1. Introduction
Potassium channels allow the passage of potassium ions through the membrane, as well as blocking the flow of other ions – particularly, sodium ions. They are composed of two parts: a part that makes the selection and allows the passage of potassium ions, and the gate, which opens and closes the channel based on environmental signals [
3,
28]. Voltage-gated potassium channels are involved in various physiological processes, from repolarization of neuronal or cardiac action potentials, upregulation of calcium signaling and cell volume, to stimulation of cell proliferation and migration [
11,
13,
26]. It also provides opportunities for the development of new drugs for various diseases and physiological processes such as scarring [
2,
3,
17]. Voltage-gated potassium channels form a large and diverse family that is evolutionarily conserved. There are 40 human voltage-gated potassium channel genes belonging to 12 subfamilies [
12,
19,
27]. These voltage-gated potassium channels show wide distributions in the nervous system and other tissues [
10,
11,
12,
13,
16]. For excitable cells such as neurons, cardiomyocytes, and muscles, voltage-gated potassium channels regulate the waveform and firing pattern of action potentials. Voltage-gated potassium channels can regulate cell volume, proliferation and migration of a wide range of cell types [
13,
18,
20,
25].
Data from published papers suggests that at the level of skin wounds, an electrical potential difference develops between the edges of the wound and the center of the wound, which favors the migration of cells in the process of their healing [
3,
9,
15]. In principle, cells migrate in an electric field because they have a certain electrical membrane potential [
2,
3,
9]. This potential is due to differences in the transmembrane electrochemical gradient. In turn, the transmembrane electrochemical gradient is due to the migration of sodium, potassium and calcium ions into the corresponding ion channels. If this is the case, the modification of the functionality of these ion channels should influence the membrane potential and, as a consequence, the wound healing process [
3,
4,
10,
15].
In the present experiment, we aimed to investigate to what extent amiodarone influences the wound healing process [
1].
Amiodarone is a substance that blocks several types of ion channels but at different concentrations: at low concentrations it blocks only potassium channels, at medium concentrations potassium and calcium channels, and at high concentrations it blocks potassium, calcium and sodium channels [
1,
7,
8,
21,
24].
We worked on rats that were given experimental skin lesions and evaluated the influence of the healing of these lesions upon the topical administration of amiodarone in 3 concentrations, respectively the concentration of 200nM which blocks only potassium channels, 2000nM which blocks both calcium channels and channels of potassium and the concentration of 200000nM which blocks potassium channels, calcium channels and sodium channels, compared to an untreated group and a group treated with benzyl alcohol, the amiodarone solvent [
6,
8,
14,
22].
2. Materials and Methods
All experiments were conducted in accordance with the protocols approved by Carol Davila University of Medicine Bucharest institutional Animal care and use Committee.
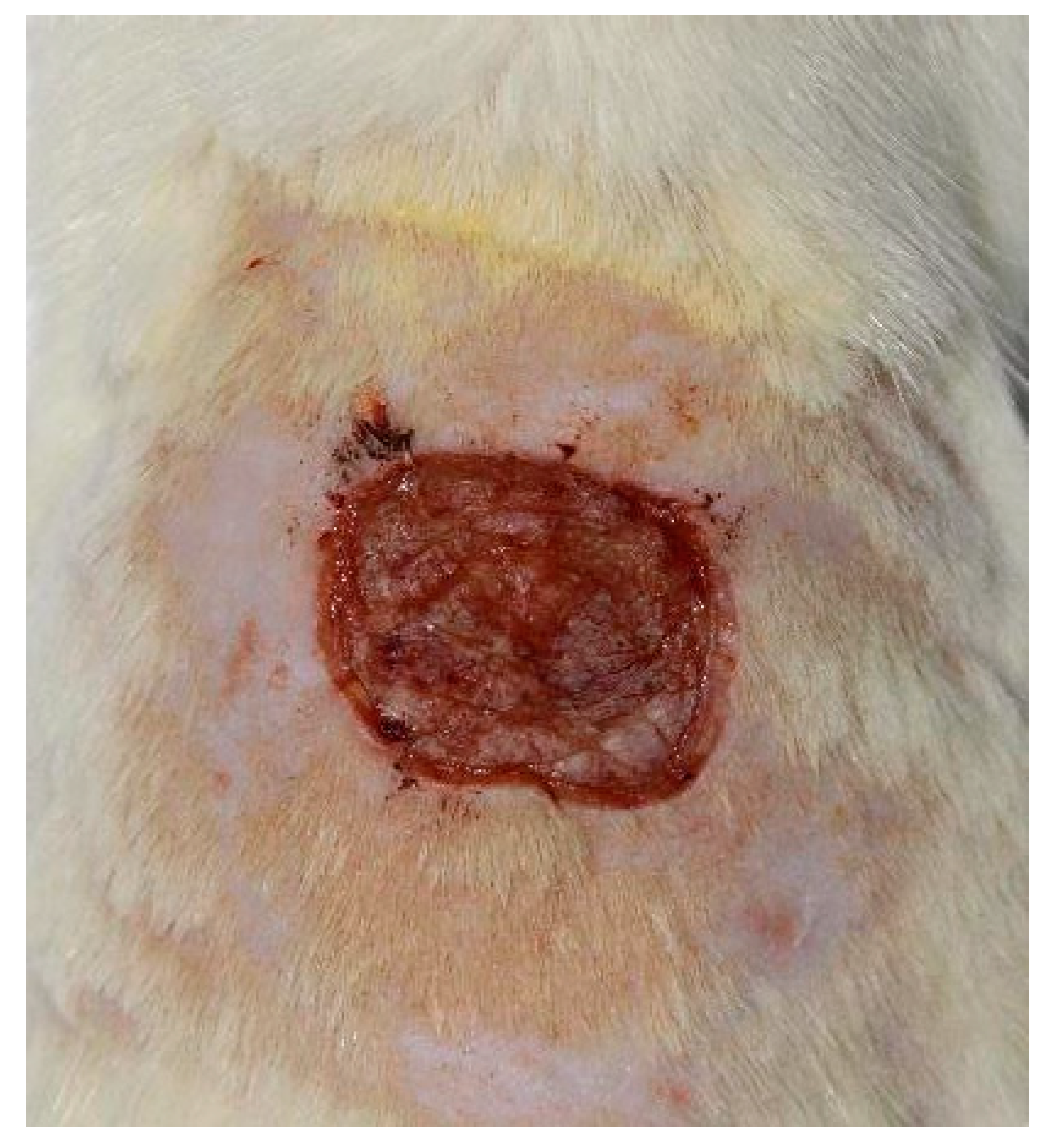
A total of 40 albino male Wistar rats were worked on. In each animal, under general anesthesia with Ketamine and Xylazine, a square lesion with a side of 1 cm was performed by skin excision after depilation (
Figure 1).
The animals were divided into 5 batches: batch number 1 was untreated, batch number 2 was treated with benzyl alcohol, amiodarone solvent, batch number 3 was treated with amiodarone at a concentration of 200 nM (nanomolar), batch number 4 a was treated with amiodarone at a concentration of 2000 nM, batch number 5 was treated with amiodarone at a concentration of 200 000 nM. Each rat was treated twice daily by topical administration of the substance corresponding to each batch until the lesions were healed.
