1. Introduction
Post-mortem fish undergo rapid spoilage because of biochemical and microbial breakdown mechanisms. Immediately refrigeration is necessary right after the capture to slow down this damage and keep its nutritional value and qualitative characteristics [
1]. The shelf life of fish preserved in ice can be extended depending on the raw material, temperature, atmospheric conditions, and different intrinsic factors such as species, age, size, fat content, the qualitative and quantitative composition of the initial microbiota, and external factors such as the seasonal period and the fishing method can also interfere.
Fresh fish and other marine products are highly susceptible to spoilage from post-mortem microbial growth and enzymatic activity [
2]. Usually, the deterioration of fish comprises four stages: rigor mortis, dissolution of rigor, autolysis (loss of freshness), and bacterial spoilage. These postmortem changes, which directly affect its quality and shelf life, are associated with protein and ATP degradation, a drop of pH, lipid oxidation, undesirable production of compounds, such as trimethylamine (TMA-N), and molecular low-weight volatile bases (TVB-N), which are produced by bacterial action [
3,
4].
Even though Chilean jack mackerel is an underutilized fatty fish captured in large volumes by countries like Chile and Perú, a great interest has been posed in its commercialization [
5]. In contrast to other muscle tissue foods, the main issues the seafood sector experiences are how quickly the flesh softens and the need to find a technique to preserve a firm and consistent texture. When the postmortem degenerative process occurs, the degradation of proteins creates ideal conditions for the growth of microorganisms [
4]. Microorganisms are present on the skin, digestive tract, and some internal organs of live fish which, postmortem, might contaminate the muscle of the fish causing degradation and thus resulting in the release of unpleasant odors and discoloration [
4,
6].
Among all the different non-thermal food-processing methods, high hydrostatic pressure (HHP) was shown to produce foods with natural-like characteristics [
1]. As a food preservation method, HHP has been increasingly employed for commercial seafood processing, such as oysters and fish [
7,
8]. The suitable selection of processing parameters like temperature, time, and pressure can ensure that the processing goal is reached without extensive detrimental effects [
9]. It has been reported that diverse HHP effects might be obtained depending on specific factors related to marine species, for instance, protein denaturation resulting in the inhibition of inherent enzymatic activity and the biogenic activity some microorganisms undergo [
10,
11]. Some research has revealed that HHP treatments can positively affect the texture, stabilize the color, and oxidize the lipids for the inactivation of endogenous enzymes, as well as control or deactivate enzymes involved in the breakdown of fish muscle [
8,
9].
To date, several studies have been published on using HHP as a method for fish preservation; however, only some studies have examined the effects of this technology on the fish muscle behavior during the pre-rigor mortis stage. Therefore, this study aims to evaluate and compare the effect of HHP applied to Chilean jack mackerel under the stage of pre- and post-rigor mortis and further assessment of its quality features such as pH, TVB-N, TMA-N, color (L*, a*, and b*), water holding capacity (WHC) and texture (TPA and shearing force).
2. Materials and Methods
2.1. Sample preparation
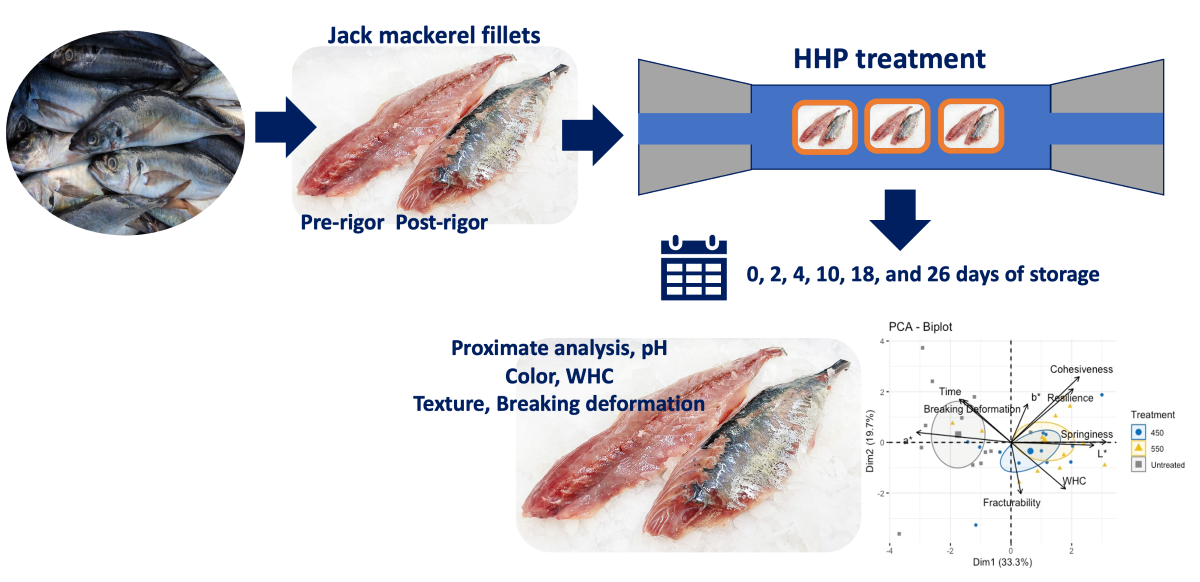
Fresh Chilean jack mackerel (Trachurus murphyi) fish (70 individuals, weight range: 390-420 g) were obtained from the Coquimbo coast (IV Region, Chile), sacrificed in a water-ice mixture, and transported to our laboratory within one hour after the catch. Fish were cut into fillets (weight: 80-90 g). The pre-rigor was less than 6 h after and the post-rigor process was greater than 24 h after the catch. The samples were individually packed and hermetically sealed in high-density polyethylene bags. Two batches were prepared to study the behaviors of the pressurized pre- and post-rigor fish. Then, they were pressurized. Samples were kept under chilling conditions (traditional flake ice) in a refrigerated room (4±1°C) until further analysis.
