Submitted:
28 November 2023
Posted:
29 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The Objectives and Hypothesis of the Work
3. Results
3.1. Calculation of the Result of Extracted Gelatin
3.2. Antioxidant Activity
3.3. Molecular Weight
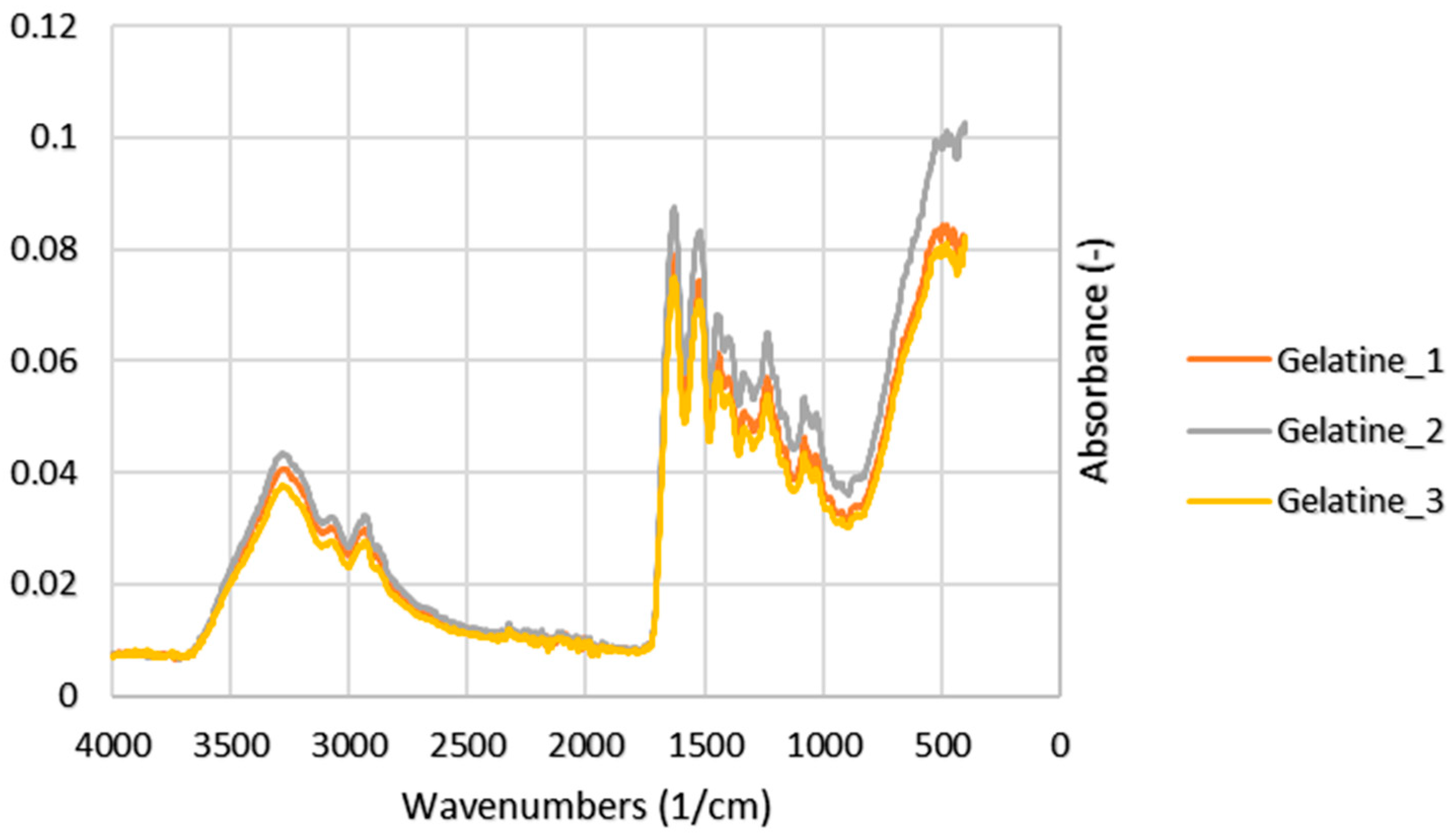
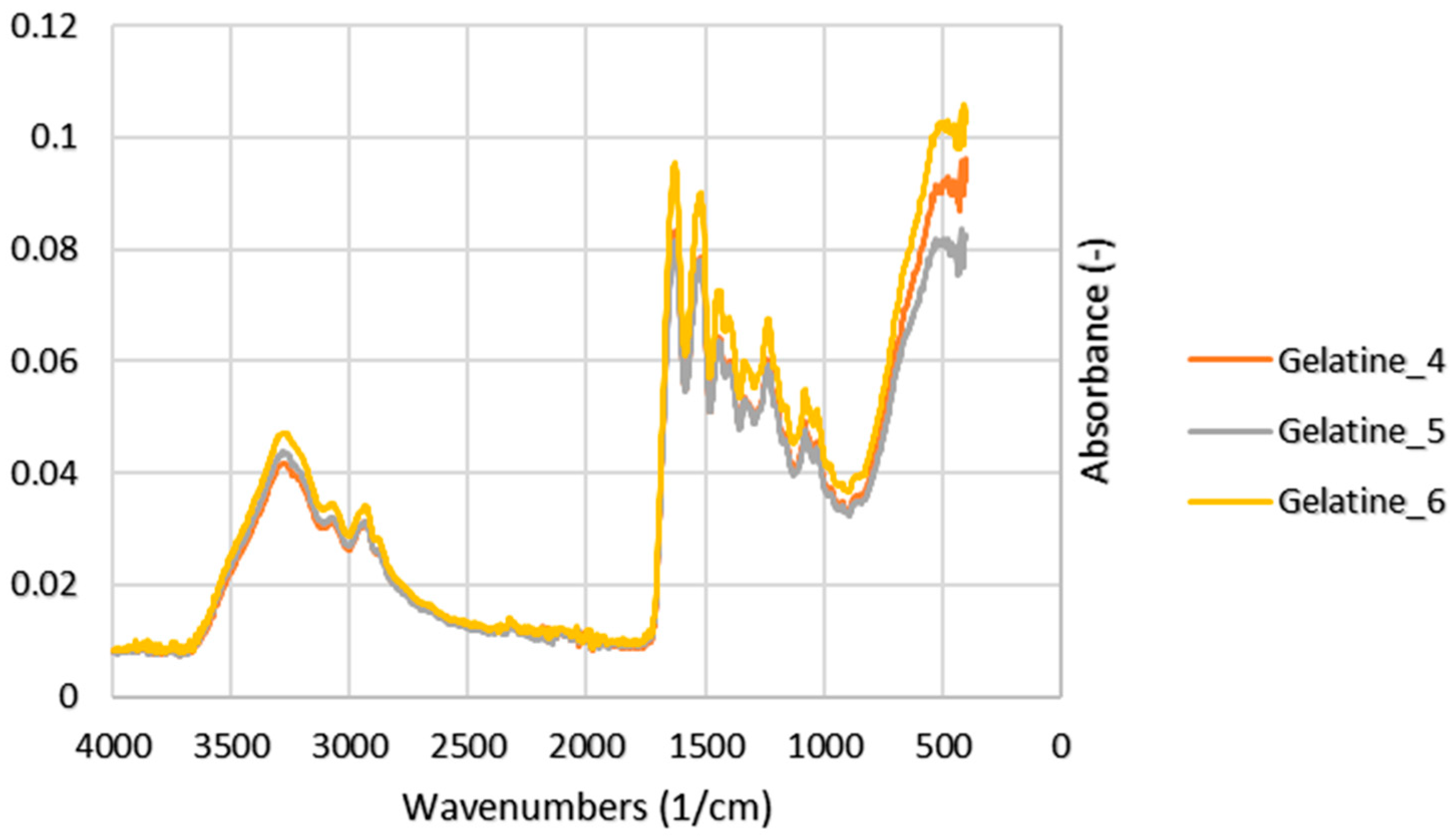
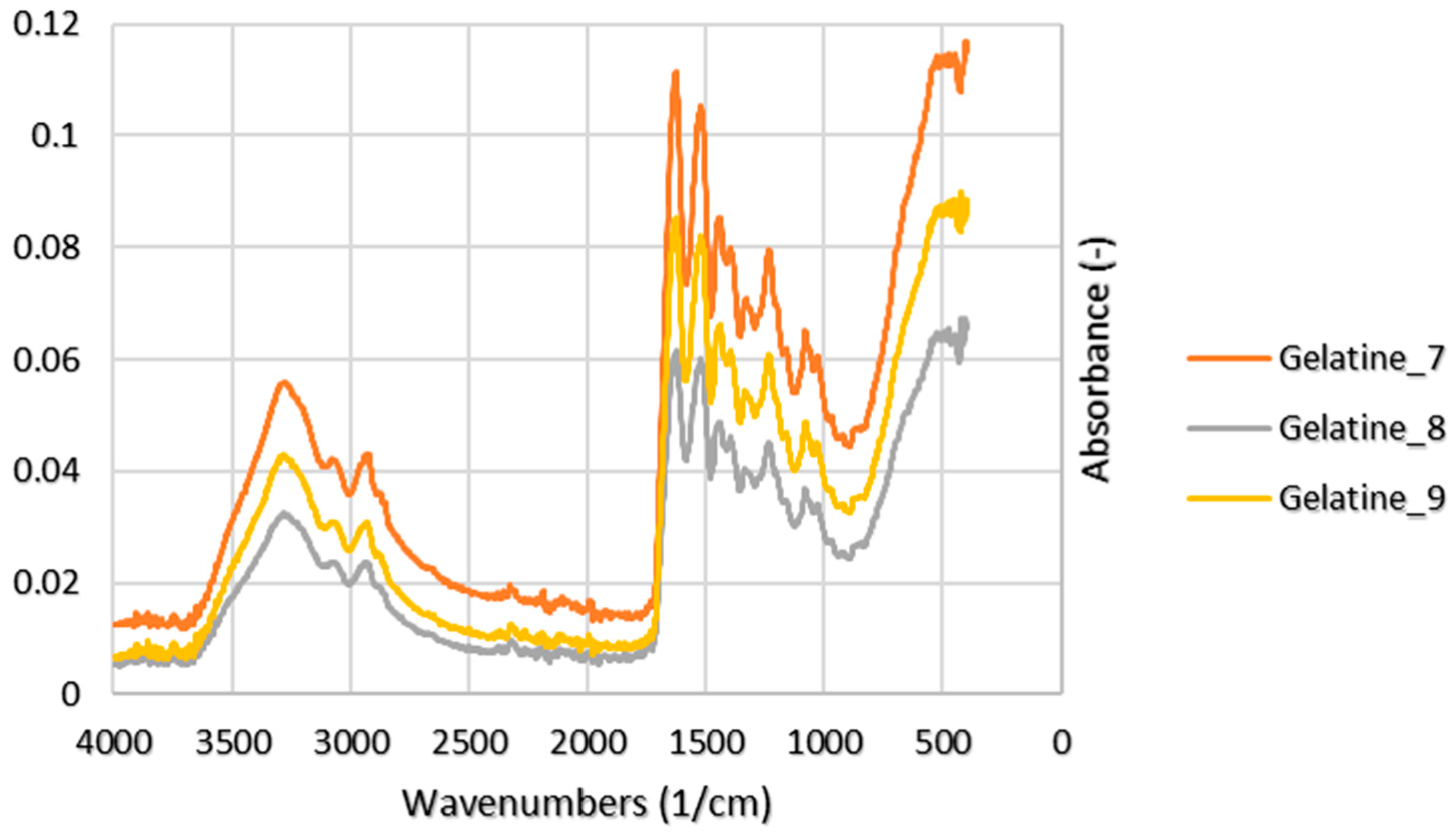
3.4. Functional Groups
3.5. Microbiological Properties
4. Discussion
4.1. Antioxidant Activity
4.2. Molecular Weight
4.3. Functional Groups
4.4. Microbiological Properties
5. Materials and Methods
5.1. Apparatus, Tools and Chemicals
5.2. Samples of Extracted Gelatins
5.2.1. Antioxidant Activity
5.2.2. Molecular Weight Distribution
5.2.3. Functional Groups
5.2.4. Microbiological Properties
5.3. Statistical Analysis
6. The Practical Relevance of the Work
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Díaz-Calderón, P.; Flores, E.; González-Muñoz, A.; Pepczynska, M.; Quero, F.; Enrione, J. Influence of extraction variables on the structure and physical properties of salmon gelatin. Food Hydrocoll. 2017, 71, 118–128. [Google Scholar] [CrossRef]
- Cho, S.H.; Jahncke, M.L.; Chin, K.B.; Eun, J.B. The effect of processing conditions on the properties of gelatin from skate (Raja Kenojei) skins. Food Hydrocoll. 2006, 20, 810–816. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Characteristics and gel properties of gelatin from skin of seabass (Lates calcarifer) as influenced by extraction conditions. Food Chem. 2014, 152, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.CH.; Huang, Q.Y.; Ding, W.; Xiao, X.H.; Zhang, H.Y.; Xiong, L.X. Fish gelatin: The novel potential applications. J. Funct. Foods 2019, 63, 103581. [Google Scholar] [CrossRef]
- Saenmuang, S.; Phothiset, S.; Chumnanka, CH. Extraction and characterization of gelatin from black-bone chicken by-products. Food Sci. Biotechnol. 2020, 29, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Hafidz, R.M.R.N.; Yaakob, C.M.; Amin, I.; Noorfaizan, A. Chemical and functional properties of bovine and porcine skin gelatin. Int. Food Res. J. 2011, 18, 787–791. [Google Scholar]
- Saidi, S.G.; Rahman, M.S.; Guizani, N. Fourier transform infrared (FTIR) spectroscopic study of extracted gelatin from shaari (Lithrinus microdon) skin: Effects of extraction conditions. Int. Food Res. J. 2012, 19, 1167–1173. [Google Scholar]
- Silva, R.S.G.; Bandeira, S.F.; Pinto, L.A.A. Characteristics and chemical composition of skins gelatin from cobia (Rachycentron canadum). LWT – Food Sci. Technol. 2014, 57, 580–585. [Google Scholar] [CrossRef]
