Submitted:
07 December 2023
Posted:
11 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental materials
Tea Residue
Tea residue filtrate
Cell Lines and Plasmids
2.2. Animal Ethics Statement
2.3. Animals and experimental design
2.4. Sample collection and measurement
2.5. Determination of nutrient digestibility
2.6. Plasma biochemical indicators
2.7. Morphological structure of the intestine
2.8. Determination of mineral fractions in feces
2.9. RNA extraction and cDNA synthesis
2.10. Real-time fluorescence quantitative PCR
2.11. Target gene cloning and plasmid recombination
2.12. Transformation, extraction and purification of recombinant plasmids
2.13. Transient transfection of cells
2.14. Electrophysiological recordings
2.15. Statistical analysis
3. Results
3.1. Effect of growth performance
3.2. Diarrhea rate
3.3. Nutrient digestibility
3.4. Plasma biochemical indexes
3.5. Effect on plasma antioxidant indexes
3.6. Effect on plasma immune indexes
3.7. Effect on the morphological structure of the intestine
3.8. Effect on fecal mineral fraction
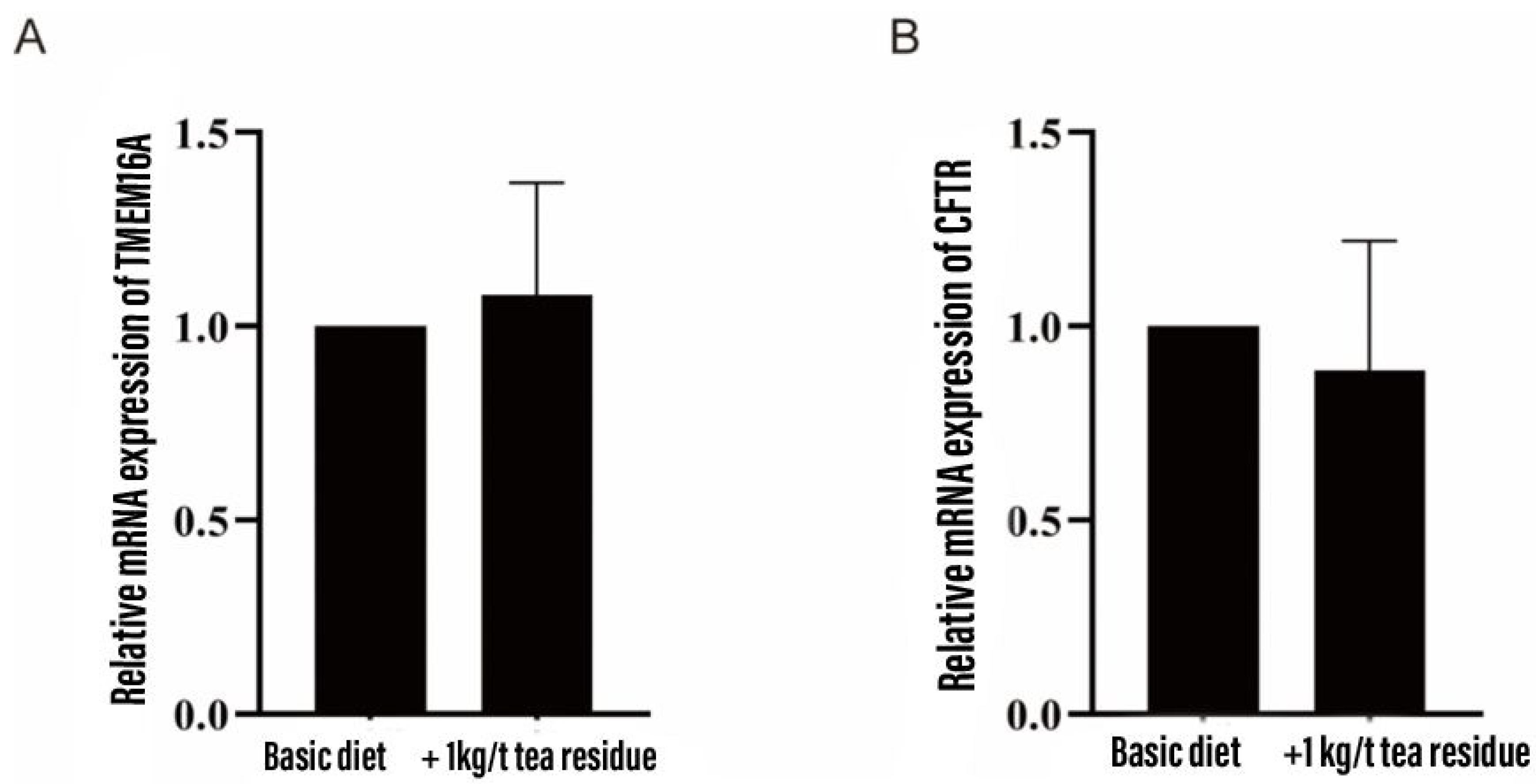
3.9. Effect on mRNA expression of colonic chloride channels
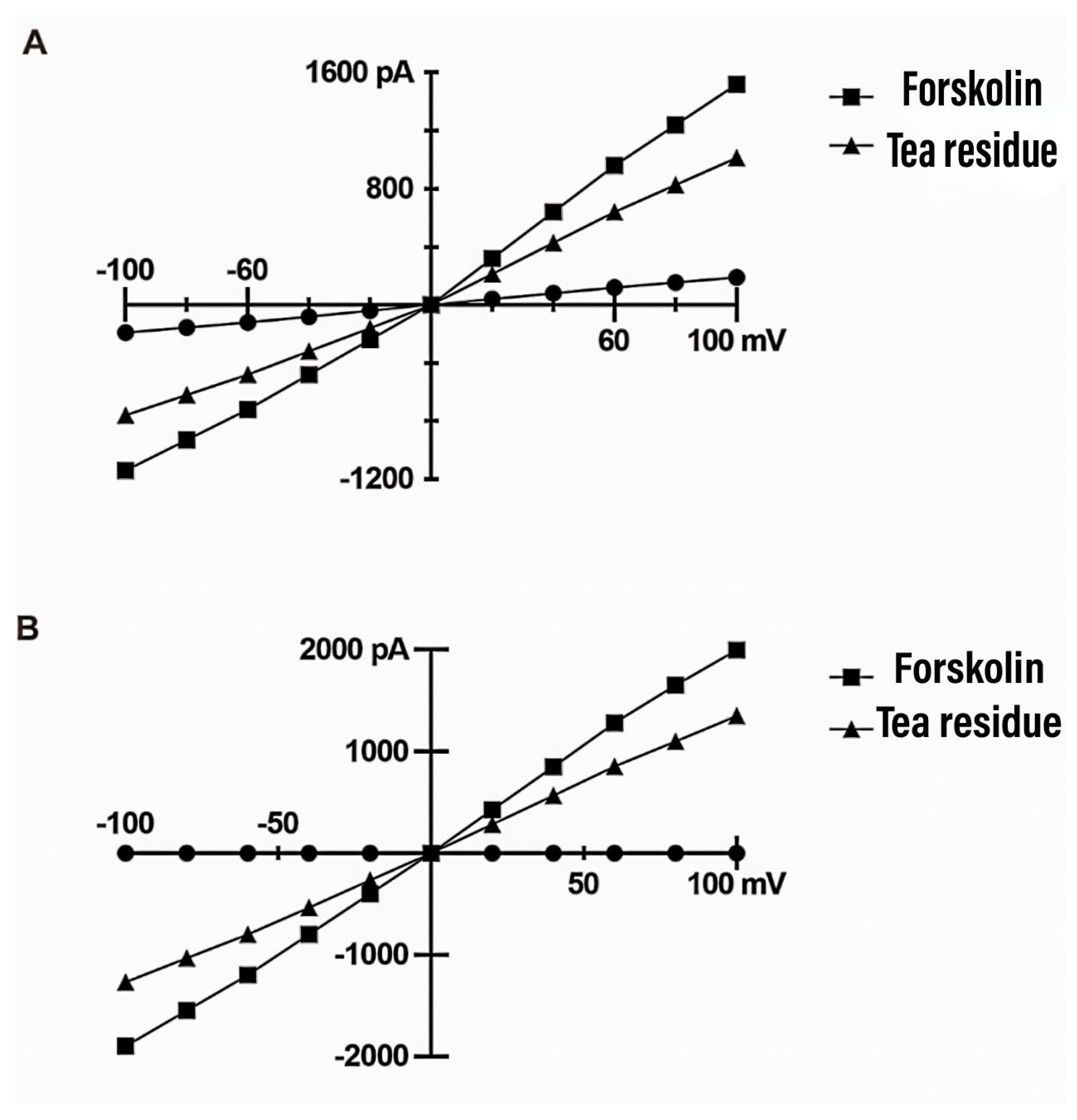
3.10. Effect on electrophysiological activity of colonic chloride channels
4. Discussion
5. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Gao, Y.; et al. Changes in gut microbial populations, intestinal morphology, expression of tight junction proteins, and cytokine production between two pig breeds after challenge with Escherichia coli K88: A comparative study. J. Anim. Sci. 2013, 91, 5614–5625. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.M.; et al. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J Anim Physiol Anim Nutr 2013, 97, 207–237. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, A.; Tugnoli, B.; Piva, A.; Grilli, E. Towards Zero Zinc Oxide: Feeding Strategies to Manage Post-Weaning Diarrhea in Piglets. Animals 2021, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; et al. Nutritional Intervention for the Intestinal Development and Health of Weaned Pigs. Front Vet Sci 2019, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Das, S., R. Jayaratne, and K.E. Barrett, The Role of Ion Transporters in the Pathophysiology of Infectious Diarrhea. Cell Mol Gastroenterol Hepatol 2018, 6, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Müllhaupt, B. [Diarrhea]. Praxis (Bern 1994) 2002, 91, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, V.; et al. Clinical Management of Infectious Diarrhea. Rev Recent Clin Trials 2020, 15, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Schiller, L.R. , Secretory diarrhea. Curr Gastroenterol Rep 1999, 1, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S. , Potential Bioactive Components and Health Promotional Benefits of Tea (Camellia sinensis). J Am Nutr Assoc 2022, 41, 65–93. [Google Scholar] [CrossRef]
- Zhang, Y.; et al. Antioxidant and functional properties of tea protein as affected by the different tea processing methods. J Food Sci Technol 2015, 52, 742–752. [Google Scholar] [CrossRef]
- Liang, J.; et al. Study on extraction of tea residue protein and antioxidant properties of enzymatic hydrolysis products. Food Science and Technolog 2020.
- Xingfei, L.; Shunshun, P.; Wenji, Z.; Lingli, S.; Qiuhua, L.; Ruohong, C.; Shili, S. Properties of ACE inhibitory peptide prepared from protein in green tea residue and evaluation of its anti-hypertensive activity. Process. Biochem. 2020, 92, 277–287. [Google Scholar] [CrossRef]
- Zhang, H., R. Qi, and Y. Mine, The impact of oolong and black tea polyphenols on human health. Food Bioscience 2019, 29, 55–61. [Google Scholar] [CrossRef]
- Yan, Z.; et al. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim Nutr 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Wan, C.; Ouyang, J.; Li, M.; Rengasamy, K.R.R.; Liu, Z. Effects of green tea polyphenol extract and epigallocatechin-3-O-gallate on diabetes mellitus and diabetic complications: Recent advances. Crit. Rev. Food Sci. Nutr. 2022, 1–29. [Google Scholar] [CrossRef]
- Hong, M.; Yu, J.; Wang, X.; Liu, Y.; Zhan, S.; Wu, Z.; Zhang, X. Tea Polyphenols as Prospective Natural Attenuators of Brain Aging. Nutrients 2022, 14, 3012. [Google Scholar] [CrossRef]
- Daneshvar, D.; et al. The effect of restricted milk feeding through conventional or step-down methods with or without forage provision in starter feed on performance of Holstein bull calves. J Anim Sci 2015, 93, 3979–3989. [Google Scholar] [CrossRef]
- Guo, W., Y. Shu, and X. Yang, Tea Dietary Fiber Improves Serum and Hepatic Lipid Profiles in Mice Fed a High Cholesterol Diet. Plant Foods Hum Nutr 2016, 71, 145–150. [Google Scholar] [CrossRef]
