Submitted:
09 November 2023
Posted:
10 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Elasticity/Viscoelasticity Imaging Using Particles Located within Tissue
2.1. Bubble Located within Tissue
2.2. Sphere Located within Tissue
3. Elasticity/Viscoelasticity Imaging Using Particles Located at Tissue Interface
3.1. Bubble Located at Tissue Interface
3.2. Sphere Located at Tissue Interface
4. Evaluation of Elasticity/Viscoelasticity Imaging Using Particles Located within Tissue and at Tissue Interface
4.1. Evaluation of Mathematical Models
4.2. Evaluation of Tissue Identification Systems
5. Concluding Remarks
- The acoustic radiation force-based elasticity imaging methods that remotely palpate tissue produce a complicated displacement field. The displacement field depends on the transducer focusing characteristics and target geometry. For these absorption-based techniques, the focal volume affects elasticity reconstruction. For elasticity imaging based on the use of small particles located within the tissue and at the tissue interface, particle displacement is independent of the focal zone of the transducer and is affected only by the viscoelastic properties of the local tissue and simple and accurate elasticity reconstruction can be performed. As sophisticated mathematical models for a particle located within the tissue and at the tissue interface can be developed, in addition to elastic properties, density and viscosity of tissue can be determined in the elasticity imaging based on the use of small particles.
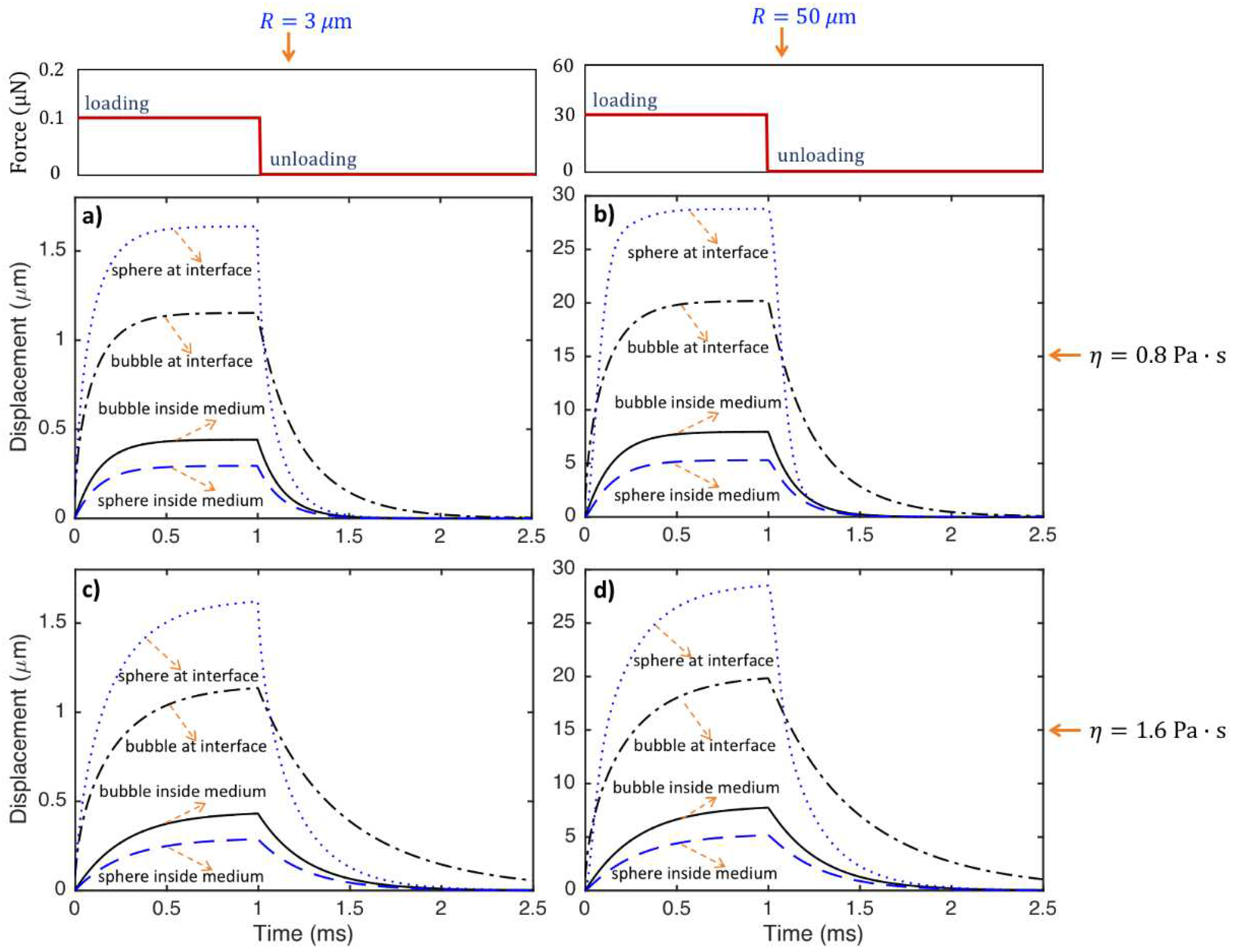
- Because the radiation force applied to the particle located within the tissue and at the tissue interface is generally much greater than that arising in purely absorbing media, lower acoustic power can be used in moderately attenuating media for the elasticity imaging techniques based on the use of small particles.
