1. Introduction
Banana (
Musa spp.) is a major staple food crop with great economic importance; globally, ~124.98 million tons of bananas are produced annually [
1]. Banana plants are widely distributed in tropical and subtropical regions, including Africa, Latin America, the Caribbean, Asia, and the Pacific. In some parts of Africa, bananas provide up to 25–35 % of daily caloric intake to inhabitants [
2]. However, banana is highly susceptible to Fusarium wilt, which is caused by
Fusarium oxysporum f. sp.
cubense tropical race 4 (
Foc TR4). Fusarium wilt has a devastating economic impact on the banana industry and has gradually spread to major banana-producing countries; it was identified in Mozambique in 2013, Colombia in 2019, and Peru in 2021 (ProMusa,
https://www.promusa.org/).
Efficient strategies to control Fusarium wilt have not yet been identified. Chemical controls are ineffective because the fungi are soil-borne and affect the vascular bundle of host plants. The repeated use of chemical fungicides also has negative impacts on the environment and human health. However, biological controls have proven unsatisfactory in field experiments [
3]. Therefore, disease-resistance breeding is the most promising strategy for
Foc TR4 management. This approach will require additional research into host species
Foc TR4 resistance mechanisms, particularly those involving autophagy-related proteins.
The eukaryotic autophagy pathway is a system of controlled cellular degradation that is highly conserved among animals, fungi, and plants [
4,
5,
6]. Autophagy in plants involves a series of five steps: induction, elongation, completion, fusing, and degradation [
7]. Kinase signaling activates the ATG1/ATG13 complex to form autophagosomes [
8,
9,
10]. Autophagosomes then transport intracellular components to the vacuoles for degradation by various autophagy-related proteins at different stages of the process [
11,
12].
The key gene
ATG1 was the first autophagy-related gene discovered [
13]. It regulates autophagy initiation and autophagosome formation [
14,
15]. In
Arabidopsis thaliana and
Camellia sinensis, at least 49 ATG genes have been discovered thus far [
16]. Proteins involved in autophagy tend to be highly conserved across many eukaryotic species [
17,
18]. One such protein, ATG8, is initially processed by a cysteine protease (ATG4) to expose a glycine residue at the C-terminus [
19]. Phosphatidylethanolamine (PE) is then attached to this residue by ATG7 and the E2-like enzyme ATG3. Finally, PE is removed from ATG8 by ATG4, leaving free ATG8 available to form autophagosomes [
14]). Many reports have indicated that autophagosome formation fails in the absence of ATG8 [
20].
Plant biotic stress responses to pathogens involve activation of numerous genes, including those encoding autophagy-related proteins [
21,
22,
23]. Some pathogens suppress host defenses by interfering with autophagy. For example, SDE3 effector of Candidatus Liberibacter undermines autophagy-mediated immunity by the specific degradation of Citrus ATG8 family proteins [
24]. ATG8 also participates in regulating host plant disease resistance, and disruption of normal ATG8 function can therefore reduce plant defenses and promote infection. For example, the γb protein of barley stripe mosaic virus (BSMV) inhibits autophagy in the host plant
Hordeum vulgare by directly interacting with ATG7, preventing ATG–ATG8 interactions and thus promoting successful infection [
25]. In apple (
Malus domestica), MdATG8i decreases pathogen sensitivity by interacting with the target protein MdEF-Tu. The
Valsa Mali effector Vm1G-1794 inhibits autophagy by competitively binding to MdATG8i, thus weakening plant resistance. In contrast, MdATG8i overexpression significantly improves pathogen resistance [
21]. Prior studies have suggested that the causative agent of apple rot manipulates the apple autophagy pathway through secretion of specific effector proteins. ATG4 and ATG6/Beclin-1 have also been shown to participate in autophagy induced by biotic and abiotic stressors [
26]. Such studies have demonstrated the important roles of ATG8s in host plant infection resistance. However, previous reports have not identified banana ATG8s at the genome scale or characterized their roles in
Foc TR4 resistance.
In the present study, pathogen-induced ATG8 family members were identified throughout the banana genome. Gene structures, conserved domains, phylogenetic relationships, chromosomal locations and expression patterns of MaATG8 genes in response to Foc TR4 infection were analyzed. Furthermore, MaATG8 functions were established through assessment of interactions with other ATGs and the effects of gene silencing in banana during Foc TR4 infection. This study provides valuable new insights that will inform future research into the molecular mechanisms of autophagy and resistance breeding in banana.
2. Materials and Methods
2.1. Culture conditions for plants and fungi
The Cavendish banana (Musa spp. AAA group) cultivars ‘ZhongJiao No. 6’ (ZJ6) and ‘Brazilian’ (BX) were grown to the five- to six-leaf stage in a controlled greenhouse at 28 °C under a 14/10 h light/dark cycle. Nicotiana. benthamiana plants were grown for ~28 d in a greenhouse at 24 °C under a 16/8 h light/dark cycle. Prior to plant inoculation, Foc TR4 strain II5 (NRRL#54006) was cultured on potato dextrose agar (PDA) for 6 d or in potato dextrose broth (PDB) for 3 d.
2.2. Identification of ATG8 genes in multiple species
The amino acid (aa), genomic DNA (gDNA), and coding sequences (CDS) of
Musa.
acuminata were downloaded from the Banana Genome Database (
http://banana-genome-hub.southgreen.fr/). A Hidden Markov Model (HMM) for ATG8 (PF02991) was downloaded from the Pfam database (
http://pfam.xfam.org/). The
M.
acuminata genome was then searched using PF02991 as a query with the Simple HMM Search in TBtools. NCBI CDD search (
https://www.ncbi.nlm. nih.gov/cdd) was used for domain validation in the resulting sequences. Using candidate protein IDs, aa, gDNA and CDS were obtained with the TBtools sequence extraction tool (Fasta Extract).
2.3. Chromosomal locations of MaATG8s
Chromosome location mapping was conducted with the Gene Location Visualize from GTF/GFF function of TBtools based on the MaATG8 gene IDs from the M. acuminata gff3 files. M. acuminata genome sequences were analyzed in pairwise comparisons using One Step MCScanX in TBtools. MaATG8 gene family collinearity was visualized in other species with Multiple Synteny Plot in TBtools. The chromosomal coordinates of MaATG8 genes were extracted from the annotated M. acuminata genome files and visualized using TBtools.
2.4. RNA extraction and quantitative reverse transcription (qRT)-PCR
RNA of banana roots was extracted with an RNA extraction kit (Accurate Biotechnology, Hunan, China) following the manufacturer’s instructions. The kit contained RNase-free Recombinant DNase I to eliminate genomic DNA. Reverse transcription was performed with the Evo M-MLV One Step RT-PCR Kit (Accurate Biotechnology, Hunan, China). qRT-PCR was performed on a StepOne real-time PCR system (Applied Biosystems, USA) with the ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) following the manufacturer’s instructions. Gene relative expression levels were determined using the 2
-∆∆Ct method with the endogenous reference gene
MaTUB (β-tubulin). There were three biological replicates of each sample type and qRT-PCR was performed in technical triplicate. All primer pairs are shown in
Table S2.
2.5. Banana transcriptomic analysis
The roots of BX and ZJ6 plants were inoculated with Foc TR4 strain II5 and collected after 18, 32, and 56 h. There were three biological replicates per cultivar for each time point. The Illumina HiSeq X Ten platform was used to generate 150-bp paired-end reads. Filtered reads were aligned to the M. acuminata genome using HISAT2 v2.0.5. Differential expression between cultivars was calculated using the ‘DESeq2’ R package. Genes were considered significantly differentially expressed at | log2(fold change) | > 1 (p < 0.05).