- Zhuang, Y.L.; Sun, L.P.; Zhao, X.; Hou, H.; Li, B.F. Investigation of gelatin polypeptides of jellyfish (Rhopilema esculentum) for their antioxidant activity in vitro. Food Technol. Biotechnol. 2010, 48, 222–228. [Google Scholar]
- Ho, T.C.; Lim, J.S.; Kim, S.J.; Kim, S.Y.; Chun, B.S. In vitro biodegradation, drug absorption, and physical properties of gelatin-fucoidan microspheres made of subcritical-water-modified fish gelatin. Marine Drugs 2023, 21, 287. [Google Scholar] [CrossRef]
- Yang, J.I.; Ho, H.Y.; Chu, Y.J.; Chow, Ch.J. Characteristic and antioxidant activity of retorted gelatin hydrolysates from cobia (Rachycentron canadum) skin. Food Chem. 2008, 110, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Physico-chemical properties, morphology and antioxidant activity of film from fish skin gelatin incorporated with root essential oils. J. Food Eng. 2013, 117, 350–360. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Nurilmala, M.; Hizbullah, H.H.; Karnia, E.; Kusumaningtyas, E.; Ochiai, Y. Characterization and antioxidant activity of collagen, gelatin, and the derived peptides from yellowfin tuna (Thunnus albacares) skin. Marine Drugs 2020, 18, 98. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Qian, Z.J.; Ryu, B.M.; Park, J.W.; Kim, S.K. In vitro antioxidant activity of a peptide isolated from Nile tilapia (Oreochromis niloticus) scale gelatin in free radical-mediated oxidative systems. J. Funct. Foods 2010, 2, 107–117. [Google Scholar] [CrossRef]
- Herawati, E.; Akhsanitaqwim, Y.; Agnesia, P.; Listyawati, S.; Pangastuti, A.; Ratriyanto, A. In vitro antioxidant and antiaging activities of collagen and its hydrolysate from mackerel scad skin (Decapterus macarellus). Marine Drugs 2022, 20, 516. [Google Scholar] [CrossRef]
- Wu, J.; Chen, S.; Ge, S.; Miao, J.; Li, J.; Zhang, Q. Preparation, properties and antioxidant activity of an active film from silver carp (Hypophthalmichthys molitrix) skin gelatin incorporated with green tea extract. Food Hydrocoll. 2013, 32, 42–51. [Google Scholar] [CrossRef]
- Gál, R.; Čmiková, N.; Prokopová, A.; Kačániová, M. Antilisterial and antimicrobial effect of Salvia officinalis essential oil in beef sous-vide meat during storage. Foods 2023, 12, 2201. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Main Groups of Microorganisms of Relevance for Food Safety and Stability: General Aspects and Overall Description. In Innovative Technologies for Food Preservation, 1st ed.; Barba, F.J., Sant’Ana, A.D.S., Orlien, V., Koubaa, M., Eds.; Elsevier Inc.: London, United Kingdom, 2018; pp. 53–107. [Google Scholar] [CrossRef]
