Submitted:
20 November 2023
Posted:
22 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Samples
COI Amplification and sequencing
Sequence analysis
Morphology analysis
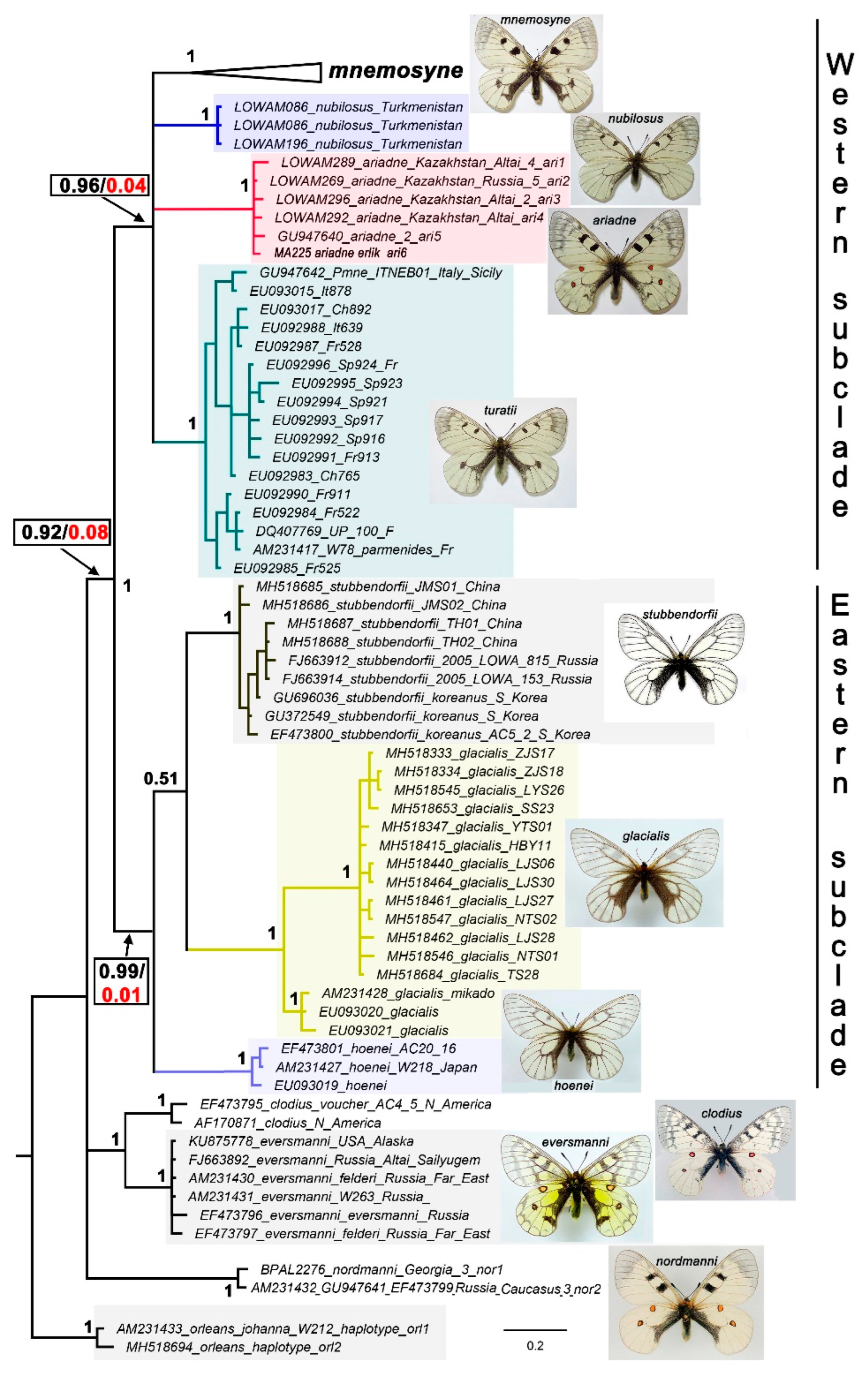
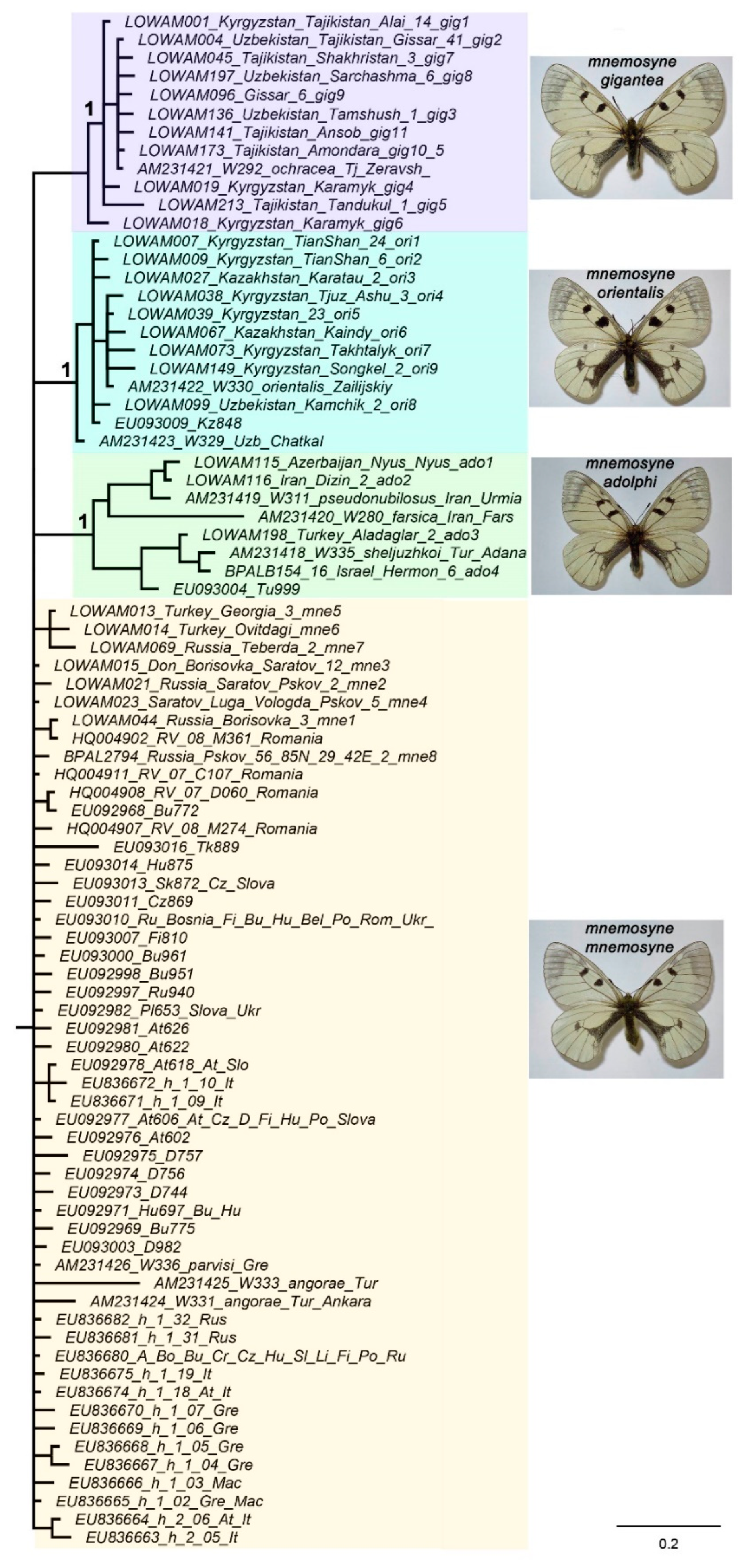
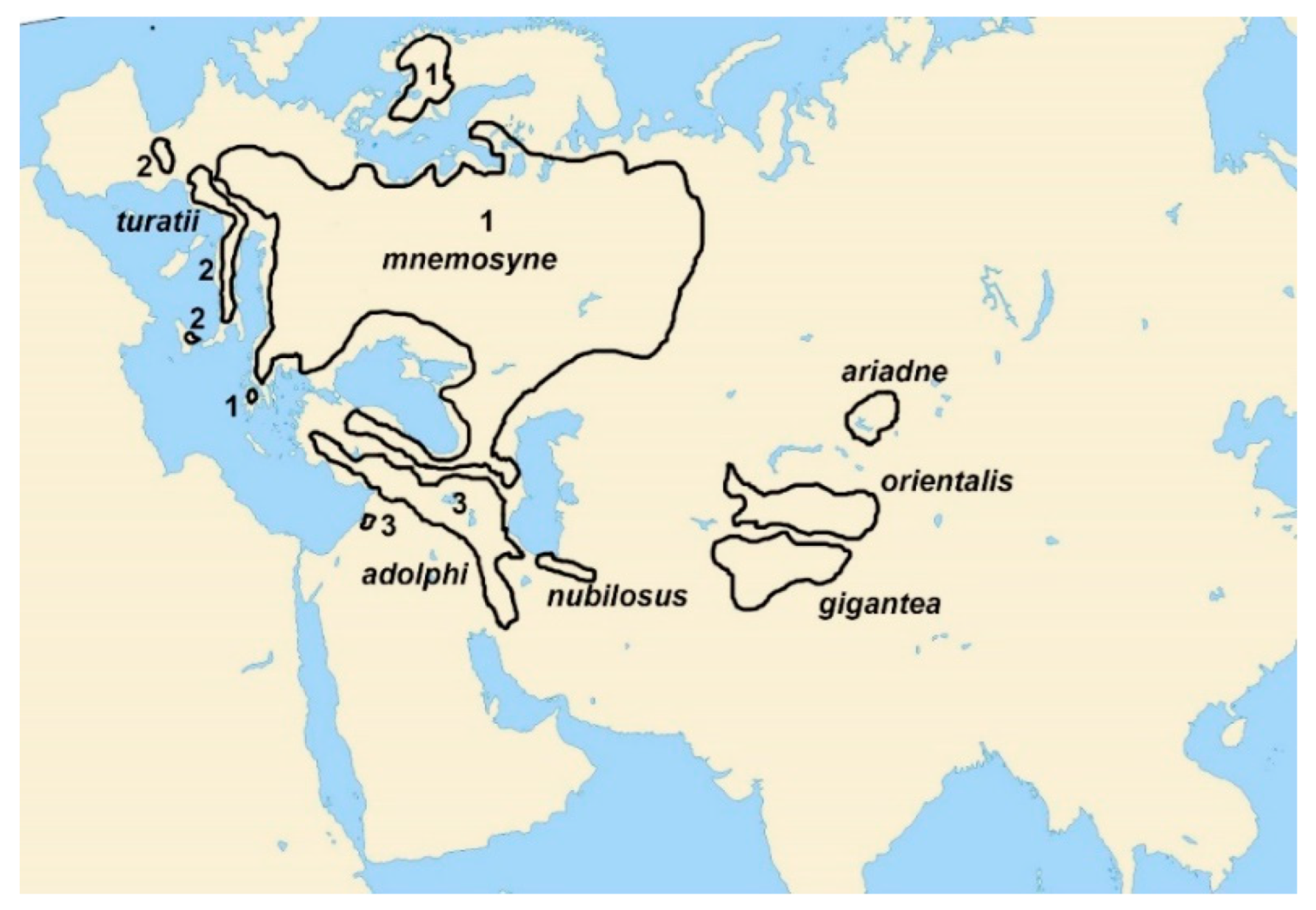
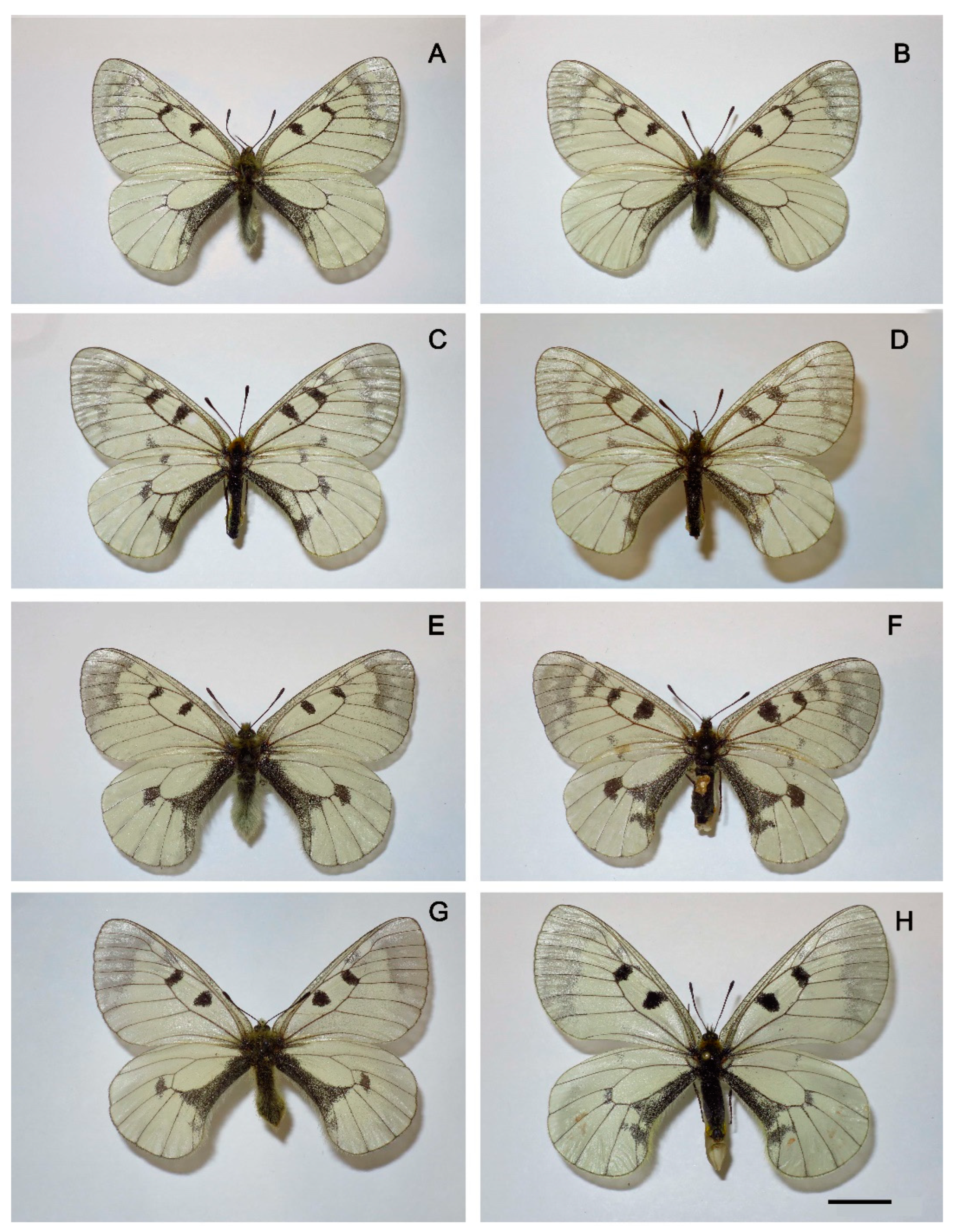
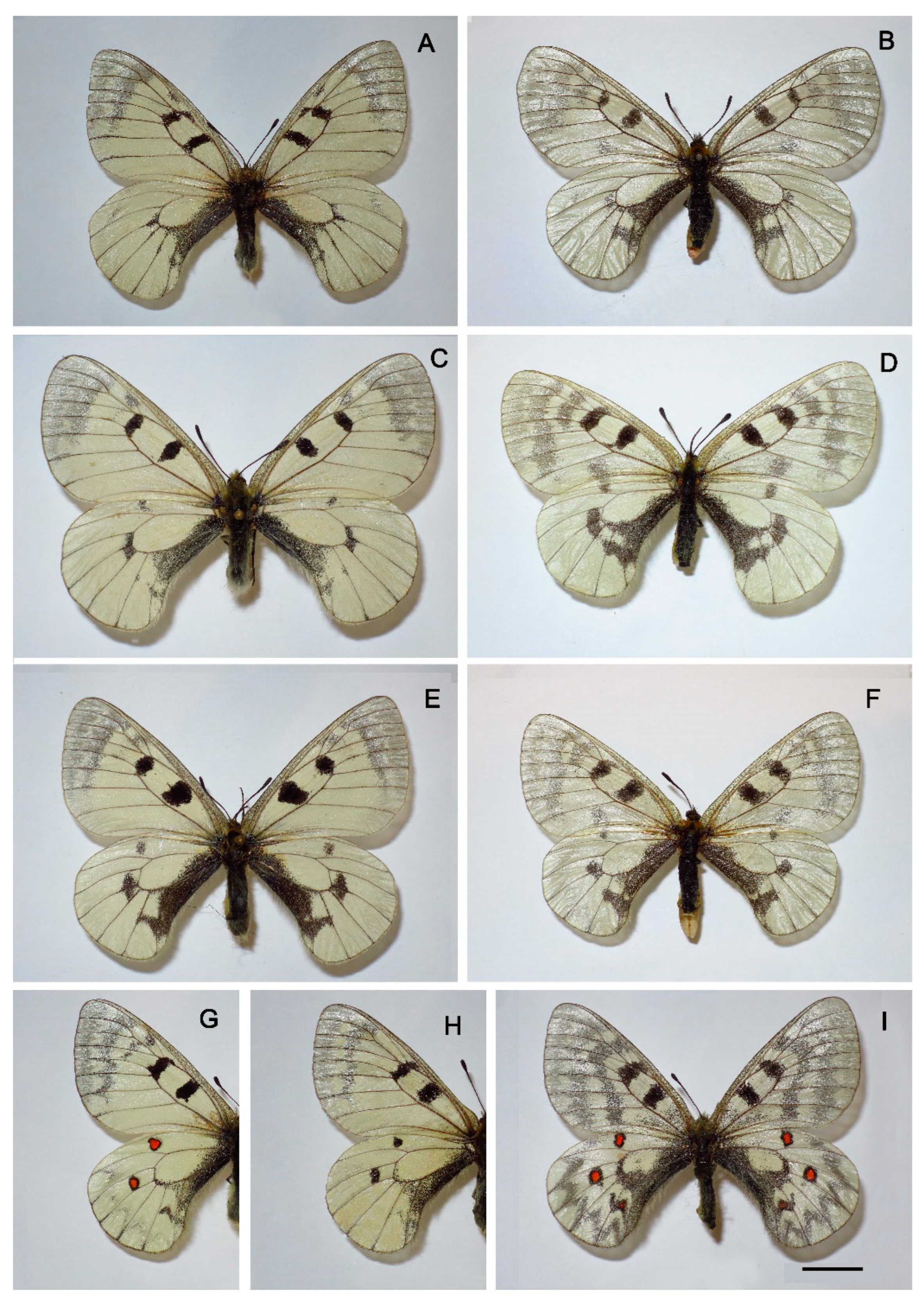
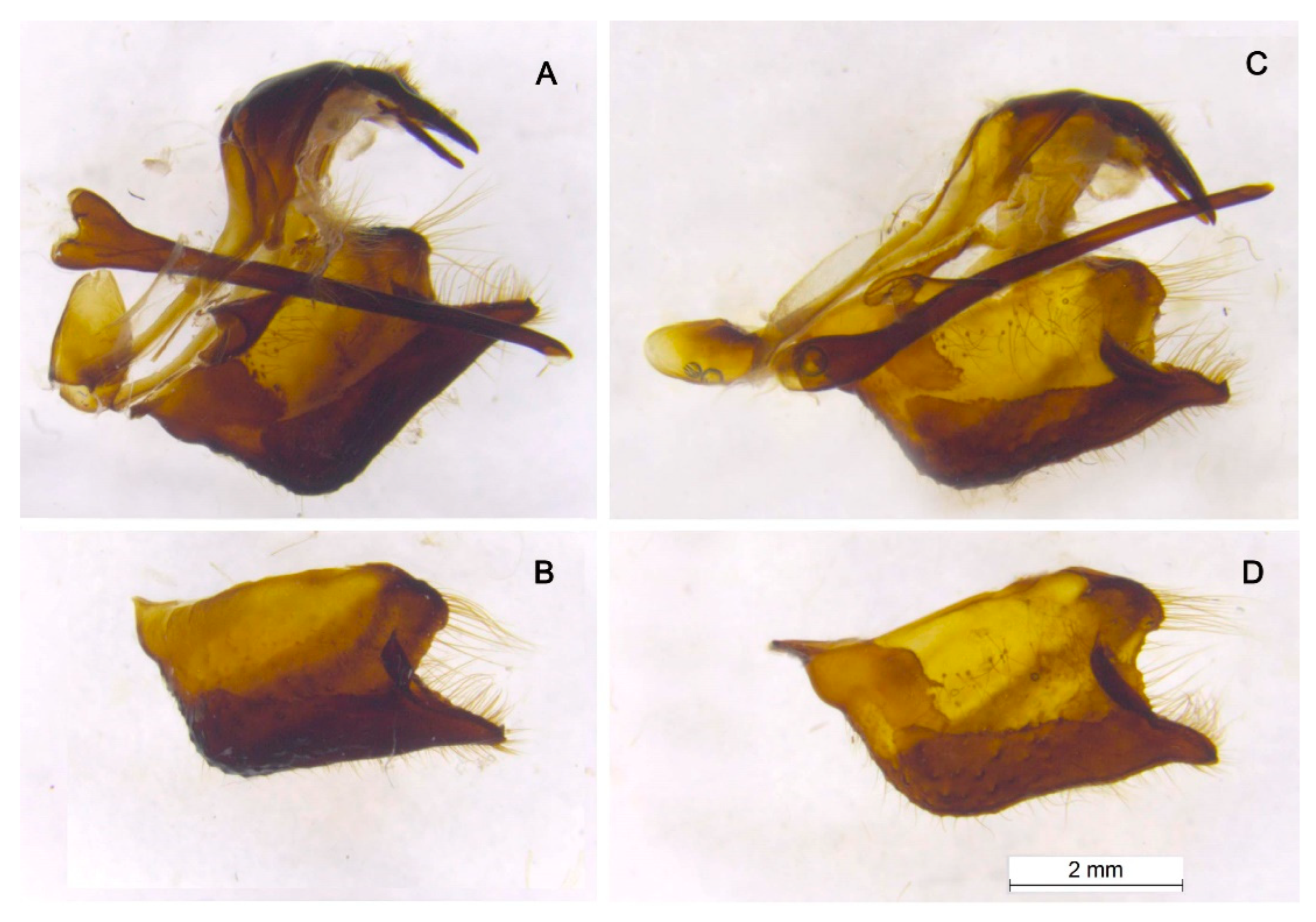
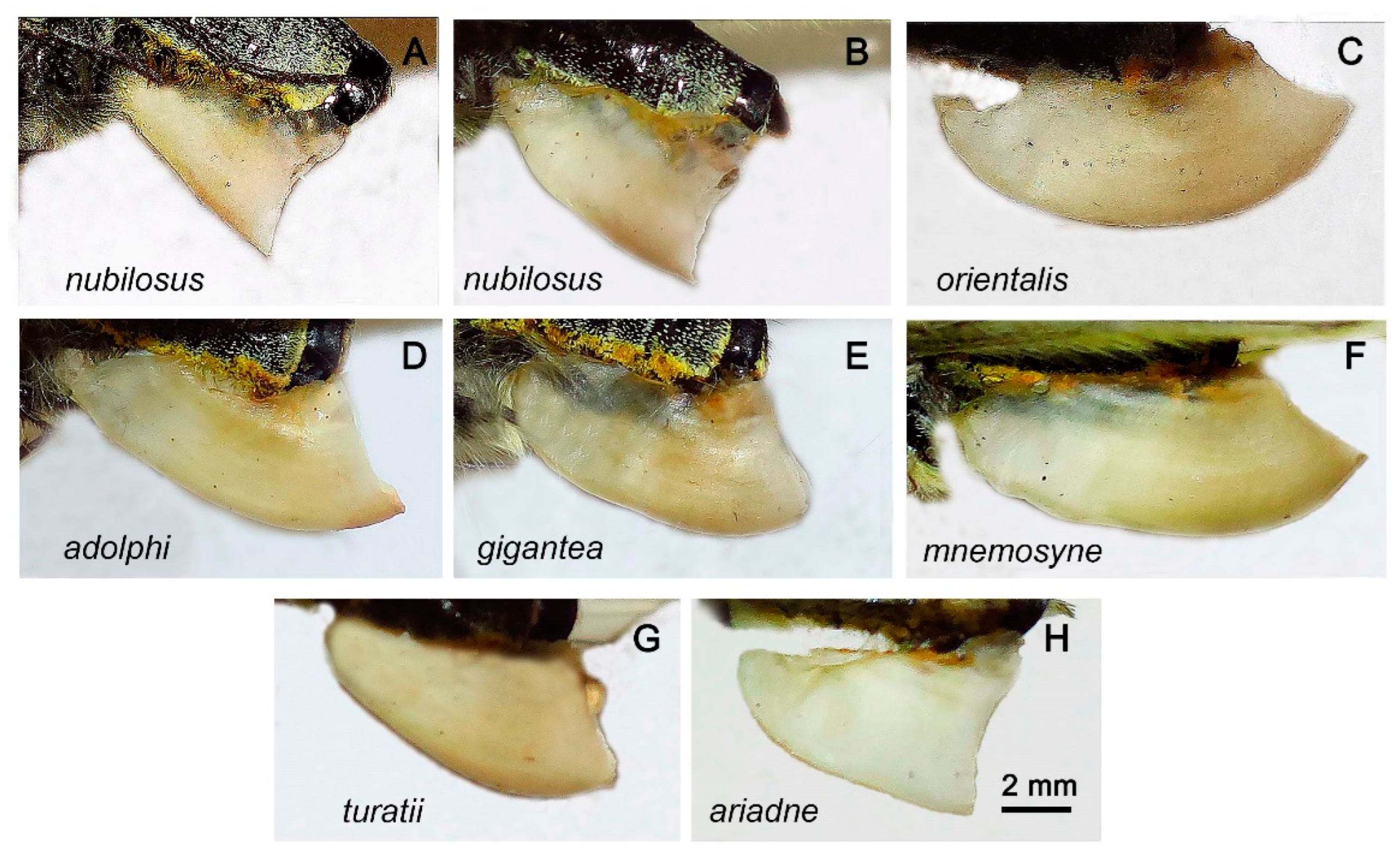
3. Results
Phylogenetic analysis
Morphology
Ancestral state reconstruction
Nomenclature and Lectotype designation of P. nubilosus
Taxonomy and nomenclature of P. ariadne
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bryk, F. Lepidoptera, Parnassidae pars II. (Subfam. Parnassiinae); W. de Gruyter: Berlin-Leipzig, Germany, 1935; pp. 1–790. [Google Scholar]
- Ackery, P.R. A guide to the genera and species of Parnassiinae (Lepidoptera: Papilionidae). Bull. Br. Mus. 1975, 31, 71–105. [Google Scholar] [CrossRef]
- Carvalho, A.P.S.; Orr, A.G.; Kawahara, A.Y. A review of the occurrence and diversity of the sphragis in butterflies (Lepidoptera, Papilionoidea). Zookeys 2017, 694, 41–70. [Google Scholar] [CrossRef] [PubMed]
- Vlasanek, P.; Konvicka, M. Sphragis in Parnassius mnemosyne (Lepidoptera: Papilionidae): Male-derived insemination plugs loose efficiencywith progress of female flight. Biologia 2009, 64, 1206–1211. [Google Scholar] [CrossRef]
- Bryk, F. Geographische und individuelle Variabilität der Sphragisbildung (Lepidoptera: Parnassiidae). Entomol. tidskr. 1950, 71, 230–234. [Google Scholar]
- Gór, Á.; Lang, Z.; Pásztor, K.; Szigeti, V.; Vajna, F.; Kis, J. Mate-guarding success depends on male investment in a butterfly. Ecol Evol. 2023, 13, e10533. [Google Scholar] [CrossRef] [PubMed]
