Background
The COVID-19 vaccines are remarkable.
They were developed in record time by many countries, e.g., Russia, Great Britain, and the United States.
They were deployed around the world more rapidly than any previous vaccine.
1 However, deployment lagged and continues to lag in the underdeveloped countries.
Though they wane and require updates for the relentless march of variants, they have been astoundingly effective and safe.
They are estimated to have averted 14·4 million (95% confidence interval 13·7–15·9) deaths from COVID-19 in 185 countries and territories between Dec 8, 2020, and Dec 8, 2021.
2
There are projects underway, such as the United States’ Project NexGen, to develop better COVID-19 vaccines.
3 These vaccines would hopefully be more variant resistant and provide better protection against SARS-CoV-2 infection and COVID-19 severity. These vaccines will likely be based on the attributes described in the hundreds of next generation vaccine papers, e.g., nasal administration, adjuvant, multi-epitope, and with luck, pan-coronavirus.
The question, of course, is what could be done to advance vaccine development and deployment for Disease X, the name given by the World Health Organization in 2018 for the unknown pathogen that could cause the next pandemic.
Here are examples of deadly viruses for which vaccines are not yet available.
4
| Disease |
Transmission |
R0
|
CFR |
| Hantavirus |
Respiratory droplets and body fluids |
1·2 |
36% |
| Ebola |
Contact and body fluids |
1·8 |
25-90% |
| HIV/AIDS1
|
Body fluids |
2–5 |
90% |
| Marburg |
Contact and body fluids |
1·6 |
23-90% |
| MERS |
Respiratory droplets |
0·5 |
34% |
| Mpox |
Physical contact, body fluids, respiratory droplets |
2·1 |
1-10% |
| Nipah virus |
Body fluids |
0·5 |
45-75% |
| SARS |
Respiratory droplets |
3 |
11% |
|
Viruses on this list, and variants of them, might cause the next pandemic; however, a virus not on this list might be the culprit just like SARS-CoV-2 wasn’t on the horizon before it was reported in China.
100. Day Vaccine Development
Even with the record COVID-19 vaccine development and deployment intervals, the pandemic still inflicted major damage around the world.
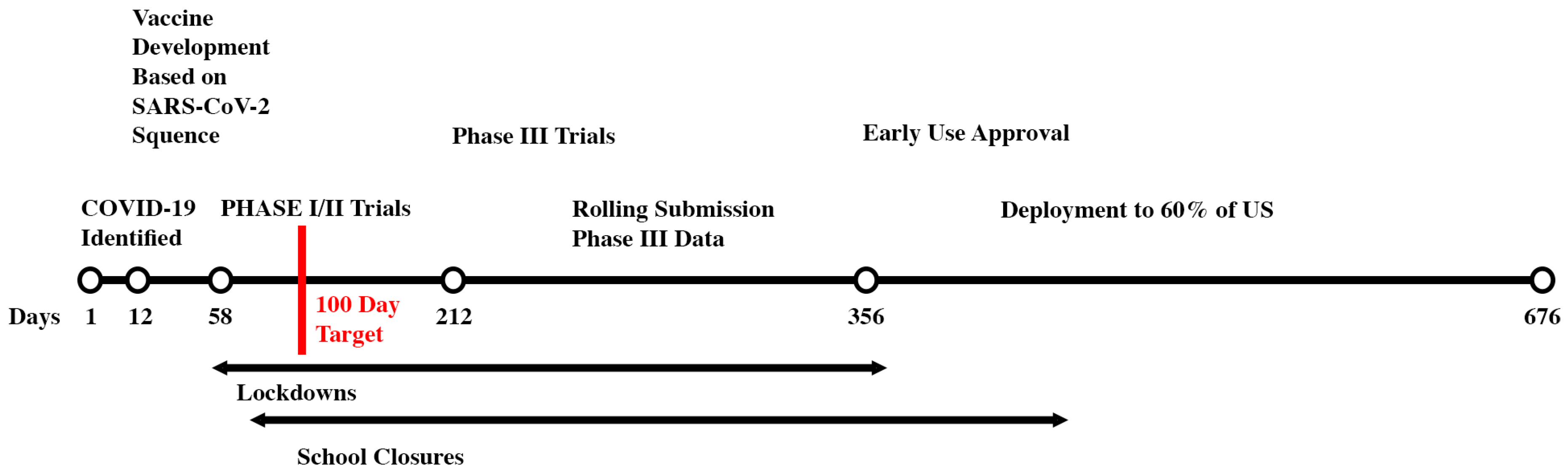
Figure 1 shows the Moderna mRNA vaccine development timeline as well as actions taken in the US to try to minimize COVID-19 cases and deaths before the COVID-19 vaccines were available. Also shown in
Figure 1 is the proposed 100 Day Vaccine Development goal.