- Jensen, G.B.; Hansen, B.M.; Eilenberg, J.; Mahillon, J. The hidden lifestyles of Bacillus cereus and relatives. Environ. Microbiol. 2003, 5, 631–640. [Google Scholar] [CrossRef]
- Clerck, E.D.; Vanhoutte, T.; Hebb, T.; Geerinck, J.; Devos, J.; Vos, P.D. Isolation, characterization, and identification of bacterial contaminants in semifinal gelatin extracts. Appl. Environ. Microbiol. 2004, 70, 3664–3672. [Google Scholar] [CrossRef]
- Coburn, B.; Grassl, G.A.; Finlay, B.B. Salmonella, the host and disease: a brief review. Immunol. Cell Biol. 2007, 85, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.A.T.; Elias, W.P.; Scaletsky, I.C.A.; Guth, B.E.C.; Rodrigues, J.F.; Piazza, R.M.F.; Ferreira, L.C.S.; Martinez, M.B. Diarrheagenic Escherichia coli. Braz. J. Microbiol. 2016, 47, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Gasanov, U.; Hughes, D.; Hansbro, P.M. Methods for the isolation and identification of Listeria spp. and Listeria monocytogenes: a review. FEMS Microbiol. Rev. 2005, 29, 851–875. [Google Scholar] [CrossRef] [PubMed]
- Rasko, D.A.; Altherr, M.R.; Han, C.S.; Ravel, J. Genomics of the Bacillus cereus group of organisms. FEMS Microbiol. Rev. 2005, 29, 303–329. [Google Scholar] [CrossRef] [PubMed]
- Deurenberg, R.H.; Stobberingh, E.E. The evolution of Staphylococcus aureus. Infect. Genet. Evol. 2008, 8, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Prokopová, A.; Pavlačková, J.; Mokrejš, P.; Gál, R. Collagen Hydrolysate prepared from chicken by-product as a functional polymer in cosmetic formulation. Molecules 2021, 26, 2021. [Google Scholar] [CrossRef] [PubMed]
- Rather, J.A.; Akhter, N.; Ashraf, Q.S.; Mir, S.A.; Makroo, H.A.; Majid, D.; Barba, F.J.; Khaneghah, A.M.; Dar, D.N. A comprehensive review on gelatin: understanding impact of the sources, extraction methods, and modifications on potential packaging applications. Food Packag. Shelf Life 2022, 34, 100945. [Google Scholar] [CrossRef]
- Rasli, H.I.; Sarbon, N.M. Effects of different drying methods on the rheological, functional and structural properties of chicken skin gelatin compared to bovine gelatin. Int. Food Res. J. 2015, 22, 584–592. [Google Scholar]
- Fatima, S.; Mir, M.I.; Khan, M.R.; Sayyed, R.Z.; Mehnaz, S.; Abbas, S.; Sadiq, M.B.; Masih, R. The optimization of gelatin extraction from chicken feet and the development of gelatin based active packaging for the shelf-life extension of fresh grapes. Sustainability 2022, 14, 7881. [Google Scholar] [CrossRef]
