1. Introduction
As described in literature [
1,
2], COVID-19 infection is burdened from a high rate of thrombotic complications. The status of increased blood hypercoagulabity, probably due to systemic hyper-inflammation and endothelial dysfunction, is associated with both venous and arterial thrombosis [
3,
4]. Arterial thrombosis account for about 4% of thromboembolic complications during COVID-19. Native aortic acute thrombosis related to Sars-CoV2 infection is largely described [
5,
6], but, to our knowledge, no case of acute stent-graft occlusion has been previously described in literature and there are no clear international guidelines regarding peri-operative management of patients with Sars-Cov2, even though it’s been four years since it appeared.
We report a case of a patient recently treated for abdominal aorto-iliac aneurysm by EVAR with Bolton Treo stent-graft implantation (Bolton Medical Inc., Sunrise, FL, USA), four days after resolution of an asymptomatic Sars-Cov2 infection. At the time of emergency admission, the clinical suspicion of aortic stent-graft thrombosis was confirmed by computed tomography angiography (CTA).
Informed consent of the patient was obtained. Patient gave his consent to the publication.
2. Case Presentation Section
A 73-years-old male patient underwent elective EVAR by Bolton Treo stent-graft implantation for an aorto-iliac aneurysm through bilateral percutaneous common femoral arteries (CFA) access.
Patient’s medical history reported hypertension, hypercholesterolemia, chronic obstructive pulmonary disease, previous open abdominal surgery, and a right popliteal artery aneurysm with a maximum transversal diameter of 23 mm. The patient had all the arterial pulses at the lower extremities.
The sizing of the endoprosthesis was chosen following the IFU, the proximal aortic neck had a length of 15 mm and a diameter of 24 mm, therefore a Bolton Treo with a proximal diameter of 28 mm was implanted.
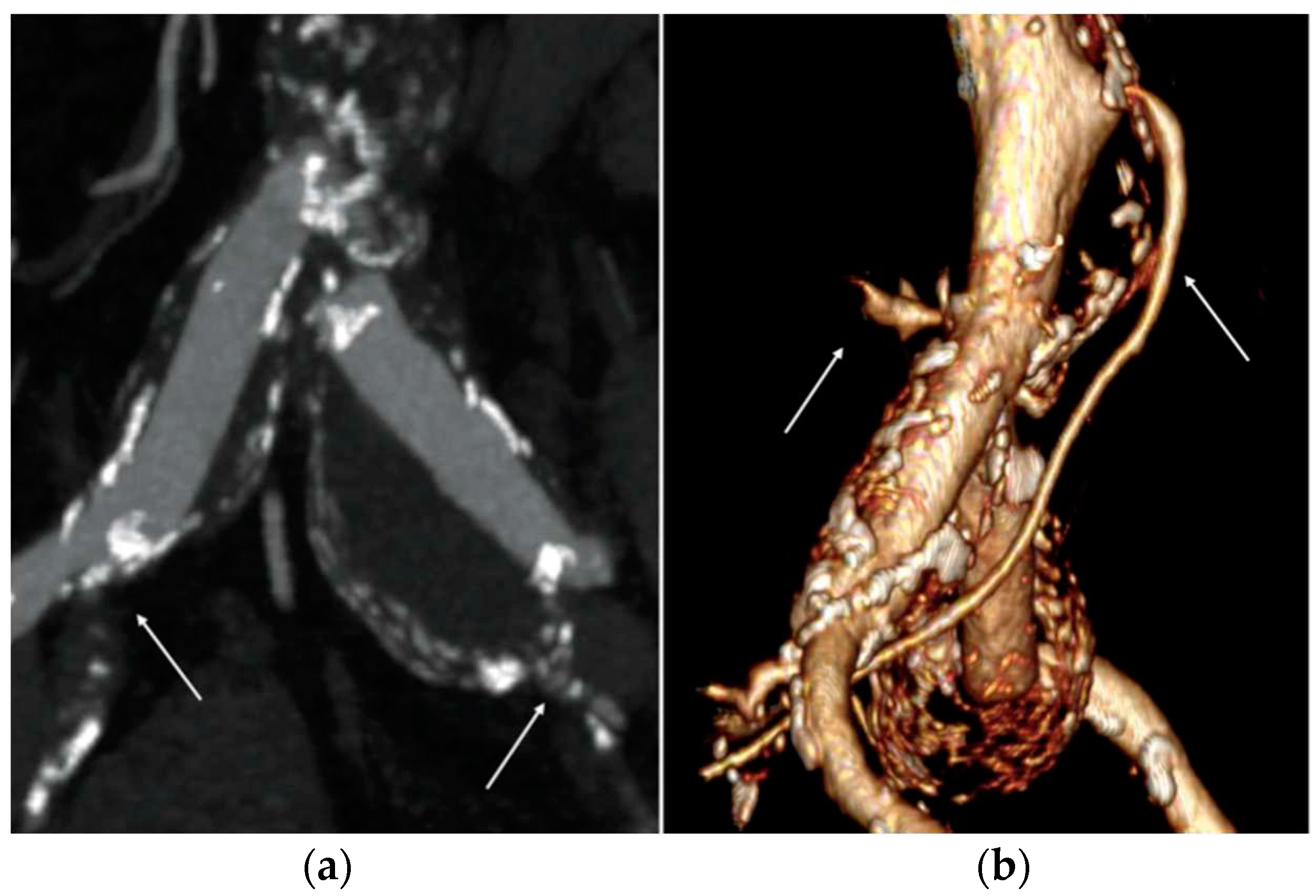
Based on the anatomical findings of preoperative CTA, the distal sealing zone was obtained at the level of the external iliac arteries (EIA). We decided to land in the external iliac artery bilaterally given the presence, on the left, of iliac aneurysm involving the carrefour with concomitant occlusion of the hypogastric artery and, on the right, of common iliac artery aneurysm with circumferential calcium plaque at the level of the carrefour and concomitant severe stenosis of the hypogastric artery (
Figure 1a); therefore landing at the level of the external iliac arteries, which were free of major parietal appositions, seemed the best choice. Iliac artery tortuosity index7 (X) and EIAs’ diameter are listed in
