Submitted:
21 December 2023
Posted:
22 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
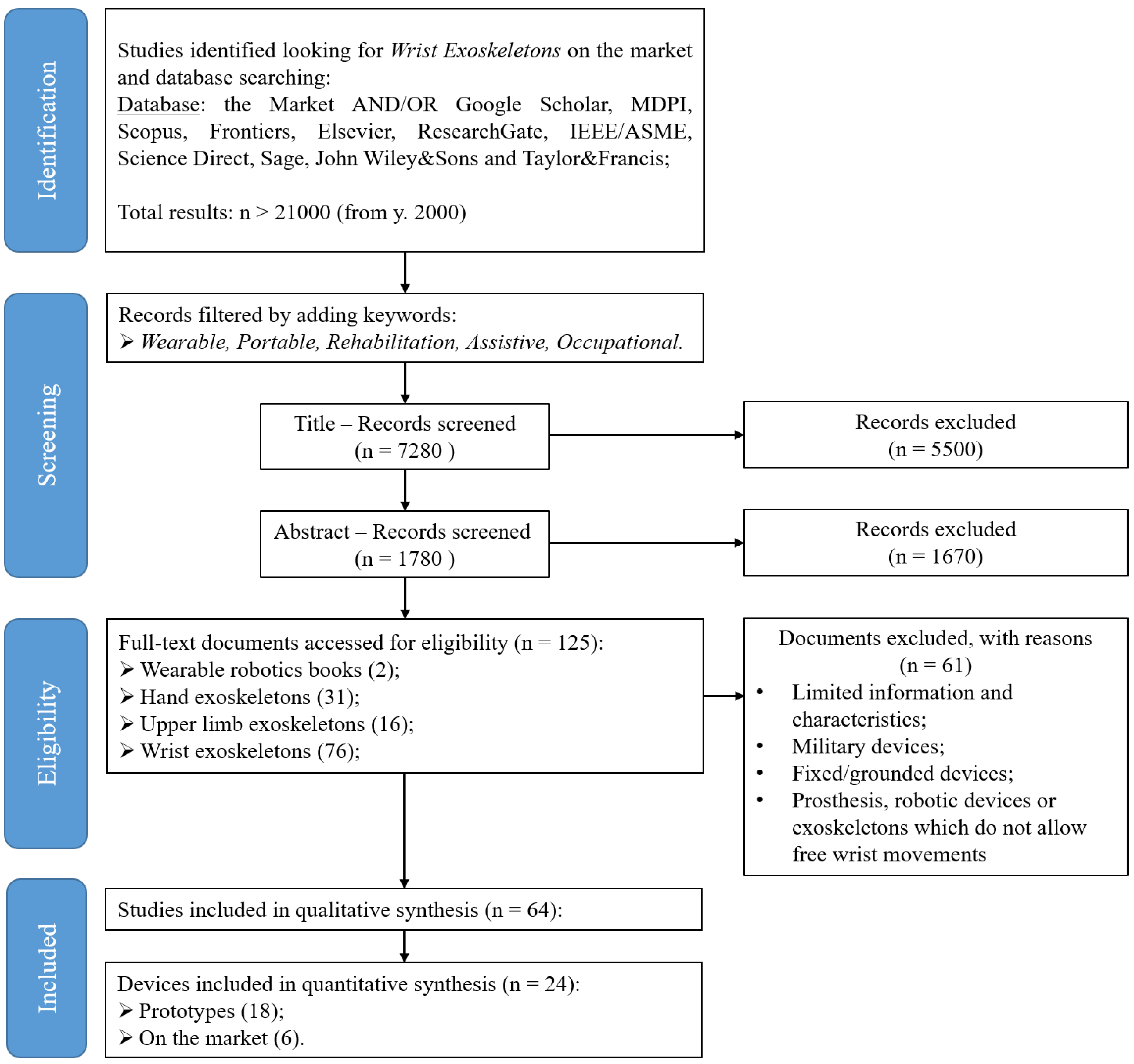
2. Materials and Methods
- Upper limb exoskeletons which include the wrist in their design;
- Devices able to relieve pain or mitigate fatigue by supporting at least one wrist movement;
- Devices intended for rehabilitation, assistance and occupational purposes;
- Portable devices;
- All studies must be accessible by the authors in English.
- Prosthesis or exoskeletons which do not allow free wrist movements;
- Military devices;
- Fixed/grounded devices;
- Studies in other languages or with insufficient information, which made the analysis unclear.
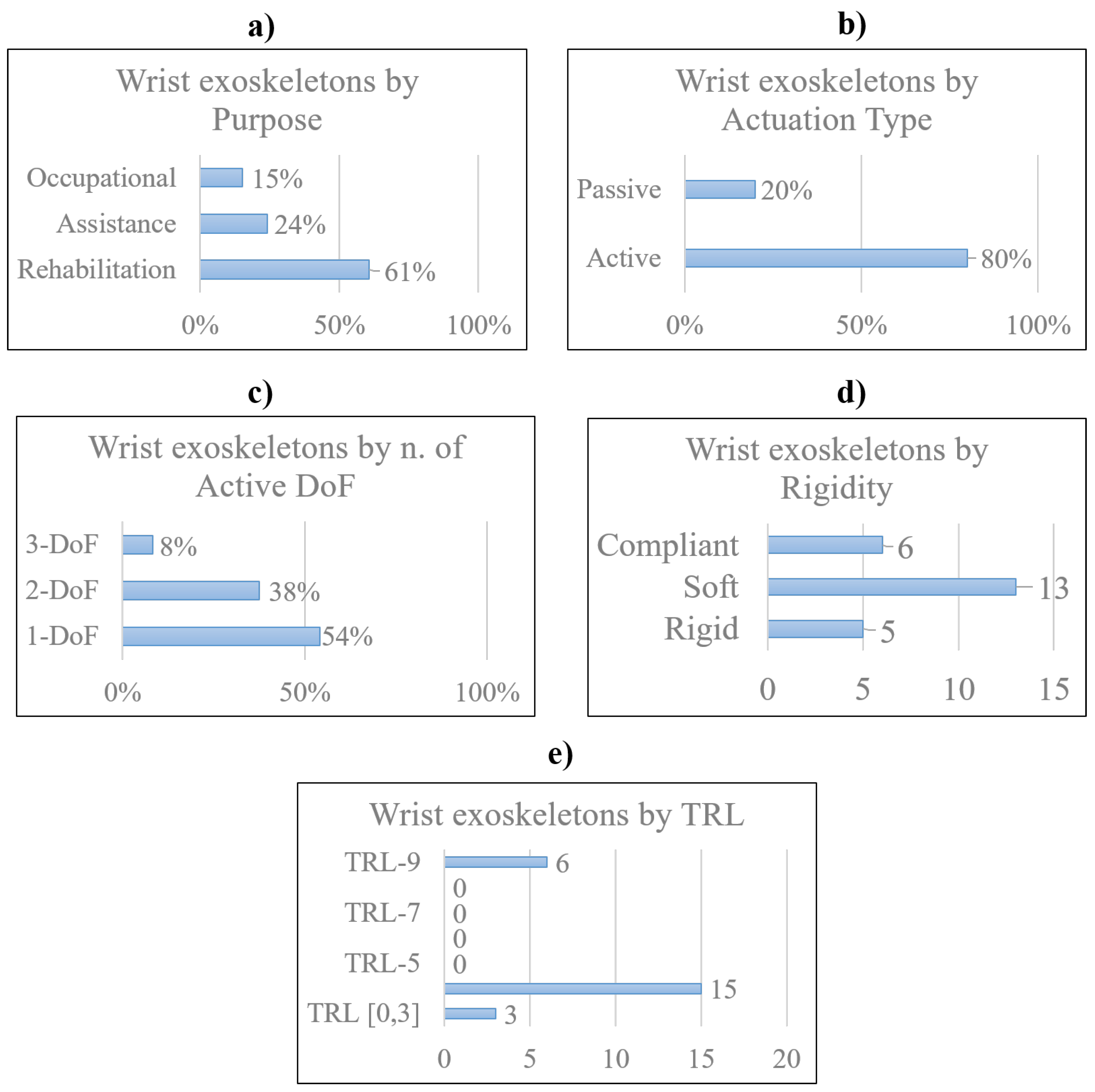
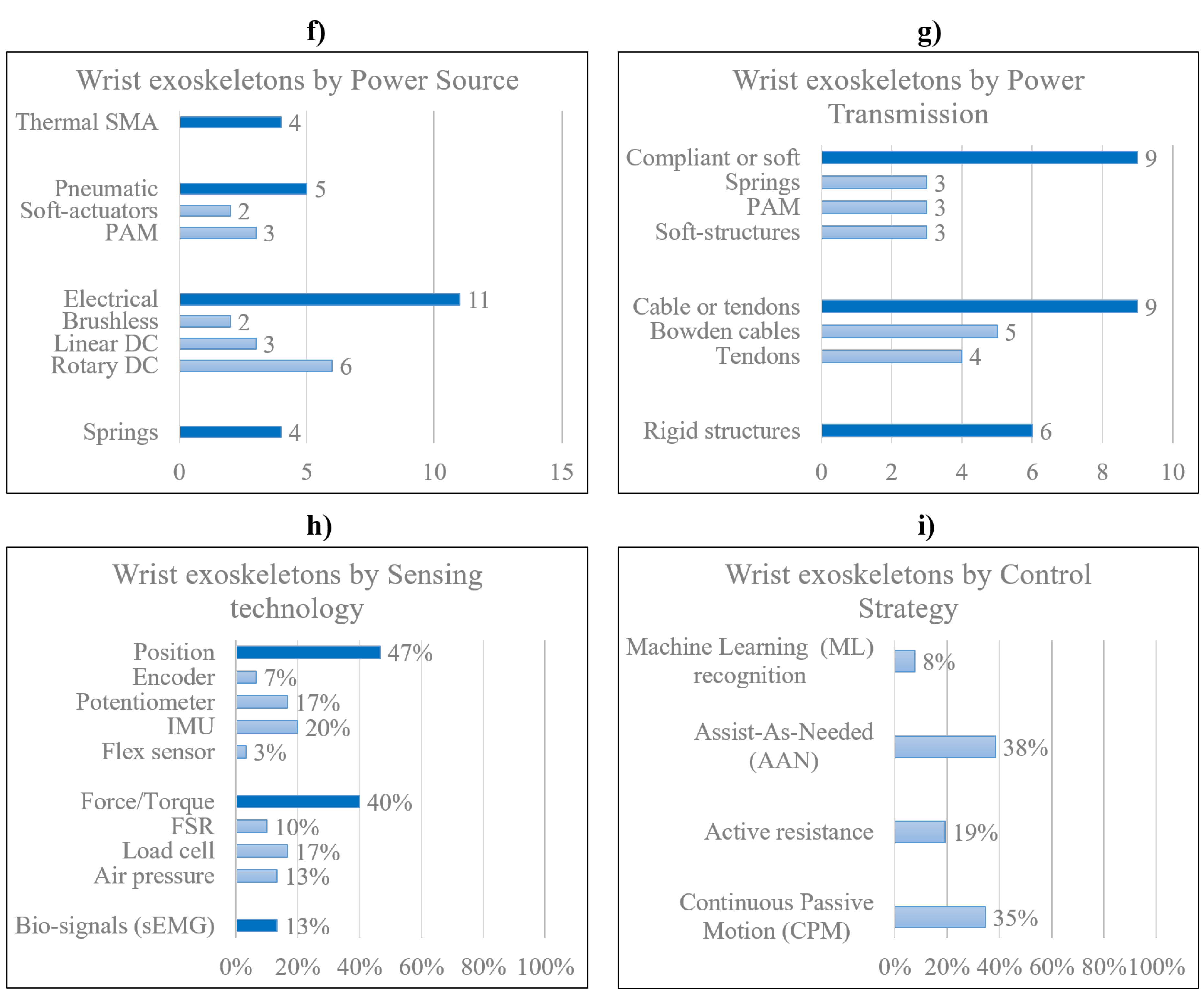
3. Research and Pre-commercial Devices

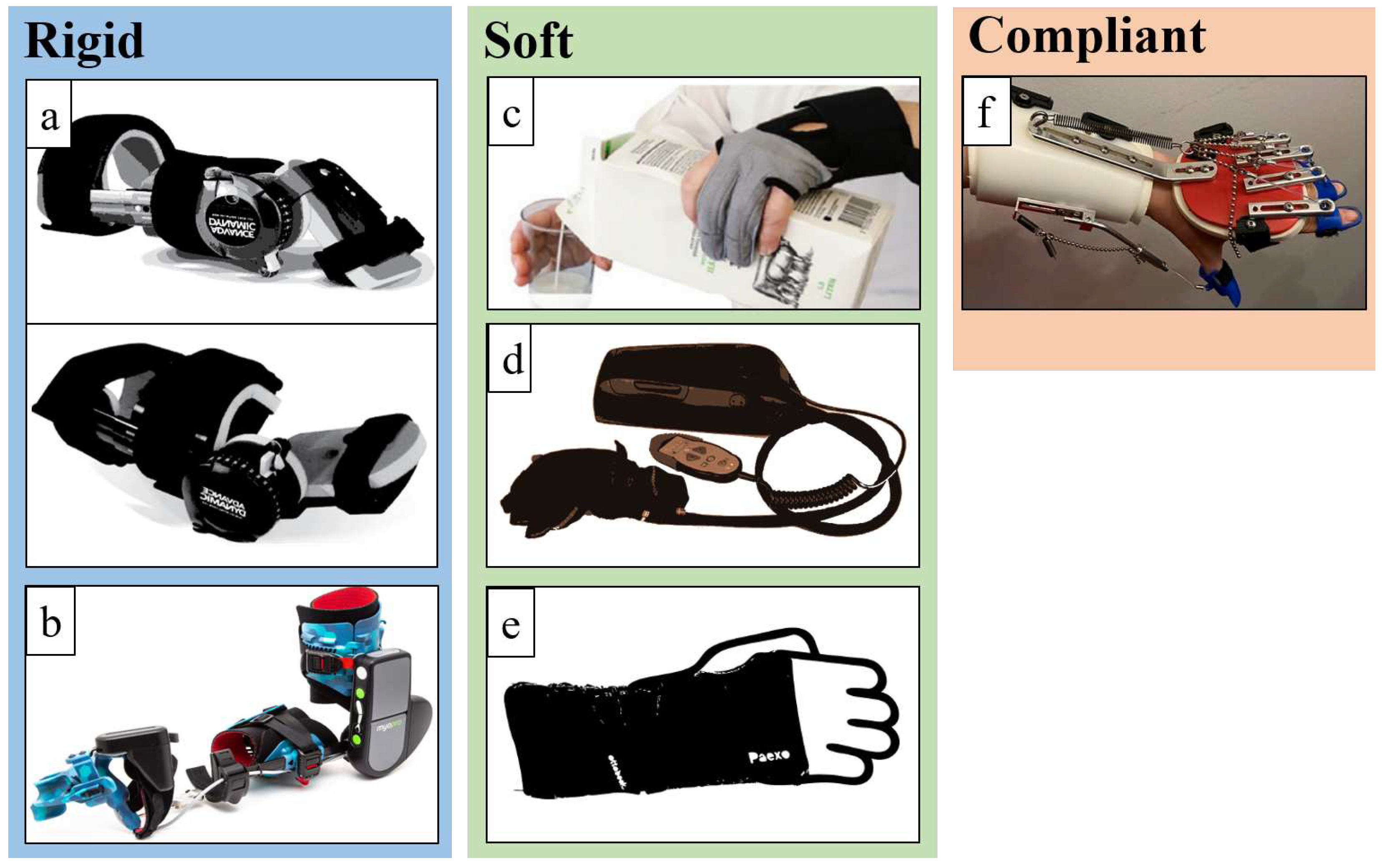
3.1. Rigid devices
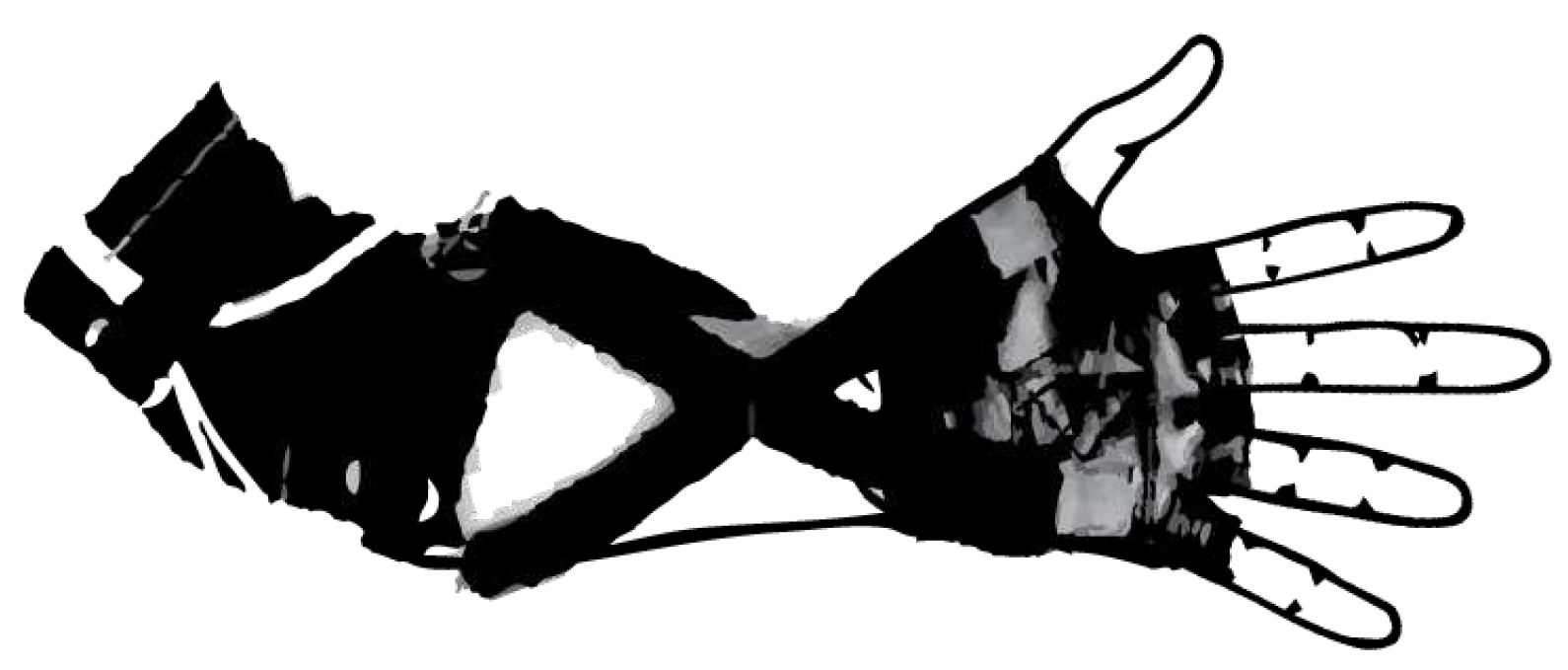
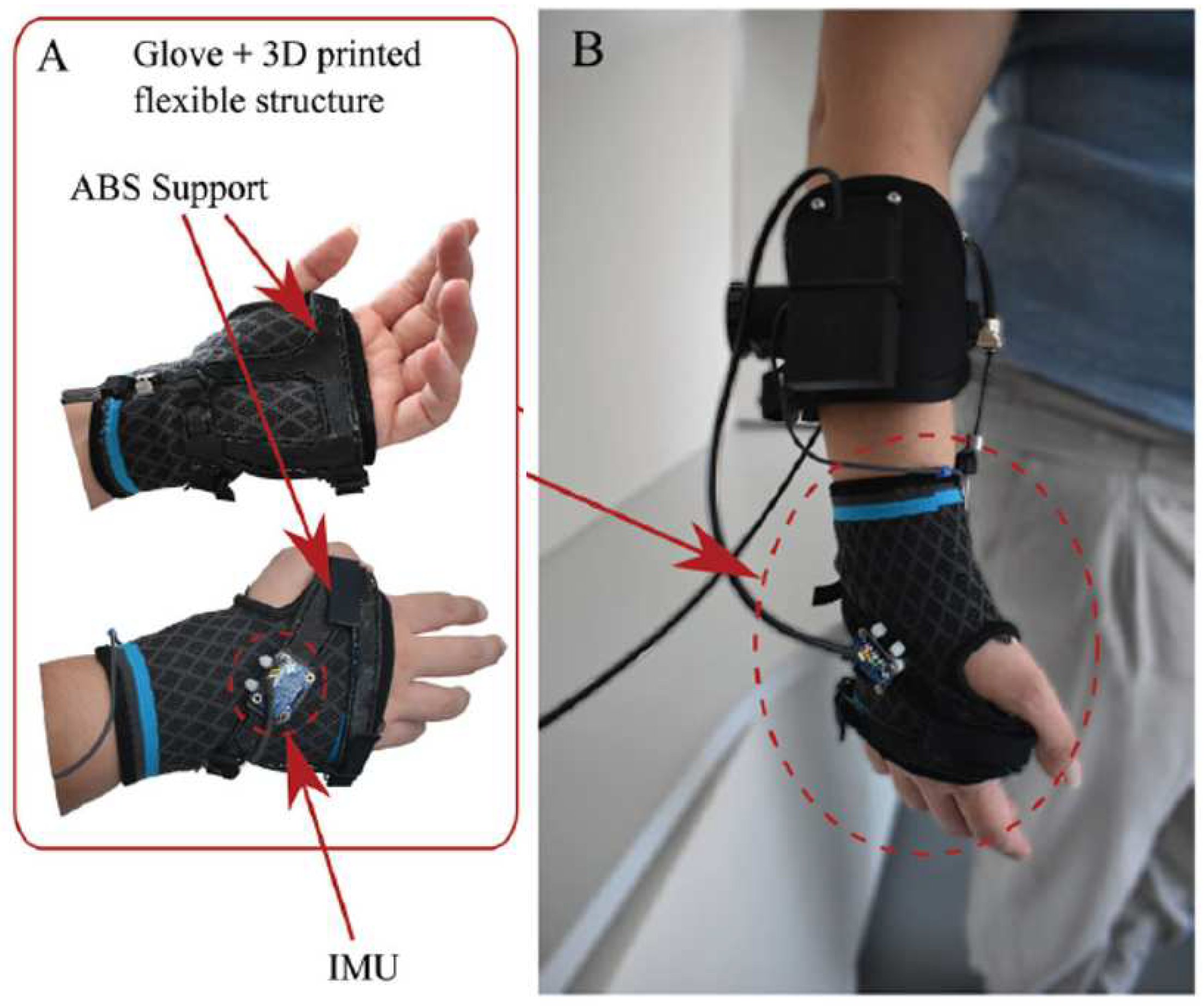
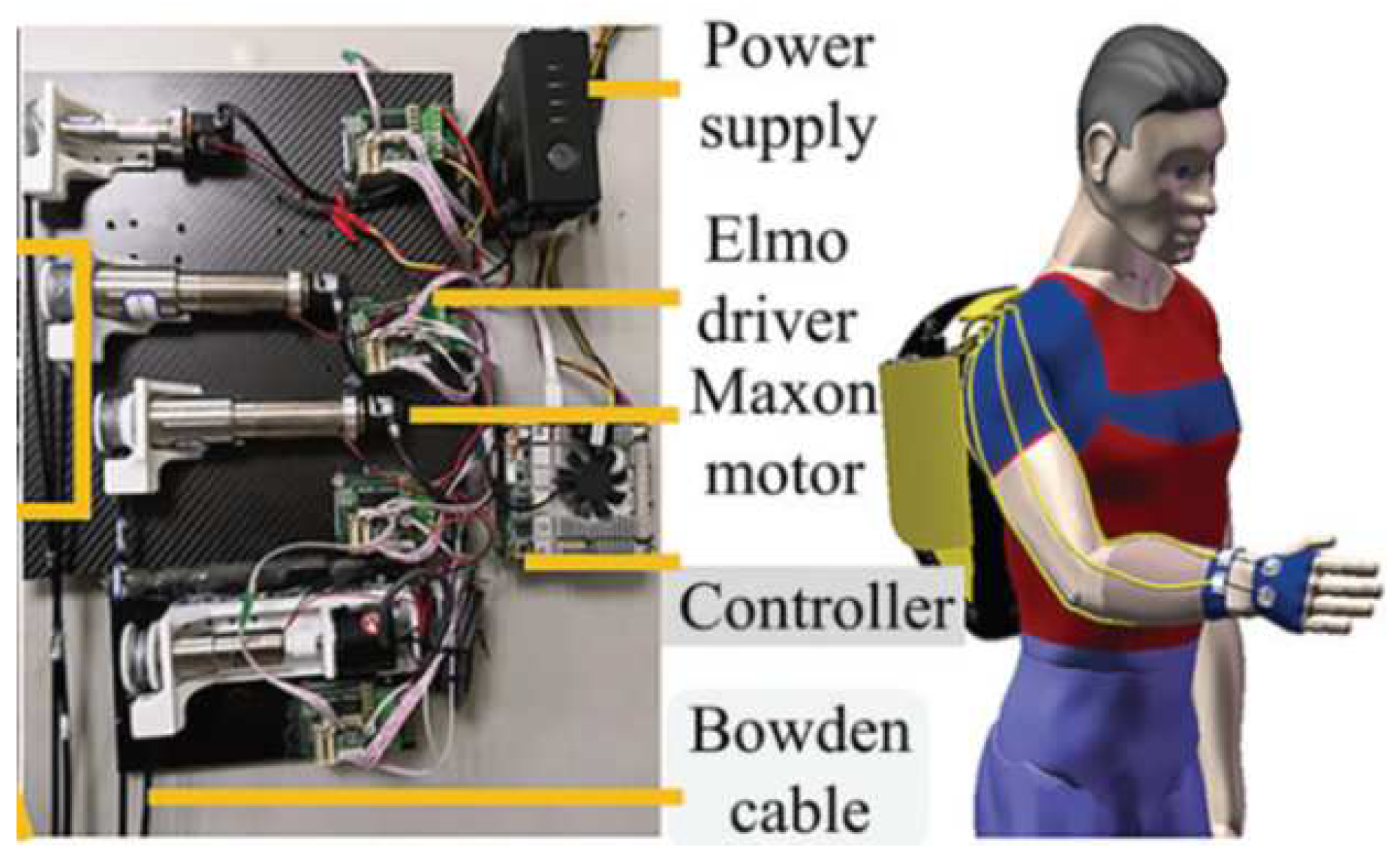
3.1.1. Development of a Portable Wrist Exoskeleton (PWE)
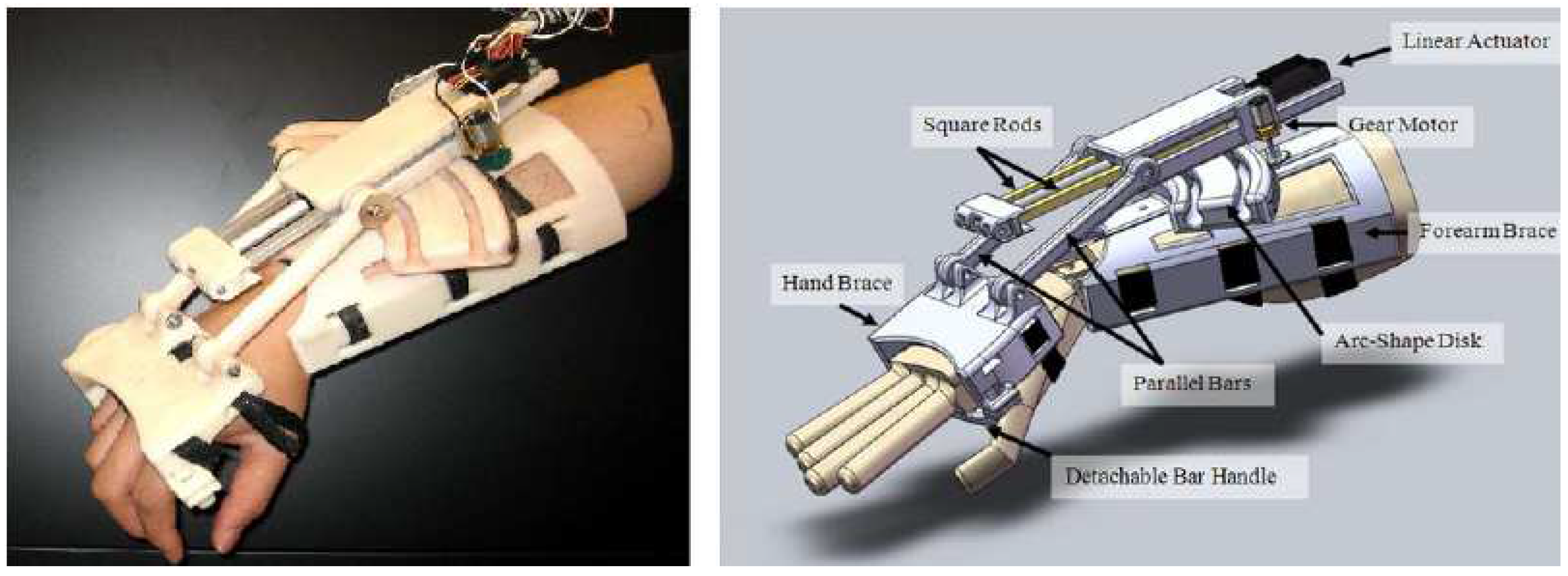
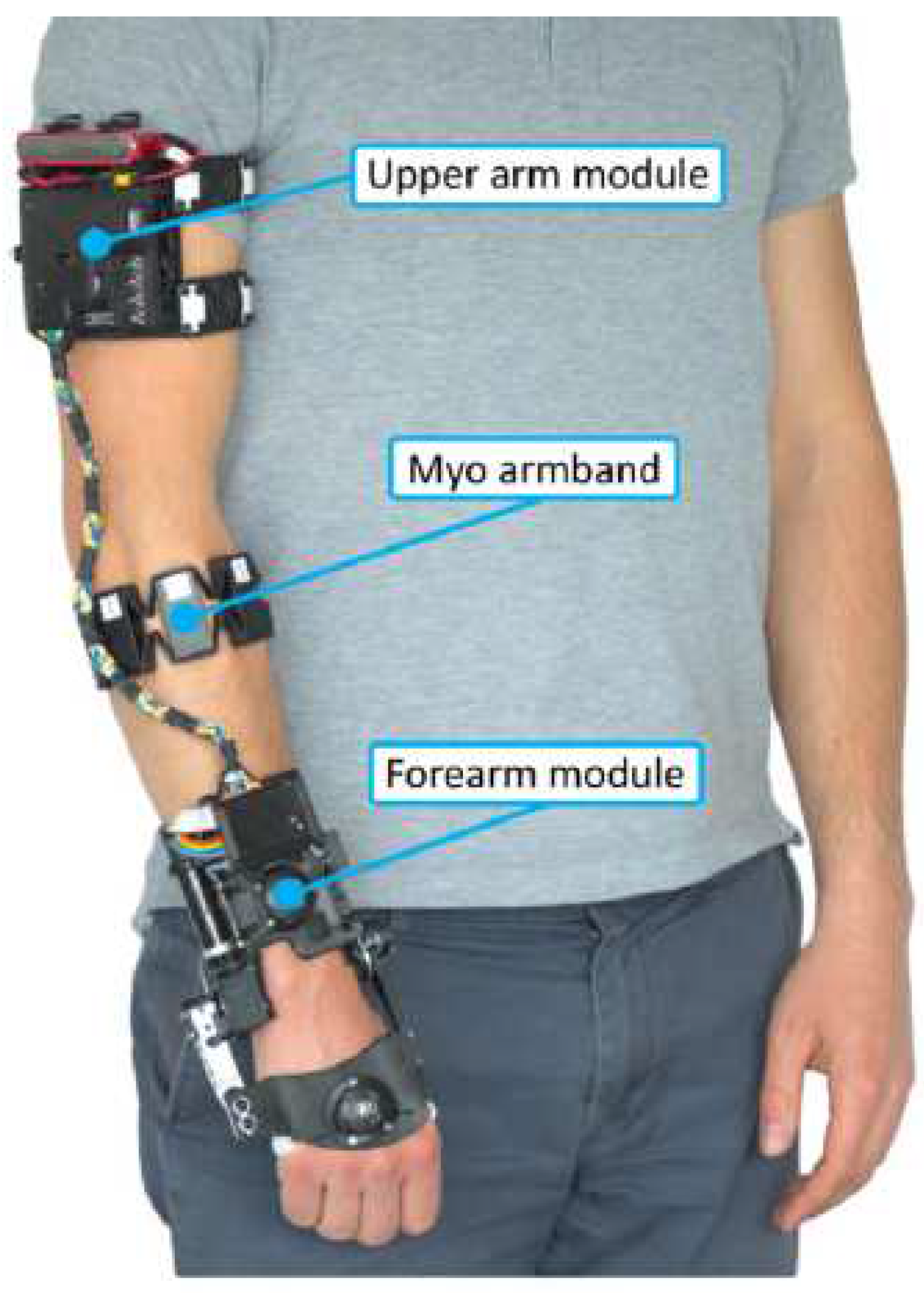
3.1.2. The eWrist - A Wearable Wrist Exoskeleton
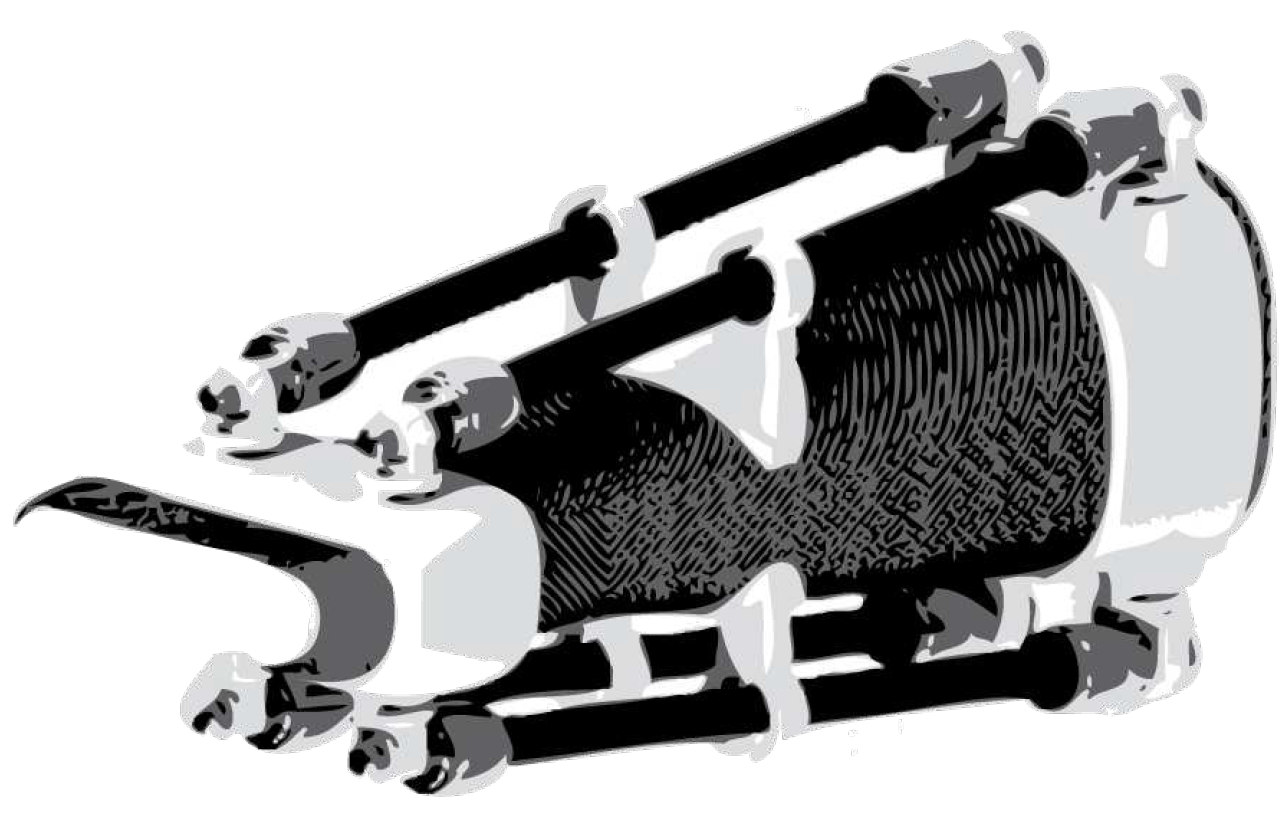
3.1.3. Robotic Orthosis for Wrist Assistance
3.2. Soft devices
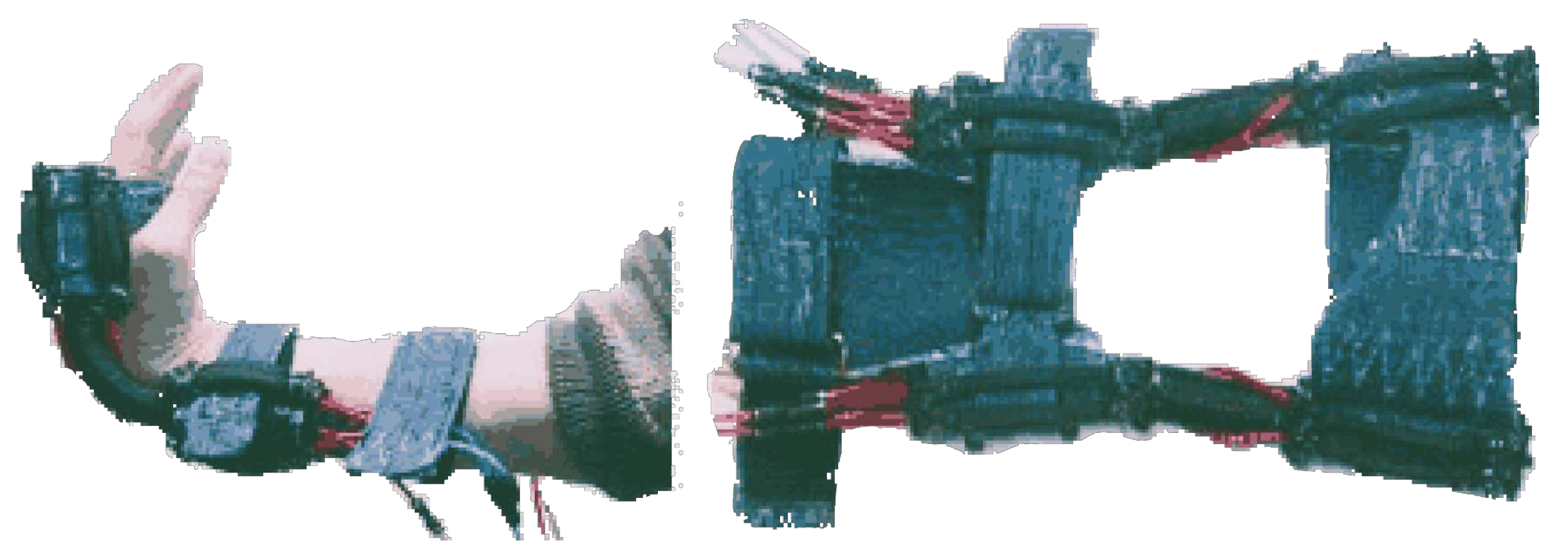
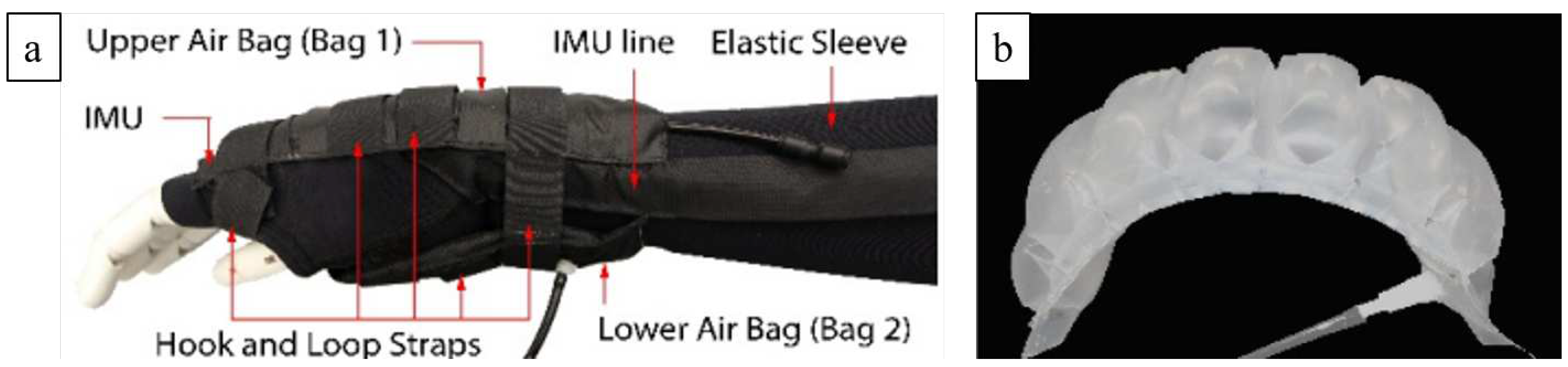
3.2.1. Soft Wrist Exosuit
3.2.2. ExoWrist - a soft tendon-driven wrist wearable robot for dart-throwing motion
3.2.3. A soft robotic orthosis for wrist rehabilitation
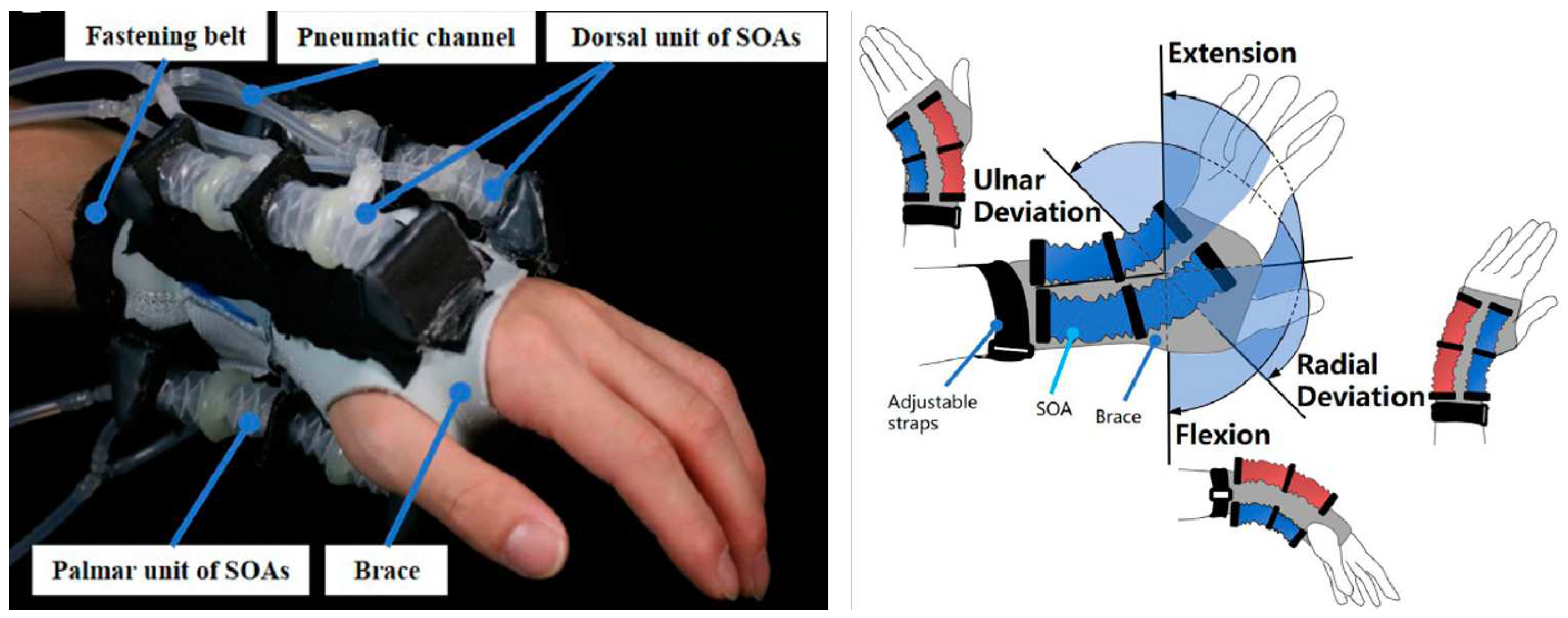
3.2.4. Active Support Splint driven by Pneumatic Soft Actuator (ASSIST)
3.2.5. A soft robotic wrist brace with origami actuators
3.2.6. Bioinspired Musculoskeletal Model-based Soft Wrist Exoskeleton
3.2.7. EXOWRIST: a wrist exoskeleton actuated by pneumatic muscle actuators
3.2.8. Carpal Tunnel Syndrome Soft Relief Device
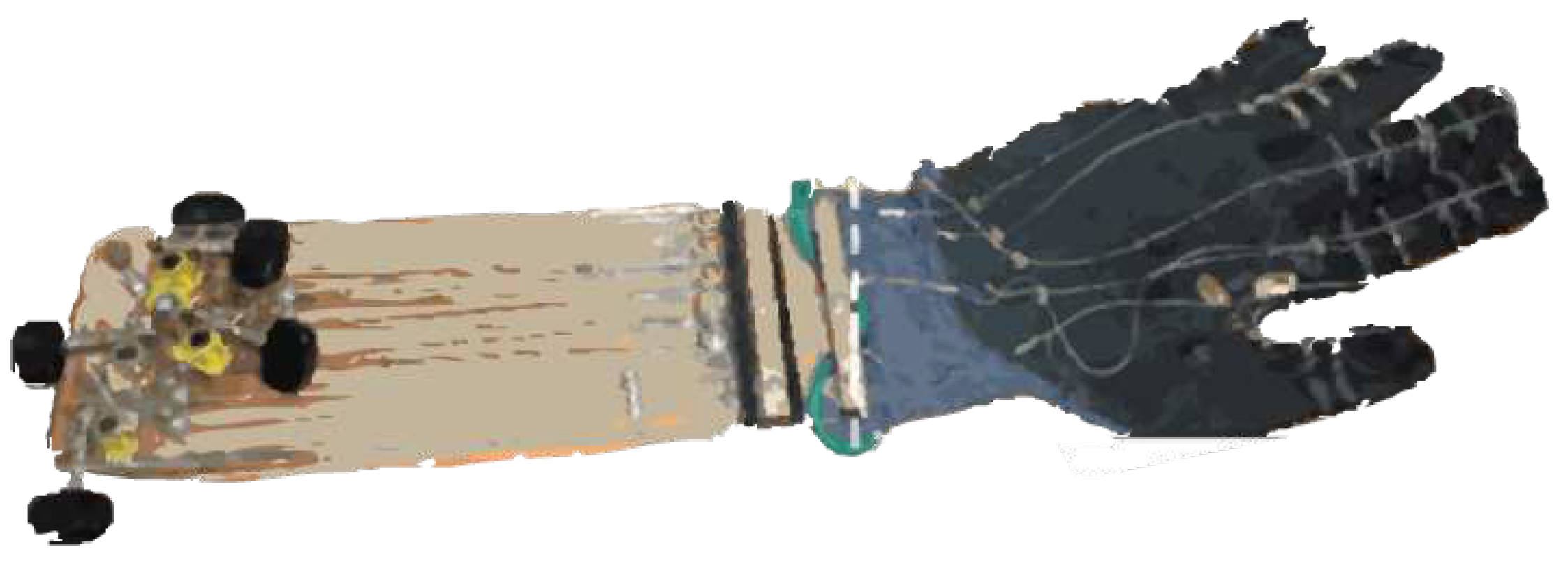
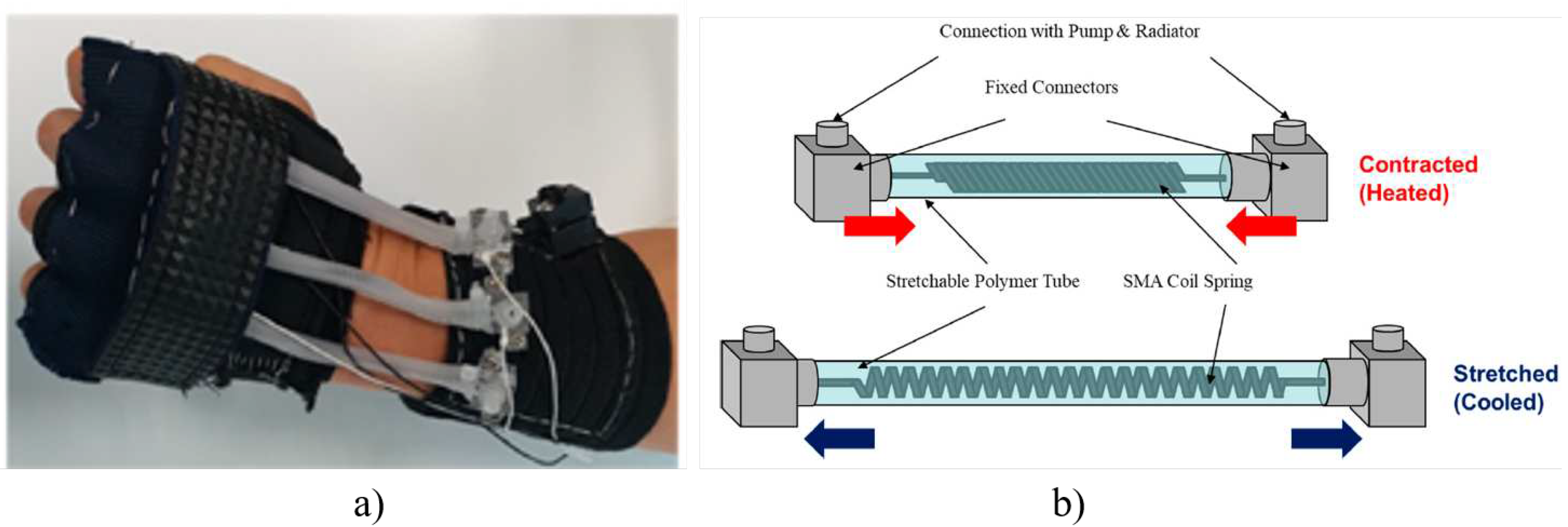
3.2.9. Wrist Assisting Soft Wearable Robot with integrated SMA Muscle
3.2.10. Wearable SMA-based Wrist and Forearm Exoskeleton

3.2.11. ASR: a wearable glove for hand grasping
3.3. Compliant devices
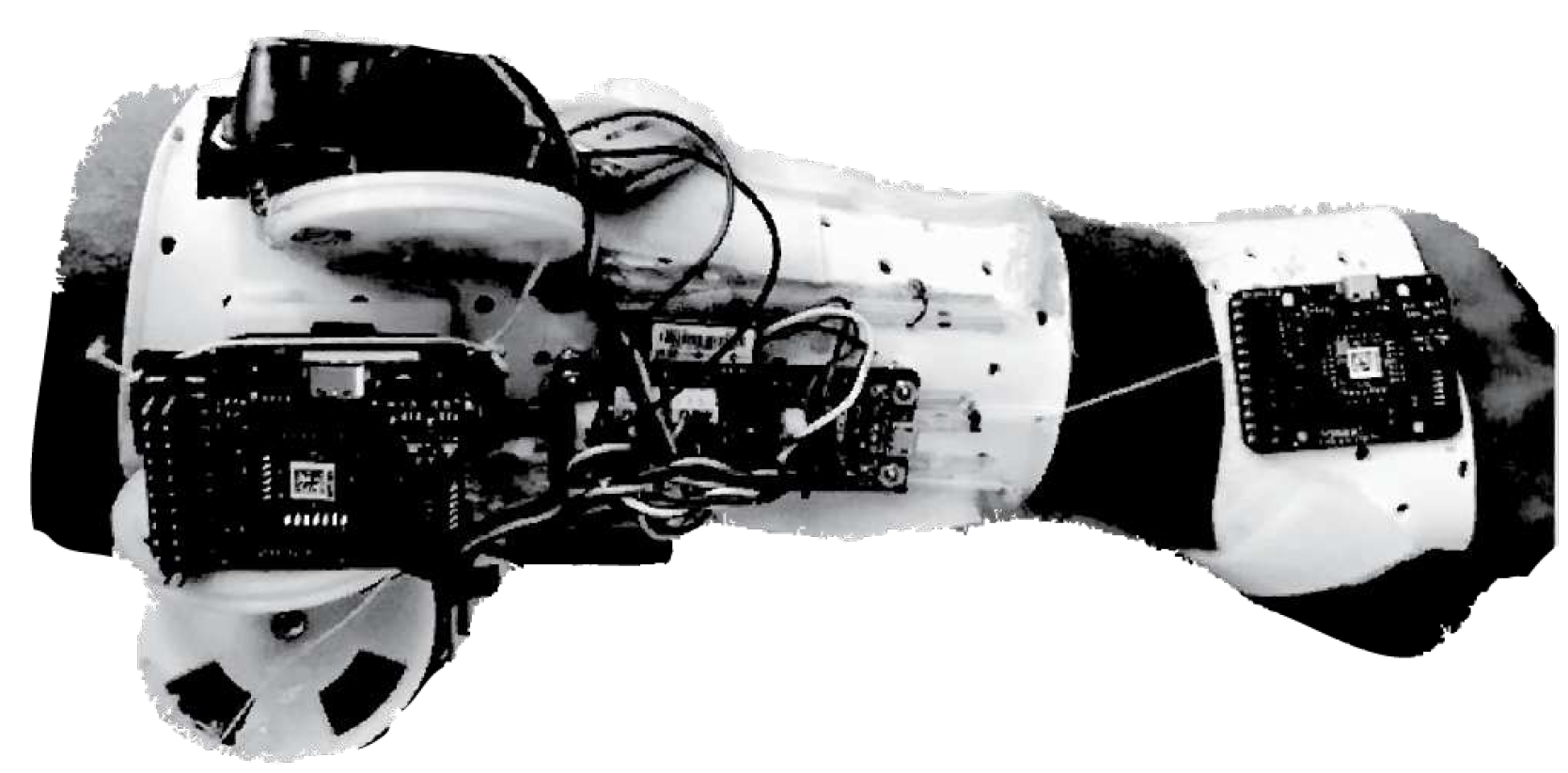
3.3.1. SMA based wrist exoskeleton
3.3.2. SCRIPT: a passive orthosis
3.3.3. Hand and Wrist actuated Exoskeleton for Rehabilitation and Training
3.3.4. Low-Profile Two-DoF Wrist Exoskeleton
4. Commercial Devices
4.1. Rigid devices
4.1.1. JAS Wrists
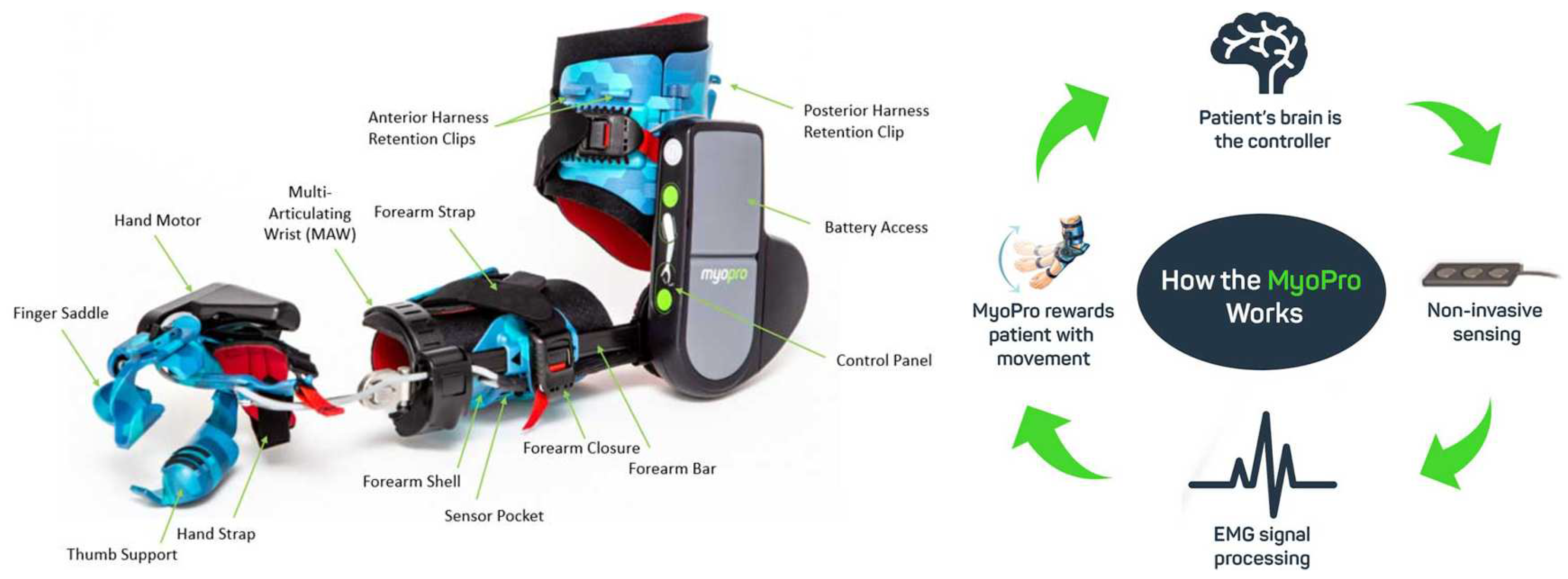
4.1.2. MyoPro Orthosis
4.2. Soft devices
4.2.1. Carbonhand®

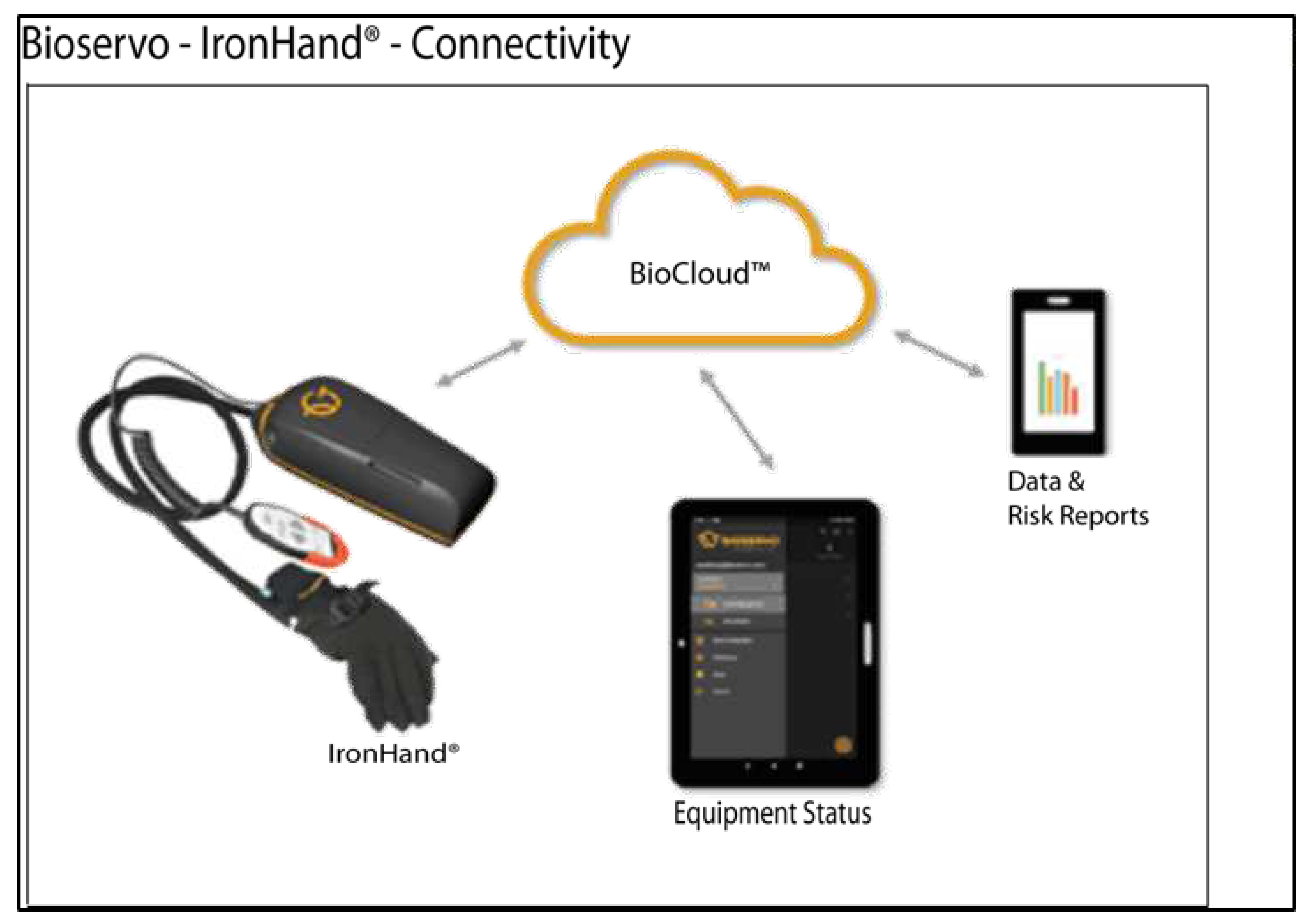