2.2. HHP treatment
A preliminary study was conducted before selecting the proper HHP treatment settings for the current experiment. Then, two independent variables were considered (pressure and holding time). HHP treatments were performed in an isostatic pressing system (Avure Technologies Incorporated, Kent, WA, USA) with a cylindrical pressure chamber (700 mm length, 60 mm diameter). Water was used as the pressure-transmitting medium (working at 17 MPa/s ramp rate). End closures, a pressure pump, and a hydraulic unit made up the system, which produced high pressure for system compression. Samples were HHP-treated at 450 and 550 MPa for 3 min at room temperature (20 °C) and compared to untreated samples (control).
2.3. Quality parameters
2.3.1. Proximate, pH, and aw
Moisture content was determined by AOAC method number 34.06 [
12] employing a vacuum oven (Gallenkamp, OVL570, Leicester, UK) and an analytical balance with an accuracy of ± 0.0001 g (CHYO, Jex120, Kyoto, Japan). Crude protein content was determined using the Kjeldahl method with a conversion factor 6.25 (AOAC 960.52). Lipid content was analyzed gravimetrically following Soxhlet extraction (AOAC 960.39). Crude ash content was estimated by incineration in a muffle furnace at 550 °C (AOAC 923.03). All methodologies followed the recommendations of the Association of Official Analytical Chemists [
12]. Changes in fish muscle pH values during storage were determined with a pH meter (HANNA HI, 99163, RI, EE. UU), while the water activity of homogenized fish muscle was determined with the Aw-meter Sprint Novasina (TH-500, Pfaffikon, Lachen Switzerland). Also, solvents and reagents were analytical grade and purchased from Sigma-Aldrich Company Ltd. (St. Louis, MO, USA). All measurements were done in triplicate.
2.3.2. Color measurement
The surface color of the fish fillets was measured using a colorimeter (HunterLab, model MiniScan XE Plus, Reston, VA, USA). The colorimeter was previously calibrated using white and black glass standards. Three equidistant spots were examined on the major axis of each fish sample. Since the spot diameter of the instrument was 31.4 mm, data was taken from 7.7 cm
2 of each sample, representing the total area of the samples. Color changes were measured according to the following parameters: L* (lightness, black = 0; white = 100), a* (redness > 0; greenness < 0), and b* (yellowness > 0; blue < 0) according to the Commission Internationale de l'Éclairage (CIE) [
1]. CIE coordinates were determined according to standard illuminant D
65 and observer 10°. The findings of three replicate measurements were averaged.
2.3.3. Water-holding capacity analysis
WHC was determined according to the methodology described by Kristensen and Purslow (2001) [
13], with modifications. Fish fillets were cut into 1 cm
3 cubes. Each cube was placed on a 50 mL falcon tube with a centrally placed plastic mesh which allowed water to drain freely from the sample during centrifugation. Samples were centrifuged at 2204 ×g for 15 min at 20 °C. The water holding capacity was calculated from the amount of water removed from the sample (collected at the bottom of the tube), following Eq. (1) expressed as g retained water/100 g water. All determinations were done in triplicate.
where M is the sample moisture content on a wet basis, W
s is the weight of the sample and W
c is the weight of the water collected in the tube after centrifugation.
2.3.4. Texture profile analysis
The texture profile analysis (TPA) of Chilean jack mackerel was evaluated instrumentally using a texture analyzer (Texture Technologies Corp, TA-XT2, Scardale, NY, USA) equipped with a 100 mm cylindrical probe. Fillets were cut into 2 × 2 cm cubes and the probe was pressed into the samples at a constant speed of 0.8 mm/s and a 50% penetration depth of the cube height. The force required to penetrate the surface of the cubes (breaking force) was recorded. The following texture parameters were obtained from the TPA curves: fracturability, springiness, cohesiveness, and resilience. Each fillet underwent ten measurements, and the mean of these measurements was employed for data analyses.
2.3.5. Trimethylamine nitrogen measurement
The quantification of TMA-N was determined according to the methodology described by the AOAC [
14]. This technique is based on the colorimetric reaction when trimethylamine and picric acid react. Homogenized samples (25 g) were weighed in ultra turrax homogenizer (IKA T18 basic), blended with 50 mL of 7.5% trichloroacetic acid (TCA) solution, centrifuged (CENTAUR 2 MSE) at 3000 rpm and furtherly filtrated. Four milliliters of the obtained extract were transferred into a test tube containing 1 mL formaldehyde (20%), 10 mL anhydrous toluene and 3 mL of a saturated potassium carbonate solution. Test tubes were shaken vigorously, and the obtained toluene phase was placed into a test tube containing 0.1 g of anhydrous sodium sulphate and then agitated to remove water residues. A 5 mL aliquot of the water-free toluene extract was transferred to a new test tube and mixed with 5 mL of picric acid solution (0.02%). The absorbance of the samples was measured at 410 nm using a spectrophotometer (Spectronic Instruments, Spectronic
® 20 Genesys
®). TMA-N concentration was calculated from a standard curve using trimethylamine as the standard compound. Results of TMA-N were expressed as mg per 100 g of muscle. All determinations were done in triplicate.
2.3.6. Total volatile basic nitrogen measurement
TVB-N (mg TVB-N/100 g) was determined according to the Botta methodology [
15]. The TVB-N was determined on 5-10 g fish muscle samples using direct distillation with MgO in a Kjeldahl distillation apparatus (Velp Scientifica, model UDK 129). The distillate titrated with 0.1 N H2SO4. Results were expressed as mg per 100 g of muscle. All measurements were done in triplicate.