Achieving 100 Day Vaccine Development could reduce lockdown and school closure times. This would result in dramatic reduction of stress, mental health problems, and the impacts to our children’s educational and social development. If countries implemented non-pharmaceutical interventions as they historically did, having COVID-19 vaccines in 100 days would have averted an estimated 9·88 million (95% confidence interval 7·56 – 10·58 million) deaths globally, mostly in low to middle income countries.
5
Mount Sinai’s Florian Krammera proposed an approach for 100 Day Vaccine Development in December 2020.
6 His key ingredients were to enhance surveillance for emerging viruses, proactively develop vaccines for related human viruses, and run phase I/II trials for them. He also proposed dramatically shorter phase III trials of just 60 days.
The Coalition for Epidemic Preparedness Innovations (CEPI) is intensely pursing the 100 Day Vaccine Development goal in its 100 Days Mission project. CEPI is well funded, has a global network, and an impressive track record of vaccine development and deployment.
As of October 2023, CEPI was following the 100-day strategy that Krammera proposed, which they summarized as, “CEPI will advance vaccines against known threats through proof-of-concept and safety testing in humans and will establish investigational vaccine stockpiles before epidemics begin - ‘just in case’. ”
7 They have 25 viral families of interest.
8 Examples of their “neighbour from hell” viral families are:
The paramyxovirus family which includes measles, mumps, Nipah and Hendra viruses.
The Coronavirus family which includes the common cold, MERS, SARS-CoV-2, and SARS-CoV-2 viruses.
The filovirus family which includes the Ebola and Marburg viruses.
The orthomyxovirus family which includes influenza viruses that have caused at least four pandemics since 1918.
The flavivirus family which includes Yellow Fever, Dengue, and Zika viruses.
The pneumoviridae family which includes the RSV virus.
The retroviridae family which includes the HIV virus.
There are two potentially fatal flaws in their plan. First, what if none of the vaccines are effective against the new virus? We have a clue that this could happen. In two small studies, previous SARS-CoV-1 infection offered some protection against SARS-CoV-2; however, previous MERS infection resulted in more severe SARS-CoV-2 cases in one of the studies!
9, 10 Perhaps, and it is only a perhaps, a vaccine based on MERS would have had little effect on SARS-CoV-2.
Second, as a New England Journal of Medicine
11 paper noted, it is not possible to achieve the 100-day goal within current regulatory practices. Given the heightened concerns regarding vaccine safety and the growing antivax movement, it is unreasonable to expect governments will reduce their understandable requirements for extensive phase III trials. Perhaps, “compassionate use” could be granted part way through the phase III trials for those most at risk.
Alternative Approach
There is an alternative approach for 100 Day Vaccine development that should be considered.
