Submitted:
08 December 2023
Posted:
11 December 2023
You are already at the latest version
Abstract
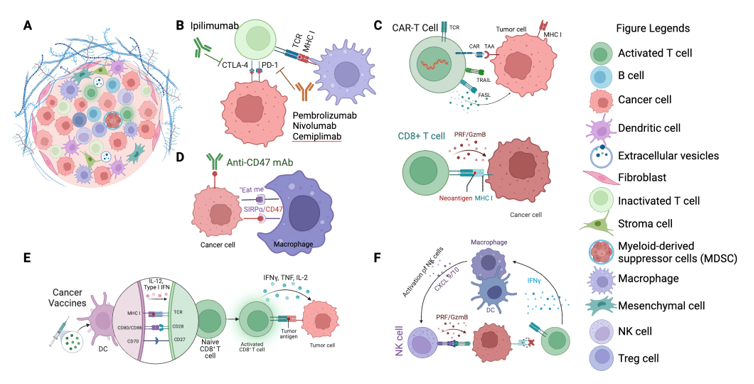
Keywords:
1. Introduction
2. The Immune Maze: Understanding the Complex Landscape
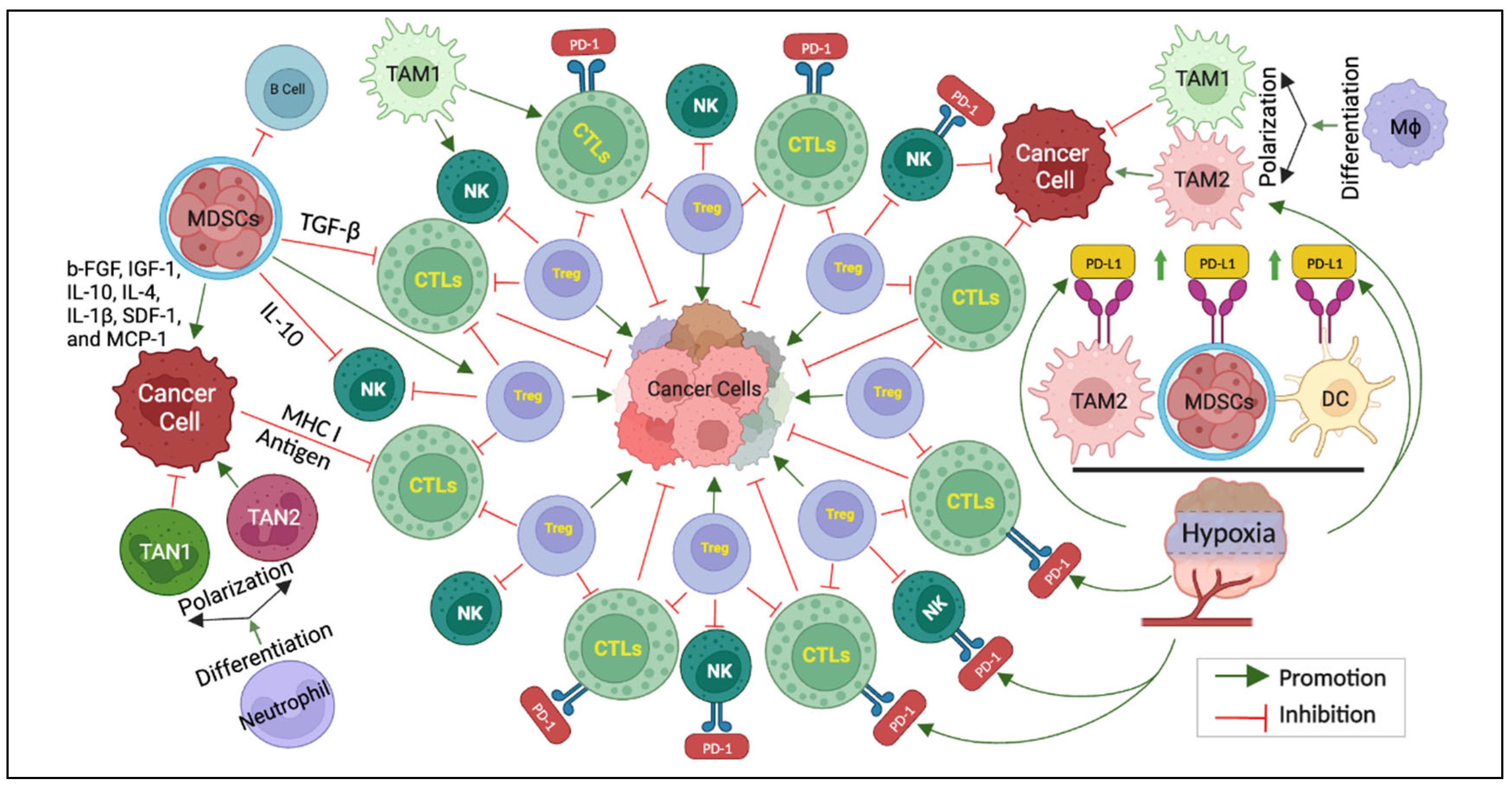
3. Frontline Foes: Decoding the Architects of Immunotherapy Resistance
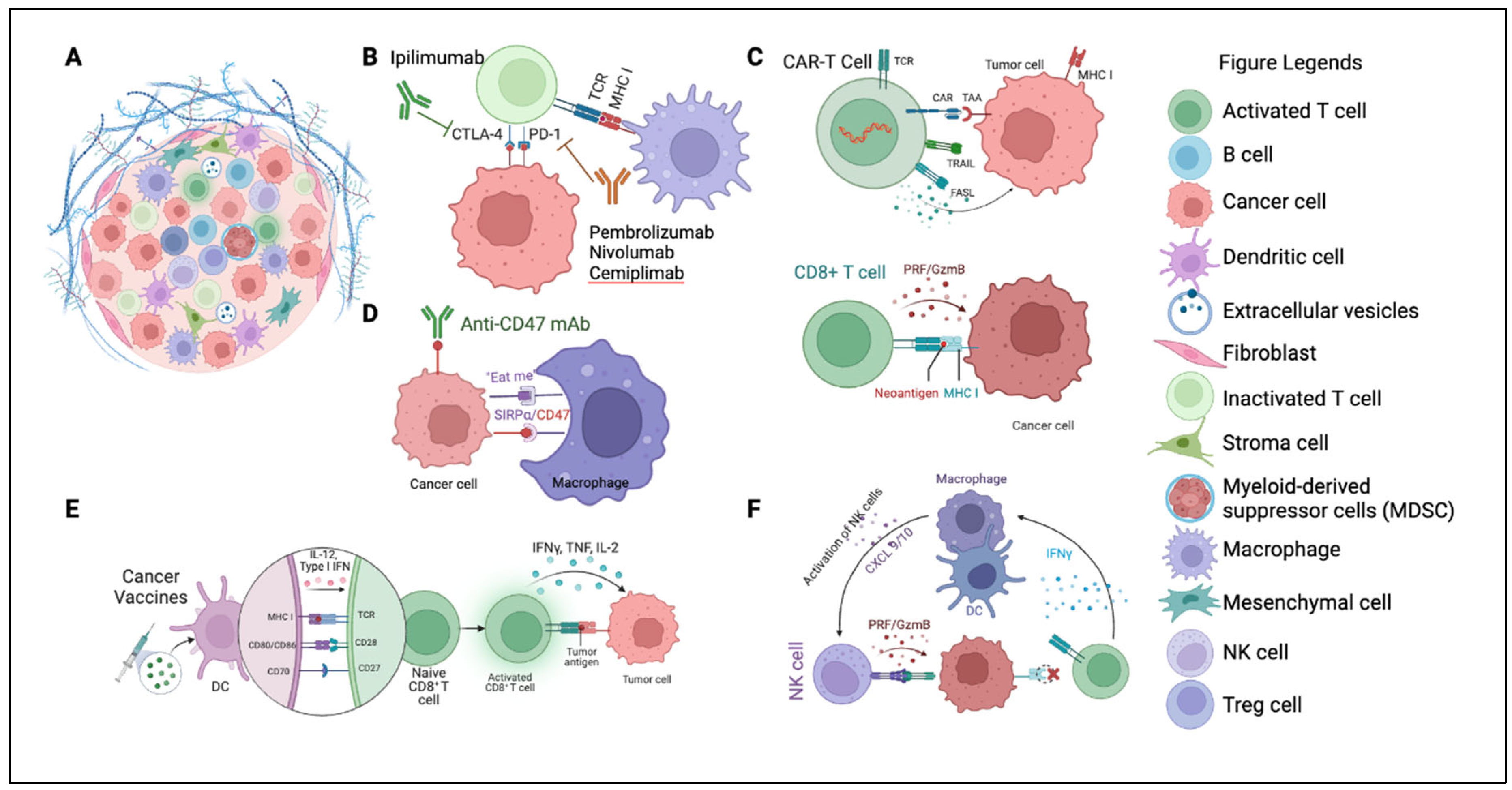
4. Pioneering Strategies to Overcome Resistance
4.1. Combination Therapies
4.2. Tumor Microenvironment (TME)
4.3. Emerging Immune Checkpoints
4.4. Enhancing Immunotherapy with Oncolytic Viruses
4.5. Cell Therapy (ACT)
4.6. Cancer Vaccines
4.7. Navigating Medication-Induced Resistance in Immunotherapy
4.8. Integrated Strategies for Overcoming Resistance
| Strategies | Description | Key components and benefits | Representative Drugs/Cells/Vaccines | References |
| Combination Therapies | Integration of several therapeutic modalities to optimize oncological outcomes. | Synergistic modalities enhancing response. Versatility against varying tumor behaviors. Potential for prolonged patient benefits | Anti-NKG2A: Monalizumab,Anti-PD-1: Nivolumab, PembrolizumabAnti-PD-L1: Atezolizumab, Avelumab, Anti-CTLA-4: Ipilimumab, Durvalumab | [94,95,96,110] |
| TME | Considers the composite of stromal and immune cells intertwined with signaling pathways. Affects tumor progression and anti-tumor immunity. | Stroma including ECM and fibroblast, mesenchymal stromal cells, and immune cells such as TAMs, TANs, and Tregs, signaling pathways that influence tumor progression. | Anti-LOXL2: Simtuzumab, Anti-Hyaluronic acid: PEGPH20, Anti-CTGF: Pamrevlumab, Anti-Integrin: Cilengitide, ATN-161, MEDI-522, Anti-TGF-β: Fresolimumab, etc. | [97,98,128] |
| Immune Checkpoints (ICIs) | Novel checkpoints open promising therapeutic possibilities. They modulate immune functions. | Potential checkpoints like TIGIT, TIM-3, LAG-3 receptors, expanding therapeutic avenues. | Anti-LAG-3 mAbs: Relatlimab, Favezelimab, REGN3767, GSK2831781, LAG525, TSR-033, Relatlimab + Nivolumab, etc. Anti-TIM3: Sabatolimab, spartalizumab | [128,129] |
| Adoptive Cell Therapy (ACT) | Capitalizes on individual’s immune cells. Offers a tailored therapeutic approach. | Precision with techniques like TILs extraction; Potential of CAR-T cells provide tailored therapeutic approach. Enhanced therapeutic results when combined with other modalities. | Tumor-infiltrating lymphocytes (TILs), T cell receptor-engineered T (TCR-T) cells, Natural killer T (NKT) cells | [107,108,109] |