- For the particles located inside the tissue, there is a need to use a high-powered laser to generate the bubble inside the medium which limits its application to shallow targets and requires local destruction of the material or it is needed to locate a bubble or rigid sphere inside the tissue in a way, which may not be permissible in human tissue. However, thanks to the models recently proposed for the particles located at the tissue interfaces, it is believed that tissue identification for in vivo can be possible in future. Furthermore, thanks to well-developed mathematical models and the ease of their use, the particles located at the medium interface can be conveniently used to identify tissue-mimicking materials and ex vivo in laboratory.
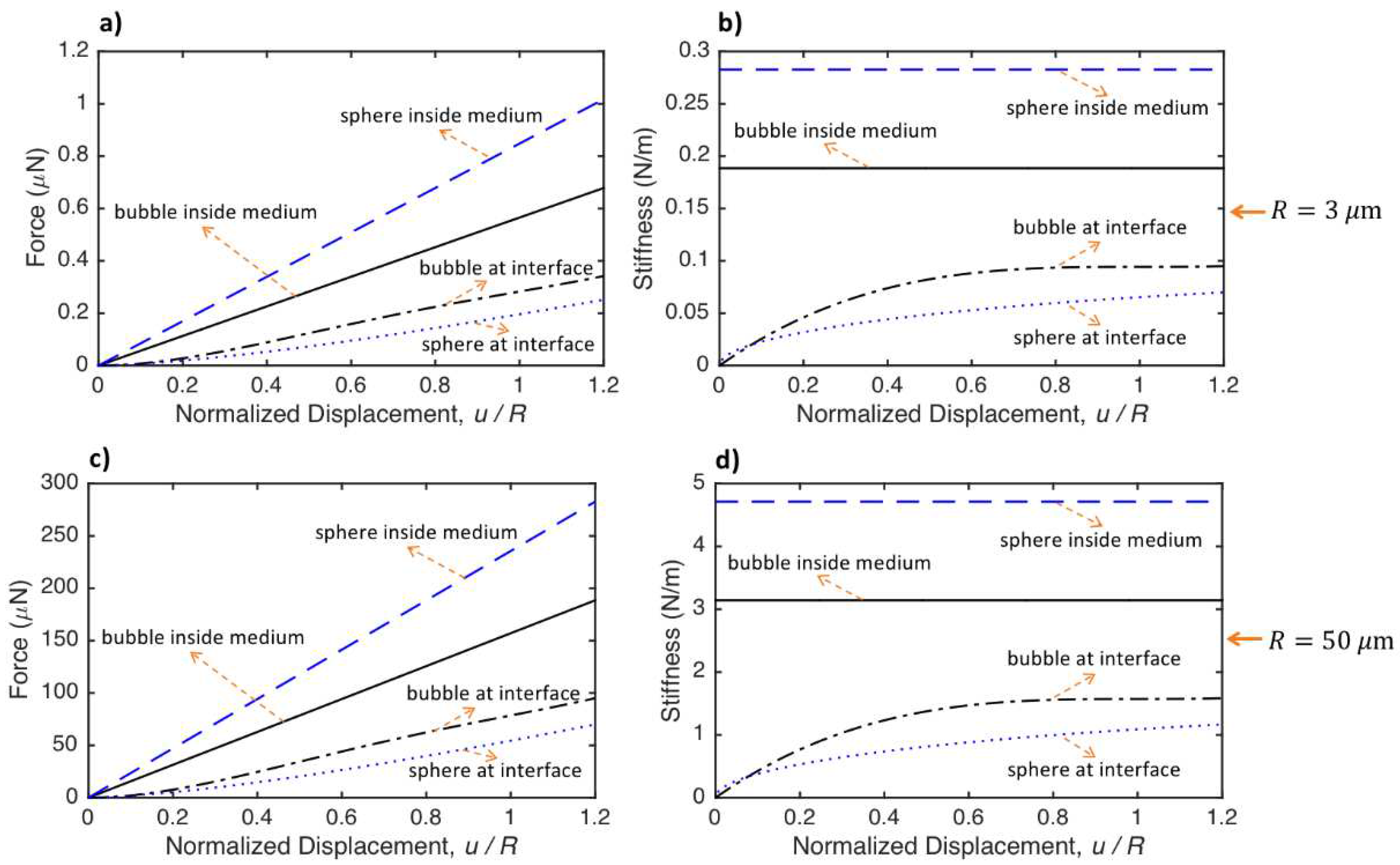
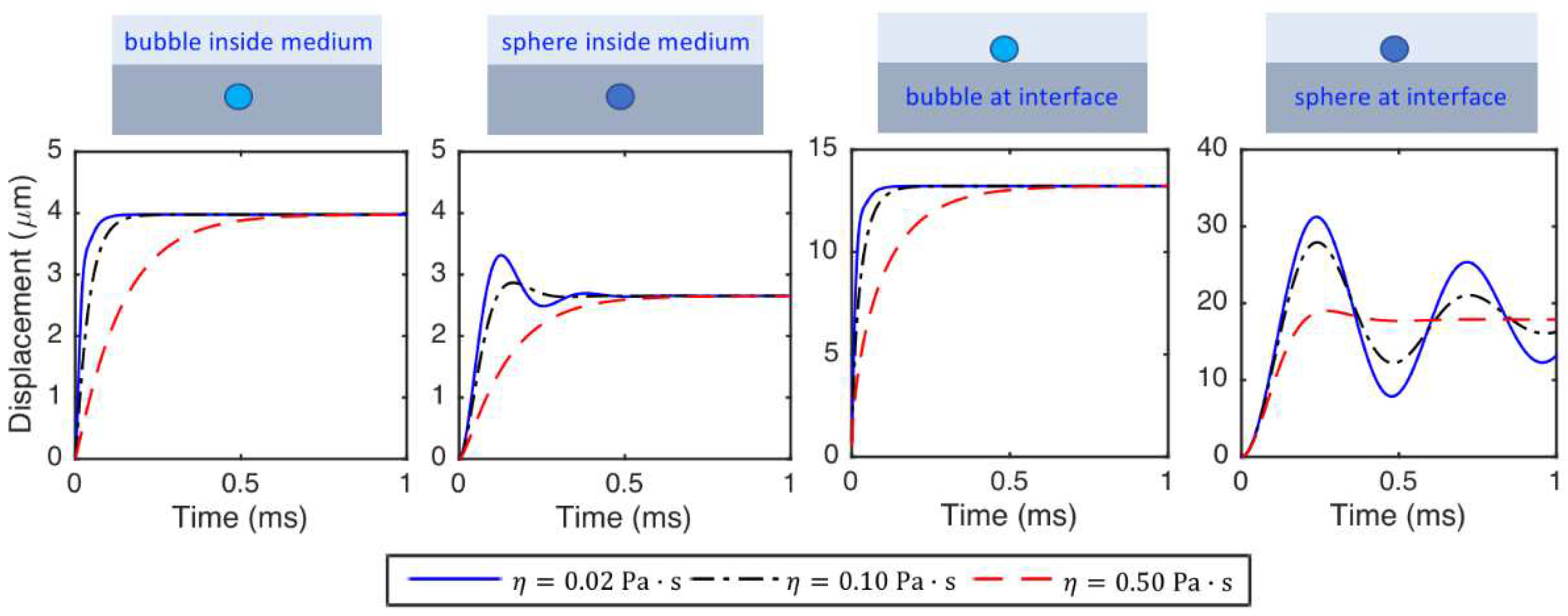
- The stiffness of the bubble and sphere inside the medium is constant, while the stiffness changes nonlinearly with the displacement for the particles located at the medium interfaces. The stiffness, hence, the force required for a specific displacement of the particle, increases from the sphere located at the medium interface to bubble located at the medium interface, bubble inside the medium and sphere inside the medium. The value of force