1. Introduction
Cancer is a very complex chain of diseases that starts slowly and spreads throughout the body, impairing growth regulation. It is a diseases with a global health challenges that claims one out of every six lives worldwide [
1]. In most organs and tissues, there is a usual maintenance of balance between cell renewal and cell death. The lifespan of various types of adult cells in the body varies; as old cells die, new cells are created through the proliferation and differentiation of various types of stem cells. The generation of new cells is normally regulated such that the number of any single type of cell remains constant. Cells that no longer respond to normal growth-control mechanisms do appear on occasion. These cells give rise to clones of cells that can grow to be quite large, resulting in a tumor, or neoplasm [
2]. A benign tumor cannot develop continuously and does not significantly invade the healthy tissue in nearby areas. Malignant tumors enlarge and spread more and more; this type of tumor represents what is essentially meant by the term "Cancer." Malignant cells show the following characteristics: cell cycle acceleration; genomic mutations; aggressive growth; increased cell mobility; chemotaxis; changes in the cellular surface; and lytic factor secretion [
3].
Cancer can develop almost everywhere in the human body, which contains trillions of cells. Human cells normally grow and divide (a process known as cell division) to generate new cells as the body requires them. Cells die as they become old or injured, and new cells replace them. This ordered process can sometimes break down, allowing abnormal or damaged cells to grow and reproduce when they should not. Tumors, which are masses of tissue, can form from these cells. Cancer cells are different from normal cells in numerous ways. For example, proliferate in the absence of growth signals. Normal cells only expand when such signals are received, disregard signals that typically instruct cells to stop growing or die (a process known as programmed cell death, or apoptosis), invade neighbouring areas, and spread to other parts of the body [
4].
Tumors are complex tissues made up of malignant cells that are surrounded by a diverse cellular micro-environment with which they interact. Single-cell sequencing allows for the molecular characterization of individual cells inside a tumor [
5]. However, the current level of study in single-cell biology for molecular characterization of overall tumor cell heterogeneity for each cell should be well-known for successful cancer treatment.
This review focuses on recent advances in molecular characterization of tumor cells and cancer immunotherapy, with an emphasis on the challenges, potential risks, and opportunities associated with their use. The review also outlines the key characteristics that set tumour cells apart from healthy cells.
2. Molecular and Cellular characteristics of Cancer cell
Cancer is now understood to be caused by mutations in genes that govern cell growth. Thus, genetic mutations are found in all cancers. These mutations change the amount or function of protein products that control cell growth, division, and DNA repair. Oncogenes and tumor suppressor genes are the two most common types of altered genes [
6,
7].
Sustained proliferative signalling, eluding growth inhibitors, preventing cell death, permitting replicative immortality, inducing angiogenesis, and activating invasion and metastasis are characteristics that set tumour cells apart from normal cells [
8].
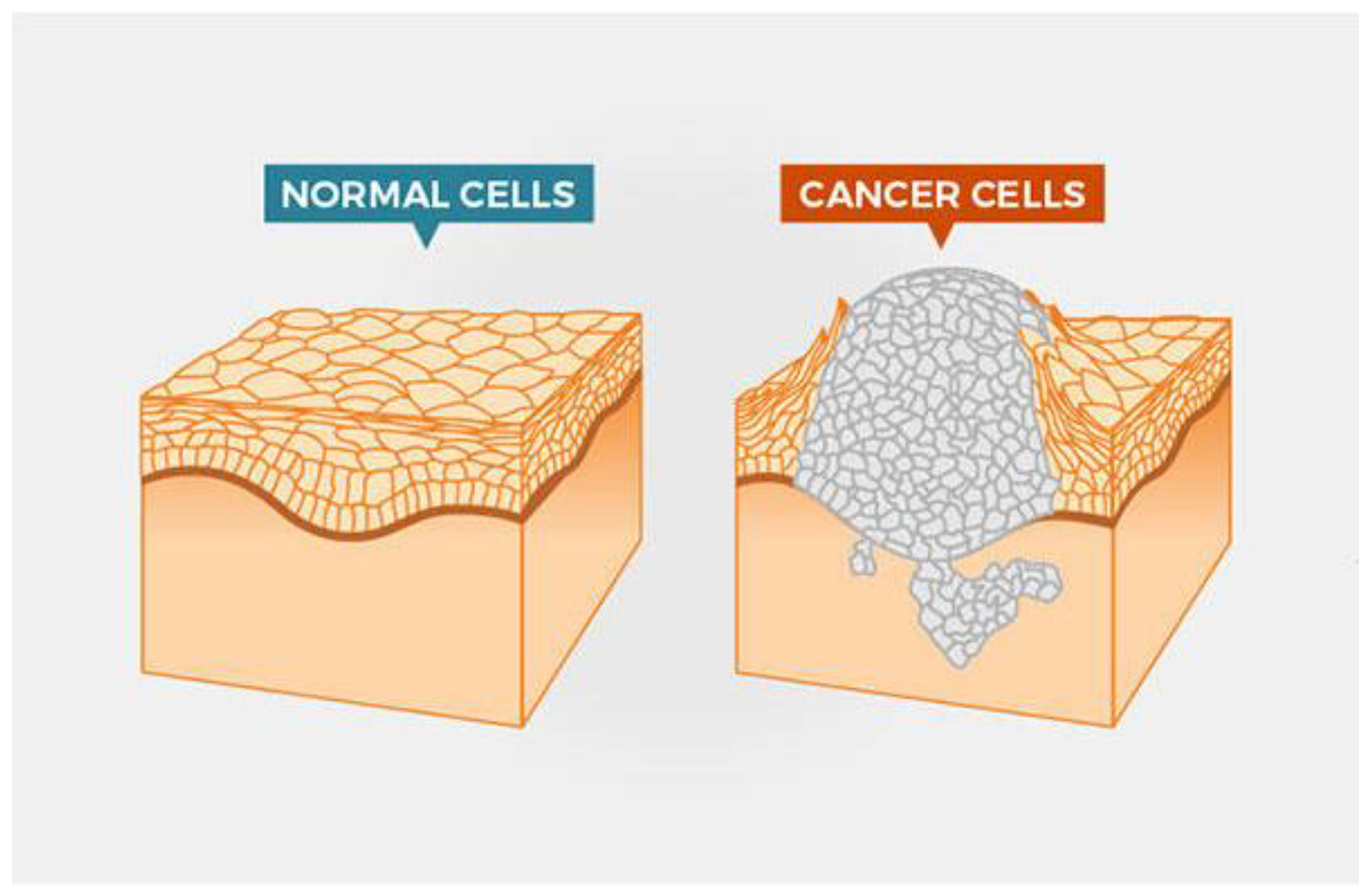
Figure 1.