2.4. Statistical analysis
All measurements were performed in triplicate. The results were reported as mean values ± standard deviation. Measurement data were analyzed using multifactorial analysis of variance ANOVA, considering all possible differences between the pre- and post-rigor stage of the samples, HHP treatments, and the different variables under study. Statgraphics plus 5.0 (Statistical Graphics Corp., Herndon, VA, USA) calculated significant differences between samples with a confidence interval of 95% (p ≤ 0.05).
3. Results and discussion
3.1. Proximate analysis
As shown in
Table S1, results for the proximate composition of jack mackerel were determined and recorded on day 0 of storage for the pre- and post-rigor mortis samples. Mean values and standard deviation for moisture, ash, protein, and fat content are shown for the untreated and the treated samples. Overall, moisture content showed significant differences between the post-rigor treated samples, the ash content showed significant differences between pre-rigor samples from the control and the treated samples, and proteins showed significant differences between treatments (450 MPa/3 min vs 550 MPa/3 min), with a greater protein content achieved for the pre-rigor sample treated at 450 MPa/3 min compared to the sample processed at 550 MPa/3 min. The increased protein content is not a direct effect of the pressure but rather a side effect of how HHP alters the protein's structure, since after the application of HHP structural modifications are carried out, including changes in tertiary and quaternary structures that may have undergone conformational modifications. On this basis, the hydration of proteins might have been impacted by HHP [
16,
17] and may account for the observed variations. Also, since the pressure level eventually leads to denaturation, with a stronger treatment a lower protein content could be expected [
16].
The moisture content found in all the samples was comparable to Celik's (2008) earlier research, which stated that the moisture content of chub and horse mackerel varied between 75.1% and 78.9% [
18]. As for ash content, according to previously published results, the Indian mackerel (
Rastrelliger kanagurta), for instance, had an ash content of roughly 1.42 [
19]. In the present study, the species under analysis showed an ash content between 1.0 and 1.40, like the reported ash content of the Indian mackerel.
Regarding lipids, the most notable result was the rise in the content between the treated post-rigor mortis samples and the control sample. The aforementioned provides evidence in favor of what has already been found regarding the possible prevention of lipid degradation by avoiding high temperatures for extended periods if proper pressure and holding time were used [
20]. Even if non-significant differences were found, a significant inhibition of the lipid oxidation for HHP-treated mackerel has been established over time [
21]. Malga et al. (2023) obtained higher lipid contents for the post-rigor chilled treated palm ruff (
Seriolella violacea) samples and higher average lipid levels for fish treated at 450 MPa, as our results showed [
22]. Nonetheless, it must be considered that previously reported findings regarding variations in the nutritional value of fish before and after rigor need to be clarified.
3.2. Determination of pH
Table S2 shows the changes in pH of jack mackerel muscle during storage at 4.0 ± 1 °C for the control and HHP-treated samples in the pre- and post-rigor stages. Every sample showed an increase in the pH value over time, however, the greatest increases in the pH values were observed for the untreated sample over time, for which a rise of almost one unit per measurement was observed. The pH of HHP-treated fish barely increases compared to untreated fish, which is probably associated with the denaturation of protein fractions [
16]. It has been established that high pressure treatments kill certain types of microorganisms that might be responsible for the degradation of fish muscle due to the production of volatile substances such as acids, amines, and bases [
9]. For these reasons, pH rises during the spoilage of meat and fish; therefore, the relative stability of the pH in the pressure-treated samples was expected [
9]. Although it is typical to see a drop in pH values immediately following death, due to the generation of lactic acid, pH rises in later post-mortem phases [
23]. A pH value between 6.8 and 7 is recommended as the accepted range for fish meat and values above 7 are considered unsafe for human consumption [
24]. This value was already met by day 10 for the untreated sample, while the treated samples remained safe until the last day of the study (day 26). It's interesting to note that HHP, besides successfully lowering the value, might also have a significant impact (p < 0.05) on most of the treated samples in pre- and post-rigor stage.
3.3. Determination total volatile basic nitrogen
Overall, all samples showed a constant TVB-N increase throughout the study time, nevertheless, it is evident that treated samples showed a lower value at the end of the storage time, which demonstrated that the control and treated samples differed significantly (p < 0.05) (
Table S3). The initial rise in TVB-N in the control samples may be attributed to the autolytic deamination of free amino acids generated by proteolysis and nucleotide degradation, which is also related to the rise in the pH of the control sample. A combination of microbial and autolytic processes likely caused the larger increase in TVB-N from day 18 to day 26 for the treated samples, since, typically, TVB-N is formed due to different biochemical activities produced in the fish [
25]. HHP has been successfully applied to prevent the rotting of various food matrices, and as a result, its use helps to postpone the development of nitrogen-based volatile chemicals [
26]. Control and treated samples showed significant differences (p < 0.05) in the TVB-N content between the pre and post rigor stages, particularly from day four in advance for the control sample, day 10 for the 450 MPa/3 min sample. Interestingly, the sample treated at 550 MPa/3 min only showed a significant difference on day zero, meaning that the storage time did not directly affect the TVB-N between the pre and post rigor stage. The above might suggest that a 550 MPa/3 min treatment might stabilize the rise in TVB-N content between pre and post rigor samples more effectively. The effect of the fish rigor stage on the production of volatile amines has been studied and a higher TVB-N value has been found for post-rigor Chilean jack mackerel, as well as for other species, such as rainbow trout (
Oncorhynchus mykiss) and mirror carp (
Cyprinus carpio) under refrigeration [
22].