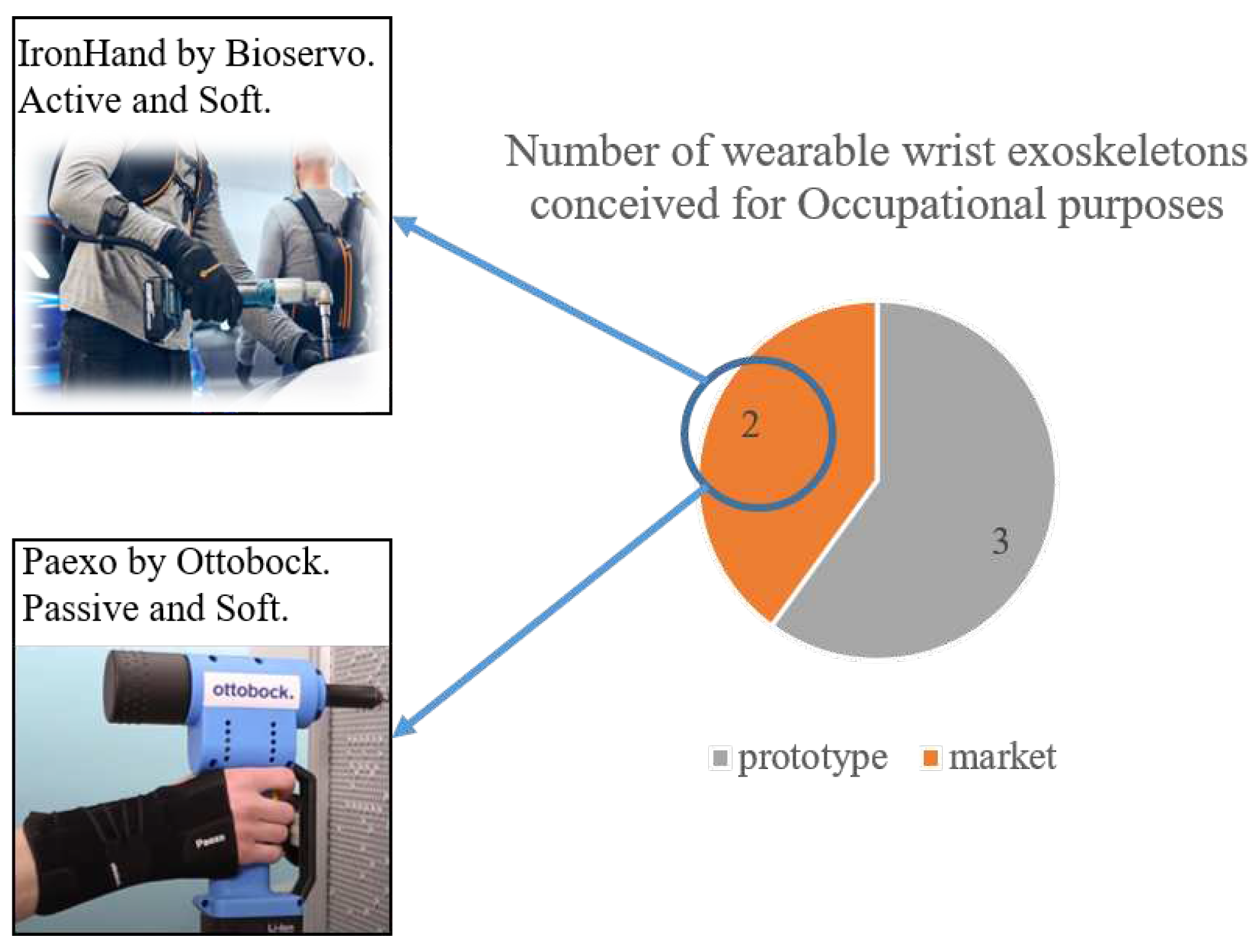
4.2.2. Ironhand®
4.2.3. Paexo Wrist®
4.3. Compliant devices
4.3.1. SaeboFlex
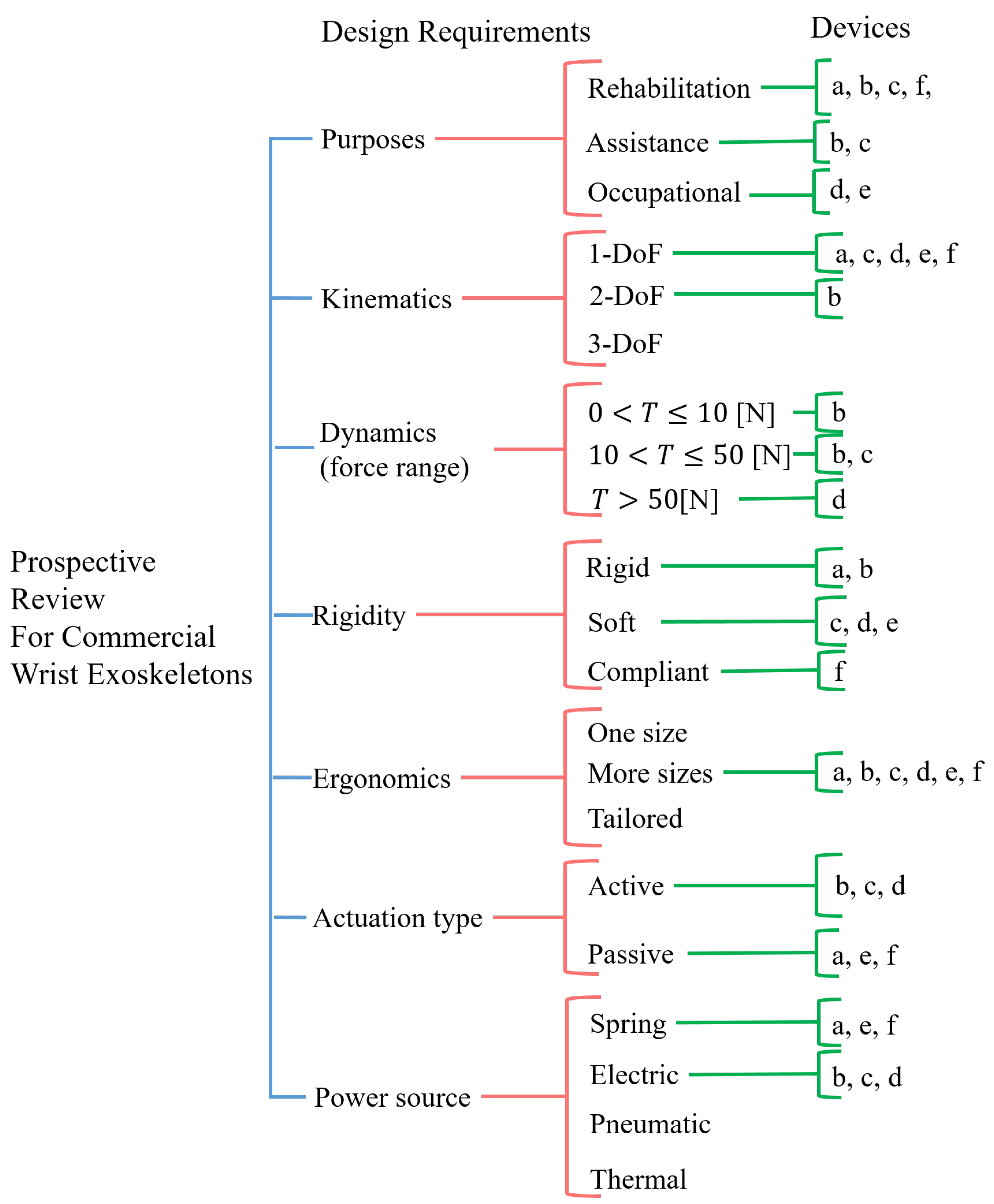
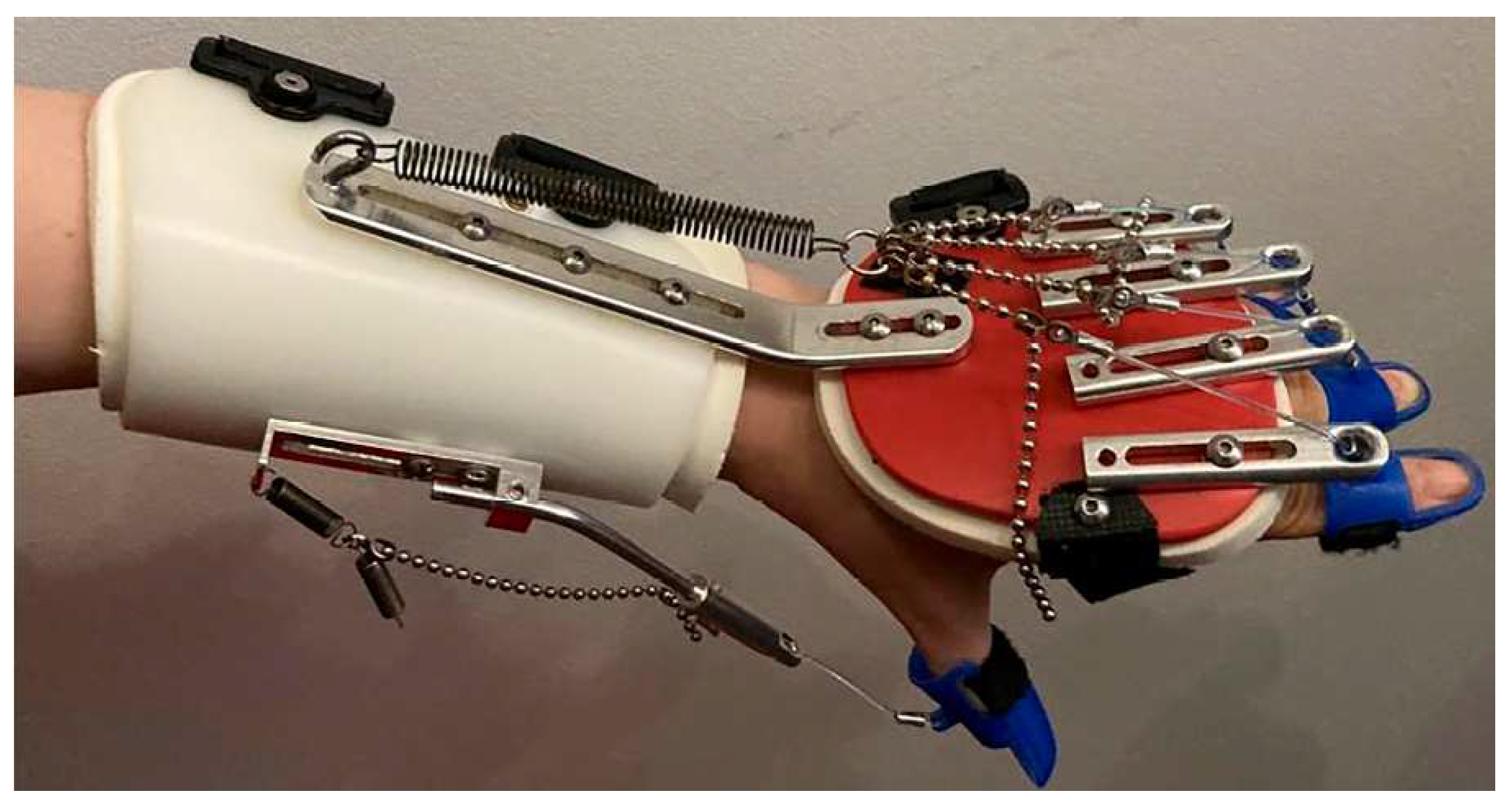
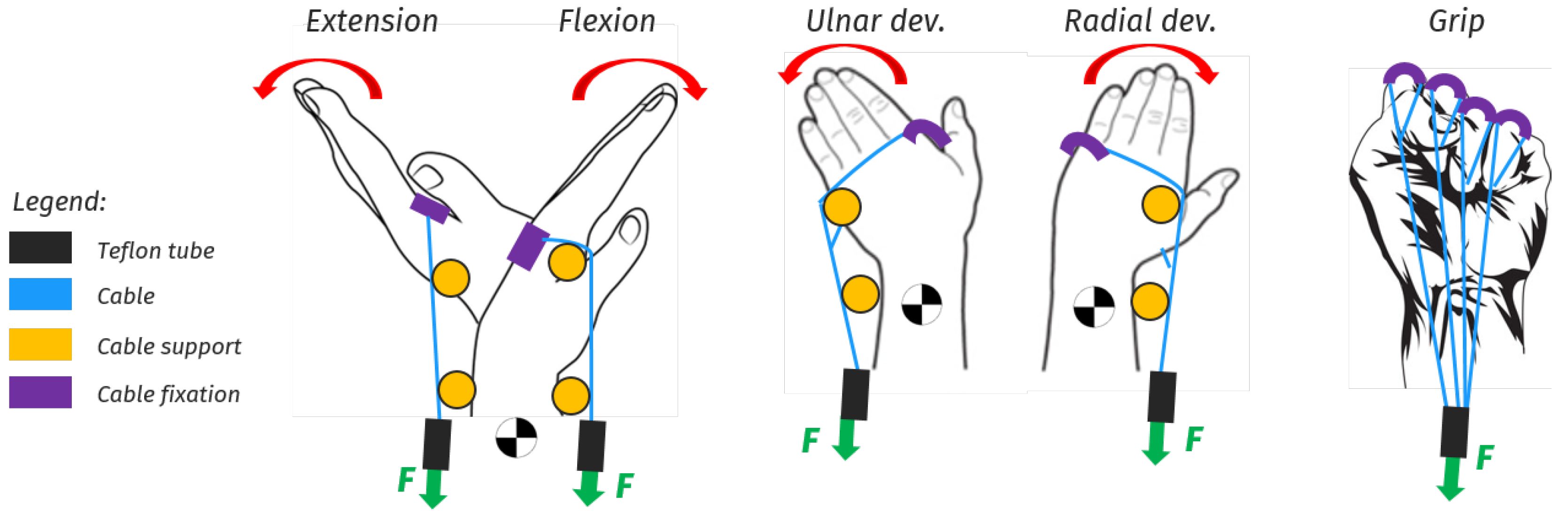
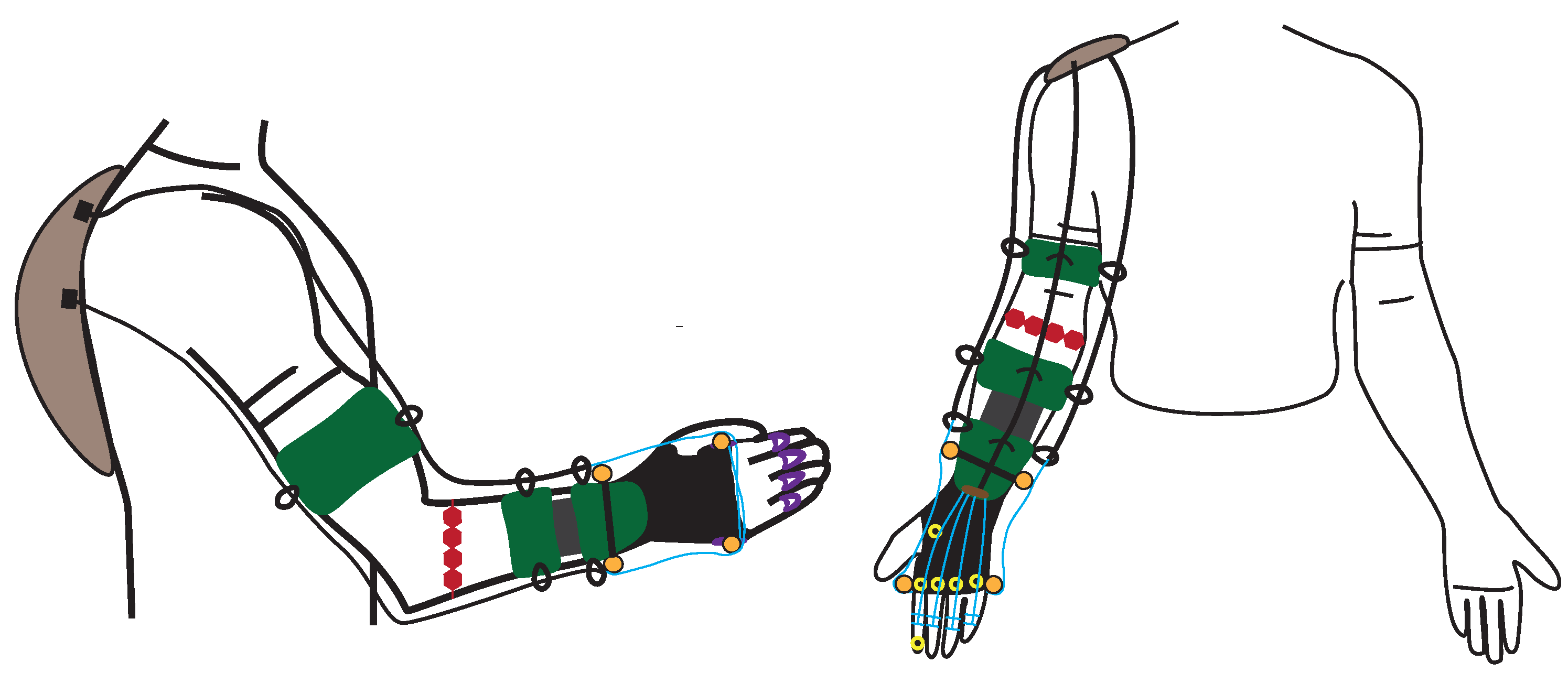
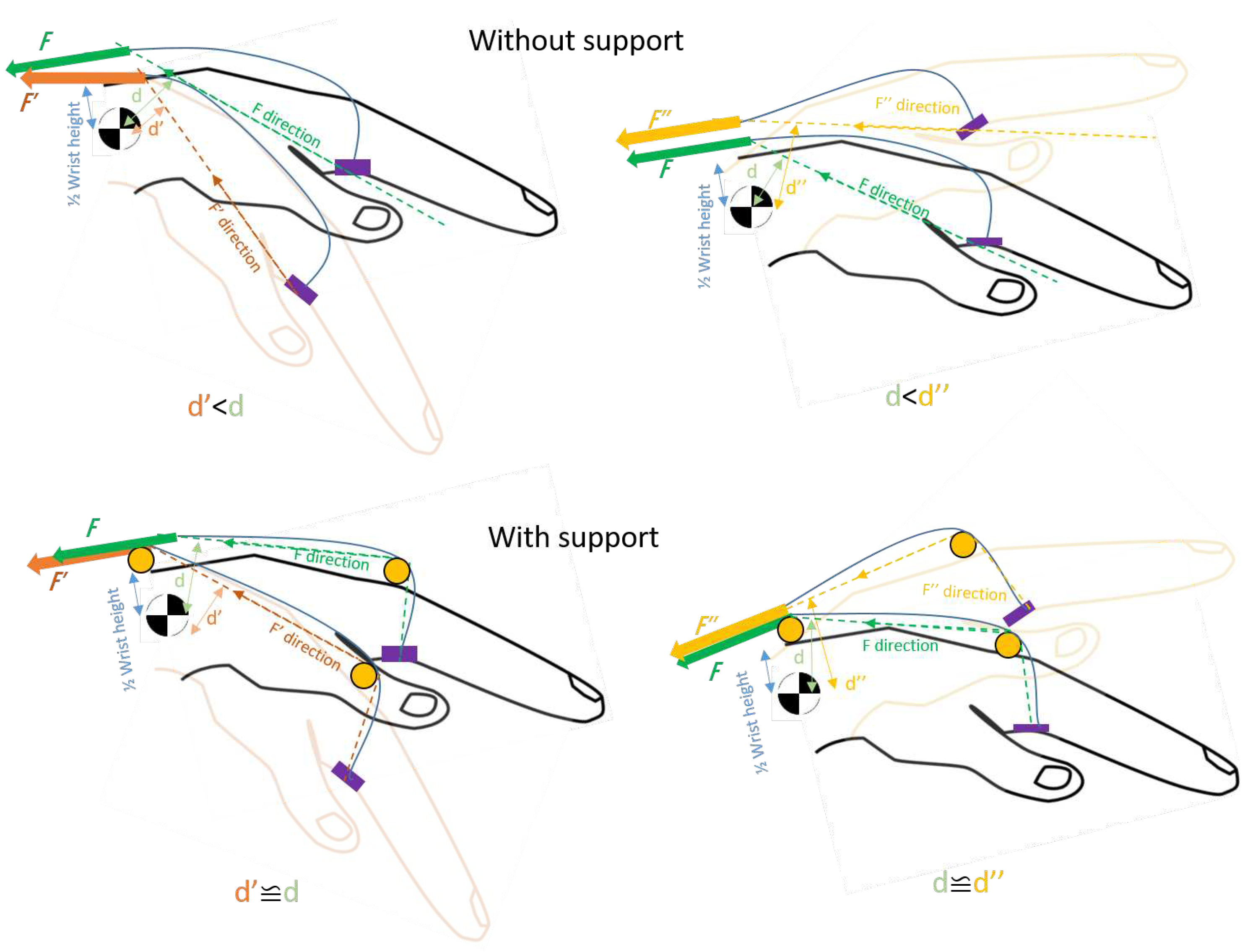
5. Design proposal for a portable wrist exoskeleton
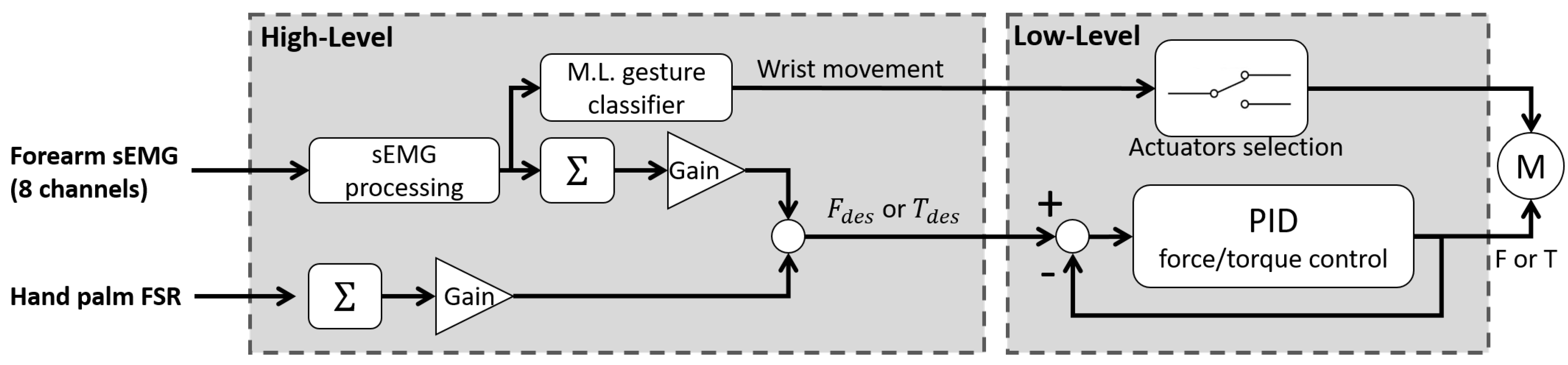
6. Discussion and conclusions
7. Appendix 1
| Reference | Device Name | Application Field | Actuation Type | Power Source | Power transmission | Force/Torque output [N][Nm] | Active DoF | Assisted motion | RoM | Sensing | Control | TRL | Weight | Price($) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Research prototypes | ||||||||||||||
| [16] | PWE | Rehab/Assistance | Active | DC motor | Links, gears | 2.3 Nm | 2 | Flex/Extension | ± 60° | Potentiometer | CPM | **4 | 0.36 kg | - |
| Occupational | 2.5 Nm | Rad/Ulnar dev. | ± 25° | sEMG | M.L. | |||||||||
| [13,17] | eWrist | Rehab/Assistance | Active | DC motor | Links, gears | up to 3.7 Nm | 1 | Flex/Extension | range 154° | sEMG, load cell, | AAN | **4 | 0.56 kg | - |
| encoder | ||||||||||||||
| [18] | *- | Rehabilitation | Passive | DC motor | Links, gears | up to 1.12 Nm | 1 | Flex/Extension | range 120° | Triggered | CPM | **4 | 0.33 kg | *- |
| Active | Force (FSR) | M.L. | ||||||||||||
| [11] | Wrist exosuit | Occupational | Active | Brushless motor | Bowden cables | 3 Nm | 1 | Flex/Extension | 70°/80° | Load cell,IMU | AAN | **4 | 0.3 kg (hand) | - |
| [19] | Exo-Wrist | Rehabilitation | Active | DC motor | Bowden cables | ≥ 0.5 Nm | 1 | Extension | ≥ 50° | sEMG | AAN | **4 | 1 kg | - |
| [20] | *- | Rehabilitation | Active | Pneumatic | Artificial muscles | 120 N | 3 | Flex/Extension | 91° | Pressure | Triggered | **4 | 2.26 kg | - |
| Rad/Ulnar dev. | 32° | |||||||||||||
| Pron/supination | 78° | |||||||||||||
| [21] | ASSIST | Rehabilitation | Active | Pneumatic | Artificial muscles | up to 1 Nm | 1 | Flex/Extension | 80° | Flex sensor | AAN | **4 | 0.39 kg | - |
| Pressure | ||||||||||||||
| [22] | SOA-wrist | Rehab/Assistance | Active | Pneumatic | Flexible joint-less | up to 0.76 Nm | 2 | Flex/Extension | 31°/30° | Pressure, | AAN | **4 | 1.76 kg | - |
| structures | Rad/Ulnar dev. | 33°/22° | IMU | |||||||||||
| [23] | *- | Rehab/Assistance | Active | Brushless motors | Bowden cables | **≥ 5 Nm | 2 | Flex/Extension | range 115° | IMU | CPM | **4 | 0.65 kg | - |
| Rad/Ulnar dev. | range 70° | |||||||||||||
| [37] | EXOWRIST | Rehabilitation | Active | Pneumatic | Artificial muscles | 630 N | 2 | Flex/Extension | ±30° | IMU | CPM | **4 | 0.43 kg | - |