- Mokrejš, P.; Mrázek, P.; Gál, R.; Pavlačková, J. Biotechnological preparation of gelatines from chicken feet. Polymers 2019, 11, 1060. [Google Scholar] [CrossRef]
- Prokopová, A.; Mokrejš, P.; Pavlačková, J.; Gál, R. Preparation of gelatin from broiler chicken stomach collagen. Foods 2023, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Razavizadeh, R.S.; Farmani, J.; Motamedzadegan, A. Enzyme-assisted extraction of chicken skin protein hydrolysates and fat: Degree of hydrolysis affects the physicochemical and functional properties. J. Am. Oil Chem. Soc. 2022, 99, 621–632. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Li, M.Y.; Tian, G.; Zhang, T.H.; Ren, H.; Quek, S.Y. Effects of ultrasonic pretreatment on the structure and functionality of chicken bone protein prepared by enzymatic method. Food Chem. 2019, 299, 125103. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.B.; Li, X.; Zhang, Ch.H.; Wang, J.Z.; Tang, Ch.H.; Sun, H.M.; Jia, W.; Li, Y.; Chen, L.L. Development of a novel method for hot-pressure extraction of protein from chicken bone and the effect of enzymatic hydrolysis on the extracts. Food Chem. 2014, 157, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lu, F.; Wu, Y.; Wang, D.; Xu, W.; Zou, Y.; Sun, W. Enzymatic extraction and functional properties of phosphatidylcholine from chicken liver. Poultry Sci. 2022, 101(6), 101689. [Google Scholar] [CrossRef] [PubMed]
- Mokrejš, P.; Gál, R.; Pavlačková, J. Enzyme conditioning of chicken collagen and taguchi design of experiments enhancing the yield and quality of prepared gelatins. Int. J. Mol. Sci. 2023, 24, 3654. [Google Scholar] [CrossRef] [PubMed]
- Mokrejš, P.; Gál, R.; Pavlačková, J.; Janáčová, D. Valorization of a by-product from the production of mechanically deboned chicken meat for preparation of gelatins. Molecules 2021, 26, 349. [Google Scholar] [CrossRef] [PubMed]
- Rogošić, M.; Mencer, H.J.; Gomzi, Z. Polydispersity index and molecular weight distributions of polymers. Eur. Polym. J. 1996, 32, 1337–1344. [Google Scholar] [CrossRef]
- Yu, H.; Huang, N.; Wang, Ch.; Tang, Z. Modeling of poly(L-lactide) thermal degradation: Theoretical prediction of molecular weight and polydispersity index. J. Appl. Polym. Sci. 2003, 88, 2557–2562. [Google Scholar] [CrossRef]