- Wu, Y.; et al. Traditional Chinese medicine Gegen Qinlian decoction ameliorates irinotecan chemotherapy-induced gut toxicity in mice. Biomed Pharmacother 2019, 109, 2252–2261. [Google Scholar] [CrossRef]
- Wiese, F.; et al. Green tea and green tea extract in oncological treatment: A systematic review. Int J Vitam Nutr Res 2023, 93, 72–84. [Google Scholar] [CrossRef]
- Kon, R.; et al. Green tea extract prevents CPT-11-induced diarrhea by regulating the gut microbiota. Sci Rep 2023, 13, 6537. [Google Scholar] [CrossRef]
- Mowat, A.M. and W.W. Agace, Regional specialization within the intestinal immune system. Nat Rev Immunol 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Chen, L.; et al. Effects of beta-alanine on intestinal development and immune performance of weaned piglets. Anim Nutr 2023, 12, 398–408. [Google Scholar] [CrossRef]
- Cao, S.T.; et al. Weaning disrupts intestinal antioxidant status, impairs intestinal barrier and mitochondrial function, and triggers mitophagy in piglets. J Anim Sci 2018, 96, 1073–1083. [Google Scholar] [CrossRef]
- Jing; et al. What Is the Impact of Diet on Nutritional Diarrhea Associated with Gut Microbiota in Weaning Piglets: A System Review. BioMed Res. Int. 2019, 2019, 6916189. [CrossRef]
- Su, W.; Li, Z.; Gong, T.; Wang, F.; Jin, M.; Wang, Y.; Lu, Z. An alternative ZnO with large specific surface area: Preparation, physicochemical characterization and effects on growth performance, diarrhea, zinc metabolism and gut barrier function of weaning piglets. Sci. Total. Environ. 2023, 882, 163558. [Google Scholar] [CrossRef]
- Guo, X.; et al. Seaweed polysaccharide mitigates intestinal barrier dysfunction induced by enterotoxigenic Escherichia coli through NF-kappaB pathway suppression in porcine intestinal epithelial cells. J Anim Physiol Anim Nutr 2021, 105, 1063–1074. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; et al. Understanding Kombucha Tea Fermentation: A Review. J Food Sci 2018, 83, 580–588. [Google Scholar] [CrossRef]
- Negi, T.; et al. Advances in bioconversion of spent tea leaves to value-added products. Bioresour Technol 2022, 346, 126409. [Google Scholar] [CrossRef]
- Zhuang, X.; et al. Fermentation quality of herbal tea residue and its application in fattening cattle under heat stress. BMC Vet Res 2021, 17, 348. [Google Scholar] [CrossRef]