Table 1.
Sac embolization with coils was deemed necessary to prevent type II endoleak due to the large diameter of the inferior mesenteric artery and lumbar arteries (
Figure 1b). It was performed from the left femoral access with coaxial catheter insertion into the aneurysmal sac and subsequent release of 2 semi-controlled coils Interlock (Boston Scientific, Marlborough, Massachusetts, USA) after completion of endoprosthesis implantation.
The procedure lasted 100 minutes, with no technical difficulties. Final intraoperative angiography showed no endoleak images and an excellent outflow in EIAs.
The EVAR procedure was performed four days after the resolution of an asymptomatic Sars-Cov2 infection, confirmed by reverse transcriptase-polymerase chain analysis. It was decided to treat the patient in the immediate post-infection period as he had been experiencing non-specific lower back pain since the previous week; as there was no other definite cause for this, it seemed more prudent to treat the aneurysmal pathology rather than discharge the patient.
Preoperative Oxygen saturation (SaO2) was 98% in air, arterial blood gas (ABG) in air showed pH 7.44, PaCO2 29 mmHg, PaO2 66 mmHg, P/F ratio 314. Blood tests showed normal platelet count (154x109/L), fibrinogen 0,22 g/dL, D-dimer 500 ng/mL, and hs-CRP 4,7 mg/L. Post-operative course was regular but characterized by post-implantation syndrome (PSI). Although no fever>38°C was observed, the leukocyte count was>12,000/mL and hs-CPR was>10 mg/L. No groin complications occurred. After two days, blood tests showed normal leukocyte count and a significative trend-towards of hs-CRP. As per protocol at our center it is performed a pre-discharge control duplex ultrasound (DUS) that showed normal flow in the absence of significant hemodynamic accelerations upstream, inside and downstream of the endoprosthesis and absence of pseudoaneurysms or arterio-venous fistulas at the level of femoral accesses. At physical examination all the arterial pulses at the lower extremities were present. The patient was discharged on third post-operative days under antiplatelet therapy.
After twenty days, the patient was admitted to our Emergency Department reporting pallor, pain, and hypothermia of lower limbs. Femoral and distal pulses were not present at physical examination. Blood tests showed fibrinogen>0,55 g/dL (range, 0,2-0,4), D-dimer >4509 ng/mL (range, 0-550), creatine phosphokinase 16623 IU/L, myoglobin >30000 ng/mL, lactate dehydrogenase 256 IU/L, IL-6 >200 pg/mL (range, 2-6) and hs-CRP 24,7 mg/L. These values are collected in
Table 2.