- Abedinia, A.; Nafchi, A.M.; Sharifi, M.; Ghalambor, P.; Oladzadabbasabadi, N.; Ariffin, F.; Huda, N. Poultry gelatin: Characteristics, developments, challenges, and future outlooks as a sustainable alternative for mammalian gelatin. Trends Food Sci. Technol. 2020, 104, 14–26. [Google Scholar] [CrossRef]
- Rigueto, C.V.T.; Rosseto, M.; Alessandretti, I.; Oliveira, R.D.; Wohlmuth, D.A.R.; Menezes, J.F.; Loss, R.A.; Dettmer, A.; Pizzutti, I.R. Gelatin films from wastes: A review of production, characterization, and application trends in food preservation and agriculture. Food Res. Int. 2022, 162, 112114. [Google Scholar] [CrossRef]
- Chakka, A.K.; Ali, A.M.M.; Sakhare, P.Z.; Bhaskar, N. Poultry processing waste as an alternative source for mammalian gelatin: extraction and characterization of gelatin from chicken feet using food grade acids. Waste Biomass Valor. 2017, 8. [Google Scholar] [CrossRef]
- Almeida, P.F.; Lannes, S.C.S.; Calarge, F.A.; Farias, T.M.B.; Santana, J.C.C. FTIR characterization of gelatin from chicken feet. J. Chem. Chem. Eng. 2012, 6, 1029–1032. [Google Scholar]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Cebi, N.; Dogan, C.E.; Mese, A.E.; Ozdemir, D.; Arıcı, M.; Sagdic, O. A rapid ATR-FTIR spectroscopic method for classification of gelatin gummy candies in relation to the gelatin source. Food Chem. 2019, 277, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Widyasari, R.; Rawdkuen, S. Extraction and characterization of gelatin from chicken feet by acid and ultrasound assisted extraction. Food Appl. Biosci. J. 2014, 2, 85–97. [Google Scholar] [CrossRef]
- Santana, J.C.C.; Gardim, R.B.; Almeida, P.F.; Borini, G.B.; Quispe, A.P.B.; Llanos, S.A.V.; Heredia, J.A.; Zamuner, S.; Gamarra, F.M.C.; Farias, T.M.B.; Ho, L.L.; Berssaneti, F.T. Valorization of chicken feet by-product of the poultry industry: high qualities of gelatin and biofilm from extraction of collagen. Polymers 2020, 12, 529. [Google Scholar] [CrossRef] [PubMed]
- Enrione, J.; Char, C.; Pepczynska, M.; Padilla, Ch.; González-Muñoz, A.; Olguín, Y.; Quinzio, C.; Iturriaga, L.; Díaz-Calderón, P. Rheological and structural study of salmon gelatin with controlled molecular weight. Polymers 2020, 12, 1587. [Google Scholar] [CrossRef]
- Sinel, C.; Augagneur, Y.; Sassi, M.; Bronsard, J.; Cacaci, M.; Guérin, F.; Sanguinetti, M.; Meignen, P.; Cattoir, V.; Felden, B. Small RNAs in vancomycin-resistant Enterococcus faecium involved in daptomycin response and resistance. Sci. Rep. 2017, 7, 11067. [Google Scholar] [CrossRef]
