Submitted:
01 December 2023
Posted:
04 December 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
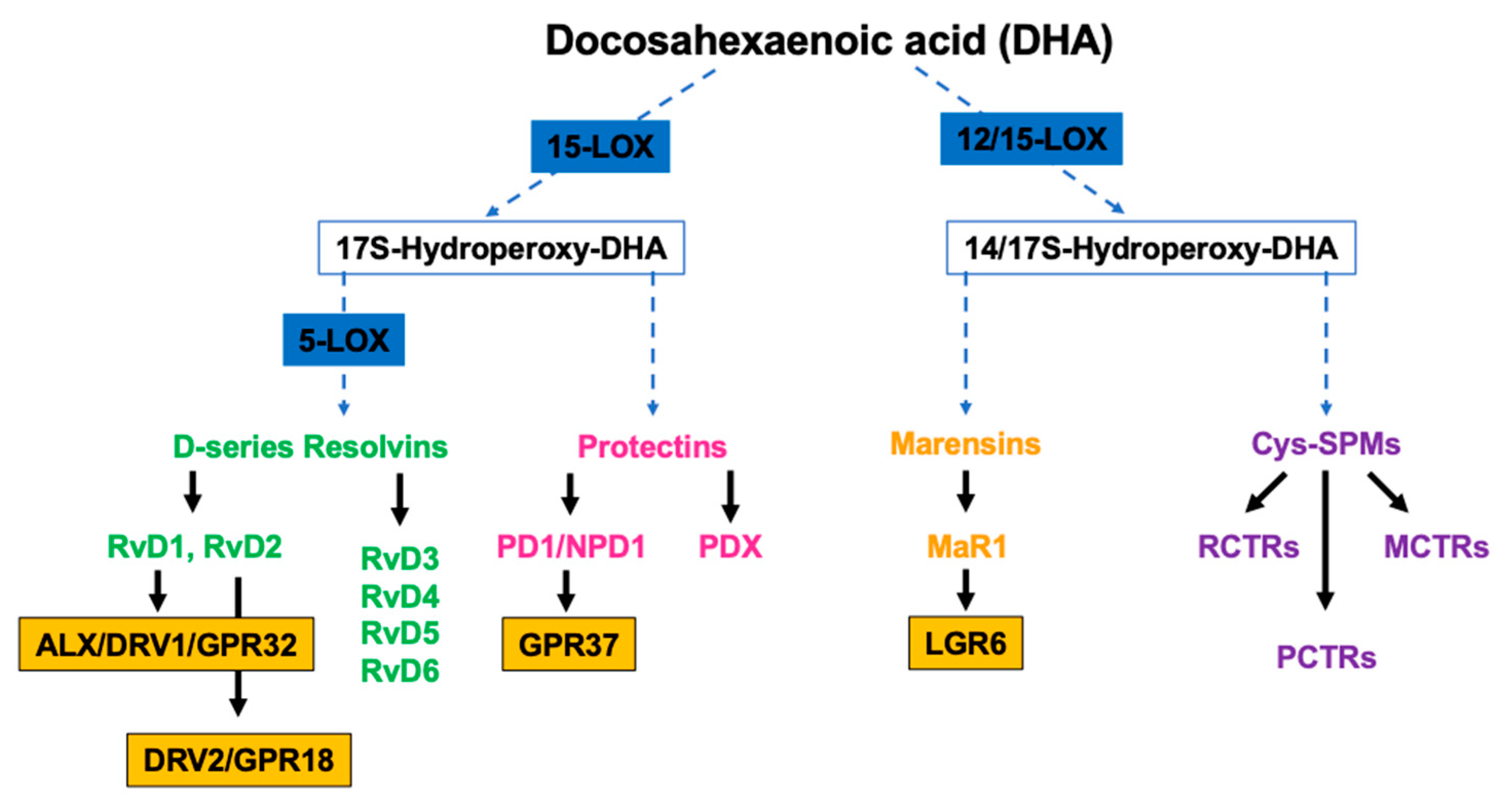
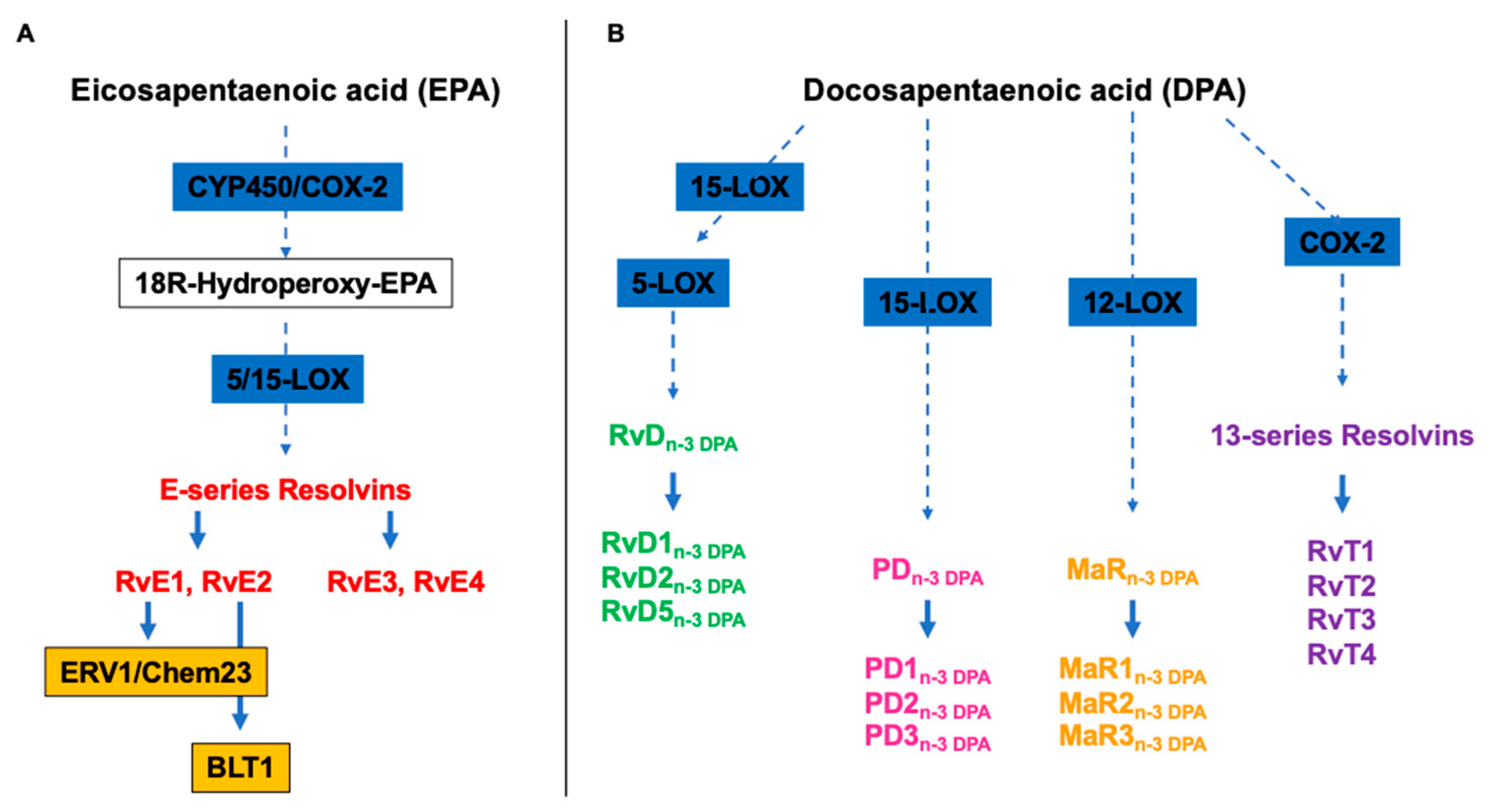
2. Omega-3 polyunsaturated fatty acids (n-3 PUFAs) and specialized pro-resolving mediators (SPMs)
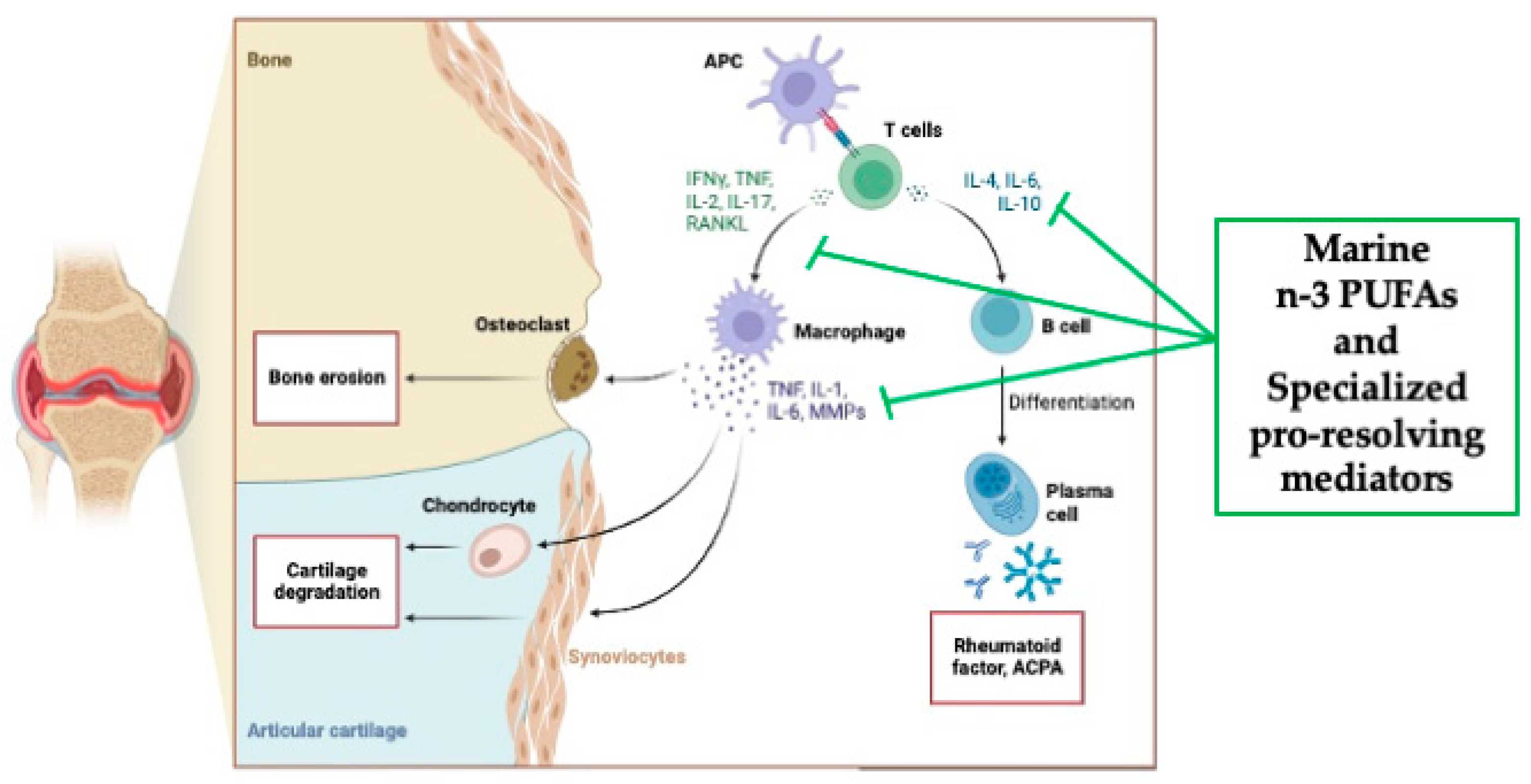
3. Rheumatoid arthritis (RA)
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization, Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases.
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019, 25, 1822-1832. [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018, 15, 505-522. [CrossRef]
- Calder, P.C.; Albers, R.; Antoine, J-M.; Blum, S.; Bourdet-Sicard, R.; Ferns, G.A.; Folkerts, G.; Friedmann, P.S.; Frost, G.S.; Guarner, F.; et al. Inflammatory disease processes and interactions with nutrition. Br J Nutr. 2009, 101, S1-45. [CrossRef]
- Netea, M.G.; Balkwill, F.; Chonchol, M.F.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat Immunol. 2017, 18, 826-831. [CrossRef]
- Larsen, G.L.; Henson, P.M. Mediators of inflammation. Annu Rev Immunol. 1993, 1, 335-359. [CrossRef]
- Serhan, C.N. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. Faseb J. 2017, 31, 1273-1288. [CrossRef]
- Barnig, C.; Bezema, T.; Calder, P.C.; Charloux, A.; Frossard, N.; Garssen, J.; Haworth, O.; Dilevskaya, K.; Levi-Schaffer, F.; Lonsdorfer, E.; et al. Activation of Resolution Pathways to Prevent and Fight Chronic Inflammation: Lessons from Asthma and Inflammatory Bowel Disease. Front Immunol. 2019, 10, 1699. [CrossRef]
- Dalli, J.; Serhan, C.N. Pro-Resolving Mediators in Regulating and Conferring Macrophage Function. Front Immunol. 2017, 8, 1400. [CrossRef]
- Smith, T.D.; Nagalla, R.R.; Chen, E.Y.; Liu, W.F. Harnessing macrophage plasticity for tissue regeneration. Adv Drug Deliv Rev. 2017, 114, 193-205. [CrossRef]
- Li, J.; Tan, J.; Martino, M.M.; Lui, K.O. Regulatory T-Cells: Potential Regulator of Tissue Repair and Regeneration. Front Immunol. 2018, 9 585. [CrossRef]
- Rauber, S.; Luber. M.; Weber, S.; Maul, L.; Soare, A.; Wohlfahrt, T.; Lin, N-Y.; Dietel, K.; Bozec, A.; Herrmann, M.; et al. Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat Med. 2017, 23, 938-944. [CrossRef]
- Poe, S.L.; Arora, M.; Oriss, T.B.; Yarlagadda, M.; Isse, K.; Khare, A.; Levy, D.E.; Lee, J.S.; Mallampalli, R.K.; Chanet, Y.R. et al. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal Immunol. 2013, 6, 189-199. [CrossRef]