| Rad/Ulnar dev. | ±30° | |||||||||||||
| [34] | *- | Occupational | Active | Pneumatic | Flexible joint-less | *- | 1 | Flex/Extension | ± 65° | IMU | AAN | **3 | *- | - |
| structure | Pressure | |||||||||||||
| [24,25] | SWA | Rehab/Assistance | Active | Thermal SMA | Compliant | up to 1.32 Nm | 2 | Flex/Extension | 38°/50° | Encoder | AAN | **4 | 1.9 kg | - |
| ≥ 0.6 Nm | Rad/Ulnar dev. | 34°/35° | Load cell | |||||||||||
| [26] | *- | Rehabilitation | Active | Thermal SMA | Pulley-tendons | *- | 3 | Flex/Extension | **up to 50°/48° | Potentiometer | CPM | **3 | 0.95 kg | *- |
| Rad/Ulnar dev. | **up to 16°/29° | Load cell | ||||||||||||
| Pron/Supination | **up to 52° | |||||||||||||
| [38] | ASR | Rehab/Assistance | Active | Thermal SMA | Tendons | 40 N | 1 | Gripping | - | Load cell | Triggered | **3 | 300 g | - |
| [27] | SMA-wrist exo | Rehabilitation | Active | Thermal SMA | Linkages | ≥ 0.5 Nm | 2 | Flex/Extension | range 50° | Potentiometer | CPM | **4 | 1 kg | 1060 |
| Rad/Ulnar dev. | range 40° | |||||||||||||
| [28] | SCRIPT | Rehabilitation | Passive | Springs | Links, tendons | 0.5-2 Nm | 1 | Flex/Extension | 45°/30° | Potentiometer | - | **4 | 0.65 kg | - |
| [14,29] | *- | Rehabilitation | Active | DC motor | Tendons | 0.4 Nm | 2 | Flex/Extension | All | IMU | CPM | 4 | 0.3 kg | 160 |
| Rad/Ulnar dev. | ||||||||||||||
| [30] | *- | Rehabilitation | Active | Linear DC motor | Leaf springs | 0.26 to 2.47 Nm | 2 | Flex/Extension | 57°/68 | Potentiometer | CPM | **4 | 0.51 kg | - |
| 0.65 Nm | Rad/Ulnar dev. | 40°/14° | ||||||||||||
| Commercial devices | ||||||||||||||
| [31] | JAS wrist | Rehabilitation | Passive | **Springs | Links | - | 1 | Flex/Extension | up to 95° | - | - | 9 | *- | *- |
| [32] | MyoPro orthosis | Rehab/Assistance | Active | DC motor | Likages | 10 to 20 N | 2 | Flex/Extension | improve up to 35° | sEMG | AAN | 9 | 1.8 kg | 10000 |
| Rad/Ulnar dev. | ||||||||||||||
| [35] | Carbonhand | Assistance | Active | DC motor | Bowden cables | **60 N | 1 | Gripping | All | Force (FSR) | AAN | 9 | 0.7 kg | 7000 |
| [35] | Ironhand | Occupational | Active | DC motor | Bowden cables | 80 N | 1 | Gripping | All | Force (FSR) | AAN | 9 | 2.5 kg | 6500 |
| [36] | Paexo wrist | Occupational | Passive | Leaf spring | Elastic | - | *1 | Holding | All | - | - | 9 | *- | 160 |
| [33] | SaeboFlex | Rehabilitation | Passive | Springs | Links, elastics | - | 1 | Flex/Extension | improve up to 6° | - | - | 9 | 1.6 kg | 600 |
| Experimental tests conducted on the device in a real environment or laboratory | ||||||