2.6. Plasmid construction
MaATG8 genes were cloned from cDNA generated from Cavendish banana roots. The amplified fragments were ligated into the BamHI-digested pCAMBIA1300-GFP empty vector or the SmaI-digested pGBKT7 or pGADT7 empty vector using the In-Fusion Cloning Kit (Vazyme Biotech, Nanjing, China). Individual colonies containing each construct were verified via PCR and sequencing.
2.7. Subcellular localization
The MaATG8F CDS was cloned into the pCAMBIA1300-35S:GFP empty vector and the resulting construct was transformed into Agrobacterium tumefaciens strain GV3101. Transformed cells were cultured for 8 hours in liquid Luria-Bertani (LB) medium at 28 °C. Bacterial cells were harvested via centrifugation, then resuspended in infiltration medium containing 10 mM 2-(4-Morpholino) ethanesulfonic acid, 10 mM MgCl2, and 200 μM acetosyringone (pH = 5.6). The concentration of the cell suspension was adjusted to an OD600 of 1.0, then the suspension was incubated for 2–3 h at room temperature prior to infiltration into the leaves of four-week-old N. benthamiana plants. The leaves were observed under a confocal microscope at 72 hours post infiltration.
2.8. Inoculation assays
To determine banana plant phenotypes after Foc TR4 infection, banana cultivars of two different resistance levels were selected: BX and ZJ6. ZJ6 is a resistant cultivar whereas BX is highly susceptible to Foc TR4. These two banana cultivars were grown in a greenhouse to the five- to six-leaf stage for use in inoculation assays. Banana cultivars were inoculated with Foc TR4 at a concentration of 1×107 conidia/L. Banana corms were observed and photographed at 35 d post inoculation and graded for disease appearance as follows: 0 (no symptoms), 1 (some brown spots in the inner rhizome), 2 (less than 25 % of the inner rhizome browning), 3 (up to 75 % of the inner rhizome browning), and 4 (entire inner rhizome and pseudostem dark brown and dead). The disease index was calculated as follows:
2.9. Y2H assays
The Invitrogen Y2H system was used to identify protein–protein interactions in vitro. The MaATG4B CDS was cloned in-frame into the bait vector pGBKT7 and the CDSs of MaATG8 genes were cloned in-frame into the prey vector pGADT7. The Y2H Gold yeast strain was co-transformed with the resulting bait and prey vectors to identify positive interactions following the manufacturer’s protocol.
2.10. Gene silencing
dsRNAs of ~300 bp in size that were complementary to
MaATG8F and
MaATG4B were generated with the T7 RNAi Transcription Kit (Vazyme Biotech, Nanjing, China). PCR technique was used to add the T7 promoter sequence to both ends of the RNA interference (RNAi) target fragments. Sequences including the T7 promoters were purified and used as the templates for dsRNA amplification. Infection assays were then performed to establish the effects of knocking out target genes. Specifically, banana leaves were detached, then treated with 10 μL dsRNA at a concentration of 500 ng/μL with 0.02 % Silwet L-77. The dsRNA solution was allowed to dry for ~1 h, then mycelial plugs of 5 mm in diameter were taken from plates containing 6-d-old
Foc TR4 strain II5. Plugs were taken only from the growing edges and were placed onto the portion of banana leaves treated with dsRNA. All leaves were then moved to plastic trays, each of which was lined with two layers of moistened paper towel. To maintain sufficiently high humidity, each tray was covered with plastic film. Trays containing inoculated leaves were incubated at 28 °C in the dark for 10 days. Leaves were then photographed and the fungal lesion size was quantified in ImageJ (
https://imagej.nih.gov/ij/). Leaves treated with GFP dsRNA were used as controls.
2.11. Trypan blue staining
Trypan blue staining was performed as previously described [
27] with some modifications. Briefly,
N.
benthamiana and banana leaves were harvested and soaked in trypan blue solution (0.02 % trypan blue in 1:1:1:1:8 phenol : glycerol : lactic acid : water : ethanol [v/v/v/v/v]) at room temperature overnight. Leaf samples were destained in 75 % alcohol several times until the destaining solution remained clear.
3. Results
3.1. Genome-wide identification of MaATG8 genes
Analysis of the
M.
acuminata genome revealed 10 putative MaATG8 genes (
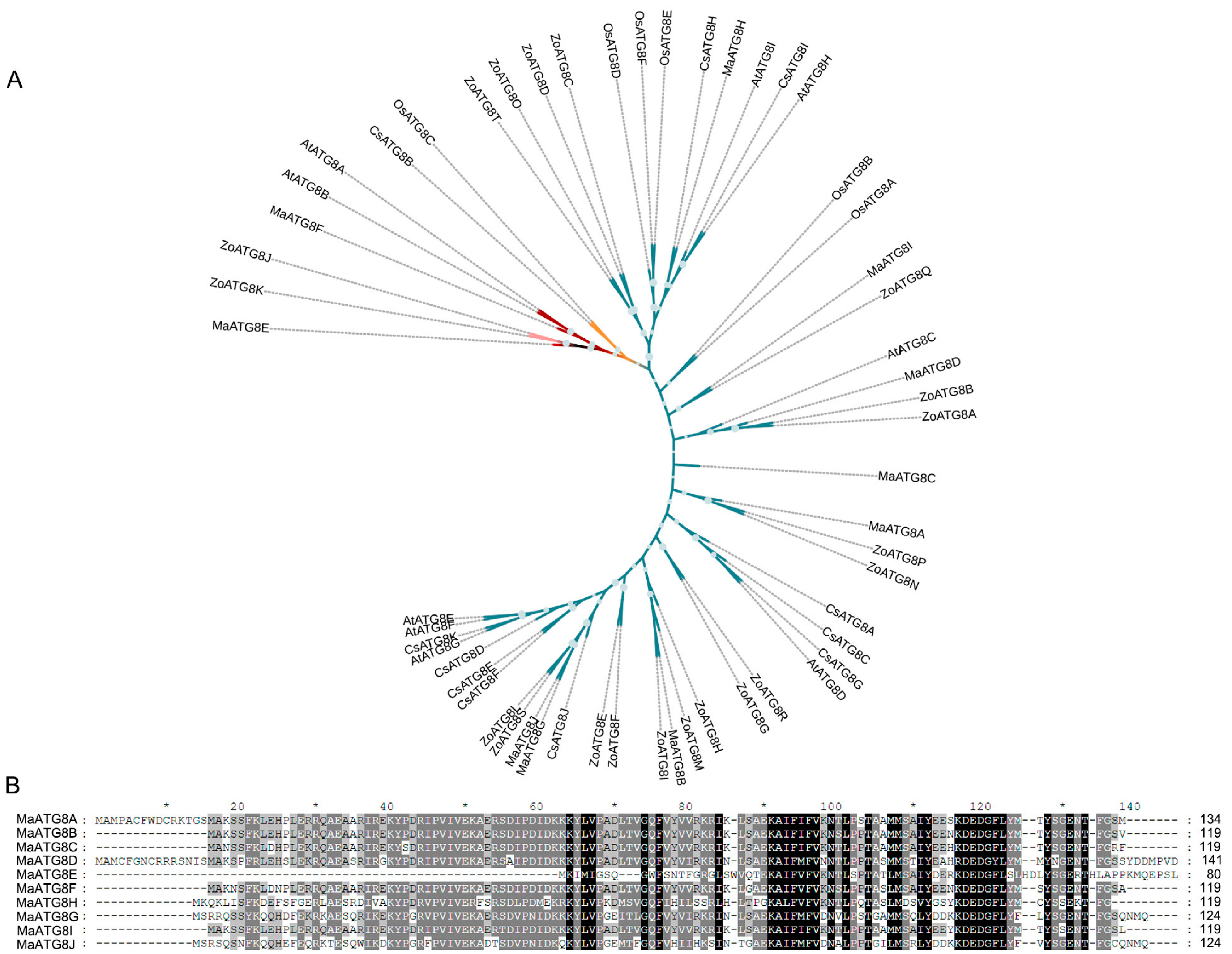
Table S1). The longest MaATG8 protein (MaATG8D) was 141 amino acids (aa) in length, and the shortest (MaATG8E) was 80 aa. A neighbor-joining phylogenetic tree constructed from ATG8 genes in banana,
A. thaliana, rice, citrus, and ginger showed five major phylogenetic clades, which were conserved in banana (10 genes),
A.
thaliana (8 genes), rice (6 genes), citrus (11 genes), and ginger (20 genes) (
Table S1,
Figure 1A). MaATG8E alone formed a separate branch (
Figure 1A). Overall, this phylogenetic analysis showed that ATG8 homologs were conserved among several plant species of varying evolutionary distances from one another. Furthermore, aa sequence alignment showed that the MaATG8 proteins were highly evolutionarily conserved (
Figure 1B).