- Ray, A.; Chakraborty, K.; Ray, P. Immunosuppressive MDSCs induced by TLR signaling during infection and role in resolution of inflammation. Front Cell Infect Microbiol. 2013, 3, 52. [CrossRef]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001, 2, 612-619. [CrossRef]
- Sugimoto, M.A.; Vago, J.P.; Perretti, M.; Teixeira, M.M. Mediators of the Resolution of the Inflammatory Response. Trends Immunol. 2019, 40, 212-227. [CrossRef]
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016, 15, 551-567. [CrossRef]
- Gilroy, D.W.; Lawrence, T.; Perretti, M.; Rossi, A.G. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004, 3, 401-416. [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA. 2018, 320, 1360-1372. [CrossRef]
- Figus, F.A.; Piga, M.; Azzolin, I.; McConnell, R.; Iagnocco, A. Rheumatoid arthritis: Extra-articular manifestations and comorbidities. Autoimmun Rev. 2021, 20, 102776. [CrossRef]
- Illiano, P.; Brambilla, R.; Parolini, C. The mutual interplay of gut microbiota, diet and human disease. Febs J. 2020, 287, 833-855. [CrossRef]
- Parolini, C. Effects of Fish n-3 PUFAs on Intestinal Microbiota and Immune System. Mar Drugs. 2019, 17, 374. [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015, 4, 469-484. [CrossRef]
- Parolini, C. Marine n-3 polyunsaturated fatty acids: Efficacy on inflammatory-based disorders. Life Sci. 2020, 263, 118591. [CrossRef]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001, 276, 16683-16689. [CrossRef]
- Kumar, A.; Takada, Y.; Boriek, A.M.; Aggarwal, B.B. Nuclear factor-kappaB: its role in health and disease. Mol Med (Berl). 2004, 82, 434-448. [CrossRef]
- Perkins, N.D. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007, 8, 49-62. [CrossRef]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv Nutr. 2015, 6, 513-540. [CrossRef]
- Spite, M.; Norling, L.V.; Summers, L.; Yang, R.; Cooper, D.; Petasis, N.A.; Flower, R.J.; Perretti, M.; Serhan, C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009, 461, 1287-1291. [CrossRef]
- Panigrahy, D.; Gilligan, M.M.; Serhan, C.N.; Kashfi, K. Resolution of inflammation: An organizing principle in biology and medicine. Pharmacol Ther. 2021, 227, 107879. [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014, 510, 92-101. [CrossRef]
- Serhan, C.N.; Brain, S.D.; Buckley, C.D.; Gilroy, D.W.; Haslett, C.; O’Neill, L.A.J.; Perretti, M.; Rossi, A.G.; Wallace, J.L.; Resolution of inflammation: state of the art, definitions and terms. Faseb J. 2007, 21, 325-332. [CrossRef]