Each lesion was photographed from the same distance and with the same degree of image magnification, every other day for the first nine days and then every three days, respectively at time t
1 - day 1 from the practice of the injury, t
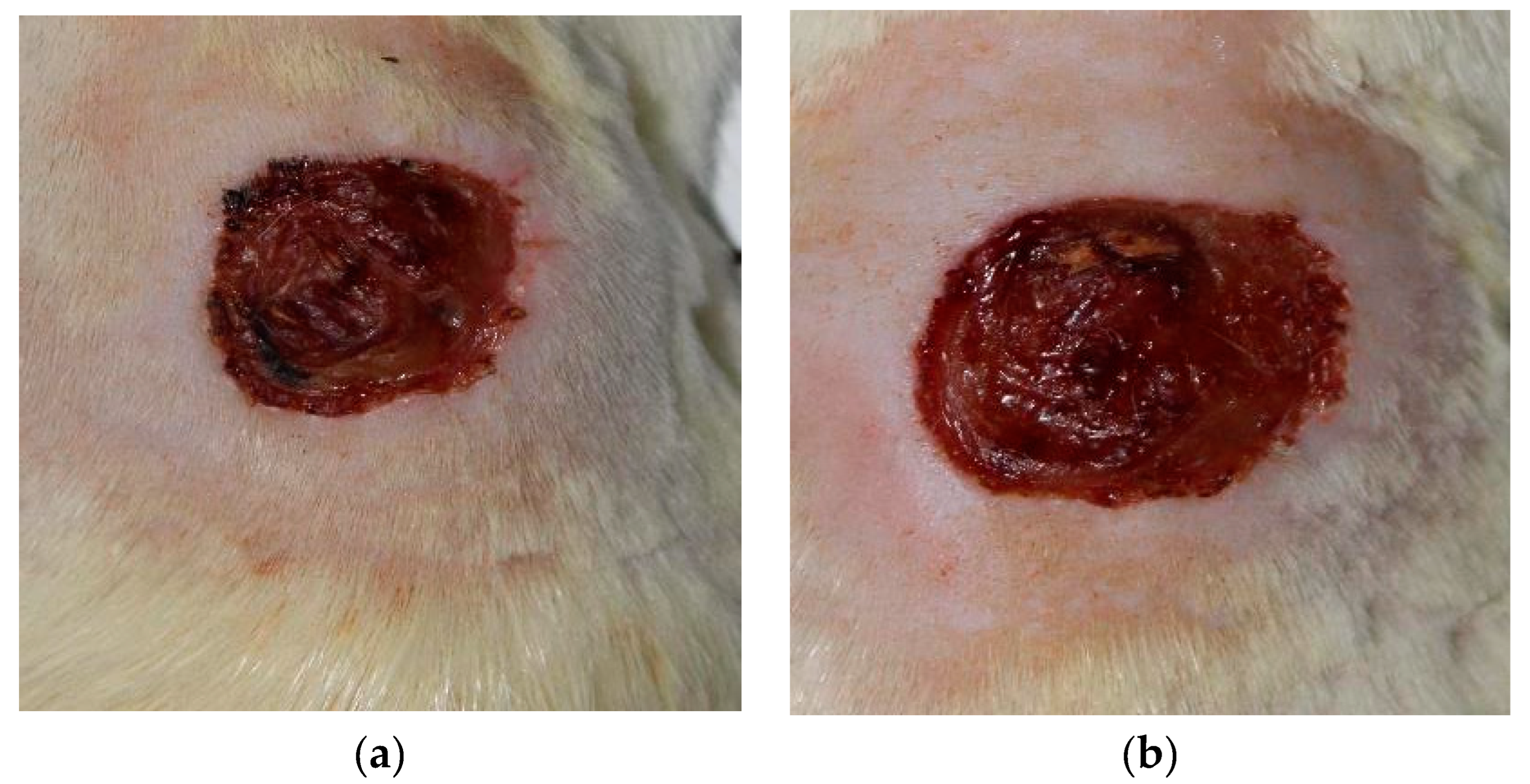
2 - day 3 (
Figure 2), t3 - day 5, t
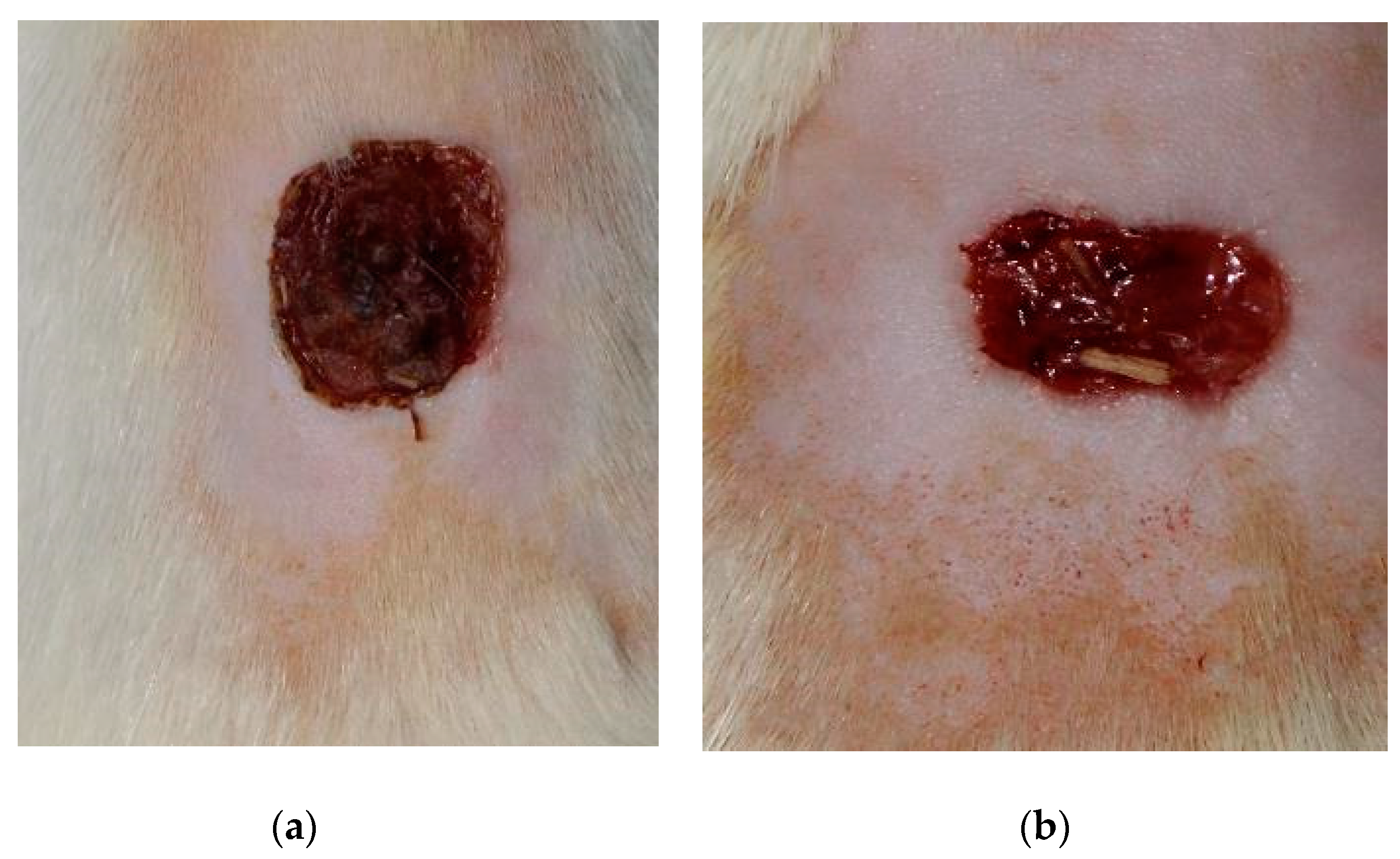
4 - day 7 (
Figure 3), t
5 - day 9, t
6 - day 12, t
7 - day 15 (
Figure 4) [
23]. Using an Image J program, the area of each lesion measured in pixels was calculated at each time of the recording.