Cancer cell vs normal cell [
4].
Figure 1.
Cancer cell vs normal cell [
4].
The molecular machinery in cells that drives the fundamental processes of proliferation (growth of the cell population by cell division), differentiation (specialisation of the cell into distinct tissue types), and apoptosis (planned cell death) is always necessary to determine the aetiology of malignancies. The genetic and epigenetic codes, two intrinsic programmes in cells, direct those. All those codes eventually change in cancer, whether it was an internal or external trigger that caused the illness in the first place. To be sure, the fact that a cancer cell proliferates itself is one of its basic traits. Stated differently, cancer is a self-perpetuating sickness that, once it manifests, becomes an inherited condition of cells [
9].
Discovering and characterising metabolic programmes essential to cancer has been made possible by notable advancements in analytical techniques that assist the study of cellular metabolism. The metabolite profile of a sample has been significantly increased by optimising Mass-Spectroscopy based methods; additionally, the measurement of metabolite engagement and compartmental exchange has been made easier by the advent of stable isotope tracing and intra- or extracellular metabolic-sensors [
10].
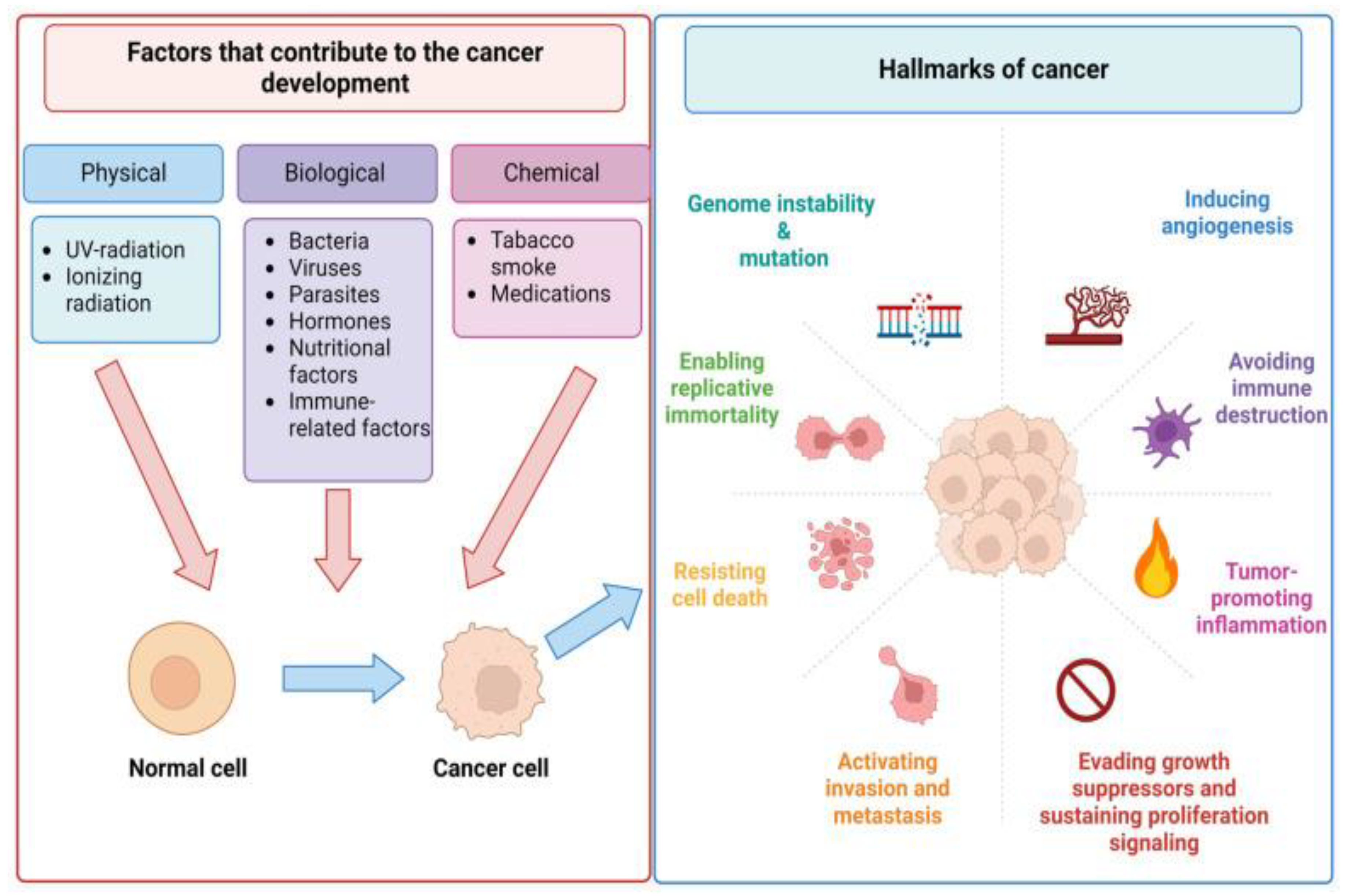
Figure 2.
