1. Introduction
Tako-tsubo syndrome (TTS) is an acute cardiac syndrome, mainly affecting women, often associated with a physical or emotional stressor and with a clinical presentation that can mimic an acute myocardial infarction [
1] (AMI). Unlike AMI, full recovery of left ventricular ejection fraction (LVEF) at echocardiogram and absence of myocardial scar at cardiac magnetic resonance (CMR) imaging have been described as key features of TTS [
2]. Notwithstanding, even in the long-term and after recovery of LVEF, patients with previous TTS may display persistent limiting symptoms, reduced myocardial strain and increased native T1 mapping values at CMR [
3,
4] as compared to control subjects. Furthermore, TTS patients with a physical trigger have been described as having a worse prognosis both in the short as well as in the long-term [
5,
6]. In these patients, the underlying acute illness could act in a synergistic fashion with TTS functional abnormalities, potentially resulting in a greater damage at cardiac level and long-term exercise intolerance.
The cardiopulmonary exercise testing (CPET) provides a non-invasive assessment of the functional capacity and exercise limitation, including information on individual responses to physical efforts at cardiac and non-cardiac level [
7,
8,
9]. A single study in a population of TTS patients with variable stressful triggers showed persistent subclinical CPET abnormalities as compared to controls [
3].
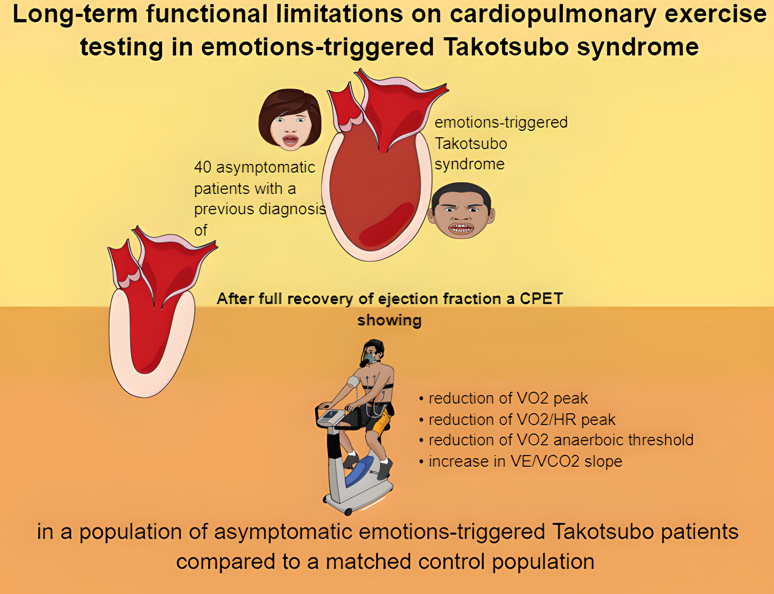
The aim of the present study was to investigate the role of CPET variables in the follow-up of a homogeneous cohort of patients with prior emotions-triggered TTS (E-TTS) and complete recovered left ventricular ejection fraction.
2. Materials and Methods
We performed an observational, retrospective case-control study of patients admitted for E-TTS at Vannini Hospital, Rome, Italy. This institution is part of the German-Italian-Spanish Tako-tsubo (GEIST) registry and shares enrollment criteria and protocols with other participating centers [
1]. From 2010 to 2021 we followed up 146 TTS patients [
10], amongst which we selected all those with emotional triggers (79 patients). Among the latter group, we included in the study those patients with recovered left ventricular ejection fraction and without diastolic dysfunction, moderate to severe valvulopathies nor overt clinical symptoms of dyspnea. Control subjects were matched for age, sex, body mass index and cardiovascular risk factors distribution and compared with the patients’ cohort. We excluded patients with ventilatory disorders.
2.1. Cardiopulmonary Exercise Test
Patients underwent CPET between 2 and 4 years from the acute hospitalization for E-TTS event. All patients receiving beta-blocker drugs were discontinued from their therapy 48 hours before performing CPET in order not to affect the VO
2 peak results [
11].
All patients performed a symptom-limited incremental cycle ergometer CPET. As standard, before each testing we performed CPET calibration procedures.