3.4. Determination of trimethylamine
Trimethylamine (TMA-N), such as TVB-N, is generated as a volatile compound as part of the nonprotein nitrogen fraction (NPN) after degradation during fish storage [
26]. The quantitative level of TMA-N in fish is considered an essential index of the quality of fish [
27].
Table S4 shows the TMA-N content (mg/100 g) for the control and treated samples, for which the increase of TMA-N was expected over time.
From all the treated samples, 66.6% (8/12) from the 450 MPa/3 min treatment and 66% (8/12) of the samples treated at 550 MPa/3 min showed significant differences compared to the control samples. Notably, the maximum TMA-N value obtained for the treated samples was 5.38 mg/100 g for the post-rigor sample treated at 450 MPa/3 min on day 26, while the maximum TMA-N obtained for the control sample (22.92 mg/100 g) was 4.26 times higher. This result, in concordance with the previously discussed TVB-N values, demonstrates the effectiveness of HHP treatments in reducing the production of volatile nitrogen-based compounds and the growth of microorganisms [
26,
28].
Since, according to the FAO, appropriate quality fish contains less than 1.5 mg/100 g TMA-N, with a limit of acceptability of 10-15 mg/100 g TMA-N [
27], the control sample would not be suitable for consumption by day 26 of storage at 4 °C. Contrary to our findings, other authors have reported lower TMA-N values for control samples. Wallner-Pendleton et al. (1994) reported a TMA-N concentration of 4.3 mg/100 g on the last day of the study of vacuum-packed hake (
Merluccius capensis), nonetheless, the type of treatment applied must be considered, as well as the length of the study (14 days) [
29]. Gram-negative microbes, which are the primary reducers of trimethylamine oxide (TMAO) to TMA in anaerobic circumstances, are primarily responsible for TMA-N increases [
27].
3.5. Color Measurement
Within the visible effects caused by the HHP treatment, one of the most apparent modifications was the color. The impact of high pressure on color aspect of the Chilean jack mackerel is shown in
Table S5. Fish fillets were noticeably lighter for the treated samples (p < 0.05), which is consistent with previous results where HHP-treated fish fillets showed an increase in the L* value compared to control samples [
1,
4]. The increase in the L* value has been described for treatments starting at 100 MPa/15 min with successive increases related to the intensification of the pressure and the holding time, as from 100 MPa for 15 min with subsequent increases as pressure and holding time increase, thus, the degree to which L* measurement rises appear to be correlated with the severity of the treatment, which may result in conformational changes in proteins, for instance, denaturation of the globin and myofibrillar proteins [
1,
4].
Also, in concordance with other studies, the yellowness of the fillets increased with the intensity of the treatment [
1]. In our results, the post-rigor sample treated at 550 MPa/3 min showed the most significant increase in the b* values. Moreover, contrary to results published by Castrica et al. (2021) for HHP-treated
Salmo salar and
Pleuronectes platessa, increases in the a-value of treated samples were observed [
1]. As the pressure was raised during the treatment, the muscle developed a cooked appearance, which would also cause an increase in b-values as the muscle coagulates forming a whitish-yellowish appearance. Myoglobin oxidation and denaturation have been linked to muscle discoloration, decreased myoglobin extractability, and the release of nonheme iron, thereby accelerating lipid oxidation in sardine and mackerel muscle during ice storage [
10,
30]. Overall, significant color changes were observed between control and treated samples, with a more evident lighter and greener outcome for the treatments. The pigmentation of the fish is an important attribute since the color of a food product is associated with the consumer’s acceptance [
31].
3.6. Water holding capacity
Apart from samples treated at 450 MPa/3 min at day 0, the WHC of jack mackerel was not significantly different depending on the rigor stage from the same sample (p > 0.05). In all cases, samples showed a high WHC value (>98 g/100 g) (
Table S6). Some effects, such as changes in the WHC, have been previously described as possible outcomes of intensive HHP treatments [
4].
Effects, including modifications in the WHC, have been mentioned as possible outcomes of more aggressive therapies [
4]. In contrast to post-rigor-treated samples, where the WHC values increased over time, treated samples' WHC values decreased over time for pre-rigor samples. Regarding this finding, authors have reported that the high-pressure treatment helps to reduce water loss, probably due to the breakdown and deployment of the protein and the pH of the mixture [
32]. Overall, water retention capacity is related to protein denaturation and evidence supports that proteolysis of cytoskeletal proteins, such as desmin, can be related to fluid retention myofibrils [
33]. Changes in intracellular fibrils architecture can be induced by pressure and may influence the ability of the muscle cells to retain water [
22,
33]. Skjervold et al. (2001) further explain that water content might also increase due to ice storage [
34].
3.7. Textural characterization
Results for the textural measurements (fracturability, springiness, cohesiveness, and resilience) and cut (breaking deformation) are summarized in
Tables S7 and S8, respectively. Fracturability, defined as the amount of fracturability in a sample after biting [
35] was noticeably different for most of the pressurized pre- and post-rigor samples (p < 0.05). As for springiness, cohesiveness, and resilience results did not evidence apparent differences between pre and post rigor treated and control samples (p > 0.05). The changes between pre- and post-rigor are likely caused by both the filleting time and the storage period, as both gaping and texture vary more with storage time [
34].
Several factors influence the texture of fish meat, among these are the rate and extent of post-mortem muscle shortening (
rigor mortis), the rate and extent of post-mortem pH decline, and the rate and extent of proteolysis causing myofibril breakdown [
36,
37]. The muscle of the HHP-treated samples remained more compact than in the control sample and did not display cracks. It has been described that high pressures induce the denaturation of myosin leading to the development of hydrogen bonds-containing structures which were thought to be stabilized by disulfide bonds. Also, in some cases, high pressure-treated samples have shown a harder muscle consistency [
38].
