Submitted:
25 November 2023
Posted:
28 November 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Grape
2.2. Standards and chemicals
2.3. Winemaking
2.3.1. Traditional processing
2.3.2. Innovative processing
2.4. HPLC anaysis
2.4.1. Phenols
2.4.2. Anthocyanins
2.5. Determination of titratable acidity
2.6. Determination of the sugar content
2.7. Statistical analysis
3. Results
3.1. Stilbenes
3.2. Quercetin
3.3. Biologically active phenols
3.4. Phenolic acids
3.5. Anthocyanins
3.6. Wine after storage
3.6.1. Principal component analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Langcake, P.; Pryce, R. J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9(1), 77-86. [CrossRef]
- Téguo, P.; Hawthorne, M. E.; Cuendet, M.; Mérillon, J. M.; Kinghorn, A. D.; Pezzuto, J. M.; Mehta, R. G. Potential cancer-chemopreventive activities of wine stilbenoids and flavans extracted from grape (Vitis vinifera) cell cultures. Nutr. Cancer. 2001, 40(2), 173-179. [CrossRef]
- Balga, I.; Lesko, A.; Ladanyi, M.; Kallay, M. Influence of ageing on changes in polyphenolic compounds in red wines. Czech J. Food Sci. 2015, 32, 563-569. http://dx.doi.org/10.17221/138/2014-CJFS.
- Cvejic, J. M.; Djekic, S. V.; Petrovic, A. V.; Atanackovic, M. T.; Jovic, S. M.; Brceski, I. D.; Gojkovic-Bukarica, L. C. Determination of trans-and cis-resveratrol in Serbian commercial wines. J. Chromatogr. Sci. 2010, 48(3), 229-234. [CrossRef]
- Atanacković, M.; Petrović, A.; Jović, S.; Gojković-Bukarica, L.; Bursać, M.; Cvejić, J. Influence of winemaking techniques on the resveratrol content, total phenolic content and antioxidant potential of red wines. Food Chem. 2012, 131(2), 513-518. [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M. T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8(3), 167. [CrossRef]
- Cantos, E.; Espín, J. C.; Fernández, M. J.; Oliva, J.; Tomás-Barberán, F. A. Postharvest UV-C-irradiated grapes as a potential source for producing stilbene-enriched red wines. J. Agric. Food Chem. 2003, 51(5), 1208-1214. [CrossRef]
- Zhang, P.; Ma, W.; Meng, Y.; Zhang, Y.; Jin, G.; Fang, Z. Wine phenolic profile altered by yeast: Mechanisms and influences. Compr. Rev. Food Sci. Food Saf., 2021, 20(4), 3579-3619. [CrossRef]
- Tristezza, M.; di Feo, L.; Tufariello, M.; Grieco, F.; Capozzi, V.; Spano, G.; Mita, G. Simultaneous inoculation of yeasts and lactic acid bacteria: Effects on fermentation dynamics and chemical composition of Negroamaro wine. LWT-Food Sci. Technol. 2016, 66, 406-412. [CrossRef]
- Minnaar, P. P.; Du Plessis, H. W.; Jolly, N. P.; Van Der Rijst, M.; Du Toit, M. Non-Saccharomyces yeast and lactic acid bacteria in Co-inoculated fermentations with two Saccharomyces cerevisiae yeast strains: A strategy to improve the phenolic content of Syrah wine. Food Chem. 2019, 4, 100070. [CrossRef]
- Ngqumba, Z.; Ntushelo, N.; Jolly, N. P.; Ximba, B. J.; Minnaar, P. P. Effect of Torulaspora delbrueckii yeast treatment on flavanols and phenolic acids of Chenin blanc wines. S. Afr. J. Enol. 2017, 38(2), 192-200. http://dx.doi.org/10.21548/38-2-1391.