Tests were considered maximal if peak respiratory exchange ratio (RER) was >1.1. Oxygen uptake (VO2), carbon dioxide output (VCO2), minute ventilation (VE), and end-tidal carbon dioxide partial pressure (PETCO2) were measured breath-by-breath (Quark CPET, Rome, Italy) and averaged every 5 seconds for subsequent analysis. Heart rate (HR) was monitored via 12-lead electrocardiography (ECG). The oxygen pulse was calculated as the VO2/HR ratio at peak exercise. Heart Rate Reserve (HRR) was calculated from the difference between predicted peak HR (220 – age) and observed peak. The anaerobic threshold (AT) was identified by three methods: the V-slope, the ventilatory equivalent, and the end-tidal methods [
12]. Peak VO2 was defined as the highest VO2 that could be sustained for at least 15 seconds during the last stage of incremental exercise. The slope of VE over VCO2 (ΔVE/ΔVCO2) during incremental testing was measured from unloaded pedaling to the ventilatory compensation point (VCP). Omitting the latter phase from calculation of the slope, make the slope independent of the duration of the test and of the individual’s effort and response to metabolic acid [
13]. For patients who did not reached the VCP, it was measured from unloaded pedaling to peak exercise. The dead-space volume of the facemask was subtracted from the total VE before calculating individual VE/VCO2 slopes and ratios.
The CPET was safe for all patients, with no adverse events reported during the test.
Before exercise testing, all E-TTS patients underwent basic spirometry in order to calculate the maximum ventilatory ventilation (MVV) to exclude any possible reason of exercise limitation due to ventilatory abnormalities. Informed consent was obtained from each patient. The study was in line with the ethical guidelines of the 1975 Declaration of Helsinki.
2.2. Statistical Analysis
Continuous variables were expressed as mean ± standard deviation and compared using student’s t-test, when normal distributed. We used Kolmogorov-Smirnov test to assess normal distribution of variables. We reported the confidence interval for the mean difference at 95%. Non-normally distributed variables were expressed as median [interquartile range] and compared using Mann-Whitney non-parametric test. Statistical significance was set at p<0.01, in order to reduce the risk of type I errors resulting from multiple hypothesis tests. Categorical variables were compared with the Chi-squared test or Fisher’s exact test, as appropriate. All data were analyzed using SPSS Statistics 26 software.
3. Results
Among 79 E-TTS patients, 61 were asymptomatic in NYHA functional class I. E-TTS patients who performed an exercise with an RER greater than 1.1 were 39 (64%). This cohort was considered for the analysis.
The median time from acute E-TTS and the CPET was 30 [12–40] months. Patients were predominantly middle-aged (65.2 ±10.8 years) and women (85%). The demographic characteristics of E-TTS patients and controls at the time of CPET, and the characteristics of E-TTS acute event are listed respectively in
Table 1 and 2. The most frequent presenting symptom was angina (86%), mean LVEF at admission was 41 ± 7%.
As expected, on CPET we did not find any sign of exercise reduction due to ventilatory limitation (47.6 ± 13
vs. 34.1 ± 9.8; P <0.001). No desaturation occurred in the two groups. Compared with control subjects, patients with prior E-TTS had lower peak VO2 and percentage of predicted peak VO2 (17.8 ± 3.6
vs. 22.5 ± 6.5; P < 0.001 and 75.2 ± 14.1 %
vs. 100.6 ± 17.1%, P <0.001), VO2 at anaerobic threshold (AT) (11.1 [10.1-12.9]
vs. 14.4 [12.5-18.7]; P <0.001), peak O2 pulse (9.7 ± 2.5
vs. 13.1 ± 3.5; P <0.001) and higher VE/VCO2 slope (30.4 ± 3.7
vs. 27.2 ± 3.5; P <0.001) compared with matched controls (
Figure 1). We didn’t find any statistically significant difference between E-TTS patients and controls in heart rate reserve (HRR) (33.3 ± 19.4
vs. 26.3 ± 13.8; P=0.07), resting systolic blood pressure (120 [110–130]
vs. 120 [110–130]; P=0.98), resting diastolic blood pressure (80 [70–80]
vs. 80 [70–90]); P=0.21) , peak systolic blood pressure (165 [160–180]
vs. 170 [160–180]; P=0.46), peak diastolic blood pressure (100 [90–100]
vs. 100 [90–100]; P=0.3) peak end-tidal Pco
2 (36.2 ± 4
vs. 37 ± 5; P=0.4) and respiratory equivalent ratio (1.13 [1.1-1.2]
vs. 1.13 [1.1-1.2]; P=0.6).
4. Discussion
Long-term effects directly attributable to a TTS attack remain controversial, due to the paucity of available data and concomitant presence of comorbidities, acute diseases and other confounding factors that can impact the outcomes within this heterogeneous population. In our selected cohort of asymptomatic NYHA functional class I patients with E-TTS we found lower peak VO
2 and VO
2 pulse, lower VO
2 at AT and higher VE/VCO
2 slope values as compared with matched controls, highlighting the ability of CPET to identify impaired cardiovascular response to physical activity in the long-term after the acute event. When compared with reference values [
14], E-TTS patients had slightly lower percentage of predicted peak VO
2 and absolute peak VO
2 values and slightly higher VE/VCO
2 slope values. Lower peak VO
2 and VO
2 pulse, VO
2 at AT and higher VE/VCO
2 slope values without ventilatory limitations are all features of a heart failure (HF) phenotype [
15], with several studies showing that peak VO
2 and VE/VCO
2 slope are cardiovascular predictors of morbidity and mortality in HF of different etiologies [
16,
17]. The abnormalities found in our E-TTS population were less evident than those in HF populations [
18], possibly explaining the low symptoms burden referred by the patients.