| Cancer Vaccines | Utilization of neoantigens to boost immune responses targeting tumors. | Innovation with DC vaccines and viral vector vaccines; enhancing immune response. | Peptide vaccines: Gardasil®, gp96, OSE2101, DSP-7888, etc.; DNA vaccines: HER2, VGX-3100, WT1, P, MA, hTERT, etc. mRNA vaccines: BNT112, BNT113, MAGE-A3, KRAS, etc.; Virus-based vaccine: PROSTVAC-V/F, TG4010, BT-001; Cell-based vaccines: DC vaccines; GVAX, etc. | [111,112,113,114] |
5. Recent Insights & Developments in Overcoming Immunotherapy Resistance
5.1. Genetic Alterations and Immunotherapy Resistance
5.2. Epigenetic Dynamics and Their Role in Resistance
5.3. The Microbiome’s Influence on Immunotherapy Efficacy
6. Clinical Implications & Translational Approaches
7. Future Perspectives in Immunotherapy
8. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflict of Interest
Publisher’s Note
Abbreviations
| ACT | adoptive cell therapy |
| AI | artificial intelligence |
| CAR | chimeric antigen receptor |
| CTLs | cytotoxic T cells |
| CTLA-4 | cytotoxic T-lymphocyte–associated antigen 4 |
| cfDNA | cell-free DNA |
| CTCs | circulating tumor cells |
| EGFR | epidermal growth factor receptor |
| LAG-3 | lymphocyte activation gene-3 |
| MDSCs | myeloid-derived suppressor cells |
| MHC | major histocompatibility complex |
| NK | natural killer |
| NSCLC | non-small cell lung cancer |
| PBMC | peripheral blood mononuclear cells |
| PD-1 | programmed cell death protein 1 |
| PD-L1 | programmed death-ligand 1 |
| PDO | patient-derived organoids |
| PDX | patient-derived xenograft |
| TAMs | tumor-associated macrophages |
| TAM1 | type-1 TAM |
| TAM2 | type-2 TAM |
| TAN1 | type-1 TAN |
| TAN2 | type-2 TAN |
| TANs | tumor-associated neutrophils |
| TIGIT | T cell immunoreceptor with immunoglobulin and ITIM domain |
| TIM-3 | T cell immunoglobulin and mucin-domain-containing-3 |
| TGF | transforming growth factor |
| Tregs | regulatory T cells |
| TMB | tumor mutational burden |
| TME | tumor microenvironment |
References
- Shin, Y.H.; Bang, S.; Park, S.M.; Ma, X.; Cassilly, C.; Graham, D.; Xavier, R.; Clardy, J. Revisiting Coley’s Toxins: Immunogenic Cardiolipins from Streptococcus pyogenes. J Am Chem Soc 2023, 145, 21183-21188. [CrossRef]
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J 2006, 26, 154-158.