| Reference | Subjects | Age | Gender | Clinical Condition | Setting | Testing Period |
| Research prototypes | ||||||
| [16] | 1 | n.d. | n.d. | Healthy | Lab. | n.d. |
| [13,17] | 15 | 26±3.4 | 7 M, 8 F | Healthy | Lab. | n.d. |
| 2 | 60±8 | M | Stroke | Lab. | n.d. | |
| [18] | 1 | n.d. | n.d. | Healthy | Lab. | n.d. |
| [11] | 4 | 28±4.0 | M | Healthy | Lab. | n.d. |
| [19] | 3 | n.d. | n.d. | Healthy | Lab. | n.d. |
| [20] | 1 | n.d. | n.d. | Healthy | Lab. | n.d. |
| [21] | 5 | n.d. | M | Healthy | Lab. | n.d. |
| [22] | - | n.d. | n.d. | n.d. | Lab. | n.d. |
| [23] | 1 | n.d. | n.d. | Healthy | Lab. | n.d. |
| 3 | 47±10.0 | M | Stroke | Lab. | ||
| [37] | 1 | n.d. | M | Healthy | Lab. | n.d. |
| [34] | - | n.d. | n.d. | n.d. | Lab. | n.d. |
| [24,25] | 5 | n.d. | 3 M, 2 F | Healthy | Lab. | n.d. |
| [26] | - | n.d. | n.d. | n.d. | Lab. | n.d. |
| [38] | 1 | n.d. | M | Healthy | Lab. | n.d. |
| [27] | 3 | n.d. | M | Healthy | Lab. | n.d. |
| [28] | 33 | n.d. | n.d. | Stroke | Lab. | n.d. |
| [14,29] | 1 | n.d. | M | Impaired Hand | Lab. | 60 trials |
| [30] | - | n.d. | n.d. | n.d. | Lab. | n.d. |
| Commercial devices | ||||||
| [31,59,60,61] | 133 | 53±17.6 | 55 M, 78 F | Radius fractures | Clinic | 3-20 weeks |
| [32,62,63,64,65,66,67,68] | 1 | 62 | M | Stroke | Clinic/Home | 3 years |
| 2 | 53±22 | M | SCI | Clinic | 6 weeks | |
| 18 | 55.5±21.5 | 11 M, 7 F | Stroke | Clinic | 1 x day | |
| 13 | 51±19.9 | 5 M, 8 F | 7 Stroke, 6 TBI | Clinic/Home | 18 weeks | |
| 18 | 52.5±14.7 | 13 M, 5 F | Stroke | Home | 3 months | |
| [35,70,71] | 15 | 41.5±23.5 | n.d. | SCI | Home | 12 weeks |
| 63 | 54±36 | n.d. | Impaired Hand | Home | 6 weeks | |
| [35,72] | 8 | n.d. | 4 M, 4 F | Healthy | Industry | 2 weeks |
| [36] | - | n.d. | n.d. | n.d. | Industry | n.d. |
| [33,56,74,75,76,77,78] | 10 | n.d. | 7 M, 3 F | Stroke | Clinic | 4 weeks |
| 8 | 70±15 | 4 M, 4 F | Stroke | Clinic | 12 weeks | |
| 11 | 60.2±7.89 | 7 M, 5 F | Stroke | Clinic | n.d. | |
| 1 | 43 | F | Stroke | Clinic | 16 weeks | |
| n.d. = Not Defined | ||||||
| M = Male | ||||||
| F = Female | ||||||
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSD | Musculoskeletal Disorder |
| WRMSD | Work-Related Musculoskeletal Disorder |
| WHO | World Health Organization |
| INAIL | Italian’s Workers Compensatory Authority |
| DARPA | Defense Advanced Research Projects Agency |
| OE | Occupational Exoskeletons |
| CTS | Carpal Tunnel Syndrome |
| DoF | Degree of Freedom |
| RoM | Range of Motion |
| CoR | Centre of Rotation |
| ADL | Activities of Daily Living |
| DTM | Dart Throwing Motion |
| TRL | Technology Readiness Level |
