1. Introduction
Citrus maxima (Burm.)
Merr, commonly known as pomelo, is an evergreen tree belonging to the Rutaceae family. Its fruit has a smooth surface with small oil glands and is typically round or pear-shaped. The flesh of the fruit can be either white or red, with a few variants exhibiting a creamy yellow color. Pomelo cultivation can be found in various Southeast Asian regions such as Malaysia, Thailand, and Vietnam [
1,
2]. In China, it is primarily cultivated in Guangdong, Guangxi, Fujian and Zhejiang [
2].
Pomelo has been widely used in food and cosmetic industry due to its special aroma, nutritional value and pharmacological activity [
3]. Pomelo, in China, is used traditionally as functional food with the potential to balance insulin and glucose levels, thereby contributing to the management of diabetes [
4]. Furthermore, pomelo products could be processed into pectin, essential oils and dried pomelo peel. [
5]. In recent years, the pomelo processing industrial products, such as beverages, canned foods and wines have been developing rapidly.
The pulp portion of pomelo is used as fresh food to supplement the nutrients such as vitamins. The peel, accounting for approximately 30% of the whole pomelo, is consumed directly in the form of sweets, tea and medicine by locals on the southeast coast of China [
2].The bioactivities and phytochemistry of pomelo have been reviewed, including flavonoids, essential oils, coumarin classes, and triterpenes. The antioxidation, antibacterial, anticancer, and alleviation of depression have been reported [
6,
7,
8,
9]. However, there is relatively limited research on the classification of different species of pomelo.
The main pomelo species in China can be divided into three categories: Wendan pomelo, Shatian pomelo, and inter-specific pomelo [
2]. Especially Wendan pomelo is well-known for its crisp and tender flesh, large size, and rich flavor. It was first cultivated in Zhangzhou, Fujian and later introduced to other regions. Currently, the most famous Wendan pomelo species in China are Yuhuan Wendan from Taizhou, Zhejiang, Duwei Wendan from Putian, Fujian, and Madou Wendan from Tainan, Taiwan. With the widespread cultivation of Wendan pomelo, there has been a continuous increase in its production. However, there is a lack of systematic research on Wendan pomelo peel, especially in terms of comparing the chemical composition and biological activities of Wendan pomelo peel from different cultivation regions.
Therefore, the present work was to investigate the composition and antioxidant activity of Wendan pomelo peel grown in China from four different origins.
2. Materials and Methods
2.1. Plant Materials
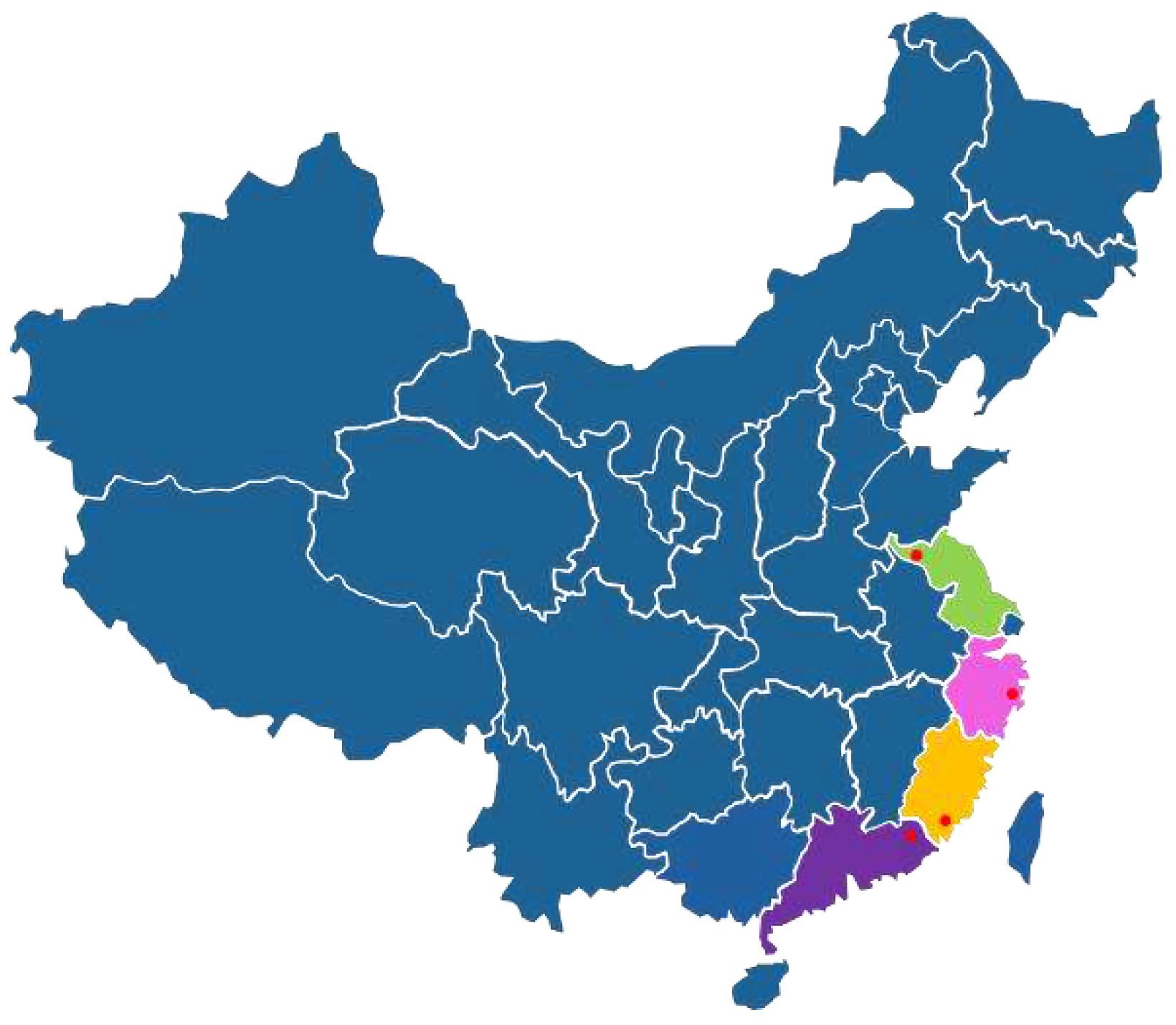
The Wendan (
Citrus maxima cv.
Wentan Buntan) peels were obtained from four different regions of China (
Table 1,
Figure 1), including Xuzhou (Jiangsu), Taizhou (Zhejiang), Zhangzhou (Fujian), Meizhou (Guangdong). These materials were identified by Professor Chun-lin Long (College of Life and Environmental Sciences, Minzu University of China) and the Flora of China, and then the fresh Wendan peels were dried, crushed and stored at cool place.
The extractions were carried out by the Soxhlet extraction method. The ground peel of Wendan pomelo (6.0g) was tightly wrapped in filter paper and placed into a Soxhlet extractor, which was then placed in a round-bottom flask (500ml) containing 70% ethanol solution to obtain the extract. The extract was subjected to vacuum filtration, with the addition of 25% ethanol solution for washing. The resulting filtrate was transferred to a rotary evaporator and distilled under reduced pressure at 60°C until no alcohol odor remained. Finally, the extract was dried in a fume hood.