First, consider the fall 2023, COVID-19
booster timeline.
| Activity |
Date |
Added Days |
Total Days |
| Pick Strain, Develop and Quick Test |
6/15/23 |
1 |
1 |
| FDA Approval |
9/11/23 |
88 |
89 |
| CDC Approval |
9/12/23 |
1 |
90 |
| Shots Available |
9/13/23 |
1 |
91 |
This COVID-19 booster development interval is the same as that for the annual flu vaccine. Governments allow
updates to approved vaccines with limited testing. Perhaps the approach to 100 Day Vaccine Development should be based on boosters for phase III trialed “pan viral family vaccines.” The first step would be to find common, conserved parts of viruses in viral families, e.g., the SARS-CoV-1, SARS-CoV-2, and MERS betacoronaviruses, and develop a vaccine based on these conserved parts. This is what CEPI is pursuing in their “Broadly Protective Betacoronavirus” project. Many researchers have proposed pan-corona virus vaccines for next generation COVID vaccines.
12, 13, 14, 15
It will be difficult to pick the “right” viral families. How this would be done would probably be a combination of virus risk and the likelihood of developing a pan viral family-based vaccine. Already people are speculating on the source of the next pandemic. For example, the University of Sydney suggests it could come from one of these families:
16
Coronaviridae – SARS-CoV-2, MERS, and SARS-CoV-1
Flaviviridae - Dengue fever, Japanese encephalitis, Zika, West Nile fever
Orthomyxoviridae - Influenza
Paramyxoviridae - Nipah virus, Hendra virus
Togaviridae (alphaviruses) - Chikungunya fever, Ross River fever, Eastern equine encephalitis, Western equine encephalitis, Venezuelan equine encephalitis
If there is viral outbreak some place in the world for a virus in one of the selected families, run a phase III trial there, e.g., the “Ebola family” in Africa, “Dengue family” in Vietnam, and “Nipah family” in India. If not, early vaccine effectiveness estimates could come from neutralization studies and/or challenge experiments like those run for COVID
17, ZIKA
18 and Dengue.
19 The ZIKA challenge experiments were run in 2022 after ZIKA vaccine candidates were available but there were no ZIKA outbreaks at which to trial the vaccine. Regardless, run the phase III trials for safety. If a virus in the virus family emerges for which the trialed vaccine isn’t effective, produce a booster for the vaccine that addresses this new virus.
Given the range of protection offered by approved vaccines, we can’t speculate what the vaccine’s effectiveness will be. Nor can we speculate what the vaccine’s wanning might be. In 2021, researchers finally discovered why the vaccine for the modestly mutating measles virus doesn’t wane. It takes multiple mutations of a particular type to defeat the vaccine, and these hadn’t happened and were unlikely to happen.
20 To date, SARS-CoV-2 and flu vaccines lose their effectiveness in the face of the never ending march of variants. Unprecedented world cooperation, led by CEPI and WHO, would be required for nations to participate in the phase III trials and then, most importantly, act on their results. Some countries, e.g., the United States, would have to change their regulatory requirements for in country phase III trials.