- Gór, Á.; Fónagy, A.; Pasztor, K.; Szigeti, V.; Lang, Z.; Kis, J. Facultative male investment in prolonged mate-guarding in a butterfly. Behaviour 2023, 160, 515–557. [Google Scholar] [CrossRef]
- Yagi, T.; Katoh, T.; Chichvarkhin, A.; Shinkawa, T.; Omoto, K. Molecular phylogeny of butterflies Parnassius glacialis and P. stubbendorfii at various localities in East Asia. Genes Genet. Syst. 2001, 76, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Omoto, K.; Katoh, T.; Chichvarkhin, A.; Yagi, T. Molecular systematics and evolution of the “Apollo” butterflies of the genus Parnassius (lepidoptera: Papilionidae) based on mitochondrial DNA sequence data. Gene 2004, 326, 141–147. [Google Scholar] [CrossRef]
- Omoto, K.; Yonezawa, T.; Shinkawa, T. Molecular systematics and evolution of the recently discovered “Parnassian” butterfly (Parnassius davydovi Churkin, 2006) and its allied species (Lepidoptera, Papilionidae). Gene 2009, 441, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Sperling, F.A.H.; Nazari, V. Mitochondrial DNA divergence and phylogeography in western Palaearctic Parnassiinae (lepidoptera: Papilionidae): How many species are there? Insect Syst. Evol. 2007, 38, 121–138. [Google Scholar] [CrossRef]
- Nazari, V.; Zakharov, E.V.; Sperling, F.A.H. Phylogeny, historical biogeography, and taxonomic ranking of Parnassiinae (lepidoptera, Papilionidae) based on morphology and seven genes. Mol. Phylogenet. Evol. 2007, 42, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.; Rebourg, C.; Cosson, E.; Descimon, H. Molecular phylogeny of Parnassiinae butterflies (lepidoptera: Papilionidae) based on the sequences of four mitochondrial DNA segments. Ann. Soc. Entomol. Fr. 2008, 44, 1–36. [Google Scholar] [CrossRef]
- Rose, K.; Weiss, J.C. The Parnassiinae of the world. Part 5; Goecke & Evers: Keltern, Germany, 2011; 120p. [Google Scholar]