2.2. HPLC-Q-TOF-MS/MS analysis
The analysis [
10] was conducted using the Agilent 1260 Infinity system (Agilent, USA) and the 1200 HPLC 6520 Q-TOF-MS (Agilent, USA). The samples were separated on a Hypersill Gold C18 column (250 mm × 4.6 mm, 5 μm, SN10609405, Thermo Scientific, USA). The injection sample volume and flow rate were set at 2 µL and 1 mL/min, respectively. The gradient conditions were as follows: solvent A (H
2O-CH
2O
2, 1%) and solvent B (acetonitrile, C
2H
3N 99%). The initial setting was 0-5 min with a linear gradient of 10% B, followed by 5-35 min with 30% B, 35-45 min with 100% B, and then held at 10% B for 5 min to allow column equilibration. The detection range was set from 200 nm to 600 nm using nitrogen gas as the carrier. Mass spectrometry conditions included electrospray ionization (ESI) source, with an ion source temperature of 300°C, positive ion detection, a scanning range of m/z 80-2000 Da, and a cone voltage of 30V.
2.3. Nuclear Magnetic Resonance
1H and 13C NMR spectra (one-dimensional) were obtained on a Bruker Avance DRX-600(Bruker, Germany) spectrometer with a 5 mm TCI cryoprobe and a 14.1 T magnetic field. The chemical shifts of 1H and 13C were referenced according to the peak of the DMSO (C2H6OS) solvent used to solubilize the samples.
2.4. DPPH (2, 2-diphenyl-1-picrylhydrazyl)-Assay
The DPPH free radical scavenging activity was assessed in accordance with a modified version of a known protocol [
11]. The extract solutions were diluted with methanol to obtain a series of concentrations ranging from 10 to 500μg/mL. The 0.06 mM DPPH solution was prepared by dissolving DPPH in methanol. The 100 μL aliquot of the sample was mixed with 100 μL of the DPPH solution and kept in the dark at room temperature. After 30 minutes, the absorbance of the solution was measured at 517 nm. BHT was used as a positive control, and methanol served as the blank control, following the same experimental procedure. The radical scavenging activity was calculated using the equation:
Where A
0 represents the absorbance of the blank control (methanol and DPPH solution), A
1 is the absorbance of the sample with DPPH solution, and A
2 is the absorbance of the sample solution with methanol. The results were expressed as SC
50 (mg/mL), and each batch of samples was tested in triplicate.
2.5. ABTS (2, 2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) assay
The ABTS free radical scavenging activity was determined with slight modifications based on a previously described method [
12]. The ABTS solution was prepared by mixing equal volumes of potassium persulfate (2.45 mM) and ABTS stock solution (7 mM), and allowing the mixture to react in the dark at room temperature for 12-16 hours. The ABTS radical solution was then diluted with methanol to achieve an absorbance of 0.70 ± 0.02 at 734 nm. The Trolox (C
14H
18O
4) standard solution (10 mM) was prepared using methanol and further diluted with methanol to obtain a series of concentrations ranging from 0.1 to 0.7 mM. For the assay, 20 μL of Trolox was added to 180 μL of the ABTS solution and incubated in the dark at room temperature. After 10 minutes, the absorbance of the solution was measured at 734 nm. The same procedure was followed for the sample analysis. The standard curve was constructed using the Trolox standard solution, with the absorbance as the ordinate and the Trolox concentration as the abscissa. The absorbance value obtained from the sample was then substituted into the standard curve to calculate the ABTS antioxidant activity. Each batch of samples was tested in triplicate, and the results were expressed as Trolox equivalent (μmol TE/g).
2.6. Ferric Reducing Antioxidant Power (FRAP) assay
The FRAP assay was conducted with slight modifications based on a previously described method [
13]. The working solution was prepared by combining 300 mM/L acetate buffer (pH 3.6), 10 mM/L TPTZ (tripyridyltriazine) solution in 40 mM/L HCl, and 20 mM/L FeCl
3•6H
2O solution in a volume ratio of 10:1:1. Prior to analysis, the FRAP solution was incubated in a 37 °C water bath. The samples were diluted with methanol, and 20 μL of the diluted sample solution was added to react with 180 μL of the FRAP solution at 37 °C in the dark. After 30 minutes, the absorbance at 593 nm was measured. A standard curve was constructed using the Fe
2+ concentration as the abscissa and the absorbance as the ordinate to calculate the FRAP antioxidant activity. Each batch of samples was tested in triplicate, and the results were expressed as Fe
2+ equivalent (μmol Fe
2+/g).
2.7. α-Glucosidase inhibitory assay
The α-glucosidase inhibitory assay was conducted with slight adjustments based on a previously described method [
14]. The 1 U/mL α-glucosidase solution was prepared using phosphate buffer (0.1M, pH 6.8). For the assay, 10 μL of the sample diluted in DMSO at various concentrations and 30 μL of α-glucosidase solution were mixed in 80 μL of potassium phosphate buffer and incubated for 5 minutes at a 37 °C water bath. Then, 30 μL of 20 mM PNPG (p-Nitrophenyl-β-D-G-alactopyranoside) was added to initiate the reaction, which was further incubated in a 37 °C water bath for 30 minutes. The reaction was terminated by adding 40 μL of Na
2CO
3 solution (2M), and the absorbance was measured at 405 nm. Acarbose was used as a positive control. The inhibition ratio was calculated using the following equation:
Where A
0 represents the absorbance of the sample blank (phosphate buffer instead of PNPG), A
1 is the absorbance of the sample, A
2 is the absorbance of the control blank (phosphate buffer instead of the sample and PNPG), and A
3 is the absorbance of the control (phosphate buffer instead of the sample). The results were expressed as IC
50 (mg/mL), and each batch of samples was tested in triplicate.