Moreover, springiness, which refers to the recovery of the deformation, and cohesiveness, which is a measure of the deformation before rupture [
39], decreased for the treated samples from day 0 to day 30 of storage. Unless the material in the study has structural damage, springiness and cohesiveness are commonly found to be closely related [
39].
The texture of fish fillets is one of the most important quality parameters of fish for producers, processors, and consumers. Consumers want a texture that makes the chewiness and digestion easy and gives a high-quality experience based on high flavor [
40,
41]. Since fish muscle is very heterogeneous, sampling and measurements are difficult to reproduce and might reflect a need for more correlation among the obtained results. In addition, instrumental, and sensory evaluations differ in assessing texture [
42].
The effect of the high-pressure treatments on the breaking deformation (mm) of Chilean jack mackerel fish fillet can be observed in
Table S8. Significant differences can be observed for pre-rigor samples from day 10 of storage. Treated samples first showed an increase in the breaking deformation by day 10 and then a decrease in the value (p < 0.05), indicating that by the end of the storage period pre-rigor treated samples showed a more flexible texture. Results for post-rigor samples showed more consistent results by days 18 and 26 of storage, where most of the treated samples showed a lower breaking deformation (p < 0.05). Pressurization at 450 MPa/3 min demonstrated more constant lower deformation values. HHP causes modifications of the TPA of fish fillets and authors have reported an increase in hardness for pressurized samples [
43], nonetheless, our results did not remain constant over time.
Hardness is consumers' most crucial texture attribute and dictates the commercial value of meat [
44]. It has been reported that the spoilage caused by the activity of autolytic enzymes in tissue appeared to significantly affect texture deterioration. The reduction of textural properties might result from the weakened connective tissue [
45]. Fish muscle generally becomes softer during chilled storage after catch [
40]. The drop in the elasticity values was observed at a higher pressure and may be due to its loss because of the disruption of cohesive forces within the tissue. Even if the tissue might seem tough/hard to touch, the reduced elasticity causes it to yield abruptly and easily under compression.
Krokida et al. (2001), for instance, reported a decrease in elasticity of sea bream muscle with increasing pressure just after treatment, but the elasticity was maintained during storage in samples treated at higher pressures [
46]. The phenomenon has been related to reduced degradation of cytoskeletal proteins (assayed by western blotting) due to blockade of proteolytic activity by HPP. Pressure-induced texture modifications have been used to affect myofibrillar proteins and their gel-forming properties, raising the possibility of developing processed muscle-based food. High pressure can affect molecular interactions (hydrogen bonds, hydrophobic interactions, and electrostatic bonds), and protein conformation, leading to protein denaturation, aggregation, or gelation [
47]. Depending on the source of muscle and other parameters such as protein concentration, pH, and ionic strength, various changes occur in myofibrillar proteins depending on the combination pressure:temperature [
33].
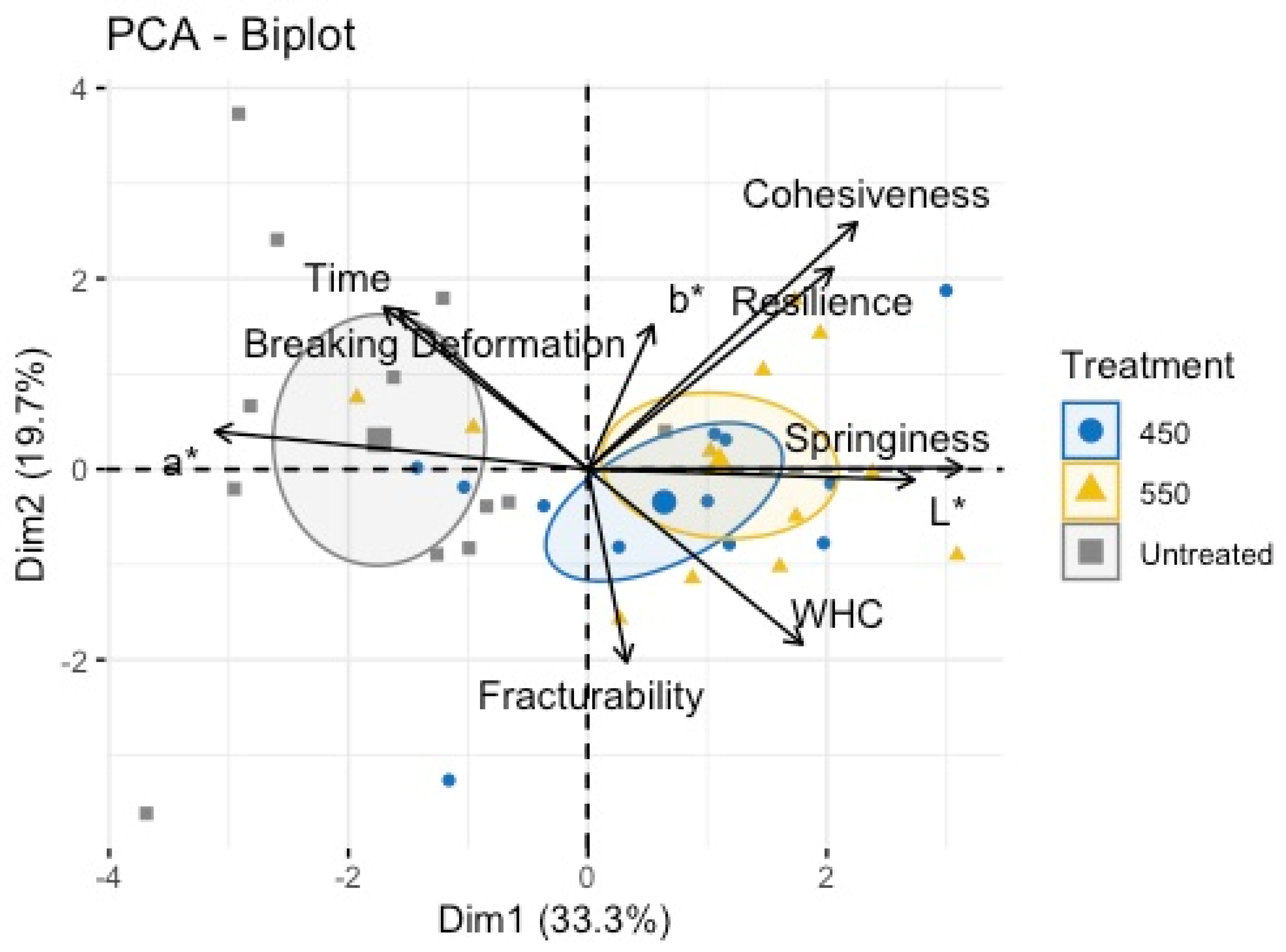
Overall, the grouped results for the color measurement, water-holding capacity and texture profile are shown in
Figure 1. The proximate component analysis (PCA) biplot performed for the untreated and treated samples did not show a specific pattern; nonetheless, treated samples were mainly related to color changes (p < 0.05) as well as textural measurements, such as cohesiveness, resilience, springiness, and fracturability.