needed for a ratio of 0.5 particle displacement to the radius of the special object and a medium with shear modulus of 5000 Pa is around 70-420 nano Newton and 20-120 micro-Newton for particles with radius of and m, respectively.
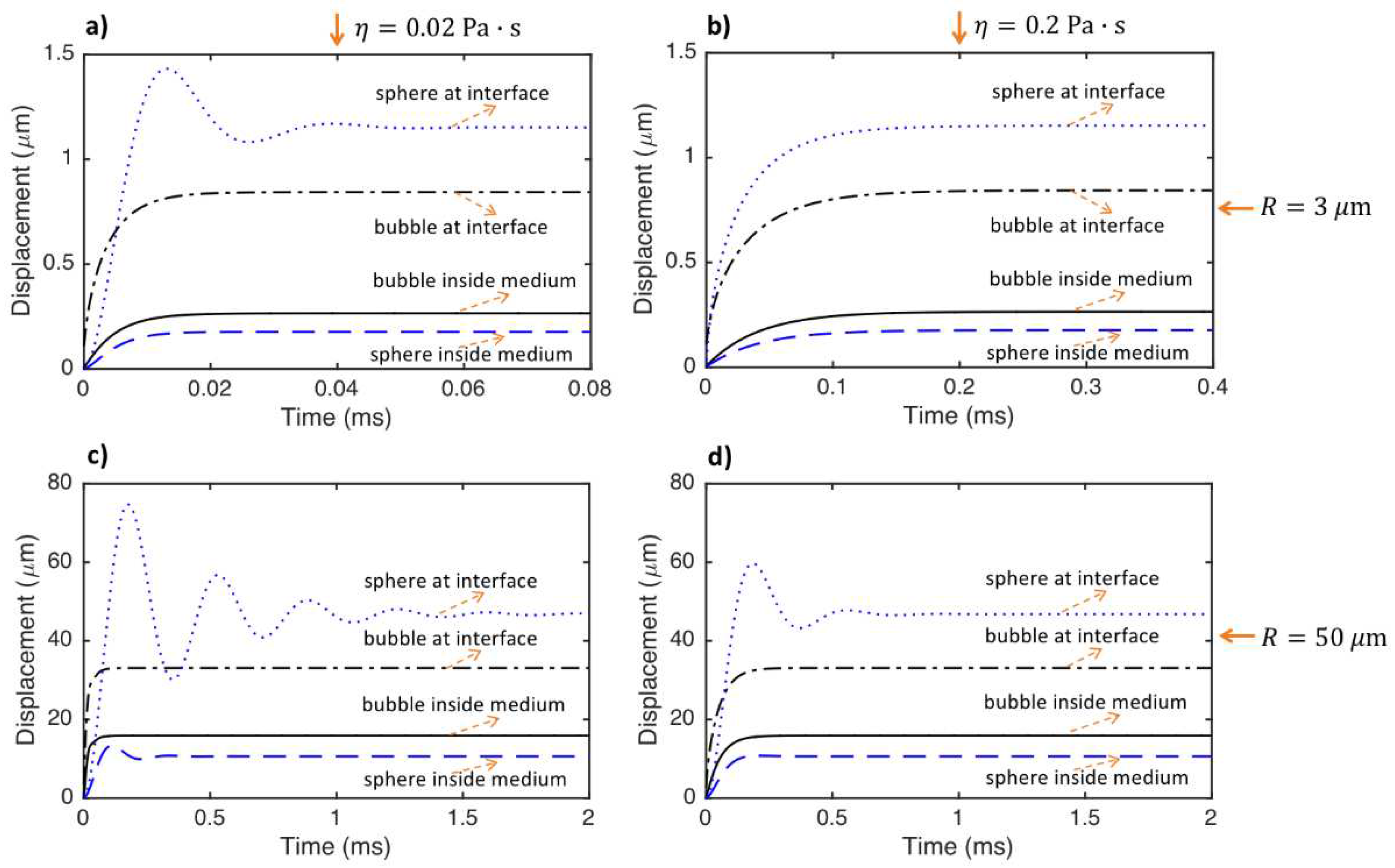
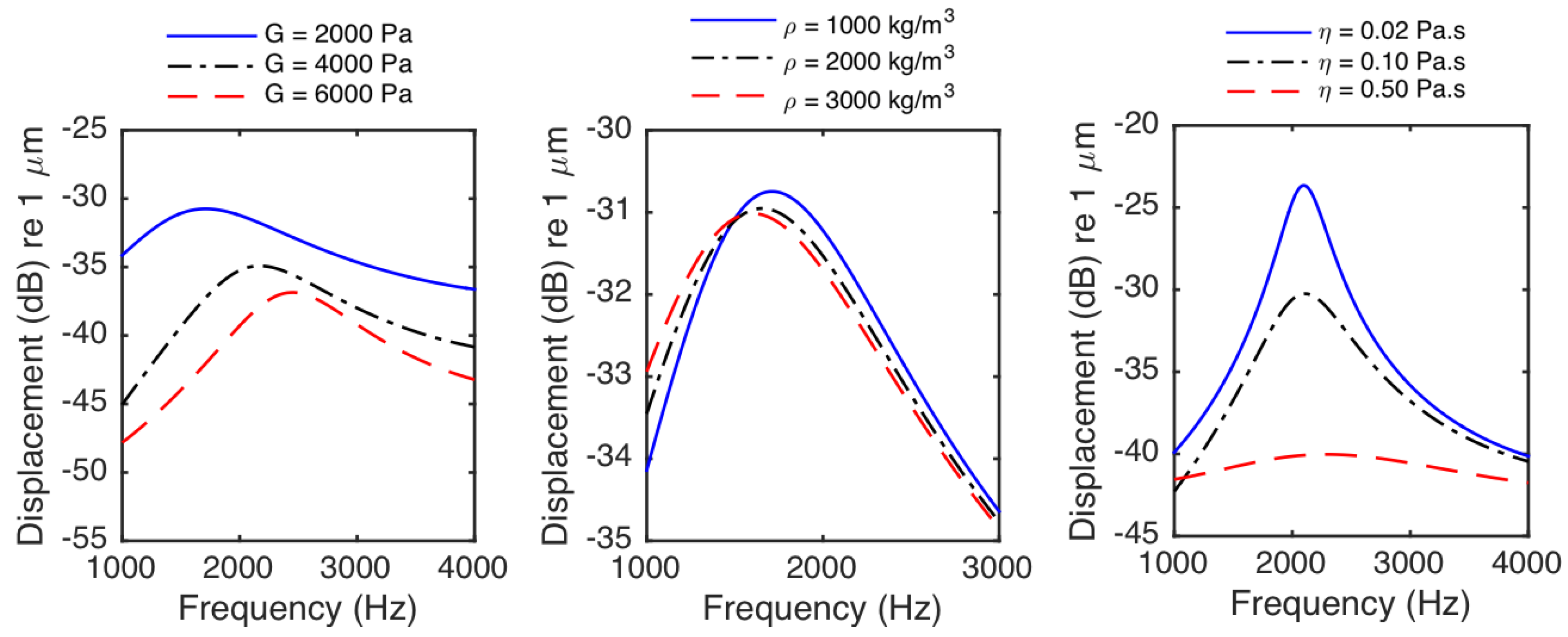
- The period of oscillations of the particle significantly increases (or the frequency of oscillation significantly decreases) as the size of the particle increases. However, even the sphere located at the medium interface does not have any oscillations if the particle size is small and the medium viscosity is considerably high.