- Zhang, L.; Qiu, C.; Yang, L.; Zhang, Z.; Zhang, Q.; Wang, B.; Wang, X. GPR18 expression on PMNs as biomarker for outcome in patient with sepsis. Life Sci. 2019, 217, 49-56. [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J-M.; Lein, P.J. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog Lipid Res. 2022, 86, 101165. [CrossRef]
- Serhan CN, Gotlinger K, Hong S, et al. (2006) Anti-inflammatory actions of neuroprotection D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol 176, 1848. [CrossRef]
- Schwarz, B.; Sharma, L.; Robert, L.; Peng, X.; Bermejo, S.; Leighton, J.; Casanovas-Massana, A.; Minasyan, M.; Farhadian, S.; Ko, A.I. Cutting Edge: Severe SARS-CoV-2 Infection in Humans Is Defined by a Shift in the Serum Lipidome, Resulting in Dysregulation of Eicosanoid Immune Mediators. J Immunol. 2021, 206, 329-334. [CrossRef]
- de la Rosa, X.; Norris, P.C.; Chiang, N.; Rodriguez, A.R.; Spur, B.W.; Serhan, C.N.; Identification and Complete Stereochemical Assignments of the New Resolvin Conjugates in Tissue Regeneration in Human Tissues that Stimulate Proresolving Phagocyte Functions and Tissue Regeneration. Am J Pathol. 2018, 188, 950-966. [CrossRef]
- Ramon, S.; Dalli, J.; Sanger, J.M.; Winkler, J.W.; Aursnes, M.; Tungen, J.E.; Hansen, T.V.; Serhan, C.N. The Protectin PCTR1 Is Produced by Human M2 Macrophages and Enhances Resolution of Infectio us Inflammation. Am J Pathol. 2016, 186, 962-973. [CrossRef]
- Dalli, J.; Sanger, J.M.; Rodriguez, A.R.; Chiang, N.; Spur, B.W.; Serhan, C.N. Identification and Actions of a Novel Third Maresin Conjugate in Tissue Regeneration: MCTR3. PloS One. 2016, 11, e0149319. [CrossRef]
- Chiang, N.; Serhan, C.N. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Aspects Med. 2017, 58, 114-129. [CrossRef]
- Dalli, J.; Colas, R.A.; Serhan, C.N. Novel n-3 immunoresolvents: structures and actions. Sci Rep. 2013, 3, 1940. [CrossRef]
- Dalli, J.; Chiang, N.; Serhan, C.N. Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat Med. 2015, 21, 1071. [CrossRef]
- Walker, M.E.; Souza, P.R.; Colas, R.A.; Dalli, J. 13-Series resolvins mediate the leukocyte-platelet actions of atorvastatin and pravastatin in inflammatory arthritis. Faseb J. 2017, 31, 3636-3648. [CrossRef]
- Firestein, G.S. Evolving concept of rheumatoid arthritis. Nature. 2003, 423, 356-361. [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011, 365, 2205-2219. [CrossRef]
