1. Introduction
The emergence and progression of natural fiber-reinforced polymer composites is viewed as a promising environmentally friendly alternative to petrochemical-based, non-renewable polymeric materials [1,2]. Concerns related to synthetic fillers include high cost, non-biodegradability, non-combustibility, and challenges associated with sustainable recycling techniques [3]. Due to these concerns, there is a focus on exploring alternative bio-based fillers. Substituting synthetic fillers with natural ones, such as cellulose fibers, offers numerous advantages. Cellulose whiskers, with their high aspect ratio, low density, cost-effectiveness, low toxicity, excellent thermal and mechanical properties, biocompatibility, and biodegradability, have gained significant attention [4]. These whiskers can be synthesized from various materials such as wood, natural fibers, fungi, algae, and bamboo. Natural fibers such as cotton, flax, jute, and hemp exhibit relatively high strength, stiffness, and low density, though their properties are influenced by factors such as geography, climate, and plant age [5].
Hemp, the industrial Cannabis sativa varietal, is gaining popularity as an industrial biomass due to its durability, cost-effectiveness, biodegradability, and sustainability. It is a fast-growing crop requiring less water and fewer pesticides than other crops [6–8]. Recent studies have explored the environmental impact and properties of hemp, revealing its superiority in terms of sustainability over other forms of cellulose biomass (such as wood, cotton, and corn stover) requiring less water, fertilizers, herbicides, and insecticides while being cultivatable in a wide range of climates and growing conditions [9,10].
Cellulose nanocrystals (CNC) derived from hemp are produced through acid hydrolysis of non-crystalline cellulose in the bast fibers using concentrated mineral acids, typically sulfuric acid. However, this method has drawbacks such as high environmental impact, recovery difficulties, low thermal stability, challenges in functionalization due to sulfate groups, toxicity, and low CNC yield [11,12]. To address environmental concerns, there is a growing interest in replacing mineral acids with organic acids for CNC preparation [13]. Organic acids are preferred for their lower acidity, reduced corrosivity, and recyclability [14,15]. Additionally, during hydrolysis, organic acids such as oxalic acid, maleic acid, and citric acid can introduce functional groups like ester and carboxylic groups, enhancing surface functionality and resulting in improved thermal and mechanical properties [16,17]. Citric acid, derived from citrus fruits, is particularly notable for its minimal impact on human health and the environment, making it a safer and more sustainable choice [18]. Oxalic acid, produced from biomass and recoverable at low temperatures through crystallization, also contributes to sustainable and economical manufacturing [19].
CNCs, with their large surface area and abundant surface hydroxyl groups, serve as excellent reinforcing nanofillers for polymer biocomposites, offering high tensile strength and Young’s modulus [20]. Achieving good dispersion in the polymer matrix is essential for enhanced performance compared to the unreinforced polymer. Cellulose whiskers, with their hydrophilicity and OH surface groups, are suitable biomaterials that can form hydrogen bonds with polar polymers, producing high-performance biocomposites. This property enables better dispersion and interaction with polymers having hydroxyl groups, leading to effective polar surface interactions. PVA, a highly polar polymer, establishes strong hydrogen-bonding interactions with CNCs. The effective preparation of PVA/CNC biocomposites using an aqueous mixing process has been reported in the literature [21–23].
PVA has emerged as an environmentally friendly and practical alternative to petroleum-based polymers like polyethylene and polypropylene, as highlighted by Arindam in 2018 [24]. Its increasing prominence in various global commercial applications such as packaging, clothing, printing, and medicine is attributed to its distinctive chemical and physical properties [25,26]. PVA is known for being non-toxic, semicrystalline, and water-soluble, as emphasized by Lu in 2008 and Roohani in 2008 [27,28]. While recognized for biodegradability, its main drawback is the relatively high cost, which can be mitigated by blending with other natural polymers for enhanced cost-effectiveness. The combination of PVA with cellulose nanocrystals (CNC) in blends enhances biodegradability, and is supported by researchers worldwide [21–23].
Considering principles of sustainability, this work aims to replace sulfuric acid with organic acids (citric acid and oxalic acid) to address environmental concerns. The morphology, chemical, and thermal properties of cellulose whiskers are characterized and reported. Additionally, the study investigates the effect of incorporating these fillers into a PVA polymer matrix at 1% and 5% filler loadings.
2. Materials and Methods
2.1. Materials
Degummed hemp fiber was acquired from Hemp Trader (Product Code: F-DG1), Sulfuric acid (98%) was purchased from Sigma-Aldrich, Citric acid (Electrophoresis Grade, 99.5%) was obtained from ThermoFisher, Oxalic acid (anhydrous, 98%) was provided from ThermoFisher, and PVA (87-89% hydrolyzed, M.W.13,000-23,000) was obtained from Arcos Organics through Fisher Scientific, cellulose nano crystals extracted from wood with sulfuric acid was purchased from the University of Maine (
https://umaine.edu/pdc/nanocellulose/) and used as a control in this study.
2.2. Cellulose Whiskers Synthesis
Cellulose nano and micro particles were synthesized using an acid hydrolysis process, following the method described by Revol, et al. [29]. The best yield was determined based on the literature considering factors such as acid concentration, process temperature, and duration, which varied depending on the type of acid used [16,20,36].
Five grams of oven-dried degummed hemp fibers, with diameters ranging from approximately 5 to 80 microns, were cut into lengths of 0.5 cm to 1 cm. These prepared fibers were placed into three-necked, round-bottomed flasks, along with 50 mL of acid and distilled water under constant stiring. In the case of sulfuric acid hydrolysis, the process was conducted using a 64% concentration at 65-70°C for 60 min. The reaction was terminated by adding 400ml distiiled water. Subsequently, the suspension was centrifuged three times with DI water in an Eppendorf Centrifuge 5810 R for 15 minutes at 9,289 x gravitational constant (g). The process continued with dialysis to remove the acid from suspension, adjusting the pH to 6-7. For sulfuric acid hydrolysis, this dialysis step extended over a period of 6-7 days. After dialysis, the suspension was recentrifuged one time to separate the particles into two phases: a clear liquid containing CNC (cellulose nano crystals) and a liquid with larger agglomerated CMF (cellulose micro fibrils).
The hydrolysis process for citric acid followed a similar protocol, with a slight variation. It involved the use of 80% citric acid, and was conducted at a temperature of 95°C to 105°C with a duration at least four hours, until all fibers were completely dissolved. Following hydrolysis, 400 mL of hot water was added, and the solution was filtered to separate the acid from the solution. A single round of centrifugation was carried out, and dialysis was performed over a period of 2-3 days, as described earlier. The resulting suspension was stored in two phases.
The process for creating cellulose whiskers with oxalic acid was similar to that with citric acid, with the only difference being the use of a 70% concentration for 60-70 min at temperatures ranging from 100°C to 110°C. Prepartation conditions and particle yields for each acid type are outlines in
Table 1.
2.3. Cast Films Preparation
A solution comprised of 15 mL of distilled water and 3 grams of PVA was placed in a beaker, along with a suspension of either CNC or CMF, each with a corresponding concentration of 1% and 5%. The mixture was heated to 80°C with continuous stirring until the PVA was fully dissolved, which took approximately 40-60 minutes. The final solution was vacuum-filtered for 2 minutes to remove bubbles. Subsequently, the homogeneous mixture was cast onto polystyrene weighing dishes and left to dry in an oven at 40°C overnight. The resulting films were then carefully removed and stored in a desiccator filled with sodium bromide at a relative humidity of 57% before undergoing characterization. The typical sample thickness was found to be within the range of 0.20 to 0.35 mm.
Due to the lower yield as indicated in
Table 1 for CNC derived using the organic acids, cast films were prepared only with the CMF material obtained from the organic acid hydrolysis. Furthermore, for the sake of comparison, cast films were also prepared using CNC extracted from wood with sulfuric acid (obtained from the University of Maine), and these were compared with CNC obtained from hemp synthesized using sulfuric acid. The cast films containing cellulose nano crystals sourced from wood with concentrations of 1% and 5% were denoted as M-N-1 and M-N-5. The cast films produced in this project with cellulose nano crystals using sulfuric acid were labeled as S-N-1 and S-N-5. In addition, cast films containing 1% and 5% cellulose micro fibrils hydrolyzed with sulfuric acid, citric acid, and oxalic acid were labeled S-M-1, S-M-5, C-M-1, C-M-5, O-M-1, and O-M-5, respectively.
2.4. Characterizations
The yield of prepared cellulose whiskers was calculated using equation (1) and reported in
Table 1.
where m
2 is the mass of the weighing dish (g), m
1 is the total mass of the CNC/CMF powder and the weighting dish (g), m
3 is the initial mass of hemp used (g), v
1 is the total volume of CNC/CMF suspension synthesized, and v
2 is the volume of the suspension analyzed [31].
2.4.1. Morphological Characterization
A Scanning Electron Microscope (SEM) (FlexSEM 1000II) was employed to investigate the morphology, shape, and structure of the cellulose whiskers and cast films. Additionally, for size measurements, the SEM images were evaluated using ImageJ software. In this technique, a drop of cellulose suspension in water (at a ratio of 1:10) was placed on aluminum foil and dried in an oven. Samples of the cast film were cut to dimensions of 8x8 mm and placed on carbon tape. High-voltage conditions of 15-20 kV were applied to capture the images. No significant differences in cast film morphology were observed.
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
The functional groups in the samples were analysed by FTIR (NICOLET iS10 Smart iTR) to identify the molecular and intermolecular bonds present in the samples. The test was conducted for cellulose particle powders and small pieces of cast films in the mid-IR region, ranging from 4000 to 650 cm−1, at room temperature and a resolution of 4 cm−1, with an average of 64 scans.