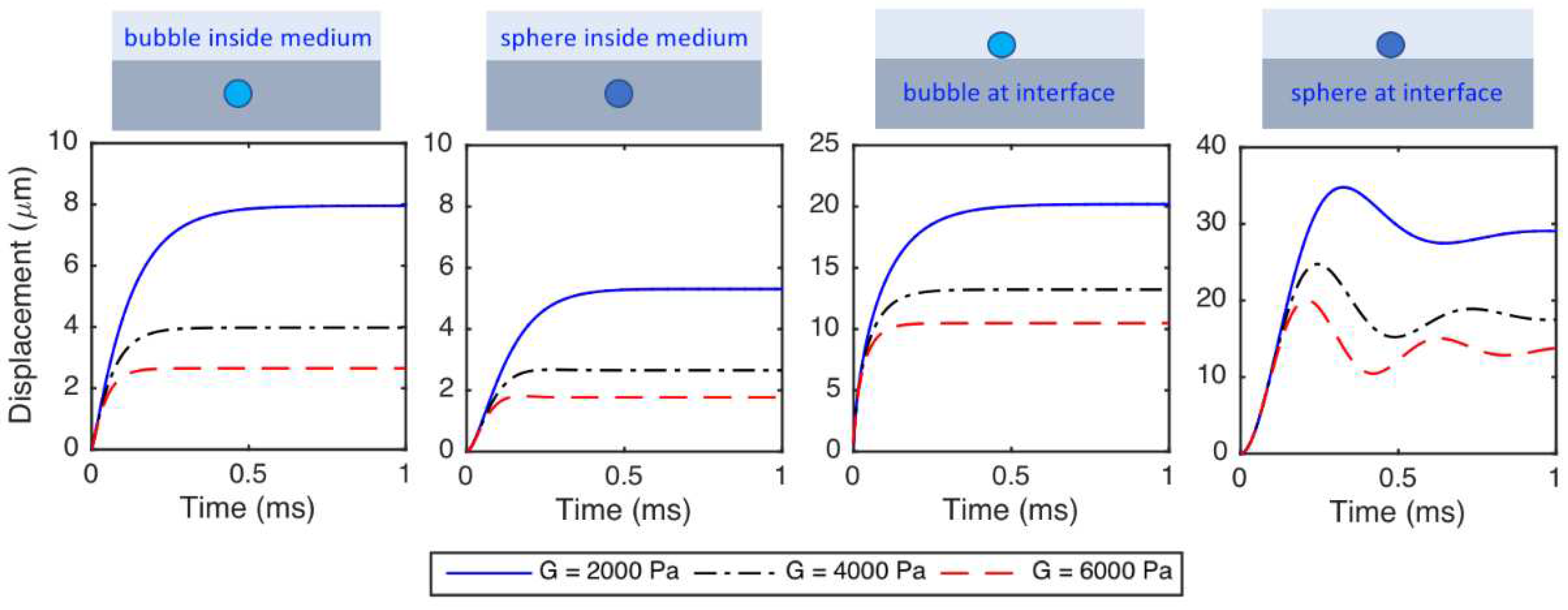
- The displacements of the particles decrease as the medium shear modulus increases. The particles react faster and the time to reach to the steady-state decreases as the medium shear modulus increases. The period of oscillations decreases (or the frequency of oscillation increases) as the medium shear modulus increases.
- The displacements of the particles at a specific time before steady-state decreases with increasing medium viscosity. The particles react faster, and the time needed for the particle to reach the steady state decreases as medium viscosity decreases. The viscosity does not change the steady-state displacements of the particles. The frequency of oscillation of the particle interacting with tissue slightly decreases as medium viscosity increases.
- The frequency of oscillations of the particle interacting with tissue deceases with increasing medium density.
- If only the identification of the elasticity (or Young’s modulus) or shear modulus of tissue is required (i.e., elasticity imaging), it is only needed to measure the displacement of the particle exposed to a static force or the steady-state displacement of the particle exposed to a dynamic force. Using the corresponding mathematical models for a static external force, the elastic properties of the tissue can be identified. However, in addition to the elasticity or shear modulus, if the identification of the density and/or viscosity of tissue is required (i.e., viscoelasticity imaging), there is a need to measure the dynamic response of the particle and to use the corresponding mathematical models for dynamic loading. By performing curve fitting using experimental data and the mathematical models, the Young’s or shear modulus, density and viscosity of tissue can be identified.
- Alternatively, the elastic properties can be determined from the steady-state displacement and the viscosity can be identified from the part of the measured curve corresponding to loading or unloading and the density of the tissue can be determined by matching the measured and theoretical oscillating frequency of the particle. Furthermore, as it may be difficult to know the magnitude of the applied force in practice, without needing the amplitude of the applied force, the Young’s modulus or shear modulus and/or the density of tissue can be identified by matching the measured and theoretical oscillating frequency of the particle and the viscosity of tissue can be identified from the part of the measured curve corresponding to loading or unloading.
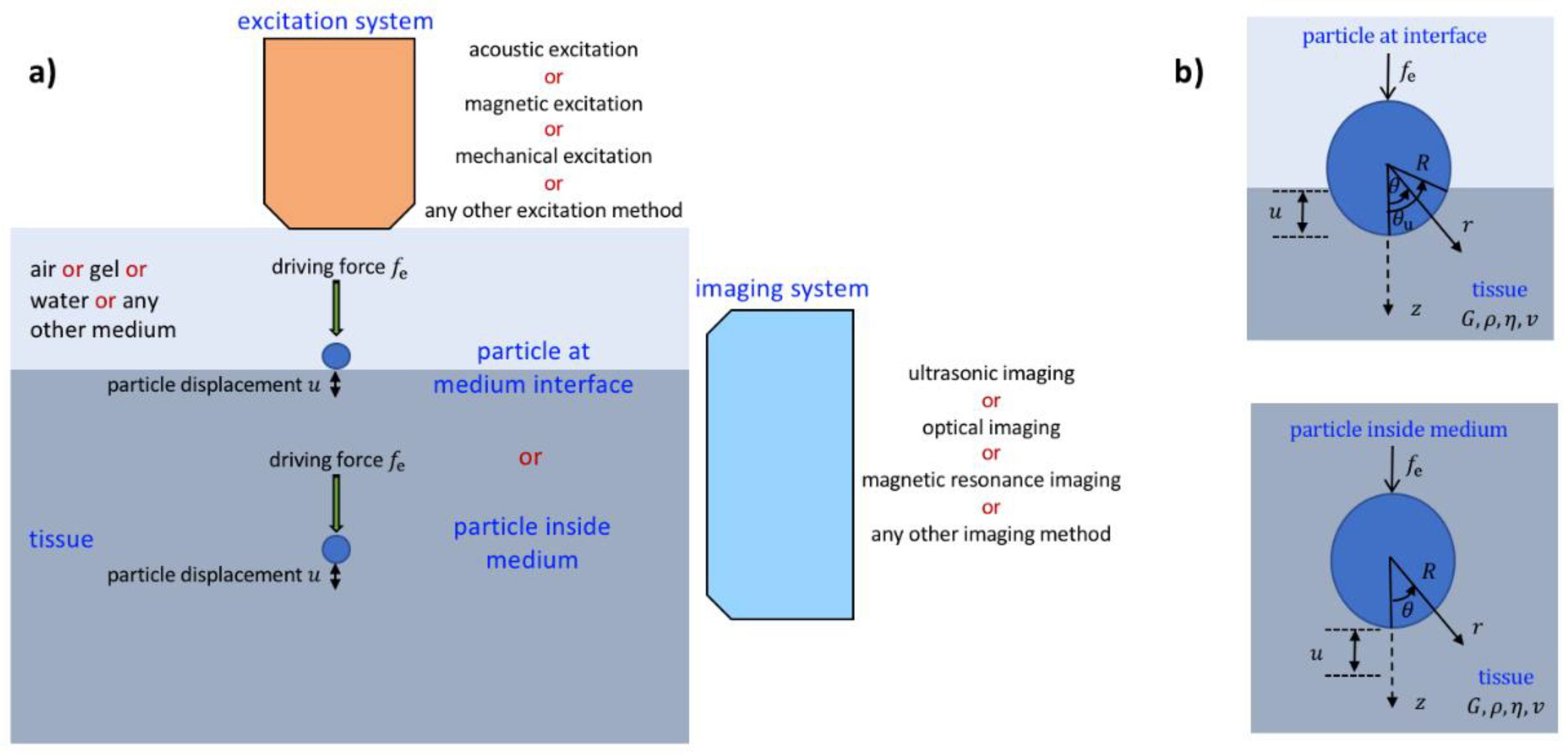
- Although ultrasonic excitation and monitoring is mostly used to identify material properties based on the use of small particles, magnetic and mechanical excitation and optical and MRI imaging can be used for elasticity and viscoelastic imaging based on the use of small particles.
Funding
Data Availability Statement
Conflicts of Interest
References
- Konofagou, E.E. Quo Vadis Elasticity Imaging? Ultrasonics 2004, 42, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Manduca, A.; Oliphant, T.E.; Dresner, M.A.; Mahowald, J.L.; Kruse, S.A.; Amromin, E.; Felmlee, J.P.; Greenleaf, J.F.; Ehman, R.L. Magnetic Resonance Elastography: Non-Invasive Mapping of Tissue Elasticity. Med Image Anal 2001, 5, 237–254. [Google Scholar] [CrossRef]
- Lee, S.; Eun, L.Y.; Hwang, J.Y.; Eun, Y. Ex Vivo Evaluation of Mechanical Anisotropic Tissues with High-Frequency Ultrasound Shear Wave Elastography. Sensors 2022, 22. [Google Scholar] [CrossRef]