| ICR | Instantaneous Centre of Rotation |
| OA | Hand Osteoarthritis |
| HAL | Hand Activity Level |
| IMU | Inertial Magnetic Unit |
| FSR | Force Resistive Sensor |
| sEMG | Surface Electromyography signals |
| CPM | Continuous Passive Motion |
| AAN | Assistance-As-Needed |
| ML | Machine Learning |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| WearRA | Wearable Robotics Association |
| SEM | Soft Extra Muscle |
| SCI | Spinal Cord Injury |
| JAS | Joint Active Systems Inc. |
| PWE | Portable Wrist Exoskeleton |
| NN | Neural Network |
| SVM | Support Vector Machine |
| FMG | Force-myography signal |
| CIMT | Constraint-Induced Movement Therapy |
| MVC | Maximum Voluntary Contraction |
| SOA | Soft Origami Actuator |
| PMA | Pneumatic Muscle Actuator |
| SMA | Shape Memory Alloy |
| SWA | Soft Wrist Assist |
| RMSE | Root Means Squared Error |
| SPS | Static Progressive Stretch |
| TBI | Traumatic Brain Injury |
| OT | Occupational Therapy |
References
- Gonçalves, H.; Cardoso, A.; Mattos, D.; Deola Borges, G.; Anacleto, P.; Colim, A.; Carneiro, P.; Arezes, P.M. Assessment of Work-Related Musculoskeletal Disorders by Observational Methods in Repetitive Tasks—A Systematic Review. Occupational and Environmental Safety and Health III 2022, 455–463. [Google Scholar] [CrossRef]
- Ramazzini, B. De morbis artificum diatriba [diseases of workers]. American journal of public health 2001, 91, 1380–1382. [Google Scholar] [CrossRef]
- Armstrong, T.J.; Fine, L.J.; Goldstein, S.A.; Lifshitz, Y.R.; Silverstein, B.A. Ergonomics considerations in hand and wrist tendinitis. The Journal of hand surgery 1987, 12, 830–837. [Google Scholar] [CrossRef]
- Sluiter, J.K.; Rest, K.M.; Frings-Dresen, M.H. Criteria document for evaluating the work-relatedness of upper-extremity musculoskeletal disorders. Scandinavian journal of work, environment & health 2001, pp. 1–102. http://www.jstor.org/stable/40967163.
- Franco, G.; Franco, F. Bernardino Ramazzini: The father of occupational medicine. American Journal of Public Health 2001, 91, 1382–1382. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Musculoskeletal conditions, Key facts. https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions, 2021.
- INAIL-Italian Workers’ Compensatory Authority). OCCUPATIONAL EXOSKELETONS:WEARABLE ROBOTIC DEVICES TO PREVENT WORK RELATED MUSCULOSKELETAL DISORDERS IN THE WORKPLACE OF THE FUTURE, 2020. Available online:http://www.osha.europa.eu.
- European Agency for Safety and Health at Work (EU-OSHA). Work-related musculoskeletal disorders – Facts and figures. Standard, European Agency for Safety and Health at Work (EU-OSHA), Publications Office of the European Union, Luxembourg, 2020. [CrossRef]
