. [Google Scholar] [CrossRef]
- Kolomeets, N.S. [Astroglia of the hippocampus in schizophrenia]. Zh Nevrol Psikhiatr Im S S Korsakova 2008, 108, 70–76. [Google Scholar]
- Feresten, A.H.; Barakauskas, V.; Ypsilanti, A.; Barr, A.M.; Beasley, C.L. Increased Expression of Glial Fibrillary Acidic Protein in Prefrontal Cortex in Psychotic Illness. Schizophr Res 2013, 150, 252–257. [Google Scholar] [CrossRef]
- Laskaris, L.E.; Di Biase, M.A.; Everall, I.; Chana, G.; Christopoulos, A.; Skafidas, E.; Cropley, V.L.; Pantelis, C. Microglial Activation and Progressive Brain Changes in Schizophrenia. Br J Pharmacol 2016, 173, 666–680. [Google Scholar] [CrossRef]
- Vallée, A. Neuroinflammation in Schizophrenia: The Key Role of the WNT/β-Catenin Pathway. Int J Mol Sci 2022, 23, 2810. [Google Scholar] [CrossRef]
- Radhakrishnan, R.K.; Kandasamy, M. SARS-CoV-2-Mediated Neuropathogenesis, Deterioration of Hippocampal Neurogenesis and Dementia. Am J Alzheimers Dis Other Demen 2022, 37, 15333175221078418. [Google Scholar] [CrossRef]
- Kandasamy, M.; Couillard-Despres, S.; Raber, K.A.; Stephan, M.; Lehner, B.; Winner, B.; Kohl, Z.; Rivera, F.J.; Nguyen, H.P.; Riess, O.; et al. Stem Cell Quiescence in the Hippocampal Neurogenic Niche Is Associated with Elevated Transforming Growth Factor-Beta Signaling in an Animal Model of Huntington Disease. J Neuropathol Exp Neurol 2010, 69, 717–728. [Google Scholar] [CrossRef]
- Ekdahl, C.T.; Kokaia, Z.; Lindvall, O. Brain Inflammation and Adult Neurogenesis: The Dual Role of Microglia. Neuroscience 2009, 158, 1021–1029. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult Hippocampal Neurogenesis Is Abundant in Neurologically Healthy Subjects and Drops Sharply in Patients with Alzheimer’s Disease. Nat Med 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Marxreiter, F.; Regensburger, M.; Winkler, J. Adult Neurogenesis in Parkinson’s Disease. Cell Mol Life Sci 2013, 70, 459–473. [Google Scholar] [CrossRef]
- Winner, B.; Kohl, Z.; Gage, F.H. Neurodegenerative Disease and Adult Neurogenesis. Eur J Neurosci 2011, 33, 1139–1151. [Google Scholar] [CrossRef]
- Eisch, A.J.; Cameron, H.A.; Encinas, J.M.; Meltzer, L.A.; Ming, G.-L.; Overstreet-Wadiche, L.S. Adult Neurogenesis, Mental Health, and Mental Illness: Hope or Hype? J Neurosci 2008, 28, 11785–11791. [Google Scholar] [CrossRef]
- Apple, D.M.; Fonseca, R.S.; Kokovay, E. The Role of Adult Neurogenesis in Psychiatric and Cognitive Disorders. Brain Res 2017, 1655, 270–276. [Google Scholar] [CrossRef]
- Jin, K.; Peel, A.L.; Mao, X.O.; Xie, L.; Cottrell, B.A.; Henshall, D.C.; Greenberg, D.A. Increased Hippocampal Neurogenesis in Alzheimer’s Disease. Proc Natl Acad Sci U S A 2004, 101, 343–347. [Google Scholar] [CrossRef]
- Boekhoorn, K.; Joels, M.; Lucassen, P.J. Increased Proliferation Reflects Glial and Vascular-Associated Changes, but Not Neurogenesis in the Presenile Alzheimer Hippocampus. Neurobiol Dis 2006, 24, 1–14. [Google Scholar] [CrossRef]
- Terreros-Roncal, J.; Moreno-Jiménez, E.P.; Flor-García, M.; Rodríguez-Moreno, C.B.; Trinchero, M.F.; Cafini, F.; Rábano, A.; Llorens-Martín, M. Impact of Neurodegenerative Diseases on Human Adult Hippocampal Neurogenesis. Science 2021, 374, 1106–1113. [Google Scholar] [CrossRef]
- Ruzo, A.; Croft, G.F.; Metzger, J.J.; Galgoczi, S.; Gerber, L.J.; Pellegrini, C.; Wang, H.; Fenner, M.; Tse, S.; Marks, A.; et al. Chromosomal Instability during Neurogenesis in Huntington’s Disease. Development 2018, 145, dev156844. [Google Scholar] [CrossRef]
- Sheu, J.-R.; Hsieh, C.-Y.; Jayakumar, T.; Tseng, M.-F.; Lee, H.-N.; Huang, S.-W.; Manubolu, M.; Yang, C.-H. A Critical Period for the Development of Schizophrenia-Like Pathology by Aberrant Postnatal Neurogenesis. Front Neurosci 2019, 13, 635. [Google Scholar] [CrossRef]
- Couillard-Despres, S.; Winner, B.; Schaubeck, S.; Aigner, R.; Vroemen, M.; Weidner, N.; Bogdahn, U.; Winkler, J.; Kuhn, H.-G.; Aigner, L. Doublecortin Expression Levels in Adult Brain Reflect Neurogenesis. Eur J Neurosci 2005, 21, 1–14. [Google Scholar] [CrossRef]
- Kandasamy, M.; Aigner, L. Neuroplasticity, Limbic Neuroblastosis and Neuro-Regenerative Disorders. Neural Regen Res 2018, 13, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, M.; Aigner, L. Reactive Neuroblastosis in Huntington’s Disease: A Putative Therapeutic Target for Striatal Regeneration in the Adult Brain. Front Cell Neurosci 2018, 12, 37. [Google Scholar] [CrossRef]