- Morata, A.; Escott, C.; Loira, I.; Del Fresno, J. M.; González, C.; Suárez-Lepe, J. A. Influence of Saccharomyces and non-Saccharomyces yeasts in the formation of pyranoanthocyanins and polymeric pigments during red wine making. Molecules 2019, 24(24), 4490. http://dx.doi.org/10.3390/molecules24244490.
- Majkić, T. M.; Torović, L. D.; Lesjak, M. M.; Četojević-Simin, D. D.; Beara, I. N. Activity profiling of Serbian and some other European Merlot wines in inflammation and oxidation processes. Food Res. Int. 2019, 121, 151-160. [CrossRef]
- Tanner, H.; Brunner, H.R. Gentranke-Analytik; Verlag Heller-Chemie und Verwaltunsgesellschaft mbH: Darmstadt, Germany, 1979.
- Escribano-Viana, R.; Portu, J.; Garijo, P.; López, R.; Santamaría, P.; López-Alfaro, I.; Gutiérrez, A. R.; González-Arenzana, L. Effect of the sequential inoculation of non-Saccharomyces/Saccharomyces on the anthocyans and stilbenes composition of tempranillo wines. Front. Microbiol. 2019, 10, 773. https://doi.10.3389/fmicb.2019.00773.
- Kostadinović, S.; Wilkens, A.; Stefova, M.; Ivanova, V.; Vojnoski, B.; Mirhosseini, H.; Winterhalter, P. Stilbene levels and antioxidant activity of Vranec and Merlot wines from Macedonia: Effect of variety and enological practices. Food Chem. 2012, 135(4), 3003-3009. [CrossRef]
- Gaensly, F.; Agustini, B. C.; da Silva, G. A.; Picheth, G.; Bonfim, T. M. B. Autochthonous yeasts with β-glucosidase activity increase resveratrol concentration during the alcoholic fermentation of Vitis labrusca grape must. J. Funct. Foods 2015, 19, 288-295. http://dx.doi.org/10.1016/j.jff.2015.09.041.
- Hernández, T.; Estrella, I.; Pérez-Gordo, M.; Alegría, E. G.; Tenorio, C.; Ruiz-Larrrea, F.; Moreno-Arribas, M. V. Contribution of malolactic fermentation by Oenococcus oeni and Lactobacillus plantarum to the changes in the nonanthocyanin polyphenolic composition of red wine. J. Agric. Food Chem. 2007, 55, 5260-5266. [CrossRef]
- Poussier M.; Guilloux-Benatier M.; Torres M.; Heras E.; Adrian M. Influence of different maceration techniques and microbial enzymatic activities on wine stilbene content. Am. J. Enol. Vitic. 2003, 54, 261-266. http://dx.doi.org/10.5344/ajev.2003.54.4.261.
- Tıraş,Z. S. E.; Okur, H. H.; Günay, Z.; Yıldırım, H. K. Different approaches to enhance resveratrol content in wine. Ciência Téc. Vitiv. 2022, 37, 13-28. [CrossRef]
- Sato, M.; Suzuki, Y.; Okuda, T.; Yokotsuka, K. Contents of resveratrol, piceid, and their isomers in commercially available wines made from grapes cultivated in Japan. Biosci. Biotechnol. Biochem. 1997, 61(11), 1800-1805. [CrossRef]
- Gerogiannaki-Christopoulou, M.; Athanasopoulos, P.; Kyriakidis, N.; Gerogiannaki, I. A.; Spanos, M. trans-Resveratrol in wines from the major Greek red and white grape varieties. Food Control 2006, 17(9), 700-706. [CrossRef]
- Kocabey, N.; Yilmaztekin, M.; Hayaloglu, A. A. Effect of maceration duration on physicochemical characteristics, organic acid, phenolic compounds and antioxidant activity of red wine from Vitis vinifera L. Karaoglan. J. Food Sci. Technol. 2016, 53(9), 3557-3565. http://dx.doi.org/10.1007/s13197-016-2335-4.
