Submitted:
05 December 2023
Posted:
06 December 2023
You are already at the latest version
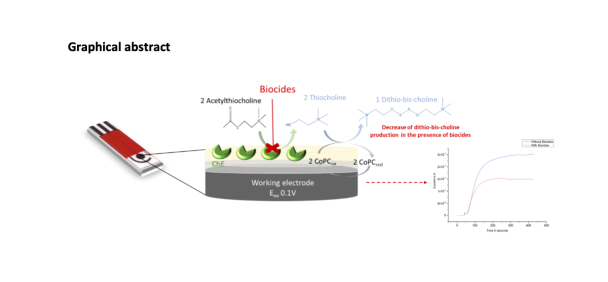
Abstract
Keywords:
1. Introduction
2. Materials and Methods
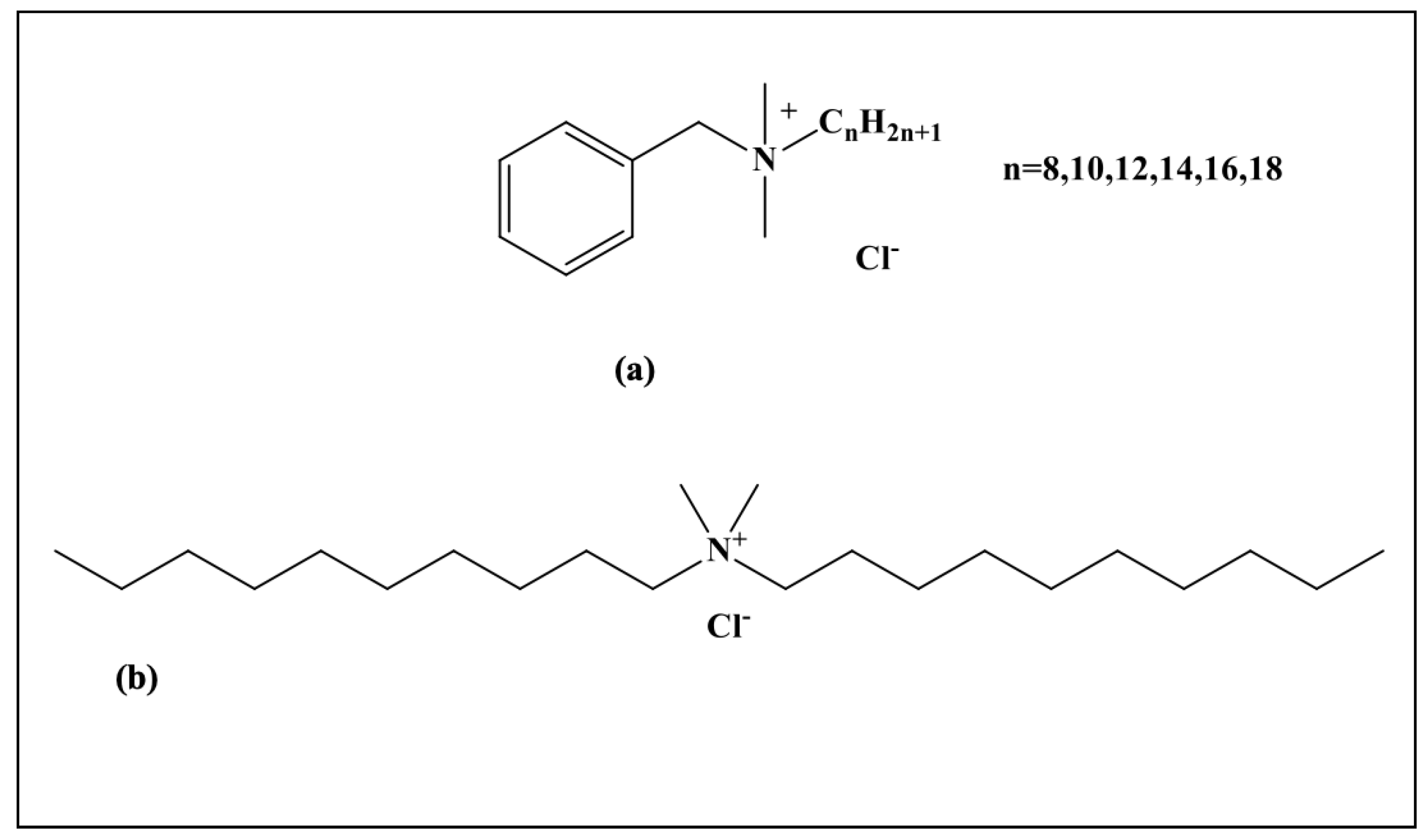
2.1. Materials
2.2. Methods
2.2.1. Determination of cholinesterases activity by colorimetry
2.2.2. Biosensor implementation
2.2.3. Sample pretreatment by solid-phase extraction
3. Results
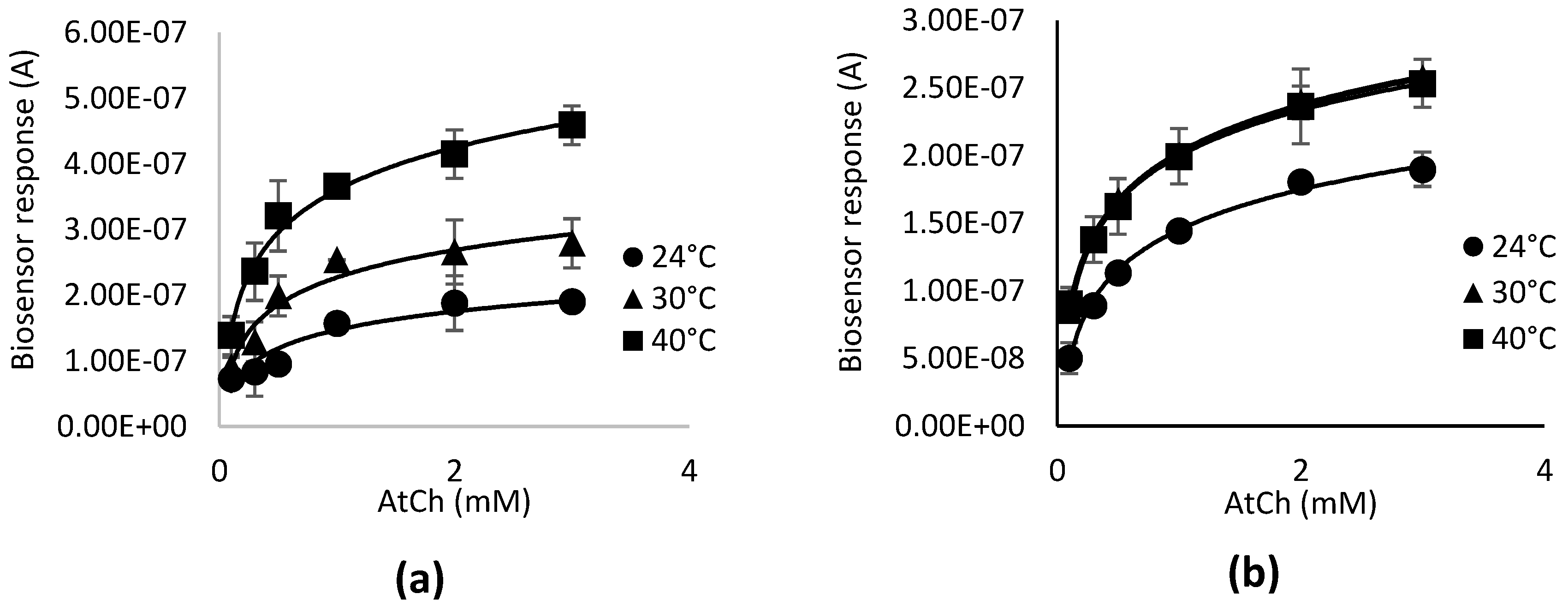
3.1. Optimization of Operating Temperature
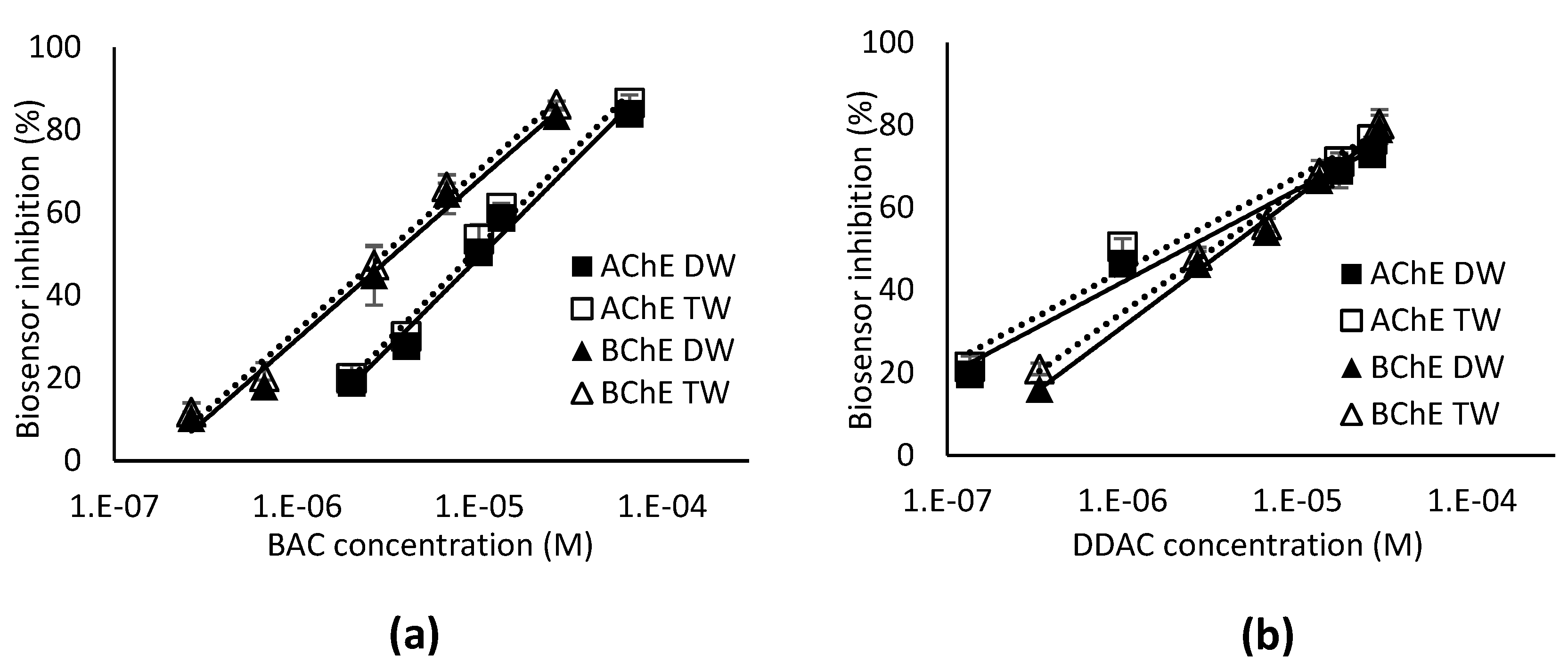