2.4.3. Thermal Analysis
Thermal degradation for both cellulose particle powders and cast films was investigated through thermogravimetric analysis (TGA) using a TGA Q50 instrument from TA Instruments. The samples were heated to 600 °C at a rate of 10 °C/min in a N2 flow of 60 mL/min. Based on the ASTM E2550–11, onset temperature was measured and reported as Td.
Differential scanning calorimetry analyses (DSC) using a DSC Q20 instrument, also from TA Instruments, were conducted to assess changes in the thermal properties of the cast films. The heat-cool-heat method was employed, cycing the temperature from 20-225°C, which is lower than the thermal degradation temperature of the samples. The heating and cooling rates were set at 10 °C/min, and a N2 flow of 50 mL/min was maintained.
The degree of crystalinity was calculated as:
where, ∆H
m represents the enthalpy of the sample film’s melting point, ∆H
0m is the enthalpy of melting for a 100% crystalline PVA sample (determined as 161.6 Jg⁻¹ according to Roohani et al. [32]), and w signifies the weight fraction of PVA in the sample.
2.4.4. Mechanical Propertise of Cast Films
A universal tensile testing machine (INSTRON 5543A) was utilized to measure the stress, strain at maximum load, Young’s modulus of the cast films. The test was conducted at a crosshead speed of 17.5 mm/min and a gauge length of 50 mm, following ASTM D882 standards. Ten film specimens for each sample, with a width of 5 mm, were tested under environmental conditions of 20°C and 65% relative humidity.
3. Results & Discussion
3.1. CNC/CMF Characterization
3.1.1. Morphological Characterization
The data presented in
Table 1 are the highest yields achieved under optimal hydrolysis conditions. Citric acid hydrolysis yielded the highest total yield with 95.5%, followed by sulfuric acid, and oxalic acid with 87.3% and 68.4%, respectively. Sulfuric acid hydrolysis produced the greatest quantity of CNC, 41.9%, while oxalic acid exhibited a higher CMF yield compared to CMF produced with sulfuric acid. Furthermore, the percentage of CMF generated with citric acid surpassed that of the other acids at 91.9%.
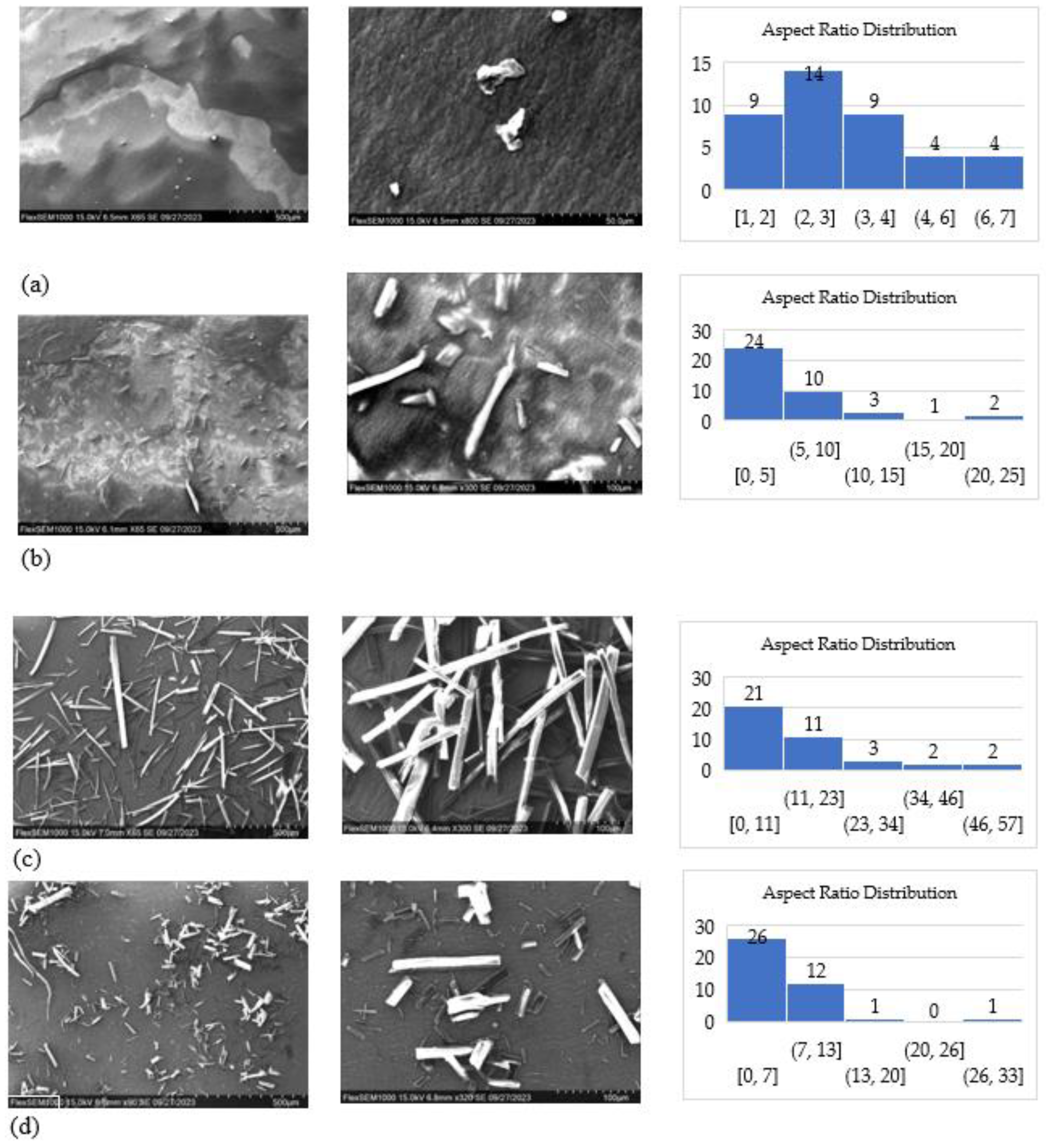
In
Figure 1 and
Table 2, SEM images illustrate the various sizes and morphologies of cellulose particles resulting from the hydrolysis of hemp cellulose using different acids. Two levels of magnification were employed to provide a more comprehensive depiction of size, morphology, and distribution.
Post-hydrolysis, the cellulose nano crystals produced with sulfuric acid were thinner and shorter compared to the other particles. They exhibited shapes resembling needles, worms, and tiny spheres, as depicted in
Figure 1(a). Their sizes ranged from 20 to 300 nm in length and 15 to 100 nm in width. In contrast, the S-CMF particles, produced from the same process, exhibited microscale dimensions of 150 nm–20 μm in width and 300 nm–120 μm in length, with a longer needle-like shape and an aspect ratio of 2.5 (
Figure 1(b)). Notably, the aspect ratio distribution of S-CNC and S-CMF was narrower than that of the other samples. These differences can be attributed to the preferential attack of hydrogen ions on the amorphous regions of cellulose, resulting in a rapid hydrolysis process that releases soluble sugars like cellobiose and polysaccharides [33]
CMF produced through hydrolysis with citric acid displayed a rod-shaped sheet structure, with lengths ranging from 1 to 400 μm and the widest aspect ratio distribution with a mean of 5.5. This could be due to fewer hydrogen ions targeting the amorphous regions, leading to a slower hydrolysis rate and the formation of larger particles (
Figure 1(c)) [33].
The morphology of CMF produced with oxalic acid was a combination of shapes, including needle-like, worm-like, and small rod-like structures. These structures were longer than those generated with sulfuric acid but shorter than those created with citric acid, spanning a range from 900 nm to 140 μm in
Figure 1 (d).
To summarize, the data from
Table 1 and
Table 2 indicate that citric acid yields the highest output, resulting in CMF with larger particle sizes and a wider aspect ratio distribution, characterized by rod-like shapes. Sulfuric acid generates the highest CNC yield, resulting in smaller sizes and a narrower aspect ratio distribution with needle-like and worm-like morphologies. CMF produced with oxalic acid exhibits a variety of particle sizes and shapes, forming a mixture of needles and rods, with 62.5% CMF of the 68.4% total yield.
The variation in size and shape of cellulose particles across samples can be attributed to differences in the degree of hydrolysis. This is dependent on factors such as the type of acid used, whether it is a strong or weak acid, acid concentration, temperature, and process duration. Ultimately, the choice of whether to use organic or mineral acid for cellulose particle production clearly depends on the intended application.