- Ličen, U.; Kozinc, Ž. Using Shear-Wave Elastography to Assess Exercise-Induced Muscle Damage: A Review. Sensors 2022, 22, 7574. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, F.; F. , G.J. Ultrasound-Stimulated Vibro-Acoustic Spectrography. Science (1979) 1998, 280, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Sarvazyan, A.P.; Rudenko, O. V; Swanson, S.D.; Fowlkes, J.B.; Emelianov, S.Y. Shear Wave Elasticity Imaging: A New Ultrasonic Technology of Medical Diagnostics. Ultrasound Med Biol 1998, 24, 1419–1435. [Google Scholar] [CrossRef]
- Nightingale, K.R.; Palmeri, M.L.; Nightingale, R.W.; Trahey, G.E. On the Feasibility of Remote Palpation Using Acoustic Radiation Force. J Acoust Soc Am 2001, 110, 625–634. [Google Scholar] [CrossRef]
- Konofagou, E.E.; Hynynen, K. Localized Harmonic Motion Imaging: Theory, Simulations and Experiments. Ultrasound Med Biol 2003, 29, 1405–1413. [Google Scholar] [CrossRef]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic Shear Imaging: A New Technique for Soft Tissue Elasticity Mapping. IEEE Trans Ultrason Ferroelectr Freq Control 2004, 51, 396–409. [Google Scholar] [CrossRef]
- Deffieux, T.; Montaldo, G.; Tanter, M.; Fink, M. Shear Wave Spectroscopy for in Vivo Quantification of Human Soft Tissues Visco-Elasticity. IEEE Trans Med Imaging 2009, 28, 313–322. [Google Scholar] [CrossRef]
- Palmeri, M.L.; Nightingale, K.R. Acoustic Radiation Force-Based Elasticity Imaging Methods. Interface Focus 2011, 1, 553–564. [Google Scholar] [CrossRef]
- Doherty, J.; Trahey, G.; Nightingale, K.; Palmeri, M. Acoustic Radiation Force Elasticity Imaging in Diagnostic Ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control 2013, 60, 685–701. [Google Scholar] [CrossRef]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.-H.; Cosgrove, D.; et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 1: Basic Principles and Terminology. Ultrasound Med Biol 2015, 41, 1126–1147. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; Nakashima, K.; Amy, D.; Cosgrove, D.; Farrokh, A.; Schafer, F.; Bamber, J.C.; Castera, L.; Choi, B.I.; Chou, Y.-H.; et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 2: Breast. Ultrasound Med Biol 2015, 41, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Filice, C.; Castera, L.; Choi, B.I.; Sporea, I.; Wilson, S.R.; Cosgrove, D.; Dietrich, C.F.; Amy, D.; Bamber, J.C.; et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 3: Liver. Ultrasound Med Biol 2015, 41, 1161–1179. [Google Scholar] [CrossRef]
- Cosgrove, D.; Barr, R.; Bojunga, J.; Cantisani, V.; Chammas, M.C.; Dighe, M.; Vinayak, S.; Xu, J.-M.; Dietrich, C.F. WFUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography: Part 4. Thyroid. Ultrasound Med Biol 2017, 43, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; Cosgrove, D.; Brock, M.; Cantisani, V.; Correas, J.M.; Postema, A.W.; Salomon, G.; Tsutsumi, M.; Xu, H.-X.; Dietrich, C.F. WFUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography: Part 5. Prostate. Ultrasound Med Biol 2017, 43, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fatemi, M.; Greenleaf, J.F. Remote Measurement of Material Properties from Radiation Force Induced Vibration of an Embedded Sphere. J Acoust Soc Am 2002, 112, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Ilinskii, Y.A.; Meegan, G.D.; Zabolotskaya, E.A.; Emelianov, S.Y. Gas Bubble and Solid Sphere Motion in Elastic Media in Response to Acoustic Radiation Force. J Acoust Soc Am 2005, 117, 2338–2346. [Google Scholar] [CrossRef]
- Erpelding, T.N.; Hollman, K.W.; O’Donnell, M. Bubble-Based Acoustic Radiation Force Elasticity Imaging. Ultrasonics, Ferroelectrics, and Frequency Control, IEEE Transactions on 2005, 52, 971–979. [Google Scholar] [CrossRef]