- Generalić Mekinić, I.; Skračić, Ž.; Kokeza, A.; Soldo, B.; Ljubenkov, I.; Banović, M.; Skroza, D. Effect of winemaking on phenolic profile, colour components and antioxidants in Crljenak kaštelanski (sin. Zinfandel, Primitivo, Tribidrag) wine. J. Food Sci. Technol. 2019, 56(4), 1841–1853. [CrossRef]
- Alencar, N. M. M.; Cazarin, C. B. B.; Corrêa, L. C.; Maróstica Junior, M. R.; Biasoto, A. C. T.; Behrens, J. H. Influence of maceration time on phenolic compounds and antioxidant activity of the Syrah must and wine. J. Food Biochem. 2017, 42, e12471. http://dx.doi.org/10.1111/jfbc.12471.
- Ginjom, I.; D’Arcy, B.; Caffin, N.; Gidley, M. Phenolic compound profiles in selected Queensland red wines at all stages of the wine-making process. Food Chem. 2011, 125(3), 823-834. [CrossRef]
- Gambuti, A.; Strollo, D.; Ugliano, M.; Lecce, L.; Moio, L. trans-Resveratrol, quercetin,(+)-catechin, and (−)-epicatechin content in south Italian monovarietal wines: relationship with maceration time and marc pressing during winemaking. J. Agric. Food Chem. 2004, 52(18), 5747-5751. [CrossRef]
- Careri, M.; Corradini, C.; Elviri, L.; Nicoletti, I.; Zagnoni, I. Direct HPLC analysis of quercetin and trans-resveratrol in red wine, grape, and winemaking by products. J. Agric. Food Chem. 2003, 51(18), 5226-5231. [CrossRef]
- Lisov, N.; Petrovic, A.; Čakar, U.; Jadranin, M.; Tešević, V.; Bukarica-Gojković, L. Extraction kinetic of some phenolic compounds during Cabernet Sauvignon alcoholic fermentation and antioxidant properties of derived wines. Maced. J. Chem. Chem. 2020, 39(2), 185-196. [CrossRef]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911-1922. [CrossRef]
- Radovanović, B. C.; Radovanović, A. N.; Souquet, J. M. Phenolic profile and free radical-scavenging activity of Cabernet Sauvignon wines of different geographical origins from the Balkan region. J. Agric. Food Chem. 2010, 90(14), 2455-2461. [CrossRef]
- Devi, A.; Anu-Appaiah, K. A.; Lin, T. F. Timing of inoculation of Oenococcus oeni and Lactobacillus plantarum in mixed malo-lactic culture along with compatible native yeast influences the polyphenolic, volatile and sensory profile of the Shiraz wines. LWT 2022, 158, 113130. [CrossRef]
- Bautista-Ortín, A. B.; Martínez-Cutillas, A.; Ros-García, J. M.; López-Roca, J. M.; Gómez-Plaza, E. Improving colour extraction and stability in red wines: the use of maceration enzymes and enological tannins. Int. J. Food Sci. Technol. 2005, 40(8), 867-878. [CrossRef]
- Artem, V.; Antoce, A. O.; Geana, E. I.; Ranca, A. Effect of grape yield and maceration time on phenolic composition of ‘Fetească neagră’organic wine. Not. Bot. Horti Agrobot. 2021, 49, 12345-12345. [CrossRef]
- Xia, E. Q.; Deng, G. F.; Guo, Y. J.; Li, H. B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11(2), 622-646. [CrossRef]
- Heras-Roger, J.; Díaz-Romero, C.; Darias-Martín, J. A comprehensive study of red wine properties according to variety. Food Chem. 2016, 196, 1224-1231. [CrossRef]
- Soto Vázquez, E.; Río Segade, S.; Orriols Fernández, I. Effect of the winemaking technique on phenolic composition and chromatic characteristics in young red wines. Eur. Food Res. Technol. 2010, 231(5), 789-802. http://dx.doi.org/10.1007/s00217-010-1332-5.