The main parameter analyzed was the mean duration of wound healing in each group.
In addition to this, secondary parameters were also analyzed, namely the percentage decrease of the lesional surfaces and the average speed percentage per day of the lesional surfaces.
The following parameters were calculated for each rat and time of measurement:
- (a)
The main parameter - the duration of wound healing measured in days
- (b)
Secondary parameters
where S is the percentage decrease in area, S
t1 is the initial area measured in pixels, and S
t is the area at the time of measurement in pixels.
- 2.
The percentage speed decrease of the lesion surface according to the formula
where V represents the percentage decrease rate per day of the surface, S
t represents the surface of the lesion at time t, measured in pixels, S
t+1 represents the surface of the lesion at time t+1 measured in pixels, and t represents the time of surface measurement expressed in days from the beginning of the experiment.
For each batch, the averages and standard deviations of the 3 parameters were calculated at each moment of the measurement, the statistical significance was investigated by the T-Student test. It was considered that the differences between the groups for each moment of the measurement are statistically significant if p <0.05 for the main parameter and for p<0.02 for secondary parameters because the Bonferroni method was applied in order not to produce an alpha risk inflation.
3. Results
3.1. Main parameter: Average duration of wound healing
Average duration of wound healing
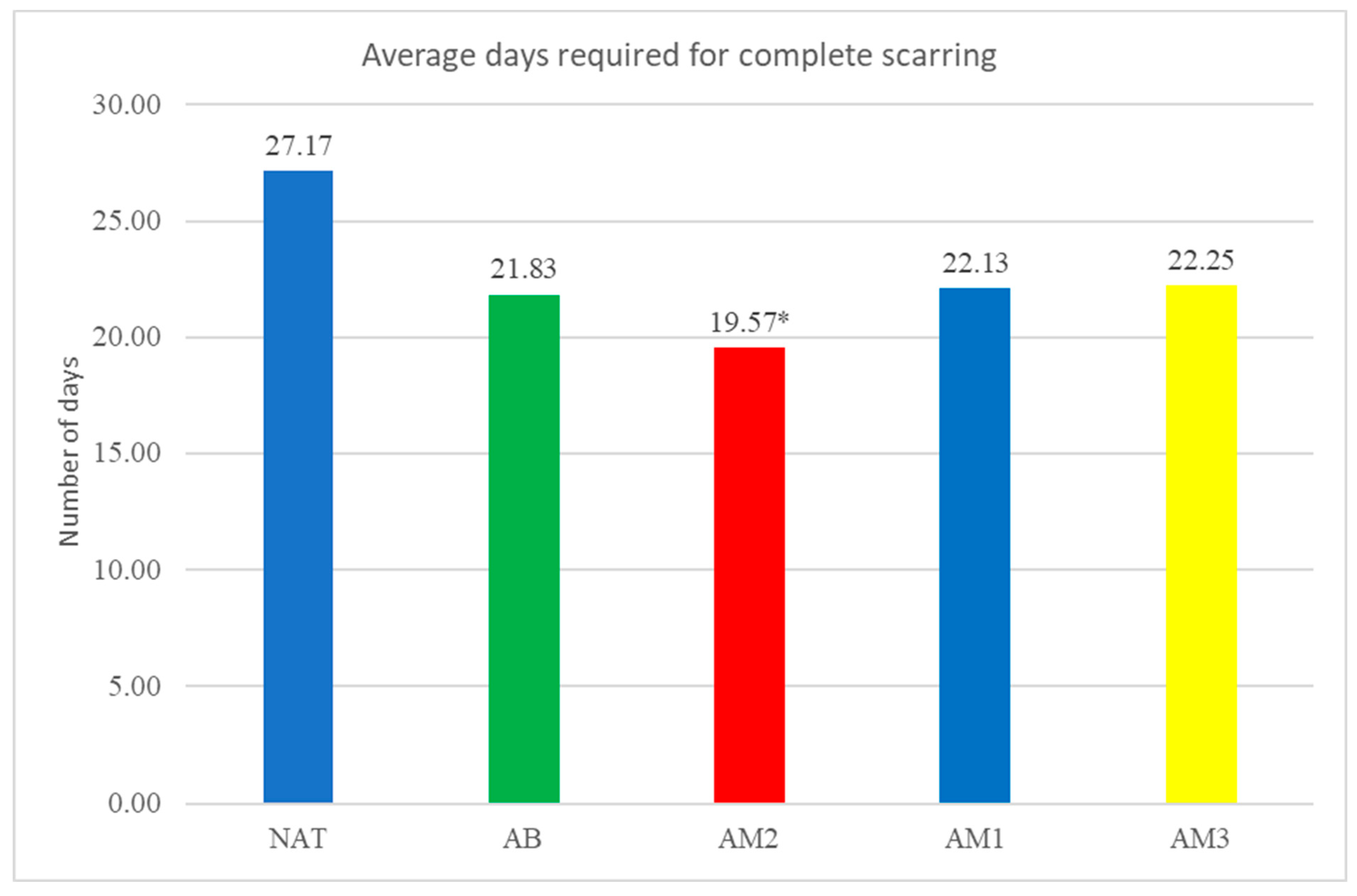
The average duration of wound healing was: in the untreated group 27.17±5.19 days, in the group treated with benzyl alcohol 21.83±2.71 days, in the group treated with low concentration amiodarone 19.57±3.05 days , in the group treated with amiodarone in medium concentration 22.13±5.17 days and in the group treated with amiodarone in high concentration 22.25±4.53 days. There was only one statistically significant difference compared to the natural batch, namely for the group treated with low concentration amiodarone (p<0.03).
The results are presented in
Table 1 and Graph 1.
Graph 1.
The time required for complete scarring. Each column represents the average number of days required healing of the wounds. *=p=0,03.