3.2. MaATG8 gene structures and conserved motif analysis
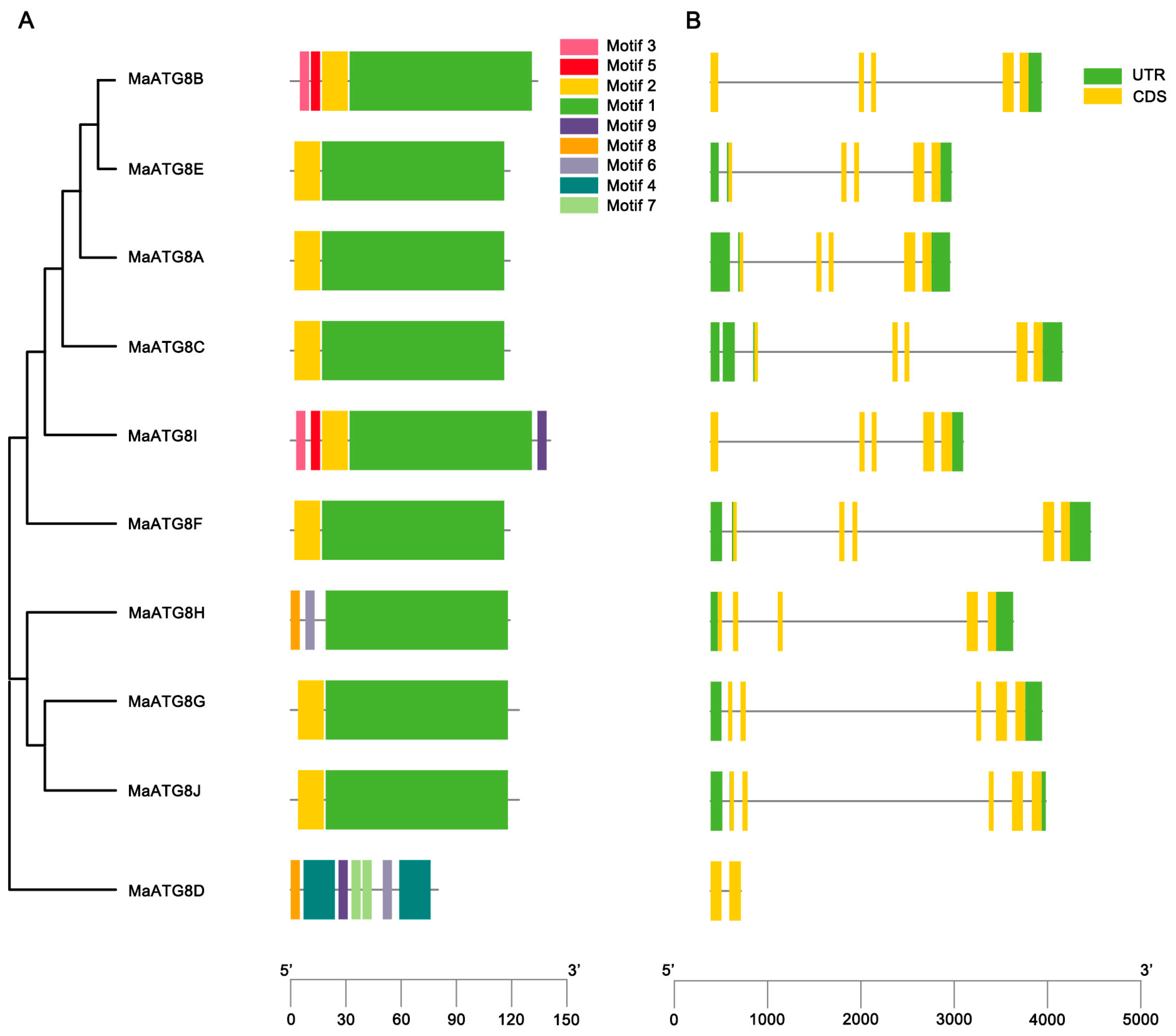
Gene structure analysis showed notable differences in the numbers and lengths of exons in
MaATG8 genes. There were nine motifs identified across the
MaATG8s (
Figure 2A).
MaATG8C,
MaATG8A,
MaATG8F,
MaATG8J,
MaATG8E, and
MaATG8G contained just two motifs each (motifs 1 and 2).
MaATG8H had three motifs (2, 6, and 8);
MaATG8B contained four motifs (1, 2, 3, and 5); and
MaATG8I had five motifs (1, 2, 3, 5, and 9).
MaATG8D contained the largest number of motifs at seven (motifs 2, 6, and 9 and two each of motifs 4 and 7) (
Figure 2A).
MaATG8D also contained two exons and no untranslated region (UTR), whereas the other nine MaATG8 genes had five exons each (
Figure 2B) and one (
MaATG8B and
MaATG8I), two (
MaATG8G,
MaATG8J, and
MaATG8H), or three (
MaATG8E,
MaATG8C,
MaATG8A, and
MaATG8F) UTRs (
Figure 2B). These results indicated varying degrees of evolutionary conservation and divergence between the
MaATG8 genes.