3.1.2. FTIR
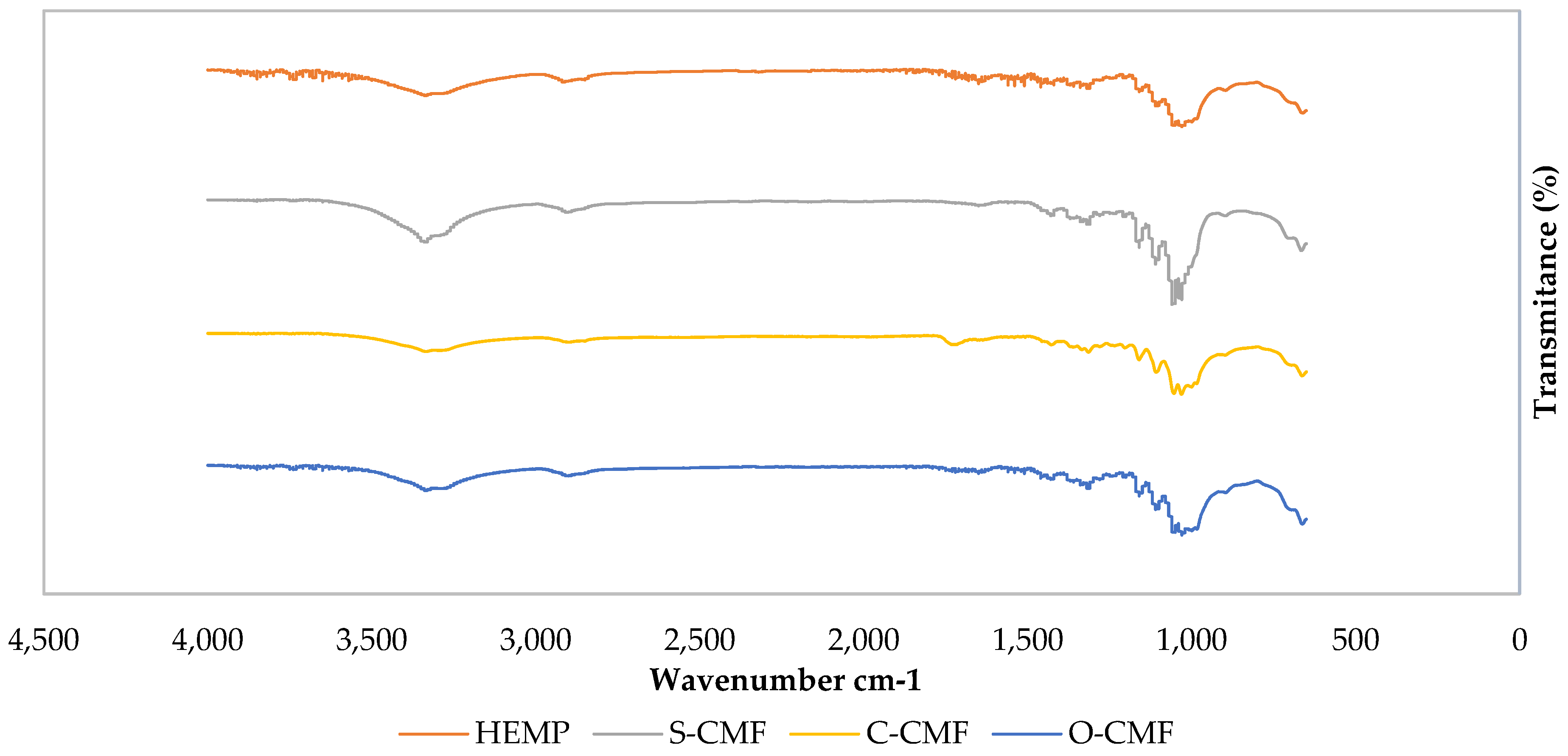
The FTIR spectra of of Hemp, S-CMF, C-CMF, and O-CMF are displayed in
Figure 2 to compare the effect of the acid used in synthesis on cellulose particle chemistry.
In all samples, a broad peak around 3335 cm-1 indicates the O-H stretch in cellulose, and the subsequent peak near 2900 cm-1 represents the C-H groups, indicating the presence of the cellulose unit [34].
A distinctive peak at 1730 cm-1 is exclusively observed in C-CMF spectra, corresponding to the C=O stretch associated with the ester carboxylic, or carbonyl group formed through the esterification process, involving the hydroxyl groups of cellulose and the carboxyl groups of citric acid. This surface modification has been reported in studies by Yuho et al, and Bondanisa et al [35,36], providing evidence of successful surface modification. Conversely, the other samples exhibit small peaks around 1720 cm-1, representing the C=O stretching frequency related to the carbonyl group in hemicellulose and lignin [37]. Both can be associated with the use of oxalic acid.
An O-H group of adsorbed water around 1640 cm-1 is observed in the S-CMF and O-CMF spectrum, due to the interaction between adsorbed water and the hydrophilic surface group (–OH) of cellulose [38]
The absence of absorption bands at around the 1500-1600 cm-1 range in the C-CMF spectra (related to aromatic ring vibrations) indicate the removal of lignin in these microfibrils [39]. However, small extra peaks in this region can be observed for cellulose microfibrils made using sulfuric and oxalic acid.
The peak around 1430 cm-1 represents the O-CH3 bend for methoxyl-O-CH3 compounds in lignin, followed by a peak at approximately 1320 cm-1 associated with CH2 stretching from cellulose, signifying the exposure of the crystalline surface of cellulose resulting from the removal of non-cellulosic components in all samples [40,41], with a stronger intensity in S-CMF spectra compared to other spectra.
The absorption phenomenon in all spectra around 1205 cm-1 refers to the bending frequency of C-O phenol in lignin. Another possibility is attributed to the S=O stretching vibration from sulfate groups, demonstrating esterification of the hydroxyl groups, as seen in the S-CMF graph [42,43].
The absorption peaks around 1170 cm-1 and 1030 cm-1 are ascribed to C-O-C group vibrations of pyranose ring skeletal compounds in cellulose [44]. Similar peaks, but with higher intensity, are observed in S-CMF spectra at around 1108, 1053, and 663 cm-1, corresponding to O-H group and C–O stretching and C–O deformation in C-OH compound of lignin and hemicellulose, and C-C stretching, respectively, in all samples [42].
In the spectral range of 3500-4000 cm-1 and 1400-1800 cm-1 associated with the stretching and bending vibration of hydroxyl groups likely represent alkene, esters, aromatics, ketones, and alcohols in the degummed hemp [45]. However, these peaks disappear in all samples’ spectra, indicating the successful removal of hemicellulose and lignin following all acid hydrolysis treatments.
3.1.3. Thermal Analysis
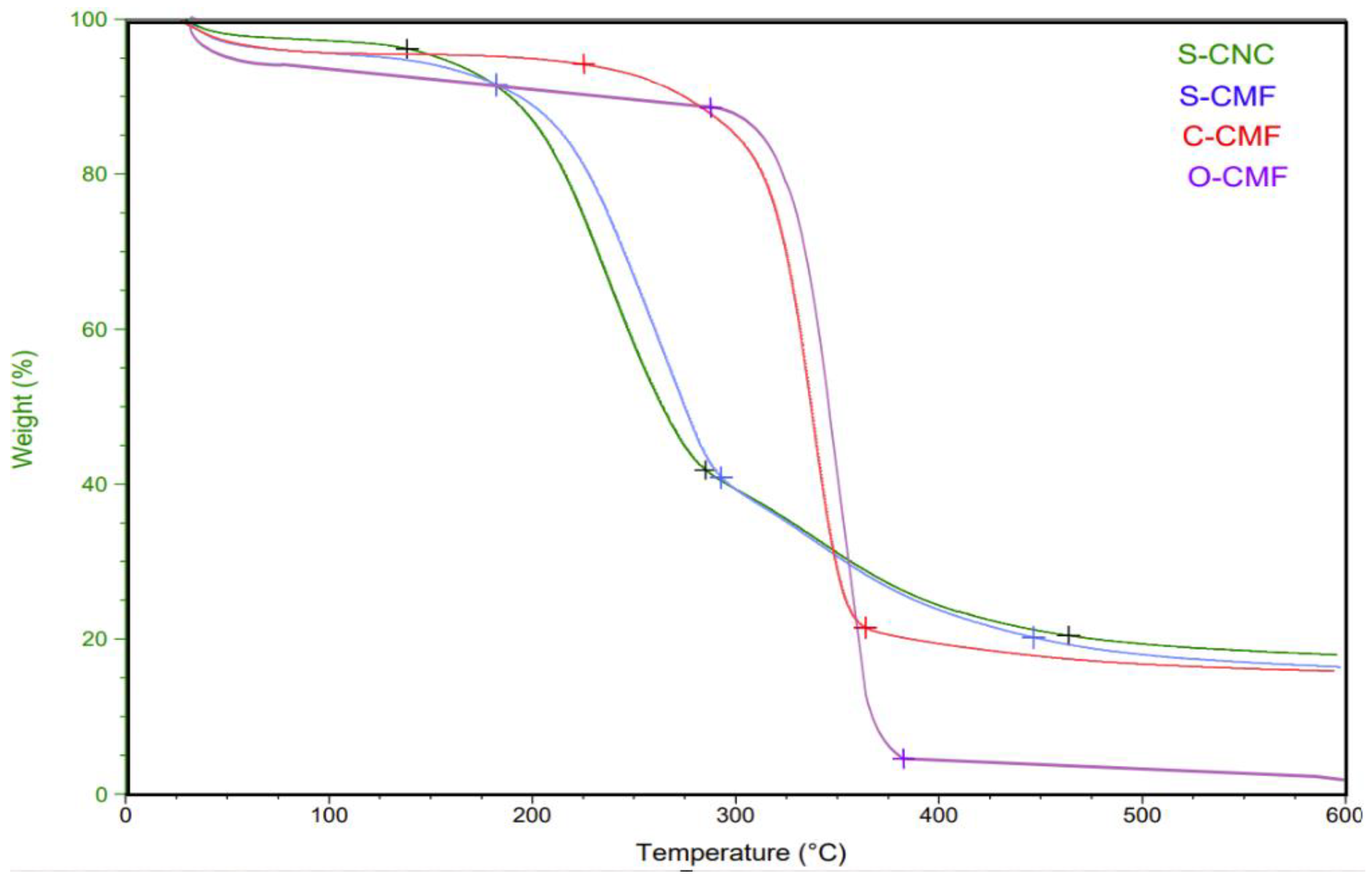
Thermal degradation tests were conducted to assess the thermal stability of the materials. The obtained graphs indicated a negligible weight loss around 100°C, signifying the removal of moisture along with the disruption of intermolecular hydrogen bonds in water [46,47]. It can be seen from
Figure 3 that samples treated with sulfuric acid exhibited three main weight loss steps, contrasting with two steps observed in samples treated with organic acids. The second weight loss step, characterized by the highest degradation peak within the temperature range of 150-290°C for all samples, was attributed to cellulose depolymerization, dehydration, and glycosyl unit decomposition [48,49]
The third region, spanning from 290°C to 460°C for S-CNC and S-CMF curves, was associated with the breakdown of carbon-containing substances [31]. S-CNC showed the lowest decomposition temperature at 150°C, losing less weight (around 80%) than the other three samples. S-CMF exhibited a thermal degradation point at 170°C, with an 82% weight loss. This discrepancy may be attributed to reduced thermal stability linked to increased specific surface area and decreased dimensions [50] [51]
Furthermore, the introduction of sulfated groups into the crystals during the acid hydrolysis process, as indicated by FTIR testing, could reduce the thermal stability of nanocrystals, as reported in earlier literature [52]. These functional groups may weaken hydrogen bonds between adjacent cellulose chains and intramolecular hydrogen bonds, contributing to decreased thermal stability. CMF with oxalic acid exhibited a maximum degradation temperature of approximately 290°C, showcasing excellent colloidal stability and the smallest interaction with water, thus achieving high thermal stability [11]. Conversely, sulfuric acid, is known for its strong reaction with water. The curve for C-CMF shows considerable thermal stability with less weight loss compared to O-CMF (86% vs. 95%).