3. Results and Discussion
3.1. Identification Components of Wendan pomelo peel
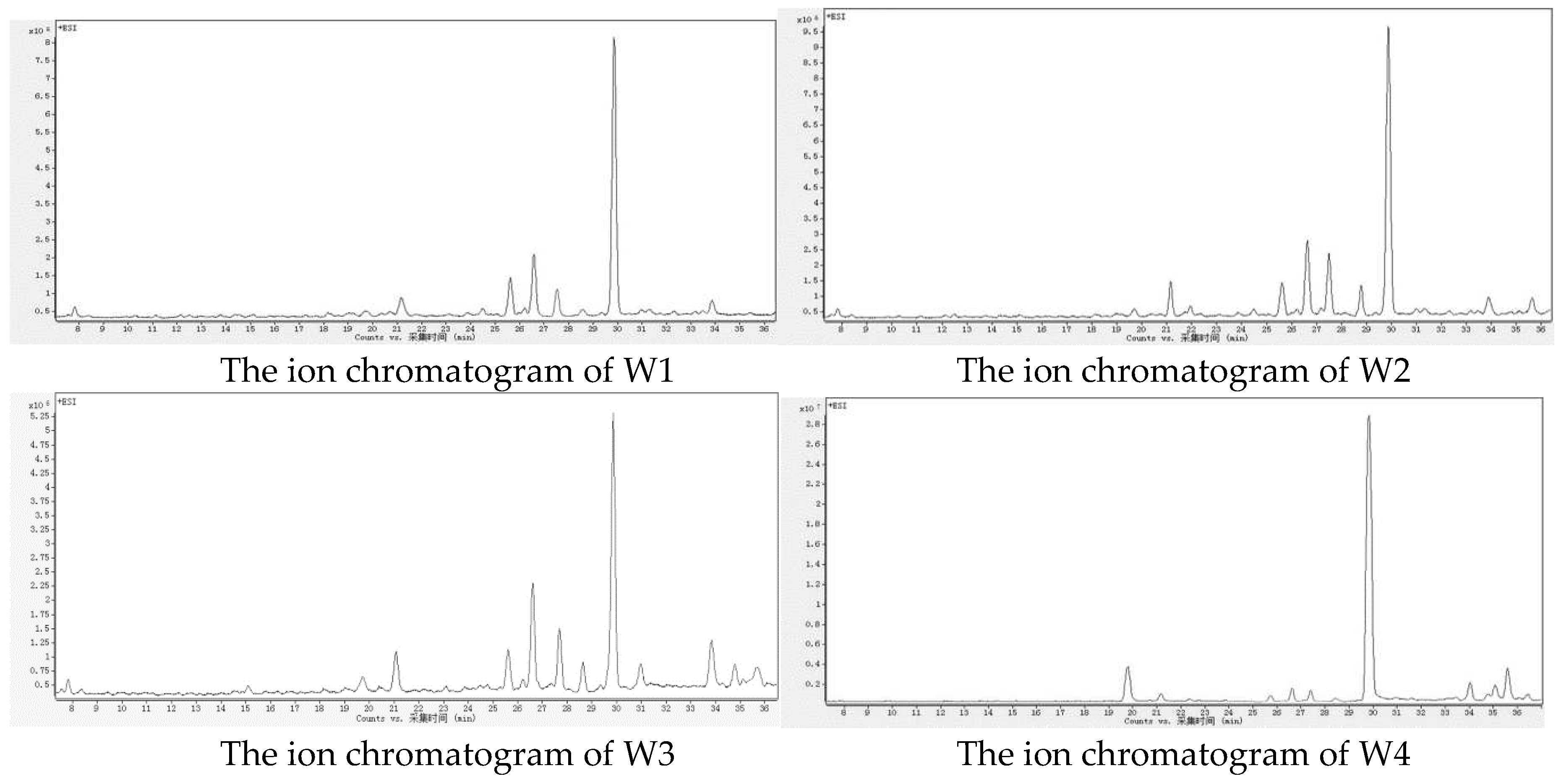
The ion chromatograms in positive ion mode obtained by HPLC-MS/MS for the pericarp of four different origins of Wendan pomelo peels are shown in
Figure 2. The chemical composition results are presented in
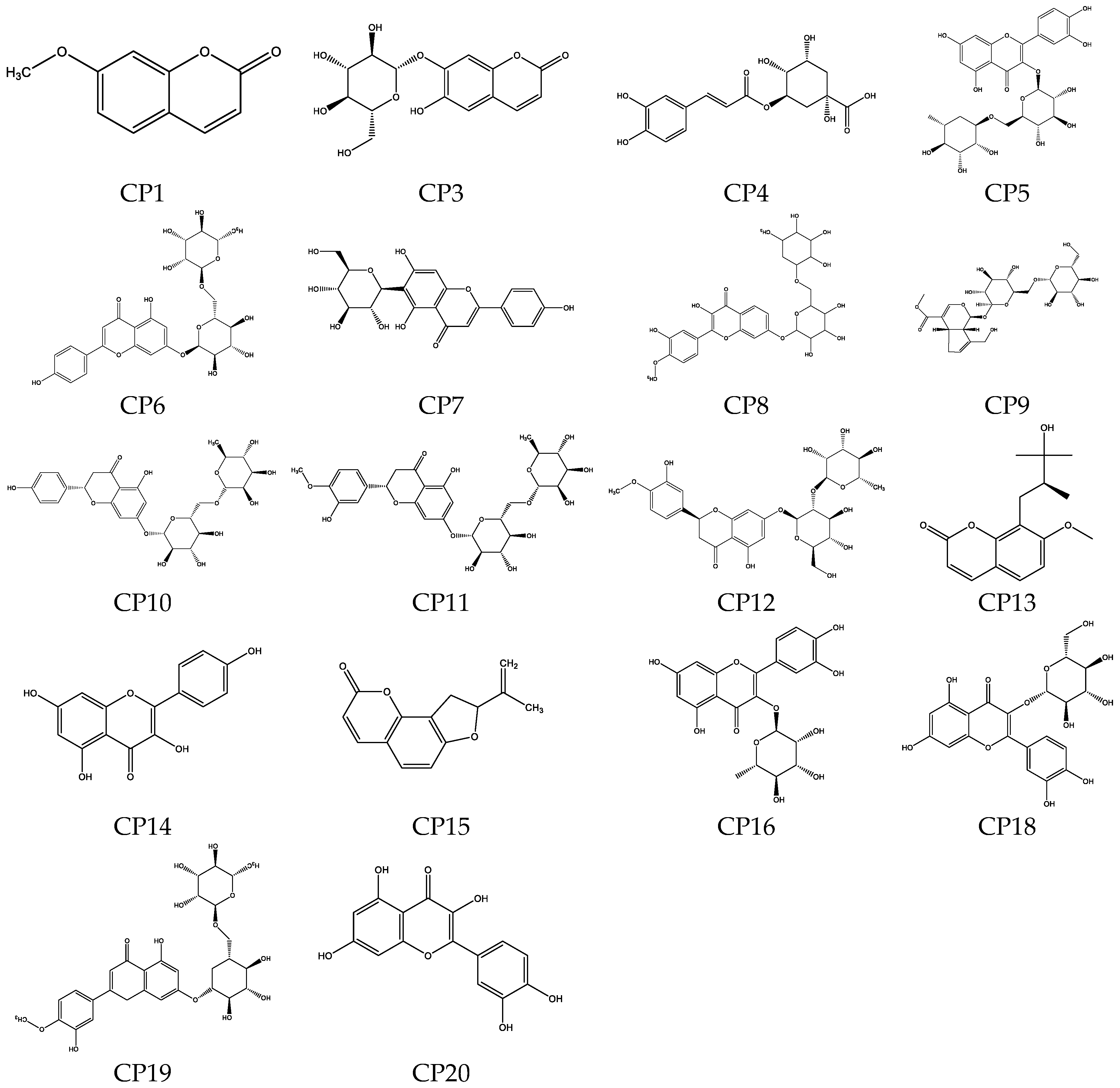
Table 2. A total of 20 compounds (
Figure 3) were detected in the samples from the four origins, with 8 compounds common to all four samples and 2 compounds were identified but their names remain unknown. The major compounds were coumarins and flavonoids. The compound meranzin hydrate F13 (CPF13), which belongs to the coumarin compounds, exhibited the highest peak area in the chromatogram. This compound was first discovered in the root of Prangos ferulacea in 1972 [
15] and is commonly studied in the context of Chaihu-Shugan-San [
16]. It was the first time that this compound was discovered in the Wendan pomelo peel. The flavonoid compounds with higher peak areas in the chromatogram were identified as rutin (CP5), narirutin (CP10), hesperidin (CP11), neohesperidin (CP12), and kaempferol (CP14). There were significant differences in peak areas among samples from different origins, with the proportions of coumarin compounds and flavonoid compounds in Zhangzhou samples being similar, while the coumarins were much more abundant than the flavonoids in the samples from Xuzhou and Meizhou.