3.3. Predicted cis-acting elements in MaATG8 promoters
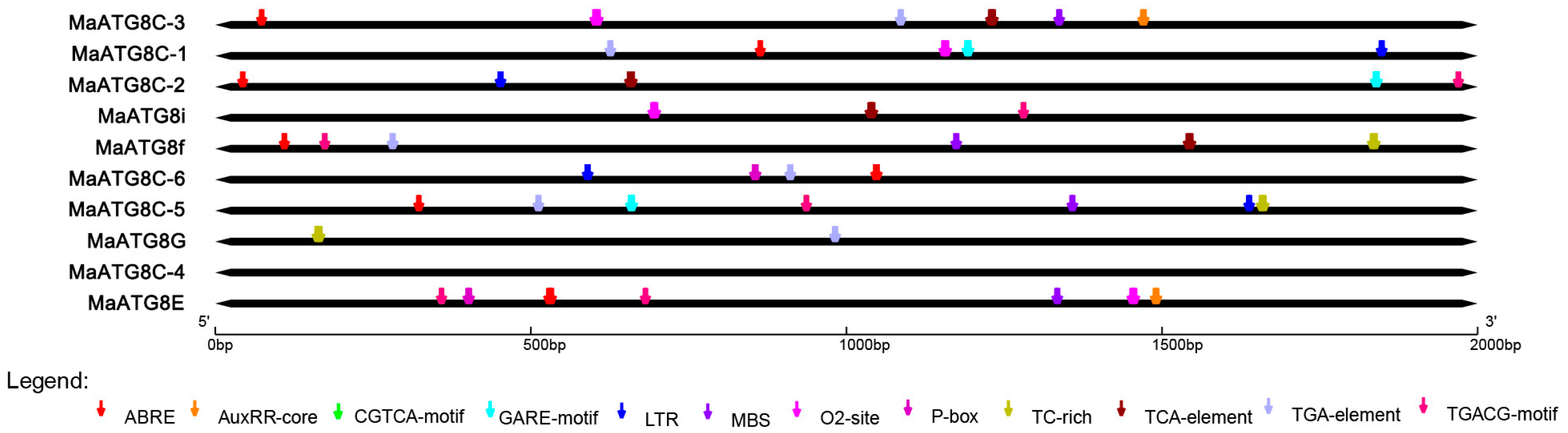
To understand the putative functional properties of
MaATG8 gene family members, the PlantCARE database was used to predict cis-acting elements in the promoter regions (classified as 2,000 bp upstream of the transcription start site for each gene). This analysis revealed many cis-acting elements, including low temperature responsiveness, defense and stress responsiveness, and hormone responsiveness elements in addition to MYB binding sites. Six
MaATG8s had abscisic acid (ABA) response elements and six had auxin responsiveness elements (
Figure 3). For example,
MaATG8A contained multiple ABRE (ABA-responsive) elements. Five
MaATG8s were predicted to respond to methyl jasmonate (MeJA) (
Figure 3);
MaATG8D,
MaATG8E, and
MaATG8J each contained multiple binding sites for MeJA-responsiveness. Four
MaATG8s were predicted to be salicylic acid (SA)-responsive. The
MaATG8s also included elements for stress responses (
Figure 3). These results suggested that
ATG8 genes played important roles in banana stress and hormone responses.
3.4. Chromosomal distribution of MaATG8s
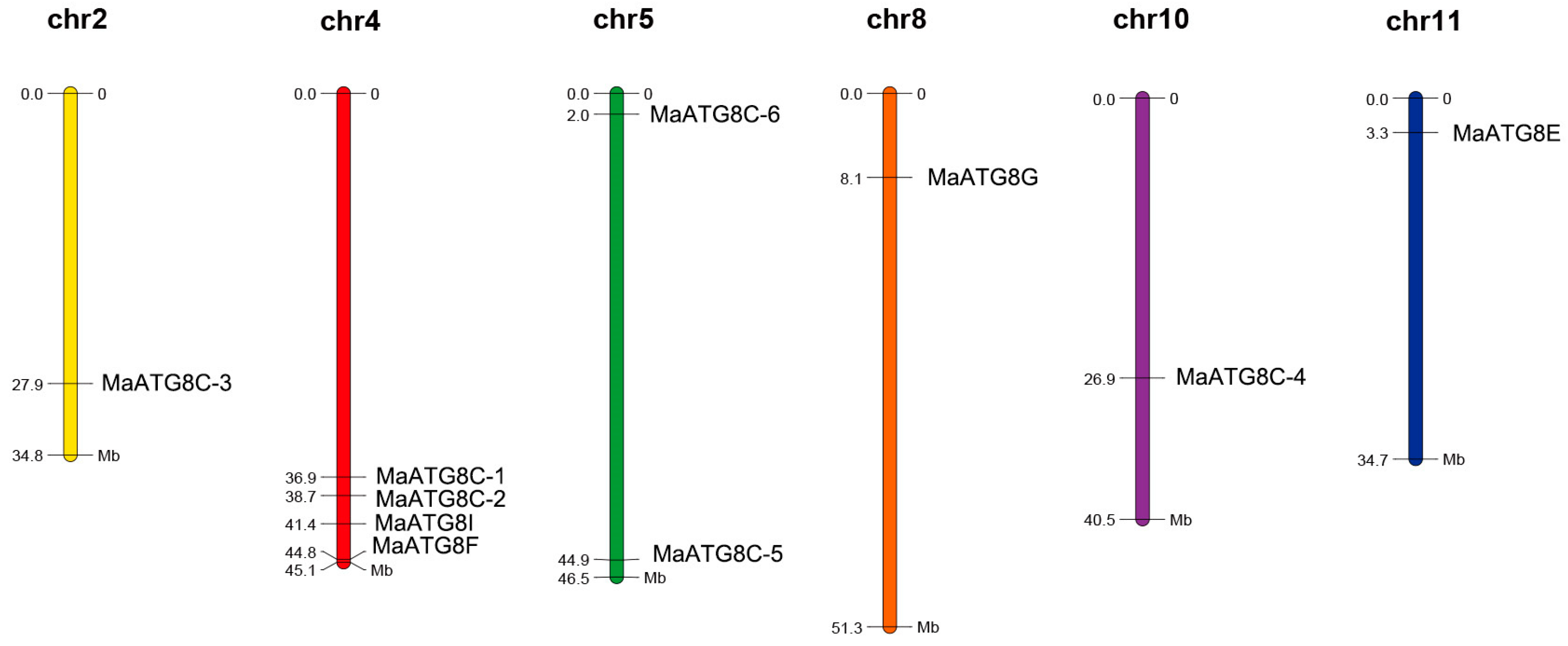
Analysis of the 10
MaATG8 locations showed that they were distributed across chromosome (Chr) 2, Chr 4, Chr 5, Chr 8, Chr 10, and Chr 11. The other banana chromosomes did not contain any
ATG8 genes (
Figure 4). Chr4 contained more MaATG genes (four) than any other chromosome:
MaATG8B,
MaATG8C,
MaATG8D, and MaATG8E. Chr5 contained two
MaATG8 genes (
MaATG8H and
MaATG8F); the remaining
MaATG8s were present on Chr2, Chr8, Chr10, and Chr11, which contained just one
MaATG8 each (
MaATG8A,
MaATG8G,
MaATG8I, and
MaATG8J, respectively). These results indicated that
MaATG8s were distributed unevenly throughout the genome.