Accelerated Deployment
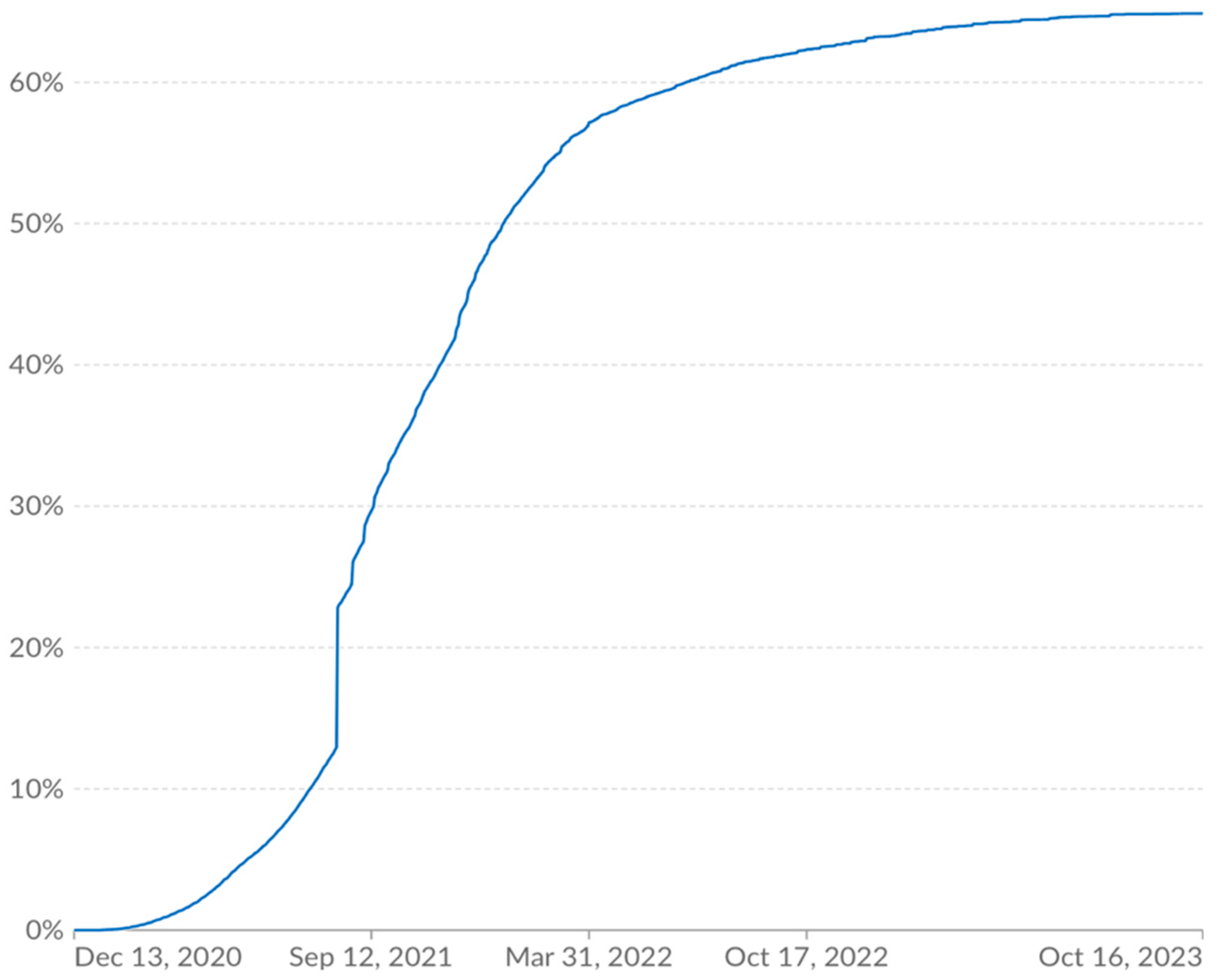
Manufacturing constraints contributed to limiting vaccine deployment to roughly 60% of the world two years after the vaccines had been developed.
21 Further, particularly in the underdeveloped countries, the difficulty of getting shots to the arms added to slowed deployment.
Figure 2 shows the vaccination rate from Our World in Data.
Much of the early delay from inadequate manufacturing capacity was overcome in 2022. By February 2022, 12 billion doses had been delivered and there was manufacturing capacity for 20 billion annual doses.
22
Fortunately, costs to vaccinate people did not and would not deter rapid deployment. A WHO report estimated it would cost
$3·70 per person vaccinated with two doses (after accounting for vaccine wastage) in the 92 low and middle income countries.
23 The IMF estimated that the marginal social value of existing COVID-19 vaccine capacity in early 2021 was
$500 to
$1,000 per course, compared with
$6 to
$40 cost per course.