Graph 1.
The time required for complete scarring. Each column represents the average number of days required healing of the wounds. *=p=0,03.
3.2. Secondary parameters
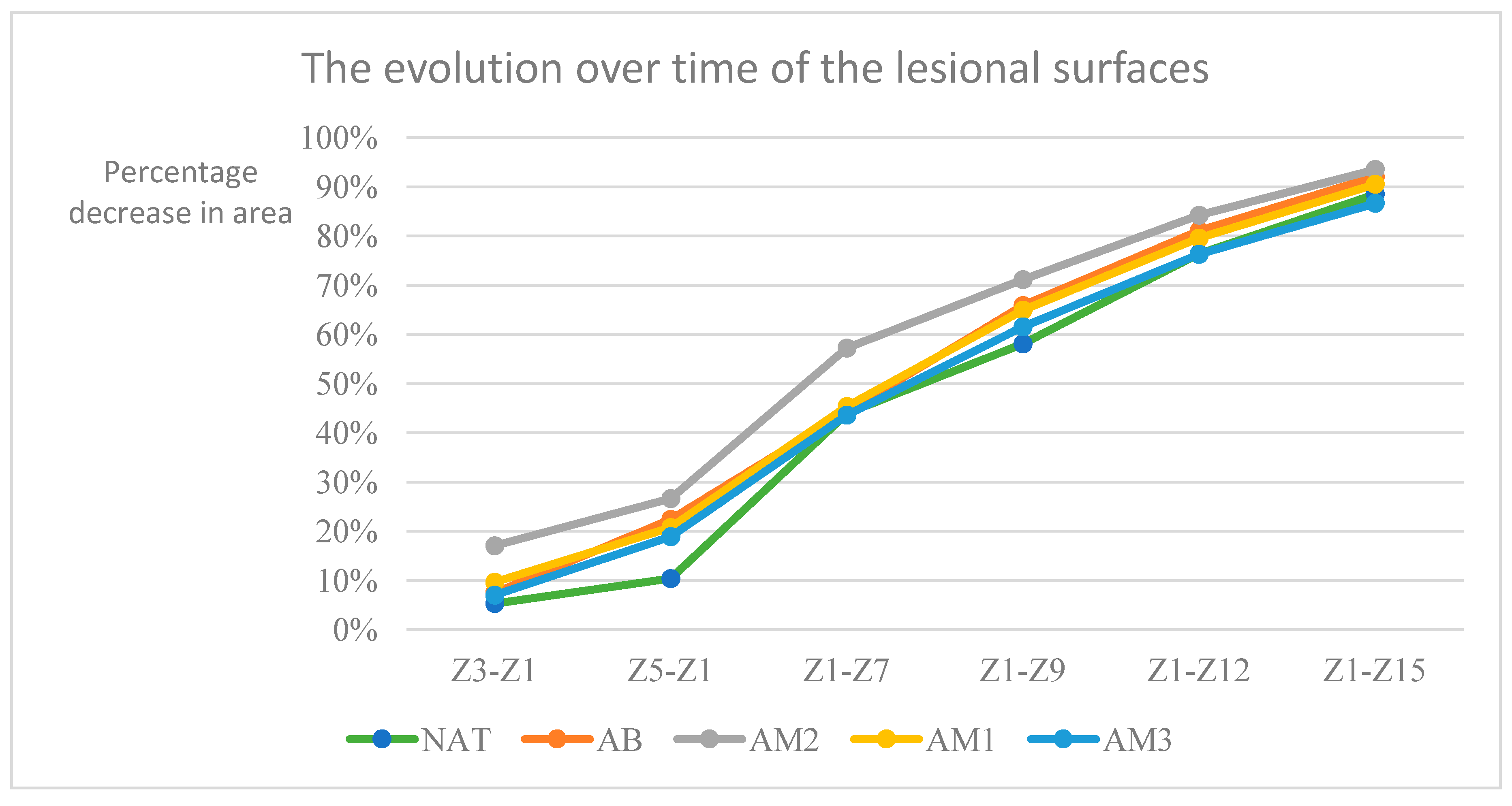
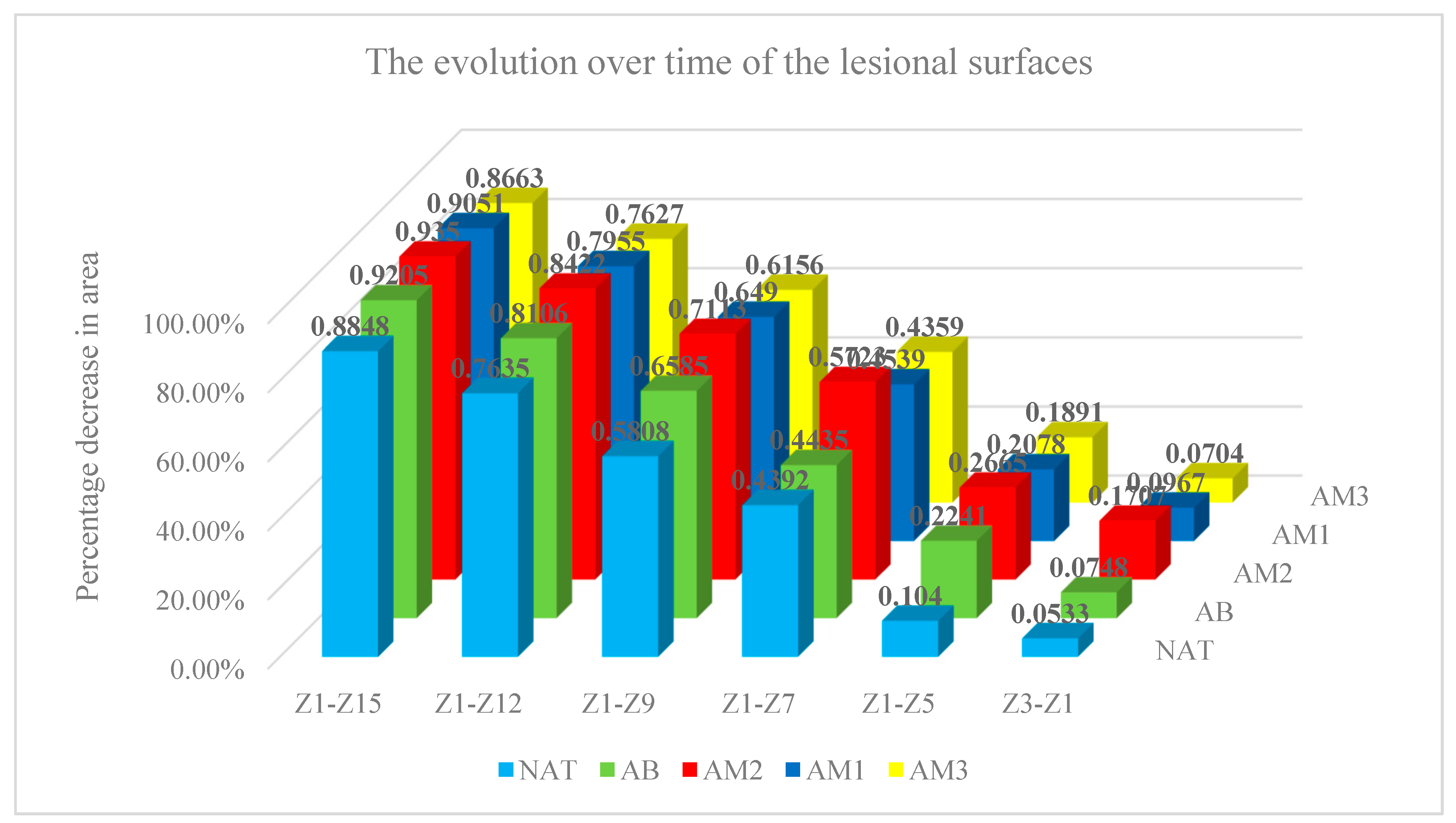
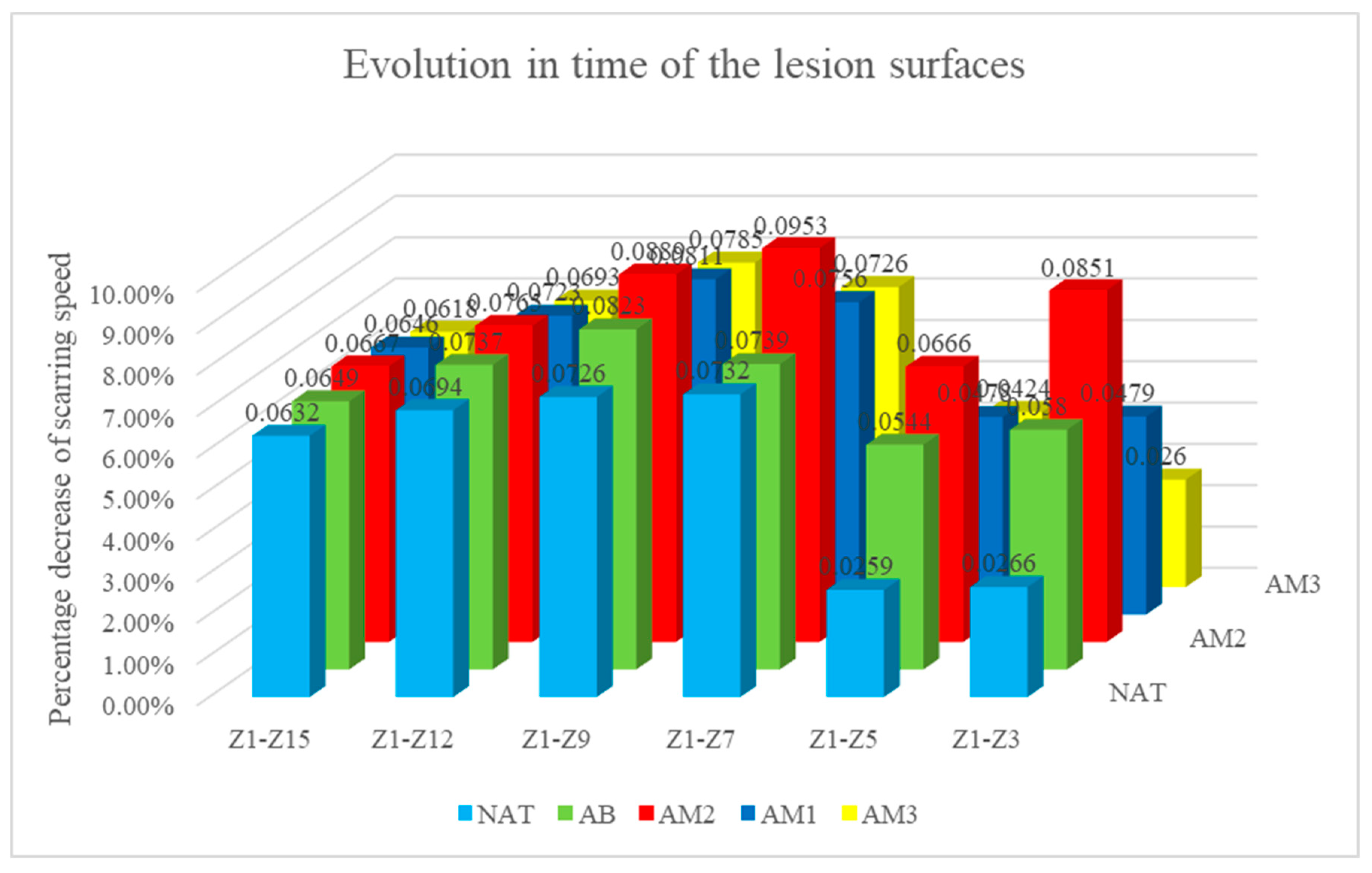
The percentage decrease of the lesion surfaces compared to the initial surface recorded the following values:
- -
in the untreated batch at time t1 -5.33% ±3.63, t2– 10.40%±8.09, t3– 43.92%±15.81, t4-58.08%±14.20, t5- 76,36%±8,20, t6- 88,48%±5,92
- -
in the batch treated with benzyl alcohol at time t1 -7.49% ±11,09, t2 – 22,41%±13,00, t3 – 44,35%±18,38, t4-65,85%±14,13, t5- 81,06%±4,34, t6- 92,05%±4,33
- -
in the batch treated with amiodarone in a concentration of 200 nM at time t1 -17.03% ±7,38, t2 – 26,65%±6,20, t3 – 57,23%±11,82, t4-71,13%±7,75, t5- 84,22%±4,18, t6- 93,50%±4,46
- -
in the batch treated with amiodarone in a concentration of 2000 nM at time t1 -9.68% ±7,57, t2 – 20,78%±10,74, t3–45,39%±11,48, t4-64,90%±14,69, t5- 79,55%±6,09, t6- 90,51%±5,83
- -
in the batch treated with amiodarone in a concentration of 200,000 nM at time t1 -7.05% ±6,98, t2 – 18,91%±6,96, t3 – 43,59%±5,59, t4-61,56%±10,35, t5- 76,27%±9=6,97, t6- 86,63%±7,23
Statistical analysis showed that:
At time t2 compared to t1: the greatest decrease in surface area was in the batch treated with low concentration amiodarone, the difference compared to the untreated batch being statistically significant for a p<0.001
At time t3 compared to t1: the biggest decrease was in the batch treated with low dose amiodarone, the difference compared to the untreated batch being statistically significant for p<0.0003, but also the group treated with high concentration amiodarone recorded the difference of the untreated batch statistically significant although lower for p<0.02.
At time t4 compared to t1, amiodarone in low concentration also registered the greatest decrease in surfaces, but the differences compared to the untreated batch are not statistically significant.
At times t5 and t6 compared to t1, the only statistically significant difference from the untreated batch was recorded for the low dose of amiodarone (p<0.02 at time t5, respectively p<0.01 at time t6).
The results are presented in
Table 2 and Graphs 2 and 3.
Graph 2.