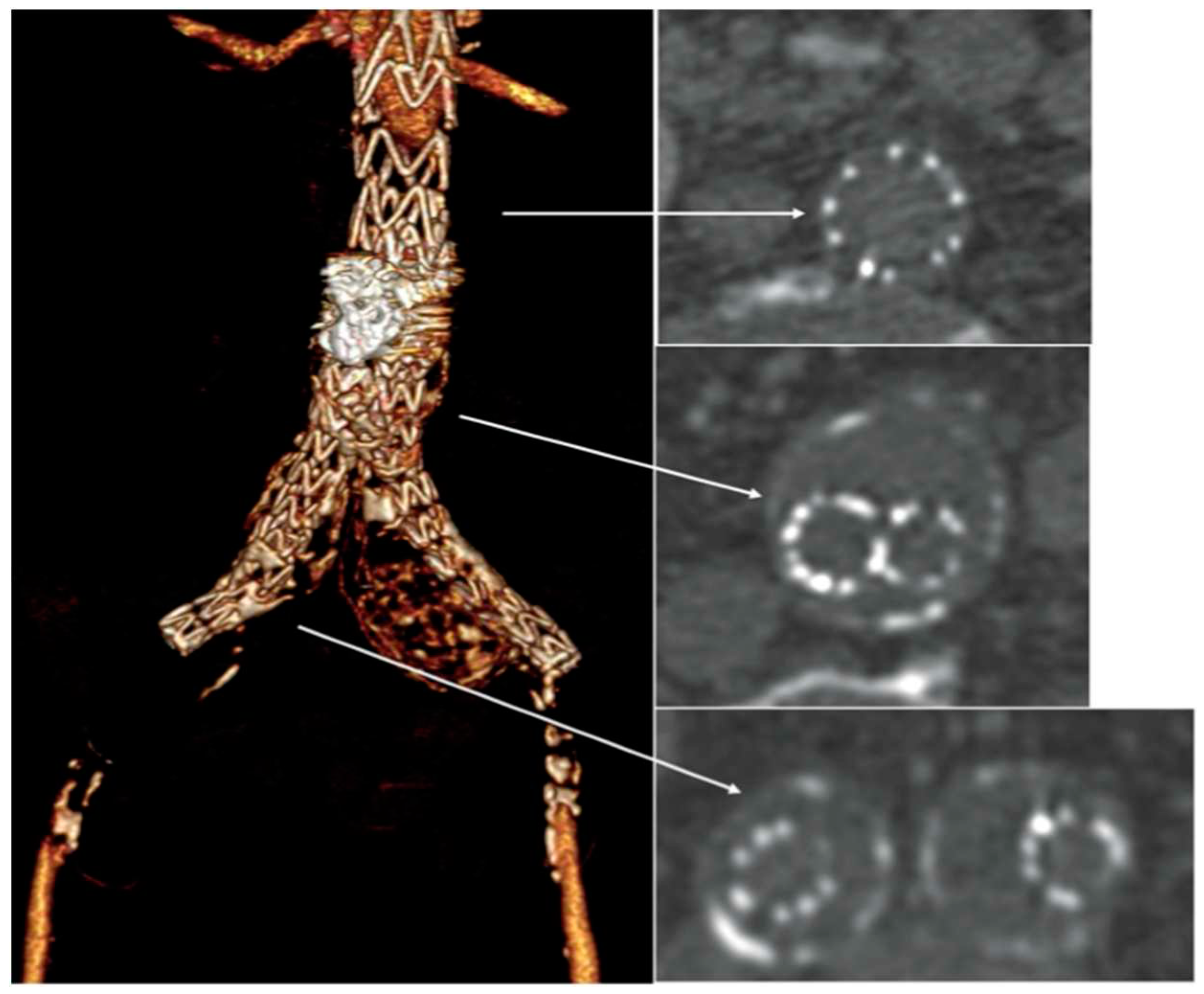
A bedside DUS examination showed the patency of the suprarenal aorta, and the complete thrombosis of the aortic stent-graft without detectable blood flow at the femoral level. An urgent CTA confirmed the complete endograft’s thrombosis with common femoral arteries’reconstitution through collateral circulations (
Figure 2), and the thrombosis of the right popliteal artery aneurysm.