24 Neither cost estimate is onerous.
To vaccinate the world will take about 15 billion doses assuming a two-dose regime. With the alternative approach to One Hundred Day Vaccine Development strategy proposed here, only one type of vaccine would likely be produced. If it were a protein subunit vaccine, then there is enough capacity to vaccinate the world in a year. If it were mRNA, then additional capacity would be needed to meet the one-year deployment goal. Sanofi is spending
$2·2 billion to beef up its mRNA program which would add to the world’s mRNA manufacturing capacity.
25 If more mRNA manufacturing capacity is needed, the cost to build mRNA vaccine manufacturing to produce 100 million doses has been estimated to be US
$127·1 million for the BNT162b2 vaccine and
$270 million for the mRNA-1273 vaccine.
26 Thus, with a bit of luck and some investment, from the time the Disease X virus emerges until the world is vaccinated could be a year! That would cut
two years off the COVID-19 timeline.
There Likely Is No Free Lunch
We can’t predict Disease X’s ongoing impact after the world is vaccinated against it. We don’t know how it will mutate, how effective the vaccines will be, and equally importantly whether our political and behavioral responses will have any pandemic memory.
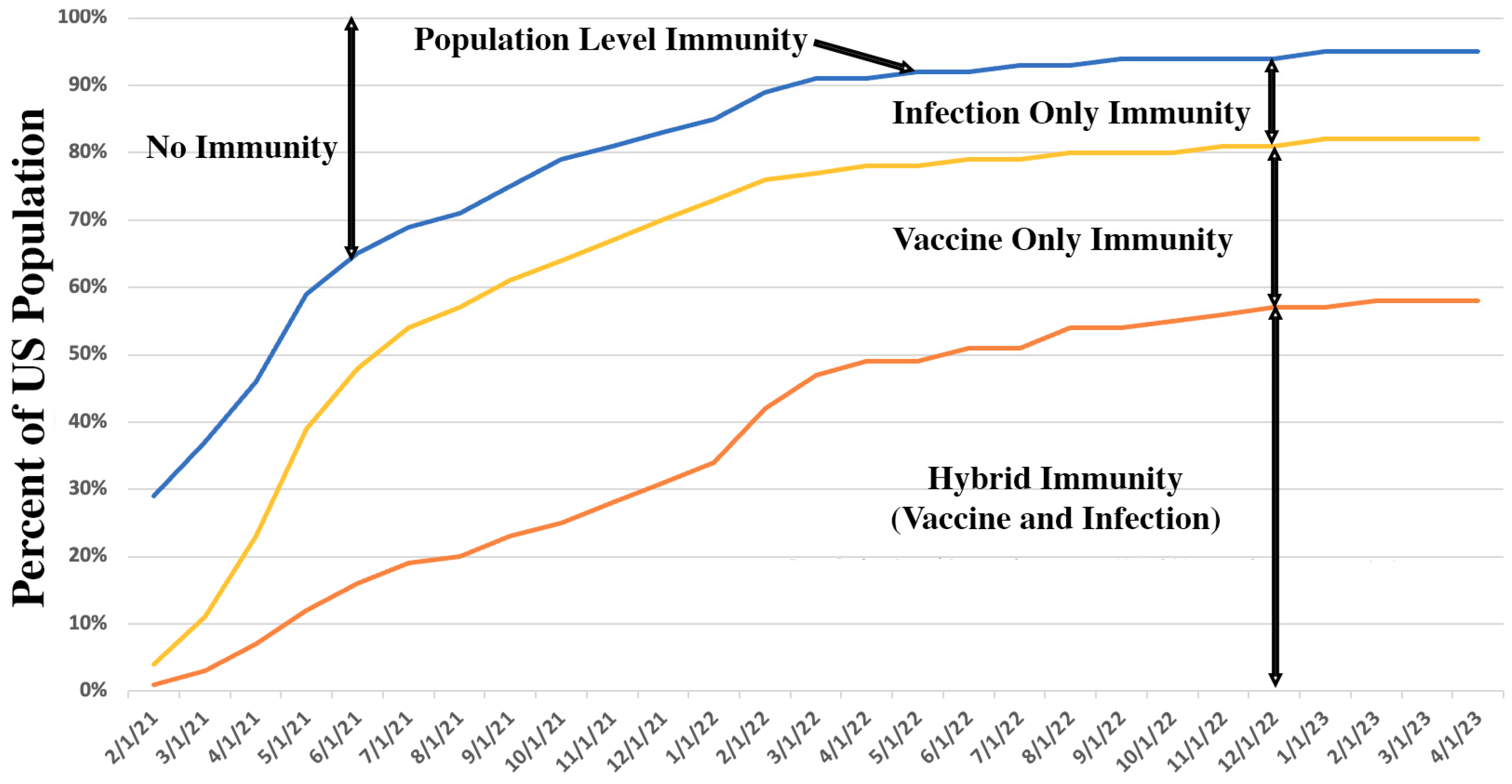
Vaccination, infection, and hybrid (vaccination and infection) immunity contribute to
population immunity which is the percent of people in a population who have some form of immunity. Emory University and Georgia Tech developed algorithms for population immunity based on infection and vaccination rates.