- Pons, J.L. Wearable robots: biomechatronic exoskeletons; John Wiley & Sons, 2008; pp. 4,11,219,332. ISBN:9780470512944.
- Du Plesis, T.; et al. Review of Active Hand Exoskeletons for Rehabilitation and Assistance. Robotics 2021, 10, 1–42. [Google Scholar] [CrossRef]
- Chiaradia, D.; Tiseni, L.; Xiloyannis, M.; Solazzi, M.; Masia, L.; Frisoli, A. An assistive soft wrist exosuit for flexion movements with an ergonomic reinforced glove. Frontiers in Robotics and AI 2021, 7, 595862. [Google Scholar] [CrossRef]
- Crea, S.; Beckerle, P.; De Looze, M.; De Pauw, K.; Grazi, L.; Kermavnar, T.; Masood, J.; O’Sullivan, L.W.; Pacifico, I.; Rodriguez-Guerrero, C.; et al. Occupational exoskeletons: A roadmap toward large-scale adoption. Methodology and challenges of bringing exoskeletons to workplaces. Wearable Technologies 2021, 2. [Google Scholar] [CrossRef]
- Lambelet, C.; Temiraliuly, D.; Siegenthaler, M.; Wirth, M.; Woolley, D.G.; Lambercy, O.; Gassert, R.; Wenderoth, N. Characterization and wearability evaluation of a fully portable wrist exoskeleton for unsupervised training after stroke. Journal of NeuroEngineering and Rehabilitation 2020, 17, 1–16. [Google Scholar] [CrossRef]
- Dragusanu, M.; Iqbal, M.Z.; Baldi, T.L.; Prattichizzo, D.; Malvezzi, M. Design, Development, and Control of a Hand/Wrist Exoskeleton for Rehabilitation and Training. IEEE Transactions on Robotics 2022. [Google Scholar] [CrossRef]
- Pitzalis, R.F.; Park, D.; Caldwell, D.G.; Berselli, G.; Ortiz, J. State of the Art in Wearable Wrist Exoskeletons Part I: Background Needs and Design Requirements. Machines 2023, 11, 458. [Google Scholar] [CrossRef]
- Xiao, Z.G.; Menon, C. Towards the development of a portable wrist exoskeleton. 2011 IEEE International Conference on Robotics and Biomimetics. IEEE, 2011, pp. 1884–1889. [CrossRef]
- Lambelet, C.; Lyu, M.; Woolley, D.; Gassert, R.; Wenderoth, N. The eWrist—A wearable wrist exoskeleton with sEMG-based force control for stroke rehabilitation. 2017 International Conference on Rehabilitation Robotics (ICORR). IEEE, 2017, pp. 726–733. [CrossRef]
- Sangha, S.; Elnady, A.M.; Menon, C. A compact robotic orthosis for wrist assistance. 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob). IEEE, 2016, pp. 1080–1085. [CrossRef]
- Choi, H.; Kang, B.B.; Jung, B.K.; Cho, K.J. Exo-wrist: A soft tendon-driven wrist-wearable robot with active anchor for dart-throwing motion in hemiplegic patients. IEEE Robotics and Automation Letters 2019, 4, 4499–4506. [Google Scholar] [CrossRef]