The significant variations were observed in the chemical composition of samples from different origins. For instance, the sample from Zhejiang Taizhou (W3) exhibited the highest number of detected compounds with a total of 16 compounds. The sample from Guangdong Meizhou (WF4) had the lowest number of detected compounds, with 11 compounds identified only. For these compounds, CP2, CP3, CP6, and CP16 were exclusively found in the sample of Taizhou, while CP14 was detected only in the sample of Zhangzhou, and CP15 was present solely in the sample of Xuzhou, compound CP8 was not detected in the sample of Meizhou. Previous studies have suggested that variations in plant chemical composition may be attributed to ecological differences [
17,
18]. The chemical composition differences have been observed in samples of the same plant species collected from different regions or even different locations within the same region as described by Zhang et al. [
19]
Table 2.
The chemical constituents of Wendan pomelo peel from four areas.
Table 2.
The chemical constituents of Wendan pomelo peel from four areas.
| Number1
|
Rt (Min) |
Molecular formula |
Fragments (m/z) |
Compound |
W1 |
W2 |
W3 |
W4 |
| 1a
|
2 |
3 |
| CP1 |
7.81 |
C10H8O3
|
177.2405 |
133.1216 |
121.1081 |
7-methoxycoumarin*
|
+ |
+ |
+ |
- |
| CP2 |
12.13 |
C15H16O9
|
341.2512 |
323.1103 |
305.0924 |
Unknown |
- |
+ |
- |
- |
| CP3 |
12.52 |
C15H16O9
|
341.0807 |
179.0340 |
151.0388 |
Esculetin-6-O-glucoside c
|
- |
+ |
- |
- |
| CP4 |
19.73 |
C16H18O9
|
355.1029 |
163.0392 |
145.0287 |
Chlorogenic acid c
|
+ |
+ |
+ |
+ |
| CP5 |
21.17 |
C27H30O16
|
611.1611 |
465.1029 |
303.0501 |
Rutin*
|
+ |
+ |
+ |
+ |
| CP6 |
21.95 |
C27H30O14
|
579.1710 |
433.1132 |
271.0603 |
Apigenin-O-(deoxyhexosyl)hexoside c
|
- |
+ |
- |
- |
| CP7 |
22.31 |
C21H20O10
|
433.1135 |
415.1034 |
397.0923 |
Isovitexin c
|
- |
- |
- |
+ |
| CP8 |
24.49 |
C28H32O16
|
625.2113 |
607.1564 |
589.3071 |
Lucenin-2 4′-methylether d
|
+ |
+ |
+ |
- |
| CP9 |
25.61 |
C23H34O15
|
573.1811 |
551.2143 |
227.1936 |
Genipin-β-gentiobiosidee
|
+ |
+ |
+ |
+ |
| CP10 |
26.68 |
C27H32O14
|
581.1881 |
419.1349 |
273.0763 |
Narirutin*
|
+ |
+ |
+ |
+ |
| CP11 |
27.55 |
C28H34O15
|
611.1994 |