- Ramdani, D.; Jayanegara, A.; Chaudhry, A.S. Biochemical Properties of Black and Green Teas and Their Insoluble Residues as Natural Dietary Additives to Optimize In Vitro Rumen Degradability and Fermentation but Reduce Methane in Sheep. Animals 2022, 12, 305. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Yin, Y.; Duan, Y.; Wang, W.; Zhang, L.; Guo, Q.; Chen, Q.; Li, F. Effects of Dietary Tea Powder on the Growth Performance, Carcass Traits, and Meat Quality of Tibetan Pig × Bama Miniature Pigs. Animals 2021, 11, 3225. [Google Scholar] [CrossRef]
- Jayaraman, B.; Nyachoti, C.M. Husbandry practices and gut health outcomes in weaned piglets: A review. Anim. Nutr. 2017, 3, 205–211. [Google Scholar] [CrossRef]
- Chowdhury, M.R.; et al. Effect of heat-treated green tea waste feeding on fermentation kinetics, in vitro degradability, in vivo apparent digestibility, nitrogen balance, and blood metabolites in Black Bengal goat. Anim Sci J 2022, 93, e13704. [Google Scholar] [CrossRef]
- Ran, L.; et al. Effects of Fermented Tea Residue on Growth Performance, Rumen Fermentation Parameters and Nutrient Apparent Digestibility of Mutton Sheep. Chin. J. OF Anim. Nutr. 2022, 34, 1768–1776. [Google Scholar] [CrossRef]
- Yang, H.; et al. The relative antioxidant activity and steric structure of green tea catechins—A kinetic approach. Food Chem 2018, 257, 399–405. [Google Scholar] [CrossRef]
- de Oliveira, C.C.; et al. Statistical Approaches to Assess the Association between Phenolic Compounds and the in vitro Antioxidant Activity of Camellia sinensis and Ilex paraguariensis Teas. Crit Rev Food Sci Nutr 2015, 55, 1456–1473. [Google Scholar] [CrossRef]
- Ding, X.; Li, H.; Wen, Z.; Hou, Y.; Wang, G.; Fan, J.; Qian, L. Effects of Fermented Tea Residue on Fattening Performance, Meat Quality, Digestive Performance, Serum Antioxidant Capacity, and Intestinal Morphology in Fatteners. Animals 2020, 10, 185. [Google Scholar] [CrossRef]
- Bai, Y.; et al. Effects of polysaccharides from Fuzhuan brick tea on immune function and gut microbiota of cyclophosphamide-treated mice. J Nutr Biochem 2022, 101, 108947. [Google Scholar] [CrossRef]
- Suzuki, T. , Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim Sci J 2020, 91, e13357. [Google Scholar] [CrossRef]