- Kandasamy, M.; Anusuyadevi, M.; Aigner, K.M.; Unger, M.S.; Kniewallner, K.M.; de Sousa, D.M.B.; Altendorfer, B.; Mrowetz, H.; Bogdahn, U.; Aigner, L. TGF-β Signaling: A Therapeutic Target to Reinstate Regenerative Plasticity in Vascular Dementia? Aging Dis 2020, 11, 828–850. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Kokaia, Z. Neurogenesis Following Stroke Affecting the Adult Brain. Cold Spring Harb Perspect Biol 2015, 7, a019034. [Google Scholar] [CrossRef]
- Kandasamy, M.; Rosskopf, M.; Wagner, K.; Klein, B.; Couillard-Despres, S.; Reitsamer, H.A.; Stephan, M.; Nguyen, H.P.; Riess, O.; Bogdahn, U.; et al. Reduction in Subventricular Zone-Derived Olfactory Bulb Neurogenesis in a Rat Model of Huntington’s Disease Is Accompanied by Striatal Invasion of Neuroblasts. PLoS One 2015, 10, e0116069. [Google Scholar] [CrossRef]
- Zheng, W.; ZhuGe, Q.; Zhong, M.; Chen, G.; Shao, B.; Wang, H.; Mao, X.; Xie, L.; Jin, K. Neurogenesis in Adult Human Brain after Traumatic Brain Injury. J Neurotrauma 2013, 30, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, S.; Parent, J.M. Epilepsy and Adult Neurogenesis. Cold Spring Harb Perspect Biol 2015, 7, a020677. [Google Scholar] [CrossRef]
- Roshan, S.A.; Elangovan, G.; Gunaseelan, D.; Jayachandran, S.K.; Kandasamy, M.; Anusuyadevi, M. Pathogenomic Signature and Aberrant Neurogenic Events in Experimental Cerebral Ischemic Stroke: A Neurotranscriptomic-Based Implication for Dementia. J Alzheimers Dis 2023, 94, S289–S308. [Google Scholar] [CrossRef]
- Walker, E.; Kestler, L.; Bollini, A.; Hochman, K.M. Schizophrenia: Etiology and Course. Annu Rev Psychol 2004, 55, 401–430. [Google Scholar] [CrossRef]
- Gejman, P.V.; Sanders, A.R.; Duan, J. The Role of Genetics in the Etiology of Schizophrenia. Psychiatr Clin North Am 2010, 33, 35–66. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, R.A.; Reis Marques, T.; Howes, O.D. Schizophrenia-An Overview. JAMA Psychiatry 2020, 77, 201–210. [Google Scholar] [CrossRef]
- Tandon, R.; Keshavan, M.S.; Nasrallah, H.A. Schizophrenia, “Just the Facts” What We Know in 2008. 2. Epidemiology and Etiology. Schizophr Res 2008, 102, 1–18. [Google Scholar] [CrossRef]
- Stilo, S.A.; Murray, R.M. Non-Genetic Factors in Schizophrenia. Curr Psychiatry Rep 2019, 21, 100. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kulhara, P. What Is Schizophrenia: A Neurodevelopmental or Neurodegenerative Disorder or a Combination of Both? A Critical Analysis. Indian J Psychiatry 2010, 52, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Hartenstein, V.; Stollewerk, A. The Evolution of Early Neurogenesis. Dev Cell 2015, 32, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Derkits, E.J. Prenatal Infection and Schizophrenia: A Review of Epidemiologic and Translational Studies. Am J Psychiatry 2010, 167, 261–280. [Google Scholar] [CrossRef]
- Muraki, K.; Tanigaki, K. Neuronal Migration Abnormalities and Its Possible Implications for Schizophrenia. Front Neurosci 2015, 9, 74. [Google Scholar] [CrossRef]
- Javitt, D.C. Glutamate and Schizophrenia: Phencyclidine, N-Methyl-D-Aspartate Receptors, and Dopamine-Glutamate Interactions. Int Rev Neurobiol 2007, 78, 69–108. [Google Scholar] [CrossRef]
- Beck, K.; Hindley, G.; Borgan, F.; Ginestet, C.; McCutcheon, R.; Brugger, S.; Driesen, N.; Ranganathan, M.; D’Souza, D.C.; Taylor, M.; et al. Association of Ketamine With Psychiatric Symptoms and Implications for Its Therapeutic Use and for Understanding Schizophrenia: A Systematic Review and Meta-Analysis. JAMA Netw Open 2020, 3, e204693. [Google Scholar] [CrossRef]
- Dietz, A.G.; Goldman, S.A.; Nedergaard, M. Glial Cells in Schizophrenia: A Unified Hypothesis. Lancet Psychiatry 2020, 7, 272–281. [Google Scholar] [CrossRef]
- Brisch, R.; Saniotis, A.; Wolf, R.; Bielau, H.; Bernstein, H.-G.; Steiner, J.; Bogerts, B.; Braun, K.; Jankowski, Z.; Kumaratilake, J.; et al. The Role of Dopamine in Schizophrenia from a Neurobiological and Evolutionary Perspective: Old Fashioned, but Still in Vogue. Front Psychiatry 2014, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.L.; Kahn, R.S.; Ko, G.; Davidson, M. Dopamine in Schizophrenia: A Review and Reconceptualization. Am J Psychiatry 1991, 148, 1474–1486. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.P.; Bozzi, Y. The Role of Dopaminergic and Serotonergic Systems in Neurodevelopmental Disorders: A Focus on Epilepsy and Seizure Susceptibility. Bioimpacts 2015, 5, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Kokkinou, M.; Ashok, A.H.; Howes, O.D. The Effects of Ketamine on Dopaminergic Function: Meta-Analysis and Review of the Implications for Neuropsychiatric Disorders. Mol Psychiatry 2018, 23, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, J.D.; Roth, R.H. The Neuropsychopharmacology of Phencyclidine: From NMDA Receptor Hypofunction to the Dopamine Hypothesis of Schizophrenia. Neuropsychopharmacology 1999, 20, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wong, A.H.C. GABAergic Inhibitory Neurons as Therapeutic Targets for Cognitive Impairment in Schizophrenia. Acta Pharmacol Sin 2018, 39, 733–753. [Google Scholar] [CrossRef] [PubMed]