- Condamine, F.L.; Rolland, J.; Höhna, S.; Sperling, F.A.H.; Sanmartín, I. Testing the role of the red queen and court jester as drivers of the macroevolution of Apollo butterflies. Syst. Biol. 2018, 67, 940–964. [Google Scholar] [CrossRef]
- Zhao, Y.; He, B.; Tao, R.; Su, C.; Ma, J.; Hao, J.; Yang, Q. Phylogeny and biogeographic history of Parnassius butterflies (Papilionidae: Parnassiinae) reveal their origin and deep diversification in West China. Insects 2022, 13, 406. [Google Scholar] [CrossRef]
- Tian, X.; Mo, S.; Liang, D.; Wang, H.; Zhang, P. Amplicon capture phylogenomics provides new insights into the phylogeny and evolution of alpine Parnassius butterflies (Lepidoptera: Papilionidae). Syst. Entomol. 2023, 48, 571–584. [Google Scholar] [CrossRef]
- Todisco, V.; Gratton, P.; Cesaroni, D.; Sbordoni, V. Phylogeography of Parnassius apollo: Hints on taxonomy and conservation of a vulnerable glacial butterfly invader. Biol. J. Linn. Soc. 2010, 101, 169–183. [Google Scholar] [CrossRef]
- Condamine, F.L.; Sperling, F. Anthropogenic threats to high-altitude parnassian diversity. News of The Lepidopterists’ Society 2018, 60, 94–99. Available online: https://hal.science/hal-02323624/.
- The IUCN Red List. Available online: https://www.iucnredlist.org/search?query=Parnassius&searchType=species (accessed on 18 October 2023).
- Talla, V.; Mrazek, V.; Höglund, J.; Backström, N. Whole genome re-sequencing uncovers significant population structure and low genetic diversity in the endangered clouded Apollo (Parnasssius mnemosyne) in Sweden. Conserv. Genet. 2023, 24, 305–314. [Google Scholar] [CrossRef]
- Condamine, F.L.; Sperling, F.A.H.; Wahlberg, N.; Rasplus, J.; Kergoat, G.J. What causes latitudinal gradients in species diversity? Evolutionary processes and ecological constraints on swallowtail biodiversity. Ecol. Lett. 2012, 15, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.; Hubert, M.K.; Schoville, S.D. Testing the role of ecological selection on color pattern variation in the butterfly Parnassius clodius. Mol Ecol. 2019, 28, 5086–5102. [Google Scholar] [CrossRef]