4. Conclusions
High hydrostatic pressure (HHP) (450 and 550 MPa for 3 min) treatments applied to Chilean jack mackerel fillets demonstrated significant changes in the physicochemical properties, such as the nutritional value, pH, TVB-N, TMA, WHC, color, and texture, during storage at 4 °C. While both treatments were beneficial, the 450 MPa/3 min treatment demonstrated a higher retention of the fish fillet quality characteristics, since this pressure and holding time combination not only preserved fillets until the last day of study (day 26) but reduced unwanted associated HHP effects, such as the loss of the natural color of raw meat. Nevertheless, the 550 MPa/3 min treatment provided superior control over several undesirable characteristics, such as the rise in pH and TBV-N. Additionally, HHP has been demonstrated to control the spoilage of pre- and post-rigor fillets, particularly important for post-rigor fillets that are more prone to rotting. Thereby, Chilean jack mackerel fillets can be considered acceptable for consumption up until day 10 of storage at 4 °C without high-pressure processing, as opposed to 26 days for the HHP-treated samples. The increase in the storage time of the fillets makes this technique a real treatment alternative for the conservation, preservation of the nutritional composition, and commercialization of Chilean jack mackerel under refrigeration conditions.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Proximate analysis results of unpressurised and pressurized Jack Mackerel in Day 0; Table S2: Effect of pressurized treatments on the pH value of pre and post rigor jack mackerel; Table S3: Evaluation of the total volatile basic nitrogen (TVB-N) content (mg 100-1 g muscle) in unpressurized and pressurized jack mackerel in pre and post rigor stage; Table S4: TMA-N (mg 100 g) analysis results of unpressurized and pressurized jack mackerel in pre and post-rigor; Table S5: Color analysis results of unpressurized and pressurized jack mackerel in pre and post rigor; Table S6: WHC values results from unpressurized and pressurized jack mackerel in pre- and post-rigor; Table S7: Texture analysis results for unpressurized and pressurized jack mackerel in pre and post rigor; Table S8: Breaking deformation (mm) results of unpressurized and pressurized Jack mackerel in pre and post rigor.
Author Contributions
Conceptualization, LZ-B and RL-M.; methodology, LZ-B.; formal analysis, LZ-B, KM, DS and RL-M.; writing-original draft preparation, DS and RL-M; writing-review and editing, DS, KM, GT-M, MP-W and RL-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the ANID-FONDECYT N°1110782 project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cropotova, J.; Mozuraityte, R.; Standal, I.B.; Ojha, S.; Rustad, T.; Tiwari, B. Influence of high-pressure processing on quality attributes of haddock and mackerel minces during frozen storage, and fishcakes prepared thereof. Innov Food Sci Emerg Technol 2020, 59, 102236. [Google Scholar] [CrossRef]
- Hassoun, A.; Jagtap, S.; Trollman, H.; Garcia-Garcia, G.; Abdullah, N.A.; Goksen, G.; Bader, F.; Ozogul, F.; Barba, F.J.; Cropotova, J.; et al. Food processing 4.0: current and future developments spurred by the fourth industrial revolution. Food Control 2023, 145, 109507. [Google Scholar] [CrossRef]
- Alasalvar, C.; Taylor, K.D.A.; Shahidi, F. Comparative quality assessment of cultured and wild sea bream (Sparus Aurata) stored in ice. J Agric Food Chem 2002, 50, 2039–2045. [Google Scholar] [CrossRef]
- Castrica, M.; Pavlovic, R.; Balzaretti, C.M.; Curone, G.; Brecchia, G.; Copelotti, E.; Panseri, S.; Pessina, D.; Arnoldi, C.; Chiesa, L.M. Effect of high-pressure processing on physico-chemical, microbiological and sensory traits in fresh fish fillets (Salmo Salar and Pleuronectes Platessa). Foods 2021, 10. [Google Scholar] [CrossRef]
- Quitral, V.; Donoso, M.L.; Ortiz, J.; Herrera, M.V.; Araya, H.; Aubourg, S.P. Chemical changes during the chilled storage of chilean jack mackerel (Trachurus Murphyi): effect of a plant-extract icing system. LWT - Food Sci Technol 2009, 42, 1450–1454. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, L. The microbial safety of fish and fish products: recent advances in understanding its significance, contamination sources, and control strategies. Compr Rev Food Sci Food Saf 2021, 20, 738–786. [Google Scholar] [CrossRef]