- Scarr, E.; Gibbons, A.S.; Neo, J.; Udawela, M.; Dean, B. Cholinergic Connectivity: It’s Implications for Psychiatric Disorders. Front Cell Neurosci 2013, 7, 55. [Google Scholar] [CrossRef]
- Mäki-Marttunen, V.; Andreassen, O.A.; Espeseth, T. The Role of Norepinephrine in the Pathophysiology of Schizophrenia. Neurosci Biobehav Rev 2020, 118, 298–314. [Google Scholar] [CrossRef]
- Salleh, M.R. The Genetics of Schizophrenia. Malays J Med Sci 2004, 11, 3–11. [Google Scholar]
- Stefansson, H.; Sigurdsson, E.; Steinthorsdottir, V.; Bjornsdottir, S.; Sigmundsson, T.; Ghosh, S.; Brynjolfsson, J.; Gunnarsdottir, S.; Ivarsson, O.; Chou, T.T.; et al. Neuregulin 1 and Susceptibility to Schizophrenia. Am J Hum Genet 2002, 71, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.M.; Preece, A.; Morris, D.W.; Spurlock, G.; Bray, N.J.; Stephens, M.; Norton, N.; Williams, H.; Clement, M.; Dwyer, S.; et al. Identification in 2 Independent Samples of a Novel Schizophrenia Risk Haplotype of the Dystrobrevin Binding Protein Gene (DTNBP1). Arch Gen Psychiatry 2004, 61, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.-C.; Wassink, T.H.; O’Leary, D.S.; Sheffield, V.C.; Andreasen, N.C. Catechol-O-Methyl Transferase Val158Met Gene Polymorphism in Schizophrenia: Working Memory, Frontal Lobe MRI Morphology and Frontal Cerebral Blood Flow. Mol Psychiatry 2005, 10, 229, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Wise, C.D.; Stein, L. Dopamine-Beta-Hydroxylase Deficits in the Brains of Schizophrenic Patients. Science 1973, 181, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Mirnics, K.; Middleton, F.A.; Stanwood, G.D.; Lewis, D.A.; Levitt, P. Disease-Specific Changes in Regulator of G-Protein Signaling 4 (RGS4) Expression in Schizophrenia. Mol Psychiatry 2001, 6, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, C.A.; Goldman, D.; Jaeger, J.; Persaud, S.; Kane, J.M.; Lipsky, R.H.; Malhotra, A.K. Disrupted in Schizophrenia 1 (DISC1): Association with Schizophrenia, Schizoaffective Disorder, and Bipolar Disorder. The American Journal of Human Genetics 2004, 75, 862–872. [Google Scholar] [CrossRef] [PubMed]
- De Luca, V.; Tharmalingam, S.; Zai, C.; Potapova, N.; Strauss, J.; Vincent, J.; Kennedy, J.L. Association of HPA Axis Genes with Suicidal Behaviour in Schizophrenia. J Psychopharmacol 2010, 24, 677–682. [Google Scholar] [CrossRef]
- Matthysse, S.; Sugarman, J. Neurotransmitter Theories of Schizophrenia. In Handbook of Psychopharmacology: Volume 10: Neuroleptics and Schizophrenia; Iversen, L.L., Iversen, S.D., Snyder, S.H., Eds.; Springer US: Boston, MA, 1978; pp. 221–242. ISBN 978-1-4613-4042-3. [Google Scholar]
- Jacobi, W.; Winkler, H. Encephalographische Studien an chronisch Schizophrenen. Archiv f. Psychiatrie 1927, 81, 299–332. [Google Scholar] [CrossRef]
- Ellis, J.K.; Walker, E.F.; Goldsmith, D.R. Selective Review of Neuroimaging Findings in Youth at Clinical High Risk for Psychosis: On the Path to Biomarkers for Conversion. Front Psychiatry 2020, 11, 567534. [Google Scholar] [CrossRef]
- Johnstone, E.C.; Crow, T.J.; Frith, C.D.; Husband, J.; Kreel, L. Cerebral Ventricular Size and Cognitive Impairment in Chronic Schizophrenia. Lancet 1976, 2, 924–926. [Google Scholar] [CrossRef]
- Vita, A.; De Peri, L.; Deste, G.; Sacchetti, E. Progressive Loss of Cortical Gray Matter in Schizophrenia: A Meta-Analysis and Meta-Regression of Longitudinal MRI Studies. Transl Psychiatry 2012, 2, e190. [Google Scholar] [CrossRef]
- Cropley, V.L.; Klauser, P.; Lenroot, R.K.; Bruggemann, J.; Sundram, S.; Bousman, C.; Pereira, A.; Di Biase, M.A.; Weickert, T.W.; Weickert, C.S.; et al. Accelerated Gray and White Matter Deterioration With Age in Schizophrenia. Am J Psychiatry 2017, 174, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Tovar, M.; Rodríguez-Ramírez, A.M.; Rodríguez-Cárdenas, L.; Sotelo-Ramírez, C.E.; Camarena, B.; Sanabrais-Jiménez, M.A.; Solís-Chagoyán, H.; Argueta, J.; López-Riquelme, G.O. Insights into Myelin Dysfunction in Schizophrenia and Bipolar Disorder. World J Psychiatry 2022, 12, 264–285. [Google Scholar] [CrossRef]