- Coley, I. WB 1893. The treatment of malignant tumors by repeated inoculations of erysipelas; with a report of ten original cases. Am. J. Med. Sci, 487-511.
- Brunet, J.F.; Denizot, F.; Luciani, M.F.; Roux-Dosseto, M.; Suzan, M.; Mattei, M.G.; Golstein, P. A new member of the immunoglobulin superfamily--CTLA-4. Nature 1987, 328, 267-270. [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies (Basel) 2020, 9. [CrossRef]
- Lee, H.T.; Lee, S.H.; Heo, Y.S. Molecular Interactions of Antibody Drugs Targeting PD-1, PD-L1, and CTLA-4 in Immuno-Oncology. Molecules 2019, 24. [CrossRef]
- Yao, L.; Jia, G.; Lu, L.; Bao, Y.; Ma, W. Factors affecting tumor responders and predictive biomarkers of toxicities in cancer patients treated with immune checkpoint inhibitors. Int Immunopharmacol 2020, 85, 106628. [CrossRef]
- Bae, J.; Parayath, N.; Ma, W.; Amiji, M.; Munshi, N.; Anderson, K.C. BCMA peptide-engineered nanoparticles enhance induction and function of antigen-specific CD8(+) cytotoxic T lymphocytes against multiple myeloma: clinical applications. Leukemia 2020, 34, 210-223. [CrossRef]
- Chen, Q.; Lu, L.; Ma, W. Efficacy, Safety, and Challenges of CAR T-Cells in the Treatment of Solid Tumors. Cancers (Basel) 2022, 14. [CrossRef]
- Jogalekar, M.P.; Rajendran, R.L.; Khan, F.; Dmello, C.; Gangadaran, P.; Ahn, B.C. CAR T-Cell-Based gene therapy for cancers: new perspectives, challenges, and clinical developments. Front Immunol 2022, 13, 925985. [CrossRef]
- Murciano-Goroff, Y.R.; Warner, A.B.; Wolchok, J.D. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res 2020, 30, 507-519. [CrossRef]
- Li, X.; Zhang, S.; Guo, G.; Han, J.; Yu, J. Gut microbiome in modulating immune checkpoint inhibitors. EBioMedicine 2022, 82, 104163. [CrossRef]
- Jackson, C.M.; Choi, J.; Lim, M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat Immunol 2019, 20, 1100-1109. [CrossRef]
- Khalaf, K.; Hana, D.; Chou, J.T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front Immunol 2021, 12, 656364. [CrossRef]
- Haist, M.; Stege, H.; Grabbe, S.; Bros, M. The Functional Crosstalk between Myeloid-Derived Suppressor Cells and Regulatory T Cells within the Immunosuppressive Tumor Microenvironment. Cancers (Basel) 2021, 13. [CrossRef]
- Iliadi, C.; Verset, L.; Bouchart, C.; Martinive, P.; Van Gestel, D.; Krayem, M. The current understanding of the immune landscape relative to radiotherapy across tumor types. Front Immunol 2023, 14, 1148692. [CrossRef]
- Piper, M.; Kluger, H.; Ruppin, E.; Hu-Lieskovan, S. Immune Resistance Mechanisms and the Road to Personalized Immunotherapy. Am Soc Clin Oncol Educ Book 2023, 43, e390290. [CrossRef]
- Fountzilas, E.; Tsimberidou, A.M.; Vo, H.H.; Kurzrock, R. Clinical trial design in the era of precision medicine. Genome Med 2022, 14, 101. [CrossRef]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer 2022, 21, 47. [CrossRef]
- Liao, J.; Li, X.; Gan, Y.; Han, S.; Rong, P.; Wang, W.; Li, W.; Zhou, L. Artificial intelligence assists precision medicine in cancer treatment. Front Oncol 2022, 12, 998222. [CrossRef]
- Brown, C.E.; Bucktrout, S.; Butterfield, L.H.; Futer, O.; Galanis, E.; Hormigo, A.; Lim, M.; Okada, H.; Prins, R.; Marr, S.S.; et al. The future of cancer immunotherapy for brain tumors: a collaborative workshop. J Transl Med 2022, 20, 236. [CrossRef]
- Luo, J.; Li, X.; Wei, K.L.; Chen, G.; Xiong, D.D. Advances in the application of computational pathology in diagnosis, immunomicroenvironment recognition, and immunotherapy evaluation of breast cancer: a narrative review. J Cancer Res Clin Oncol 2023, 149, 12535-12542. [CrossRef]
- Abaza, A.; Sid Idris, F.; Anis Shaikh, H.; Vahora, I.; Moparthi, K.P.; Al Rushaidi, M.T.; Muddam, M.R.; Obajeun, O.A.; Jaramillo, A.P.; Khan, S. Programmed Cell Death Protein 1 (PD-1) and Programmed Cell Death Ligand 1 (PD-L1) Immunotherapy: A Promising Breakthrough in Cancer Therapeutics. Cureus 2023, 15, e44582. [CrossRef]
- Yu, J.; Guo, Z.; Wang, L. Progress and Challenges of Immunotherapy Predictive Biomarkers for Triple Negative Breast Cancer in the Era of Single-Cell Multi-Omics. Life (Basel) 2023, 13. [CrossRef]
- Lei, Z.N.; Tian, Q.; Teng, Q.X.; Wurpel, J.N.D.; Zeng, L.; Pan, Y.; Chen, Z.S. Understanding and targeting resistance mechanisms in cancer. MedComm (2020) 2023, 4, e265. [CrossRef]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front Pharmacol 2022, 13, 868695. [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front Immunol 2021, 12, 636568. [CrossRef]
- Liu, Z.; Zhou, Z.; Dang, Q.; Xu, H.; Lv, J.; Li, H.; Han, X. Immunosuppression in tumor immune microenvironment and its optimization from CAR-T cell therapy. Theranostics 2022, 12, 6273-6290. [CrossRef]
- Tie, Y.; Tang, F.; Wei, Y.Q.; Wei, X.W. Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J Hematol Oncol 2022, 15, 61. [CrossRef]