Another factor contributing to the higher thermal stability of nanocellulose particles treated with organic acids compared to those treated with mineral acid is the presence of functional surface groups. For instance, citric acid hydrolysis of cellulose generates ester groups, releasing carboxylic groups on the nanocellulose surface, as evidenced by the FT-IR transmittance spectra at the 1730 cm-1 peak. This molecular bond is strong and highly resistant to breakage. Conversely, in the case of sulfuric acid, the hydroxyl groups on the CNC surface are randomly replaced by sulfate esters, leading to negatively charged S-CNC/CMF and relatively low thermal stability due to the presence of sulfate groups on the surface [53].
3.2. Cast Fillms Characterization
3.2.1. FTIR
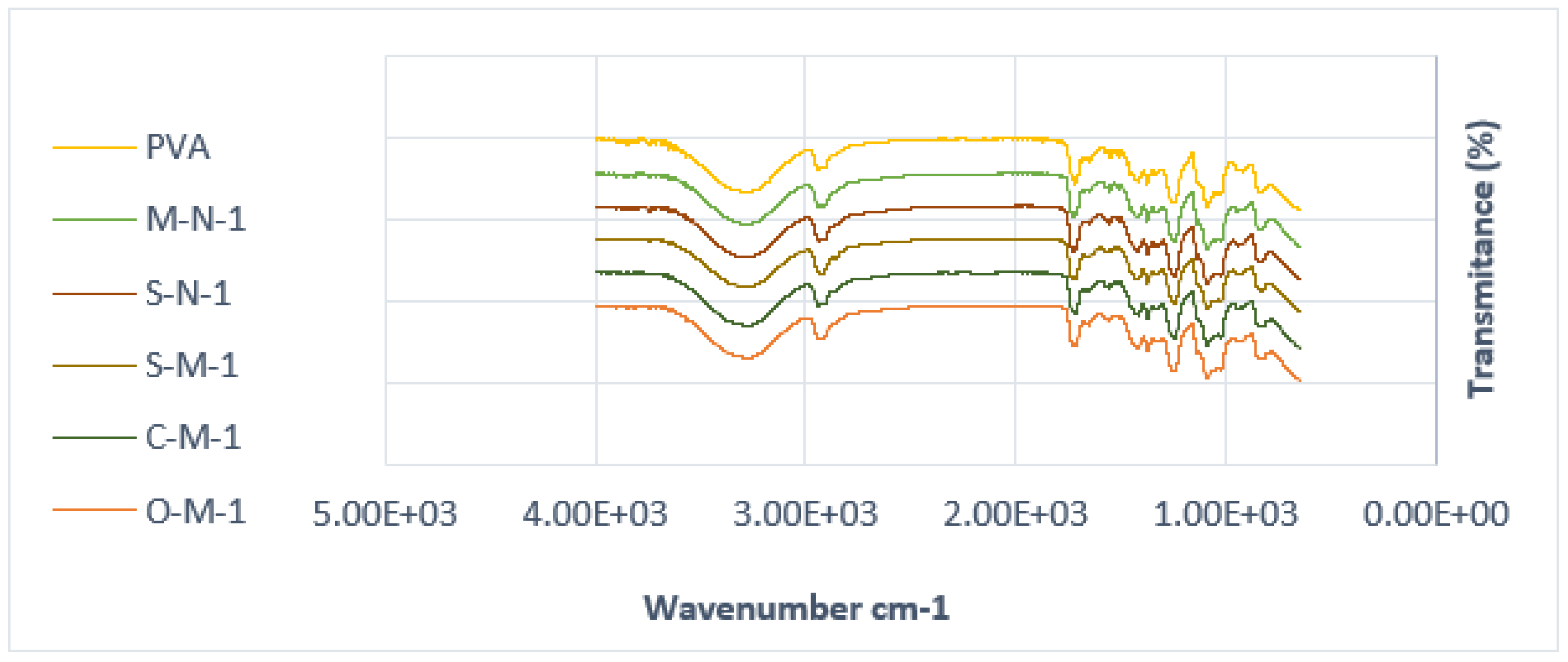
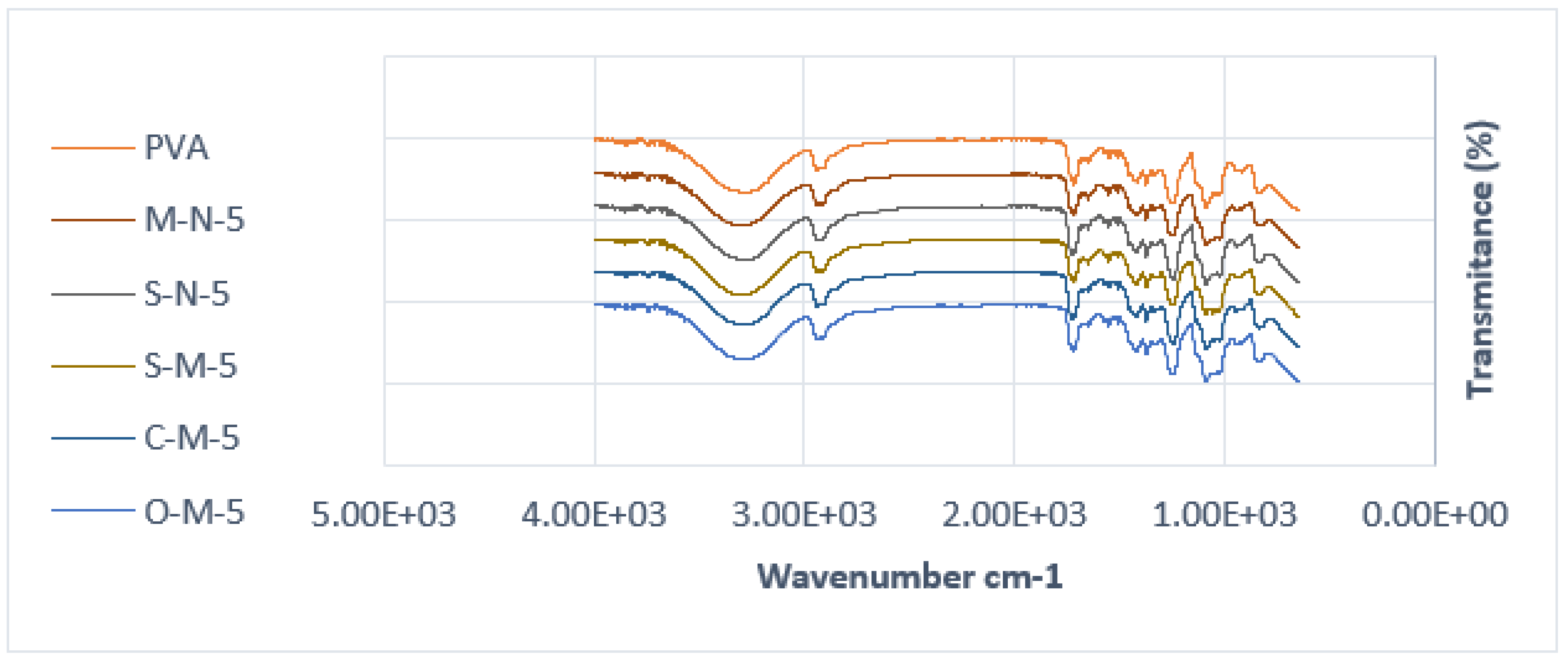
The chemical behavior of both neat PVA and PVA biocomposites was investigated through FTIR analysis, as illustrated in
Figure 4 and
Figure 5. The FTIR spectra of PVA reveals several prominent absorption bands. It is evident that all the spectra are similar, except for a few small changes. The peak at around 3274 cm⁻¹ corresponds to the stretching vibration of (–OH), resulting from intramolecular hydrogen bonds within the PVA and intermolecular hydrogen bonding between hydroxyl groups of PVA and CNC. Notably, in cast films with reinforcement, the addition of CNC intensifies the peak at 3274 cm⁻¹ [54].
Additional peaks at 2941 cm⁻¹ (–CH stretching) arise from alkyl groups, and 1716 cm⁻¹ (C=O stretching) is attributed to the C=O and C-O stretching from residual acetate groups in the PVA matrix. This peak shifts to a higher peak at 1732 cm⁻¹ in cast films, indicating an increased amount of C-O groups in the matrix, possibly due to the presence of hemicelluloses in the CNC. Another possibility could be the existence of a crosslinking structure between hydroxyl groups of PVA and CNC at around 1740 cm⁻¹ [54] (though this has yet to be verified in our samples).
The absorption band at 1653 cm⁻¹ corresponds to observed water molecules, while additional bands at 1418 cm⁻¹ and 1374 cm⁻¹ (C-H stretching) result from the bending vibration of C-H bonds, appearing in almost all spectra [55].