- Turetsky, B.; Cowell, P.E.; Gur, R.C.; Grossman, R.I.; Shtasel, D.L.; Gur, R.E. Frontal and Temporal Lobe Brain Volumes in Schizophrenia. Relationship to Symptoms and Clinical Subtype. Arch Gen Psychiatry 1995, 52, 1061–1070. [Google Scholar] [CrossRef]
- Kaur, A.; Basavanagowda, D.M.; Rathod, B.; Mishra, N.; Fuad, S.; Nosher, S.; Alrashid, Z.A.; Mohan, D.; Heindl, S.E. Structural and Functional Alterations of the Temporal Lobe in Schizophrenia: A Literature Review. Cureus 12, e11177. [CrossRef]
- Jaaro-Peled, H.; Ayhan, Y.; Pletnikov, M.V.; Sawa, A. Review of Pathological Hallmarks of Schizophrenia: Comparison of Genetic Models With Patients and Nongenetic Models. Schizophr Bull 2010, 36, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Glausier, J.R.; Lewis, D.A. Dendritic Spine Pathology in Schizophrenia. Neuroscience 2013, 251, 90–107. [Google Scholar] [CrossRef]
- Sprooten, E.; Papmeyer, M.; Smyth, A.M.; Vincenz, D.; Honold, S.; Conlon, G.A.; Moorhead, T.W.J.; Job, D.; Whalley, H.C.; Hall, J.; et al. Cortical Thickness in First-Episode Schizophrenia Patients and Individuals at High Familial Risk: A Cross-Sectional Comparison. Schizophr Res 2013, 151, 259–264. [Google Scholar] [CrossRef]
- Sheffield, J.M.; Barch, D.M. Cognition and Resting-State Functional Connectivity in Schizophrenia. Neuroscience & Biobehavioral Reviews 2016, 61, 108–120. [Google Scholar] [CrossRef]
- Jiang, S.; Huang, H.; Zhou, J.; Li, H.; Duan, M.; Yao, D.; Luo, C. Progressive Trajectories of Schizophrenia across Symptoms, Genes, and the Brain. BMC Med 2023, 21, 237. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ouyang, P.; Zheng, Y.; Mi, L.; Zhao, J.; Ning, Y.; Guo, W. A Selective Review of the Excitatory-Inhibitory Imbalance in Schizophrenia: Underlying Biology, Genetics, Microcircuits, and Symptoms. Front Cell Dev Biol 2021, 9, 664535. [Google Scholar] [CrossRef]
- Horga, G.; Bernacer, J.; Dusi, N.; Entis, J.; Chu, K.; Hazlett, E.A.; Mehmet Haznedar, M.; Kemether, E.; Byne, W.; Buchsbaum, M.S. Correlations between Ventricular Enlargement and Gray and White Matter Volumes of Cortex, Thalamus, Striatum, and Internal Capsule in Schizophrenia. Eur Arch Psychiatry Clin Neurosci 2011, 261, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, L.G.; Green, A.E.; Babakchanian, S.; Hwang, K.S.; Chou, Y.-Y.; Toga, A.W.; Thompson, P.M. Hippocampal Atrophy and Ventricular Enlargement in Normal Aging, Mild Cognitive Impairment and Alzheimer’s Disease. Alzheimer Dis Assoc Disord 2012, 26, 17–27. [Google Scholar] [CrossRef]
- Mak, E.; Su, L.; Williams, G.B.; Firbank, M.J.; Lawson, R.A.; Yarnall, A.J.; Duncan, G.W.; Mollenhauer, B.; Owen, A.M.; Khoo, T.K.; et al. Longitudinal Whole-Brain Atrophy and Ventricular Enlargement in Nondemented Parkinson’s Disease. Neurobiol Aging 2017, 55, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Stephan, K.E.; Friston, K.J.; Frith, C.D. Dysconnection in Schizophrenia: From Abnormal Synaptic Plasticity to Failures of Self-Monitoring. Schizophr Bull 2009, 35, 509–527. [Google Scholar] [CrossRef]
- Crow, T.J. Schizophrenia as a Transcallosal Misconnection Syndrome. Schizophr Res 1998, 30, 111–114. [Google Scholar] [CrossRef]
- Osimo, E.F.; Beck, K.; Reis Marques, T.; Howes, O.D. Synaptic Loss in Schizophrenia: A Meta-Analysis and Systematic Review of Synaptic Protein and mRNA Measures. Mol Psychiatry 2019, 24, 549–561. [Google Scholar] [CrossRef]
- Najjar, S.; Pearlman, D.M. Neuroinflammation and White Matter Pathology in Schizophrenia: Systematic Review. Schizophrenia Research 2015, 161, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The Role of Adult Hippocampal Neurogenesis in Brain Health and Disease. Mol Psychiatry 2019, 24, 67–87. [Google Scholar] [CrossRef]
- Kempermann, G.; Song, H.; Gage, F.H. Neurogenesis in the Adult Hippocampus. Cold Spring Harb Perspect Biol 2015, 7, a018812. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Girgis, R.R.; Brucato, G.; Moore, H.; Provenzano, F.; Kegeles, L.; Javitt, D.; Kantrowitz, J.; Wall, M.M.; Corcoran, C.M.; et al. Hippocampal Dysfunction in the Pathophysiology of Schizophrenia: A Selective Review and Hypothesis for Early Detection and Intervention. Mol Psychiatry 2018, 23, 1764–1772. [Google Scholar] [CrossRef]