- Upadhaya, S.-D.; Kim, I.-H. The Impact of Weaning Stress on Gut Health and the Mechanistic Aspects of Several Feed Additives Contributing to Improved Gut Health Function in Weanling Piglets—A Review. Animals 2021, 11, 2418. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; et al. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livestock Science 2010, 134, 124–134. [Google Scholar] [CrossRef]
- Papp, R.; Nagaraj, C.; Zabini, D.; Nagy, B.M.; Lengyel, M.; Maurer, D.S.; Sharma, N.; Egemnazarov, B.; Kovacs, G.; Kwapiszewska, G.; et al. Targeting TMEM16A to reverse vasoconstriction and remodelling in idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1800965. [Google Scholar] [CrossRef] [PubMed]
- Cil, O.; et al. A small molecule inhibitor of the chloride channel TMEM16A blocks vascular smooth muscle contraction and lowers blood pressure in spontaneously hypertensive rats. Kidney Int 2021, 100, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; et al. Cell-specific mechanisms of TMEM16A Ca(2+)-activated chloride channel in cancer. Mol Cancer 2017, 16, 152. [Google Scholar] [CrossRef] [PubMed]
- Ayon, R.J.; et al. Molecular mechanism of TMEM16A regulation: Role of CaMKII and PP1/PP2A. Am J Physiol Cell Physiol 2019, 317, C1093–C1106. [Google Scholar] [CrossRef]
- Yibcharoenporn, C.; et al. Discovery of a novel chalcone derivative inhibiting CFTR chloride channel via AMPK activation and its anti-diarrheal application. J Pharmacol Sci 2019, 140, 273–283. [Google Scholar] [CrossRef]
| Projects | Content |
|---|---|
| Component, % | |
| Corn | 61.39 |
| Whey powder | 5.00 |
| Puffed soybeans | 5.00 |
| Soybean meal | 15.33 |
| Fish Meal | 3.00 |
| Plasma Protein Powder | 3.00 |
| Calcium hydrogen phosphate | 1.70 |
| Zeolite powder | 1.00 |
| Stone Powder | 1.00 |
| Active yeast | 2.28 |
| Lysine hydrochloride | 0.23 |
| DL-Methionine | 0.07 |
| Compound premixes1 | 1.00 |
| Total | 100 |
| Nutritional level | |
| Crude protein, % | 20.00 |
| Digestive energy, Mcal/kg | 3.25 |
| Lysine2, % | 1.30 |
| Methionine2 + cysteine2, % | 0.75 |
| Tryptophan2, % | 0.29 |
| Threonine2, % | 0.85 |
| Ca, % | 1.00 |
| Total phosphorus2, % | 0.75 |
| Na, % | 0.27 |
| Genes | Primers | Sequence,5'-3' | Size,bp |
|---|---|---|---|
| β-actin | Forward | AGTTGAAGGTGGTCTCGTGG | 216 |
| Reverse | TGCGGGACATCAAGGAGAAG | ||
| TMEM16A | Forward | CAAAACCCGGAGCACAATCG | 149 |
| Reverse | CGTGCTCCCCTTCGTAGTC | ||