- [38]Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54(2), 1844-1858. [CrossRef]
- Chen, K.; Escott, C.; Loira, I.; Del Fresno, J. M.; Morata, A.; Tesfaye, W.; Calderon, F.; Suárez-Lepe, J. A.; Han. S.; Benito, S.. Use of non-Saccharomyces yeasts and oenological tannin in red winemaking: Influence on colour, aroma and sensorial properties of young wines. Food Microbiol. 2018, 69, 51-63. [CrossRef]
- Burns, T. R.; Osborne, J. P. Loss of Pinot noir wine color and polymeric pigment after malolactic fermentation and potential causes. Am. J. Enol. Vitic. 2015, 66(2), 130-137. http://dx.doi.org/10.5344/ajev.2014.14061.
- Álvarez, I.; Aleixandre, J. L.; García, M. J.; Lizama, V. Impact of prefermentative maceration on the phenolic and volatile compounds in Monastrell red wines. Anal. Chim. Acta. 2006, 563(1-2), 109-115. [CrossRef]
- Pérez-Lamela, C.; García-Falcón, M. S.; Simal-Gándara, J.; Orriols-Fernández, I. Influence of grape variety, vine system and enological treatments on the colour stability of young red wines. Food Chem. 2007, 101(2), 601-606. [CrossRef]