Our results are in line with those from Scally et al. [
3], which showed a reduction in peak VO
2 and increase in VE/VCO
2 slope at long-term follow up in a heterogeneous population of twenty patients with previous TTS compared with matched subjects. Of note, in that study approximately one third of the TTS patients did not have an identifiable emotional trigger, a marker of worse prognosis which identifies a subset of high-risk comorbid patients [
6]. Furthermore, that mixed cohort of patients was still symptomatic long-term after the acute event, with the majority in NYHA functional class I-II.
Our findings highlight how CPET abnormalities can be detected even in the low-risk and asymptomatic E-TTS subset long time after the acute event, and hence suggest that this non-invasive tool could aid a more comprehensive characterization of TTS patients across the whole clinical spectrum, potentially providing an evidence-based reason to prospectively follow-up this subset of patients. Our preliminary results support further investigation of CPET in this clinical context, however leaving several areas of uncertainty. Firstly, detected abnormalities were not clearly pathologic and it cannot be excluded that they preceded the development of the TTS attack rather than being its consequence, in keeping with the highly vulnerable background of this population [
19]. Moreover, prognostic relevance of CPET in this scenario, where mortality in the long-term is mainly driven by non-cardiovascular events [
20], remains to be assessed.
Our study has some limitations. Despite peak VO
2 has consistently been found to be a robust variable in heart failure patients, it might be underestimated due to reduced patient effort. Nevertheless, VO
2 at AT and VE/VCO
2 slope below the VCP are variables in the submaximal range that showed a prognostic value in patients with chronic heart failure [
21]. Remembering the trigger of the acute event can be a source of recall bias that we cannot exclude in our study. However, we accurately reviewed medical records to exclude all TTS possibly related to medical conditions, procedures or neurologic disorders [
6].
5. Conclusions
Despite their overall favorable outcome, asymptomatic patients with E-TTS were found to have long-term subclinical functional cardiac impairments. CPET proved to be a useful tool in the long-term evaluation of E-TTS patients after recovery of LVEF. Further studies are needed to fully clarify the origin of the detected CPET abnormalities as well as their prognostic relevance in patience with recovered E-TTS.
Author Contributions
Conceptualization, Jean Pierre Jabbour and Silvia Papa; Data curation, Damiano Magrì, Tommaso Recchioni and Silvia Papa; Formal analysis, Jean Pierre Jabbour and Enrico Maggio; Investigation, Luca Arcari, Luca Cacciotti and Enrico Maggio; Methodology, Luca Arcari, Luca Cacciotti and Livia Valeri; Project administration, Silvia Papa; Supervision, Carmine Vizza, Roberto Badagliacca and Silvia Papa; Visualization, Carmine Vizza and Roberto Badagliacca; Writing – original draft, Jean Pierre Jabbour, Enrico Maggio and Silvia Papa.
Institutional Review Board Statement
“The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of La Sapienza University.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Acknowledgments
This work was supported by the Department of Clinical, Anesthesiologic and Cardiovascular Sciences of the Sapienza University of Rome.
Conflicts of Interest
The authors state that they do not have any conflict of interest related with the submitted study.