| CFTR | Forward | CTACGCTGGTTCCAAATGCG | 244 |
| Reverse | TAGGTTGATCTCCTTCTGCCG |
| Genes | Primers | Sequence 5’-3’ |
|---|---|---|
| TMEM16A | Forward | ATTGCTAGCGCCACCATGGAGGAGACGACGCTGGA |
| Reverse | AATTATAGGATCCGCAGGGGGCCCCCGGAA | |
| CFTR | Forward | CCGCTCGAGGCCACCATGTTCTATGGAATCATATTATAT |
| Reverse | TAGACCGGTCAAGTCTTGTTTCTTGCA |
| Projects | Basic diet | +1 kg/t tea residue |
P value |
|---|---|---|---|
| BW, kg | |||
| d 1 | 6.52±0.12 | 6.56 ± 0.14 | 0.396 |
| d 7 | 6.77±0.14 | 6.75 ± 0.17 | 0.783 |
| d 28 | 10.25±0.24 | 10.21 ± 0.36 | 0.734 |
| ADG, g/d | |||
| 1~7 d | 13.82±2.89 | 13.46 ± 2.61 | 0.699 |
| 8~28 d | 173.13±6.85 | 170.79 ± 6.70 | 0.309 |
| 1~28 d | 133.13±8.57 | 130.56 ± 14.47 | 0.520 |
| ADFI, g/d | |||
| 1~7 d | 78.38±3.66 | 78.52±3.73 | 0.911 |
| 8~28 d | 420.09±11.69 | 415.85±10.26 | 0.256 |
| 1~28 d | 381.14±7.56 | 384.30±7.87 | 0.227 |
| G:F, g/g | |||
| 1~7 d | 5.91±1.24 | 6.03±1.14 | 0.765 |
| 8~28 d | 2.43±0.12 | 2.44±0.13 | 0.832 |
| 1~28 d | 2.87±0.18 | 2.98±0.35 | 0.265 |
| Projects | Basic diet | +1 kg/t tea residue |
P value |
|---|---|---|---|
| 1-7 d | 32.34±4.16 | 26.35±2.08 | <0.001 |
| 8-28 d | 14.70±1.12 | 15.55±2.19 | 0.151 |
| 1-28 d | 19.94±0.60 | 17.98±2.42 | 0.002 |
| Projects | Basic diet | +1 kg/t tea residue |
P value |
|---|---|---|---|
| Crude protein | |||
| d 7 | 37.88±3.67 | 36.90±3.41 | 0.413 |
| d 28 | 73.63±3.83 | 72.31±2.71 | 0.240 |
| Crude fat | |||
| d 7 | 57.91±2.47 | 58.62±1.80 | 0.333 |
| d 28 | 59.53±2.52 | 60.53±2.17 | 0.209 |
| Crude fiber | |||
| d 7 | 17.14±3.00 | 16.54±2.30 | 0.500 |
| d 28 | 38.06±2.51 | 36.95±3.13 | 0.248 |
| Total energy | |||
| d 7 | 59.12±2.67 | 59.61±2.46 | 0.570 |
| d 28 | 84.20±2.13 | 83.81±2.05 | 0.579 |
| Projects | Basic diet | +1 kg/t tea residue |
P value |
|---|---|---|---|
| TP, g/L | |||
| d 7 | 49.88±0.59 | 49.94±0.67 | 0.763 |
| d 28 | 45.29±2.98 | 43.96±2.57 | 0.159 |
| ALB, g/L | |||
| d 7 | 28.65±0.37 | 28.80±0.38 | 0.244 |
| d 28 | 23.69±0.36 | 23.81±0.33 | 0.304 |
| GLO, g/L | |||
| d 7 | 21.56±0.49 | 21.35±0.57 | 0.245 |
| d 28 | 21.44±0.61 | 21.60±0.68 | 0.473 |
| TC, mmol/L | |||
| d 7 | 1.59±0.03 | 1.60±0.03 | 0.229 |
| d 28 | 1.77±0.09 | 1.79±0.09 | 0.491 |
| TG, mmol/L | |||
| d 7 | 0.40±0.03 | 0.41±0.03 | 0.090 |
| d 28 | 0.38±0.02 | 0.38±0.02 | 0.568 |
| AST, U/L | |||