- Tao, R.; Xu, C.; Wang, Y.; Sun, X.; Li, C.; Ma, J.; Hao, J.; Yang, Q. Spatiotemporal differentiation of alpine butterfly Parnassius glacialis (Papilionidae: Parnassiinae) in China: Evidence from mitochondrial DNA and nuclear single nucleotide polymorphisms. Genes 2020, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Xie, T.; Wang, Y.; Si, C.; Li, L.; Ma, J.; Li, C.; Sun, X.; Hao, J.; Yang, Q. Miocene diversification and high-altitude adaptation of Parnassius butterflies (lepidoptera: Papilionidae) in Qinghai–Tibet plateau revealed by large-scale transcriptomic data. Insects 2020, 11, 754. [Google Scholar] [CrossRef] [PubMed]
- Allio, R.; Nabholz, B.; Wanke, S.; Chomicki, G.; Pérez-Escobar, O.A.; Cotton, A.M.; Clamens, A.L.; Kergoat, G.J.; Sperling, F.A.H.; Condamine, F.L. Genome-wide macroevolutionary signatures of key innovations in butterflies colonizing new host plants. Nat Commun. 2021, 12, 354. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhao, Y.; Su, C.; Lin, G.; Wang, Y.; Li, L.; Ma, J.; Yan, Q.; Hao, J. Phylogenomics reveal extensive phylogenetic discordance due to incomplete lineage sorting following the rapid radiation of alpine butterflies (Papilionidae: Parnassius). Syst. Entomol. 2023, 48, 585–599. [Google Scholar] [CrossRef]
- Lukhtanov, V.A.; Sourakov, A.; Zakharov, E.V. DNA barcodes as a tool in biodiversity research: Testing pre-existing taxonomic hypotheses in Delphic Apollo butterflies (Lepidoptera, Papilionidae). Syst. Biodivers. 2016, 14, 599–613. [Google Scholar] [CrossRef]
- Weiss, J.C. The Parnassiinae of the world. Part 3. Venette, France: Sciences Nat. 1999. Canterbury: Hillside Books 98 pp.