- Ivanova, V.; Vojnoski, B.; Stefova, M. Effect of winemaking treatment and wine aging on phenolic content in Vranec wines. J. Food Sci. Technol. 2012, 49(2), 161-172. [CrossRef]
- Castillo-Sánchez, J. X.; García-Falcón, M. S.; Garrido, J.; Martínez-Carballo, E.; Martins-Dias, L. R.; Mejuto, X. C. Phenolic compounds and colour stability of Vinhao wines: Influence of wine-making protocol and fining agents. Food Chem. 2008, 106(1), 18-26. [CrossRef]
- Gómez-Plaza, E.; Gil-Muñoz, R.; López-Roca, J. M.; Martı́nez-Cutillas, A.; Fernández-Fernández, J. I. Maintenance of colour composition of a red wine during storage. Influence of prefermentative practices, maceration time and storage. LWT-Food Sci Technol. 2002, 35(1), 46-53. [CrossRef]
- Bimpilas, A.; Panagopoulou, M.; Tsimogiannis, D.; Oreopoulou, V. Anthocyanin copigmentation and color of wine: The effect of naturally obtained hydroxycinnamic acids as cofactors. Food Chem. 2016, 197, 39-46. [CrossRef]
- Carew, A. L.; Smith, P.; Close, D. C.; Curtin, C.; Dambergs, R. G. Yeast effects on Pinot noir wine phenolics, color, and tannin composition. J. Agric. Food Chem. 2013, 61(41), 9892-9898. [CrossRef]