The main causes of human cancers and hallmarks of cancer established [
11].
Figure 2.
The main causes of human cancers and hallmarks of cancer established [
11].
3. Malignant Transformation (tumorgenesis)
The process by which normal, metaplastic, or benign neoplastic tissue transforms into cancer is referred to as malignant transformation. The process is typically carried out in a succession of steps, with the damaged tissue gradually accumulating genetic changes that show a malignant phenotype. The genetic stages are slowly becoming revealed [
12].
With the development of novel deep sequencing technologies, new avenues for investigating the underlying molecular events that lead to cancer have opened up. A large number of studies have looked into tumor mutations, such as the Cancer Genome Atlas's analysis of mRNA expression, micro RNA expression, and DNA copy number in a large number of Tumors [
13].
4. Tumor micro-environment complexity
Cancer cells and their surroundings microenvironment interact closely through molecular communication, which determines their biological properties and behaviour. The tumour microenvironment plays an important role in tumour maintenance and spread. As a result, understanding the interactions between the microenvironment and the tumour is critical for the investigation of novel promising therapeutics [
14].
Cancer is a complex and heterogeneous illness with a dynamic interplay between cancer cells and their surroundings. The extracellular matrix, fibroblasts, immune cells, pericytes, blood vessels, and cancer cells interact to form the tumour microenvironment [
15]. Tumor microenvironment is made up of a variety of cellular and non-cellular components, including endothelial cells, immune cells, adipocytes, stromal cells, fibroblasts, extracellular matrix, and blood vasculature, among many others. Tumour cells and its micro-environment components such as cytokines, chemokines, growth factors, immune cells, proteins, and enzymes interact and crosstalk with one another [
16].
The hypoxic microenvironment has been linked to cancer as well as drug resistance. Understanding the processes involved in hypoxia regulation may lead to the identification of new therapeutic targets. Exosomes have been identified as valuable diagnostic and therapeutic tools in this area, disclosing tumor-derived secretomes and delivering medicines to tumour cells [
17]
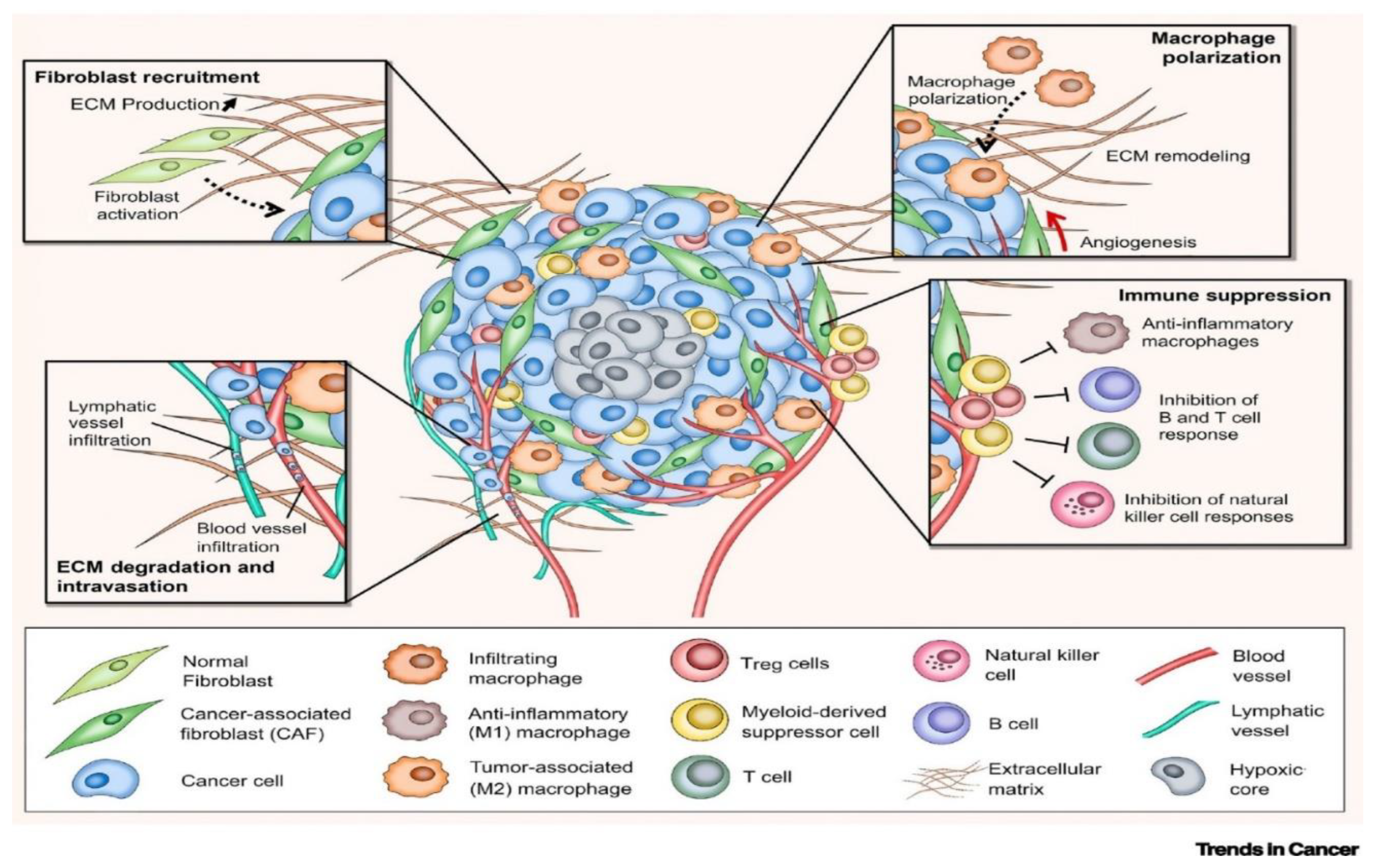
Figure 3.