449.1457 |
303.0870 |
Hesperidin*
|
+ |
+ |
+ |
+ |
| CP12 |
28.91 |
C28H34O15
|
611.1987 |
449.1447 |
303.0871 |
Neohesperidin*
|
+ |
+ |
+ |
+ |
| CP13 |
29.87 |
C15H18O5
|
279.1243 |
261.1294 |
243.1027 |
Meranzin hydrate*,x
|
+ |
+ |
+ |
+ |
| CP14 |
30.88 |
C15H10O6
|
287.0553 |
258.0522 |
213.0543 |
Kaempferol *,c
|
- |
+ |
+ |
- |
| CP15 |
31.14 |
C11H6O3
|
187.0401 |
159.0444 |
143.0499 |
Angelicin c
|
+ |
- |
- |
- |
| CP16 |
31.36 |
C21H20O11
|
449.1078 |
303.0501 |
229.0495 |
Quercitrin c
|
- |
- |
+ |
- |
| CP17 |
32.08 |
C20H24O11
|
441.2495 |
352.3446 |
282.2769 |
Unknown |
+ |
+ |
- |
- |
| CP18 |
33.88 |
C21H20O12
|
465.4033 |
303.2861 |
274.3367 |
Isoquercitrin*
|
+ |
+ |
+ |
+ |
| CP19 |
34.83 |
C28H32O15
|
609.1820 |
463.1244 |
301.1069 |
Diosmetin-7-O-rutinoside c
|
- |
- |
+ |
+ |
| CP20 |
35.62 |
C15H10O7
|
303.0507 |
285.0409 |
257.0453 |
Quercetin c
|
- |
+ |
+ |
+ |
3.2. NMR
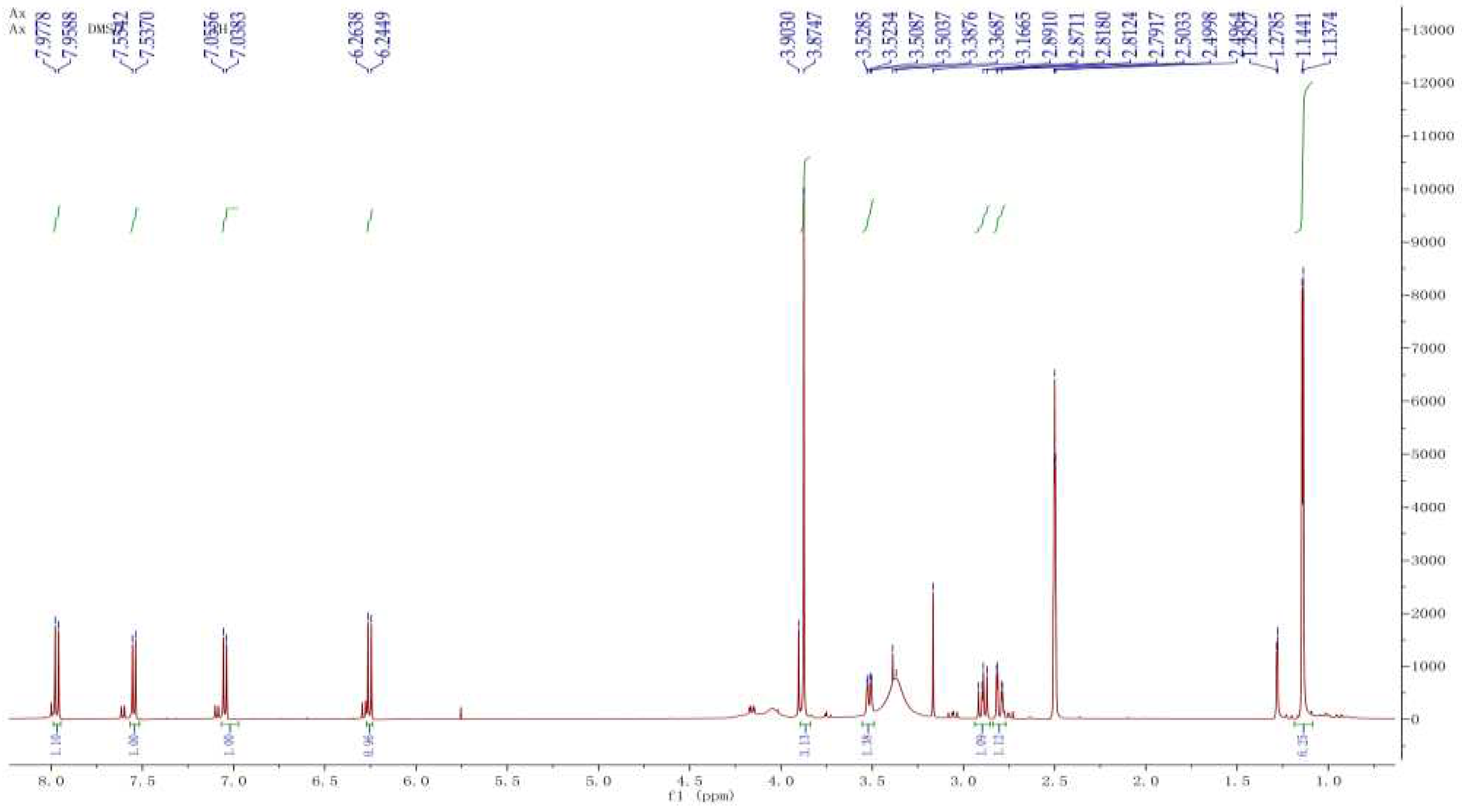
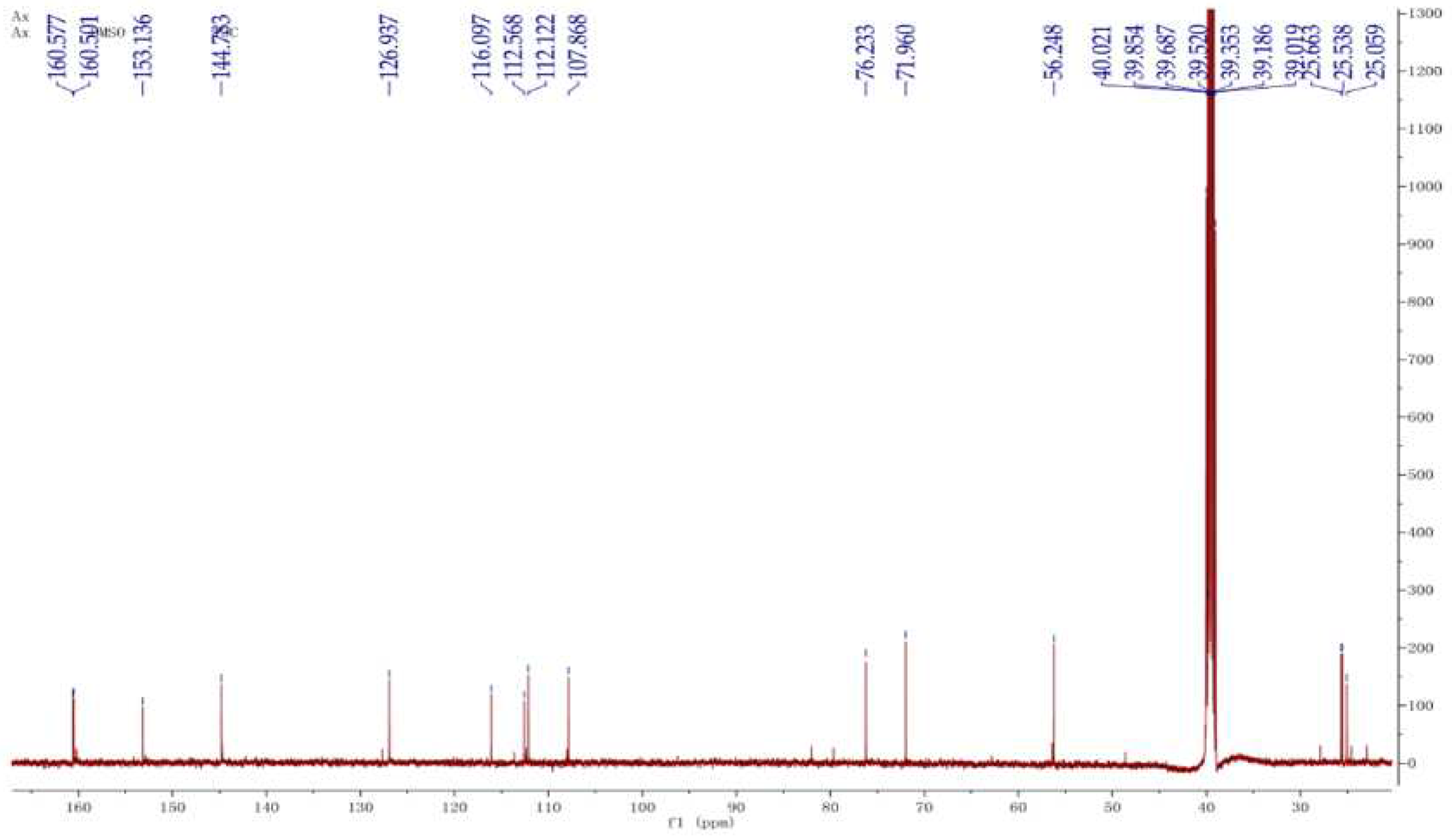
The compound CP13 was characterized based on the analysis of 1H NMR and 13C NMR spectra. In the
1H NMR spectrum (
Figure 4), methyl signals at δ 1.13 (s, H-17), 1.14 (s, H-18), and 3.87 (s, H-13) were observed on three carbon atoms. Additionally, four olefinic signals were detected at δ 6.24 (t, H-2), 7.95(t, H-3), 7.53 (t, H-7), and 7.03 (t, H-8). Analysis of the
13C NMR spectrum (
Figure 5) revealed a total of 15 carbon atoms in the compound, including three signals of CH
3, one signal of CH
2, five signals of CH, and six signals of C. The
1H NMR and
13C NMR spectra displayed characteristic signals for a coumarin nucleus substituted on 7 by a methoxyl group. The molecular formula of CP13 was determined as C
15H
18O
5 based on the results obtained from MS, in conjunction with the analysis of the
1H NMR and
13C NMR spectra. The compound was identified as a meranzin hydrate through structural elucidation. The compound meranzin hydrate, identified as a major constituent of the traditional Chinese medicine Chaihu-Shugan-San, has been proven to possess anti-atherosclerotic and antidepressant properties [
16]. In the four samples analyzed, this compound exhibited the highest proportion, indicating its significant research potential.