3.5. Involvement of MaATG8 family members in the Foc TR4 infection response
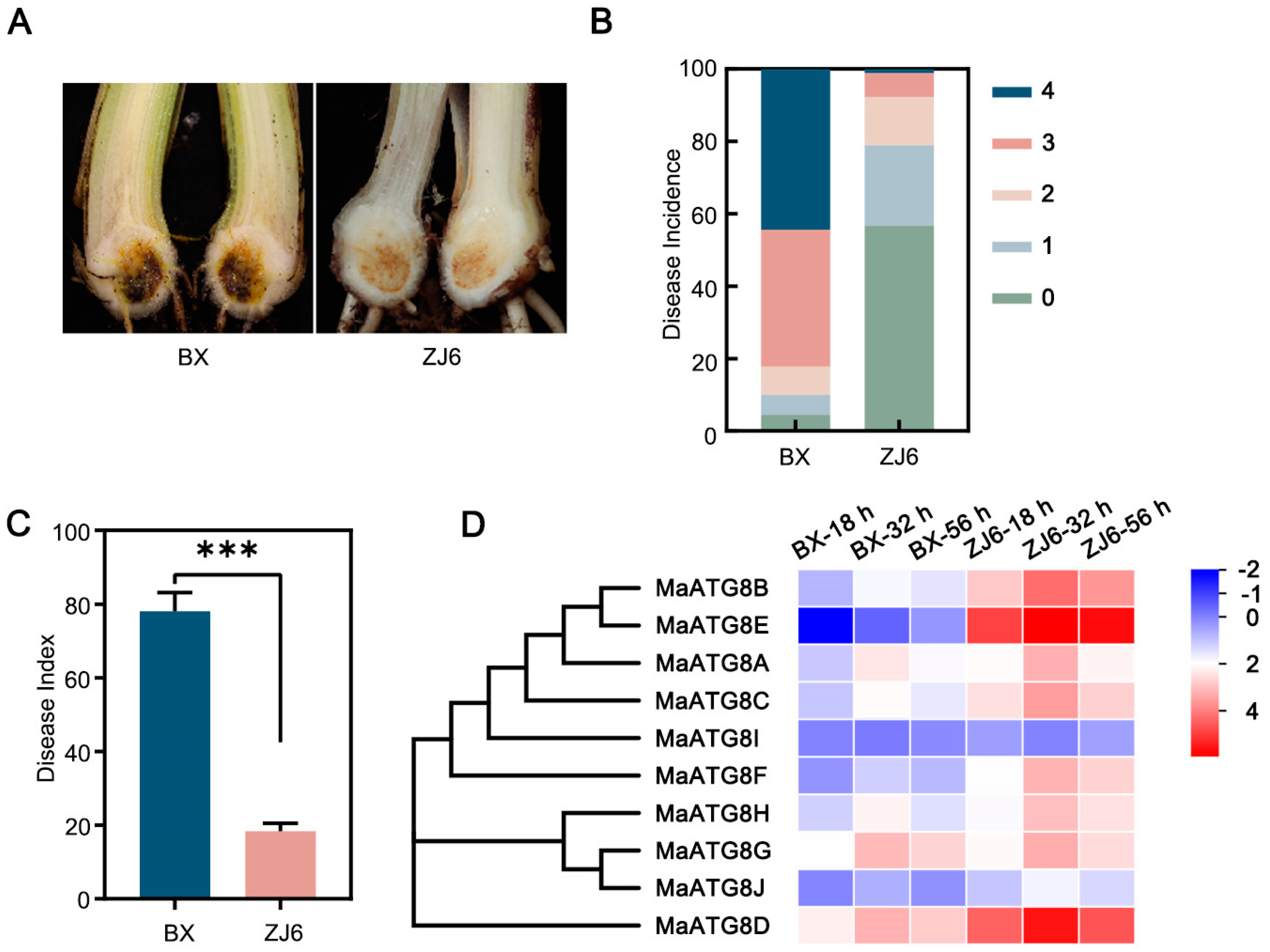
The resistant
M.
acuminata cultivar ‘ZhongJiao No. 6’ (ZJ6) and the susceptible cultivar ‘Brazilian’ (BX) were inoculated with
Foc TR4 strain II5 at the five- to six-leaf stage. At 35 d after inoculation, the corms of BX plants showed significant browning symptoms; the disease incidence exceeded 95 % and the disease index was higher than 78 % (
Figure 5A–C). Among ZJ6 plants, only 43.3 % showed disease symptoms, which were mild, and the disease index reached just 18.3 % (
Figure 5A–C). This demonstrated ZJ6 resistance to
Foc TR4. To investigate
MaATG8 gene expression profiles during the early infection process (at 18, 32, and 56 h post inoculation), we performed transcriptomic analyses of the susceptible and resistance cultivars. Nine of the
MaATG8 genes were induced in both the resistant and susceptible cultivars; only MaATG8I was not induced (
Table S3;
Figure 5D). Furthermore, the remaining nine genes were all highly expressed in the resistant cultivar, but only
MaATG8C (
Macma4_04_g32300),
MaATG8G (
Macma4_08_g10850),
MaATG8A (
Macma4_02_g16130),
MaATG8D (
Macma4_04_g36560), and
MaATG8H (
Macma4_05_g30830) were continuously induced during the infection process (
Table S3;
Figure 5D).
MaATG8F (Macma4_05_g02860),
MaATG8J (
Macma4_11_g04160), and
MaATG8E (
Macma4_04_g42290) were most strongly induced in the resistant cultivar (
Table S3;
Figure 5D). These results showed that MaATG8 genes were responsive to
Foc TR4 infection and were more highly expressed in the resistant cultivar (ZJ6) than in the susceptible cultivar (BX), clearly demonstrating participation of
MaATG8 genes in the banana immune response.
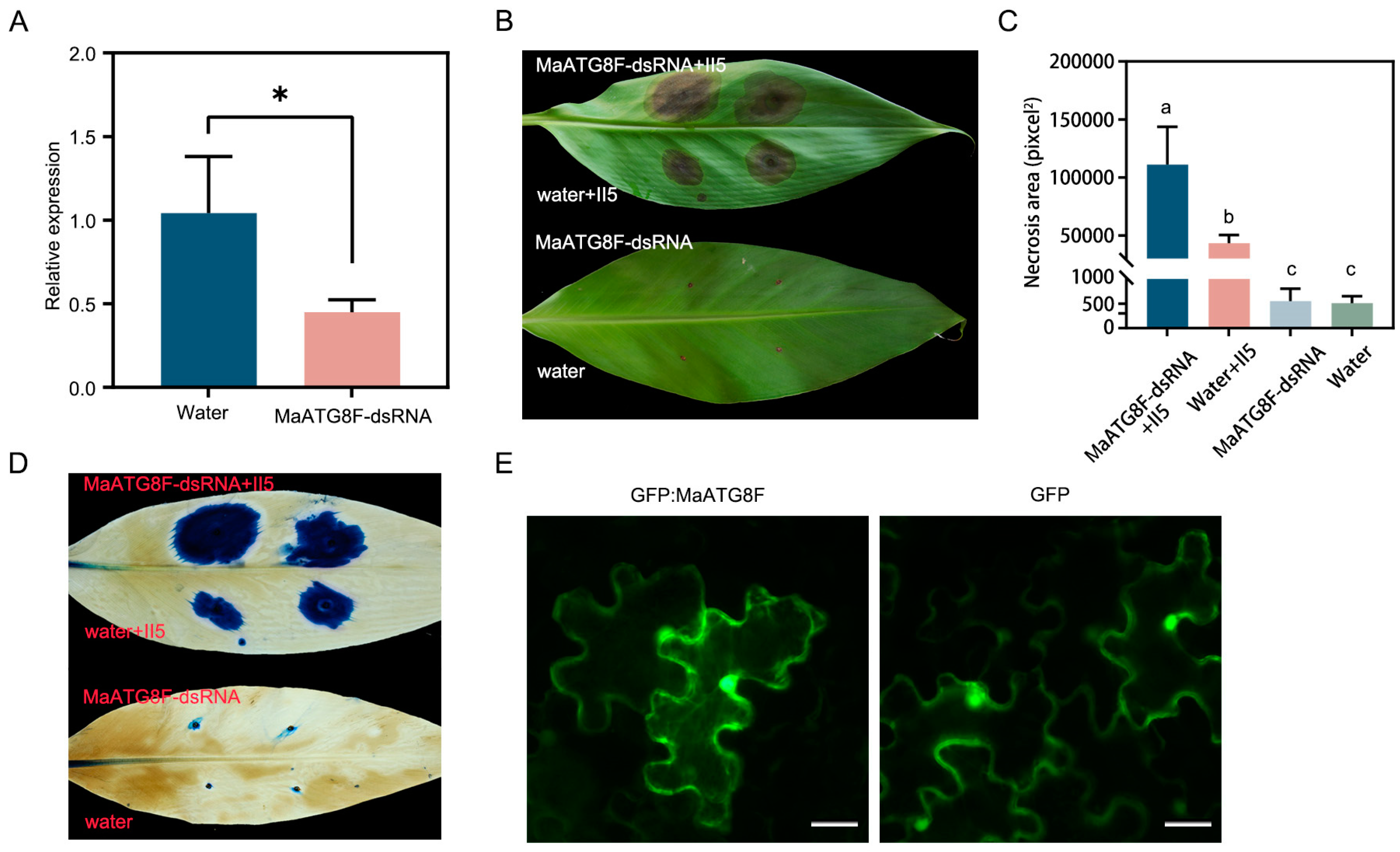
3.6. MaATG8F silencing reduced Foc TR4 resistance
To investigate the biological function of
MaATG8F in the
Foc TR4 infection response, wounded banana leaves were treated with
MaATG8F-dsRNA or a water control, then plugs of
Foc TR4 II5 were placed on the same areas. As expected,
MaATG8F was strongly downregulated after treatment with
MaATG8F-dsRNA, demonstrating successful
MaATG8F silencing (
Figure 6A). Furthermore, leaves treated with
MaATG8F-dsRNA showed more extensive necrosis than water-treated control leaves, as confirmed with both a visual comparison of the leaves (
Figure 6B,C) and trypan blue staining (
Figure 6D). To determine MaATG8F subcellular localization, a MaATG8F–green fluorescent protein (GFP) construct was generated with the pCAMBIA1300-35S:GFP vector, which was transiently expressed in
N.