- Trujillo, J.A.; Luke, J.J.; Zha, Y.; Segal, J.P.; Ritterhouse, L.L.; Spranger, S.; Matijevich, K.; Gajewski, T.F. Secondary resistance to immunotherapy associated with beta-catenin pathway activation or PTEN loss in metastatic melanoma. J Immunother Cancer 2019, 7, 295. [CrossRef]
- Cendrowicz, E.; Sas, Z.; Bremer, E.; Rygiel, T.P. The Role of Macrophages in Cancer Development and Therapy. Cancers (Basel) 2021, 13. [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal 2020, 18, 59. [CrossRef]
- Chakravarthi, B.V.; Nepal, S.; Varambally, S. Genomic and Epigenomic Alterations in Cancer. Am J Pathol 2016, 186, 1724-1735. [CrossRef]
- Dai, E.; Zhu, Z.; Wahed, S.; Qu, Z.; Storkus, W.J.; Guo, Z.S. Epigenetic modulation of antitumor immunity for improved cancer immunotherapy. Mol Cancer 2021, 20, 171. [CrossRef]
- Martinez-Jimenez, F.; Priestley, P.; Shale, C.; Baber, J.; Rozemuller, E.; Cuppen, E. Genetic immune escape landscape in primary and metastatic cancer. Nat Genet 2023, 55, 820-831. [CrossRef]
- Cao, J.; Yan, Q. Cancer Epigenetics, Tumor Immunity, and Immunotherapy. Trends Cancer 2020, 6, 580-592. [CrossRef]
- Liang, Y.; Turcan, S. Epigenetic Drugs and Their Immune Modulating Potential in Cancers. Biomedicines 2022, 10. [CrossRef]
- Yang, J.; Xu, J.; Wang, W.; Zhang, B.; Yu, X.; Shi, S. Epigenetic regulation in the tumor microenvironment: molecular mechanisms and therapeutic targets. Signal Transduct Target Ther 2023, 8, 210. [CrossRef]
- Lindau, D.; Gielen, P.; Kroesen, M.; Wesseling, P.; Adema, G.J. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 2013, 138, 105-115. [CrossRef]
- Ma, T.; Renz, B.W.; Ilmer, M.; Koch, D.; Yang, Y.; Werner, J.; Bazhin, A.V. Myeloid-Derived Suppressor Cells in Solid Tumors. Cells 2022, 11. [CrossRef]
- Sun, R.; Zhao, H.; Gao, D.S.; Ni, A.; Li, H.; Chen, L.; Lu, X.; Chen, K.; Lu, B. Amphiregulin couples IL1RL1(+) regulatory T cells and cancer-associated fibroblasts to impede antitumor immunity. Sci Adv 2023, 9, eadd7399. [CrossRef]
- Shi, H.; Li, K.; Ni, Y.; Liang, X.; Zhao, X. Myeloid-Derived Suppressor Cells: Implications in the Resistance of Malignant Tumors to T Cell-Based Immunotherapy. Front Cell Dev Biol 2021, 9, 707198. [CrossRef]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther 2021, 6, 362. [CrossRef]
- Kopecka, J.; Salaroglio, I.C.; Perez-Ruiz, E.; Sarmento-Ribeiro, A.B.; Saponara, S.; De Las Rivas, J.; Riganti, C. Hypoxia as a driver of resistance to immunotherapy. Drug Resist Updat 2021, 59, 100787. [CrossRef]
- Wang, B.; Zhao, Q.; Zhang, Y.; Liu, Z.; Zheng, Z.; Liu, S.; Meng, L.; Xin, Y.; Jiang, X. Targeting hypoxia in the tumor microenvironment: a potential strategy to improve cancer immunotherapy. J Exp Clin Cancer Res 2021, 40, 24. [CrossRef]
- Shi, T.; Zhu, J.; Zhang, X.; Mao, X. The Role of Hypoxia and Cancer Stem Cells in Development of Glioblastoma. Cancers (Basel) 2023, 15. [CrossRef]
- Bhattacharya, S.; Calar, K.; de la Puente, P. Mimicking tumor hypoxia and tumor-immune interactions employing three-dimensional in vitro models. J Exp Clin Cancer Res 2020, 39, 75. [CrossRef]
- Shurin, M.R.; Umansky, V. Cross-talk between HIF and PD-1/PD-L1 pathways in carcinogenesis and therapy. J Clin Invest 2022, 132. [CrossRef]
- Wu, Q.; You, L.; Nepovimova, E.; Heger, Z.; Wu, W.; Kuca, K.; Adam, V. Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape. J Hematol Oncol 2022, 15, 77. [CrossRef]
- Li, H.; Shao, S.; Cai, J.; Burner, D.; Lu, L.; Chen, Q.; Minev, B.; Ma, W. Artificial human antigen-presenting cells are superior to dendritic cells at inducing cytotoxic T-cell responses. Immunology 2017, 152, 462-471. [CrossRef]
- Lu, L.; Ma, W.; Johnson, C.H.; Khan, S.A.; Irwin, M.L.; Pusztai, L. In silico designed mRNA vaccines targeting CA-125 neoantigen in breast and ovarian cancer. Vaccine 2023, 41, 2073-2083. [CrossRef]
- Ma, W.; Smith, T.; Bogin, V.; Zhang, Y.; Ozkan, C.; Ozkan, M.; Hayden, M.; Schroter, S.; Carrier, E.; Messmer, D.; et al. Enhanced presentation of MHC class Ia, Ib and class II-restricted peptides encapsulated in biodegradable nanoparticles: a promising strategy for tumor immunotherapy. J Transl Med 2011, 9, 34. [CrossRef]
- Kallingal, A.; Olszewski, M.; Maciejewska, N.; Brankiewicz, W.; Baginski, M. Cancer immune escape: the role of antigen presentation machinery. J Cancer Res Clin Oncol 2023, 149, 8131-8141. [CrossRef]
- Haddad, A.F.; Young, J.S.; Gill, S.; Aghi, M.K. Resistance to immune checkpoint blockade: Mechanisms, counter-acting approaches, and future directions. Semin Cancer Biol 2022, 86, 532-541. [CrossRef]
- Wang, B.; Han, Y.; Zhang, Y.; Zhao, Q.; Wang, H.; Wei, J.; Meng, L.; Xin, Y.; Jiang, X. Overcoming acquired resistance to cancer immune checkpoint therapy: potential strategies based on molecular mechanisms. Cell Biosci 2023, 13, 120. [CrossRef]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev 2018, 32, 1267-1284. [CrossRef]