- Myasoedova, E.; Davis, J.MIII.; Crowson, C.S.; Gabriel, S.E. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010, 5, 379-385. [CrossRef]
- Mason, J.C.; Libby, P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J. 2015, 36, 482-489. [CrossRef]
- Gioxari, A.; Kaliora, A.C.; Marantidou, F.; Panagiotakos, D.P. Intake of ω-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A systematic review and meta-analysis. Nutrition. 2018, 45, 114. [CrossRef]
- MacGregor, A.J.; Snieder, H.; Rigby, A.S.; Koskenvuo, M.; Kaprio, J.; Aho, K.; Silman, A.J. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 2000, 43, 30-37. [CrossRef]
- Okada, Y.; Wu, D.; Trynka, G.; Raj, T.; Terao, C.; Ikari, K.; Kochi, Y.; Ohmura, K.; Suzuki, A.; Yoshida, S.; et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014, 506, 376-381. [CrossRef]
- Gregersen, P.K.; Silver, J.; Winchester, R.J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987, 30, 1205-1213. [CrossRef]
- Felix, N.J.; Suri, A.; Salter-Cid, L.; Nadler, S.G.; Gujrathi, S.; Corbo, M.; Aranda, R. Targeting lymphocyte co-stimulation: from bench to bedside. Autoimmunity. 2010, 43, 514-525. [CrossRef]
- De Rycke, L.; Peene, I.; Hoffman, I.E.A.; Kruithof, E.; Union, A.; Meheus, L.; Lebeer K.; Wyns, B.; Vincent, C.; Mielants, H.; et al. Rheumatoid factor and anticitrullinated protein antibodies in rheumatoid arthritis: diagnostic value, associations with radiological progression rate, and extra-articular manifestations. Ann Rheum Dis. 2004, 63, 1587-1593. [CrossRef]
- Kallberg, H.; Padyukov, L.; Plenge, R.M.; Ronnelid, J.; Gregersen, P.K.; van der Helm-van Mil, A.H.M.; Toes, R.E.M.; Huizinga, T.W.; Klareskog, L.; Alfredsson, L.; et al. Gene-gene and gene-environment interactions involving HLA-DRB1, PTPN22, and smoking in two subsets of rheumatoid arthritis. Am J Hum Genet. 2007, 80, 867-875. [CrossRef]
- Remmers, E.F.; Plenge, R.M.; Lee, A.T.; Graham, R.R.; Hom, G.; Behrens, T.W.; de Bakker, P.I.W.; Le, J.M.; Lee, H-S.; Batliwalla, F.; et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N Engl J Med. 2007, 357, 977-986. [CrossRef]
- Klareskog, L.; Stolt, P.; Lundberg, K.; Källberg, H.; Bengtsson, C.; Grunewald, J.; Rönnelid, J.; Harris, H.E.; Ulfgren, A-K.; Rantapää-Dahlqvist, S.; et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006, 54, 38-46. [CrossRef]
- Stolt, P.; Yahya, A.; Bengtsson, C.; Källberg, H.; Rönnelid, J.; Lundberg, I.; Klareskog, L.; Alfredsson, L.; EIRA Study Group. Silica exposure among male current smokers is associated with a high risk of developing ACPA-positive rheumatoid arthritis. Ann Rheum Dis. 2010, 69, 1072-1076. [CrossRef]