- Bolotov, I.N.; Gofarov, M.Y.; Gorbach, V.V.; Kolosova, Y.S.; Zheludkova, A.A.; Kondakov, A.V.; Spitsyn, V.M. Parnassius nebrodensis: A threatened but neglected Apollo butterfly species from Southern Europe (Lepidoptera: Papilionidae). Ecol. Montenegrina 2021, 40, 140–163. [Google Scholar] [CrossRef]
- Cotton, A.M.; Bolotov, I.N.; Gofarov, M.Y.; Gorbach, V.V.; Kolosova, Y.S.; Zheludkova, A.A.; Kondakov, A.V.; Spitsyn, V.M. The correct name for the South Western European species recently separated from Parnassius mnemosyne (Linnaeus, 1758) (Lepidoptera: Papilionidae). Ecol. Montenegrina 2021, 43, 56–58. [Google Scholar] [CrossRef]
- Gratton, P.; Konopiński, M.K.; Sbordoni, V. Pleistocene evolutionary history of the Clouded Apollo (Parnassius mnemosyne): Genetic signatures of climate cycles and a ‘time-dependent’ mitochondrial substitution rate. Mol Ecol. 2008, 17, 4248–4262. [Google Scholar] [CrossRef] [PubMed]
- Scalercio, S.; Cini, A.; Menchetti, M.; Vodă, R.; Bonelli, S.; Bordoni, A.; Casacci, L.P.; Dincă, V.; Balletto, E.; Vila, R.; et al. How long is 3 km for a butterfly? Ecological constraints and functional traits explain high mitochondrial genetic diversity between Sicily and the Italian Peninsula. J. Anim. Ecol. 2020, 89, 2013–2026. [Google Scholar] [CrossRef]
- Hajibabaei, M.; deWaard, J.R.; Ivanova, N.V.; Ratnasingham, S.; Dooh, R.T.; Kirk, S.L.; Mackie, P.M.; Hebert, P.D. Critical factors for assembling a high volume of DNA barcodes. Philos Trans R Soc Lond B Biol Sci. 2005, 360, 1959–1967. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Dapporto, L.; Cini, A.; Vodă, R.; Dincă, V.; Wiemers, M.; Menchetti, M.; Magini, G.; Talavera, G.; Shreeve, T.; Bonelli, S.; et al. Integrating three comprehensive data sets shows that mitochondrial DNA variation is linked to species traits and paleogeographic events in European butterflies. Mol Ecol Resour. 2019, 19, 1623–1636. [Google Scholar] [CrossRef] [PubMed]
- Dincă, V.; Zakharov, E.V.; Hebert, P.D.; Vila, R. Complete DNA barcode reference library for a country’s butterfly fauna reveals high performance for temperate Europe. Proc. Biol Sci. 2011, 278, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Dincă, V.; Dapporto, L.; Somervuo, P.; Vodă, R.; Cuvelier, S.; Gascoigne-Pees, M.; Huemer, P.; Mutanen, M.; Hebert, P.D.N.; Vila, R. High resolution DNA barcode library for European butterflies reveals continental patterns of mitochondrial genetic diversity. Commun Biol. 2021, 4, 315. [Google Scholar] [CrossRef] [PubMed]
- Espeland, M.; Breinholt, J.; Willmott, K.R.; Warren, A.D.; Vila, R.; Toussaint, E.F.A.; Maunsell, S.C.; Aduse-Poku, K.; Talavera, G.; Eastwood, R.; et al. A comprehensive and dated phylogenomic analysis of butterflies. Curr. Biol. 2018, 28, 770–778.e5. [Google Scholar] [CrossRef] [PubMed]
- Litman, J.; Chittaro, Y.; Birrer, S.; Praz, C.; Wermeille, E.; Fluri, M.; Stalling, T.; Schmid, S.; Wyler, S.; Gonsethet, Y. A DNA barcode reference library for Swiss butterflies and forester moths as a tool for species identification, systematics and conservation. PLoS ONE 2018, 13, e0208639. [Google Scholar] [CrossRef] [PubMed]
- Mutanen, M.; Kivelä, S.M.; Vos, R.A.; Doorenweerd, C.; Ratnasingham, S.; Hausmann, A.; Huemer, P.; Dincă, V.; van Nieukerken, E.J.; Lopez-Vaamonde, C.; et al. Species-level para- and polyphyly in DNA barcode gene trees: Strong operational bias in European Lepidoptera. Syst Biol. 2016, 65, 1024–1040. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Vershinina, A.O.; Lukhtanov, V.A. Geographical distribution of the cryptic species Agrodiaetus alcestis alcestis, A. alcestis karacetinae and A. demavendi (Lepidoptera, Lycaenidae) revealed by cytogenetic analysis. Comp. Cytogen. 2010, 4, 1–11. [Google Scholar] [CrossRef]
- Lukhtanov, V.A.; Gagarina, A.V. Molecular phylogeny and taxonomy of the butterfly subtribe Scolitantidina with special focus on the genera Pseudophilotes, Glaucopsyche and Iolana (Lepidoptera, Lycaenidae). Insects 2022, 13, 1110. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Gorbunov, P.J. The butterflies of Russia: Classification, genitalia, keys for identification (Lepidoptera: Hesperioidea and Pappilionoidea). Thesis: Ekaterinburg, Russia, 2001, 320 pp.
- Christoph, H. Weiterer Beitrag zum Verzeichnisse der in Nord-Persien einheimischen Schmetterlinge. Horae Soc. Ent. Ross. 1873, 10, 3–55. [Google Scholar]
- Staudinger, O. Macrolepidoptera. In Catalog der Lepidopteren des palaearctischen Faunengebites; Staudnger, O., Rebel, H., Eds.; Friedländer: Berlin, Germany, 1901; pp. 1–411. [Google Scholar]
- Horn, W.; Kahle, I.; Friese, G.; Gaedike, R. Collectiones entomologicae. Ein Kompendium über den Verbleib entomologischer Sammlungen der Welt bis 1960. Akademie der Landwirtschaftswissenschaften der Deutschen Demokratischen Republik: Berlin, 1990. Teil 1: A bis K, pp. 1−220. Teil 2: L bis Z, pp. 221−573.
- Verity, R. Rhopalocera palaearctica: Iconographie et description des papilions diurnes de la region paléarctique. Papilionidae et Pieridae. Landi Florence, Italy, 1905-1911. 368p.
- Ackery, P.R. A list of the type-specimens of Parnassius (Lepidoptera: Papilionidae) in the British Museum (Natural History). Bull. Br. Mus. Nat. 1973, 29, 3–35. [Google Scholar]
- Tshikolovets, V.; Naderi, A.; Eckweiler, W. The butterflies of Iran and Iraq. Series: Butterflies of Palaearctic Asia, Volume: 10; Tshikolovets Publications: Pardubice, Czech Republic, 2014; 366p. [Google Scholar]
- International Commission on Zoological Nomenclature. International Code of Zoological Nomenclature; The International Trust for Zoological Nomenclature: London, UK, 1999; Available online: https://www.iczn.org/the-code/the-code-online/ (accessed on 15 November 2023).
- Nekrutenko, Y.P. The butterflies of the Caucasus. The Guide. Paplionidae, Pieridae, Satyridae, Danaidae. Naukova Dumka: Kiev, Ukraine, 1990; pp. 1–216. (in Russian).
- Grum-Grshimailo, G.E. Le Pamir et sa faune lépidoptérologiae. In Mémoires sur les Lépidoptères; Rimonoff, N.M., Ed.; St.-Pétersbourg Imprimerie de M.M. Stassuléwitch: St.-Petersburg, 1890; Volume 4, 575p. [Google Scholar]
- Eversmann, E. Quedam Lepidopterorum species novae, in montibus Uralensibus et Altaicis habitantes, nunc descriptae et depictae. Bull. Soc. Nat. Moscou 1843, 16, 535–555, + pls. vii–x. [Google Scholar]
- Lederer, J. Lepidopterologisches aus Sibirien. Verh. Zool.-Bot. Ver. Wien 1853, 3, 351–386. [Google Scholar]