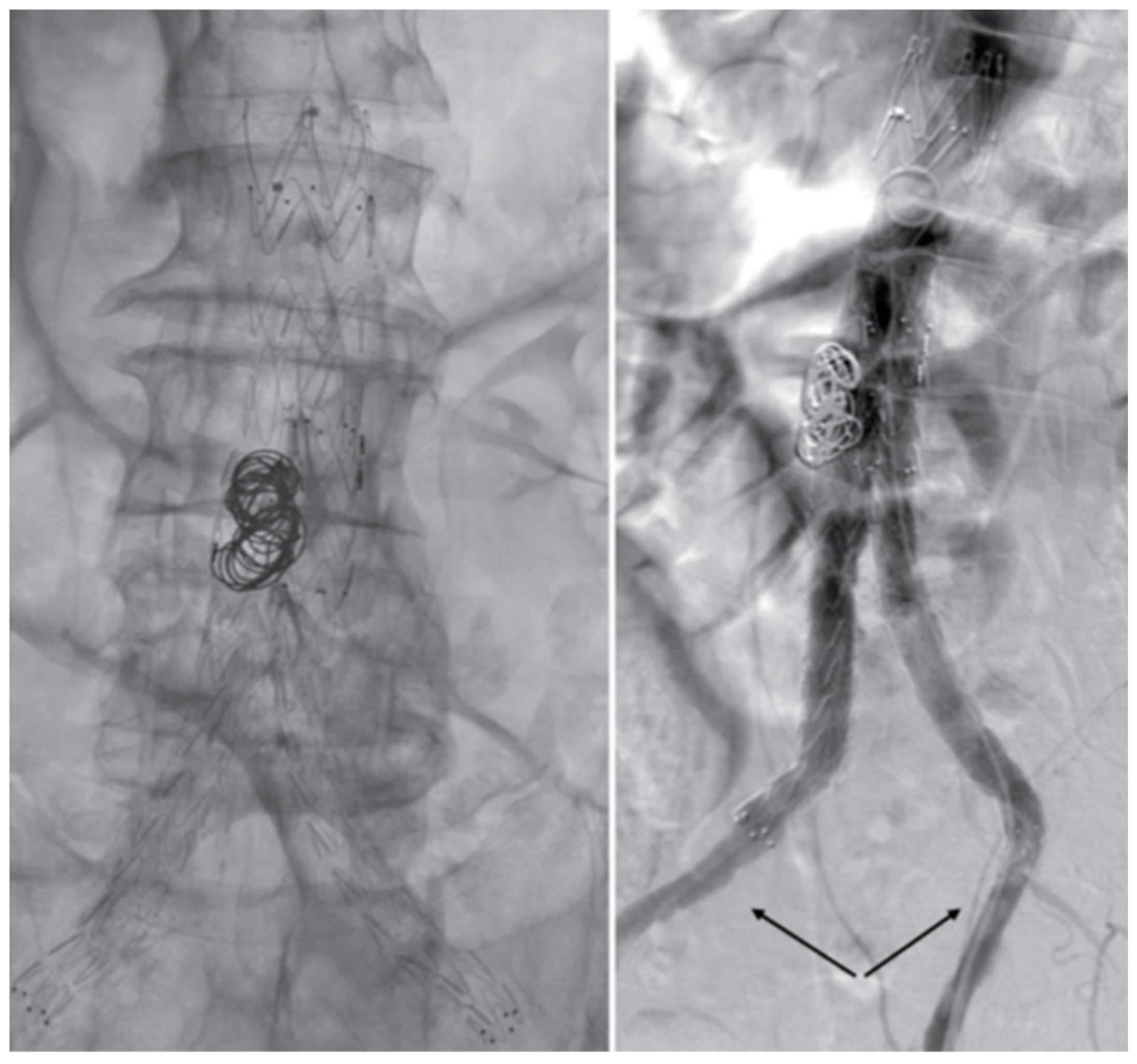
After consulting a team of interventional radiologists, an endovascular treatment with fibrinolytic agent infusion was preferred. Catheter directed thrombolysis with urokinase infusion (60000 IU/h) was started through bilateral percutaneous CFA access. No fabric’s infolding, stent fracture or stenosis of the native EIAs were noted (
Figure 3).