3.2. Biosensor detection of BAC and DDAC
3.2.1. Calibration curves
3.2.2. Sample pre-concentration and biosensor analysis
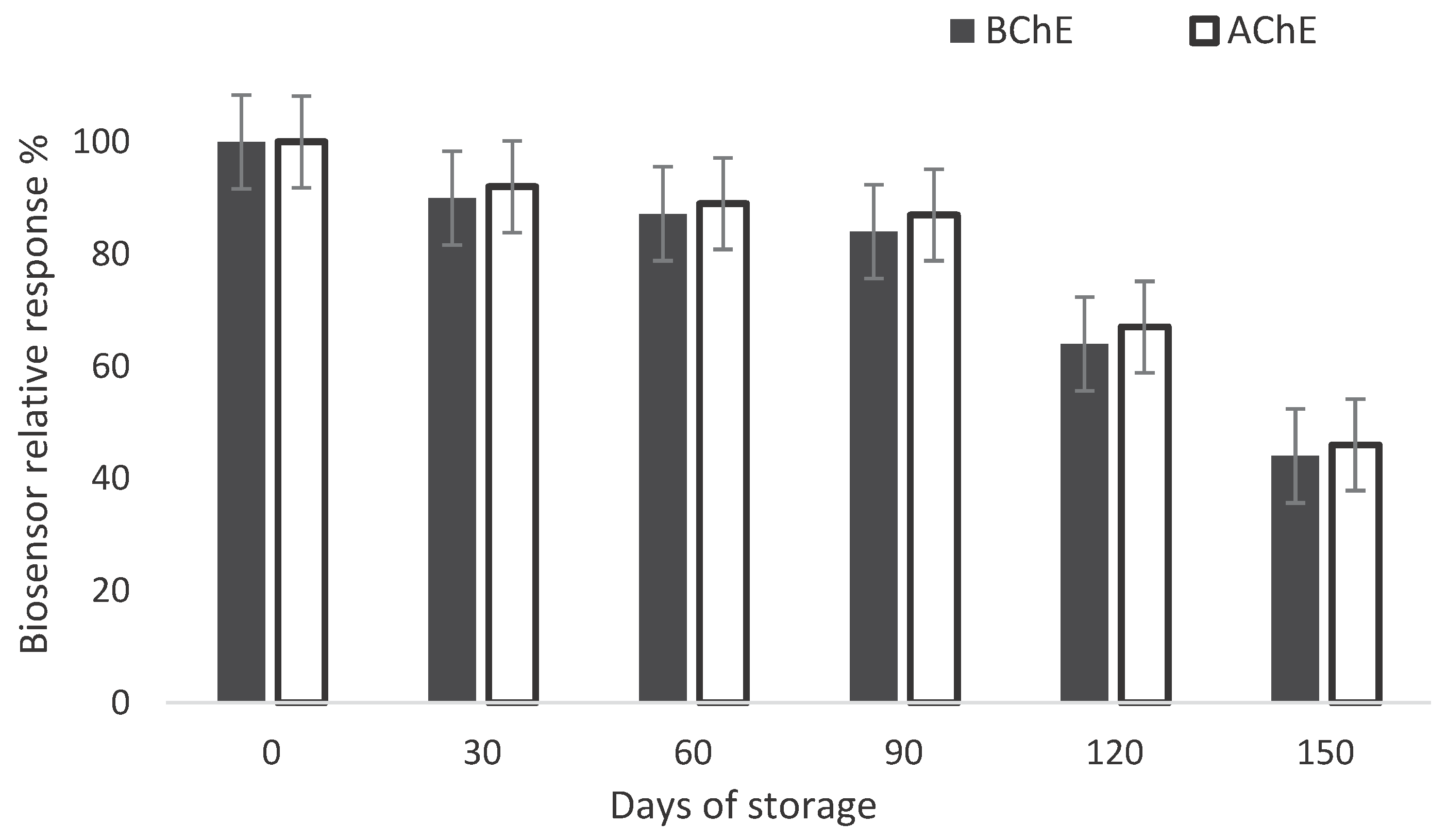
3.3. Biosensor Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jones, I.A.; Joshi, L.T. Biocide Use in the Antimicrobial Era: A Review. Molecules 2021, 26, 2276. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. Introduction of biocides into clinical practice and the impact on antibiotic-resistant bacteria. J. Appl. Microbiol. 2002, 92. [Google Scholar] [CrossRef]
- Rutala, W.; Weber, D. Infection control: the role of disinfection and sterilization. J. Hosp. Infect. 1999, 43, S43–S55. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.-Y. Antimicrobial biocides in the healthcare environment: efficacy, usage, policies, and perceived problems. Ther. Clin. Risk Manag. 2005, 1, 307–320. [Google Scholar] [PubMed]
- Chauret, C.P. Sanitization. In Encyclopedia of Food Microbiology (Second Edition); Academic Press: Oxford, UK, 2014; pp. 360–364. [Google Scholar]
- Choi, Y.-H.; Kang, M.-S.; Huh, D.-A.; Chae, W.-R.; Moon, K.W. Priority Setting for Management of Hazardous Biocides in Korea Using Chemical Ranking and Scoring Method. Int. J. Environ. Res. Public. Health 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- EU Reference Laboratory for Pesticides. Analysis of BACs and DDAC in milk using QuEChERS method and LC-MS/MS. Available online: www.eurl-pesticides.eu (Accessed on 7 June 2023).
- EU chemical agency. Guidance on Biocidal Products Regulation: Volume IV Environment - Assessment.Available online: www.echa.europa.eu (Accessed on 21 June 2023).