- Pidot, S.J.; Gao, W.; Buultjens, A.H.; Monk, I.R.; Guerillot, R.; Carter, G.P.; Lee, J.Y.H.; Lam, M.M.C.; Grayson, M.L.; Ballard, S.A.; Mahony, A.A.; Grabsch, E.A.; Kotsanas, D.; Korman, T.M.; Coombs, G.W.; Robinson, J.O.; Gonçalves da Silva, A.; Seemann, Z.; Howden, B.P.; Johnson, P.D.R.; Stinear, T.P. Increasing tolerance of hospital Enterococcus faecium to handwash alcohols. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Siepert, B.; Reinhardt, N.; Kreuzer, S.; Bondzio, A.; Twardziok, S.; Brockmann, G.; Nöckler, K.; Szabó, I.; Janczyk, P.; Pieper, R.; Tedin, K. Enterococcus faecium NCIMB 10415 supplementation affects intestinal immune-associated gene expression in post-weaning piglets. Vet. Immunol. Immunopathol. 2014, 157, 65–77. [Google Scholar] [CrossRef]
- Razali, A.N.; Amin, A.M.; Sarbon, N.M. Antioxidant activity and functional properties of fractionated cobia skin gelatin hydrolysate at different molecular weight. Int. Food Res. J. 2015, 22, 651–660. [Google Scholar]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Skin gelatin from bigeye snapper and brownstripe red snapper: chemical compositions and effect of microbial transglutaminase on gel properties. Food Hydrocoll. 2006, 20, 1216–1222. [Google Scholar] [CrossRef]
- Mohtar, N.F.; Perera, C.; Quek, S.Y. Optimisation of gelatine extraction from hoki (Macruronus novaezelandiae) skins and measurement of gel strength and SDS–PAGE. Food Chem. 2010, 122, 307–313. [Google Scholar] [CrossRef]
- Tewari, A.; Abdullah, S. Bacillus cereus food poisoning: international and Indian perspective. J. Food Sci. Technol. 2015, 52, 2500–2511. [Google Scholar] [CrossRef] [PubMed]
- Giraffa, G. Enterococci from foods. FEMS Microbiol. Rev. 2002, 26, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Verma, T.; Chaves, B.D.; Howell Jr., T.; Subbiah, J. Thermal inactivation kinetics of Salmonella and Enterococcus faecium NRRL B-2354 on dried basil leaves. Food Microbiol. 2021, 96, 103710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Chen, J.C.; Kirby, E.D. Surface roughness optimization in an end-milling operation using the Taguchi design method. J. Mater. Process. Technol. 2007, 184, 233–239. [Google Scholar] [CrossRef]
| Exp. No. |
DPPH (%) | ABTS (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gelatins concentration (mg/ml) | ||||||||||
| 2 | 4 | 6 | 8 | 10 | 2 | 4 | 6 | 8 | 10 | |
| 1 | 72 ± 1 | 76 ± 1 | 82 ± 1 | 85 ± 2 | 87 ± 2 | 84 ± 2 | 87 ± 2 | 91 ± 2 | 93 ± 1 | 97 ± 1 |
| 2 | 71 ± 1 | 72 ± 2 | 80 ± 1 | 82 ± 1 | 87 ± 1 | 83 ± 2 | 85 ± 1 | 88 ± 2 | 93 ± 1 | 94 ± 1 |
| 3 | 71 ± 2 | 76 ± 2 | 82 ± 1 | 82 ± 2 | 86 ± 1 | 80 ± 1 | 85 ± 1 | 88 ± 1 | 93 ± 2 | 95 ± 1 |