The Tumor Microenvironment (TME) [
18].
Figure 3.
The Tumor Microenvironment (TME) [
18].
In addition to a cancer cell that has undergone malignant transformation, tumours also contain a variety of other cell types, such as immune system cells, fibroblasts, and endothelial cells. The extracellular matrix (ECM) of the tumour is made up of all of these cells plus cancer cells. The term "tumour stroma" refers to the ECM and non-cancerous cells within the tumour [
19]. Significant progress has been made in discovering the molecular processes that allow cancer cells to manipulate the immunological component of the microenvironment in order to promote tumour growth and dissemination [
20].
5. Tumor angiogenesis
Angiogenesis, the process of creating new blood vessels from pre-existing ones, has three basic mechanisms: sprouting, intussusception, and looping. Because abnormalities in the angiogenic process might result in major clinical disorders, the mechanism of angiogenesis is strictly regulated by highly specific antigenic stimulators and inhibitors. Tumour angiogenesis is essential for the survival and growth of tumour cells and is crucial for their expansion, invasion, and metastasis [
21,
22].
Normal vasculature have an organised and hierarchical branching arrangement of arteries, veins, and capillaries. Endothelial cells in healthy arteries are supported by basal membrane and pericytes and are closely linked by stable cell-cell junctions. Tumour vessels differ from normal vessels in both morphology and functionality. Tumour vasculature exhibit an unorganised network lacking of hierarchical vessel division in response to chronic and unbalanced expression of angiogenic factors and inhibitors. Tumour blood vessels have decreased blood flow, endothelial cell sprouting, and abnormal endothelial cell basal membrane, all of which contribute to tissue hypoxia and tumour cell intravasation [
23].
Tumour vascularization is caused by multiple separate biological processes that not only differ by tumour type and anatomic location, but also occur concurrently within the same cancer tissue. These activities are controlled by a variety of secreted proteins and signalling pathways, and non-endothelial cells, such as progenitors or cancer stem cells, may participate. Controlling tumor-associated angiogenesis is a viable strategy for slowing cancer progression because angiogenesis is required for tumour growth and metastasis [
23,
24].
6. Cancer Invasion and Metastasis
Most cancer patients die as a result of cancer metastasis, which is the spread of cancer cells to tissues and organs outside of the area of the original tumour and the development of new tumours. The procedure, known as the "metastatic cascade," consists of a series of steps that must be completed for the tumour cell to properly metastasis. As a multiplex disease, cancer is complicated in part due of this process. Changes in cell-cell adhesion and cell-matrix adhesion play a crucial role in the metastatic cascade. The loss of cell adhesion, which causes the cell to become detached from the parent tumour, and the cell's subsequent capacity to develop a motile phenotype through modifications in cell-matrix interaction are therefore necessary for the metastatic cascade to occur [
25].
Advances in scientific and clinical research are focused more on metastatic disease, the primary cause of death for cancer patients. However, little is still known about the mechanisms and there are few effective ways to stop metastasis. It is, therefore, encouraging to see some of the significant advancements in this crucial field of cancer research. New opportunities have emerged for applying some of the observations to the diagnosis, prognosis, and therapy of metastatic disease due to our growing understanding of gene expression, cellular behaviour, and biological events in the spread routes of cancer cells [
26].
7. The cells of Immune System and Cancer cell
Cancer cells implement diverse mechanisms that imitate peripheral immune tolerance in order to avoid tumoricidal attack as the tumour progresses from neoplastic tissue to clinically identifiable tumours. Cytotoxic innate and adaptive immune cells can regulate the growth of tumours, however when they progress from neoplastic tissue to clinically identifiable tumours, cancer cells develop new defence mechanisms that imitate peripheral immunological tolerance to fight against tumor-curing attacks [
27].