- Bartlett, N.W.; Lyau, V.; Raiford, W.A.; Holland, D.; Gafford, J.B.; Ellis, T.D.; Walsh, C.J. A soft robotic orthosis for wrist rehabilitation. Journal of Medical Devices 2015, 9. [Google Scholar] [CrossRef]
- Sasaki, D.; Noritsugu, T.; Takaiwa, M. Development of active support splint driven by pneumatic soft actuator (ASSIST). Proceedings of the 2005 IEEE international conference on robotics and automation. IEEE, 2005, pp. 520–525. [CrossRef]
- Liu, S.; Fang, Z.; Liu, J.; Tang, K.; Luo, J.; Yi, J.; Hu, X.; Wang, Z. A compact soft robotic wrist brace with origami actuators. Frontiers in Robotics and AI 2021, 34. [Google Scholar] [CrossRef]
- Li, N.; Yang, T.; Yang, Y.; Yu, P.; Xue, X.; Zhao, X.; Song, G.; Elhajj, I.H.; Wang, W.; Xi, N.; et al. Bioinspired musculoskeletal model-based soft wrist exoskeleton for stroke rehabilitation. Journal of Bionic Engineering 2020, 17, 1163–1174. [Google Scholar] [CrossRef]
- Jeong, J.; Yasir, I.B.; Han, J.; Park, C.H.; Bok, S.K.; Kyung, K.U. Design of shape memory alloy-based soft wearable robot for assisting wrist motion. Applied Sciences 2019, 9, 4025. [Google Scholar] [CrossRef]
- Jeong, J.; Hyeon, K.; Han, J.; Park, C.H.; Ahn, S.Y.; Bok, S.K.; Kyung, K.U. Wrist assisting soft wearable robot with stretchable coolant vessel integrated SMA muscle. IEEE/ASME Transactions on Mechatronics 2021, 27, 1046–1058. [Google Scholar] [CrossRef]
- Hope, J.; McDaid, A. Development of wearable wrist and forearm exoskeleton with shape memory alloy actuators. Journal of Intelligent & Robotic Systems 2017, 86, 397. [Google Scholar] [CrossRef]
- Serrano, D.; Copaci, D.S.; Moreno, L.; Blanco, D. SMA based wrist exoskeleton for rehabilitation therapy. 2018 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS). IEEE, 2018, pp. 2318–2323. [CrossRef]
- Ates, S.; Haarman, C.J.; Stienen, A.H. SCRIPT passive orthosis: design of interactive hand and wrist exoskeleton for rehabilitation at home after stroke. Autonomous Robots 2017, 41, 711–723. [Google Scholar] [CrossRef]
- Dragusanu, M.; Baldi, T.L.; Iqbal, Z.; Prattichizzo, D.; Malvezzi, M. Design, development, and control of a tendon-actuated exoskeleton for wrist rehabilitation and training. 2020 IEEE International Conference on Robotics and Automation (ICRA). IEEE, 2020, pp. 1749–1754. [CrossRef]
- Higuma, T.; Kiguchi, K.; Arata, J. Low-profile two-degree-of-freedom wrist exoskeleton device using multiple spring blades. IEEE Robotics and Automation Letters 2017, 3, 305–311. [Google Scholar] [CrossRef]
- JAS-Joint Active Systems. JAS Wrist. https://www.jointactivesystems.com/products/areas/wrist, 2017. Online; accessed 5th of August 2022.