- Erkan, N.; Üretener, G. The effect of high hydrostatic pressure on the microbiological, chemical and sensory quality of fresh gilthead sea bream (Sparus Aurata). Eur Food Res Technol 2010, 230, 533–542. [Google Scholar] [CrossRef]
- Perez-Won, M.; Lemus-Mondaca, R.; Herrera-Lavados, C.; Reyes, J.E.; Roco, T.; Palma-Acevedo, A.; Tabilo-Munizaga, G.; Aubourg, S.P. Combined treatments of high hydrostatic pressure and co2 in coho salmon (Oncorhynchus Kisutch): effects on enzyme inactivation, physicochemical properties, and microbial shelf life. Foods 2020, 9. [Google Scholar] [CrossRef]
- Tsevdou, M.; Dimopoulos, G.; Limnaios, A.; Semenoglou, I.; Tsironi, T.; Taoukis, P. High pressure processing under mild conditions for bacterial mitigation and shelf life extension of european sea bass fillets. Applied Sciences 2023, 13, 3845. [Google Scholar] [CrossRef]
- Yagiz, Y.; Kristinsson, H.G.; Balaban, M.O.; Marshall, M.R. Effect of high pressure treatment on the quality of rainbow trout (Oncorhynchus Mykiss) and mahi mahi (Coryphaena Hippurus). J Food Sci 2007, 72. [Google Scholar] [CrossRef]
- Yagiz, Y.; Kristinsson, H.G.; Balaban, M.O.; Welt, B.A.; Ralat, M.; Marshall, M.R. Effect of high pressure processing and cooking treatment on the quality of atlantic salmon. Food Chem 2009, 116, 828–835. [Google Scholar] [CrossRef]
- AOAC Official Methods of Analysis. 15th Ed. 1990.
- Kristensen, L.; Purslow, P.P. The Effect of Ageing on the water-holding capacity of pork: role of cytoskeletal proteins. Meat Sci 2001, 58, 17–23. [Google Scholar] [CrossRef]
- Bethea, S.; Hillig, F. Determination of trimethylamine nitrogen in extracts and in volatile fractions of fish. J AOAC Int 1965, 48, 731–735. [Google Scholar] [CrossRef]
- Botta, J.R.; Lauder, J.T.; Jewer, M.A. Effect of methodology on total volatile basic nitrogen (TVB-N) determination as an index of quality of fresh atlantic cod (Gadus Morhua). J Food Sci 1984, 49, 734–736. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Zu, S.; Wu, X.; Shi, A.; Zhang, J.; Wang, Q.; He, N. Effects of high hydrostatic pressure on the conformational structure and gel properties of myofibrillar protein and meat quality: a review. Foods 2021, 10. [Google Scholar] [CrossRef]
- Nikbakht Nasrabadi, M.; Sedaghat Doost, A.; Mezzenga, R. Modification approaches of plant-based proteins to improve their techno-functionality and use in food products. Food Hydrocoll 2021, 118, 106789. [Google Scholar] [CrossRef]
- Celik, M. Seasonal changes in the proximate chemical compositions and fatty acids of chub mackerel (Scomber Japonicus) and horse mackerel (Trachurus Trachurus) from the north eastern mediterranean sea. Int J Food Sci Technol 2008, 43, 933–938. [Google Scholar] [CrossRef]
- Sonavane, A.E. Proximate Composition and fatty acid profiling of indian mackerel (Rastrelliger Kanagurta) off Ratnagiri, West Coast of India. Int J Pure Appl Biosci 2017, 5, 920–924. [Google Scholar] [CrossRef]
- Medina-Meza, I.G.; Barnaba, C.; Barbosa-Cánovas, G. V Effects of high pressure processing on lipid oxidation: a review. Innov Food Sci Emerg Technol 2014, 22, 1–10. [Google Scholar] [CrossRef]
- Maluenda, D.; Roco, T.; Tabilo-Munizaga, G.; Pérez-Won, M.; Aubourg, S.P. Effect of a previous high hydrostatic pressure treatment on lipid damage in chilled chilean jack mackerel (Trachurus Murphyi). Grasas y Aceites 2013, 64, 472–481. [Google Scholar] [CrossRef]
- Malga, J.M.; Roco, T.; Silva, A.; Tabilo-Munizaga, G.; Pérez-Won, M.; Aubourg, S.P. Effect of rigor stage and pressurisation on lipid damage, total volatile amine formation and autolysis development in palm ruff stored on ice. Foods 2023, 12. [Google Scholar] [CrossRef]
- Briones, L.S.; Reyes, J.E.; Tabilo-Munizaga, G.E.; Pérez-Won, M.O. Microbial shelf-life extension of chilled coho salmon (Oncorhynchus Kisutch) and abalone (Haliotis Rufescens) by high hydrostatic pressure treatment. Food Control 2010, 21, 1530–1535. [Google Scholar] [CrossRef]
- Said Talab, A. effect of cooking methods and freezing storage on the quality characteristics of fish cutlets. Adv J Food Sci Technol 2014, 6, 468–479. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Srivastav, P.P.; Pathak, S.S.; Das, K. Mathematical modeling of total volatile basic nitrogen and microbial biomass in stored rohu (Labeo Rohita) fish. Front Sustain Food Syst 2021, 5. [Google Scholar] [CrossRef]
- Lan, Q.; Tappi, S.; Braschi, G.; Picone, G.; Rocculi, P.; Laghi, L. Effect of high hydrostatic pressure on the metabolite profile of striped prawn (Melicertus Kerathurus) during chilled storage. Foods 2022, 11. [Google Scholar] [CrossRef]