Furthermore, the peak at 1373 cm⁻¹, 1338 cm⁻¹ (O-H stretching) indicative of hydroxyl crosslinking by hydrogen bonding of CNC/CMF, 1247 cm⁻¹ (–CO of carbonate). A double peak is observed in the region of 1089–1023 cm⁻¹, where the peak at 1089 cm⁻¹ (–O–C=O carbonate) corresponds to CO stretching vibration, and the peak at 1023 cm⁻¹ could be attributed to the CH bending vibration of CH2 groups. The second peak at 1023 cm⁻¹ becomes sharper with the addition of CNC, reducing the intensity of the first peak at 1086 cm⁻¹, indicating potential interaction between PVA and the cellulose particles [56,57].
Another double peak is observed around 917 cm⁻¹ (C-H stretching bonding), as reported by Alemdar and Sain, 2008a, which undergoes a shape change and shifts to a higher peak at around 945 cm⁻¹ in the composite compared to neat PVA. Additionally, an absorption band at 833 cm⁻¹ (C–C methyl group) is gradually reduced with the increase of CNC, supporting the possible interaction between CNC and PVA [58,59].
It is noteworthy that in S-M-5, three peaks are observed instead of a double peak around 1058 cm⁻¹, and the spectra of S-M-1 present a single peak instead of two, with a smaller peak at 2822 cm⁻¹.
3.2.3. Thermal Analysis
TGA
Thermogravimetric analysis was conducted to assess the thermal stability of PVA and biocomposite cast films reported in
Table 4. No discernible trend was identified among all samples; however, enhanced thermal stability was observed in samples incorporating CMF and 5 percent of CNC.
A consistent pattern of two main weight losses was observed across all samples. A minor reduction with small weight loss in the 100–150°C range was noted, attributed to the evaporation of moisture or low molecular weight compounds [60]. The peak temperature corresponding to the primary decomposition step for neat PVA and PVA/CNC/CMF biocomposites fell within the range of 257–271°C. Notably, O-M-1 exhibited the highest thermal stability, followed closely by S-M-1 and C-M-1, with temperatures of 271°C, 270°C, and 269°C, respectively. With the addition of cellulose particles (5%), the rate of weight loss increased. S-N-1 displayed the minimum weight loss at 54 percent, approximately 40% less than the others. Consequently, the incorporation of CMF and CNC with 5% in the PVA matrix resulted in only a slight enhancement in the thermal stability of the matrix reinforcement
DSC
Differential scanning calorimetry was employed to assess the glass transition temperature (T
g), melting temperature (T
m), and crystallization temperature (T
c) of pristine PVA and biocomposite films reinforced with cellulose particles, as detailed in
Table 4. To eliminate moisture and ensure consistent thermal history, all samples underwent a 30-minute treatment at 130°C.
Common thermal behavior patterns were observed among all samples, featuring two endothermic peaks in the second heating cycle and one exothermic peak during cooling. However, the thermal response exhibited no consistent pattern following the incorporation of CNC/CMF in the PVA cast film. A noteworthy increase in specific heat around 66°C, corresponding to the glass-rubber transition (Tg) of PVA, was observed. While the glass transition temperature of PVA experienced a slight decrease with the addition of reinforcement, this effect was more pronounced at higher CNC and CMF contents. However, only the O-M-5 sample demonstrated a higher Tg compared to PVA, increasing from 66 to 68°C. The initial reduction in Tg, attributed to the restriction of PVA chain mobility due to the adsorptive forces of the reinforcement, aligns with previous findings [55,61].
The melting temperature of PVA showed an increase of approximately 10°C for all samples with both 1% and 5% reinforcement. C-M-5 displayed a slight increase to 179°C compared to neat PVA at 175°C, wheras O-M-5 exhibited the maximum Tm at 188°C.
Table 4 illustrates a slight enhancement in the crystallinity of cast films with the addition of 1% and 5% percent of CNC and CMF. M-N-1 and S-M-5 demonstrated a higher increase in crystallinity, reaching 18% compared to pure PVA (11%). This rise in crystallinity is attributed to the nucleating effect of nano-sized fibers, supporting previous studies [27].
A parallel trend was observed in the crystallization temperature during the cooling scan with the increased concentration of CNC/CMF. This elevation in Tc, associated with cellulose particle addition, is attributed to variations in crystal size and morphology in cast films. The PVA membrane with more uniform crystals experiences dominant homogeneous phase nucleation during cooling [61].
3.4. Mechanical Propertise of Cast Films
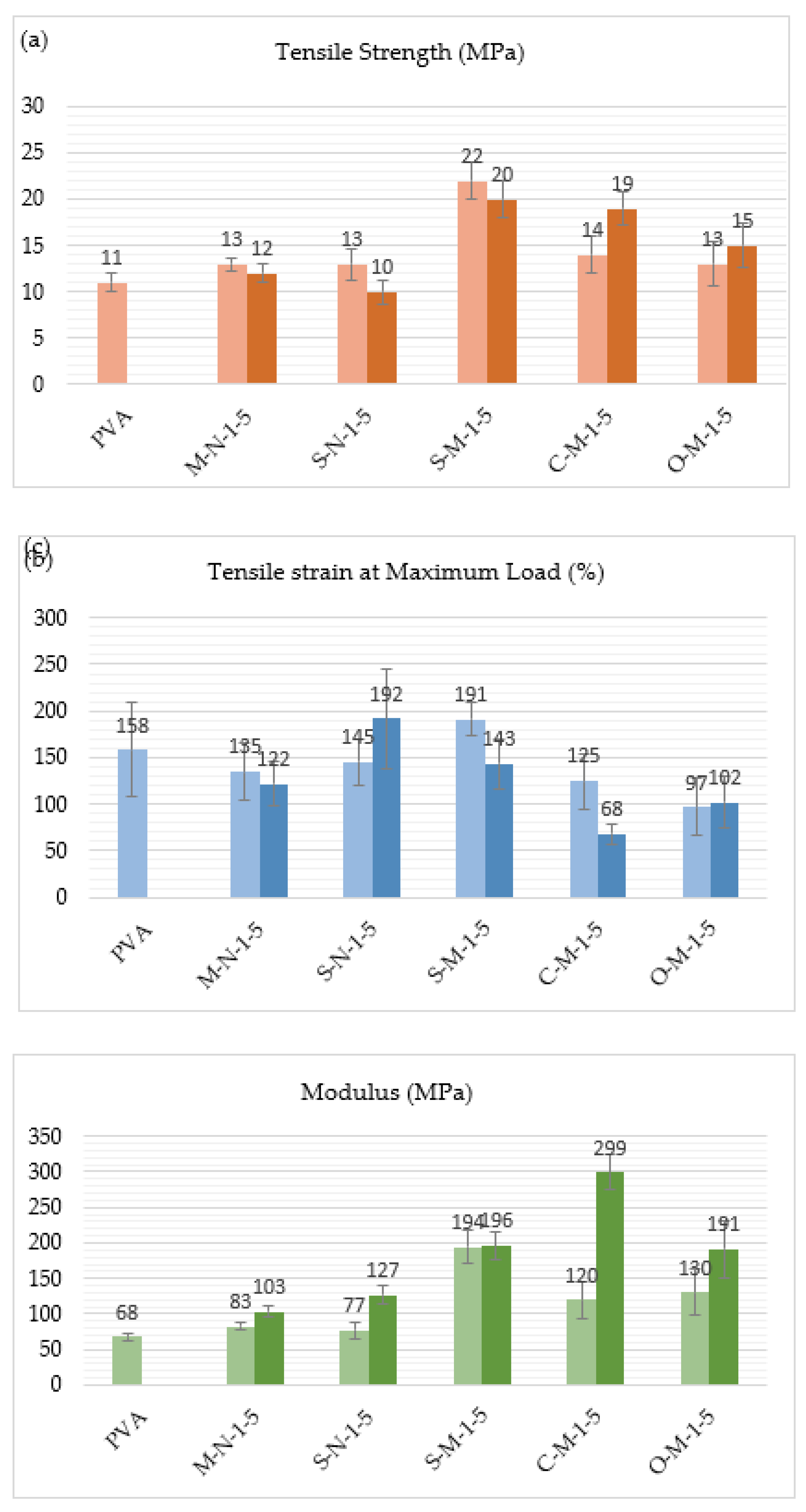
Figure 6 (a) displays the tensile strength results of PVA and the reinforced films with nano and micro cellulose at 1% and 5% reinforcement. The first bar is for films with 1% reinforcement, and the second bar is for films with 5% reinforcement. It is observed that the tensile strength for all reinforced films increased compared to unreinforced PVA, except for the sample with 5% CNC derived from hemp using sulfuric acid. For the other samples, there is an overall enhancement in the film’s tensile strength, consistent with previous studies [62,63]. It’s mentioned that the strong affinity between the PVA matrix and CNC/CMF, both carrying hydroxyl-rich surfaces, makes for relatively strong interfacial interactions [28]. These interactions, characterized by hydrogen bonding, significantly strengthen the interface, impacting the mechanical properties of the composite material [1,27]. This enhancement is more pronounced in CMF reinforced samples as compared to CNC reinforced samples, with strengths approximately 2, 1.7 and 1.3 times larger for CMF reinforced samples prepared with sulfuric acid, citric acid, and oxalic acid, respectively. This could be attributed to the larger particles present in these samples, as shown in
Figure 1, with higher aspect ratios. Furthermore, Lee et al. (2009) [64], described that using 1 wt% of cellulose nanocrystals led to an increase in tensile strength, but when the CNC loading was increased to 5 wt% in a PVA matrix, the tensile strength slightly reduced, from 13 to 12 MPa and from 13 to 10 MPa for CNC with wood and CNC with hemp, respectively. This behavior is not observed in the case of CMF samples due to differences in particle characteristics, such as size, aspect ratio, crystallinity, morphology, and distribution, which can impact the mechanical properties [65].