Cancer vaccines based on tumour antigens may be able to induce more focused and effective anti-tumor immune responses with fewer toxicities and side effects. Designing a cancer vaccine is primarily determined by the choice of tumour antigens. Current tumour antigens and recent advancements in tumour antigen-based cancer vaccines, such as traditional antigen vaccines, neoantigen vaccines, shared antigen vaccines, and oral antigen vaccines, are summarised with the goal of serving as a fundamental building block for the further development of novel cancer vaccine-based therapies [
28].
Viral infection is thought to be a significant contributor to the global cancer burden, accounting for an estimated 15% of all human malignancies globally. Cancer in humans can be brought on by viruses with DNA or RNA, it has been demonstrated. Epstein-Barr virus, human papilloma virus, hepatitis B virus, and human herpes virus-8 are the four DNA viruses that can lead to the emergence of cancer in people. The two RNA viruses that cause cancer in humans are the hepatitis C and human T lymphotrophic virus type 1 viruses [
29].
Genes having a tissue-specific expression code for differentiation antigens. As a result, they are displayed by specific tumour types as well as the equivalent healthy tissue. The enhanced transcription or gene amplification that occurs in tumours causes some genes to be overexpressed. Promiscuous gene expression, which is the expression of tissue-specific self-antigens in the thymus, imposes T cell tolerance and guards against autoimmune disorders. Additionally, this antigen pool contains a number of tumor-associated antigens (TAA) that were previously believed to be immune system-separate [
30,
31].
Neoantigen that activate CD8 T cells to fight cancer can be produced by tumor-specific mutations. The identification of mutations and the prediction of neoantigens have been accomplished with success using next-generation sequencing and computational techniques. The identification of a new class of tumour antigens formed from somatic mutations, also known as neoantigens or tumor-specific antigens and which we have named mutation-derived tumour antigens (MTAs), has been made possible by recent improvements in genetic sequencing technologies [
32].
7.1. T- Lymphocytes, Antibodies, NK Cells and Macrophages cells and cancer cell reactions
T cells are adaptive immune system components that function as coordinators and effectors of immunity. Depending on the immunological milieu, T cells can develop functional and effector phenotypes whose activity has direct inflammatory or anti-inflammatory implications. Naive T cells will be primed in the draining lymph nodes during the early phases of tumour start, followed by their concurrent activation and migration to the TME, if sufficient immunogenic antigens are generated. They mount a protective effector immune response from there, eradicating immunogenic cancer cells [
27].
Recent developments in antibody engineering techniques have made it feasible to design various antibody-associated tumor-targeted delivery systems for chemoprevention, chemotherapy, and early cancer detection [
33]. Understanding the roles of natural killer (NK) cells in immunity to viruses and tumours has advanced significantly in recent years. Natural killer (NK) cells are cytotoxic innate lymphoid cells that release chemokines and cytokines that cause inflammation. They inhibit the growth of tumours and viral infections by lysing altered or infected cells [
34].
The ability of natural killer (NK) cells to eliminate cancer cells without purposeful immunisation or activation led to their initial discovery. They were then discovered to possess the ability to eliminate specific virus-infected cells as well as to target cells that do not exhibit major histocompatibility complex (MHC) class I antigens. The molecular processes governing NK activation and function have been revealed by the recent identification of novel NK receptors and their ligands. It has been discovered that NK cells can identify tumours and virus-infected cells thanks to a number of activating NK cell receptors and costimulatory molecules [
35]. Macrophages have the potential to kill tumour cells, mediate antibody-dependent cellular cytotoxicity and phagocytosis, elicit vascular damage and tumour necrosis, and activate innate or adaptive lymphoid cell-mediated mechanisms of tumour resistance [
36].
7.2. Immune Responses to Tumors
Due to its ability to identify aberrant antigens on the cell surface as "nonself," or foreign, the immune system is able to recognise and eliminate newly formed cancer cells. Since foreign substances typically pose a threat to the body, the immune system is designed to eliminate them. Collectively, the immune system can both support and inhibit tumour growth, which contributes to cancer. Against cancer, infectious agents, and external invaders, the immune system is a network of organs, cells, and signals [
9].
Cancer immune responses are intricate. Immuno-editing is the process by which immune system cells identify and reject cancerous cells, thereby halting the formation and progression of tumours. However, by causing oncogenic inflammation, immune responses can also encourage the proliferation, survival, and angiogenesis of tumour cells. Both spontaneous and virally generated cancer can be predisposed to by immunodeficiency, and established tumours frequently create immunosuppressive microenvironments that can impede the formation of effective antitumor immunity [
37].
Certain lymphocytes that are specific to an antigen proliferate as a result of a particular immunological response. Through the production and release of lymphokines, chemokines, and cytokines, antibodies and T-cell receptors are produced and up-regulated, leading to the development of immunity. When antigenic responses against carcinomas are triggered, the innate and acquired immune systems work together [
38]. Immunosuppressed and immunodeficient patients have a higher incidence of malignancies, which illustrates the role of the immune system in avoiding cancer. Tumours change to evade immune-mediated destruction as a result of the immune response's selective pressure [
39].