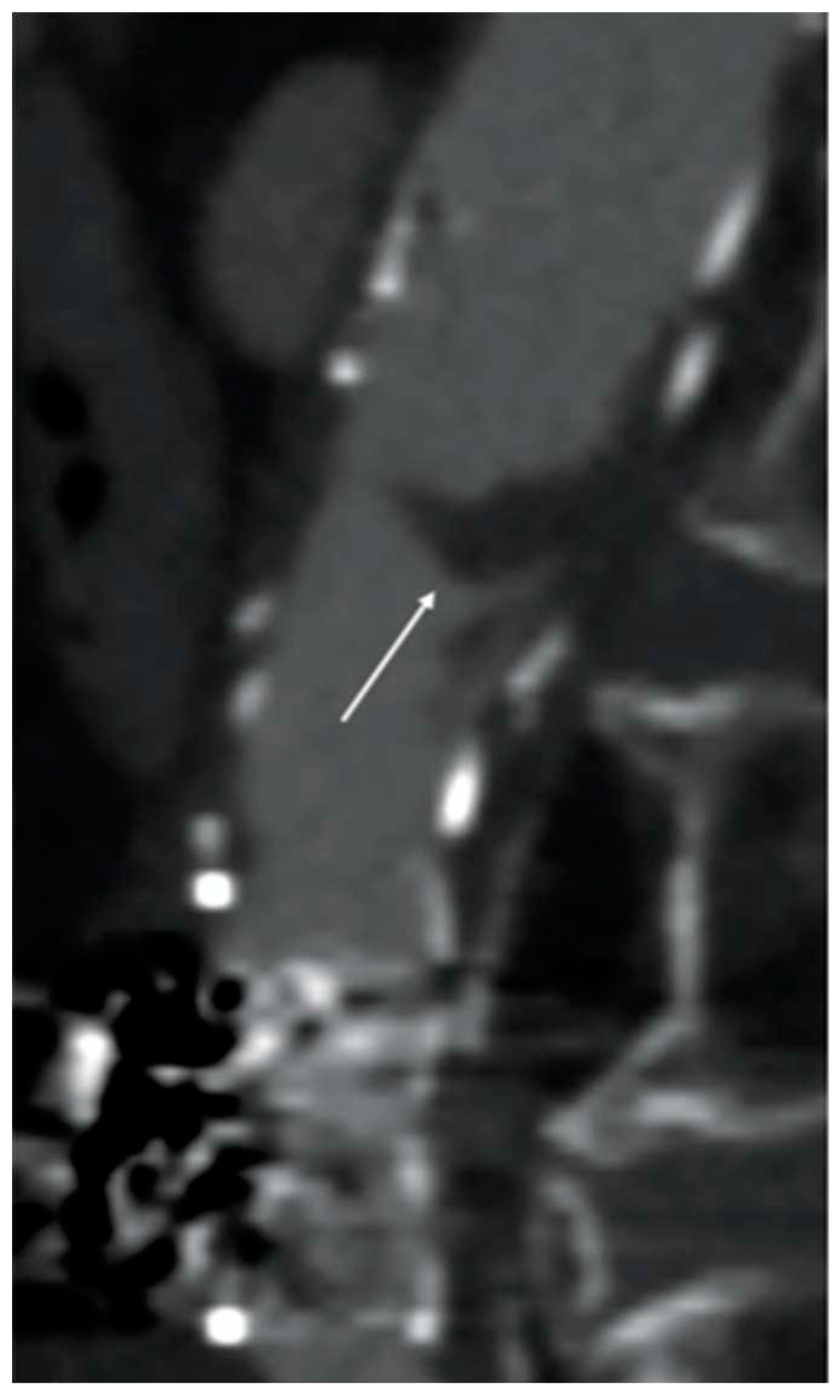
Although fibrinolytic infusion dissolved almost the entire clot, residual floating thrombosis of the main body of the stent-graft was observed at the control CTA (
Figure 4).
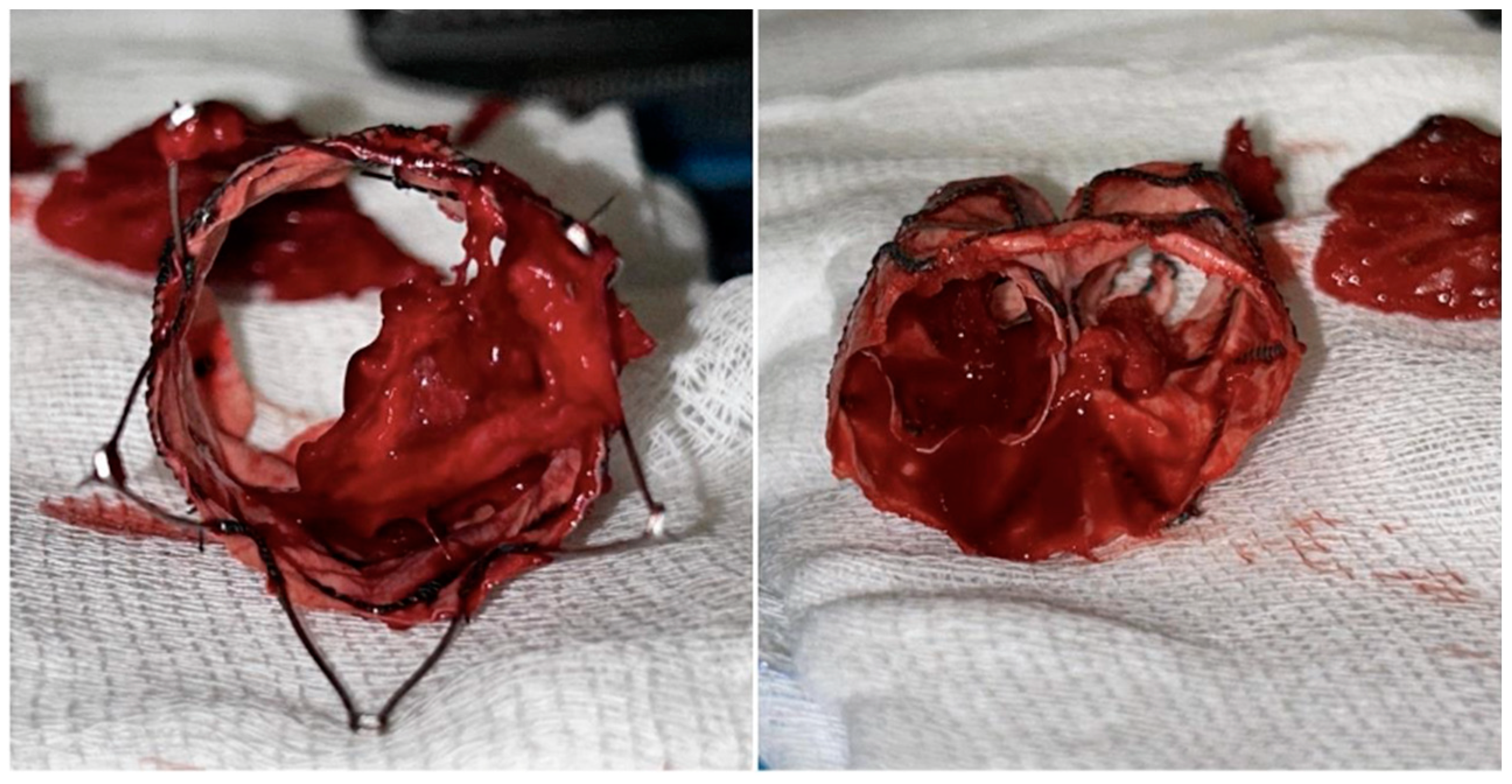
Mechanical thrombectomy with AngioJetTM (Possis, Minneapolis, MN, USA) was attempted but failed because of the tenacious adhesion of the residual clot to the fabric (
Figure 5).
Therefore, a proximal aortic cuff deployment to exclude the floating thrombosis was planned. Unfortunately, the cuff was deployed across the free-flow’s stents of the bifurcated stent-graft. Thus, any additional bailout endovascular procedures were deemed unfeasible, and patient underwent an urgent explantation and an aorto-bifemoral prosthetic bypass. A mechanical thrombectomy of the thrombosed right popliteal artery aneurysm with Penumbra/Indigo Systems (Penumbra Inc, CA, USA) was performed after the restoration of the aortic flow. A Gore Viabahn 6-50 mm (WL Gore & Associates, Flagstaff, AZ, USA) was deployed to exclude the aneurysm and to prevent distal embolization.
Four months after open conversion, a follow-up CTA showed the patency of the prosthetic aortic bypass and the complete exclusion of the right popliteal aneurysm.
3. Discussion
Novel COVID-19 first originated from Wuhan, China and has spread rapidly to all over the world. It spreads via respiratory droplets between close contacts and patients usually present with respiratory symptoms, however, it can manifest with a large variety of symptoms, even very serious, due to the possible involvement of various systems such as the cardiovascular system, the gastrointestinal system, the nervous system and the musculo-skeletal system.
Coagulopathy and hypercoagulation are widely known severe complications of COVID-19 infection which can have unfavorable outcomes and lead to high mortality rate. Approximately one-third of patients with COVID-19 infection may experience venous or arterial thrombosis [
7], this state has been termed COVID-19-associated coagulopathy (CAC) [
8].
The estimated incidence of arterial thrombosis during Sars-Cov2 infection is about 4%, but coagulopathy may persist even after the acute phase of infection and even after the negativity [
9,
10].
Arterial thrombosis involved more often the lower limb arteries than the large vessels, whereas prosthetic graft [
11] and stent-graft thrombosis are rare. Aortic stent-graft thrombosis is favored by concurrent conditions such as stenosis or severe occlusive disease of the outflow vessels [
12,
13].