27 They provide options to parametrically simulate the impact of case rate underreporting. They provide population immunity estimates for the United States and each of its states. From their data, one can estimate US population immunity trends as shown in
Figure 3.
Their model’s results are consistent with a CDC study that used seroprevalence, that is, blood sampling, to determine that by the third quarter of 2022, an estimated 96·4% of persons aged ≥16 years had SARS-CoV-2 antibodies from previous infection and/or vaccination, including 22.6% from infection alone and 26·1% from vaccination alone; 47·7% had hybrid immunity.
28
Disease X’s population immunity when 70% of the US is vaccinated will likely be significantly lower than when 70% of the US was vaccinated for COVID-19 because not as many people will have been infected; thus, there will likely be less protection from infection and from hybrid immunity. One can only speculate what Disease X’s population immunity trajectory will be.
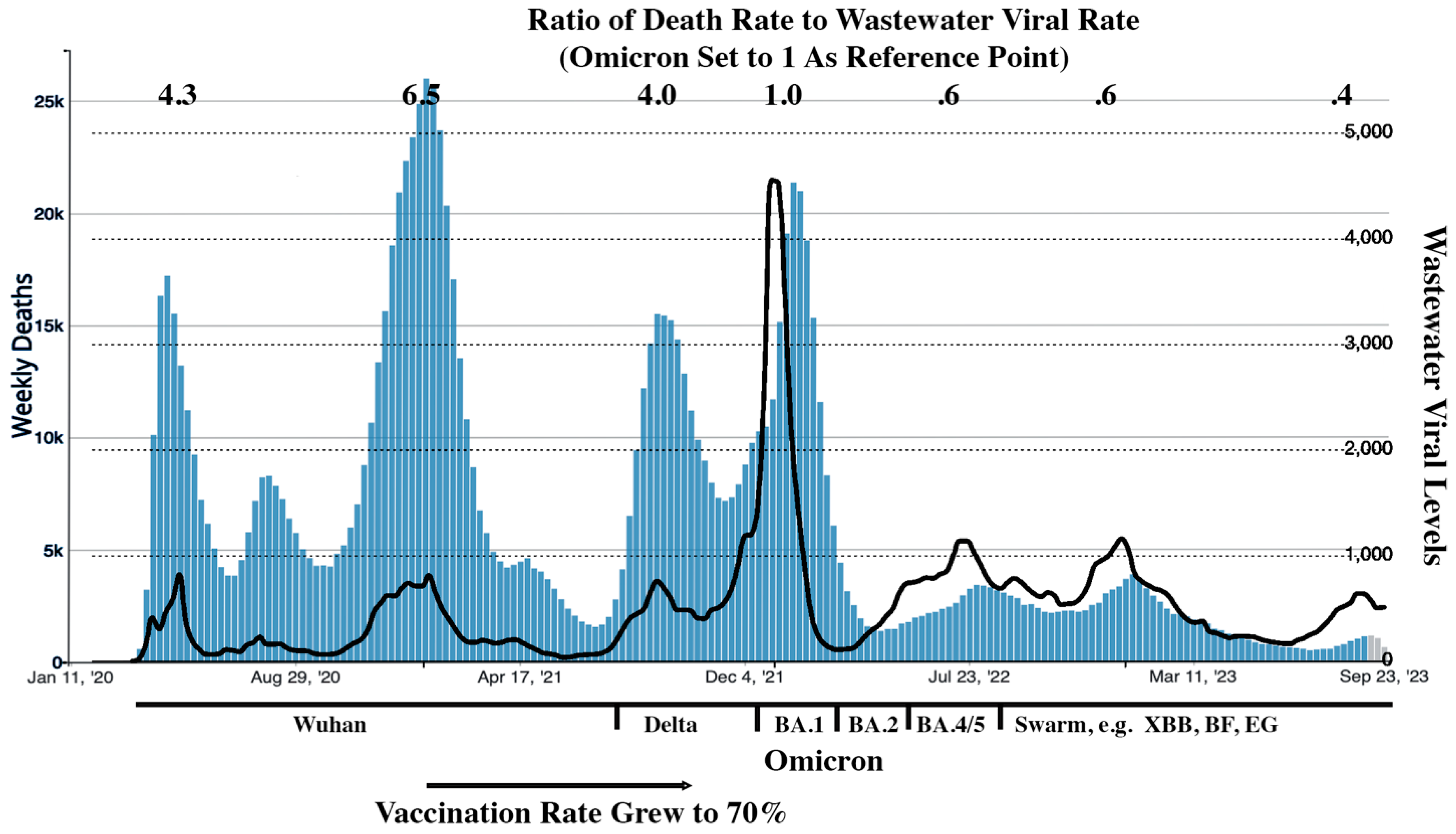
The following is perhaps the Godzilla of all back-of-the envelope estimates to get some insight into the impact to case fatality rates of growing population immunity and weaker variants. CDC statistics were used for death rates. Wastewater monitoring data was used as a surrogate for case rates.
29 The wastewater and the death rate curves were arbitrarily scaled so that their Omicron peaks matched. From this back-of-the envelope estimate, as shown in
Figure 4, we can see that US case death rates have dropped by roughly a factor of 10 since the pandemic began. Fortunately, the SARS-CoV-2 variants,