8. Cancer therapy
Cancer treatment has been a difficult and time-consuming process. Traditional treatment approaches, such as surgery, chemotherapy, and radiotherapy, have been used in the past, but significant advances in recent years have included stem cell therapy, targeted therapy, ablation therapy, nanoparticles, natural antioxidants, radionics, chemodynamic therapy, sonodynamic therapy, and ferroptosis-based therapy. Current oncology strategies are centred on the creation of cancer nanomedicines that are both safe and effective. Stem cell therapy has shown promise in regenerating and repairing diseased or damaged tissues by targeting both primary and metastatic cancer foci, while nanoparticles have provided new diagnostic and therapeutic possibilities [
1].
Cancer Immunotherapy has made steady progress towards improving human health during the past ten years. Chimeric antigen receptor (CAR) T-cell therapy, an emerging method, has the advantages of precise cancer cell elimination, a high incidence of symptom remission from cancer, quick tumour eradication, and long-lasting tumour immunity, opening a new avenue for tumour treatment. Due to target diversity, tumour heterogeneity, and the intricate microenvironment, CAR T-cell therapy for solid tumours still faces some difficulties [
40]. Understanding of the immune system's function in preventing cancers and the strategies used by cancer cells to evade immunosurveillance has advanced significantly during the last 20 years. Certain forms of leukaemia and lymphomas, as well as melanoma, lung cancer, bladder cancer, and other cancers, have all responded remarkably well to cancer immunotherapy treatment [
11].
Cancer immunotherapy has become a cutting-edge cancer treatment, despite the fact that recent immunotherapy trials have yielded less than ideal results and that only a tiny percentage of patients have experienced persistent responses. It has been demonstrated that tumour immune evasion and treatment failure are caused by the tumour microenvironment (TME). Tumor-associated macrophages (TAMs) are essential to the tumour microenvironment (TME). TAMs have emerged as interesting targets for cancer immunotherapy and are typically linked to poor prognosis and treatment resistance, including immunotherapies [
41].
A new approach to the treatment of cancer has been immunotherapy, which aims to up-regulate the immune system in order that it may better control carcinogenesis. Currently, several forms of immunotherapy that use natural biological substances to activate the immune system are being explored therapeutically. The various forms of immunotherapy fall into three main categories: monoclonal antibodies, immune response modifiers, and vaccines [
38].
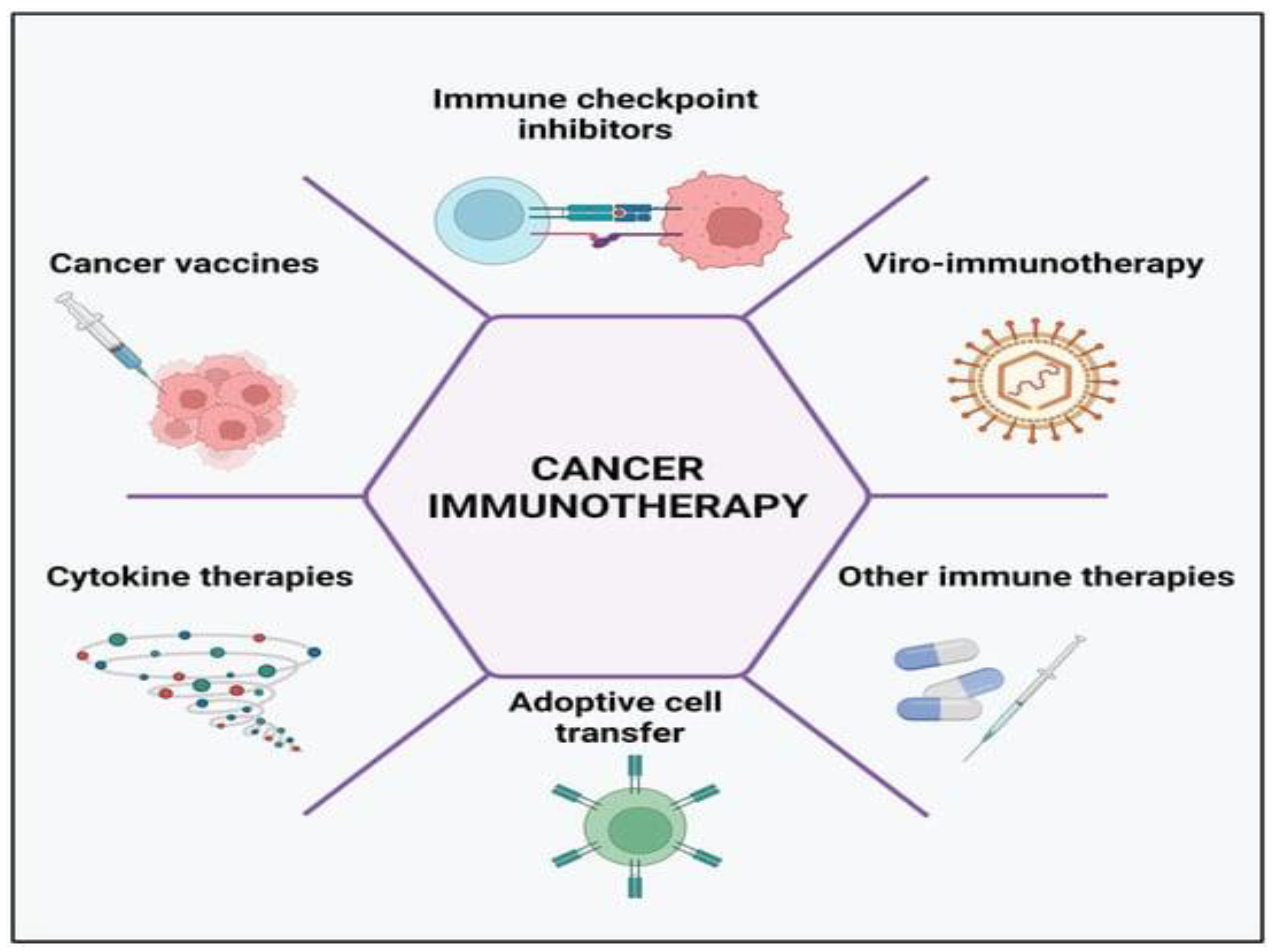
Cancer immunotherapy has become preferable among researchers in recent years compared to conventional therapeutic options, such as chemotherapy, surgery, and radiotherapy. The control of immunological checkpoints, oncolytic viral therapies, cancer vaccines, cytokine therapies, and adoptive cell transfer are among the five main categories into which scientists have lately divided the processes of cancer immune therapies. A number of stimulatory and inhibitory factors, when combined, control the immune response and prevent the excessive reaction that could result in autoimmune illness, which is the hallmark of the cancer-immunity cycle. The study of tumour immunology has been improved and treatment possibilities have been transformed by immunotherapy [
11].