Abbreviations
| TTS |
Tako-tsubo syndrome |
| AMI |
Acute myocardial infarction |
| LVEF |
Left ventricular ejection fraction |
| CMR |
Cardiac magnetic resonance |
| CPET |
Cardiopulmonary exercise testing |
| E-TTS |
Emotions-triggered Tako-tsubo syndrome |
| GEIST |
German-Italian-Spanish Tako-tsubo registry |
| RER |
Respiratory exchange ratio |
| VO2 |
Oxygen uptake |
| VCO2 |
Carbon dioxide output |
| VE |
minute ventilation |
| PETCO2 |
end-tidal carbon dioxide partial pressure |
| HR |
heart rate |
| VO2/HR |
oxygen pulse |
| VCP |
Ventilatory compensation point |
| MVV |
maximum voluntary ventilation |
| AT |
anaerobic threshold |
| HF |
heart failure |
References
- Arcari, L., Núñez Gil, I. J., Stiermaier, et al. Gender differences in takotsubo syndrome. J. Am. Coll. Cardiol. 2022, 79, 2085–2093. [CrossRef]
- Dias, A., Núñez Gil, I. J., Santoro, et al. Takotsubo syndrome: State-of-the-art review by an expert panel - Part 2. Cardiovasc. Revasc. Med. 2019, 20, 153–166. [CrossRef]
- Scally, C., Rudd, A., Mezincescu, A. et al. Persistent long-term structural, functional, and metabolic changes after stress-induced (takotsubo) cardiomyopathy. Circulation 2018, 137, 1039–1048. [CrossRef]
- Schwarz, K., Ahearn, T., Srinivasan, J. et al. Alterations in cardiac deformation, timing of contraction and relaxation, and early myocardial fibrosis accompany the apparent recovery of acute stress-induced (takotsubo) cardiomyopathy: An end to the concept of transience. J. Am. Soc. Echocardiogr. 2017, 30, 745–755. [CrossRef]
- Uribarri, A., Núñez-Gil, I. J., Conty, D., et al. Short- and long-term prognosis of patients with Takotsubo Syndrome based on different triggers: Importance of the physical nature. J. Am. Heart Assoc. 2019, 8, e013701. [CrossRef]
- Ghadri, J. R., Kato, K., Cammann, V. L., et al. Long-term prognosis of patients with Takotsubo syndrome. J. Am. Coll. Cardiol. 2018, 72, 874–882. [CrossRef]
- Laveneziana, P., Di Paolo, M., & Palange, P. The clinical value of cardiopulmonary exercise testing in the modern era. Eur. Respir. Rev. 2021, 30, 200187. [CrossRef]
- Badagliacca R, Rischard F, Giudice FL, et al. Incremental value of cardiopulmonary exercise testing in intermediate-risk pulmonary arterial hypertension. J Heart Lung Transplant. 2022, 41, 780–790. [CrossRef]
- Ghio S, Acquaro M, Agostoni P, et al. Right heart failure in left heart disease: imaging, functional, and biochemical aspects of right ventricular dysfunction. Heart Fail Rev. 2023, 28, 1009–1022. [CrossRef]
- Cacciotti, L., Passaseo, I., Marazzi, G., et al. Observational study on Takotsubo-like cardiomyopathy: clinical features, diagnosis, prognosis and follow-up. BMJ Open 2012, 2, e001165. [CrossRef]
- Cramer, S.P., "Effects of Beta-Blockers on Maximal Oxygen Consumption". Master’s Theses. 2003, 4681. https://scholarworks.wmich.edu/masters_theses/4681.
- Sietsema, K. E., Stringer, W. W., Sue, D. Y., Ward, S., Wasserman & Whipp’s Principles of Exercise Testing and Interpretation. Measurements during integrative cardiopulmonary exercise testing. Sixth edition, Wolters Kluwer, NL, 2021 pp 71-73.
- Sietsema, K. E., Stringer, W. W., Sue, D. Y., Ward, S., Wasserman & Whipp’s Principles of Exercise Testing and Interpretation. Approaches to Data Summary and interpretation. Sixth edition. Wolters Kluwer, NL, 2021; pp 143-144.
- Wasserman, K., Zhang, Y. Y., Gitt, A., et al. Lung function and exercise gas exchange in chronic heart failure. Circulation 1997, 96, 2221–2227. [CrossRef]
- Sietsema, K. E., Stringer, W. W., Sue, D. Y., Ward, S., Wasserman & Whipp’s Principles of Exercise Testing and Interpretation. Diagnostic Specificity of Exercise Intolerance: a flowchart approach. Sixth edition. Wolters Kluwer, NL, 2021; pp 226-227.
- Stelken, A. M., Younis, L. T., Jennison, et al. Prognostic value of cardiopulmonary exercise testing using percent achieved of predicted peak oxygen uptake for patients with ischemic and dilated cardiomyopathy. J. Am. Coll. Cardiol. 1996, 27, 345–352. [CrossRef]
- Chua, T. P., Ponikowski, P., Harrington, D., et al. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J. Am. Coll. Cardiol. 1997, 29, 1585–1590. [CrossRef]
- Agostoni, P., Corrà, U., Cattadori, G., et al. Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: a multiparametric approach to heart failure prognosis. Int. J. Cardiol. 2013, 167, 2710–2718. [CrossRef]
- Limite, L. R., Arcari, L., Cacciotti, L., Russo, D., & Musumeci, M.B. Cardiogenic shock in takotsubo syndrome: A clue to unravel what hides behind the curtain? JACC. Heart Fail. 2019, 7, 175–176. [CrossRef]
- Scudiero, F., Arcari, L., Cacciotti, L., et al. Prognostic relevance of GRACE risk score in Takotsubo syndrome. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 721–728. [CrossRef]
- Sun, X.-G., Hansen, J. E., Beshai, J. F., & Wasserman, K. Oscillatory breathing and exercise gas exchange abnormalities prognosticate early mortality and morbidity in heart failure. J. Am. Coll. Cardiol. 2010, 55, 1814–1823. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).