3.3. Antioxidant Activities
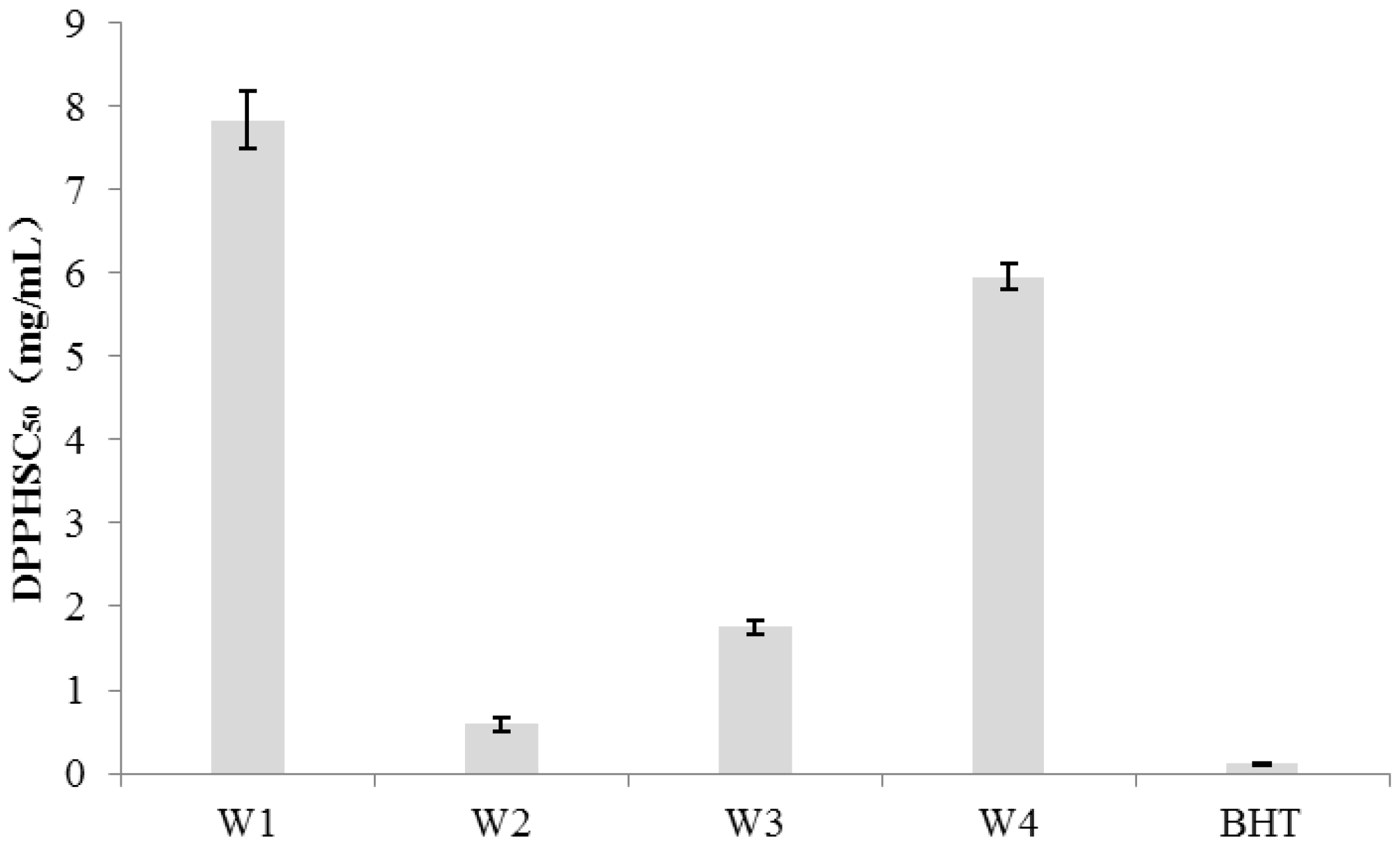
The DPPH assay, as an in vitro antioxidant activity assessment, exhibits a lower value indicating a more significant antioxidant activity. The concentration of BHT corresponding to a 50% DPPH radical scavenging rate (SC
50) was found to be 0.11 mg/mL (
Figure 6). The Sample of W2 and W3 demonstrated values of 0.59 mg/mL and 1.75 mg/mL respectively, indicating better antioxidant activity among samples. Additionally, sample of W3 exhibited slightly lower antioxidant activity compared to sample of W1. Both sample W1 and W4 exhibited relatively weaker antioxidant capabilities with SC
50 values of 7.84 mg/mL and 5.96 mg/mL respectively.