- Pujol, N.; Penadés, R.; Junqué, C.; Dinov, I.; Fu, C.H.Y.; Catalán, R.; Ibarretxe-Bilbao, N.; Bargalló, N.; Bernardo, M.; Toga, A.; et al. Hippocampal Abnormalities and Age in Chronic Schizophrenia: Morphometric Study across the Adult Lifespan. Br J Psychiatry 2014, 205, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Heckers, S.; Konradi, C. Hippocampal Neurons in Schizophrenia. J Neural Transm 2002, 109, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Poornimai Abirami, G.P.; Radhakrishnan, R.K.; Johnson, E.; Roshan, S.A.; Yesudhas, A.; Parveen, S.; Biswas, A.; Ravichandran, V.R.; Muthuswamy, A.; Kandasamy, M. The Regulation of Reactive Neuroblastosis, Neuroplasticity, and Nutraceuticals for Effective Management of Autism Spectrum Disorder. Adv Neurobiol 2020, 24, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Polner, B.; Hupuczi, E.; Kéri, S.; Kállai, J. Adaptive and Maladaptive Features of Schizotypy Clusters in a Community Sample. Sci Rep 2021, 11, 16653. [Google Scholar] [CrossRef]
- Babcock, K.R.; Page, J.S.; Fallon, J.R.; Webb, A.E. Adult Hippocampal Neurogenesis in Aging and Alzheimer’s Disease. Stem Cell Reports 2021, 16, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, H.; Takao, K.; Walton, N.M.; Matsumoto, M.; Miyakawa, T. Immature Dentate Gyrus: An Endophenotype of Neuropsychiatric Disorders. Neural Plast 2013, 2013, 318596. [Google Scholar] [CrossRef] [PubMed]
- Tavitian, A.; Song, W.; Schipper, H.M. Dentate Gyrus Immaturity in Schizophrenia. Neuroscientist 2019, 25, 528–547. [Google Scholar] [CrossRef] [PubMed]
- Boksa, P. On the Neurobiology of Hallucinations. J Psychiatry Neurosci 2009, 34, 260–262. [Google Scholar] [PubMed]
- Hare, S.M. Hallucinations: A Functional Network Model of How Sensory Representations Become Selected for Conscious Awareness in Schizophrenia. Front Neurosci 2021, 15, 733038. [Google Scholar] [CrossRef]
- Jardri, R.; Pouchet, A.; Pins, D.; Thomas, P. Cortical Activations during Auditory Verbal Hallucinations in Schizophrenia: A Coordinate-Based Meta-Analysis. Am J Psychiatry 2011, 168, 73–81. [Google Scholar] [CrossRef]
- Wu, J.L.; Haberman, R.P.; Gallagher, M.; Koh, M.T. Probing for Conditioned Hallucinations Through Neural Activation in a Ketamine Mouse Model of Schizophrenia. Neurosci Bull 2020, 36, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Telles-Correia, D.; Moreira, A.L.; Gonçalves, J.S. Hallucinations and Related Concepts—Their Conceptual Background. Front Psychol 2015, 6, 991. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, N.C. Concept of Schizophrenia: Past, Present, and Future. In Schizophrenia; John Wiley & Sons, Ltd, 2010; pp. 1–8. ISBN 978-1-4443-2729-8.
- Ibor, J.J.L. Lecciones de psicologia medica: según apuntes tomados en la cátedra. In Lecciones de psicologia medica: según apuntes tomados en la cátedra; 1964; pp. 427–427.
- Feinberg, I. Corollary Discharge, Hallucinations, and Dreaming. Schizophr Bull 2011, 37, 1–3. [Google Scholar] [CrossRef]
- Jackson, H. The Selected Writings of John Hughlings Jackson. Volume 1. On Epilepsy and Epileptiform Convulsions. Archives of Neurology & Psychiatry 1932, 27, 757. [Google Scholar] [CrossRef]
- Penfield, W.; Boldrey, E. SOMATIC MOTOR AND SENSORY REPRESENTATION IN THE CEREBRAL CORTEX OF MAN AS STUDIED BY ELECTRICAL STIMULATION. Brain 1937, 60, 389–443. [Google Scholar] [CrossRef]
- Catani, M. A Little Man of Some Importance. Brain 2017, 140, 3055–3061. [Google Scholar] [CrossRef] [PubMed]
- Perez-Costas, E.; Melendez-Ferro, M.; Roberts, R.C. BASAL GANGLIA PATHOLOGY IN SCHIZOPHRENIA: DOPAMINE CONNECTIONS and ANOMALIES. J Neurochem 2010, 113, 287–302. [Google Scholar] [CrossRef]
- Kumar, S.; Soren, S.; Chaudhury, S. Hallucinations: Etiology and Clinical Implications. Ind Psychiatry J 2009, 18, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.M.; Cousins, L.; Deakin, J.; Lennox, B.R.; Yolken, R.; Jones, P.B. Inflammation and Immunity in Schizophrenia: Implications for Pathophysiology and Treatment. Lancet Psychiatry 2015, 2, 258–270. [Google Scholar] [CrossRef] [PubMed]