after Delta, became weaker. Viruses mutations do not always lead to weaker variants.
30
These uncertainties in the Disease X post-vaccinated world should not reduce the commitment to develop vaccines in 100 days and deploy them within a year. Rather, they should just help set realistic expectations.
Conclusion
One 100 day vaccine development and one-year deployment could save millions of lives and reduce human suffering. The present approach being taken by CEPI appears to be flawed in two ways, a surprise virus, and a shortened phase III trial. An alternative approach based on developing boosters for phase III trialed vaccines for virus families such as Filoviridae, Coronaviridae, and Bunyaviridae appears to address these problems.
References
- Amanda Glassman, Charles Kenny, and George Yang. COVID-19 Vaccine Development and Rollout in Historical Perspective. Center for Global Development, February 2023.
- Oliver J Watson, et. al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet, June 2022. [CrossRef]
- Project Next Gen is discussed at https://aspr.hhs.gov/NextGen/Pages/Default.aspx.
- Wikipedia – articles on R0 and case fatality rates.
- Andrew A. Torkleson et. al. Modelling the Potential Impact of 100 Days Mission and Broader Investments on the Covid-19 Pandemic. SSRN, September 2023.
- Florian Krammer. Pandemic Vaccines: How Are We Going to Be Better Prepared Next Time? Med, December 2020. [CrossRef]
- From CEPI Mission Statement. https://cepi.net/about/whyweexist/.