- Hemming, F. Revisional notes on some species of Rhopalocera (Lepidoptera). Stylops 1934, 3, 193–200. [Google Scholar]
- Lukhtanov, V.A.; Pelham, J.P.; Cotton, A.M.; Calhoun, J.V. Case 3767 – Papilio phoebus Fabricius, 1793 (currently Parnassius phoebus; Insecta, Lepidoptera): Proposed conservation of prevailing usage of the specific name and that of Doritis ariadne Lederer, 1853 (currently Parnassius ariadne) by the designation of a neotype. Bull. zool. nomencl. 2019, 78, 14–22. [Google Scholar] [CrossRef]
- Elwes, H. On the Lepidoptera of the Altai mountains. Trans. ent. Soc. Lond. 1899, 47, 295–367. [Google Scholar] [CrossRef]
- Yakovlev, R.V. A new subspecies of Parnassius ariadne (Lederer, 1853) from the South-Eastern part of Russian Altai (Lepidoptera, Papilionidae). Atalanta 2009, 40, 201. [Google Scholar]
- Ritter, S.; Michalski, S.G.; Settele, J.; Wiemers, M.; Fric, Z.F.; Sielezniew, M.; Šašić, M.; Rozier, Y.; Durka, W. Wolbachia infections mimic cryptic speciation in two parasitic butterfly species, Phengaris teleius and P. nausithous (Lepidoptera: Lycaenidae). PLoS ONE 2013, 8, e78107. [Google Scholar] [CrossRef]
- Sucháčková Bartoňová, A.; Konvička, M.; Marešová, J.; Wiemers, M.; Ignatev, N.; Wahlberg, N.; Schmitt, T.; Faltýnek Fric, Z. Wolbachia affects mitochondrial population structure in two systems of closely related Palaearctic blue butterflies. Sci. Rep. 2021, 11, 3019. [Google Scholar] [CrossRef] [PubMed]
- Dapporto, L. Speciation in Mediterranean refugia and post-glacial expansion of Zerynthia polyxena (Lepidoptera, Papilionidae). J. Zool. Syst. Evol. Res. 2010, 48, 229–237. [Google Scholar]
- Kryzhanovsky, O.L. Composition and Distribution of Insect Faunas of the World; KMK Press: Moscow, Russia, 2002; 237p, [in Russian]. [Google Scholar]
- Kolesnichenko, K.A.; Kotlobay, A.A. Review of the fritillary species systematically close to Melitaea lutko Evans, 1932 (Lepidoptera: Nymphalidae) with analysis of their geographic distribution and interrelations with host plants. Eur. J. Taxon. 2022, 830, 1–60. [Google Scholar] [CrossRef]
- Brower, A.V.Z. Rapid morphological radiation and convergence among races of the butterfly Heliconius erato inferred from patterns of mitochondrial DNA evolution. Proc. Natl. Acad. Sci. USA 1994, 91, 6491–6495. [Google Scholar] [CrossRef] [PubMed]
- Farrell, B.D. Evolutionary assembly of the milkweed fauna: Cytochrome oxidase I and the age of Tetraopes beetles. Mol. Phylogenet. Evol. 2001, 18, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Quek, S.P.; Stuart, J.D.; Itino, T.; Pierce, N.E. Codiversification in an ant –plant mutualism: Stem texture and the evolution of host use in Crematogaster (Formicidae: Myrmicinae) inhabitants of Macaranga (Euphorbiaceae). Evolution 2004, 58, 554–570. [Google Scholar] [CrossRef] [PubMed]
- Young, R.G.; Abbott, C.L.; Therriault, T.W.; Adamowicz, S.J. Barcode-based species delimitation in the marine realm: A test using Hexanauplia (Multicrustacea: Thecostraca and Copepoda). Genome 2017, 60, 169–182. [Google Scholar] [CrossRef]
- Yakovlev, R.V. New information about Parnassius stubbendorfii (Ménétriés, 1948) and Parnassius clarius (Eversman, 1843) (Lepidoptera, Papilionidae) from South-West and Central Altai. Entomol. News Russ. 1998, 1, 20–25. [Google Scholar]
- Lukhtanov, V.A.; Kandul, N.P.; Plotkin, J.B.; Dantchenko, A.V.; Haig, D.; Pierce, N.E. Reinforcement of pre-zygotic isolation and karyotype evolution in Agrodiaetus butterflies. Nature 2005, 436, 385–389. [Google Scholar] [CrossRef]


| BOLD ID | GenBank ID | Haplotype | Identification | Country | Region |
| LOWAM001-08 | OR822545 | gig1 | mnemosyne | Kyrgyzstan | Alai Mts |
| LOWAM002-08 | OR822537 | gig1 | mnemosyne | Kyrgyzstan | Alai Mts |
| LOWAM003-08 | OR822507 | gig1 | mnemosyne | Kyrgyzstan | Alai Mts |
| LOWAM004-08 | OR822401 | gig2 | mnemosyne | Uzbekistan | Gissar Mts |
| LOWAM005-08 | OR822544 | gig2 | mnemosyne | Uzbekistan | Gissar Mts |
| LOWAM006-08 | OR822581 | gig2 | mnemosyne | Uzbekistan | Gissar Mts |
| LOWAM007-08 | OR822585 | ori1 | mnemosyne | Kyrgyzstan | Moldatoo Mts |
| LOWAM008-08 | OR822475 | ori1 | mnemosyne | Kyrgyzstan | Moldatoo Mts |
| LOWAM009-08 | OR822518 | ori2 | mnemosyne | Kyrgyzstan | Moldatoo Mts |
| LOWAM010-08 | OR822548 | gig2 | mnemosyne | Tajikistan | Turkestanski Mts |
| LOWAM011-08 | OR822588 | gig2 | mnemosyne | Tajikistan | Turkestanski Mts |
| LOWAM012-08 | OR822423 | gig2 | mnemosyne | Tajikistan | Turkestanski Mts |
| LOWAM013-08 | OR822478 | mne5 | mnemosyne | Turkey | Ovitdagi Gecidi |
| LOWAM014-08 | OR822546 | mne6 | mnemosyne | Turkey | Ovitdagi Gecidi |
| LOWAM015-08 | OR822577 | mne3 | mnemosyne | Russia | Don River |
| LOWAM016-08 | OR822394 | mne3 | mnemosyne | Russia | Don River |
| LOWAM017-08 | OR822413 | gig1 | mnemosyne | Kyrgyzstan | Alai Mts |
| LOWAM018-08 | OR822525 | gig6 | mnemosyne | Kyrgyzstan | Alai Mts |
| LOWAM019-08 | OR822524 | gig4 | mnemosyne | Kyrgyzstan | Alai Mts |
| LOWAM020-08 | OR822403 | mne3 | mnemosyne | Russia | Saratovskaya Oblast |
| LOWAM021-08 | OR822586 | mne2 | mnemosyne | Russia | Saratovskaya Oblast |
| LOWAM022-08 | OR822563 | mne3 | mnemosyne | Russia | Saratovskaya Oblast |
| LOWAM023-08 | OR822554 | mne4 | mnemosyne | Russia | Saratovskaya Oblast |
| LOWAM024-08 | OR822454 | mne3 | mnemosyne | Russia | Saratovskaya Oblast |
| LOWAM025-08 | OR822543 | mne3 | mnemosyne | Russia | Saratovskaya Oblast |
| LOWAM026-08 | OR822532 | gig2 | mnemosyne | Uzbekistan | W Gissar Mts |
| LOWAM027-08 | OR822515 | ori3 | mnemosyne | Kazakhstan | Karatau Mts |
| LOWAM028-08 | OR822520 | ori3 | mnemosyne | Kazakhstan | Karatau Mts |
| LOWAM029-08 | OR822502 | gig2 | mnemosyne | Tajikistan | Taribak |
| LOWAM030-08 | OR822471 | gig2 | mnemosyne | Tajikistan | Taribak |
| LOWAM031-08 | OR822490 | gig2 | mnemosyne | Tajikistan | Taribak |
| LOWAM032-08 | OR822523 | gig2 | mnemosyne | Uzbekistan | Nuratau Mts |
| LOWAM033-08 | OR822421 | gig2 | mnemosyne | Uzbekistan | Nuratau Mts |
| LOWAM034-08 | OR822419 | gig2 | mnemosyne | Uzbekistan | Nuratau Mts |
| LOWAM036-08 | OR822448 | mne2 | mnemosyne | Russia | Pskovskaya Oblast |
| LOWAM037-08 | OR822459 | mne4 | mnemosyne | Russia | Pskovskaya Oblast |
| LOWAM038-08 | OR822452 | ori4 | mnemosyne | Kyrgyzstan | Kirgizsky Khrebet |
| LOWAM039-08 | OR822428 | ori5 | mnemosyne | Kyrgyzstan | Kirgizsky Khrebet |
| LOWAM040-08 | OR822506 | ori4 | mnemosyne | Kyrgyzstan | Kirgizsky Khrebet |