- Marin-Acevedo, J.A.; Dholaria, B.; Soyano, A.E.; Knutson, K.L.; Chumsri, S.; Lou, Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol 2018, 11, 39. [CrossRef]
- Naimi, A.; Mohammed, R.N.; Raji, A.; Chupradit, S.; Yumashev, A.V.; Suksatan, W.; Shalaby, M.N.; Thangavelu, L.; Kamrava, S.; Shomali, N.; et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun Signal 2022, 20, 44. [CrossRef]
- Webb, E.S.; Liu, P.; Baleeiro, R.; Lemoine, N.R.; Yuan, M.; Wang, Y.H. Immune checkpoint inhibitors in cancer therapy. J Biomed Res 2018, 32, 317-326. [CrossRef]
- Xie, Q.; Zhang, P.; Wang, Y.; Mei, W.; Zeng, C. Overcoming resistance to immune checkpoint inhibitors in hepatocellular carcinoma: Challenges and opportunities. Front Oncol 2022, 12, 958720. [CrossRef]
- Fares, C.M.; Van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am Soc Clin Oncol Educ Book 2019, 39, 147-164. [CrossRef]
- Passaro, A.; Brahmer, J.; Antonia, S.; Mok, T.; Peters, S. Managing Resistance to Immune Checkpoint Inhibitors in Lung Cancer: Treatment and Novel Strategies. J Clin Oncol 2022, 40, 598-610. [CrossRef]
- Metropulos, A.E.; Munshi, H.G.; Principe, D.R. The difficulty in translating the preclinical success of combined TGFbeta and immune checkpoint inhibition to clinical trial. EBioMedicine 2022, 86, 104380. [CrossRef]
- Tiwari, A.; Trivedi, R.; Lin, S.Y. Tumor microenvironment: barrier or opportunity towards effective cancer therapy. J Biomed Sci 2022, 29, 83. [CrossRef]
- Chen, M.L.; Pittet, M.J.; Gorelik, L.; Flavell, R.A.; Weissleder, R.; von Boehmer, H.; Khazaie, K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A 2005, 102, 419-424. [CrossRef]
- Huang, L.; Guo, Y.; Liu, S.; Wang, H.; Zhu, J.; Ou, L.; Xu, X. Targeting regulatory T cells for immunotherapy in melanoma. Mol Biomed 2021, 2, 11. [CrossRef]
- Itahashi, K.; Irie, T.; Nishikawa, H. Regulatory T-cell development in the tumor microenvironment. Eur J Immunol 2022, 52, 1216-1227. [CrossRef]
- Nishikawa, H.; Koyama, S. Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. J Immunother Cancer 2021, 9. [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther 2021, 6, 263. [CrossRef]
- Wang, S.; Zhao, X.; Wu, S.; Cui, D.; Xu, Z. Myeloid-derived suppressor cells: key immunosuppressive regulators and therapeutic targets in hematological malignancies. Biomark Res 2023, 11, 34. [CrossRef]
- Zalfa, C.; Paust, S. Natural Killer Cell Interactions With Myeloid Derived Suppressor Cells in the Tumor Microenvironment and Implications for Cancer Immunotherapy. Front Immunol 2021, 12, 633205. [CrossRef]
- Jakos, T.; Pislar, A.; Jewett, A.; Kos, J. Myeloid-Derived Suppressor Cells Hamper Natural Killer Cell Activity in Cancer: Role of Peptidases. Crit Rev Immunol 2021, 41, 77-99. [CrossRef]
- Li, Y.; He, H.; Jihu, R.; Zhou, J.; Zeng, R.; Yan, H. Novel Characterization of Myeloid-Derived Suppressor Cells in Tumor Microenvironment. Front Cell Dev Biol 2021, 9, 698532. [CrossRef]
- Mehdizadeh, R.; Shariatpanahi, S.P.; Goliaei, B.; Ruegg, C. Targeting myeloid-derived suppressor cells in combination with tumor cell vaccination predicts anti-tumor immunity and breast cancer dormancy: an in silico experiment. Sci Rep 2023, 13, 5875. [CrossRef]
- He, Z.; Zhang, S. Tumor-Associated Macrophages and Their Functional Transformation in the Hypoxic Tumor Microenvironment. Front Immunol 2021, 12, 741305. [CrossRef]
- Chen, S.; Saeed, A.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther 2023, 8, 207. [CrossRef]
- Li, M.; He, L.; Zhu, J.; Zhang, P.; Liang, S. Targeting tumor-associated macrophages for cancer treatment. Cell Biosci 2022, 12, 85. [CrossRef]
- Mehta, A.K.; Kadel, S.; Townsend, M.G.; Oliwa, M.; Guerriero, J.L. Macrophage Biology and Mechanisms of Immune Suppression in Breast Cancer. Front Immunol 2021, 12, 643771. [CrossRef]
- Feng, Y.; Ye, Z.; Song, F.; He, Y.; Liu, J. The Role of TAMs in Tumor Microenvironment and New Research Progress. Stem Cells Int 2022, 2022, 5775696. [CrossRef]
- Yan, M.; Zheng, M.; Niu, R.; Yang, X.; Tian, S.; Fan, L.; Li, Y.; Zhang, S. Roles of tumor-associated neutrophils in tumor metastasis and its clinical applications. Front Cell Dev Biol 2022, 10, 938289. [CrossRef]
- Que, H.; Fu, Q.; Lan, T.; Tian, X.; Wei, X. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochim Biophys Acta Rev Cancer 2022, 1877, 188762. [CrossRef]
- Wicks, E.E.; Semenza, G.L. Hypoxia-inducible factors: cancer progression and clinical translation. J Clin Invest 2022, 132. [CrossRef]
- Li, Y.; Zhao, L.; Li, X.F. Hypoxia and the Tumor Microenvironment. Technol Cancer Res Treat 2021, 20, 15330338211036304. [CrossRef]
- Patsoukis, N.; Wang, Q.; Strauss, L.; Boussiotis, V.A. Revisiting the PD-1 pathway. Sci Adv 2020, 6. [CrossRef]
- Yi, M.; Jiao, D.; Xu, H.; Liu, Q.; Zhao, W.; Han, X.; Wu, K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer 2018, 17, 129. [CrossRef]
- Zhang, Y.; Coleman, M.; Brekken, R.A. Perspectives on Hypoxia Signaling in Tumor Stroma. Cancers (Basel) 2021, 13. [CrossRef]