- Marquez, A.; Serratosa, M. P.; Merida, J. Influence of bottle storage time on colour, phenolic composition and sensory properties of sweet red wines. Food Chem. 2014, 146, 507-514. [CrossRef]
- Bimpilas, A.; Tsimogiannis, D.; Balta-Brouma, K.; Lymperopoulou, T.; Oreopoulou, V. Evolution of phenolic compounds and metal content of wine during alcoholic fermentation and storage. Food Chem. 2015, 178, 164-171. [CrossRef]
- Castellari, M.; Matricardi, L.; Arfelli, G.; Galassi, S.; Amati, A. Level of single bioactive phenolics in red wine as a function of the oxygen supplied during storage. Food Chem. 2000, 69(1), 61-67. [CrossRef]
- Suprun, A. R.; Dubrovina, A. S.; Tyunin, A. P.; Kiselev, K. V. Profile of stilbenes and other phenolics in Fanagoria white and red Russian wines. Metabolites 2021, 11(4), 231. [CrossRef]
- García-Falcón, M. S.; Pérez-Lamela, C.; Martínez-Carballo, E.; Simal-Gándara, J. Determination of phenolic compounds in wines: Influence of bottle storage of young red wines on their evolution. Food Chem. 2007, 105(1), 248-259. [CrossRef]