Figure 4.
Cancer immunotherapy types include the use of immune-checkpoint inhibitors, cancer vaccines, cytokines, viruses, and adoptive cell transfer [
11].
Figure 4.
Cancer immunotherapy types include the use of immune-checkpoint inhibitors, cancer vaccines, cytokines, viruses, and adoptive cell transfer [
11].
9. Conclusion
The molecular and cellular underpinnings of cancer cells have been increasingly understood during the last few decades. Nonetheless, a great deal remains unknown regarding the ways in which tumour cells modify their surroundings and impact the organisation and makeup of cells.Numerous factors, including internal cell factors and the cell microenvironment, influence tumour heterogeneity. Effective and personalised tumour treatment is limited mostly by heterogeneity. Understanding the variability of tumours in multiple dimensions and being able to employ a range of detection techniques flexibly to capture tumour changes at a time when targeted therapy and immunotherapy are developing quickly can improve cancer diagnosis and treatment plans while also benefiting patients.
The process of stable clonal selection occurs and transforms heterogeneous tumors into a therapy-resistant, fast-progressing, and metastatic stage. Our current knowledge regarding molecular principles and cellular traits during these processes is rather limited. The molecular concepts and cellular characteristics involved in these processes are mostly unknown to us at this time.
Author Contributions
Study design & writing of the manuscript done by Abas Mahammed.
Funding
There is no any funding for this review.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Author confirm publication of this review.
Availability of data and materials
Not applicable.
Competing of Interest
The author declare no competing interests or conflict of interests.
References
- Debela DT, Muzazu SG, Heraro KD, Ndalama MT, Mesele BW, Haile DC, Kitui SK, Manyazewal T: New approaches and procedures for cancer treatment: Current perspectives. SAGE open medicine 2021, 9:20503121211034366. [CrossRef]
- Hutchison VPaCJ: Adult stem cell maintenance and tissue regeneration in the ageing context: the role for A-type lamins as intrinsic modulators of ageing in adult stem cells and their niches. Journal of AnatomyA 2008, 213(1). [CrossRef]
- Alecsandru Ioan Baba CC: Comparative Oncology. In: Comparative Oncology. edn. Bucharest (RO): The Publishing House of the Romanian Academy; 2007.
- What Is Cancer? Available online: https://www.cancer.gov/about-cancer/understanding/what-is-cancer.
- Dohmen J, Baranovskii A, Ronen J, Uyar B, Franke V, Akalin A: Identifying tumor cells at the single-cell level using machine learning. Genome Biol 2022, 23(1):123. Available online: https://doi.org/10.1186/s13059-022-02683-1.
- Gale RP: Cellular and Molecular Basis of Cancer. In: MSD Manual. vol. 2023. Imperial College London; 2022.
- HAUSMAN GMCRE: THE CELL: A Molecular Approach, 4 edn; 2007.
- Kushlinskiĭ NE, Nemtsova MV: [Molecular biological characteristics of cancer]. Vestn Ross Akad Med Nauk 2014(1-2):5-15. [CrossRef]
- The immune response to tumours. Available online: https://www.britannica.com/science/cancer-disease/Oncogenes.
- Kang YP, Ward NP, DeNicola GM: Recent advances in cancer metabolism: a technological perspective. Experimental & molecular medicine 2018, 50(4):1-16. [CrossRef]
- Kciuk M, Yahya EB, Mohamed Ibrahim Mohamed M, Rashid S, Iqbal MO, Kontek R, Abdulsamad MA, Allaq AA: Recent Advances in Molecular Mechanisms of Cancer Immunotherapy. Cancers 2023, 15(10):2721. [CrossRef]
- Pütz SM, Vogiatzi F, Stiewe T, Sickmann A: Malignant transformation in a defined genetic background: proteome changes displayed by 2D-PAGE. Mol Cancer 2010, 9:254. [CrossRef]
- Network T: institution. participants are arranged by area of contribution and then by, sites d, bell d, berchucka, birrer m, chien j, cramer dw, dao f, dhir r, et al. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474:609-615.