- Dr Richard Hatchett, Developing pandemic-busting vaccines in 100 days. CEPI 100 Days, November 2021.
- Anas A. Khan et. al. Potential Cross-Reactive Immunity to COVID-19 Infection in Individuals With Laboratory-Confirmed MERS-CoV Infection: A National Retrospective Cohort Study From Saudi Arabia. Frontiers in Immunology, September 2021. [CrossRef]
- Aiman El-Saed et. al. Symptomatic MERS-CoV infection reduces the risk of future COVID-19 disease; a retrospective cohort study. BMC Infectious Diseases, November 2023. [CrossRef]
- Melanie Saville, et. al. Delivering Pandemic Vaccines in 100 Days — What Will It Take? New England Journal of Medicine, July 2022. [CrossRef]
- Deborah L Burnett et. al. Immunizations with diverse sarbecovirus receptor-binding domains elicit SARS-CoV-2 neutralizing antibodies against a conserved site of vulnerability. Immunity, October 2021. [CrossRef]
- M Gordon Joyce et. al. A SARS-CoV-2 ferritin nanoparticle vaccine elicits protective immune responses in nonhuman primates. Science Translation Medicine, February 2022. [CrossRef]
- Abhigyan Choudhury et. al. Designing AbhiSCoVac – A single potential vaccine for all ‘corona culprits’: Immunoinformatics and immune simulation approaches. Journal of Molecular Liquids, January 2022. [CrossRef]
- David R. Martinez et. al. Vaccine-mediated protection against Merbecovirus and Sarbecovirus challenge in mice. Cell Reports, October 2023. [CrossRef]
- Allen Cheng et. al. Five virus families that could cause the next pandemic. The University of Sydney, September 2022.
- Ben Killingley et. al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nature, March 2022. [CrossRef]
- Cathy Shufro. How Human Challenge Trials Accelerate Vaccine Development, Hopkins Bloomberg Public Health, March 2023. [CrossRef]
- Jon Cohen. Infecting volunteers with dengue virus shows experimental drug’s promise. Science Insider, October 2023.
- Miguel Ángel Muñoz-Alía et. al. Serotypic evolution of measles virus is constrained by multiple co-dominant B cell epitopes on its surface glycoproteins. Cell Reports Medicine, March 2021. [CrossRef]
- Our World In Data.
- Megan Van Etten. New Data: COVID-19 vaccine global production capacity projected to exceed 20 billion doses this year. RhRMA, February 2022.
- Ulla Griffiths et. al. Costs of delivering COVID-19 vaccine in 92 AMC countries - Updated estimates from COVAX Working Group on delivery costs. World Health Organization, February 2021.
- Arthur Baker et. al. Accelerating Vaccinations, Internal National Monetary Fund, December 2021.
- Sudip Kar-Gupta and Manas Mishra. Sanofi clinches $2 billion vaccines deal with Translate Bio. Reuters, December 2020.
- Zoltán Kis and Zain Rizvi. How to Make Enough Vaccine for the World in One Year, Public Citizen, May 2021.
- Benjamin A. Lopman et. al. A framework for monitoring population immunity to SARS-CoV-2. Annals of Epidemiology, November 2021. Also, see https://covid19dashboardgt.shinyapps.io/us_immunitylevel/. [CrossRef]
- Jefferson M. Jones. Estimates of SARS-CoV-2 Seroprevalence and Incidence of Primary SARS-CoV-2 Infections Among Blood Donors, by COVID-19 Vaccination Status — United States, April 2021–September 2022. CDC Morbidity and Mortality Weekly Report (MMR), June 2023. [CrossRef]
-
https://biobot.io/data/ - data taken October 2023.
- Celia Perales et. al. The increasing impact of lethal mutagenesis of viruses. Future Medicinal Chemistry, August 2019. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).