The tensile strain results are shown in
Figure 6 (b). As observed in the graph, the tensile strain of most samples, except for two, decreased when compared with the unreinforced PVA film. The minimum strain, 68%, is observed in the case of C-M-5. This significant decrease is due to the carboxylate bonds at the fiber interface, which make the interface stronger but more brittle. On the other hand, the maximum strain, 192%, is observed in the case of S-N-5 and S-M-1. These values indicate more plastic behavior with extensive plastic deformation and slippage at the fiber-matrix interface. Therefore, depending on the specific application, the appropriate sample can be selected to achieve the desired mechanical response [66,67].
In
Figure 6 (c), the impact of cellulose nano/micro particles on the Young’s modulus is demonstrated. The results indicate that the inclusion of cellulose nanocrystals and cellulose microfibrils as reinforcement leads to an increase in the modulus. The modulus increases with the addition of CNC/CMF loading, and films with CMF exhibit a higher modulus compared to CNC films, ranging from 120-299 MPa to 77-127 MPa, respectively. When the CMF concentration is 5% with citric acid, the modulus obtains its maximum value, which is 4.4 times greater than that of the unreinforced PVA film. It is important to note that these increases in modulus are significantly higher than one would expect with 1% and 5% reinforcing particles using models such as the rule-of-mixtures or the Halpin-Tsai equations. This increase can be attributed to the stiffening of the polymer matrix due to chain mobility constraints introduced by the large amount of fiber-matrix interface introduced by the addition of micro-fibril reinforcements [62,68].
These results demonstrate a significant improvement in mechanical properties through the addition of CNC/CMF in these biocomposites. It’s worth noting that one of the key considerations for such composites with natural reinforcement is uniform particle distribution, which can have an impact on mechanical properties.
4. Conclusions
This study focused on the production of cellulose whiskers from hemp through the application of both organic and mineral acids. The first step involved characterizing cellulose particles derived with different acids to compare their properties. Morphological and thermal analyses, supported by chemical analysis, revealed that cellulose particles derived from hydrolysis with citric acid had the highest total yield at 95.9%, followed by 62.5% CMF using oxalic acid. In contrast, using sulfuric acid produced the highest yield of nanocrystals at 41.9%. Particles synthesized using citric acid exhibited the highest aspect ratio with larger rod-like particles, while particles synthesized using sulfuric acid produced cellulose particles in the nano range with needle and worm-like shapes. In terms of thermal stability, particles synthesized using organic acids were significantly more stable compared with particles synthesized using sulfuric acid, which exhibited poor thermal resistance due to weaker bonds with water and esterification of sulfate surface groups present in sulfuric acid.
Characterizing cellulose fillers in biocomposite films involved several tests. The morphology of cast films exhibited consistent surface modification. FTIR results confirmed interfacial forces between the filler and the polymer. In terms of thermal properties, the addition of the reinforcements to PVA increased the melting and crystallization temperatures and increased the degree of crystallinity compared to unreinforced PVA. Thermal stability slightly increased in nearly all reinforced samples.
Tensile tests yielded significant results. Tensile strength and Young’s modulus increased in almost all samples, with CMF-containing samples showing more significant increases. Reinforcing with particles synthesized using citric acid exhibited the highest modulus, with an increase of more than 4 times the value for unreinforced PVA. This composite also demonstrated the lowest strain to failure due to the presence of carboxylic groups at the fiber-matrix interface, making for a stronger, but more brittle interface.
The dispersion of these fillers into the composite and its impact on the results will be further investigated using Dynamic Light Scattering (DLS) in future studies. Additional tests, such as XRD and Z-potential, will also be used to complete the characterization of these cellulose particles synthesized from organic and mineral acids. The latter test will help to interrogate the fiber matrix interface and will be complemented with surface energy measurement using experiments such as the Wilhelmy single fiber wetting technique.
Author Contributions
Conceptualization: F.M. and R.K.; writing—original draft preparation: F.M.; writing—review and editing: F.M., G.B. and R.K.; supervision: G.B. and R.K.; funding acquisition: R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Thomas Jefferson University through a foundation grant provided by the Lambert Innovation Fund.
Institutional Review Board Statement
N/A
Informed Consent Statement
N/A
Data Availability Statement
All date is presented in the manuscript.
Acknowledgments
The authors acknowledge Professor Lucas Guz, Assistant Research Professor at Universidad Nacional de San Martin, Argentina for his advice and assistance in the synthesis and characterization phases of this project. The authors also acknowledge the Bruner Materials Characterization Laboratory at Thomas Jefferson University for use of their SEM and mechanical testing frame, and the Jefferson College of Life Sciences for use of their FTIR.
Conflicts of Interest
The authors declare no conflict of interest.
References
- W. Li, Q. Wu, X. Zhao, Z. Huang, J. Cao, J. Li and S. Liu, ’"Enhanced thermal and mechanical properties of PVA composites formed with filamentous nanocellulose fibrils," Carbohydr.Polym., vol. 113, pp. 403-410. [CrossRef]
- Y.C. Ching, A. Rahman, K.Y. Ching, N.L. Sukiman and H.C. Cheng, ’"Preparation and characterization of polyvinyl alcohol-based composite reinforced with nanocellulose and nanosilica," BioResources, vol. 10, no. 2, pp. 3364-3377. [CrossRef]
- A. Bhatnagar and M. Sain, ’"Processing of cellulose nanofiber-reinforced composites," J Reinf Plast Compos, vol. 24, no. 12, pp. 1259-1268. [CrossRef]
- M. Börjesson and G. Westman, ’"Crystalline nanocellulose—preparation, modification, and properties," Cellulose-fundamental aspects and current trends, vol. 7.
- G. Siqueira, J. Bras and A. Dufresne, ’"Cellulosic bionanocomposites: a review of preparation, properties and applications," Polymers, vol. 2, no. 4, pp. 728-765.
- J.P. Manaia, A.T. Manaia and L. Rodriges, ’"Industrial hemp fibers: An overview," Fibers, vol. 7, no. 12, pp. 106. [CrossRef]
- G. Crini, E. Lichtfouse, G. Chanet and N. Morin-Crini, ’"Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review," Environmental Chemistry Letters, vol. 18, no. 5, pp. 1451-1476. [CrossRef]
- G. Kaur and R. Kander, ’"The Sustainability of Industrial Hemp: A Literature Review of Its Economic, Environmental, and Social Sustainability," Sustainability (Basel, Switzerland), vol. 15, no. 6457, pp. 6457.
- J.G. Crowley, ’"The Performance of Cannabis Sativa (HEMP) as a Fibre Source for Medium Density Fibre Board (MDF).", 2001.
- S. González-García, A. Hospido, G. Feijoo and M.T. Moreira, ’"Life cycle assessment of raw materials for non-wood pulp mills: Hemp and flax," Resour.Conserv.Recycling, vol. 54, no. 11, pp. 923-930. [CrossRef]
- L. Chen, J.Y. Zhu, C. Baez, P. Kitin and T. Elder, ’"Highly thermal-stable and functional cellulose nanocrystals and nanofibrils produced using fully recyclable organic acids," Green Chem., vol. 18, no. 13, -06-27, pp. 3835-3843. [CrossRef]
- M. Roman and W.T. Winter, ’"Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose," Biomacromolecules, vol. 5, no. 5, pp. 1671-1677. [CrossRef]
- L.A. Worku, R.K. Bachheti and M.G. Tadesse, ’"Preparation and characterization of carboxylated cellulose nanocrystals from Oxytenanthera abyssinica (Ethiopian lowland bamboo) cellulose via citric acid anhydrous hydrolysis catalyzed by sulfuric acid," Biomass conversion and biorefinery. [CrossRef]
- J. Jiang, Y. Zhu and F. Jiang, ’"Sustainable isolation of nanocellulose from cellulose and lignocellulosic feedstocks: Recent progress and perspectives," Carbohydr.Polym., vol. 267, pp. 118188. [CrossRef]
- H. Xie, H. Du, X. Yang and C. Si, ’"Recent Strategies in Preparation of Cellulose Nanocrystals and Cellulose Nanofibrils Derived from Raw Cellulose Materials," International journal of polymer science, vol. 2018, pp. 1-25. [CrossRef]
- H. Ji, Z. Xiang, H. Qi, T. Han, A. Pranovich and T. Song, ’"Strategy towards one-step preparation of carboxylic cellulose nanocrystals and nanofibrils with high yield, carboxylation and highly stable dispersibility using innocuous citric acid," Green Chem., vol. 21, no. 8, pp. 1956-1964.
- H. Wang, H. Du, K. Liu, H. Liu, T. Xu, S. Zhang, X. Chen, R. Zhang, H. Li, H. Xie, X. Zhang and C. Si, ’"Sustainable preparation of bifunctional cellulose nanocrystals via mixed H2SO4/formic acid hydrolysis," Carbohydr.Polym., vol. 266, pp. 118107.
- H. Ji, Z. Xiang, H. Qi, T. Han, A. Pranovich and T. Song, ’"Strategy towards one-step preparation of carboxylic cellulose nanocrystals and nanofibrils with high yield, carboxylation and highly stable dispersibility using innocuous citric acid," Green Chem., vol. 21, no. 8, pp. 1956-1964.
- W. Jia and Y. Liu, ’"Two characteristic cellulose nanocrystals (CNCs) obtained from oxalic acid and sulfuric acid processing," Cellulose, vol. 26, pp. 8351-8365. [CrossRef]
- R.J. Moon, A. Martini, J. Nairn, J. Simonsen and J. Youngblood, ’"Cellulose nanomaterials review: structure, properties and nanocomposites," Chem.Soc.Rev., vol. 40, no. 7, pp. 3941-3994.