The pathogenesis of hypercoagulability in COVID-19 is ill defined. All three components of Virchow’s triad appear to be involved, including endothelial injury, stasis, and hypercoagulable state. Endothelial injury is evident from the direct invasion of endothelial cells by SARS-CoV-2; endothelial cells have a high number of angiotensin-converting enzyme 2 (ACE-2) receptors. SARS-CoV-2 enters the cell through the ACE-2 receptor [
14]. Increased angiogenesis was also seen in these patients [
15]. Increased cytokines are released, such as interleukin (IL)-6, and various acute-phase reactants in COVID-19 can lead to endothelial injury [
16]. The use of intravascular catheters can cause direct endothelial cell injury, too. Stasis is due to immobilization in all hospitalized patients, especially those who are critically ill. A hypercoagulable state is seen due to several coagulation abnormalities from elevated circulating prothrombotic factors such as elevated von Willebrand factor (vWF), factor VIII, D-dimer, fibrinogen, neutrophil extracellular traps, prothrombotic microparticles, and anionic phospholipids [
17].
We analyzed several variables to determine the aetiology of this complication. No native EIAs stenosis was detected at preoperative CTA, at completion angiography and at postoperative CFAs Doppler waveform analysis. Before the admission to the Emergency Department, the patient did not refer intermittent or buttock claudication. The most recent outpatient examination demonstrated the patency of the stent-graft without significant distal disease.
Moreover, no evidence of compromise to the integrity of the device was observed. We also excluded congenital or acquired thrombophilia, as well as infection, as the potential cause of thrombosis because laboratory studies for these conditions were negative.
Consequently, the possible explanation of acute stent-graft thrombosis was hypercoagulability and systemic inflammation related to the recent COVID-19 infection, as supported by the laboratory tests [
18]. In addition, thrombosis of popliteal aneurysms is reported in the literature as a consequence of a Sars-Cov2 infection [
18].
Complete blood counts, inflammation and coagulation tests were evaluated. A significant increase in IL-6, hs-CRP, fibrinogen, and D-dimer serum level was observed. Moreover, the CWA profile and literature showed a significant higher aPTT, close to the upper limit of the reference range [
19], characterized by increases in the density, velocity, and acceleration of clot formation. This higher density of clot can explain it tenacious adhesion to the fabric of the stent-graft and the difficulties to completely remove the clot with fibrinolytic infusion and mechanical thrombectomy. Is well known that inflammatory systemic conditions, confirmed by the high serum level of inflammatory biomarkers, lead to interactions between neutrophils and platelets, as platelet and complement activation. Although platelet count was normal, these inflammatory factors are collectively involved in immune-thrombosis, which promotes thrombin-mediated fibrin generation and local blood clot formation.
In our case, another additional risk factor for thrombosis, compared with other COVID-19 patients, was the presence of the aortic stent-graft, which may have been the ideal substrate to platelet aggregation. There are some studies in the literature about coronary stent thrombosis in patients with Sars-Cov2 infection [
20,
21,
22,
23], the mechanism involved in aortic graft thrombosis is most likely the same that occurs in coronary stents, with the much lower difference in incidence that would be explained by the much larger diameter of an aortic graft compared to a coronary stent. So, the thrombogenic mechanisms activated by covid and that remain even months after the negativization associated with the presence of intravasal devices are a perfect combination capable of causing potentially catastrophic thrombosis. Certainly, a careful antiplatelet and anticoagulant therapy can balance, at least partially, the high thrombotic risk, however, in a patient recently undergoing surgery, attention must also be paid to the risk of bleeding.
Further experience and larger studies are needed to better define both etiology and treatment.
4. Conclusions
Although rare, acute aortic stent-grafts thrombosis is a possible and serious complication of COVID-19 infection.
No specific guidelines on medical treatment and surgical management are now available of this condition, and literature is mostly relegated to single case reports, although it has been four years since the appearance of Sars-Cov2. In these cases, it would be advisable to delay the aneurysm treatment and/or prescribe a more aggressive antithrombotic therapy during and immediately after Sars-Cov2 infection.
Despite the presence of less Sars-Cov2 aggressive forms and increasingly updated vaccines, continue to investigate still unclear aspects of covid remains fundamental given the still high spread of the virus and the possibility of new pandemics caused by pathogens with characteristics similar to Sars-Cov2.
Author Contributions
All authors contributed equally to the manuscript and have read and agreed to the published version of the manuscript.
Funding
Please add: This research received no external funding.
Institutional Review Board Statement
Our Institution doesn’t request an ethics commission for this kind of paper.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020, 191, 145–147. [CrossRef] [PubMed]
- Kashi M, Jacquin A, Dakhil B, Zaimi R, Mahé E, Tella E, et al. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res. 2020, 192, 75–77. [CrossRef] [PubMed]
- Galyfos G, Sianou A, Frountzas M, Vasilios K, Vouros D, Theodoropoulos C, et al. Acute limb ischemia among patients with COVID-19 infection. J Vasc Surg. 2022, 75, 326–342. [CrossRef] [PubMed]
- Naouli H, Jiber H, Bouarhroum A. Acute Limb Ischemia in COVID-19 Patients: A Single University Center Experience. Cureus. 2022, 14.