Evolution over time of the lesional surfaces. The percentage difference between the initial surface and the surface at the time of the measurement relative to the initial surface is represented on the vertical axis. The days in which the measurements were taken counted from the start of the experiment are represented on the horizontal axis.
Graph 2.
Evolution over time of the lesional surfaces. The percentage difference between the initial surface and the surface at the time of the measurement relative to the initial surface is represented on the vertical axis. The days in which the measurements were taken counted from the start of the experiment are represented on the horizontal axis.
Graph 3.
The evolution over time of the lesional surfaces. Each column represents the difference between the initial surface and the surface at the time of measurement relative to the initial surface, all measured in pixels.
Graph 3.
The evolution over time of the lesional surfaces. Each column represents the difference between the initial surface and the surface at the time of measurement relative to the initial surface, all measured in pixels.
- 2.
The rate of daily percentage decrease of the lesion surface
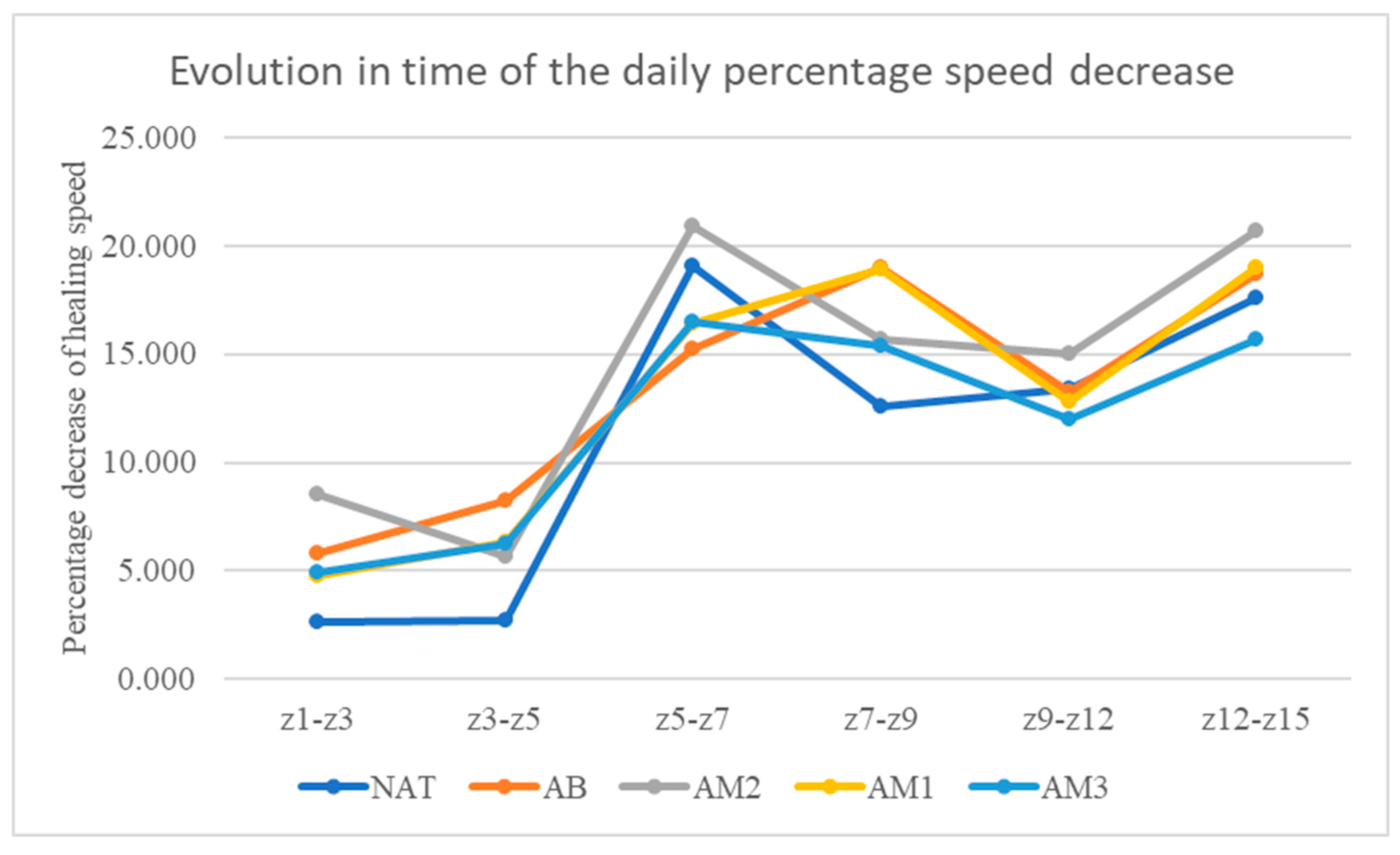
At time t2, the velocities were: in the untreated batch 2.66±1.81/day, in the batch treated with benzyl alcohol 5.80%±3.71/day, in the batch treated with low concentration amiodarone 8.51%± 3.69/day, in the batch treated with amiodarone in medium concentration 4.79%±3.97/day, and in the batch treated with amiodarone in high concentration 4.96%±3.31/day.
At time t3, the velocities were: in the untreated batch 2.72±3.16/day, in the batch treated with benzyl alcohol 8.23%±2.52/day, in the batch treated with low concentration amiodarone 5.70%± 2.89/day, in the batch treated with amiodarone in medium concentration 6.32%±3.08/day, and in the batch treated with amiodarone in high concentration 6.24%±3.93/day.
At time t4, the velocities were: in the untreated batch 19.06±6.84/day, in the batch treated with benzyl alcohol 15.25%±5.76/day, in the batch treated with low concentration amiodarone 20.94%± 7.11/day, in the batch treated with amiodarone in medium concentration 16.42%±3.66/day, and in the batch treated with amiodarone in high concentration 16.49%±4.33/day.
At time t5, the velocities were: in the untreated batch 12.61±5.83/day, in the batch treated with benzyl alcohol 18.99%±8.98/day, in the batch treated with low concentration amiodarone 15.68%± 5.87/day, in the batch treated with amiodarone in medium concentration 18.91%±8.31/day, and in the batch treated with amiodarone in high concentration 15.37%±11.41/day.
At time t6, the velocities were: in the untreated batch 13.41±6.02/day, in the batch treated with benzyl alcohol 13.22%±6.12/day, in the batch treated with low concentration amiodarone 15.04%± 2.52/day, in the batch treated with amiodarone in medium concentration 12.82%±4.18/day, and in the batch treated with amiodarone in high concentration 11.98%±6.10/day.
At time t7, the velocities were: in the untreated batch 17.60±4.88/day, in the batch treated with benzyl alcohol 18.69%±7.10/day, in the batch treated with low concentration amiodarone 20.73%± 6.74/day, in the batch treated with amiodarone in medium concentration 18.98%±7.46/day, and in the batch treated with amiodarone in high concentration 15.72%±5.30/day.