- Aglyamov, S.R.; Karpiouk, A.B.; Ilinskii, Y.A.; Zabolotskaya, E.A.; Emelianov, S.Y. Motion of a Solid Sphere in a Viscoelastic Medium in Response to Applied Acoustic Radiation Force: Theoretical Analysis and Experimental Verification. J Acoust Soc Am 2007, 122, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.W.; Nenadic, I.Z.; Mitchell, S.A.; Chen, S.; Greenleaf, J.F. Generalized Response of a Sphere Embedded in a Viscoelastic Medium Excited by an Ultrasonic Radiation Force. J Acoust Soc Am 2011, 130, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Koruk, H.; El Ghamrawy, A.; Pouliopoulos, A.N.; Choi, J.J. Acoustic Particle Palpation for Measuring Tissue Elasticity. Appl Phys Lett 2015, 107. [Google Scholar] [CrossRef] [PubMed]
- Bezer, J.H.; Koruk, H.; Rowlands, C.J.; Choi, J.J. Elastic Deformation of Soft Tissue-Mimicking Materials Using a Single Microbubble and Acoustic Radiation Force. Ultrasound Med Biol 2020, 46, 3327–3338. [Google Scholar] [CrossRef] [PubMed]
- Rus, G.; Faris, I.H.; Torres, J.; Callejas, A.; Melchor, J. Why Are Viscosity and Nonlinearity Bound to Make an Impact in Clinical Elastographic Diagnosis? Sensors 2020, 20, 2379. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, K.; Soo, M.S.; Nightingale, R.; Trahey, G. Acoustic Radiation Force Impulse Imaging: In Vivo Demonstration of Clinical Feasibility. Ultrasound Med Biol 2002, 28, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, M.; Greenleaf, J.F. Vibro-Acoustography: An Imaging Modality Based on Ultrasound-Stimulated Acoustic Emission. Proceedings of the National Academy of Sciences 1999, 96, 6603 LP – 6608. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Church, C.C. A Model for the Dynamics of Gas Bubbles in Soft Tissue. J Acoust Soc Am 2005, 118, 3595–3606. [Google Scholar] [CrossRef]
- Zabolotskaya, E.A.; Ilinskii, Y.A.; Meegan, G.D.; Hamilton, M.F. Modifications of the Equation for Gas Bubble Dynamics in a Soft Elastic Medium. J Acoust Soc Am 2005, 118, 2173–2181. [Google Scholar] [CrossRef]
- Barajas, C.; Johnsen, E. The Effects of Heat and Mass Diffusion on Freely Oscillating Bubbles in a Viscoelastic, Tissue-like Medium. Journal of the Acoustical Society of America 2017, 141, 908–918. [Google Scholar] [CrossRef]
- Hamaguchi, F.; Ando, K. Linear Oscillation of Gas Bubbles in a Viscoelastic Material under Ultrasound Irradiation. Physics of Fluids 2015, 27, 113103. [Google Scholar] [CrossRef]
- Landau, L.D. ; E. M. Lifshitz Fluid Mechanics; 2nd ed.; Pergamon: New York, 1987. [Google Scholar]
- Koruk, H. Assessment of the Models for Predicting the Responses of Spherical Objects in Viscoelastic Mediums and at Viscoelastic Interfaces. IOP Conf Ser Mater Sci Eng 2021, 1150, 012016. [Google Scholar] [CrossRef]
- Erpelding, T.N.; Hollman, K.W.; O’Donnell, M. Mapping Age-Related Elasticity Changes in Porcine Lenses Using Bubble-Based Acoustic Radiation Force. Exp Eye Res 2007, 84, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Hollman, K.W.; O’Donnell, M.; Erpelding, T.N. Mapping Elasticity in Human Lenses Using Bubble-Based Acoustic Radiation Force. Exp Eye Res 2007, 85, 890–893. [Google Scholar] [CrossRef]
- Mikula, E.; Hollman, K.; Chai, D.; Jester, J.V.; Juhasz, T. Measurement of Corneal Elasticity with an Acoustic Radiation Force Elasticity Microscope. Ultrasound Med Biol 2014, 40, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Mikula, E.R.; Jester, J. V; Juhasz, T. Measurement of an Elasticity Map in the Human Cornea. Invest Ophthalmol Vis Sci 2016, 57, 3282–3286. [Google Scholar] [CrossRef]
- Yoon, S.; Aglyamov, S.R.; Karpiouk, A.B.; Kim, S.; Emelianov, S.Y. Estimation of Mechanical Properties of a Viscoelastic Medium Using a Laser-Induced Microbubble Interrogated by an Acoustic Radiation Force. J Acoust Soc Am 2011, 130, 2241–2248. [Google Scholar] [CrossRef]
- Yoon, S.; Aglyamov, S.; Karpiouk, A.; Emelianov, S. The Mechanical Properties of Ex Vivo Bovine and Porcine Crystalline Lenses: Age-Related Changes and Location-Dependent Variations. Ultrasound Med Biol 2013, 39, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Oestreicher, H.L. Field and Impedance of an Oscillating Sphere in a Viscoelastic Medium with an Application to Biophysics. J Acoust Soc Am 1951, 23, 707–714. [Google Scholar] [CrossRef]