- Källberg, H.; Ding, B.; Padyukov, L.; Bengtsson, C.; Rönnelid, J.; Klareskog, L.; Alfredsson; L.; EIRA Study Group. Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis. 2011, 70, 508-511. [CrossRef]
- van der Woude, D.; Rantapää-Dahlqvist, S.; Ioan-Facsinay, A.; Onnekink, C.; Schwarte, C.M.; Verpoort, K.N.; Drijfhout, J.W.; Huizinga, T.W.J.; Toes, R.E.M.; Pruijn, G.J.M. Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis. Ann Rheum Dis. 2010, 69, 1554-1561. [CrossRef]
- Mahdi, H.; Fisher, B.A.; Källberg, H.; Plant, D.; Malmström, V.; Rönnelid, J.; Charles, P.; Ding, B.; Alfredsson, L.; Padyukov, L.; et al. Specific interaction between genotype, smoking and autoimmunity to citrullinated alpha-enolase in the etiology of rheumatoid arthritis. Nat Genet. 2009, 41, 1319-1324. [CrossRef]
- Takei, M.; Kitamura, N.; Nagasawa, Y.; Tsuzuki, H.; Iwata, M.; Nagatsuka, Y.; Nakamura, H.; Imai, K.; Fujiwara, S. Are Viral Infections Key Inducers of Autoimmune Diseases? Focus on Epstein-Barr Virus. Viruses. 2022, 14, 1900. [CrossRef]
- Kamphuis, S.; Kuis, W.; de Jager, W.; Teklenburg, G.; Massa, M.; Gordon, G.; Boerhof, M.; Rijkers, G.T.; Uiterwaal, C.S.; Otten, H.G.; et al. Tolerogenic immune responses to novel T-cell epitopes from heat-shock protein 60 in juvenile idiopathic arthriti. Lancet. 2005, 366, 50-56. [CrossRef]
- Wegner, N.; Wait, R.; Sroka, A.; Eick, S.; Nguyen, K-A.; Lundberg, K.; Kinloch, A.; Culshaw, S.; Potempa, J.; Venables, P.J. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010, 62, 2662-2672. [CrossRef]
- Manasson, J.; Blank, R.B.; Scher, J.U. The microbiome in rheumatology: Where are we and where should we go? Ann Rheum Dis. 2020, 79, 727-733. [CrossRef]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013, 2, e01202. [CrossRef]
- Attur, M.; Scher, J.U.; Abramson, S.B.; Attur, M. Role of Intestinal Dysbiosis and Nutrition in Rheumatoid Arthritis. Cells. 2022, 11, 2436. [CrossRef]
- Sweeney, S.E.; Firestein, G.S. Rheumatoid arthritis: regulation of synovial inflammation. Int J Biochem Biol. 2004, 36, 372-378. [CrossRef]
- Komatsu, N.; Takayanagi, H. Mechanisms of joint destruction in rheumatoid arthritis – immune cell-fibroblast-bone interactions. Nat Rev Rheumatol. 2022, 18, 415-429. [CrossRef]
- Feldmann, M.; Maini, R.N. The role of cytokines in the pathogenesis of rheumatoid arthritis. Rheumatology. 1999, 38 (Suppl. 2) 3-7.