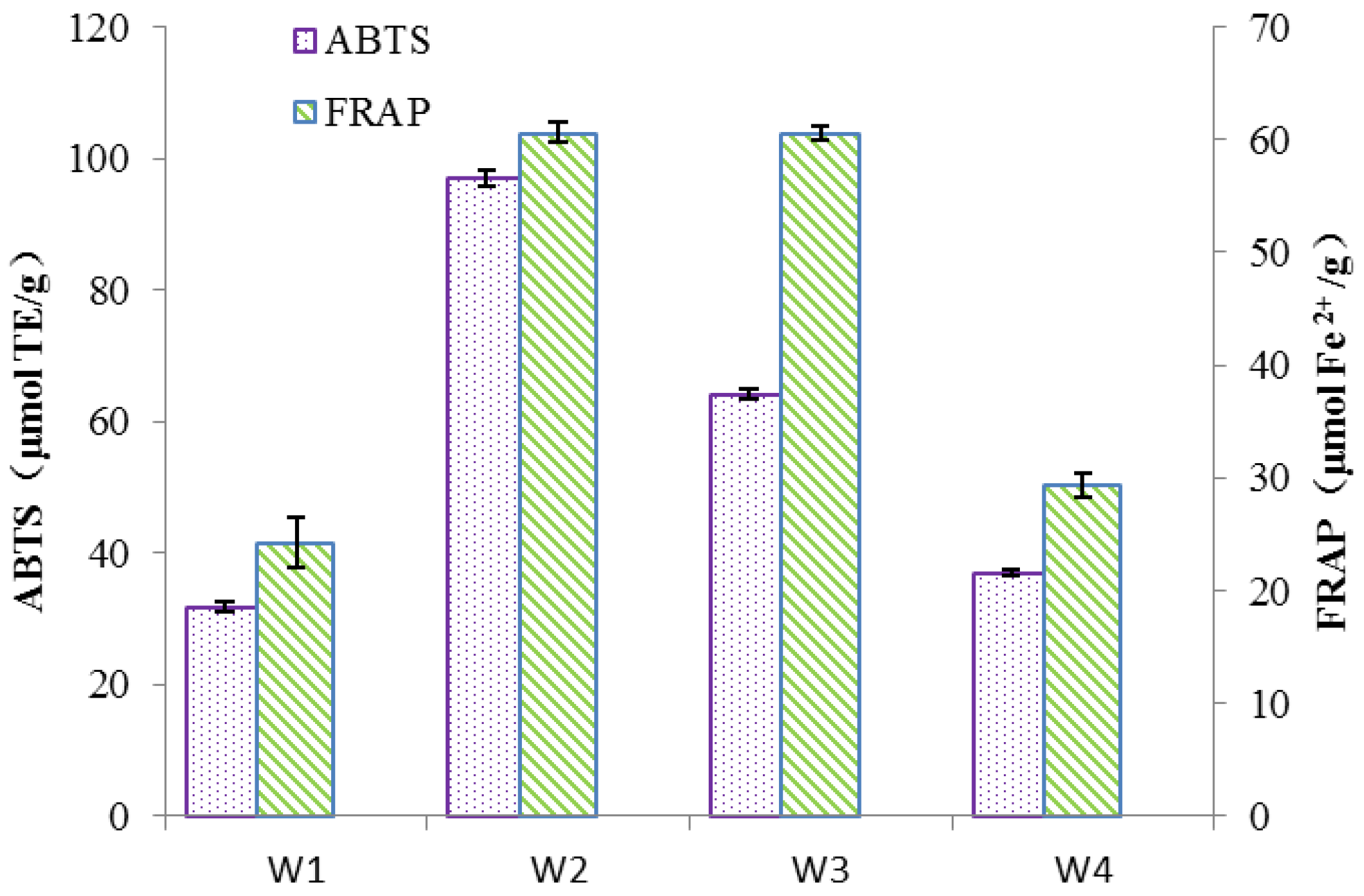
The ABTS and FRAP assay results were expressed in terms of Trolox equivalents and Fe
2+ concentrations respectively, where higher values indicate stronger antioxidant capacity. The samples of W2, according to
Figure 7, exhibited the highest antioxidant capacity in both the ABTS and FRAP assays with values of 97.06 μmol TE/g and 60.62 μmol Fe
2+/g respectively. The W3 showed a slightly lower antioxidant capacity than W2 in the ABTS and FRAP assays with values of 64.08 μmol TE/g and 45.29 μmol Fe
2+/g respectively. However, samples of W1 and W4 demonstrated relatively weaker antioxidant capacities, especially the W1 displayed the weakest antioxidant activity in both tests.
The previous studies have demonstrated the favorable antioxidant activity of flavonoid compounds in the Citrus fruits [
23]. Rutin (CP5), for instance, exerts its free radical scavenging effect through its ability to eliminate hydrogen peroxide and peroxides [
24]. Naringin (CP10) exhibits strong antioxidant properties due to its ability to eliminate free radicals [
25,
26], while hesperidin (CP11) exerts antioxidant effects by attenuating oxidative stress reactions [
27]. Neohesperidin (CP12) has also been confirmed to possess excellent free radical scavenging abilities in both superoxide and hydroxyl radical tests [
28]. These four compounds were detected in all samples, providing a comprehensive explanation for the observed antioxidant activity. However, the compound kaempferol (CP14) has been shown to possess antioxidant activity [
29] and considering the differences in compound composition among samples and the potential synergistic effects between compounds [
30], this may explain why some samples exhibit stronger antioxidant capacity while others show relatively weaker activity.
3.4. α-Glucosidase inhibitory activity
The α-glucosidase inhibition activity, according to
Table 3, was evaluated as a measure of hypoglycemic with lower values indicating stronger blood glucose-lowering ability. All samples from the four regions exhibited weaker activity compared to the positive control acarbose. But the samples of W3 (
I=7.99 mg/mL) demonstrated the highest inhibitory activity in all samples, followed by W1 with a value of 11.86 mg/mL. Sample of W2 showed moderate activity with a value of 12.38 mg/mL, while W4 exhibited the weakest inhibitory activity.
The studies have indicated that quercitrin exhibits significant inhibitory effects on α-amylase and α-glucosidase, making it an ideal compound for targeting diabetes management [
31,
32]. Based on the obtained chemical composition and without considering synergistic effects, it was observed that the compound quercitrin (CPF16) detected in sample of W3 was not found in other samples. Therefore, the presence of quercitrin in sample W3 may serve as a crucial indicator of its superior inhibitory activity compared to other samples.
4. Conclusions
This study compared the chemical composition and biological activities of Wendan peel samples collected from four different regions in China. The results revealed variations in the chemical composition of the samples from different origins. A total of 20 compounds, primarily flavonoids and coumarins, were detected among the four samples. The bioactivity assays demonstrated that the sample from Taizhou (W2) exhibited the highest antioxidant activity, followed by the sample from Zhangzhou (W3). These two samples could be considered as potential natural antioxidants. Additionally, the sample from Zhangzhou (W3) could be regarded as a promising source for the research and development of natural α-glucosidase inhibitors.
Author Contributions
Conceptualization, J.L.; methodology, J.L..; data curation, J.L.; writing—original draft preparation, J.L.; writing—review and editing, J.L. and W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to acknowledge the technical guidance provided by the Institute of Microbiology, Chinese Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burana-Osot, J.; Soonthornchareonnon, N.; Chaidedgumjorn, A.; Hosoyama, S.; Toida, T. Determination of galacturonic acid from pomelo pectin in term of galactose by hpaec with fluorescence detection. Carbohydr. Polym. 2010, 81, 461–465. [Google Scholar] [CrossRef]
- Editorial Committee of the Flora of China, Chinese Academy of Sciences. Flora of China Volume 1 to Volume 65. Beijing: Science Press. 1997,43, 13–17.