- Shklovskaya, E.; Rizos, H. MHC Class I Deficiency in Solid Tumors and Therapeutic Strategies to Overcome It. Int J Mol Sci 2021, 22. [CrossRef]
- Wen, M.; Li, Y.; Qin, X.; Qin, B.; Wang, Q. Insight into Cancer Immunity: MHCs, Immune Cells and Commensal Microbiota. Cells 2023, 12. [CrossRef]
- Zhou, J.; Bashey, A.; Zhong, R.; Corringham, S.; Messer, K.; Pu, M.; Ma, W.; Chut, T.; Soiffer, R.; Mitrovich, R.C.; et al. CTLA-4 blockade following relapse of malignancy after allogeneic stem cell transplantation is associated with T cell activation but not with increased levels of T regulatory cells. Biol Blood Marrow Transplant 2011, 17, 682-692. [CrossRef]
- Chen, X.; Zhang, W.; Yang, W.; Zhou, M.; Liu, F. Acquired resistance for immune checkpoint inhibitors in cancer immunotherapy: challenges and prospects. Aging (Albany NY) 2022, 14, 1048-1064. [CrossRef]
- Said, S.S.; Ibrahim, W.N. Cancer Resistance to Immunotherapy: Comprehensive Insights with Future Perspectives. Pharmaceutics 2023, 15. [CrossRef]
- Lao, Y.; Shen, D.; Zhang, W.; He, R.; Jiang, M. Immune Checkpoint Inhibitors in Cancer Therapy-How to Overcome Drug Resistance? Cancers (Basel) 2022, 14. [CrossRef]
- Baxter, M.A.; Middleton, F.; Cagney, H.P.; Petty, R.D. Resistance to immune checkpoint inhibitors in advanced gastro-oesophageal cancers. Br J Cancer 2021, 125, 1068-1079. [CrossRef]
- Dutta, S.; Ganguly, A.; Chatterjee, K.; Spada, S.; Mukherjee, S. Targets of Immune Escape Mechanisms in Cancer: Basis for Development and Evolution of Cancer Immune Checkpoint Inhibitors. Biology (Basel) 2023, 12. [CrossRef]
- Vanneman, M.; Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012, 12, 237-251. [CrossRef]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front Oncol 2022, 12, 891652. [CrossRef]
- Flies, D.B.; Langermann, S.; Jensen, C.; Karsdal, M.A.; Willumsen, N. Regulation of tumor immunity and immunotherapy by the tumor collagen extracellular matrix. Front Immunol 2023, 14, 1199513. [CrossRef]
- Chen, C.; Liu, X.; Chang, C.Y.; Wang, H.Y.; Wang, R.F. The Interplay between T Cells and Cancer: The Basis of Immunotherapy. Genes (Basel) 2023, 14. [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov 2022, 21, 799-820. [CrossRef]
- Park, K.; Veena, M.S.; Shin, D.S. Key Players of the Immunosuppressive Tumor Microenvironment and Emerging Therapeutic Strategies. Front Cell Dev Biol 2022, 10, 830208. [CrossRef]
- Wang, Y.; Huang, T.; Gu, J.; Lu, L. Targeting the metabolism of tumor-infiltrating regulatory T cells. Trends Immunol 2023, 44, 598-612. [CrossRef]
- Bejarano, L.; Jordao, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov 2021, 11, 933-959. [CrossRef]
- Lee, J.B.; Ha, S.J.; Kim, H.R. Clinical Insights Into Novel Immune Checkpoint Inhibitors. Front Pharmacol 2021, 12, 681320. [CrossRef]
- Dulal, D.; Boring, A.; Terrero, D.; Johnson, T.; Tiwari, A.K.; Raman, D. Tackling of Immunorefractory Tumors by Targeting Alternative Immune Checkpoints. Cancers (Basel) 2023, 15. [CrossRef]
- Zhu, X.; Fan, C.; Xiong, Z.; Chen, M.; Li, Z.; Tao, T.; Liu, X. Development and application of oncolytic viruses as the nemesis of tumor cells. Front Microbiol 2023, 14, 1188526. [CrossRef]
- Chesney, J.A.; Ribas, A.; Long, G.V.; Kirkwood, J.M.; Dummer, R.; Puzanov, I.; Hoeller, C.; Gajewski, T.F.; Gutzmer, R.; Rutkowski, P.; et al. Randomized, Double-Blind, Placebo-Controlled, Global Phase III Trial of Talimogene Laherparepvec Combined With Pembrolizumab for Advanced Melanoma. Journal of Clinical Oncology 2023, 41, 528-540. [CrossRef]
- Feldman, S.A.; Assadipour, Y.; Kriley, I.; Goff, S.L.; Rosenberg, S.A. Adoptive Cell Therapy--Tumor-Infiltrating Lymphocytes, T-Cell Receptors, and Chimeric Antigen Receptors. Semin Oncol 2015, 42, 626-639. [CrossRef]
- Li, J.; Xiao, Z.; Wang, D.; Jia, L.; Nie, S.; Zeng, X.; Hu, W. The screening, identification, design and clinical application of tumor-specific neoantigens for TCR-T cells. Mol Cancer 2023, 22, 141. [CrossRef]
- Ingram, Z.; Madan, S.; Merchant, J.; Carter, Z.; Gordon, Z.; Carey, G.; Webb, T.J. Targeting Natural Killer T Cells in Solid Malignancies. Cells 2021, 10. [CrossRef]
- Zhu, S.; Zhang, T.; Zheng, L.; Liu, H.; Song, W.; Liu, D.; Li, Z.; Pan, C.X. Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol 2021, 14, 156. [CrossRef]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol 2022, 15, 28. [CrossRef]
- Kaczmarek, M.; Poznanska, J.; Fechner, F.; Michalska, N.; Paszkowska, S.; Napierala, A.; Mackiewicz, A. Cancer Vaccine Therapeutics: Limitations and Effectiveness-A Literature Review. Cells 2023, 12. [CrossRef]
- Lee, K.W.; Yam, J.W.P.; Mao, X. Dendritic Cell Vaccines: A Shift from Conventional Approach to New Generations. Cells 2023, 12. [CrossRef]