- Ohata, J.; Zvaifler, N.J.; Nishio, M.; Boyle, D.L.; Kalled, S.L.; Carson, D.A.; Kipps, T.J. Fibroblast-like synoviocytes of mesenchymal origin express functional B cell-activating factor of the TNF family in response to proinflammatory cytokines. J Immunol. 2005, 174, 864-870. [CrossRef]
- Edwards, J.C.W.; Szczepanski, L.; Szechinski, J.; Filipowicz-Sosnowska, A.; Emery, P.; Close, D.R.; Stevens, R.M., Shaw, T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004, 350, 2572-2581. [CrossRef]
- Haringman, J.J.; Gerlag, D.H.; Zwinderman, A.H.; Smeets, T.J.M.; Kraan, M.C.; Baeten, D.; McInnes, I.B.; Bresnihan, B.; Tak, P.P. Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005, 64, 834-838. [CrossRef]
- Liew, F.Y.; McInnes, I.B. The role of innate mediators in inflammatory response. Mol Immunol. 2002, 38, 887-890. [CrossRef]
- Krishnamurthy, A.; Joshua, V.; Hensvold, A.H.; Jin, T.; Sun, M.; Vivar, N.; Ytterberg, A.J.; Engström, M.; Fernandes-Cerqueira, C.; Amara, K.; et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann Rheum Dis. 2016, 75, 721-729. [CrossRef]
- McInnes, I.B.; Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017, 389, 2328-2337. [CrossRef]
- Hess, A.; Axmann, R.; Rech, J.; Finzel, S.; Heindl, C.; Kreitz, S.; Sergeeva, M.; Saake, M.; Garcia, M.; Kollias, G.; et al. Blockade of TNF-α rapidly inhibits pain responses in the central nervous system. Proc Natl Acad Sci U S A. 2011, 108, 3731-3736. [CrossRef]
- Li, F.; Ai, W.; Ye, J.; Wang, C.; Yuan, S.; Xie, Y.; Mo, X.; Li, W.; He, Z.; Chen, Y.; et al. Inflammatory markers and risk factors of RA patients with depression and application of different scales in judging depression. Clin Rheumatol. 2022, 41, 2309-2317. [CrossRef]
- McInnes, I.B.; Leung, B.P.; Liew, F.Y. Cell-cell interactions in synovitis. Interactions between T lymphocytes and synovial cells. Arthritis Res. 2000, 2, 374-378. [CrossRef]
- Marcouiller, P.; Pelletier, J-P.; Guévremont, M.; Martel-Pelletier, J.; Ranger, P.; Laufer, S.; Reboul, P. Leukotriene and prostaglandin synthesis pathways in osteoarthritic synovial membranes: regulating factors for interleukin 1beta synthesis. J Rheumatol. 2005, 32, 704-712.
- van der Heijde, D.M. Joint erosions and patients with early rheumatoid arthritis. Br J Rheumatol. 1995, 34, 74-78.
- Visser, H.; le Cessie, S.; Vos, K.; Breedveld, F.C.; Hazes, J.M.W. How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheuml. 2002, 46, 357-365. [CrossRef]
- Schett, G.; Teitelbaum, S.L. Osteoclasts and Arthritis. J Bone Miner Res. 2009, 24, 1142-1146.