| 4 | 72 ± 2 | 77 ± 2 | 82 ± 1 | 85 ± 2 | 87 ± 1 | 84 ± 1 | 88 ± 2 | 91 ± 1 | 93 ± 2 | 96 ± 1 |
| 5 | 69 ± 1 | 76 ± 1 | 77 ± 2 | 82 ± 1 | 85 ± 1 | 80 ± 2 | 87 ± 2 | 90 ± 2 | 91 ± 1 | 96 ± 1 |
| 6 | 69 ± 1 | 74 ± 1 | 78 ± 1 | 84 ± 1 | 87 ± 2 | 82 ± 1 | 84 ± 2 | 91 ± 1 | 90 ± 1 | 95 ± 1 |
| 7 | 71 ± 1 | 77 ± 1 | 82 ± 2 | 84 ± 1 | 87 ± 1 | 84 ± 2 | 88 ± 1 | 90 ± 1 | 93 ± 2 | 95 ± 1 |
| 8 | 72 ± 1 | 76 ± 2 | 79 ± 2 | 84 ± 1 | 87 ± 2 | 82 ± 1 | 88 ± 2 | 90 ± 2 | 93 ± 1 | 95 ± 2 |
| 9 | 70 ± 1 | 74 ± 2 | 77 ± 1 | 84 ± 1 | 87 ± 2 | 80 ± 1 | 86 ± 1 | 88 ± 1 | 92 ± 1 | 94 ± 1 |
| ±SD | 71 ± 1 | 75 ± 2 | 80 ± 1 | 84 ± 1 | 87 ± 1 | 82 ± 1 | 86 ± 2 | 90 ± 1 | 92 ± 1 | 95 ± 1 |
| Exp. No. | Mp 1 (kDa) | Mw 2 (kDa) | Mn 3 (kDa) | PDI (–) |
|---|---|---|---|---|
| 1 | 18.4 | 24.5 | 5.6 | 4.4 |
| 2 | 41.0 | 65.1 | 8.3 | 7.9 |
| 3 | 44.1 | 94.9 | 8.6 | 11.1 |
| 4 | 18.8 | 30.5 | 6.0 | 5.1 |
| 5 | 19.2 | 57.3 | 6.4 | 9.0 |
| 6 | 57.5 | 105.1 | 9.6 | 11.0 |
| 7 | 17.5 | 24.4 | 5.3 | 4.6 |
| 8 | 18.7 | 45.8 | 7.0 | 6.5 |
| 9 | 38.7 | 74.0 | 8.3 | 8.9 |
| Exp. No. | ML 1 (%) | Mα 2 (%) | Mβ 3 (%) | Mγ 4 (%) | MH 5 (%) |
|---|---|---|---|---|---|
| 1 | 76.2 | 11.7 | 8.3 | 3.8 | 0.0 |
| 2 | 64.7 | 10.1 | 8.2 | 6.1 | 10.9 |
| 3 | 60.3 | 9.3 | 6.7 | 6.7 | 17.0 |
| 4 | 67.8 | 9.5 | 7.4 | 8.4 | 6.9 |
| 5 | 62.2 | 9.6 | 7.9 | 6.9 | 13.4 |
| 6 | 59.9 | 9.3 | 6.5 | 7.4 | 16.9 |
| 7 | 64.6 | 10.4 | 7.0 | 7.9 | 10.1 |
| 8 | 63.4 | 9.5 | 7.5 | 6.7 | 12.9 |
| 9 | 58.3 | 10.4 | 7.9 | 6.5 | 16.9 |
| ±SD | 65.0±5.0 | 10.0±1.0 | 7.0±1.0 | 7.0±1.0 | 12.0±6.0 |
| Exp. No. | Peak | Wavenumbers (1/cm) | Reference (1/cm)1 | Note |
|---|---|---|---|---|
| 1 | 3282 | 3440–3300 | N–H stretching | |
| 2 | Amide A | 3275 | ||
| 3 | 3290 | |||
| 1 | 2937 | 3080–2899 | CH2 asymmetrical stretch | |
| 2 | Amide B | 2933 | ||
| 3 | 2927 | |||
| 1 | 1640 | 1700–1600 | C=O stretching | |
| 2 | Amide I | 1644 | ||
| 3 | 1639 | |||
| 1 | 1515 | 1580–1500 | N–H bending | |
| 2 | Amide II | 1517 | ||
| 3 | 1510 | |||
| 1 | 1241 | 1350–1200 | N–H bending and C–N stretching | |
| 2 | Amide III | 1240 | ||
| 3 | 1238 |
| Exp. No. | Peak | Wavenumbers (1/cm) | Reference (1/cm)1 | Note |
|---|---|---|---|---|
| 4 | 3289 | 3440–3300 | N–H stretching | |
| 5 | Amide A | 3272 | ||
| 6 | 3277 | |||
| 4 | 2938 | 3080–2899 | CH2 asymmetrical stretch | |
| 5 | Amide B | 2932 | ||
| 6 | 2929 | |||
| 4 | 1636 | 1700–1600 | C=O stretching | |
| 5 | Amide I | 1643 | ||
| 6 | 1637 | |||
| 4 | 1515 | 1580–1500 | N–H bending | |