| d 7 | 94.47±11.46 | 98.62±12.20 | 0.301 |
| d 28 | 105.21±5.63 | 105.88±6.03 | 0.735 |
| ALT, U/L | |||
| d 7 | 45.98±2.73 | 45.31±3.13 | 0.495 |
| d 28 | 39.55±3.32 | 38.82±2.94 | 0.488 |
| ALP, U/L | |||
| d 7 | 255.65±12.06 | 258.73±12.76 | 0.461 |
| d 28 | 181.41±19.98 | 182.90±19.14 | 0.821 |
| HDL, mmol/L | |||
| d 7 | 0.64±0.02 | 0.64±0.02 | 0.665 |
| d 28 | 0.40±0.03 | 0.39±0.03 | 0.403 |
| LDL, mmol/L | |||
| d 7 | 0.66±0.01 | 0.66±0.01 | 0.815 |
| d 28 | 0.97±0.06 | 0.96±0.06 | 0.751 |
| Projects | Basic diet | +1 kg/t tea residue |
P value |
|---|---|---|---|
| CAT, U/mL | |||
| d 7 | 51.93±5.41 | 53.08±4.95 | 0.510 |
| d 28 | 50.38±7.60 | 51.62±7.54 | 0.626 |
| GSH, μmol/L | |||
| d 7 | 4.26±1.58 | 3.59±1.39 | 0.185 |
| d 28 | 2.52±0.28 | 2.73±0.19 | 0.012 |
| GSH-Px, U/mL | |||
| d 7 | 356.07±16.43 | 352.12±16.91 | 0.482 |
| d 28 | 443.83±46.94 | 444.10±52.12 | 0.987 |
| T-SOD, U/mL | |||
| d 7 | 116.80±0.13 | 116.84±0.20 | 0.505 |
| d 28 | 116.81±0.17 | 116.77±0.17 | 0.552 |
| T-AOC, U/mL | |||
| d 7 | 5.41±1.96 | 5.25±1.97 | 0.806 |
| d 28 | 4.84±0.80 | 4.48±0.73 | 0.168 |
| MDA, nmol/mL | |||
| d 7 | 46.86±3.71 | 47.38±3.10 | 0.651 |
| d 28 | 48.08±3.99 | 49.23±4.58 | 0.431 |
| Projects | Basic diet | +1 kg/t tea residue |
P value |
|---|---|---|---|
| IgA, mg/mL | |||
| d 7 | 0.75±0.05 | 0.73±0.06 | 0.252 |
| d 28 | 1.93±0.19 | 1.95±0.20 | 0.737 |
| IgG, mg/mL | |||
| d 7 | 3.95±0.21 | 3.89±0.25 | 0.394 |
| d 28 | 2.20±0.16 | 2.22±0.18 | 0.747 |
| IgM, mg/mL | |||
| d 7 | 0.58±0.06 | 0.56±0.07 | 0.317 |
| d 28 | 0.76±0.06 | 0.71±0.06 | 0.849 |
| Projects | Basic diet | +1 kg/t tea residue |
P value |
|---|---|---|---|
| jejunum | |||
| villus height, μm | 329.57±12.49 | 334.32±13.51 | 0.282 |
| crypt depth, μm | 330.09±9.86 | 334.07±14.56 | 0.345 |
| VH/CD | 1.00±0.05 | 1.00±0.06 | 0.849 |
| ileum | |||
| villus height, μm | 262.25±7.85 | 260.04±7.82 | 0.404 |
| crypt depth, μm | 285.41±6.46 | 287.06±6.31 | 0.444 |
| VH/CD | 0.92±0.04 | 0.91±0.03 | 0.247 |
| Projects | Basic diet | +1 kg/t tea residue |
P value |
|---|---|---|---|
| Ca, mg/kg | 5072.89±565.29 | 4979.61±600.21 | 0.634 |
| P, mg/kg | 4088.00±260.73 | 4111.94±207.51 | 0.762 |
| Na, mg/kg | 4056.06±220.76 | 4112.72±233.29 | 0.459 |
| Cl, mg/kg | 4051.67±255.08 | 3205.25±153.20 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