- Schett, G.; Stach, C.; Zwerina, J.; Voll, R.; Manger, B. How antirheumatic drugs protect joints from damage in rheumatoid arthritis. Arthritis Rheuml. 2008, 58, 2936-2948. [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023, 82, 3-18. [CrossRef]
- van Nies, J.A.; Krabben, A.; Huizinga, J.W.; Huizinga, T.W.J.; Kloppenburg, M.; van der Helm-van Mil, A.H.M. What is the evidence for the presence of a therapeutic window of opportunity in rheumatoid arthritis? A systematic literature review. Ann Rheum Dis. 2014, 73, 861-870. [CrossRef]
- Burmester, G.R.; Bijlsma, J.W.J.; Cutolo, M.; McInnes, I.B. Managing rheumatic and musculoskeletal diseases - past, present and future. Nat Rev Rheumatol. 2017, 13, 443-448. [CrossRef]
- Bergstra, S.A.; Sepriano, A.; Kerschbaumer, A.; van der Heijde, D.; Caporali, R.; Edwards, C.J.; Verschueren, P.; de Souza, S.; Pope, J.E.; Takeuchi, T.; et al. Efficacy, duration of use and safety of glucocorticoids: a systematic literature review informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2023, 82, 81-94. [CrossRef]
- Sepriano, A.; Kerschbaumer, A.; Bergstra, S.A.; Smolen, J.S.; van der Heijde, D.; Caporali, R.; Edwards, C.J.; Verschueren, P.; de Souza, S.; Pope, J.E.; et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2023, 82, 107-118. [CrossRef]
- Kerschbaumer, A.; Sepriano, A.; Bergstra, S.A.; Smolen, J.S.; van der Heijde, D.; Caporali, R.; Edwards, C.J.; Verschueren, P.; de Souza, S.; Pope, J.E.; et al. Efficacy of synthetic and biological DMARDs: a systematic literature review informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2023, 82, 95-106. [CrossRef]
- Weng, H.H.; Ranganath, V.K.; Khanna, D.; Oh, M.; Furst, D.E.; Park, G.S.; Elashoff, D.A.; Sharp, J.T.; Gold, R.H.; Peter, J.B.; et al. Equivalent responses to disease-modifying antirheumatic drugs initiated at any time during the first 15 months after symptom onset in patients with seropositive rheumatoid arthritis. J Rheumatol. 2010, 37, 550-557. [CrossRef]
- Burmester, G.R.; Pope, J.E. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017, 389, 2338-2348. [CrossRef]
- Tanski, W.; Swiatoniawska-Lonc, N.; Tabin, M.; Jankowska-Polanska, B. The Relationship between Fatty Acids and the Development, Course and Treatment of Rheumatoid Arthritis. Nutrients. 2022, 14, 1030. [CrossRef]
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid arthritis. Lancet. 2010, 9746, 1094-10108. [CrossRef]
- Nikiphorou. E.; Philippou, E. Nutrition and its role in prevention and management of rheumatoid arthritis. Autoimmun Rev. 2023, 7, 103333. [CrossRef]
- Leslie, C.A.; Gonnerman, W.A.; Ullman, M.D.; Hayes, K.C.; Franzblau, C.; Cathcart, E.S. Dietary fish oil modulates macrophage fatty acids and decreases arthritis susceptibility in mice. J Exp Med. 1985, 4, 1336-1349. [CrossRef]
- Volker, D.H.; FitzGerald, P.E.; Garg, M.L. The eicosapentaenoic to docosahexaenoic acid ratio of diets affects the pathogenesis of arthritis in Lew/SSN rats. J Nutr. 2000, 3, 559-565. [CrossRef]
- Ierna, M.; Kerr, A.; Scales, H.; Berge, K.; Griinari, M. Supplementation of diet with krill oil protects against experimental rheumatoid arthritis. BMC Musculoskelet Disord. 2010, 11, 136. [CrossRef]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Jones, P.J.H. Enhanced increase of omega-3 index in healthy individuals with response to 4-week n-3 fatty acid supplementation from krill oil versus fish oil. Lipid Health Dis. 2013, 12, 178. [CrossRef]
- Di Giuseppe, D.; Vallin, A.; Bottai, M.; Askling, J.; Wolk, A. Long-term intake of dietary long-chain n-3 polyunsaturated fatty acids and risk of rheumatoid arthritis: a prospective cohort study of women. Ann Rheum Dis. 2014, 11, 1949-1953. [CrossRef]
- Rosell, M.; Wesley, A.M.; Rydin, K.; Klareskog, L.; Alfredsson, L.; EIRA study group. Dietary fish and fish oil and the risk of rheumatoid arthritis. Epidemiology. 2009, 6, 896. Doi.10.1097/EDE.0b013e3181b5f0ce.
- Pedersen, M.; Stripp, C.; Klarlund, M.; Olsen, S.F.; Tjønneland, A.M.; Frisch, M. Diet and risk of rheumatoid arthritis in a prospective cohort. J Rheumatol. 2005, 7, 1249-1252.