| LOWAM041-08 | OR822406 | ori1 | mnemosyne | Kyrgyzstan | Alabel Pass |
| LOWAM042-08 | OR822465 | mne3 | mnemosyne | Russia | Belgorodskaya oblast |
| LOWAM043-08 | OR822426 | mne3 | mnemosyne | Russia | Belgorodskaya oblast |
| LOWAM044-08 | OR822503 | mne1 | mnemosyne | Russia | Belgorodskaya oblast |
| LOWAM045-08 | OR822481 | gig7 | mnemosyne | Tajikistan | Turkestanski Mts |
| LOWAM046-08 | OR822493 | gig7 | mnemosyne | Tajikistan | Turkestanski Mts |
| LOWAM047-08 | OR822538 | gig7 | mnemosyne | Tajikistan | Turkestanski Mts |
| LOWAM048-08 | OR822444 | gig2 | mnemosyne | Tajikistan | Turkestanski Mts |
| LOWAM049-08 | OR822411 | gig2 | mnemosyne | Tajikistan | Turkestanski Mts |
| LOWAM050-08 | OR822408 | gig2 | mnemosyne | Tajikistan | Turkestanski Mts |
| LOWAM051-08 | OR822590 | gig8 | mnemosyne | Tajikistan | Revad |
| LOWAM052-08 | OR822477 | gig2 | mnemosyne | Tajikistan | |
| LOWAM055-08 | OR822420 | gig8 | mnemosyne | Tajikistan | Gissar Mts |
| LOWAM056-08 | OR822404 | gig2 | mnemosyne | Tajikistan | Gissar Mts |
| LOWAM057-08 | OR822480 | gig2 | mnemosyne | Tajikistan | Gissar Mts |
| LOWAM058-08 | OR822508 | ori1 | mnemosyne | Kazakhstan | Karzhantau Mts |
| LOWAM059-08 | OR822460 | ori1 | mnemosyne | Kazakhstan | Karzhantau Mts |
| LOWAM060-08 | OR822418 | ori1 | mnemosyne | Kazakhstan | Karzhantau Mts |
| LOWAM061-08 | OR822425 | ori1 | mnemosyne | Kazakhstan | Saryaigyr |
| LOWAM062-08 | OR822587 | gig2 | mnemosyne | Tajikistan | Gissar Mts (West) |
| LOWAM063-08 | OR822469 | gig8 | mnemosyne | Tajikistan | Gissar Mts (West) |
| LOWAM064-08 | OR822447 | ori9 | mnemosyne | Kazakhstan | Kirgizsky Khrebet |
| LOWAM065-08 | OR822517 | ori9 | mnemosyne | Kazakhstan | Kirgizsky Khrebet |
| LOWAM066-08 | OR822552 | ori9 | mnemosyne | Kazakhstan | Kirgizsky Khrebet |
| LOWAM067-08 | OR822415 | ori6 | mnemosyne | Kazakhstan | Kirgizsky Khrebet |
| LOWAM069-08 | OR822551 | mne7 | mnemosyne | Russia | W Caucasus |
| LOWAM070-08 | OR822533 | mne5 | mnemosyne | Russia | W Caucasus |
| LOWAM072-08 | OR822571 | ori1 | mnemosyne | Kyrgyzstan | Chatkalsky Khrebet |
| LOWAM073-08 | OR822453 | ori7 | mnemosyne | Kyrgyzstan | Takhtalyk |
| LOWAM074-08 | OR822491 | ori5 | mnemosyne | Kyrgyzstan | Naryn-Too Mts |
| LOWAM075-08 | OR822570 | ori1 | mnemosyne | Kyrgyzstan | Naryn-Too Mts |
| LOWAM076-08 | OR822498 | ori5 | mnemosyne | Kyrgyzstan | Ak-Muz |
| LOWAM077-08 | OR822557 | ori2 | mnemosyne | Kyrgyzstan | Moldatoo Mts |
| LOWAM078-08 | OR822516 | ori5 | mnemosyne | Kyrgyzstan | Moldatoo Mts |
| LOWAM079-08 | OR822439 | ori9 | mnemosyne | Kyrgyzstan | Moldatoo Mts |
| LOWAM080-08 | OR822500 | ori5 | mnemosyne | Kyrgyzstan | Songkel Lake |
| LOWAM081-08 | OR822463 | ori5 | mnemosyne | Kyrgyzstan | Songkel Lake |
| LOWAM082-08 | OR822436 | ori5 | mnemosyne | Kyrgyzstan | Songkel Lake |
| LOWAM086-08 | OR822567 | nub1 | nubilosus | Turkmenistan | Kopetdagh |
| LOWAM087-08 | OR822476 | gig8 | mnemosyne | Uzbekistan | Gissar Mts (West) |
| LOWAM088-08 | OR822432 | gig2 | mnemosyne | Uzbekistan | Gissar Mts (West) |
| LOWAM089-08 | OR822505 | gig2 | mnemosyne | Uzbekistan | Gissar Mts (West) |
| LOWAM090-08 | OR822528 | ori1 | mnemosyne | Kyrgyzstan | Chychkan |
| LOWAM091-08 | OR822501 | ori1 | mnemosyne | Kyrgyzstan | Chychkan |
| LOWAM092-08 | OR822510 | ori1 | mnemosyne | Kyrgyzstan | Chychkan |
| LOWAM093-08 | OR822565 | ori5 | mnemosyne | Kyrgyzstan | between Alabel and Tjuz-Ashu passes |
| LOWAM094-08 | OR822391 | ori5 | mnemosyne | Kyrgyzstan | Kirgizsky Khrebet |
| LOWAM095-08 | OR822527 | ori5 | mnemosyne | Kyrgyzstan | Kirgizsky Khrebet |
| LOWAM096-08 | OR822499 | gig9 | mnemosyne | Tajikistan | Gissar Mts (SW) |
| LOWAM097-08 | OR822574 | gig9 | mnemosyne | Tajikistan | Gissar Mts (SW) |
| LOWAM098-08 | OR822399 | gig9 | mnemosyne | Tajikistan | Gissar Mts (SW) |
| LOWAM099-08 | OR822461 | ori8 | mnemosyne | Uzbekistan | Kuraminsky Khrebet |
| LOWAM100-08 | OR822560 | ori5 | mnemosyne | Kyrgyzstan | Ferganski Khrebet |
| LOWAM101-08 | OR822450 | ori5 | mnemosyne | Kyrgyzstan | Ferganski Khrebet |
| LOWAM102-08 | OR822579 | ori5 | mnemosyne | Kyrgyzstan | Ferganski Khrebet |
| LOWAM103-08 | OR822550 | ori1 | mnemosyne | Kazakhstan | Turaigyr |
| LOWAM104-08 | OR822529 | ori1 | mnemosyne | Kazakhstan | Turaigyr |
| LOWAM105-08 | OR822561 | ori1 | mnemosyne | Kazakhstan | Turaigyr |
| LOWAM106-08 | OR822485 | ori8 | mnemosyne | Uzbekistan | S of Tashkent |
| LOWAM107-08 | OR822564 | gig2 | mnemosyne | Uzbekistan | Seravshaski Khrebet |
| LOWAM111-08 | OR822431 | ori5 | mnemosyne | Kyrgyzstan | Naryn-Too Mts |
| LOWAM112-08 | OR822430 | ori5 | mnemosyne | Kyrgyzstan | Naryn-Too Mts |
| LOWAM115-08 | OR822592 | ado1 | mnemosyne | Azerbaijan | Nakhichevan |
| LOWAM116-08 | OR822566 | ado2 | mnemosyne | Iran | Elburs Mts |
| LOWAM117-08 | OR822397 | ado2 | mnemosyne | Iran | Elburs Mts |
| LOWAM118-08 | OR822396 | mne1 | mnemosyne | Russia | Belgorod Region |
| LOWAM119-08 | OR822526 | mne1 | mnemosyne | Russia | Belgorod Region |
| LOWAM120-08 | OR822486 | mne3 | mnemosyne | Russia | Belgorod Region |
| LOWAM121-08 | OR822416 | mne3 | mnemosyne | Russia | Saratovskaya Oblast |
| LOWAM122-08 | OR822542 | mne3 | mnemosyne | Russia | Saratovskaya Oblast |
| LOWAM123-08 | OR822553 | mne3 | mnemosyne | Russia | Saratovskaya Oblast |
| LOWAM124-08 | OR822479 | ori5 | mnemosyne | Kyrgyzstan | Ak-Muz |
| LOWAM125-08 | OR822445 | ori5 | mnemosyne | Kyrgyzstan | Ak-Muz |
| LOWAM126-08 | OR822539 | gig1 | mnemosyne | Kyrgyzstan | Alai Mts |
| LOWAM127-08 | OR822511 | gig1 | mnemosyne | Kyrgyzstan | Alai Mts |
| LOWAM128-08 | OR822578 | gig1 | mnemosyne | Kyrgyzstan | Alai Mts |
| LOWAM129-08 | OR822457 | gig8 | mnemosyne | Tajikistan | Turkestanski Mts |
| LOWAM130-08 | OR822575 | gig2 | mnemosyne | Tajikistan | Turkestanski Mts |
| LOWAM131-08 | OR822562 | gig2 | mnemosyne | Tajikistan | Turkestanski Mts |
| LOWAM132-08 | OR822496 | gig2 | mnemosyne | Tajikistan | Turkestanski Mts |
| LOWAM133-08 | OR822488 | gig2 | mnemosyne | Uzbekistan | Gissar Mts West |
| LOWAM134-08 | OR822559 | gig2 | mnemosyne | Uzbekistan | Gissar Mts West |
| LOWAM135-08 | OR822462 | gig9 | mnemosyne | Uzbekistan | Gissar Mts West |
| LOWAM136-08 | OR822451 | gig3 | mnemosyne | Uzbekistan | Gissar Mts |
| LOWAM137-08 | OR822449 | gig2 | mnemosyne | Uzbekistan | Gissar Mts |
| LOWAM138-08 | OR822556 | gig9 | mnemosyne | Uzbekistan | Gissar Mts |