benthamiana leaves. Microscopic observations of
N.
benthamiana leaves collected at 3 d post agro-infiltration revealed nuclear and cytoplasmic localization of GFP:MaATG8, identical to the results of leaves infiltrated with the GFP empty vector (
Figure 6E). Overall, these results indicated that MaATG8F positively regulated banana disease resistance to
Foc TR4.
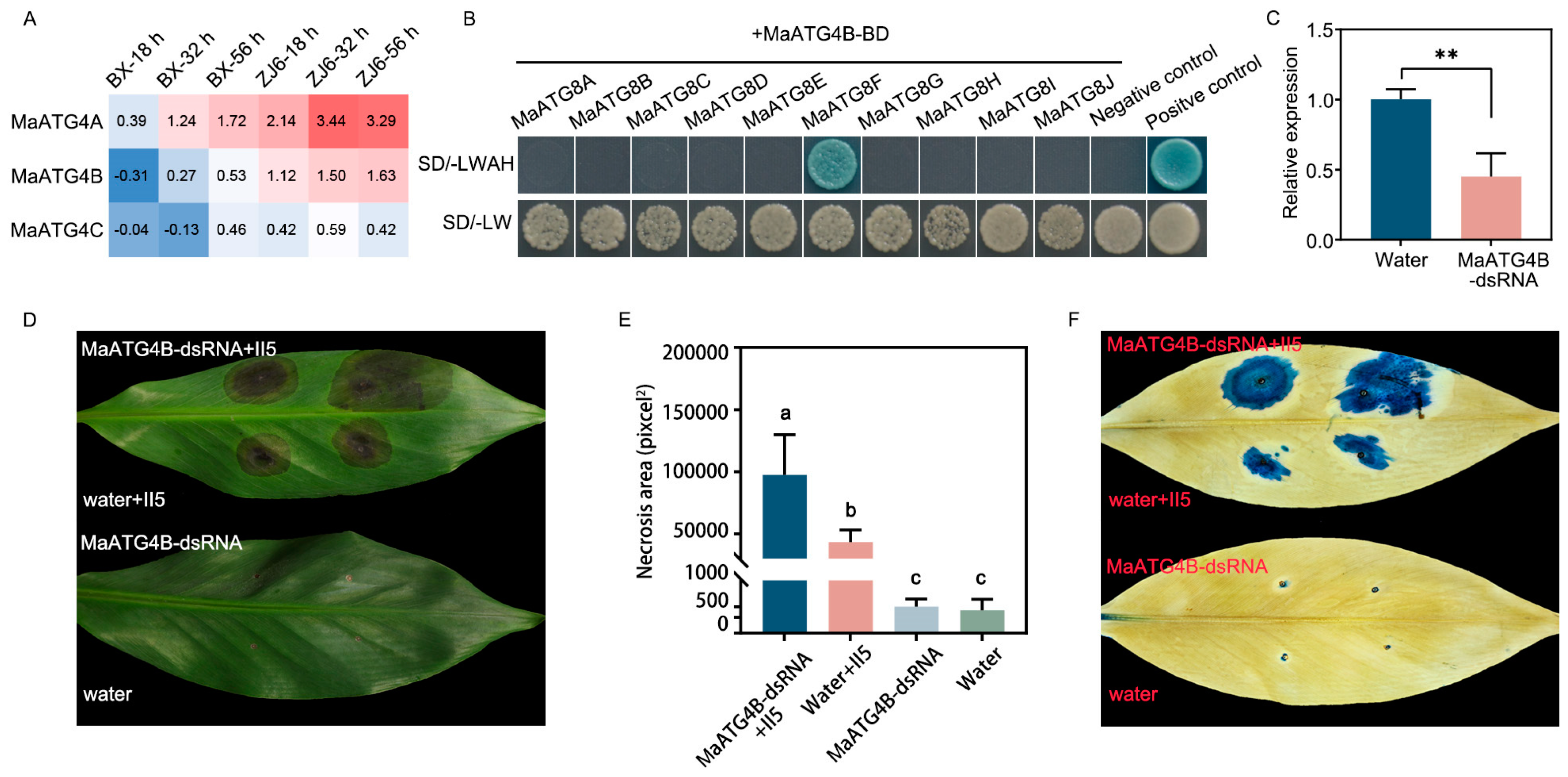
3.7. MaATG4B interacted with MaATG8F
To investigate the molecular mechanism underlying MaATG8-mediated activation of the host immune response, yeast two-hybrid (Y2H) assays were performed to identify potential host targets of MaATG4, which are autophagy-related members of the ATG family. Interactions of ATG4s and ATG8s have previously been identified in
Arabidopsis [
28].
MaATG4A and
MaATG4B were found to be induced during
Foc TR4 infection, whereas
MaATG4C was not (
Figure 7A). Notably,
MaATG4B was only induced in the resistant cultivar ZJ6, whereas
MaATG4A was induced in both ZJ6 and BX (
Figure 7A). Thus, MaATG4B was selected for confirmation of physical interactions with MaATG8 homologs. The full-length coding sequences (CDSs) of
MaATG4B and
MaATG8 genes were cloned into the bait and prey vectors, respectively. Colony growth of co-transformants on selection medium containing X-α-gal confirmed pairwise interactions between MaATG4B and all 10 MaATG8 proteins in yeast. Only MaATG8F interacted with MaATG4B (
Figure 7B). These results indicated that the interactions of MaATG8F and MaATG4B could also happen in banana plants.
To investigate the biological function of MaATG4B in response to
Foc TR4 infection, banana leaves were treated with MaATG4B-dsRNA or a water control. qRT-PCR analysis showed significant MaATG4B downregulation in dsRNA-treated leaves (
Figure 7C), indicating that dsRNA treatment silenced MaATG4B as expected. To confirm whether silencing
MaATG4B influenced
Foc TR4 resistance, dsRNA-treated leaves were inoculated with
Foc TR4 plugs. The dsRNA-treated leaves showed larger necrotic areas than the controls (
Figure 7D–F). Taken together, these results indicated that MaATG4B functioned as a positive regulator of banana defense responses and interacted with the autophagy-related protein MaATG8F.
4. Discussion
Plant growth is a continuous process that is regulated by several genetic and environmental factors. Stressors such as salt, high temperature, drought and pathogen infection in particular have substantial influences on plant growth and yield throughout the world. Plants have evolved various mechanisms to protect themselves from environmental stressors, enabling survival. Autophagy is one of the most important strategies that allows plants to survive adverseconditions [
18,
29,
30]. The molecular mechanisms by which members of the autophagy-related ATG8 protein family function in banana have not previously been characterized. In the present study, 10
MaATG8 genes were identified throughout the banana genome. Phylogenetic analysis of
ATG8 genes in
A.
thaliana, rice, citrus, ginger, and banana demonstrated relatively high conservation between species, indicating that ATG8 proteins may perform similar functions in different species.