- Asoudeh, F.; Jayedi, A.; Kavian, Z.; Ebrahimi-Mousavi, S.; Nielsen, S.M.; Mohammadi, H. A systematic review and meta-analysis of observational studies on the association between animal protein sources and risk of rheumatoid arthritis. Clin Nut. 2021, 7, 4644. [CrossRef]
- Kremer, J.M.; Bigauoette, J.; Michalek, A.V.; Timchalk, M.A.; Lininger, L.; Rynes, R.I.; Huyck, C.; Zieminski, J.; Bartholomew L.E. Effects of manipulation of dietary fatty acids on clinical manifestations of rheumatoid arthritis. Lancet. 1985, 8422, 184-187. [CrossRef]
- Espersen, G.T.; Grunnet, N.; Lervang, H.H.; Nielsen, G.L.; Thomsen, B.S.; Faarvang, K.L.; Dyerberg, J.; Ernst, E. Decreased interleukin-1 beta levels in plasma from rheumatoid arthritis patients after dietary supplementation with n-3 polyunsaturated fatty acids. Clin Rheumatol. 1992, 11, 393-395. [CrossRef]
- Sperling, R.I.; Weinblatt, M.; Robin, J.L.; Ravalese, J3rd.; Hoover, R.L.; House, F.; Coblyn, J.S.; Fraser, P.A.; Spur, B.W.; Robinson, D.R.; et al. Effects of dietary supplementation with marine fish oil on leukocyte lipid mediator generation and function in rheumatoid arthritis. Arthritis Rheum. 1987, 9, 988-997. [CrossRef]
- Dawczynski, C.; Hackermeier, U.; Viehweger, M.; Stange, R.; Springer, M.; Jahreis, G. Incorporation of n-3 PUFA and γ-linolenic acid in blood lipids and red blood cell lipids together with their influence on disease activity in patients with chronic inflammatory arthritis--a randomized controlled human intervention trial. Lipids Health Dis. 2011, 10, 130. [CrossRef]
- Dawczynski, C.; Schubert, R.; Hein, G.; Müller, A.; Eidner, T.; Vogelsang, H.; Basu, S.; Jahreis, G. Long-term moderate intervention with n-3 long-chain PUFA-supplemented dairy products: effects on pathophysiological biomarkers in patients with rheumatoid arthritis. Br J Nutr. 2009, 10, 1517-1526. [CrossRef]
- Belch, J.J.; Ansell, D.; Madhok, R.; O'Dowd, A.; Sturrock, R.D. Effects of altering dietary essential fatty acids on requirements for non-steroidal anti-inflammatory drugs in patients with rheumatoid arthritis: a double blind placebo controlled study. Ann Rheum Dis. 1988, 2, 96-104. [CrossRef]
- Geusens, P.; Wouters, C.; Nijs, J.; Jiang, Y.; Dequeker, J. Long-term effect of omega-3 fatty acid supplementation in active rheumatoid arthritis. A 12-month, double-blind, controlled study. Arthritis Rheum. 1994, 6, 824. [CrossRef]
- Galarraga, B.; Ho, M.; Youssef, H.M.; Hill, A.; McMahon, H.; Hall, C.; Ogston, S.; Nuki, G.; Belch, J.J.F. Cod liver oil (n-3 fatty acids) as an non-steroidal anti-inflammatory drug sparing agent in rheumatoid arthritis. Rheumatology (Oxford). 2008, 5, 665-669. [CrossRef]
- Goldberg, R.J.; Katz, J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007, 129, 210. [CrossRef]
- Ritchie, D.M.; Boyle, J.A.; Mclnnes, J.M.; Jasani, M.K.; Dalakos, T.G.; Grieveson, P.; Buchanan, W.W. Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. Q J Med. 1968, 37, 393-406.
- Moreno, J.J.; Carbonell, T.; Sanchez, T.; Miret, S.; Mitjavila, M.T. Olive oil decreases both oxidative stress and the production of arachidonic acid metabolites by the prostaglandin G/H synthase pathway in rat macrophages. J Nutr. 2001, 131, 2145-2149. [CrossRef]
- Lee, Y-H.; Bae, S-C.; Song, G.G. Omega-3 polyunsaturated fatty acids and the treatment of rheumatoid arthritis: a meta-analysis. Arch Med Res. 2012, 43, 356-362. [CrossRef]
- Fatel, E.C.S.; Rosa, F.T.; Alfieri, D.F.; Flauzino, T.; Scavuzzi, B.M.; Lozovoy, M.A.B.; Iriyoda, T.M.V.; Simão, A.N.C.; Dichi, I. Beneficial effects of fish oil and cranberry juice on disease activity and inflammatory biomarkers in people with rheumatoid arthritis. Nutrition. 2021, 86, 111183. [CrossRef]
- Yamada, H.; Saegusa, J.; Sendo, S.; Ueda, Y.; Okano, T.; Shinohara, M.; Morinobu, A. Effect of resolvin D5 on T cell differentiation and osteoclastogenesis analyzed by lipid mediator profiling in the experimental arthritis. Sci Rep. 2021, 11, 17312. [CrossRef]
- Marchand, N.E.; Choi, M.Y.; Oakes, E.G.; Cook, N.R.; Stevens, E.; Gomelskaya, N.; Kotler, G.; Manson, J.E.; Lasky-Su, J.; Mora, S.; et al. Over-the-counter fish oil supplementation and pro-resolving and pro-inflammatory lipid mediators in rheumatoid arthritis. Prostaglandins Leukot Essent Fatty Acid. 2023, 190, 102542. [CrossRef]
- Sigaux, J.; Mathieu, S.; Nguyen, Y.; Sanchez, P.; Letarouilly, J-G.; Soubrier, M.; Czernichow, S.; Flipo, R-M.; Sellam, J.; Daïen C. Impact of type and dose of oral polyunsaturated fatty acid supplementation on disease activity in inflammatory rheumatic diseases: a systematic literature review and meta-analysis. Arthritis Res Ther. 2022, 24, 100. [CrossRef]
- Gkiouras, K.; Grammatikopoulou, M.G.; Myrogiannis, I.; Papamitsou, T.; Rigopoulou, E.I.; Sakkas, L.I.; Bogdanos, D.P. Efficacy of n-3 fatty acid supplementation on rheumatoid arthritis' disease activity indicators: a systematic review and meta-analysis of randomized placebo-controlled trials. Crit Rev Food Sci Nutr. 2022, 28, 1-15. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