There were statistically significant differences compared to the untreated batch at time t
2 only in the batch treated with low concentration amiodarone (p< 0.001), and at time t
2 the statistically significant difference compared to the untreated batch was for the average dose of amiodarone (p< 0.02). The results are presented in
Table 3 and Graphs 4 and 5.
Graph 4.
Time evolution of the daily percentage speed decrease. On the vertical axis the percentage speed decrease between 2 consecutive measurements is represented. On the horizontal axis the time interval between the 2 consecutive measurements is represented.
Graph 4.
Time evolution of the daily percentage speed decrease. On the vertical axis the percentage speed decrease between 2 consecutive measurements is represented. On the horizontal axis the time interval between the 2 consecutive measurements is represented.
Graph 5.
Time evolution of the daily percentage speed decrease. Each column represents the average of the percentage speed decrease between 2 consecutive measurements, related to the time interval.
Graph 5.
Time evolution of the daily percentage speed decrease. Each column represents the average of the percentage speed decrease between 2 consecutive measurements, related to the time interval.
The results are presented in
Table 3. Each value represents the average of the percentage speed decrease between 2 consecutive measurements, related to the time interval.
4. Discussion
As it can be seen from the evaluation of the duration of wound healing, only low-dose amiodarone showed a statistically significant decrease compared to the untreated batch, i.e. 19.57 days for low-dose amiodarone compared to 27.17 days for the untreated batch.
In principle, low-dose amiodarone decreased the time required for wound healing by approximately 8 days, representing 27.97% of the time required for wound healing in the untreated batch.
Considering the working hypothesis, we can assume that blocking of potassium channels accelerates wound healing under our experimental conditions, represented by the decrease in the time required for wound healing. This effect was maintained throughout the entire period of wound healing.
This implies that blocking potassium channels favors wound healing, considering that the medium dose, which in addition to potassium channels also blocks calcium channels, and the high dose, which in addition to potassium channels also blocks calcium and sodium channels, had no statistically significant effect. Furthermore, taking into consideration that medium and high doses of amiodarone had no effect on wound healing, we can presume that both blocking of calcium channels and of sodium channels antagonize the effect of blocking potassium channels.
The hypothesis seems logical because the potassium current is a repolarizing current while the calcium and sodium currents are depolarizing currents. It is very likely that blocking the repolarizing potassium current increased the membrane potential and thereby accelerated cell migration in the electric field. Under these conditions, blocking of depolarizing currents decreased the membrane potential raised by potassium channel blockade and thus antagonized the favorable effect of potassium channel blockade. Indeed, this seems to have been the case in our experiment, because the effect of amiodarone in high concentrations on wound healing was less intense than the effect of amiodarone in medium concentrations.
The secondary parameters used, respectively the percentage decrease of the lesional surfaces compared to the initial surface and the daily percentage speed decrease of the lesional surfaces tried to evaluate by which mechanisms the blocking of the ion channels influenced wound healing.
Amiodarone in high concentration reduced progressively, from one determination to another, the differences between the decrease in lesional surfaces compared to the control group, but only at time t3 these differences were statistically significant.
Regarding the daily rate of decrease in lesional area, it generally increased during the first 3 days, after which it remained relatively constant. The highest speed of daily decrease in surface area was found in the batch treated with amiodarone in low concentration, the differences being statistically significant compared to the untreated batch at time t2.
Both the batches treated with medium and high concentration of amiodarone recorded higher rates of daily percentage decrease of the lesion surfaces compared to the untreated batch, but only the average dose of amiodarone was statistically significant at time t3.
These results show that the shortening of wound healing time is produced by an acceleration of the rate of decrease of the lesional surfaces.
The fact that the differences were statistically significant only at certain times of the measurement, but not at all times, suggests that different mechanisms are involved during healing from one stage to another, and that it is likely that blocking potassium channels promotes wound healing only at certain stages of the healing process, depending on the mechanism involved in the respective stages. The fact that in the analysis of these secondary parameters and amiodarone in medium concentration had statistically significant effects at certain times of the measurement, but amiodarone in high concentration never had statistically significant effects, suggests that blocking calcium channels only partially antagonizes the effect of blocking potassium channels. The effect of blocking potassium channels appears to be completely antagonized only by simultaneously blocking both calcium and sodium channels.
From a statistical point of view, there is of course the possibility that the small number of lesions per group of animals did not confer enough statistical power to detect differences between the batches treated with different doses of amiodarone.
5. Conclusions
Low concentration amiodarone promoted wound healing under our experimental conditions, both in terms of duration of healing and speed of healing.
Blocking potassium channels promotes wound healing.
Neither medium-dose amiodarone nor high-dose amiodarone had statistically significant effects on wound healing time.
The blocking of calcium channels and blocking of sodium channels antagonize the effects of the blocking of potassium channels on wound healing.
Given that a potassium current is a depolarizing current, while calcium and sodium currents are repolarizing, it turns out that blocking the potassium current increases the membrane potential, this increase being antagonized by blocking calcium and sodium currents.
It is possible that the increase in membrane potential produced by the blocking of potassium channels accelerated the migration of cells into the wound field, which explained the acceleration of healing.
Author Contributions
D.G.A. made the experimental design, conducted the experimental protocol, and analyzed the obtained specimens and data. A.V.B made the experimental design and conducted the statistical and data analysis. S.S, P.E., M.E and A.Z. helped with the experimental protocol and animal housing. O.A.C. analyzed the final version of the manuscript. I.F. analyzed the manuscript and supervised the experiment and analyzed the results. All authors drafted the work or revised it critically for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania (13310/27 May 2021) for studies involving animals, in conformity with 43/2014 Law regarding animal protection used in scientific purposes, with further completions and 86/609/CEE Directive from 24 November 1986 regarding acts with power of law and administrative acts of member states for animal protection used in experimental purposes and other scientific purposes.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yihong Zhang, Charlotte K. Colenso, Aziza El Harchi, Hongwei Cheng, Harry J. Witchel, Chris E. Dempsey, Jules C. Hancox, Interactions between amiodarone and the hERG potassium channel pore determined with mutagenesis and in silico docking, Biochemical Pharmacology 113 (2016) 24–35.
- Carlos Gonz´alez,1 David Baez-Nieto,Ignacio Valencia, Ingrid Oyarz´un, Patricio Rojas,David Naranjo, and Ram´on Latorre, K+ Channels: Function-Structural Overview, Compr Physiol 2:2087-2149, 2012.
- Zhang W, Das P, Kelangi S, Bei M. Potassium channels as potential drug targets for limb wound repair and regeneration. Precis Clin Med. 2020;3(1):22-33. [CrossRef]
- Wengeng Zhang M.D., Ph.D., Marianna Bei D.M.D., Ph.D, Kcnh2 and Kcnj8 interactively regulate skin wound healinkg and regeneration, Original Research-Basic Science 2015.
- Luis A. Pardo, Voltage-Gated Potassium Channels in Cell Proliferation, Physiology 19: 285–292, 2004; 10.1152/physiol.00011.2004.
- Emna El Golli-Bennour, Amel Bouslimi, Olfa Zouaoui, Safa Nouira, Abdellatif Achour, Hassen Bacha, Cytotoxicity effects of amiodarone on cultured cells, Experimental and Toxicologic Pathology 64 (2012) 425– 430.