- McGuire, P.K.; Shah, G.M.; Murray, R.M. Increased Blood Flow in Broca’s Area during Auditory Hallucinations in Schizophrenia. Lancet 1993, 342, 703–706. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, H.; Li, C.; Li, Z.; Li, J.; Li, H.; Dai, L.; Dong, M.; Zhou, J.; He, J.; et al. Metabolite Differences in the Medial Prefrontal Cortex in Schizophrenia Patients with and without Persistent Auditory Verbal Hallucinations: A 1H MRS Study. Transl Psychiatry 2022, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cleghorn, J.M.; Garnett, E.S.; Nahmias, C.; Brown, G.M.; Kaplan, R.D.; Szechtman, H.; Szechtman, B.; Franco, S.; Dermer, S.W.; Cook, P. Regional Brain Metabolism during Auditory Hallucinations in Chronic Schizophrenia. Br J Psychiatry 1990, 157, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S. Hallucinations: Clinical Aspects and Management. Ind Psychiatry J 2010, 19, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Degueure, A.; Fontenot, A.; Husan, A.; Khan, M.W. An Unusual Presentation of Vivid Hallucinations. Cureus 14, e25441. [CrossRef]
- Gottlieb, J.D.; Mueser, K.T.; Rosenberg, S.D.; Xie, H.; Wolfe, R.S. Psychotic Depression, Posttraumatic Stress Disorder, and Engagement in Cognitive-Behavioral Therapy within an Outpatient Sample of Adults with Serious Mental Illness. Compr Psychiatry 2011, 52, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Bassiony, M.M.; Lyketsos, C.G. Delusions and Hallucinations in Alzheimer’s Disease: Review of the Brain Decade. Psychosomatics 2003, 44, 388–401. [Google Scholar] [CrossRef]
- Patel, S.S.; Attard, A.; Jacobsen, P.; Shergill, S. Acetylcholinesterase Inhibitors (AChEI’s) for the Treatment of Visual Hallucinations in Schizophrenia: A Review of the Literature. BMC Psychiatry 2010, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, A. Neuropsychiatry of Huntington’s Disease. Dialogues Clin Neurosci 2007, 9, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; David, A.S. Visual Hallucinations in Parkinson’s Disease: A Review and Phenomenological Survey. J Neurol Neurosurg Psychiatry 2001, 70, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Lafay-Chebassier, C.; Chavant, F.; Favrelière, S.; Pizzoglio, V.; Pérault-Pochat, M.-C. French Association of Regional Pharmacovigilance Centers Drug-Induced Depression: A Case/Non Case Study in the French Pharmacovigilance Database. Therapie 2015, 70, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Niebrzydowska, A.; Grabowski, J. Medication-Induced Psychotic Disorder. A Review of Selected Drugs Side Effects. Psychiatr Danub 2022, 34, 11–18. [Google Scholar] [CrossRef]
- Rolland, B.; Jardri, R.; Amad, A.; Thomas, P.; Cottencin, O.; Bordet, R. Pharmacology of Hallucinations: Several Mechanisms for One Single Symptom? Biomed Res Int 2014, 2014, 307106. [Google Scholar] [CrossRef] [PubMed]
- Taoufik, E.; Kouroupi, G.; Zygogianni, O.; Matsas, R. Synaptic Dysfunction in Neurodegenerative and Neurodevelopmental Diseases: An Overview of Induced Pluripotent Stem-Cell-Based Disease Models. Open Biol 2018, 8, 180138. [Google Scholar] [CrossRef] [PubMed]
- Prasad, T.; Iyer, S.; Chatterjee, S.; Kumar, M. In Vivo Models to Study Neurogenesis and Associated Neurodevelopmental Disorders—Microcephaly and Autism Spectrum Disorder. WIREs Mechanisms of Disease 2023, 15, e1603. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Sportelli, V.; Ziller, M.; Spengler, D.; Hoffmann, A. Tracing Early Neurodevelopment in Schizophrenia with Induced Pluripotent Stem Cells. Cells 2018, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Toro, C.T.; Deakin, J.F.W. Adult Neurogenesis and Schizophrenia: A Window on Abnormal Early Brain Development? Schizophrenia Research 2007, 90, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Iannitelli, A.; Quartini, A.; Tirassa, P.; Bersani, G. Schizophrenia and Neurogenesis: A Stem Cell Approach. Neurosci Biobehav Rev 2017, 80, 414–442. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.S.; Drew, M.R. Functional Neurogenesis Over the Years. Behav Brain Res 2020, 382, 112470. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Kempermann, G.; Wiskott, L.; Gage, F.H. Functional Significance of Adult Neurogenesis. Curr Opin Neurobiol 2004, 14, 186–191. [Google Scholar] [CrossRef]
- Bonfanti, L.; Seki, T. The PSA-NCAM-Positive “Immature” Neurons: An Old Discovery Providing New Vistas on Brain Structural Plasticity. Cells 2021, 10, 2542. [Google Scholar] [CrossRef]
- Kumar, A.; Pareek, V.; Faiq, M.A.; Kumar, P.; Kumari, C.; Singh, H.N.; Ghosh, S.K. Transcriptomic Analysis of the Signature of Neurogenesis in Human Hippocampus Suggests Restricted Progenitor Cell Progression Post-Childhood. IBRO Reports 2020, 9, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pareek, V.; Faiq, M.A.; Ghosh, S.K.; Kumari, C. ADULT NEUROGENESIS IN HUMANS: A Review of Basic Concepts, History, Current Research, and Clinical Implications. Innov Clin Neurosci 2019, 16, 30–37. [Google Scholar] [PubMed]