- N. El Miri, M. El Achaby, A. Fihri, M. Larzek, M. Zahouily, K. Abdelouahdi, A. Barakat and A. Solhy, ’"Synergistic effect of cellulose nanocrystals/graphene oxide nanosheets as functional hybrid nanofiller for enhancing properties of PVA nanocomposites," Carbohydr.Polym., vol. 137, pp. 239-248. [CrossRef]
- A. Jalal Uddin, J. Araki and Y. Gotoh, ’"Toward “Strong” Green Nanocomposites: Polyvinyl Alcohol Reinforced with Extremely Oriented Cellulose Whiskers," Biomacromolecules, vol. 12, no. 3, pp. 617-624. [CrossRef]
- C.E. Meree, G.T. Schueneman, J.C. Meredith and M.L. Shofner, ’"Rheological behavior of highly loaded cellulose nanocrystal/poly(vinyl alcohol) composite suspensions," Cellulose, vol. 23, no. 5, pp. 3001-3012. [CrossRef]
- A. Chakrabarty and Y. Teramoto, ’"Recent advances in nanocellulose composites with polymers: a guide for choosing partners and how to incorporate them," Polymers, vol. 10, no. 5, pp. 517. [CrossRef]
- F.M. Boroujeni and M. Sharzehee, ’"Synthetic oligomers with urea binding and their role as crosslinkers in improving the printing quality on cotton fabric," Fibers and Polymers, vol. 24, no. 3, pp. 855-867. [CrossRef]
- R.K. Ulaganathan, N.A.M. Senusi, M.A.M. Amin, M.K.A.A. Razab, A. Ismardi and N.H. Abdullah, ’"Effect of cellulose nanocrystals (CNC) on PVA/CNC bio-nanocomposite film as potential food packaging application," Materials Today: Proceedings, vol. 66, pp. 3150-3153. [CrossRef]
- J. Lu, T. Wang and L.T. Drzal, ’"Preparation and properties of microfibrillated cellulose polyvinyl alcohol composite materials," Composites.Part A, Applied science and manufacturing, vol. 39, no. 5, pp. 738-746. [CrossRef]
- M. Roohani, Y. Habibi, N.M. Belgacem, G. Ebrahim, A.N. Karimi and A. Dufresne, ’"Cellulose whiskers reinforced polyvinyl alcohol copolymers nanocomposites," European polymer journal, vol. 44, no. 8, pp. 2489-2498. [CrossRef]
- J. Revol, H. Bradford, J. Giasson, R.H. Marchessault and D.G. Gray, ’"Helicoidal self-ordering of cellulose microfibrils in aqueous suspension," Int.J.Biol.Macromol., vol. 14, no. 3, pp. 170-172. [CrossRef]
- Y. Habibi, L.A. Lucia and O.J. Rojas, ’"Cellulose nanocrystals: chemistry, self-assembly, and applications," Chem.Rev., vol. 110, no. 6, pp. 3479-3500.
- L.A. Worku, R.K. Bachheti and M.G. Tadesse, ’"Preparation and characterization of carboxylated cellulose nanocrystals from Oxytenanthera abyssinica (Ethiopian lowland bamboo) cellulose via citric acid anhydrous hydrolysis catalyzed by sulfuric acid," Biomass Conversion and Biorefinery, pp. 1-17. [CrossRef]
- M. Roohani, Y. Habibi, N.M. Belgacem, G. Ebrahim, A.N. Karimi and A. Dufresne, ’"Cellulose whiskers reinforced polyvinyl alcohol copolymers nanocomposites," European polymer journal, vol. 44, no. 8, pp. 2489-2498. [CrossRef]
- P.P. Zhang, D.S. Tong, C.X. Lin, H.M. Yang, Z.K. Zhong, W.H. Yu, H. Wang and C.H. Zhou, ’"Effects of acid treatments on bamboo cellulose nanocrystals," Asia-Pacific Journal of Chemical Engineering, vol. 9, no. 5, pp. 686-695. [CrossRef]
- A. Demirbaş, ’"Mechanisms of liquefaction and pyrolysis reactions of biomass," Energy conversion and management, vol. 41, no. 6, pp. 633-646. [CrossRef]
- H. Yu, D. Zhang, F. Lu and J. Yao, ’"New approach for single-step extraction of carboxylated cellulose nanocrystals for their use as adsorbents and flocculants," ACS Sustainable Chemistry & Engineering, vol. 4, no. 5, pp. 2632-2643. [CrossRef]
- T.J. Bondancia, J. de Aguiar, G. Batista, A.J. Cruz, J.M. Marconcini, L.H.C. Mattoso and C.S. Farinas, ’"Production of nanocellulose using citric acid in a biorefinery concept: effect of the hydrolysis reaction time and techno-economic analysis," Ind Eng Chem Res, vol. 59, no. 25, pp. 11505-11516. [CrossRef]
- R. Li, J. Fei, Y. Cai, Y. Li, J. Feng and J. Yao, ’"Cellulose whiskers extracted from mulberry: A novel biomass production," Carbohydr.Polym., vol. 76, no. 1, pp. 94-99. [CrossRef]
- J. Łojewska, P. Miśkowiec, T. Łojewski and L.M. Proniewicz, ’"Cellulose oxidative and hydrolytic degradation: In situ FTIR approach," Polym.Degrad.Stab., vol. 88, no. 3, pp. 512-520. [CrossRef]
- J.I. Morán, V.A. Alvarez, V.P. Cyras and A. Vázquez, ’"Extraction of cellulose and preparation of nanocellulose from sisal fibers," Cellulose, vol. 15, pp. 149-159. [CrossRef]
- N. Reddy and Y. Yang, ’"Structure and properties of high quality natural cellulose fibers from cornstalks," Polymer (Guilford), vol. 46, no. 15, pp. 5494-5500. [CrossRef]
- N. Sgriccia, M.C. Hawley and M. Misra, ’"Characterization of natural fiber surfaces and natural fiber composites," Composites Part A: Applied Science and Manufacturing, vol. 39, no. 10, pp. 1632-1637. [CrossRef]
- H. Yang, R. Yan, H. Chen, D.H. Lee and C. Zheng, ’"Characteristics of hemicellulose, cellulose and lignin pyrolysis," Fuel, vol. 86, no. 12-13, pp. 1781-1788.
- H. Yu, Z. Qin, B. Liang, N. Liu, Z. Zhou and L. Chen, ’"Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions," Journal of Materials Chemistry A, vol. 1, no. 12, pp. 3938-3944. [CrossRef]
- A. Alemdar and M. Sain, ’"Isolation and characterization of nanofibers from agricultural residues–Wheat straw and soy hulls," Bioresour.Technol., vol. 99, no. 6, pp. 1664-1671. [CrossRef]
- M. Rosa, E. Medeiros, J.A. Malmonge, K.S. Gregorski, D.F. Wood, L. Mattoso, G. Glenn, W.J. Orts and S.H. Imam, ’"Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior," Carbohydr.Polym., vol. 81, no. 1, pp. 83-92. [CrossRef]
- R.M. dos Santos, W.P.F. Neto, H.A. Silvério, D.F. Martins, N.O. Dantas and D. Pasquini, ’"Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste," Industrial Crops and Products, vol. 50, pp. 707-714. [CrossRef]
- X.Y. Tan, S.B. Abd Hamid and C.W. Lai, ’"Preparation of high crystallinity cellulose nanocrystals (CNCs) by ionic liquid solvolysis," Biomass Bioenergy, vol. 81, pp. 584-591. [CrossRef]
- J. Gong, J. Li, J. Xu, Z. Xiang and L. Mo, ’"Research on cellulose nanocrystals produced from cellulose sources with various polymorphs," RSC Adv., vol. 7, -07-03, pp. 33486-33493. [CrossRef]
- H. Celebi and A. Kurt, ’"Effects of processing on the properties of chitosan/cellulose nanocrystal films," Carbohydr.Polym., vol. 133, pp. 284-293. [CrossRef]
- W. Liu, H. Du, H. Liu, H. Xie, T. Xu, X. Zhao, Y. Liu, X. Zhang and C. Si, ’"Highly efficient and sustainable preparation of carboxylic and thermostable cellulose nanocrystals via FeCl3-catalyzed innocuous citric acid hydrolysis," ACS Sustainable Chemistry & Engineering, vol. 8, no. 44, pp. 16691-16700.
- X. Yang, H. Xie, H. Du, X. Zhang, Z. Zou, Y. Zou, W. Liu, H. Lan, X. Zhang and C. Si, ’"Facile extraction of thermally stable and dispersible cellulose nanocrystals with high yield via a green and recyclable FeCl3-catalyzed deep eutectic solvent system," ACS Sustainable Chemistry & Engineering, vol. 7, no. 7, pp. 7200-7208. [CrossRef]
- M.N. Angles and A. Dufresne, ’"Plasticized starch/tunicin whiskers nanocomposite materials. 2. Mechanical behavior," Macromolecules, vol. 34, no. 9, pp. 2921-2931.