- Jules C. Hancox, Amiodarone Blocks L-Type Calcium Current in Single Myocytes Isolated from the Rabbit Atrioventricular Node, Gen. Pharmac. Vol. 29, No. 3, pp. 429-435, 1997.
- Atsushi Nishida, Taichi Takizawa, Akio Matsumoto1, Takashi Miki, Susumu Seino,and Haruaki Nakaya, Inhibition of ATP-Sensitive K+ Channels and L-Type Ca2+ Channels by Amiodarone Elicits Contradictory Effect on Insulin Secretion in MIN6 Cells, J Pharmacol Sci 116, 73 – 80 (2011).
- Rolf F. Hertel, Potassium channel activation improves blood flow pattern of conscious rats in cutaneous microcirculation, Clinical and Experimental Pharmacology and Physiology (1992) 19, 243-248.
- Lynn McKeown, Lisa Swanton, Philip Robinson & Dr Owen T. Jones, Surface expression and distribution of voltage gated potassium channels in neurons (Review), ISSN: 0968-7688 (Print) 1464-5203 (Online) Journal homepage: https://www.tandfonline.com/loi/imbc20.
- Zhichao Yue , Jia Xie , Albert S Yu , Jonathan Stock , Jianyang Du , Lixia Yue, Role of TRP channels in the cardiovascular system, Yue Z, Xie J, Yu AS, Stock J, Du J, Yue L. Role of TRP channels in the cardiovascular system. Am J Physiol Heart Circ Physiol. 2015;308(3):H157-H182. [CrossRef]
- Heike Wulff, Neil A. Castle & Luis A. Pardo, Voltage-gated potassium channels as therapeutic targets, Nat Rev Drug Discov 8, 982–1001 (2009). https://doi.org/10.1038/nrd2983.
- Richard Nuccitelli, Pamela Nuccitelli, Samdeo Ramlatchan, Richard Sanger, Peter J S Smith, Imaging the electric field associated with mouse and human skin wounds, Wound Repair Regen. 2008 May-Jun;16(3):432-41. [CrossRef]
- Karami MY, Mansournia N, Bagherian N, et al. Effects of Azelnidipine-Carboxymethylcellulose Gel on Healing of Full-Thickness Skin Wounds in Streptozotocin Induced Diabetic Rats. Vet Med (Auckl). 2019;10:215-222. Published 2019 Dec 17. [CrossRef]
- Moulin VJ, Dubé J, Rochette-Drouin O, Lévesque P, Gauvin R, Roberge CJ, Auger FA, Goulet D, Bourdages M, Plante M, Germain L. Electric Potential Across Epidermis and Its Role During Wound Healing Can Be Studied by Using an In Vitro Reconstructed Human Skin. Adv Wound Care (New Rochelle). 2012 Apr;1(2):81-87. [CrossRef] [PubMed] [PubMed Central]
- Zhang W, Das P, Kelangi S, Bei M. Potassium channels as potential drug targets for limb wound repair and regeneration. Precis Clin Med. 2020 Mar;3(1):22-33. Epub 2019 Dec 30. [CrossRef] [PubMed] [PubMed Central]
- Lai IK, Valdearcos M, Morioka K, Saxena S, Feng X, Li R, Uchida Y, Lijun A, Li W, Pan J, Koliwad S, Marcucio R, Wulff H, Maze M. Blocking Kv1.3 potassium channels prevents postoperative neuroinflammation and cognitive decline without impairing wound healing in mice. Br J Anaesth. 2020 Sep;125(3):298-307. Epub 2020 Jul 2. [CrossRef] [PubMed] [PubMed Central]
- Kang D, Choi TH, Han K, Son D, Kim JH, Kim SH, Park J. Regulation of K(+) channels may enhance wound healing in the skin. Med Hypotheses. 2008 Dec;71(6):927-9. Epub 2008 Aug 29. [CrossRef] [PubMed]
- Kelkar S, Nailwal N, Bhatia NY, Doshi G, Sathaye S, Godad AP. An Update On Proficiency of Voltage-gated Ion Channel Blockers in the Treatment of Inflammation-associated Diseases. Curr Drug Targets. 2022;23(14):1290-1303. [CrossRef] [PubMed]
- Erdem Kış E, Tiftik RN, Al Hennawi K, Ün İ. The role of potassium channels in the proliferation and migration of endometrial adenocarcinoma HEC1-A cells. Mol Biol Rep. 2022 Aug;49(8):7447-7454. Epub 2022 May 13. [CrossRef] [PubMed]
- Lubic SP, Nguyen KP, Dave B, Giacomini JC. Antiarrhythmic agent amiodarone possesses calcium channel blocker properties. J Cardiovasc Pharmacol. 1994 Nov;24(5):707-14. [CrossRef] [PubMed]
- Watanabe Y, Kimura J. Inhibitory effect of amiodarone on Na(+)/Ca(2+) exchange current in guinea-pig cardiac myocytes. Br J Pharmacol. 2000 Sep;131(1):80-4. [CrossRef] [PubMed] [PubMed Central]
- Waite A, Gilliver SC, Masterson GR, Hardman MJ, Ashcroft GS.- Clinically relevant doses of lidocaine and bupivacaine do not impair cutaneous wound healing in mice. Br J Anaesth. 2010 Jun;104(6):768-73. Epub 2010 Apr 24. [CrossRef] [PubMed] [PubMed Central]
- Waldhauser KM, Brecht K, Hebeisen S, Ha HR, Konrad D, Bur D, Krähenbühl S. Interaction with the hERG channel and cytotoxicity of amiodarone and amiodarone analogues. Br J Pharmacol. 2008 Oct;155(4):585-95. Epub 2008 Jul 7. [CrossRef] [PubMed] [PubMed Central]
- Varró A, Biliczki P, Iost N, Virág L, Hála O, Kovács P, Mátyus P, Papp JG. Theoretical possibilities for the development of novel antiarrhythmic drugs. Curr Med Chem. 2004 Jan;11(1):1-11. [CrossRef] [PubMed]
- Wang SP, Wang JA, Luo RH, Cui WY, Wang H. Potassium channel currents in rat mesenchymal stem cells and their possible roles in cell proliferation. Clin Exp Pharmacol Physiol. 2008 Sep;35(9):1077-84. [CrossRef] [PubMed]
- Crociani O, Guasti L, Balzi M, Becchetti A, Wanke E, Olivotto M, Wymore RS, Arcangeli A. Cell cycle-dependent expression of HERG1 and HERG1B isoforms in tumor cells. J Biol Chem. 2003 Jan 31;278(5):2947-55. Epub 2002 Nov 12. [CrossRef] [PubMed]
- Soejima H, Kawamoto S, Akai J, Miyoshi O, Arai Y, Morohka T, Matsuo S, Niikawa N, Kimura A, Okubo K, Mukai T. Isolation of novel heart-specific genes using the BodyMap database. Genomics. 2001 May 15;74(1):115-20. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).