- Simioni C, Conti I, Varano G, Brenna C, Costanzi E, Neri LM: The complexity of the tumor microenvironment and its role in acute lymphoblastic leukemia: Implications for therapies. Frontiers in Oncology 2021, 11:673506. [CrossRef]
- Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, Kolahian S, Javaheri T, Zare P: Tumor microenvironment complexity and therapeutic implications at a glance. Cell Communication and Signaling 2020, 18:1-19. [CrossRef]
- Prasad S, Saha P, Chatterjee B, Chaudhary AA, Lall R, Srivastava AK: Complexity of tumor microenvironment: therapeutic role of Curcumin and its metabolites. Nutrition and Cancer 2022, 75(1):1-13. [CrossRef]
- Dzobo K, Senthebane DA, Dandara C: The tumor microenvironment in tumorigenesis and therapy resistance revisited. Cancers 2023, 15(2):376. [CrossRef]
- Rodrigues J, Heinrich MA, Teixeira LM, Prakash J: 3D in vitro model (R) evolution: unveiling tumor–stroma interactions. Trends in cancer 2021, 7(3):249-264. [CrossRef]
- Sund M, Kalluri R: Tumor stroma derived biomarkers in cancer. Cancer and Metastasis Reviews 2009, 28:177-183. [CrossRef]
- Poltavets V, Kochetkova M, Pitson SM, Samuel MS: The Role of the Extracellular Matrix and Its Molecular and Cellular Regulators in Cancer Cell Plasticity. Front Oncol 2018, 8:431. [CrossRef]
- Jiang X, Wang J, Deng X, Xiong F, Zhang S, Gong Z, Li X, Cao K, Deng H, He Y: The role of microenvironment in tumor angiogenesis. Journal of Experimental & Clinical Cancer Research 2020, 39(1):1-19. [CrossRef]
- Teleanu RI, Chircov C, Grumezescu AM, Teleanu DM: Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. Journal of clinical medicine 2019, 9(1):84. [CrossRef]
- Lugano R, Ramachandran M, Dimberg A: Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci 2020, 77(9):1745-1770. [CrossRef]
- Weis SM, Cheresh DA: Tumor angiogenesis: molecular pathways and therapeutic targets. Nature medicine 2011, 17(11):1359-1370.
- Tracey A. Martin LY, Andrew J. Sanders, Jane Lane, and Wen G. Jiang.: Cancer Invasion and Metastasis: Molecular and Cellular Perspective; 2013.
- Martin TA, Ye L, Sanders AJ, Lane J, Jiang WG: Cancer invasion and metastasis: molecular and cellular perspective. In: Madame Curie Bioscience Database [Internet]. edn.: Landes Bioscience; 2013.
- Gonzalez H, Hagerling C, Werb Z: Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes & development 2018, 32(19-20):1267-1284. [CrossRef]
- Shi W, Chen S, Chi F, Qiu Q, Zhong Y, Bian X, Zhang H, Xi J, Qian H: Advances in Tumor Antigen-Based Anticancer Immunotherapy: Recent Progress, Prevailing Challenges, and Future Perspective. Advanced Therapeutics 2023, 6(2):2200239. [CrossRef]
- Liao JB: Cancer issue: Viruses and human cancer. The Yale journal of biology and medicine 2006, 79(3-4):115.
- Cloosen S, Arnold J, Thio M, Bos GM, Kyewski B, Germeraad WT: Expression of tumor-associated differentiation antigens, MUC1 glycoforms and CEA, in human thymic epithelial cells: implications for self-tolerance and tumor therapy. Cancer research 2007, 67(8):3919-3926. [CrossRef]
- Vigneron N: Human tumor antigens and cancer immunotherapy. BioMed research international 2015, 2015. [CrossRef]
- Finnigan Jr JP, Rubinsteyn A, Hammerbacher J, Bhardwaj N: Mutation-derived tumor antigens: novel targets in cancer immunotherapy. Oncology 2015, 29(12):970-970.
- Attarwala H: Role of antibodies in cancer targeting. Journal of natural science, biology, and medicine 2010, 1(1):53. [CrossRef]
- Wolf NK, Kissiov DU, Raulet DH: Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nature Reviews Immunology 2023, 23(2):90-105. [CrossRef]
- Brittenden J, Heys SD, Ross J, Eremin O: Natural killer cells and cancer. Cancer: Interdisciplinary International Journal of the American Cancer Society 1996, 77(7):1226-1243. [CrossRef]
- Mantovani A, Allavena P, Marchesi F, Garlanda C: Macrophages as tools and targets in cancer therapy. Nature Reviews Drug Discovery 2022, 21(11):799-820. [CrossRef]
- Dougan M, Dranoff G: The immune response to tumors. Current Protocols in Immunology 2009, 85(1):20.11. 21-20.11. 24.
- Adam JK, Odhav B, Bhoola KD: Immune responses in cancer. Pharmacology & therapeutics 2003, 99(1):113-132. [CrossRef]
- Hastings KT, Rausch MP: Innate and adaptive immune responses to cancer. In: Fundamentals of Cancer Prevention. edn.: Springer; 2013: 81-121.
- Yan T, Zhu L, Chen J: Current advances and challenges in CAR T-Cell therapy for solid tumors: tumor-associated antigens and the tumor microenvironment. Experimental Hematology & Oncology 2023, 12(1):14. [CrossRef]
- Kumari N, Choi SH: Tumor-associated macrophages in cancer: recent advancements in cancer nanoimmunotherapies. Journal of Experimental & Clinical Cancer Research 2022, 41(1):68. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).