- Myomo Inc.. MyoPro Orthosis. https://myomo.com/what-is-a-myopro-orthosis/, 2006. Online; accessed 8th of August 2022.
- Saebo Inc.. SaeboFlex. https://www.saebo.com/shop/saeboflex/. Last accessed 30th of November 2022.
- Zhu, M.; Adams, W.; Polygerinos, P. Carpal tunnel syndrome soft relief device for typing applications. Frontiers in Biomedical Devices. American Society of Mechanical Engineers, 2017, Vol. 40672, p. V001T03A003. [CrossRef]
- Bioservo Technologies. Bioservo Strength for life - Professional and Healthcare. https://www.bioservo.com/. Last accessed 7th of September 2022.
- Ottobock. Paexo Wrist Exoskeleton. https://paexo.com/paexo-wrist/?lang=it. Last accessed 5th of August 2022.
- Andrikopoulos, G.; Nikolakopoulos, G.; Manesis, S. Motion control of a novel robotic wrist exoskeleton via pneumatic muscle actuators. 2015 IEEE 20th Conference on Emerging Technologies & Factory Automation (ETFA). IEEE, 2015, pp. 1–8. [CrossRef]
- Hadi, A.; Alipour, K.; Kazeminasab, S.; Elahinia, M. ASR glove: A wearable glove for hand assistance and rehabilitation using shape memory alloys. Journal of Intelligent Material Systems and Structures 2018, 29, 1575–1585. [Google Scholar] [CrossRef]
- Ferguson, R.; et al. Wrist pain: a systematic review of prevalence and risk factors–what is the role of occupation and activity? BMC Musculoskeletal Disorders 2019, 20. [Google Scholar] [CrossRef]
- Noronha, B.; Accoto, D. Exoskeletal devices for hand assistance and rehabilitation: A comprehensive analysis of state-of-the-art technologies. IEEE Transactions on Medical Robotics and Bionics 2021, 3, 525–538. [Google Scholar] [CrossRef]
- Gopura, R.; Kiguchi, K. Mechanical designs of active upper-limb exoskeleton robots: State-of-the-art and design difficulties. 2009 IEEE International Conference on Rehabilitation Robotics. IEEE, 2009, pp. 178–187. [CrossRef]
- Gopura, R.; Bandara, D.; Kiguchi, K.; Mann, G.K. Developments in hardware systems of active upper-limb exoskeleton robots: A review. Robotics and Autonomous Systems 2016, 75, 203–220. [Google Scholar] [CrossRef]
- Nikhil, G.; Yedukondalu, G.; Rao, S. Robotic exoskeletons: a review on development. International Journal of Mechanical and Production Engineering Research and Development 2019, 9, 529–542, https://archive.org/details/52.ijmperdaug201952. [Google Scholar]
- Tiboni, M.; Borboni, A.; Vérité, F.; Bregoli, C.; Amici, C. Sensors and actuation technologies in exoskeletons: A review. Sensors 2022, 22, 884. [Google Scholar] [CrossRef]
- Hussain, S.; Jamwal, P.K.; Van Vliet, P.; Ghayesh, M.H. State-of-the-art robotic devices for wrist rehabilitation: Design and control aspects. IEEE Transactions on Human-Machine Systems 2020, 50, 361–372. [Google Scholar] [CrossRef]
- Cornejo, J.; Huamanchahua, D.; Huamán-Vizconde, S.; Terrazas-Rodas, D.; Sierra-Huertas, J.; Janampa-Espinoza, A.; Gonzáles, J.; Cardona, M. Mechatronic exoskeleton systems for supporting the biomechanics of shoulder-elbow-wrist: An innovative review. 2021 IEEE International IOT, Electronics and Mechatronics Conference (IEMTRONICS). IEEE, 2021, pp. 1–9. [CrossRef]
- Khokhar, Z.O.; Xiao, Z.G.; Menon, C. Surface EMG pattern recognition for real-time control of a wrist exoskeleton. Biomedical engineering online 2010, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Vardakastani, V.; Bell, H.; Mee, S.; Brigstocke, G.; Kedgley, A.E. Clinical measurement of the dart throwing motion of the wrist: variability, accuracy and correction. Journal of Hand Surgery (European Volume) 2018, 43, 723–731. [Google Scholar]
- Braidotti, F.; Atzei, A.; Fairplay, T. Dart-Splint: an innovative orthosis that can be integrated into a scapho-lunate and palmar midcarpal instability re-education protocol. Journal of Hand Therapy 2015, 28, 329–335. [Google Scholar] [CrossRef]
- Kaufman-Cohen, Y.; Friedman, J.; Levanon, Y.; Jacobi, G.; Doron, N.; Portnoy, S. Wrist plane of motion and range during daily activities. The American Journal of Occupational Therapy 2018, 72, 7206205080p1–7206205080p10. [Google Scholar] [CrossRef]
- Brigstocke, G.; Hearnden, A.; Holt, C.; Whatling, G. In-vivo confirmation of the use of the dart thrower’s motion during activities of daily living. Journal of Hand Surgery (European Volume) 2014, 39, 373–378. [Google Scholar]
- Borislav, M. The Number Of Companies Making Industrial Exoskeletons Has Been Quietly Increasing For The Past Five Years. https://www.forbes.com/sites/borislavmarinov/2020/09/24/the-number-of-companies-making-industrial-exoskeletons-has-been-quietly-increasing-for-the-past-five-years/?sh=231b1c067bf4, 2020. Last accessed 5th of August 2022.
- Borislav, M. Updated Directory of Exoskeleton Companies and Industry Statistics. https://exoskeletonreport.com/2021/12/updated-directory-of-exoskeleton-companies-and-industry-statistics/, 2021. Last accessed 5th of August 2022.
- Radder, B.; Prange-Lasonder, G.B.; Kottink, A.I.; Holmberg, J.; Sletta, K.; van Dijk, M.; Meyer, T.; Melendez-Calderon, A.; Buurke, J.H.; Rietman, J.S. Home rehabilitation supported by a wearable soft-robotic device for improving hand function in older adults: A pilot randomized controlled trial. PloS one 2019, 14, e0220544. [Google Scholar] [CrossRef]
- Hyttinge, M.; Einarsson, F. Bioservo Technologies. Technical report, Redeye, Equity Research and Investment Banking Company, 2021.
- Moskiewicz, D.; Mraz, M.; Chamela-Bilińska, D. Botulinum Toxin and Dynamic Splint Restore Grasping Function after Stroke: A Case Report. International Journal of Environmental Research and Public Health 2023, 20, 4873. [Google Scholar] [CrossRef] [PubMed]
- Lucado, A.M.; Li, Z.; Russell, G.B.; Papadonikolakis, A.; Ruch, D.S. Changes in impairment and function after static progressive splinting for stiffness after distal radius fracture. Journal of Hand Therapy 2008, 21, 319–325. [Google Scholar] [CrossRef]
- Lucado, A.M.; Li, Z. Static progressive splinting to improve wrist stiffness after distal radius fracture: a prospective, case series study. Physiotherapy theory and practice 2009, 25, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Berner, S.H.; Willis, F.B. Dynamic splinting in wrist extension following distal radius fractures. Journal of orthopaedic surgery and research 2010, 5, 1–4. [Google Scholar] [CrossRef]
- Schwartz, D.A. Static progressive orthoses for the upper extremity: a comprehensive literature review. Hand 2012, 7, 10–17. [Google Scholar] [CrossRef]
- Sodhi, N.; Yao, B.; Anis, H.K.; Khlopas, A.; Sultan, A.A.; Newman, J.M.; Mont, M.A. Patient satisfaction and outcomes of static progressive stretch bracing: a 10-year prospective analysis. Annals of Translational Medicine 2019, 7. [Google Scholar] [CrossRef]
- Dunaway, S.; Dezsi, D.B.; Perkins, J.; Tran, D.; Naft, J. Case report on the use of a custom myoelectric elbow–wrist–hand orthosis for the remediation of upper extremity paresis and loss of function in chronic stroke. Military medicine 2017, 182, e1963–e1968. [Google Scholar] [CrossRef]
- Androwis, G.J.; Engler, A.; Rana, S.; Kirshblum, S.; Yue, G.H. The Rehabilitation Effects of Myoelectric Powered Wearable Orthotics on Improving Upper Extremity Function in Persons with SCI. 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). IEEE, 2021, pp. 4944–4948.
- Androwis, G.J.; Engler, A.; Rana, S.; Kirshblum, S.; Yue, G. Upper Extremity Functional Improvements in Persons with SCI Resulted from Daily Utilization of Myoelectric Powered Wearable Orthotics. 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). IEEE, 2021, pp. 4949–4952.
- Hoppe-Ludwig, S.; Armitage, J.; Turner, K.L.; O’Brien, M.K.; Mummidisetty, C.K.; Koch, L.M.; Kocherginsky, M.; Jayaraman, A. Usability, functionality, and efficacy of a custom myoelectric elbow-wrist-hand orthosis to assist elbow function in individuals with stroke. Journal of Rehabilitation and Assistive Technologies Engineering 2021, 8, 20556683211035057. [Google Scholar] [CrossRef] [PubMed]
- Pundik, S.; McCabe, J.; Skelly, M.; Salameh, A.; Naft, J.; Chen, Z.; Tatsuoka, C.; Fatone, S. Myoelectric Arm Orthosis in Motor Learning-Based Therapy for Chronic Deficits After Stroke and Traumatic Brain Injury. Frontiers in Neurology 2022, 13, 19. [Google Scholar] [CrossRef]
- Chang, S.R.; Hofland, N.; Chen, Z.; Tatsuoka, C.; Richards, L.G.; Bruestle, M.; Kovelman, H.; Naft, J. Myoelectric Arm Orthosis Assists Functional Activities: A 3-Month Home Use Outcome Report. Archives of Rehabilitation Research and Clinical Translation 2023, 5, 100279. [Google Scholar] [CrossRef]
- McCabe, J.P.; Henniger, D.; Perkins, J.; Skelly, M.; Tatsuoka, C.; Pundik, S. Feasibility and clinical experience of implementing a myoelectric upper limb orthosis in the rehabilitation of chronic stroke patients: a clinical case series report. PloS one 2019, 14, e0215311. [Google Scholar] [CrossRef]
- Nilsson, M.; Ingvast, J.; Wikander, J.; von Holst, H. The Soft Extra Muscle system for improving the grasping capability in neurological rehabilitation. 2012 IEEE-EMBS conference on biomedical engineering and sciences. IEEE, 2012, pp. 412–417. [CrossRef]
- Osuagwu, B.A.; Timms, S.; Peachment, R.; Dowie, S.; Thrussell, H.; Cross, S.; Heywood, T.; Shirley, R.; Taylor, J. Clinical Trial of the Soft Extra Muscle Glove to Assess Orthotic and Long-Term Functional Gain Following Chronic Incomplete Tetraplegia: Preliminary Functional Results. International Conference on NeuroRehabilitation. Springer, 2018, pp. 385–389. [CrossRef]
- Kottink, A.I.R.; Nikamp, C.D.; Bos, F.P.; van der Sluis, C.K.; van den Broek, M.; Onneweer, B.; Stolwijk-Swüste, J.M.; Brink, S.M.; Voet, N.B.; Buurke, J.B.; et al. Therapeutic Effect of a Soft Robotic Glove for Activities of Daily Living In People With Impaired Hand Strength: Protocol for a Multicenter Clinical Trial (iHand). JMIR research protocols 2022, 11, e34200. [Google Scholar] [CrossRef]
- Cousins, D.; Porto, R.; Bigelo, A.; Fox, R.; Libs, B.; Holmes, M.; Cort, J. Effects of the Ironhand® Soft Exoskeleton on Forearm Muscle Activity During in Field Automotive Assembly Tasks. Available at SSRN 4559929.
- Hoffman, H.B.; Blakey, G.L. New design of dynamic orthoses for neurological conditions. NeuroRehabilitation 2011, 28, 55–61. [Google Scholar] [CrossRef]
- Jeon, H.s.; Woo, Y.K.; Yi, C.h.; Kwon, O.y.; Jung, M.y.; Lee, Y.h.; Hwang, S.; Choi, B.r. Effect of intensive training with a pring-assisted hand orthosis on movement smoothness in upper extremity following stroke: A pilot clinical trial. Topics in stroke rehabilitation 2012, 19, 320–328. [Google Scholar] [CrossRef]
- Stuck, R.A.; Marshall, L.M.; Sivakumar, R. Feasibility of SaeboFlex upper-limb training in acute stroke rehabilitation: a clinical case series. Occupational therapy international 2014, 21, 108–114. [Google Scholar] [CrossRef]
- Andriske, L.; Verikios, D.; Hitch, D.; et al. Patient and therapist experiences of the SaeboFlex: A pilot study. Occupational therapy international 2017, 2017. [Google Scholar] [CrossRef]
- Barry, J.; Saabye, S.; Baker, D.; Fogarty, J.; Beck, K. Effects of the saeboflex® orthosis and a home exercise program on upper extremity recovery in individuals with chronic. Journal of Neurologic Physical Therapy 2006, 30, 207. [Google Scholar] [CrossRef]
- Lannin, N.A.; Cusick, A.; Hills, C.; Kinnear, B.; Vogel, K.; Matthews, K.; Bowring, G. Upper limb motor training using a Saebo™ orthosis is feasible for increasing task-specific practice in hospital after stroke. Australian occupational therapy journal 2016, 63, 364–372. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, J.; Li, Z.; Su, C.Y. Adaptive impedance control of robotic exoskeletons using reinforcement learning. 2016 International Conference on Advanced Robotics and Mechatronics (ICARM). IEEE, 2016, pp. 243–248. [CrossRef]
- Ren, J.L.; Chien, Y.H.; Chia, E.Y.; Fu, L.C.; Lai, J.S. Deep learning based motion prediction for exoskeleton robot control in upper limb rehabilitation. 2019 International Conference on Robotics and Automation (ICRA). IEEE, 2019, pp. 5076–5082. [CrossRef]
- Rose, L.; Bazzocchi, M.C.; Nejat, G. A model-free deep reinforcement learning approach for control of exoskeleton gait patterns. Robotica 2022, 40, 2189–2214. [Google Scholar] [CrossRef]
- Côté-Allard, U.; Fall, C.L.; Drouin, A.; Campeau-Lecours, A.; Gosselin, C.; Glette, K.; Laviolette, F.; Gosselin, B. Deep learning for electromyographic hand gesture signal classification using transfer learning. IEEE transactions on neural systems and rehabilitation engineering 2019, 27, 760–771. [Google Scholar] [CrossRef]
- Theurel, J.; Desbrosses, K. Occupational exoskeletons: overview of their benefits and limitations in preventing work-related musculoskeletal disorders. IISE Transactions on Occupational Ergonomics and Human Factors 2019, 7, 264–280. [Google Scholar] [CrossRef]
- Elprama, S.A.; Vanderborght, B.; Jacobs, A. An industrial exoskeleton user acceptance framework based on a literature review of empirical studies. Applied Ergonomics 2022, 100, 103615. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).