- K.J. Nagarajan, A.N. Balaji, S.T. Kasi Rajan and N.R. Ramanujam, ’"Preparation of bio-eco based cellulose nanomaterials from used disposal paper cups through citric acid hydrolysis," Carbohydr.Polym., vol. 235, pp. 115997. [CrossRef]
- P.S. Thomas, J. Guerbois, G.F. Russell and B.J. Briscoe, ’"FTIR study of the thermal degradation of poly (vinyl alcohol)," Journal of thermal analysis and calorimetry, vol. 64, pp. 501-508. [CrossRef]
- Z. Jahan, M.B.K. Niazi and ØW. Gregersen, ’"Mechanical, thermal and swelling properties of cellulose nanocrystals/PVA nanocomposites membranes," Journal of industrial and engineering chemistry, vol. 57, pp. 113-124. [CrossRef]
- H.N. Kumar, M.N. Prabhakar, C.V. Prasad, K.M. Rao, T.A.K. Reddy, K.C. Rao and M. Subha, ’"Compatibility studies of chitosan/PVA blend in 2% aqueous acetic acid solution at 30 C," Carbohydr.Polym., vol. 82, no. 2, pp. 251-255. [CrossRef]
- K. Choo, Y.C. Ching, C.H. Chuah, S. Julai and N. Liou, ’"Preparation and characterization of polyvinyl alcohol-chitosan composite films reinforced with cellulose nanofiber," Materials, vol. 9, no. 8, pp. 644. [CrossRef]
- B. Balavairavan and S.S. Saravanakumar, ’"Characterization of ecofriendly poly (vinyl alcohol) and green banana peel filler (GBPF) reinforced bio-films," Journal of Polymers and the Environment, vol. 29, pp. 2756-2771. [CrossRef]
- M.S. Peresin, Y. Habibi, J.O. Zoppe, J.J. Pawlak and O.J. Rojas, ’"Nanofiber composites of polyvinyl alcohol and cellulose nanocrystals: manufacture and characterization," Biomacromolecules, vol. 11, no. 3, pp. 674-681. [CrossRef]
- W. Li, J. Yue and S. Liu, ’"Preparation of nanocrystalline cellulose via ultrasound and its reinforcement capability for poly (vinyl alcohol) composites," Ultrason.Sonochem., vol. 19, no. 3, pp. 479-485. [CrossRef]
- A. Mandal and D. Chakrabarty, ’"Studies on the mechanical, thermal, morphological and barrier properties of nanocomposites based on poly (vinyl alcohol) and nanocellulose from sugarcane bagasse," Journal of Industrial and Engineering Chemistry, vol. 20, no. 2, pp. 462-473.
- E.H. Qua, P.R. Hornsby, H.S.S. Sharma, G. Lyons and R.D. McCall, ’"Preparation and characterization of poly(vinyl alcohol) nanocomposites made from cellulose nanofibers," J Appl Polym Sci, vol. 113, no. 4, pp. 2238-2247. [CrossRef]
- A. Kaboorani, B. Riedl, P. Blanchet, M. Fellin, O. Hosseinaei and S. Wang, ’"Nanocrystalline cellulose (NCC): A renewable nano-material for polyvinyl acetate (PVA) adhesive," European polymer journal, vol. 48, no. 11, pp. 1829-1837. [CrossRef]
- S. Lee, D.J. Mohan, I. Kang, G. Doh, S. Lee and S.O. Han, ’"Nanocellulose reinforced PVA composite films: Effects of acid treatment and filler loading," Fibers Polym, vol. 10, no. 1, pp. 77-82. [CrossRef]
- H.A. Silvério, W.P. Flauzino Neto, N.O. Dantas and D. Pasquini, ’"Extraction and characterization of cellulose nanocrystals from corncob for application as reinforcing agent in nanocomposites," Industrial crops and products, vol. 44, pp. 427-436. [CrossRef]
- T.W. Yee, L.J. Choy and W.A.W.A. Rahman, ’"Mechanical and water absorption properties of poly (vinyl alcohol)/sago pith waste biocomposites," J.Composite Mater., vol. 45, no. 11, pp. 1201-1207.
- E. Fortunati, D. Puglia, F. Luzi, C. Santulli, J.M. Kenny and L. Torre, ’"Binary PVA bio-nanocomposites containing cellulose nanocrystals extracted from different natural sources: Part I," Carbohydr.Polym., vol. 97, no. 2, pp. 825-836. [CrossRef]
- B. Kord, B. Malekian, H. Yousefi and A. Najafi, ’"Preparation and characterization of nanofibrillated Cellulose/Poly (Vinyl Alcohol) composite films," Maderas; Maderas, Cienc.tecnol, vol. 18, no. 4, pp. 743-752. [CrossRef]
Figure 1.
a: SEM images and aspect ratio distribution of CNC made using sulfuric acid, b: SEM images and aspect ratio distribution of CMF made using sulfuric acid, c: SEM images and aspect ratio distribution of CMF made using citric acid, d: SEM images and aspect ratio distrubution of CMF made with oxalic acid.
Figure 1.
a: SEM images and aspect ratio distribution of CNC made using sulfuric acid, b: SEM images and aspect ratio distribution of CMF made using sulfuric acid, c: SEM images and aspect ratio distribution of CMF made using citric acid, d: SEM images and aspect ratio distrubution of CMF made with oxalic acid.
Figure 2.
FTIR spectra of Hemp, S-CMF, C-CMF, and O-CMF.
Figure 2.
FTIR spectra of Hemp, S-CMF, C-CMF, and O-CMF.
Figure 3.
Thermogravimetric graph of S-CNC, S-CMF, C-CMF, and O-CMF.
Figure 3.
Thermogravimetric graph of S-CNC, S-CMF, C-CMF, and O-CMF.
Figure 4.
FTIR spectra of PVA cast films with 1% filler.
Figure 4.
FTIR spectra of PVA cast films with 1% filler.
Figure 5.
FTIR spectra of PVA cast films with 5% filler.
Figure 5.
FTIR spectra of PVA cast films with 5% filler.
Figure 6.
(a) Tensile strength, (b) Tensile strain at Maximum Load, (c) Modulus of unreinforced PVA and 1% and 5% reinforced biocomposites.
Figure 6.
(a) Tensile strength, (b) Tensile strain at Maximum Load, (c) Modulus of unreinforced PVA and 1% and 5% reinforced biocomposites.
Table 1.
Synthesis of CNC/CMF: reaction temperatures, T(°C); processing times, t(min); acid concentrations, w/w (%); and yields (%).
Table 1.
Synthesis of CNC/CMF: reaction temperatures, T(°C); processing times, t(min); acid concentrations, w/w (%); and yields (%).
| Samples |
T(°C ) |
t(min) |
w/w(%) |
CNC Yield (%) |
CMF Yield (%) |
Total-yield (%) |
| Sulfuric Acid Hydrolysis |
65-70 |
60 |
64 |
41.9 |
45.5 |
87.3 |
Citric Acid
Hydrolysis |
95-105 |
240 |
80 |
3.7 |
91.9 |
95.5 |
Oxalic Acid
Hydrolysis |
100-110 |
60-70 |
70 |
5.9 |
62.5 |
68.4 |
Table 2.
The length, width, and aspect ratio (L/W) of CNC/CMF synthesized using different acids.
Table 2.
The length, width, and aspect ratio (L/W) of CNC/CMF synthesized using different acids.
| Samples |
Length |
Width |
(L/W) |
|
Range |
Mean |
Range |
Mean |
Mode |
| S-CNC |
20-300 nm |
100 nm |
15-100 nm |
60 nm |
2.5 |
| S-CMF |
300 nm-120 μm |
20 μm |
150 nm-20 μm |
5 μm |
2.5 |
| C-CMF |
1 μm-400 μm |
100 μm |
300 nm-22 μm |
7 μm |
5.5 |
| O-CMF |
900 nm-140 μm |
23 μm |
200 nm-20 μm |
5 μm |
3.5 |
Table 3.
Degradation temperature (Td), moisture content (MC) and mass change of CNC with sulfuric acid (S-CNC), CMF with sulfuric acid (S-CMF), CMF with citric acid (C-CMF), and CMF with oxalic acid (O-CMF).
Table 3.
Degradation temperature (Td), moisture content (MC) and mass change of CNC with sulfuric acid (S-CNC), CMF with sulfuric acid (S-CMF), CMF with citric acid (C-CMF), and CMF with oxalic acid (O-CMF).
| Sample |
Td (°C) |
MC (%) |
Mass Change (%) |
| S-CNC |
150 |
4 |
80 |
| S-CMF |
170 |
6 |
82 |
| C-CMF |
230 |
5 |
86 |
| O-CMF |
290 |
9 |
95 |
Table 4.
Glass transition temperature (Tg), melting temperature (Tm), heat of fusion (ΔHm), crystallinity degree (Xc), crystallization temperature (Tc), degradation temperature (Td), moisture content (MC), and Total Mass Change (TMC) of PVA and the reinforced PVA films
Table 4.
Glass transition temperature (Tg), melting temperature (Tm), heat of fusion (ΔHm), crystallinity degree (Xc), crystallization temperature (Tc), degradation temperature (Td), moisture content (MC), and Total Mass Change (TMC) of PVA and the reinforced PVA films
| Samples |
Tg (°C) |
Tm (°C) |
∆Hm (J/g) |
Xc (%) |
Tc (°C) |
Td (°C) |
MC (%) |
TMC (%) |
| PVA |
66 |
175 |
18 |
11 |
127 |
264 |
6 |
92 |
| M-N-1 |
60 |
188 |
28 |
18 |
153 |
257 |
7 |
95 |
| S-N-1 |
65 |
185 |
22 |
14 |
147 |
261 |
4 |
59 |
| S-M-1 |
60 |
187 |
22 |
14 |
154 |
270 |
10 |
90 |
| C-M-1 |
60 |
188 |
27 |
17 |
155 |
269 |
5 |
89 |
| O-M-1 |
62 |
186 |
21 |
13 |
149 |
271 |
6 |
99 |
| M-N-5 |
48 |
185 |
26 |
17 |
153 |
264 |
13 |
94 |
| S-N-5 |
59 |
187 |
23 |
15 |
163 |
266 |
17 |
96 |
| S-M-5 |
55 |
186 |
27 |
18 |
158 |
269 |
12 |
96 |
| C-M-5 |
61 |
179 |
20 |
13 |
142 |
269 |
14 |
97 |
| O-M-5 |
68 |
188 |
19 |
12 |
150 |
258 |
11 |
99 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).