- Amrein, I. Adult Hippocampal Neurogenesis in Natural Populations of Mammals. Cold Spring Harb Perspect Biol 2015, 7, a021295. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Leal-Galicia, P.; Chávez-Hernández, M.E.; Mata, F.; Mata-Luévanos, J.; Rodríguez-Serrano, L.M.; Tapia-de-Jesús, A.; Buenrostro-Jáuregui, M.H. Adult Neurogenesis: A Story Ranging from Controversial New Neurogenic Areas and Human Adult Neurogenesis to Molecular Regulation. Int J Mol Sci 2021, 22, 11489. [Google Scholar] [CrossRef]
- Stagni, F.; Giacomini, A.; Emili, M.; Guidi, S.; Bartesaghi, R. Neurogenesis Impairment: An Early Developmental Defect in Down Syndrome. Free Radic Biol Med 2018, 114, 15–32. [Google Scholar] [CrossRef]
- Guarnieri, F.C.; de Chevigny, A.; Falace, A.; Cardoso, C. Disorders of Neurogenesis and Cortical Development. Dialogues Clin Neurosci 2018, 20, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Stangeland, H.; Orgeta, V.; Bell, V. Poststroke Psychosis: A Systematic Review. J Neurol Neurosurg Psychiatry 2018, 89, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Cascella, N.G.; Schretlen, D.J.; Sawa, A. SCHIZOPHRENIA AND EPILEPSY: IS THERE A SHARED SUSCEPTIBILITY? Neurosci Res 2009, 63, 227–235. [Google Scholar] [CrossRef]
- Kasper, B.S.; Kasper, E.M.; Pauli, E.; Stefan, H. Phenomenology of Hallucinations, Illusions, and Delusions as Part of Seizure Semiology. Epilepsy Behav 2010, 18, 13–23. [Google Scholar] [CrossRef]
- Liu, P.Z.; Nusslock, R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front Neurosci 2018, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Lessell, S. Exercise-Induced Visual Hallucinations A Symptom of Occipital Lobe Tumors. Journal of Neuro-Ophthalmology 1988, 8, 81. [Google Scholar]
- Nieto, R.; Kukuljan, M.; Silva, H. BDNF and Schizophrenia: From Neurodevelopment to Neuronal Plasticity, Learning, and Memory. Frontiers in Psychiatry 2013, 4. [Google Scholar] [CrossRef]
- Manickam, N.; Radhakrishnan, R.K.; Vergil Andrews, J.F.; Selvaraj, D.B.; Kandasamy, M. Cell Cycle Re-Entry of Neurons and Reactive Neuroblastosis in Huntington’s Disease: Possibilities for Neural-Glial Transition in the Brain. Life Sci 2020, 263, 118569. [Google Scholar] [CrossRef] [PubMed]
- Bartkowska, K.; Turlejski, K.; Koguc-Sobolewska, P.; Djavadian, R. Adult Neurogenesis in the Mammalian Hypothalamus: Impact of Newly Generated Neurons on Hypothalamic Function. Neuroscience 2023, 515, 83–92. [Google Scholar] [CrossRef]
- Kandasamy, M.; Radhakrishnan, R.K.; Poornimai Abirami, G.P.; Roshan, S.A.; Yesudhas, A.; Balamuthu, K.; Prahalathan, C.; Shanmugaapriya, S.; Moorthy, A.; Essa, M.M.; et al. Possible Existence of the Hypothalamic-Pituitary-Hippocampal (HPH) Axis: A Reciprocal Relationship Between Hippocampal Specific Neuroestradiol Synthesis and Neuroblastosis in Ageing Brains with Special Reference to Menopause and Neurocognitive Disorders. Neurochem Res 2019, 44, 1781–1795. [Google Scholar] [CrossRef]
- Jurkowski, M.P.; Bettio, L.; K. Woo, E.; Patten, A.; Yau, S.-Y.; Gil-Mohapel, J. Beyond the Hippocampus and the SVZ: Adult Neurogenesis Throughout the Brain. Front Cell Neurosci 2020, 14, 576444. [Google Scholar] [CrossRef]
- Gonzalez-Perez, O.; Quiñones-Hinojosa, A. Astrocytes as Neural Stem Cells in the Adult Brain. J Stem Cells 2012, 7, 181–188. [Google Scholar]
- Parolisi, R.; Cozzi, B.; Bonfanti, L. Humans and Dolphins: Decline and Fall of Adult Neurogenesis. Front Neurosci 2018, 12, 497. [Google Scholar] [CrossRef]
- Piumatti, M.; Palazzo, O.; La Rosa, C.; Crociara, P.; Parolisi, R.; Luzzati, F.; Lévy, F.; Bonfanti, L. Non-Newly Generated, “Immature” Neurons in the Sheep Brain Are Not Restricted to Cerebral Cortex. J Neurosci 2018, 38, 826–842. [Google Scholar] [CrossRef]
- Urbán, N.; Cheung, T.H. Stem Cell Quiescence: The Challenging Path to Activation. Development 2021, 148, dev165084. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, M.; Lehner, B.; Kraus, S.; Sander, P.R.; Marschallinger, J.; Rivera, F.J.; Trümbach, D.; Ueberham, U.; Reitsamer, H.A.; Strauss, O.; et al. TGF-Beta Signalling in the Adult Neurogenic Niche Promotes Stem Cell Quiescence as Well as Generation of New Neurons. J Cell Mol Med 2014, 18, 1444–1459. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Zhou, Y.; Yan, L.; Xuan, F.; Tong, J.; Li, Y.; Huang, J.; Feng, W.; Chen, S.; Cui, Y.; et al. TGF-Β1 Is Associated with Deficits in Cognition and Cerebral Cortical Thickness in First-Episode Schizophrenia. J Psychiatry Neurosci 2022, 47, E86–E98. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.M.; Fung, S.J.; Shannon Weickert, C. Cell Proliferation Is Reduced in the Hippocampus in Schizophrenia. Aust N Z J Psychiatry 2016, 50, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, D.; Liang, J.J.; Robitalille, Y.; Quirion, R.; Srivastava, L.K. Decreased Expression of the Embryonic Form of the Neural Cell Adhesion Molecule in Schizophrenic Brains. Proc Natl Acad Sci U S A 1995, 92, 2785–2789. [Google Scholar] [CrossRef] [PubMed]