- Gupta, M.; Wahi, A.; Sharma, P.; Nagpal, R.; Raina, N.; Kaurav, M.; Bhattacharya, J.; Rodrigues Oliveira, S.M.; Dolma, K.G.; Paul, A.K.; et al. Recent Advances in Cancer Vaccines: Challenges, Achievements, and Futuristic Prospects. Vaccines (Basel) 2022, 10. [CrossRef]
- Goodman, R.S.; Johnson, D.B.; Balko, J.M. Corticosteroids and Cancer Immunotherapy. Clin Cancer Res 2023, 29, 2580-2587. [CrossRef]
- Kalfeist, L.; Galland, L.; Ledys, F.; Ghiringhelli, F.; Limagne, E.; Ladoire, S. Impact of Glucocorticoid Use in Oncology in the Immunotherapy Era. Cells 2022, 11. [CrossRef]
- Meng, L.; Wei, Y.; Xiao, Y. Chemo-immunoablation of solid tumors: A new concept in tumor ablation. Front Immunol 2022, 13, 1057535. [CrossRef]
- Li, J.Y.; Chen, Y.P.; Li, Y.Q.; Liu, N.; Ma, J. Chemotherapeutic and targeted agents can modulate the tumor microenvironment and increase the efficacy of immune checkpoint blockades. Mol Cancer 2021, 20, 27. [CrossRef]
- Eng, L.; Sutradhar, R.; Niu, Y.; Liu, N.; Liu, Y.; Kaliwal, Y.; Powis, M.L.; Liu, G.; Peppercorn, J.M.; Bedard, P.L.; et al. Impact of Antibiotic Exposure Before Immune Checkpoint Inhibitor Treatment on Overall Survival in Older Adults With Cancer: A Population-Based Study. Journal of Clinical Oncology 2023, 41, 3122-3134. [CrossRef]
- Peng, C.; Rabold, K.; Mulder, W.J.M.; Jaeger, M.; Netea-Maier, R.T. Kinase Inhibitors’ Effects on Innate Immunity in Solid Cancers. Cancers (Basel) 2021, 13. [CrossRef]
- Castelo-Soccio, L.; Kim, H.; Gadina, M.; Schwartzberg, P.L.; Laurence, A.; O’Shea, J.J. Protein kinases: drug targets for immunological disorders. Nat Rev Immunol 2023, 23, 787-806. [CrossRef]
- Fogli, L.K.; Aurigemma, R.; Sommers, C.L.; Singh, A.; Bourcier, K.; Ernstoff, M.S.; Committee, N.C.I.C.T.W. Challenges and next steps in the advancement of immunotherapy: summary of the 2018 and 2020 National Cancer Institute workshops on cell-based immunotherapy for solid tumors. J Immunother Cancer 2021, 9. [CrossRef]
- Piper, M.; Kluger, H.; Ruppin, E.; Hu-Lieskovan, S. Immune Resistance Mechanisms and the Road to Personalized Immunotherapy. American Society of Clinical Oncology Educational Book 2023, e390290. [CrossRef]
- Xu, S.; Tan, S.; Guo, L. Patient-Derived Organoids as a Promising Tool for Multimodal Management of Sarcomas. Cancers (Basel) 2023, 15. [CrossRef]
- Seyhan, A.A.; Carini, C. Insights and Strategies of Melanoma Immunotherapy: Predictive Biomarkers of Response and Resistance and Strategies to Improve Response Rates. Int J Mol Sci 2022, 24. [CrossRef]
- Bai, R.; Lv, Z.; Xu, D.; Cui, J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res 2020, 8, 34. [CrossRef]
- Shao, J.; Jin, Y.; Jin, C. A new approach to overcoming resistance to immunotherapy: nanotechnology. Front Oncol 2023, 13, 1210245. [CrossRef]
- Xu, M.; Zhang, T.; Xia, R.; Wei, Y.; Wei, X. Targeting the tumor stroma for cancer therapy. Mol Cancer 2022, 21, 208. [CrossRef]
- Curigliano, G.; Gelderblom, H.; Mach, N.; Doi, T.; Tai, D.; Forde, P.M.; Sarantopoulos, J.; Bedard, P.L.; Lin, C.C.; Hodi, F.S.; et al. Phase I/Ib Clinical Trial of Sabatolimab, an Anti-TIM-3 Antibody, Alone and in Combination with Spartalizumab, an Anti-PD-1 Antibody, in Advanced Solid Tumors. Clin Cancer Res 2021, 27, 3620-3629. [CrossRef]
- Finck, A.V.; Blanchard, T.; Roselle, C.P.; Golinelli, G.; June, C.H. Engineered cellular immunotherapies in cancer and beyond. Nat Med 2022, 28, 678-689. [CrossRef]
- Kiaie, S.H.; Salehi-Shadkami, H.; Sanaei, M.J.; Azizi, M.; Shokrollahi Barough, M.; Nasr, M.S.; Sheibani, M. Nano-immunotherapy: overcoming delivery challenge of immune checkpoint therapy. J Nanobiotechnology 2023, 21, 339. [CrossRef]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of Cancer Resistance to Immunotherapy. Front Oncol 2020, 10, 1290. [CrossRef]
- Audia, J.E.; Campbell, R.M. Histone Modifications and Cancer. Cold Spring Harb Perspect Biol 2016, 8, a019521. [CrossRef]
- Xu, J.; Xu, H.M.; Yang, M.F.; Liang, Y.J.; Peng, Q.Z.; Zhang, Y.; Tian, C.M.; Wang, L.S.; Yao, J.; Nie, Y.Q.; et al. New Insights Into the Epigenetic Regulation of Inflammatory Bowel Disease. Front Pharmacol 2022, 13, 813659. [CrossRef]
- Wang, C.; Wang, Z.; Yao, T.; Zhou, J.; Wang, Z. The immune-related role of beta-2-microglobulin in melanoma. Front Oncol 2022, 12, 944722. [CrossRef]
- Liu, F.; Zhong, F.; Wu, H.; Che, K.; Shi, J.; Wu, N.; Fu, Y.; Wang, Y.; Hu, J.; Qian, X.; et al. Prevalence and Associations of Beta2-Microglobulin Mutations in MSI-H/dMMR Cancers. Oncologist 2023, 28, e136-e144. [CrossRef]
- Hu, Q.; Bian, Q.; Rong, D.; Wang, L.; Song, J.; Huang, H.S.; Zeng, J.; Mei, J.; Wang, P.Y. JAK/STAT pathway: Extracellular signals, diseases, immunity, and therapeutic regimens. Front Bioeng Biotechnol 2023, 11, 1110765. [CrossRef]
- Shen, H.; Huang, F.; Zhang, X.; Ojo, O.A.; Li, Y.; Trummell, H.Q.; Anderson, J.C.; Fiveash, J.; Bredel, M.; Yang, E.S.; et al. Selective suppression of melanoma lacking IFN-gamma pathway by JAK inhibition depends on T cells and host TNF signaling. Nat Commun 2022, 13, 5013. [CrossRef]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: promising targets for cancer therapy. Signal Transduct Target Ther 2023, 8, 9. [CrossRef]