| 5 | Amide II | 1522 | ||
| 6 | 1517 | |||
| 4 | 1236 | 1350–1200 | N–H bending and C–N stretching | |
| 5 | Amide III | 1240 | ||
| 6 | 1238 |
| Exp. No. | Peak | Wavenumbers (1/cm) | Reference (1/cm) 1 | Note |
|---|---|---|---|---|
| 7 | 3276 | 3440–3300 | N–H stretching | |
| 8 | Amide A | 3286 | ||
| 9 | 3290 | |||
| 7 | 2936 | 3080–2899 | CH2 asymmetrical stretch | |
| 8 | Amide B | 2934 | ||
| 9 | 2925 | |||
| 7 | 1641 | 1700–1600 | C=O stretching | |
| 8 | Amide I | 1645 | ||
| 9 | 1644 | |||
| 7 | 1519 | 1580–1500 | N–H bending | |
| 8 | Amide II | 1525 | ||
| 9 | 1525 | |||
| 7 | 1245 | 1350–1200 | N–H bending and C–N stretching | |
| 8 | Amide III | 1242 | ||
| 9 | 1235 |
| Exp. No. | Organism | Score value | Exp. No. | Organism | Score value |
|---|---|---|---|---|---|
| 1 |
Brevibacillus agri Bacillus cereus |
1997 3 1755 3 |
6 | - | - |
| 2 |
Bacillus cereus Bacillus flexus Brevibacillus agri |
2265 2 2093 2 1995 3 |
7 |
Bacillus cereus Acinetobacter radioresistens |
2239 2 1758 3 |
| 3 |
Acinetobacter radioresistens Acinetobacter baumannii |
2431 1 2004 2 |
8 | Acinetobacter baumannii | 2359 1 |
| 4 |
Enterococcus faecium Bacillus flexus Brevibacillus agri |
2065 2 1835 3 1708 3 |
9 |
Acinetobacter baumannii Brevibacillus agri |
2121 2 2012 2 |
| 5 |
Acinetobacter baumannii Staphylococcus hominis Bacillus cereus |
2071 2 1801 3 2106 2 |
|||
| Exp. | Factor A | Factor B | Exp. | Factor A | Factor B |
|---|---|---|---|---|---|
| No. | Enzyme (%) | Temperature (°C) | No. | Enzyme (%) | Temperature (°C) |
| 1 | 0.10 | 55.0 | 6 | 0.15 | 70.0 |
| 2 | 0.10 | 62.5 | 7 | 0.20 | 55.0 |
| 3 | 0.10 | 70.0 | 8 | 0.20 | 62.5 |
| 4 | 0.15 | 55.0 | 9 | 0.20 | 70.0 |
| 5 | 0.15 | 62.5 |
| Molecular weight species | Unit | Note |
|---|---|---|
| Mp | kDa | Molecular weight of the peak maxima |
| Mw | kDa | Number-average molecular weight |
| Mn | kDa | Weight-average molecular weight |
| PDI | – | Polydispersity index |
| Peak | Reference (1/cm)1 | Note |
|---|---|---|
| Amide A | 3440–3300 | N–H stretching |
| Amide B | 3080–2899 | CH2 asymmetrical stretch |
| Amide I | 1700–1600 | C=O stretching |
| Amide II | 1580–1500 | N–H bending |
| Amide III | 1350–1200 | N–H bending and C–N stretching |
| Score | Note |
|---|---|
| 2300–3000 | Highly probable species identification |
| 2000–2299 | Secure genus identification, probable species identification |
| 1700–1999 | Probable genus identification |
| <1700 | Not reliable identification |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