- Walton, N.M.; Shin, R.; Tajinda, K.; Heusner, C.L.; Kogan, J.H.; Miyake, S.; Chen, Q.; Tamura, K.; Matsumoto, M. Adult Neurogenesis Transiently Generates Oxidative Stress. PLoS One 2012, 7, e35264. [Google Scholar] [CrossRef] [PubMed]
- Walton, N.M.; Zhou, Y.; Kogan, J.H.; Shin, R.; Webster, M.; Gross, A.K.; Heusner, C.L.; Chen, Q.; Miyake, S.; Tajinda, K.; et al. Detection of an Immature Dentate Gyrus Feature in Human Schizophrenia/Bipolar Patients. Transl Psychiatry 2012, 2, e135. [Google Scholar] [CrossRef] [PubMed]
- Duchatel, R.J.; Weickert, C.S.; Tooney, P.A. White Matter Neuron Biology and Neuropathology in Schizophrenia. NPJ Schizophrenia 2019, 5. [Google Scholar] [CrossRef]
- Fung, S.J.; Joshi, D.; Allen, K.M.; Sivagnanasundaram, S.; Rothmond, D.A.; Saunders, R.; Noble, P.L.; Webster, M.J.; Shannon Weickert, C. Developmental Patterns of Doublecortin Expression and White Matter Neuron Density in the Postnatal Primate Prefrontal Cortex and Schizophrenia. PLoS One 2011, 6, e25194. [Google Scholar] [CrossRef]
- Lodge, D.; Mercier, M.S. Ketamine and Phencyclidine: The Good, the Bad and the Unexpected. Br J Pharmacol 2015, 172, 4254–4276. [Google Scholar] [CrossRef]
- Rawat, R.; Tunc-Ozcan, E.; McGuire, T.L.; Peng, C.-Y.; Kessler, J.A. Ketamine Activates Adult-Born Immature Granule Neurons to Rapidly Alleviate Depression-like Behaviors in Mice. Nat Commun 2022, 13, 2650. [Google Scholar] [CrossRef]
- Wang, C.Z.; Yang, S.F.; Xia, Y.; Johnson, K.M. Postnatal Phencyclidine Administration Selectively Reduces Adult Cortical Parvalbumin-Containing Interneurons. Neuropsychopharmacol 2008, 33, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Suzuki, T.; Seki, T.; Namba, T.; Tanimura, A.; Arai, H. Effects of Repeated Phencyclidine Administration on Adult Hippocampal Neurogenesis in the Rat. Synapse 2006, 60, 56–68. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, R.D.; Féron, F.; Perry, C.; Chant, D.C.; McLean, D.; Matigian, N.; Hayward, N.K.; McGrath, J.J.; Mackay-Sim, A. Cell Cycle Alterations in Biopsied Olfactory Neuroepithelium in Schizophrenia and Bipolar I Disorder Using Cell Culture and Gene Expression Analyses. Schizophrenia Research 2006, 82, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yi, J.H.; Lee, S.; Park, C.-H.; Ryu, J.H.; Shin, K.S.; Kang, S.J. Defective Neurogenesis and Schizophrenia-like Behavior in PARP-1-Deficient Mice. Cell Death Dis 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hoseth, E.Z.; Krull, F.; Dieset, I.; Mørch, R.H.; Hope, S.; Gardsjord, E.S.; Steen, N.E.; Melle, I.; Brattbakk, H.-R.; Steen, V.M.; et al. Exploring the Wnt Signaling Pathway in Schizophrenia and Bipolar Disorder. Transl Psychiatry 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Topol, A.; Zhu, S.; Tran, N.; Simone, A.; Fang, G.; Brennand, K.J. Altered WNT Signaling in hiPSC NPCs Derived from Four Schizophrenia Patients. Biol Psychiatry 2015, 78, e29–e34. [Google Scholar] [CrossRef]
- Romero-Luna, G.; Mejía-Pérez, S.I.; Ramírez-Cruz, J.; Aguilar-Hidalgo, K.M.; Ocampo-Díaz, K.M.; Moscardini-Martelli, J.; Ramírez-Stubbe, V.; Santellán-Hernández, J.O. Schizophrenia-Like Psychosis Presented in a Patient With a Temporal Lobe Tumor: A Case Report. Cureus 14, e29034. [CrossRef]
- Arnold, S.E.; Han, L.Y.; Moberg, P.J.; Turetsky, B.I.; Gur, R.E.; Trojanowski, J.Q.; Hahn, C.G. Dysregulation of Olfactory Receptor Neuron Lineage in Schizophrenia. Arch Gen Psychiatry 2001, 58, 829–835. [Google Scholar] [CrossRef]
- Shors, T.J.; Anderson, M.L.; Curlik, D.M.; Nokia, M.S. Use It or Lose It: How Neurogenesis Keeps the Brain Fit for Learning. Behav Brain Res 2012, 227, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.; Andrade, J.P. Adult Hippocampal Neurogenesis: Regulation and Possible Functional and Clinical Correlates. Frontiers in Neuroanatomy 2018, 12. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