- Naiker, M.; Anderson, S.; Johnson, J. B.; Mani, J. S.; Wakeling, L.; Bowry, V. Loss of trans-resveratrol during storage and ageing of red wines. Aust. J. Grape Wine Res. 2020, 26(4), 385-387. [CrossRef]
- Milovanovic, M.; Žeravík, J.; Obořil, M.; Pelcová, M.; Lacina, K.; Cakar, U.; Petrovic, A.; Glatz, Z.; Skládal, P. A novel method for classification of wine based on organic acids. Food Chem. 2019, 284, 296-302. [CrossRef]

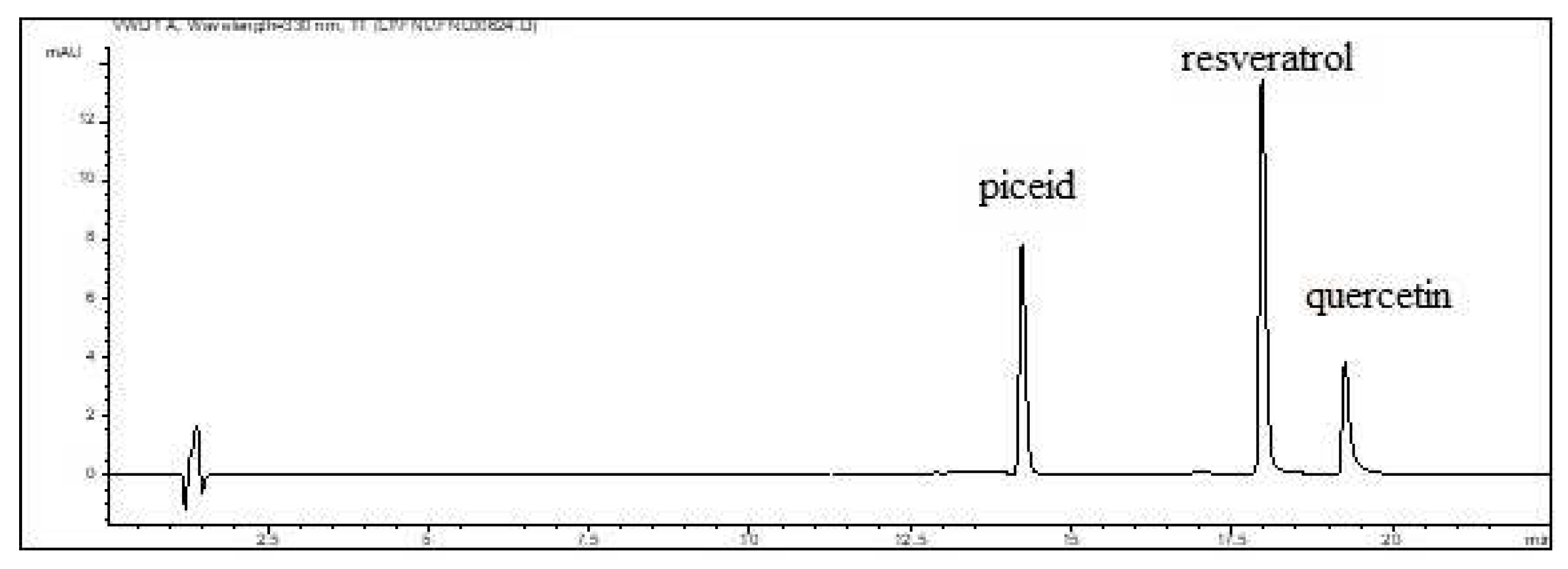
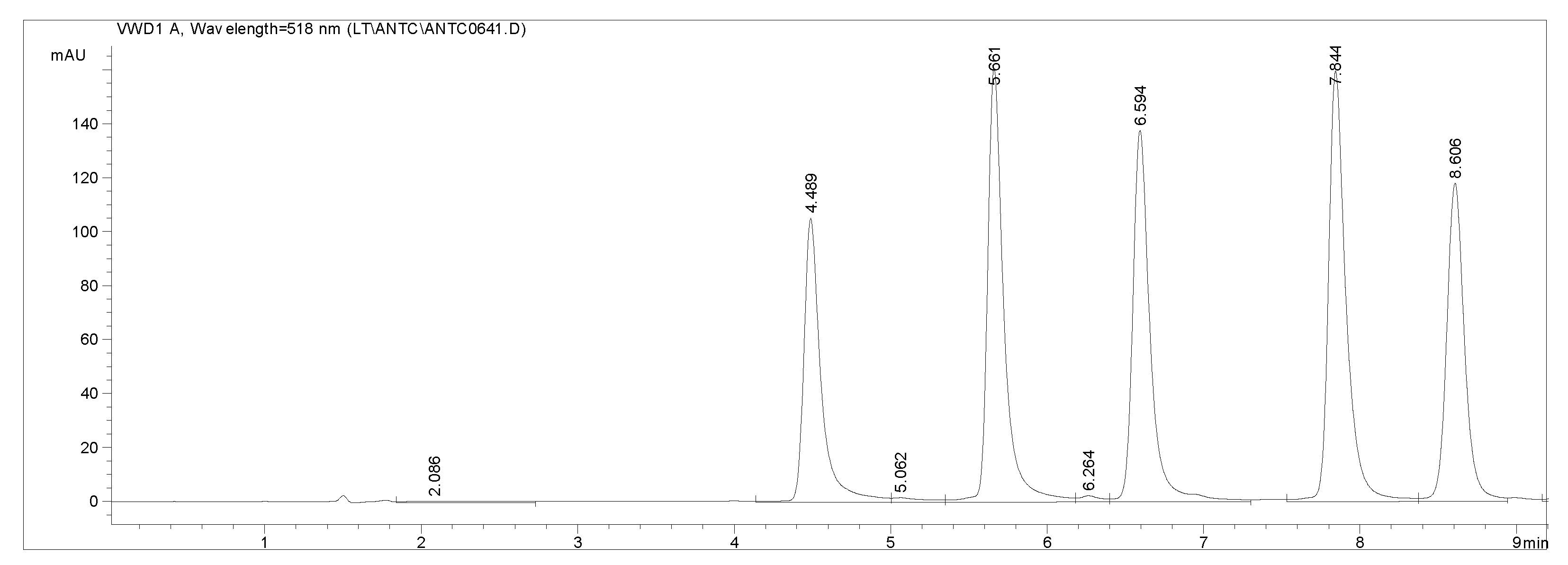
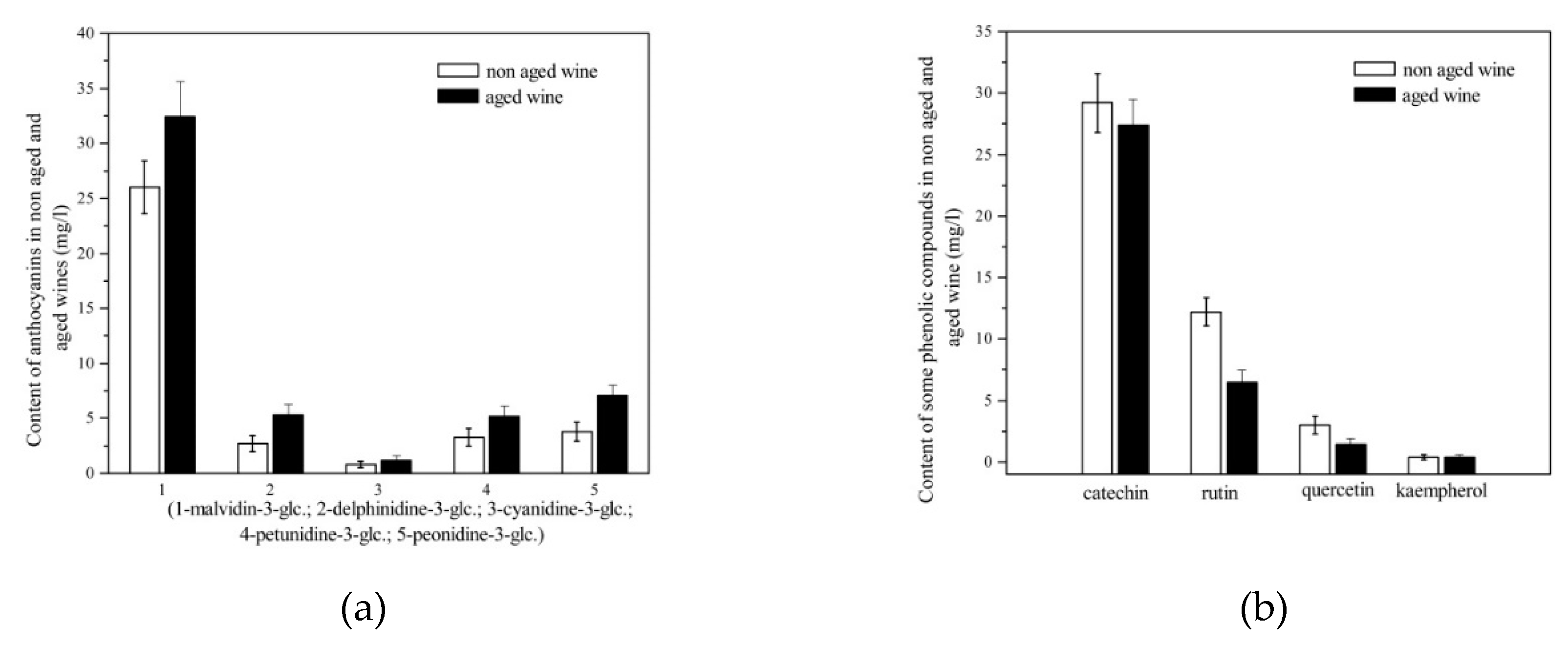
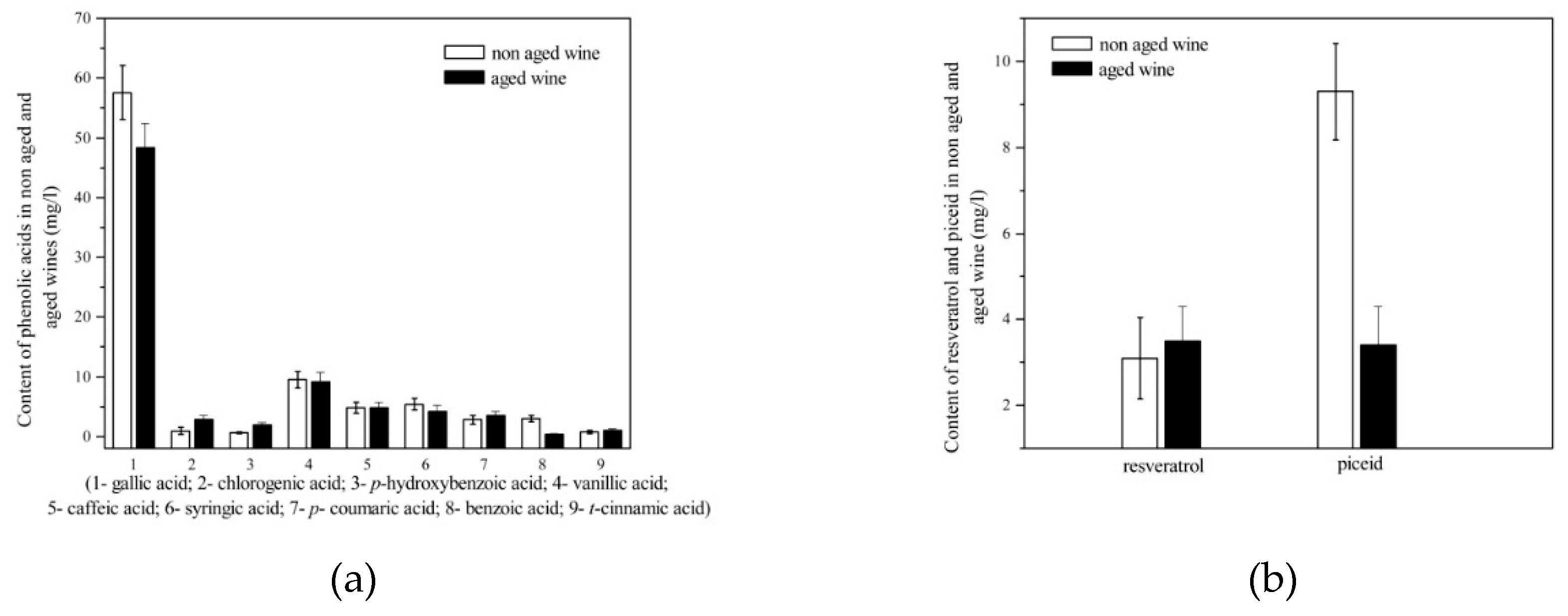
| Wine | Traditional winemaking technology |
Innovative winemaking technology |
% increase in content |
| trans-resveratrol (mg/L) | 1.30±0.09 | 3.10±0.10 | 138.5 |
| Piceid (mg/L) | 4.80±0.18 | 9.30±0.20 | 93.8 |
| Quercetin (mg/L) | 0.70±0.04 | 3.0±0.08 | 328.6 |
| Wine |
Traditional winemaking technology |
Innovative winemaking technology |
% increase in content |
| Catechin (mg/L) | 25.3±0.90 | 29.2±1.10 | 15.4 |