- Tinelli G, Sica S, Montanari F, Franceschi F, Covino M, Dionisio D, et al. Spontaneous Acute Aortic Thrombosis in SARS-CoV-2 Infection. Ann Vasc Surg. 2021, 75, 136–139. [CrossRef] [PubMed]
- Borulu F, Erkut B. Severe Aortic Thrombosis in the Early Period after COVID-19: Two Cases. Ann Vasc Surg. 2021, 73, 114–118.
- Ali EW, Ibrahim IK. Multi-factorial mechanism behind COVID-19 related thrombosis. Med Arch. 2022, 76, 62–65. [CrossRef] [PubMed]
- COVID-19 and coagulopathy: frequently asked questions. 29 May. Available online: https://www.hematology.org/covid-19/covid-19-and-coagulopath (accessed on 29 May 2020).
- Cheruiyot I, Kipkorir V, Ngure B, Misiani M, Munguti J, Ogeng’o J. Arterial thrombosis in coronavirus disease 2019 Patients: A rapid systematic review. Ann Vasc Surg. 2021, 70, 273–281. [CrossRef]
- Silva Andrade B, Siqueira S, de Assis Soares WR, de Souza Rangel F, Santos NO, Dos Santos Freitas A, et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses. 2021, 13, 700. [CrossRef]
- Giacomelli E, Dorigo W, Fargion A, Calugi G, Cianchi G, Pratesi C. Acute thrombosis of an aortic prosthetic graft in a patient with severe COVID-19-Related pneumonia. Ann Vasc Surg. 2020, 66, 8–10. [CrossRef]
- Coelho A, Nogueira C, Lobo M, Gouveia R, Campos J, Augusto R, et al. Impact of Post-EVAR Graft Limb Kinking in EVAR Limb Occlusion: Aetiology, Early Diagnosis, and Management. Eur J Vasc Endovasc Surg. 2019, 58, 681–689. [CrossRef] [PubMed]
- Daye D, Walker TG. Complications of endovascular aneurysm repair of the thoracic and abdominal aorta: evaluation and management. Cardiovasc Diagn Ther. 2018, 8 (Suppl 1), S138–S156. [CrossRef] [PubMed]
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020, 395, 1417–8. [CrossRef] [PubMed]
- Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020, 383, 120–8. [CrossRef] [PubMed]
- Jing H, Wu X, Xiang M, Liu L, Novakovic VA, Shi J. Pathophysiological mechanisms of thrombosis in acute and long COVID-19. Front Immunol. 2022, 13, 992384. [CrossRef] [PubMed]
- Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Hae- most. 2020, 18, 1747–51. [CrossRef] [PubMed]
- Kasirajan, K. Acute upper extremity ischemia and symptomatic popliteal artery aneurysm secondary to coronavirus disease 2019. J Vasc Surg Cases Innov Tech. 2021, 7, 267–270. [Google Scholar] [CrossRef]
- Tan CW, Tan JY, Wong WH, Cheong MA, Ng IM, Conceicao EP, et al. Clinical and laboratory features of hypercoagulability in COVID-19 and other respiratory viral infections amongst predominantly younger adults with few comorbidities. Sci Rep. 2021, 11, 1793. [CrossRef] [PubMed]
- Skorupski WJ, Grygier M, Lesiak M, Kałużna-Oleksy M. Coronary Stent Thrombosis in COVID-19 Patients: A Systematic Review of Cases Reported Worldwide. Viruses. 2022, 14, 260. [CrossRef] [PubMed]
- Montaseri M, Golchin Vafa R, Attar A, Ali Hosseini S, Kojuri J. Stent thrombosis during COVID-19 pandemic: A case series. Clin Case Rep. 2022, 10, e05872. [CrossRef]
- Kunal S, Pathak V, Pathak K, Mishra M, Sharma SM, Bhandari S. Very late stent thrombosis associated with COVID-19 infection: a case report and review of the literature. Monaldi Arch Chest Dis. 2021, 92.
- El-Medany A, Kandoole V, Lonsdale N, Doolub G, Felekos I. In-stent Thrombosis and COVID-19 Infection: Current Insights on the Mechanistic Relationship. Curr Cardiol Rev. 2023, 19, e120522204669. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).