MaATG8 was here found to be induced by
Foc TR4 infection, and was more highly expressed in a resistant than a susceptible banana cultivar. This led to the hypothesis that
MaATG8 expression levels in resistant cultivars may be associated with their mechanisms of disease resistance. Silencing
MaATG8 in banana leaves enhanced host susceptibility to
Foc TR4 infection, which might be one of explanations for the hypothesis. An increasing number of studies have shown that single nucleotide polymorphisms (SNPs) play important roles in various plant processes [
31,
32,
33]. A cis-acting element analysis here revealed that the MaATG8 promoter sequences contained a large number of resistance-related cis-elements. This suggested that the
MaATG8 promoter elements of resistant and susceptible cultivars contained SNPs leading to differences in the binding abilities of associated transcription factors, resulting in cultivar-specific differences in
MaATG8 gene expression. This is another possible explanation for differential
MaATG8 expression between resistant and susceptible cultivars. Due to the difficulty of banana plant transformation and the length of time required,
MaATG8 transgenic plants have not yet been obtained. To verify the biological functions of
MaATG8s, banana suspension cells should be transformed to generate transgenic plants, including those overexpressing
MaATG8s or expressing CRISPR/Cas9-edited versions of
MaATG8s. Further studies should also address the mechanisms by which
MaATG8s induce autophagy to degrade effectors secreted by
Foc TR4. These mechanisms may protect banana plants from pathogen infection.
Autophagy-related proteins are known to play important roles in plant life processes, including pathogen resistance. For example, MdATG8i can mediate disease resistance and drought tolerance in apples [
21,
34]. Many mechanisms of interactions between ATG4 and ATG8 have been revealed previously. For example, the cysteine protease ATG4 facilitates normal ATG8 functioning by first hydrolyzing the C-terminal arginine residue, exposing a glycine residue for PE binding, then by cleaving ATG8 from PE to enable autophagosome formation [
14,
19]. The results of the present study verified interactions between MaATG4 and MaATG8
in vitro, suggesting that MaATG4–MaATG8 interactions also regulated autophagy in banana. Interestingly, Y2H assays showed that MaATG4 could not interact with every one of the MaATG8 family members, indicating that some of these proteins may have evolved new functions in banana. Further studies should be conducted to determine if the other two MaATG4 homologs in
M.
acuminata can interact with all MaATG8 family members.
ATG6 is another protein with an important role in ATG8-mediated autophagosome formation. In rice, the C-terminus of a Rhabdovirus glycoprotein interacts with SnRK1B, promoting ATG6b phosphorylation. Rice ATG6b can also interact with the N-terminus of the viral glycoprotein; this connects the glycoprotein with ATG8 on the autophagosome membrane, promoting removal of the glycoprotein into the autophagosome for degradation [
22]. A prior study indicated that ATG6, ATG8, and ATG4 all interact to form a protein complex. However, whether this is true in banana, and whether such a complex participates in
Foc TR4 resistance, remains unknown. Previous research has demonstrated that interactions between ATG8 and AIM-containing proteins participate in cell-selective autophagy and regulate plant disease resistance [
35,
36]. Our research group determined with Y2H assays that an AIM-containing protein interacts with MaATG8 (unpublished data), but the relationship between this interaction and disease resistance requires further study.
In summary, a total of 10 MaATG8 genes were here identified in the banana genome. Phylogenetic relationships, gene structures, chromosomal locations, and expression pattern analyses were conducted for all 10 MaATG8 genes. Nearly all of the MaATG8 genes were upregulated in disease-resistant cultivars after Foc TR4 infection. Based on the results of MaATG8 expression analyses, the role of MaATG8 was explored further. MaATG8F silencing indicated that this gene positively regulated banana resistance to Foc TR4. Y2H results showed that MaATG4B could interact with MaATG8F in vitro, but not with the other MaATG8 homologs. This study provides novel resitant gene resources for subsequent functional research of autophagy-related proteins in banana.
5. Conclusions
In the present study, 10 MaATG8 genes were identified in the banana genome, some of which were determined to be involved in the host immune response against Foc TR4. Nine of the 10 MaATG8 genes were significantly induced during the infection process in the resistant and/or susceptible cultivars, with the exception of MaATG8I. The results of several analyses indicated that MaATG8F positively regulated banana disease resistance to Foc TR4. MaATG4A and MaATG4B were strongly upregulated in response to Foc TR4 infection, with MaATG4B only induced in the disease-resistant banana cultivar. MaATG4B was found to interact with MaATG8F (but not with other MaATG8s) and to positively regulate banana disease resistance to Foc TR4. Taken together, this comprehensive analysis of the MaATG8 family in a wild banana species provides valuable new information to facilitate mining of related resistance genes for future genetic improvement of banana cultivars.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: ATG8 homologues identified in different plant species; Table S2: Primers used in this study; Table S3: DEGs of MaATG8 genes.
Author Contributions
Huoqing Huang carried out the experiments and data analysis; Yuzhen Tian and Yile Huo provided RNA-seq data analysis and performed bioinformatics; Yushan Liu performed the subcellular localization; Wenlong Yang and Yuqing Li performed synthesis of dsRNA; Mengxia Zhuo performed the inoculation assays; Ganjun Yi and Chunyu Li conceived the project; Chunyu Li and Siwen Liu carried out experiment design and manuscript design; Huoqing Huang wrote the manuscript. Ganjun Yi, Chunyu Li and Siwen Liu revised the manuscript. All authors have read and agreed the published version of the manuscript.
Funding
This research was supported by the Science and Technology Department of Guangdong Province (2022A1515110433), the Guangdong Science and Technology Project (2019B1515120088), the Guangdong Science and Technology Project (110263295131), the Laboratory of Lingnan Modern Agriculture Project (NT2021004), the Research Fund of Maoming Branch, Guangdong Laboratory for Lingnan Modern Agriculture (2021TDQD003), the Guangdong Provincial Special Fund for Modern Agriculture Industry Technology Innovation Teams, Department of Agriculture and Rural Affairs of Guangdong Province (2023KJ109), and the Earmarked Fund for CARS (CARS-31).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAOSTAT. Food and Agricultural Organization (FAO). Retrieved from: http://www.fao.org/faostat/en/#data/QC. Accessed on Fri, March, 31, 16:07:35, 2023.