| LOWAM139-08 | OR822434 | gig9 | mnemosyne | Tajikistan | Gissar Mts SW |
| LOWAM140-08 | OR822473 | gig2 | mnemosyne | Tajikistan | Gissar Mts |
| LOWAM141-08 | OR822521 | gig11 | mnemosyne | Tajikistan | Gissar Mts |
| LOWAM142-08 | OR822417 | ori2 | mnemosyne | Kyrgyzstan | Modatoo Mts |
| LOWAM143-08 | OR822402 | ori1 | mnemosyne | Kyrgyzstan | Modatoo Mts |
| LOWAM144-08 | OR822582 | ori1 | mnemosyne | Kyrgyzstan | Modatoo Mts |
| LOWAM145-08 | OR822458 | ori2 | mnemosyne | Kyrgyzstan | Moldatoo Mts |
| LOWAM146-08 | OR822536 | ori2 | mnemosyne | Kyrgyzstan | Moldatoo Mts |
| LOWAM147-08 | OR822519 | ori2 | mnemosyne | Kyrgyzstan | Moldatoo Mts |
| LOWAM148-08 | OR822456 | ori5 | mnemosyne | Kyrgyzstan | Songkel Lake |
| LOWAM149-08 | OR822464 | ori9 | mnemosyne | Kyrgyzstan | Songkel Lake |
| LOWAM150-08 | OR822422 | ori5 | mnemosyne | Kyrgyzstan | Songkel Lake |
| LOWAM151-08 | OR822433 | ori4 | mnemosyne | Kyrgyzstan | Kirgizsky Khrebet |
| LOWAM152-08 | OR822409 | ori1 | mnemosyne | Kyrgyzstan | Kirgizsky Khrebet |
| LOWAM153-08 | OR822443 | gig1 | mnemosyne | Kyrgyzstan | Alai Mts |
| LOWAM154-08 | OR822535 | gig1 | mnemosyne | Kyrgyzstan | Alai Mts |
| LOWAM155-08 | OR822541 | gig1 | mnemosyne | Kyrgyzstan | Alai Mts |
| LOWAM156-08 | OR822522 | ori1 | mnemosyne | Kyrgyzstan | Chychkan |
| LOWAM157-08 | OR822410 | ori1 | mnemosyne | Kyrgyzstan | Chychkan |
| LOWAM158-08 | OR822470 | ori1 | mnemosyne | Kyrgyzstan | Chychkan |
| LOWAM159-08 | OR822509 | ori5 | mnemosyne | Kazakhstan | Kirgizsky Khrebet |
| LOWAM160-08 | OR822455 | ori5 | mnemosyne | Kazakhstan | Kirgizsky Khrebet |
| LOWAM161-08 | OR822580 | ori5 | mnemosyne | Kazakhstan | Kirgizsky Khrebet |
| LOWAM162-08 | OR822555 | ori2 | mnemosyne | Kazakhstan | Karzhantau Mts |
| LOWAM163-08 | OR822483 | ori1 | mnemosyne | Kazakhstan | Karzhantau Mts |
| LOWAM164-08 | OR822393 | ori1 | mnemosyne | Kazakhstan | Karzhantau Mts |
| LOWAM165-08 | OR822395 | gig2 | mnemosyne | Tajikistan | Taribak |
| LOWAM166-08 | OR822583 | gig2 | mnemosyne | Tajikistan | Taribak |
| LOWAM167-08 | OR822474 | gig10 | mnemosyne | Tajikistan | Taribak |
| LOWAM168-08 | OR822487 | gig2 | mnemosyne | Uzbekistan | Nuratau Mts |
| LOWAM169-08 | OR822576 | gig2 | mnemosyne | Uzbekistan | Nuratau Mts |
| LOWAM170-08 | OR822497 | gig10 | mnemosyne | Uzbekistan | Nuratau Mts |
| LOWAM171-08 | OR822424 | gig2 | mnemosyne | Tajikistan | Turkestanski Mts |
| LOWAM172-08 | OR822429 | gig2 | mnemosyne | Tajikistan | Turkestanski Mts |
| LOWAM173-08 | OR822530 | gig10 | mnemosyne | Tajikistan | Turkestanski Mts |
| LOWAM196-09 | OR822494 | nub1 | nubilosus | Turkmenistan | West Kopet-Dagh |
| LOWAM197-09 | OR822435 | nub1 | nubilosus | Turkmenistan | West Kopet-Dagh |
| LOWAM198-09 | OR822540 | ado3 | mnemosyne | Turkey | Taurus (gory Tavr) |
| LOWAM199-09 | OR822513 | ado3 | mnemosyne | Turkey | Taurus (gory Tavr) |
| LOWAM200-09 | OR822584 | mne5 | mnemosyne | Georgia | Mestia |
| LOWAM201-09 | OR822531 | mne5 | mnemosyne | Georgia | Mestia |
| LOWAM202-09 | OR822467 | mne4 | mnemosyne | Russia | St. Petersburg region |
| LOWAM203-09 | OR822414 | mne4 | mnemosyne | Russia | St. Petersburg region |
| LOWAM205-09 | OR822512 | ori1 | mnemosyne | Kyrgyzstan | Kungey-Alatoo Mts |
| LOWAM206-09 | OR822441 | mne4 | mnemosyne | Russia | Vologodskaya oblast |
| LOWAM207-09 | OR822534 | gig10 | mnemosyne | Tajikistan | Obihingou |
| LOWAM208-09 | OR822504 | gig8 | mnemosyne | Tajikistan | Peter The Great Mts |
| LOWAM209-09 | OR822412 | gig2 | mnemosyne | Tajikistan | Gissar Mts |
| LOWAM210-09 | OR822440 | gig2 | mnemosyne | Tajikistan | Gissar Mts |
| LOWAM212-09 | OR822427 | gig2 | mnemosyne | Tajikistan | Gissar Mts |
| LOWAM213-09 | OR822495 | gig5 | mnemosyne | Tajikistan | Alai Mts |
| BPALB154-16 | OR822438 | ado4 | mnemosyne | Israel | Hermon |
| BPALB230-17 | OR822392 | gig2 | mnemosyne | Tajikistan | |
| BPALB245-17 | OR822591 | gig2 | mnemosyne | Tajikistan | |
| BPALB258-17 | OR822442 | gig1 | mnemosyne | Tajikistan | |
| BPALB369-17 | OR822468 | gig1 | mnemosyne | Tajikistan | |
| BPALB389-17 | OR822484 | gig1 | mnemosyne | Tajikistan | |
| BPAL2225-13 | OR822589 | ado4 | mnemosyne | Israel | N. Israel |
| BPAL2226-13 | OR822405 | ado4 | mnemosyne | Israel | N. Israel |
| BPAL2794-15 | OR822572 | mne8 | mnemosyne | Russia | Pskov |
| BPAL2795-15 | OR822568 | mne8 | mnemosyne | Russia | Pskov |
| BPAL3195-16 | OR822437 | ado4 | mnemosyne | Israel | Hermon |
| BPAL3196-16 | OR822489 | ado4 | mnemosyne | Israel | Hermon |
| BPAL3358-16 | OR822547 | ado4 | mnemosyne | Israel | Hermon Mt |
| BPAL2276-13 | OR822466 | nor1 | nordmanni | Georgia | Adzharia |
| BPAL2277-13 | OR822573 | nor1 | nordmanni | Georgia | Adzharia |
| BPAL2278-13 | OR822398 | nor1 | nordmanni | Georgia | Adzharia |
| LOWAM269-11 | OR822482 | ari2 | ariadne | Kazakhstan | West Altai |
| LOWAM273-11 | OR822514 | ari2 | ariadne | Kazakhstan | West Altai |
| LOWAM287-11 | OR822407 | ari1 | ariadne | Kazakhstan | Altai |
| LOWAM288-11 | OR822446 | ari1 | ariadne | Kazakhstan | Altai |
| LOWAM289-11 | OR822472 | ari1 | ariadne | Kazakhstan | Altai |
| LOWAM290-11 | OR822400 | ari1 | ariadne | Kazakhstan | Altai |
| LOWAM292-11 | OR822558 | ari4 | ariadne | Kazakhstan | Altai |
| LOWAM295-11 | OR822492 | ari3 | ariadne | Kazakhstan | Altai |
| LOWAM296-11 | OR822569 | ari3 | ariadne | Kazakhstan | Altai |
| LOWAM301-11 | OR822549 | ari2 | ariadne clarus | Kazakhstan | Saur Mts |
| MA225 | ari6 | ariadne erlik | Russia | N of Kosh-Agach |
| ori | gig | mne | ado | nub | tur | ari | stu | hoe | gla | clo | eve | nor | |
| orientalis | 0 | ||||||||||||
| gigantea | 1.22 | ||||||||||||
| mnemosyne | 0.92 | 0.92 | |||||||||||
| adolphi | 2.45 | 2.45 | 2.14 | ||||||||||
| nubilosus | 2.91 | 2.29 | 2.60 | 3.52 | |||||||||
| turatii | 3.24 | 2.93 | 2.78 | 3.70 | 2.78 | ||||||||
| ariadne | 3.37 | 3.67 | 3.36 | 3.06 | 3.52 | 3.55 | |||||||
| stubbendorfii | 4.74 | 4.43 | 4.74 | 5.35 | 4.28 | 4.17 | 5.05 | ||||||
| hoenei | 5.09 | 5.09 | 4.78 | 5.25 | 3.70 | 4.32 | 5.25 | 4.01 | |||||
| glacialis | 6.29 | 5.98 | 6.29 | 6.76 | 5.66 | 6.51 | 7.08 | 4.38 | 6.03 | ||||
| clodius | 3.52 | 3.52 | 3.82 | 3.82 | 3.98 | 3.86 | 3.82 | 3.81 | 4.78 | 5.94 | |||
| eversmanni | 3.98 | 3.67 | 3.67 | 3.98 | 3.52 | 3.86 | 3.98 | 3.65 | 4.46 | 5.48 | 1.98 | ||
| nordmanni | 5.58 | 5.89 | 5.58 | 6.36 | 4.81 | 5.43 | 5.43 | 5.12 | 5.74 | 7.18 | 4.34 | 4.65 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