- Rhoda Mae, Simora.; Armada, C.D.; Babaran, R.P. Quality assessment of marinated flying fish (Cheilopogon Intermedius) fillets during vacuum-packed storage at 4° C. Philipp J Sci 2021, 150, 223–232. [CrossRef]
- Mitsubayashi, K.; Kubotera, Y.; Yano, K.; Hashimoto, Y.; Kon, T.; Nakakura, S.; Nishi, Y.; Endo, H. Trimethylamine biosensor with flavin-containing monooxygenase type 3 (FMO3) for fish-freshness analysis. Sens Actuators B Chem 2004, 103, 463–467. [Google Scholar] [CrossRef]
- Wallner-Pendleton, E.A.; Sumner, S.S.; Froning, G.W.; Stetson, L.E. The use of ultraviolet radiation to reduce Salmonella and psychrotrophic bacterial contamination on poultry carcasses, Poult Sci 1994, 73, 1327–1333. [Google Scholar] [CrossRef]
- Chaijan, M.; Benjakul, S.; Visessanguan, W.; Faustman, C. Changes of lipids in sardine (Sardinella Gibbosa) muscle during iced storage. Food Chem 2006, 99, 83–91. [Google Scholar] [CrossRef]
- Colihueque, N.; Araneda, C. Appearance traits in fish farming: progress from classical genetics to genomics, providing insight into current and potential genetic improvement. Front Genet 2014, 5. [Google Scholar] [CrossRef]
- Cheftel, J.C.; Culioli, J. Effects of high pressure on meat: a review. Meat Sci 1997, 46, 211–236. [Google Scholar] [CrossRef]
- Campus, M. High pressure processing of meat, meat products and seafood. Food Eng Rev 2010, 2, 256–273. [Google Scholar] [CrossRef]
- Skjervold, P.O.; Bencze Rørå, A.M.; Fjæra, S.O.; Vegusdal, A.; Vorre, A.; Einen, O. Effects of pre-, in-, or post-rigor filleting of live chilled atlantic salmon. Aquaculture 2001, 194, 315–326. [Google Scholar] [CrossRef]
- Yates, M.D.; Drake, M.A. Texture properties of gouda cheese. J Sens Stud 2007, 22, 493–506. [Google Scholar] [CrossRef]
- Ando, M.; Nishiyabu, A.; Tsukamasa, Y.; Makinodan, Y. Post-mortem softening of fish muscle during chilled storage as affected by bleeding. J Food Sci 1999, 64, 423–428. [Google Scholar] [CrossRef]
- Delbarre-Ladrat, C.; Chéret, R.; Taylor, R.; Verrez-Bagnis, V. Trends in postmortem aging in fish: understanding of proteolysis and disorganization of the myofibrillar structure. Crit Rev Food Sci Nutr 2006, 46, 409–421. [Google Scholar] [CrossRef]
- Sun, X.D.; Holley, R.A. Factors influencing gel formation by myofibrillar proteins in muscle foods. Compr Rev Food Sci Food Saf 2011, 10, 33–51. [Google Scholar] [CrossRef]
- Jonkers, N.; van Dommelen, J.A.W.; Geers, M.G.D. Intrinsic mechanical properties of food in relation to texture parameters. Mech Time Depend Mater 2022, 26, 323–346. [Google Scholar] [CrossRef]
- Bland, J.M.; Bett-Garber, K.L.; Li, C.H.; Brashear, S.S.; Lea, J.M.; Bechtel, P.J. Comparison of sensory and instrumental methods for the analysis of texture of cooked individually quick frozen and fresh-frozen catfish fillets. Food Sci Nutr 2018, 6, 1692–1705. [Google Scholar] [CrossRef]
- Johnson, A. Texture Analyses of Catfish: a Genetics approach. Master’s Thesis, Auburn University, USA, 2021. [Google Scholar]
- Sigurgisladottir, S.; Hafsteinsson, H.; Jonsson, A.; Lie, Ø.; Nortvedt, R.; Thomassen, M.; Torrissen, O. Textural properties of raw salmon fillets as related to sampling method. J Food Sci 1999, 64, 99–104. [Google Scholar] [CrossRef]
- Huang, C.-H.; Lin, C.-S.; Lee, Y.-C.; Ciou, J.-W.; Kuo, C.-H.; Huang, C.-Y.; Tseng, C.-H.; Tsai, Y.-H. quality improvement in mackerel fillets caused by brine salting combined with high-pressure processing. Biology (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Yoshioka, K.; Yamada, A.; Maki, T. Application of high pressurization to fish meat: changes in the physical properties of carp skeletal muscle resulting from high pressure thawing. Progress in Biotechnology 1996, 13. [Google Scholar] [CrossRef]
- Lakshmanan, R.; Piggott, J.R.; Paterson, A. Potential applications of high pressure for improvement in salmon quality. Trends Food Sci Technol 2003, 14, 354–363. [Google Scholar] [CrossRef]
- Krokida, M.K.; Maroulis, Z.B.; Saravacos, G.D. Rheological properties of fluid fruit and vegetable puree products: compilation of literature data. Int J Food Prop 2001, 4, 179–200. [Google Scholar] [CrossRef]
- Messens, W.; Van Camp, J.; Huyghebaert, A. The use of high pressure to modify the functionality of food proteins. Trends Food Sci Technol 1997, 8, 107–112. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).