- Bubici G, Kaushal M, Prigigallo MI, Gómez-Lama Cabanás C, Mercado-Blanco J. Biological Control Agents Against Fusarium Wilt of Banana. Front Microbiol. 2019, 10:616. [CrossRef]
- Ploetz RC. Fusarium Wilt of Banana. Phytopathology. 2015, 105(12):1512-21. [CrossRef]
- Pollack JK, Harris SD, Marten MR. Autophagy in filamentous fungi. Fungal Genet Biol. 2009, 46(1):1-8. [CrossRef]
- Liu Y, Bassham DC. Autophagy: pathways for self-eating in plant cells. Annu Rev Plant Biol. 2012, 63:215-37. [CrossRef]
- Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014, 20;20(3):460-73. [CrossRef]
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004, 6(4):463-77. [CrossRef]
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000, 103(2):253-62. [CrossRef]
- Ismayil A, Yang M, Liu Y. Role of autophagy during plant-virus interactions. Semin Cell Dev Biol. 2020, 101:36-40. [CrossRef]
- Wang Y, Cao JJ, Wang KX, Xia XJ, Shi K, Zhou YH, Yu JQ, Zhou J. BZR1 Mediates Brassinosteroid-Induced Autophagy and Nitrogen Starvation in Tomato. Plant Physiol. 2019, 179(2):671-685. [CrossRef]
- Marshall RS, Vierstra RD. Autophagy: The Master of Bulk and Selective Recycling. Annu Rev Plant Biol. 2018, 69:173-208. [CrossRef]
- Yoshimoto K, Ohsumi Y. Unveiling the Molecular Mechanisms of Plant Autophagy-From Autophagosomes to Vacuoles in Plants. Plant Cell Physiol. 2018, 59(7):1337-1344. [CrossRef]
- Harding TM, Morano KA, Scott SV, Klionsky DJ. Isolation and characterization of yeast mutants in the cytoplasm to vacuole protein targeting pathway. J Cell Biol. 1995, 131(3):591-602. [CrossRef]
- Klionsky DJ, Cregg JM, Dunn WA Jr, Emr SD, Sakai Y, Sandoval IV, Sibirny A, Subramani S, Thumm M, Veenhuis M, Ohsumi Y. A unified nomenclature for yeast autophagy-related genes. Dev Cell. 2003, 5(4):539-45. [CrossRef]
- Zhu JK. Abiotic Stress Signaling and Responses in Plants. Cell. 2016, 167(2):313-324. [CrossRef]
- Huang W, Ma DN, Liu HL, Luo J, Wang P, Wang ML, Guo F, Wang Y, Zhao H, Ni DJ. Genome-Wide Identification of CsATGs in Tea Plant and the Involvement of CsATG8e in Nitrogen Utilization. Int J Mol Sci. 2020, 21(19):7043. [CrossRef]
- Nakatogawa H, Ichimura Y, Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell. 2007, 130(1):165-78. [CrossRef]
- Kataura T, Sedlackova L, Otten EG, Kumari R, Shapira D, Scialo F, Stefanatos R, Ishikawa KI, Kelly G, Seranova E, Sun C, Maetzel D, Kenneth N, Trushin S, Zhang T, Trushina E, Bascom CC, Tasseff R, Isfort RJ, Oblong JE, Miwa S, Lazarou M, Jaenisch R, Imoto M, Saiki S, Papamichos-Chronakis M, Manjithaya R, Maddocks ODK, Sanz A, Sarkar S, Korolchuk VI. Autophagy promotes cell survival by maintaining NAD levels. Dev Cell. 2022, 57(22):2584-2598.e11. [CrossRef]
- Kirisako T, Ichimura Y, Okada H, Kabeya Y, Mizushima N, Yoshimori T, Ohsumi M, Takao T, Noda T, Ohsumi Y. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J Cell Biol. 2000, 151(2):263-76. [CrossRef]
- Doelling JH, Walker JM, Friedman EM, Thompson AR, Vierstra RD. The APG8/12-activating enzyme APG7 is required for proper nutrient recycling and senescence in Arabidopsis thaliana. J Biol Chem. 2002, 277(36):33105-14. [CrossRef]
- Che R, Liu C, Wang Q, Tu W, Wang P, Li C, Gong X, Mao K, Feng H, Huang L, Li P, Ma F. The Valsa Mali effector Vm1G-1794 protects the aggregated MdEF-Tu from autophagic degradation to promote infection in apple. Autophagy. 2023, 19(6):1745-1763. [CrossRef]
- Huang X, Wang J, Chen S, Liu S, Li Z, Wang Z, Chen B, Zhang C, Zhang Y, Wu J, Yang X, Xie Q, Li F, An H, Huang J, Li H, Liu C, Wu X, Liu DX, Yang X, Zhou G, Zhang T. Rhabdovirus encoded glycoprotein induces and harnesses host antiviral autophagy for maintaining its compatible infection. Autophagy. 2023, 1:1-20. [CrossRef]
- Liu Y, Schiff M, Czymmek K, Tallóczy Z, Levine B, Dinesh-Kumar SP. Autophagy regulates programmed cell death during the plant innate immune response. Cell. 2005, 121(4):567-577. [CrossRef]
- Shi J, Gong Y, Shi H, Ma X, Zhu Y, Yang F, Wang D, Fu Y, Lin Y, Yang N, Yang Z, Zeng C, Li W, Zhou C, Wang X, Qiao Y. 'Candidatus Liberibacter asiaticus' secretory protein SDE3 inhibits host autophagy to promote Huanglongbing disease in citrus. Autophagy. 2023, 19(9):2558-2574. [CrossRef]
- Yang M, Zhang Y, Xie X, Yue N, Li J, Wang XB, Han C, Yu J, Liu Y, Li D. Barley stripe mosaic virus γb Protein Subverts Autophagy to Promote Viral Infection by Disrupting the ATG7-ATG8 Interaction. Plant Cell. 2018, 30(7):1582-1595. [CrossRef]
- Pei D, Zhang W, Sun H, Wei X, Yue J, Wang H. Identification of autophagy-related genes ATG4 and ATG8 from wheat (Triticum aestivum L.) and profiling of their expression patterns responding to biotic and abiotic stresses. Plant Cell Rep. 2014, 33(10):1697-710. [CrossRef]
- Fernández-Bautista, N., Domínguez-Núñez, J. A., Moreno, M. M. C. and Berrocal-Lobo, M. Plant Tissue Trypan Blue Staining During Phytopathogen Infection. Bio-protocol. 2016, 6(24): e2078. [CrossRef]
- Park E, Woo J, Dinesh-Kumar SP. Arabidopsis ATG4 cysteine proteases specificity toward ATG8 substrates. Autophagy. 2014, 10(5):926-7. [CrossRef]
- Tang J, Bassham DC. Autophagy during drought: function, regulation, and potential application. Plant J. 2022, 109(2):390-401. [CrossRef]
- Avin-Wittenberg T. Autophagy and its role in plant abiotic stress management. Plant Cell Environ. 2019, 42(3):1045-1053. [CrossRef]
- Xing A, Gao Y, Ye L, Zhang W, Cai L, Ching A, Llaca V, Johnson B, Liu L, Yang X, Kang D, Yan J, Li J. A rare SNP mutation in Brachytic2 moderately reduces plant height and increases yield potential in maize. J Exp Bot. 2015, 66(13):3791-802. [CrossRef]
- Li N, Lin B, Wang H, Li X, Yang F, Ding X, Yan J, Chu Z. Natural variation in ZmFBL41 confers banded leaf and sheath blight resistance in maize. Nat Genet. 2019, 51(10):1540-1548. [CrossRef]
- Zhang P, Yan X, Gebrewahid TW, Zhou Y, Yang E, Xia X, He Z, Li Z, Liu D. Genome-wide association mapping of leaf rust and stripe rust resistance in wheat accessions using the 90K SNP array. Theor Appl Genet. 2021, 134(4):1233-1251. [CrossRef]
- Jia X, Gong X, Jia X, Li X, Wang Y, Wang P, Huo L, Sun X, Che R, Li T, Zou Y, Ma F. Overexpression of MdATG8i Enhances Drought Tolerance by Alleviating Oxidative Damage and Promoting Water Uptake in Transgenic Apple. Int J Mol Sci. 2021, 22(11):5517. [CrossRef]
- Kalvari I, Tsompanis S, Mulakkal NC, Osgood R, Johansen T, Nezis IP, Promponas VJ. iLIR: A web resource for prediction of Atg8-family interacting proteins. Autophagy. 2014, 10(5):913-25. [CrossRef]
- Nolan TM, Brennan B, Yang M, Chen J, Zhang M, Li Z, Wang X, Bassham DC, Walley J, Yin Y. Selective Autophagy of BES1 Mediated by DSK2 Balances Plant Growth and Survival. Dev Cell. 2017, 41(1):33-46.e7. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).