- Barber, O.W.; Hartmann, E.M. Benzalkonium chloride: A systematic review of its environmental entry through wastewater treatment, potential impact, and mitigation strategies. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2691–2719. [Google Scholar] [CrossRef]
- Journal of the European Union, EU law n° 1119/2014. Available online: www.eur-lex.europa.eu (Accessed on 10 September 2023).
- Núñez, O.; Moyano, E.; Galceran, M.T. Determination of quaternary ammonium biocides by liquid chromatography–mass spectrometry, Mass Spectrom. Innov. Appl. Part III 2004, 1058, 89–95. [Google Scholar]
- Bertuzzi, T.; Pietri, A. Determination of Benzalkonium Homologues and Didecyldimethylammonium in Powdered and Liquid Milk for Infants by Hydrophilic Interaction Liquid Chromatography–Mass Spectrometry. Food Anal. Methods 2014, 7, 1278–1284. [Google Scholar] [CrossRef]
- Bergmann, F.; Shimoni, A. Quaternary ammonium salts as inhibitors of acetylcholine esterase II. pH dependence of the inhibitory effects of quaternary ammonium salts, and the dissociation constant of the anionic site. Biochim. Biophys. Acta 1952, 8, 347–348. [Google Scholar] [CrossRef]
- Gaudin, V.; Soumet, C. Development of Enzymatic Biosensors to Detect Biocide Disinfectants to Strengthen Self-Monitoring in Industry. Eng. Proc. 2011, 6. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Istamboulie, G.; Sikora, T.; Jubete, E.; Ochoteco, E.; Marty, J.-L.; Noguer, T. Screen-printed poly(3,4-ethylenedioxythiophene) (PEDOT): A new electrochemical mediator for acetylcholinesterase-based biosensors. Talanta 2010, 82, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.E.; Yang, Q.; Xiao, Z.; Liu, L.; Wang, N.; Cui, X.; Yang, L. Impact of immobilization technology in industrial and pharmaceutical applications. 3 Biotech. 2019, 440. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.E.; Daniel, R.M.; Danson, M.J.; Eisenthal, R. The dependence of enzyme activity on temperature: determination and validation of parameters. Biochem. J. 2007, 402, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Istamboulié, G.; Cortina-Puig, M.; Marty, J.-L.; Noguer, T. The use of Artificial Neural Networks for the selective detection of two organophosphate insecticides: chlorpyrifos and chlorfenvinfos. Talanta 2009, 79, 507–511. [Google Scholar] [CrossRef]
|
AChE biosensor y = 19.625ln(x) + 277.62 |
BChE biosensor y = 16.952ln(x) + 265.6 |
||||
| [BAC]theoretical µM |
[BAC]measured µM |
Recovery (%) |
[BAC]theoretical µM |
[BAC]measured µM |
Recovery (%) |
| 0.27 | - | - | 0.27 | 0.29 | 109 |
| 0.67 | - | - | 0.67 | 0.61 | 91.7 |
| 2.65 | 2.30 | 86.8 | 2.65 | 2.18 | 82.2 |
| 26.5 | 22.5 | 86.5 | 26.5 | 20.3 | 78.1 |
|
AChE biosensor y = 19.625ln(x) + 277.62 |
BChE biosensor y = 16.952ln(x) + 265.6 |
||||
| [DDAC]theoretical µM |
[DDAC]measured µM |
Recovery (%) |
[DDAC]theoretical µM |
[DDAC]measured µM |
Recovery (%) |
| 0.14 | 0.11 | 81.5 | 0.14 | - | - |
| 0.34 | 0.25 | 75.8 | 0.34 | 0.27 | 74.8 |
| 0.75 | 0.62 | 80.0 | 0.75 | 0.74 | 99.3 |
| 2.70 | 2.2 | 81.5 | 2.70 | 2.60 | 96.3 |
| 29.7 | 23.7 | 89.4 | 29.7 | 27.0 | 90.9 |
| BAC | DDAC | |
|---|---|---|
| AChE-sensor | 5.3-10-8 - 5.3 10-7 M | 2.7-10-9 - 5.9 10-7 M |
| BChE sensor | 5.3-10-9- 5.3 10-7 M | 6.7-10-9 -5.9 10-7 M |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