| Rutin (mg/L) | 7.3±0.20 | 12.2±0.20 | 67.1 |
| Kaempherol (mg/L) | n.d.* | 0.40±0.04 | 100.0 |
| Phenolic acids (mg/l) |
Traditional winemaking technology |
Innovative winemaking technology |
| Gallic acid | 58.90±1.60 | 57.60±1.20 |
| Chlorogenic acid | 0.80±0.05 | 0.95±0.09 |
| p-hidroxybenzoic acid | 0.70±0.04 | 0.60±0.02 |
| Vanillic acid | 10.40±0.25 | 9.50±0.40 |
| Caffeic acid | 3.70±0.20 | 4.80±0.25 |
| Syringic acid | 6.90±0.30 | 5.40±0.20 |
| p-coumaric acid | 3.0±0.12 | 2.80±0.15 |
| Benzoic acid | 2.50±0.10 | 3.0±0.10 |
| t-cinnamic acid | 0.10±0.02 | 0.75±0.04 |
| Anthocyanin (mg/l) | Traditional winemaking technology |
Innovative winemaking technology |
% increase in content |
| delphinidin 3-O-glucoside |
1.60±0.15 | 2.70±0.25 | 68.80 |
| cyanidin 3-O-glucoside |
0.40±0.04 | 0.80±0.10 | 100.0 |
| petunidin 3-O-glucoside |
2.0±0.05 | 3.30±0.15 | 65.0 |
| peonidin 3-O-glucoside |
2.0±0.05 | 3.80±0.25 | 90.0 |
| malvidin 3-O-glucoside |
24.20±0.80 | 26.0±0.95 | 7.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