- Jiang, L.Y. Study on the Antibacterial Activity of the Extract from Peel of Pomelo. J. Anhui Agric. Sci. 2008, 36, 9354–9340. [Google Scholar] [CrossRef]
- Murunga, A.N.; Miruka, D.O.; Driver, C.; Nkomo, F.S.; Cobongela, S.Z.; Owira, P.M. Grapefruit derived flavonoid naringin improves ketoacidosis and lipid peroxidation in type 1 diabetes rat model. PLoS One 2016, 11, e0153241. [Google Scholar] [CrossRef]
- Huyen, L.T.; On, T.N.H.; Nhi, T.T.; Phat, D.T.; Cang, M.H. Product diversification from pomelo peel. Essential oil, Pectin and semi-dried pomelo peel. Pol. J. Chem. Technol. 2021, 23, 17–25. [Google Scholar] [CrossRef]
- Wu, T.; Guan, Y.; Ye, J. Determination of flavonoids and ascorbic acid in grapefruit peel and juice by capillary electrophoresis with electrochemical detection. Food Chem. 2007, 100, 1573–1579. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Koaze, H.; Karanja, P.N.; Sawamura, M. Volatile constituents of redblush grapefruit (Citrus paradisi) and pummelo (Citrus grandis) peel essential oils from Kenya. J. Agric. Food Chem. 2005, 53, 9790–9794. [Google Scholar] [CrossRef]
- Xi, W.; Fang, B.; Zhao, Q.; Jiao, B.; Zhou, Z. Flavonoid composition and antioxidant activities of Chinese local pummelo (Citrus grandis Osbeck.) varieties. Food Chem. 2014, 161, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ye, F.; Zhou, Y.; Zhao, G.; Zhao, G. Utilization of pomelo peels to manufacture value-added products: A review. Food Chem. 2021, 351, 129247. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Morabito, D.; Dugo, G. Characterization of the Anthocyanin Fraction of Sicilian Blood Orange Juice by Micro-HPLC-ESI/MS. J. Agric. Food Chem. 2003, 51, 1173–1176. [Google Scholar] [CrossRef]
- Hatano, T.; Edamatsu, R.; Hiramatsu, M.; Mori, A.; Fujita, Y.; Yasuhara, T.; Yoshida, T.; Okuda, T. Effects of the interaction of tannins with co-existing substances. VI. Effects of tannins and related polyphenols on superoxide anion radical and on 1, 1-diphenyl-2-picrylhydrazyl radical. Chem. Pharm. Bull. 2008, 37, 2016–2021. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, E.; Lee, C.M. In vitro potential of Ascophyllum nodosum phenolic antioxidant-mediated α-glycosidase and α-amylase inhibition. J. Food Sci. 2010, 75, H97–H102. [Google Scholar] [CrossRef] [PubMed]
- Abyshev, A.Z.; Denisenko, P.P.; Kostyuchenko, N.P.; Ermakov, A.I.; Sheinker, Y.N. Natural meranzin hydrate-A new component of the roots of Prangos ferulacea. Chem. Nat. Compd. 1972, 8, 577–580. [Google Scholar] [CrossRef]
- Li, L.; Yu, A.L.; Wang, Z.L.; Chen, K.; Zheng, W.; Zhou, J.J.; Xie, Q.; Yan, H.B.; Ren, P.; Huang, X. Chaihu-Shugan-San and absorbed meranzin hydrate induce anti-atherosclerosis and behavioral improvements in high-fat diet ApoE-/-mice via anti-inflammatory and BDNF-TrkB pathway. Biomed. Pharmacother. 2019, 115, 108893. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.P.D.; Pinto, J.A.O.; Santos, C.A.D.; Arie, F.B. Harvest time and geographical origin affect the essential oil of Lippia gracilis Schauer. Ind. Crop Prod. 2016, 79, 205–210. [Google Scholar] [CrossRef]
- Delfine, S.; Marrelli, M.; Conforti, F.; Formisano, C.; Rigano, D.; Menichini, F. Variation of Malva sylvestris, essential oil yield, chemical composition and biologicalactivity in response to different environments across Southern Italy. Ind. Crop Prod. 2017, 98, 29–37. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Chen, F.; Su, P.; Chen, D.; Pan, W.; Fang, Y.; Dong, C.; Zheng, X.; Du, Z. Composition and bioactivity assessment of essential oils of Curcuma longaL. collected in China. Ind. Crop Prod. 2017, 109, 60–73. [Google Scholar] [CrossRef]
- Zengin, G.; Paksoy, M.Y.; Aumeeruddy, M.Z.; Glamocilja, J.; Sokovic, M.; Diuzheva, A.; Jeko, J.; Cziaky, Z.; Rodrigues, M.J.; Custodio, L.; et al. New insights into the chemical profiling, cytotoxicity and bioactivity of four Bunium species. Food Res. Int. 2019, 123, 414–424. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Leuzzi, U.; Gattuso, G. First evidence of C-and O-glycosyl flavone in blood orange (Citrus sinensis (L.) Osbeck) juice and their influence on antioxidant properties. Food Chem. 2014, 149, 244–252. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.; Yan, X.; An, L.; Luo, K.; Shao, M.; Jiang, Y.; Xie, R.; Feng, F. An untargeted metabolomics-driven approach based on LC–TOF/MS and LC–MS/MS for the screening of xenobiotics and metabolites of Zhi-Zi-Da-Huang decoction in rat plasma. J. Pharm. Biomed. Anal. 2015, 115, 315–322. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, Y.; Shen, S.; Zhi, Z.; Cheng, H.; Chen, S.; Ye, X. Antioxidant and pancreatic lipase inhibitory effects of flavonoids from different citrus peel extracts: An in vitro study. Food Chem. 2020, 326, 126785. [Google Scholar] [CrossRef]
- Alam, P.; Alajmi, M.F.; Arbab, A.H.; Parvez, M.K.; Siddiqui, N.A.; Alqasoumi, S.I.; AI-Rehaily, A.J.; AI-Dosari, M.S.; Basudan, O.A. Comparative study of antioxidant activity and validated RP-HPTLC analysis of rutin in the leaves of different Acacia species grown in Saudi Arabia. Saudi Pharm. J. 2017, 25, 715–723. [Google Scholar] [CrossRef]
- Safari, M.R.; Sheikh, N. Effects of flavonoids on the susceptibility of low-density lipoprotein to oxidative modification. Prostaglandins Leukot. Essent. Fat. Acids 2003, 69, 73–77. [Google Scholar] [CrossRef]
- El-Desoky, A.H.; Abdel-Rahman, R.F.; Ahmed, O.K.; El-Beltagi, H.S.; Hattori, M. Anti-inflammatory and antioxidant activities of naringin isolated from Carissa carandas L.: In vitro and in vivo evidence. Phytomedicine 2018, 42, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Tirkey, N.; Pilkhwal, S.; Kuhad, A.; Chopra, K. Hesperidin, a citrus bioflavonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidney. BMC Pharmacol. 2005, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Suarez, J.; Herrera, M.D.; Marhuenda, E. In vitro scavenger and antioxidant properties of hesperidin and neohesperidin dihydrochalcone. Phytomedicine 1998, 5, 469–473. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. South Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Anagnostopoulou, M.A.; Kefalas, P.; Papageorgiou, V.P.; Assimopoulou, A.N.; Boskou, D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem. 2006, 94, 19–25. [Google Scholar] [CrossRef]
- Van, L.V.; Pham, E.C.; Nguyen, C.V.; Duong, N.T.N.; Le Thi, T.V.; Truong, T.N. In vitro and in vivo antidiabetic activity, isolation of flavonoids, and in silico molecular docking of stem extract of Merremia tridentata (L.). Biomed. Pharmacother. 2022, 146, 112611. [Google Scholar] [CrossRef]
- Oh, T.W.; Do, H.J.; Jeon, J.H.; Kim, K. Quercitrin inhibits platelet activation in arterial thrombosis. Phytomedicine 2021, 80, 153363. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).