- Blass, E.; Ott, P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol 2021, 18, 215-229. [CrossRef]
- Mehdi, A.; Rabbani, S.A. Role of Methylation in Pro- and Anti-Cancer Immunity. Cancers (Basel) 2021, 13. [CrossRef]
- Desaulniers, D.; Vasseur, P.; Jacobs, A.; Aguila, M.C.; Ertych, N.; Jacobs, M.N. Integration of Epigenetic Mechanisms into Non-Genotoxic Carcinogenicity Hazard Assessment: Focus on DNA Methylation and Histone Modifications. Int J Mol Sci 2021, 22. [CrossRef]
- Markouli, M.; Strepkos, D.; Basdra, E.K.; Papavassiliou, A.G.; Piperi, C. Prominent Role of Histone Modifications in the Regulation of Tumor Metastasis. Int J Mol Sci 2021, 22. [CrossRef]
- Xiong, D.; Zhang, L.; Sun, Z.J. Targeting the epigenome to reinvigorate T cells for cancer immunotherapy. Mil Med Res 2023, 10, 59. [CrossRef]
- Hu, C.; Liu, X.; Zeng, Y.; Liu, J.; Wu, F. DNA methyltransferase inhibitors combination therapy for the treatment of solid tumor: mechanism and clinical application. Clin Epigenetics 2021, 13, 166. [CrossRef]
- Shen, C.; Li, M.; Duan, Y.; Jiang, X.; Hou, X.; Xue, F.; Zhang, Y.; Luo, Y. HDAC inhibitors enhance the anti-tumor effect of immunotherapies in hepatocellular carcinoma. Front Immunol 2023, 14, 1170207. [CrossRef]
- Lu, G.; Jin, S.; Lin, S.; Gong, Y.; Zhang, L.; Yang, J.; Mou, W.; Du, J. Update on histone deacetylase inhibitors in peripheral T-cell lymphoma (PTCL). Clin Epigenetics 2023, 15, 124. [CrossRef]
- Derosa, L.; Routy, B.; Thomas, A.M.; Iebba, V.; Zalcman, G.; Friard, S.; Mazieres, J.; Audigier-Valette, C.; Moro-Sibilot, D.; Goldwasser, F.; et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med 2022, 28, 315-324. [CrossRef]
- Patel, P.; Poudel, A.; Kafle, S.; Thapa Magar, M.; Cancarevic, I. Influence of Microbiome and Antibiotics on the Efficacy of Immune Checkpoint Inhibitors. Cureus 2021, 13, e16829. [CrossRef]
- Najmi, M.; Tran, T.; Witt, R.G.; Nelson, K.C. Modulation of the Gut Microbiome to Enhance Immunotherapy Response in Metastatic Melanoma Patients: A Clinical Review. Dermatol Ther (Heidelb) 2022, 12, 2489-2497. [CrossRef]
- Villemin, C.; Six, A.; Neville, B.A.; Lawley, T.D.; Robinson, M.J.; Bakdash, G. The heightened importance of the microbiome in cancer immunotherapy. Trends Immunol 2023, 44, 44-59. [CrossRef]
- Sarhadi, V.K.; Armengol, G. Molecular Biomarkers in Cancer. Biomolecules 2022, 12. [CrossRef]
- Seyhan, A.A.; Carini, C. Are innovation and new technologies in precision medicine paving a new era in patients centric care? J Transl Med 2019, 17, 114. [CrossRef]
- Wang, W.; Li, Y.; Lin, K.; Wang, X.; Tu, Y.; Zhuo, Z. Progress in building clinically relevant patient-derived tumor xenograft models for cancer research. Animal Model Exp Med 2023, 6, 381-398. [CrossRef]
- Chen, K.; Li, Y.; Wang, B.; Yan, X.; Tao, Y.; Song, W.; Xi, Z.; He, K.; Xia, Q. Patient-derived models facilitate precision medicine in liver cancer by remodeling cell-matrix interaction. Front Immunol 2023, 14, 1101324. [CrossRef]
- Chitrangi, S.; Vaity, P.; Jamdar, A.; Bhatt, S. Patient-derived organoids for precision oncology: a platform to facilitate clinical decision making. BMC Cancer 2023, 23, 689. [CrossRef]
- Singh, S.; Kumar, R.; Payra, S.; Singh, S.K. Artificial Intelligence and Machine Learning in Pharmacological Research: Bridging the Gap Between Data and Drug Discovery. Cureus 2023, 15, e44359. [CrossRef]
- Dlamini, Z.; Francies, F.Z.; Hull, R.; Marima, R. Artificial intelligence (AI) and big data in cancer and precision oncology. Comput Struct Biotechnol J 2020, 18, 2300-2311. [CrossRef]
- Subbiah, V. The next generation of evidence-based medicine. Nature Medicine 2023, 29, 49-58. [CrossRef]
- Chen, Q.; Jia, G.; Zhao, X.; Bao, Y.; Zhang, Y.; Ozkan, C.; Minev, B.; Ma, W. Novel Survivin Peptides Screened With Computer Algorithm Induce Cytotoxic T Lymphocytes With Higher Cytotoxic Efficiency to Cancer Cells. Front Mol Biosci 2020, 7, 570003. [CrossRef]
- Chen, Q.; Bao, Y.; Burner, D.; Kaushal, S.; Zhang, Y.; Mendoza, T.; Bouvet, M.; Ozkan, C.; Minev, B.; Ma, W. Tumor growth inhibition by mSTEAP peptide nanovaccine inducing augmented CD8(+) T cell immune responses. Drug Deliv Transl Res 2019, 9, 1095-1105. [CrossRef]
- Ma, W.; Chen, M.; Kaushal, S.; McElroy, M.; Zhang, Y.; Ozkan, C.; Bouvet, M.; Kruse, C.; Grotjahn, D.; Ichim, T.; et al. PLGA nanoparticle-mediated delivery of tumor antigenic peptides elicits effective immune responses. Int J Nanomedicine 2012, 7, 1475-1487. [CrossRef]
- Haimi, M. The tragic paradoxical effect of telemedicine on healthcare disparities- a time for redemption: a narrative review. BMC Med Inform Decis Mak 2023, 23, 95. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