- Karpiouk, A.B.; Aglyamov, S.R.; Ilinskii, Y.A.; Zabolotskaya, E.A.; Emelianov, S.Y. Assessment of Shear Modulus of Tissue Using Ultrasound Radiation Force Acting on a Spherical Acoustic Inhomogeneity. IEEE Trans Ultrason Ferroelectr Freq Control 2009, 56, 2380–2387. [Google Scholar] [CrossRef]
- Shih, C.; Liu, T.; Huang, C. In Vitro Assessments of Viscoelastic Properties of Fibrin Clot by Using Acoustic Radiation Force on a Solid Sphere. In Proceedings of the 2010 IEEE International Ultrasonics Symposium; 2010; pp. 479–482. [Google Scholar]
- Huang, C.-C.; Shih, C.-C.; Liu, T.-Y.; Lee, P.-Y. Assessing the Viscoelastic Properties of Thrombus Using a Solid-Sphere-Based Instantaneous Force Approach. Ultrasound Med Biol 2011, 37, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.E.; Oldenburg, A.L. Single Magnetic Particle Motion in Magnetomotive Ultrasound: An Analytical Model and Experimental Validation. IEEE Trans Ultrason Ferroelectr Freq Control 2021, 68, 2635–2644. [Google Scholar] [CrossRef] [PubMed]
- Cebrecos, A.; Jiménez, N.; Tarazona, R.; Company, M.; Benlloch, J.M.; Camarena, F. Characterization of Viscoelastic Media Combining Ultrasound and Magnetic-Force Induced Vibrations on an Embedded Soft Magnetic Sphere. IEEE Trans Ultrason Ferroelectr Freq Control 2021, 68, 3540–3548. [Google Scholar] [CrossRef] [PubMed]
- Koruk, H.; Choi, J.J. Displacement of a Bubble by Acoustic Radiation Force into a Fluid–Tissue Interface. J Acoust Soc Am 2018, 143, 2535–2540. [Google Scholar] [CrossRef] [PubMed]
- Koruk, H.; Choi, J.J. Displacement of a Bubble Located at a Fluid-Viscoelastic Medium Interface. Journal of the Acoustical Society of America 2019, 145, EL410–EL416. [Google Scholar] [CrossRef] [PubMed]
- Saharkhiz, N.; Koruk, H.; Choi, J.J. The Effects of Ultrasound Parameters and Microbubble Concentration on Acoustic Particle Palpation. J Acoust Soc Am 2018, 144, 796–805. [Google Scholar] [CrossRef]
- Koruk, H. Development of a Model for Predicting Dynamic Response of a Sphere at Viscoelastic Interface: A Dynamic Hertz Model. IOP Conf Ser Mater Sci Eng 2021, 1150, 012015. [Google Scholar] [CrossRef]
- Wu, C.-E.; Lin, K.-H.; Juang, J.-Y. Hertzian Load–Displacement Relation Holds for Spherical Indentation on Soft Elastic Solids Undergoing Large Deformations. Tribol Int 2016, 97, 71–76. [Google Scholar] [CrossRef]
- Sneddon, I.N. The Relation between Load and Penetration in the Axisymmetric Boussinesq Problem for a Punch of Arbitrary Profile. Int J Eng Sci 1965, 3, 47–57. [Google Scholar] [CrossRef]
- Dimitriadis, E.K.; Horkay, F.; Maresca, J.; Kachar, B.; Chadwick, R.S. Determination of Elastic Moduli of Thin Layers of Soft Material Using the Atomic Force Microscope. Biophys J 2002, 82, 2798–2810. [Google Scholar] [CrossRef]
- Guo, Z.; Hao, M.; Jiang, L.; Li, D.; Chen, Y.; Dong, L. A Modified Hertz Model for Finite Spherical Indentation Inspired by Numerical Simulations. European Journal of Mechanics - A/Solids 2020, 83, 104042. [Google Scholar] [CrossRef]
- Kontomaris, S. V; Malamou, A. A Novel Approximate Method to Calculate the Force Applied on an Elastic Half Space by a Rigid Sphere. Eur J Phys 2021, 42, 25010. [Google Scholar] [CrossRef]
- Koruk, H. Modelling Small and Large Displacements of a Sphere on an Elastic Half-Space Exposed to a Dynamic Force. Eur J Phys 2021, 52, 055006. [Google Scholar] [CrossRef]
- Koruk, H. Development of an Improved Mathematical Model for the Dynamic Response of a Sphere Located at a Viscoelastic Medium Interface. Eur J Phys 2022, 43, 25002. [Google Scholar] [CrossRef]
- Koruk, H.; Yurdaer, S.B.; Koc, H.O.; Besli, A. Identification of the Viscoelastic Properties of Soft Materials Using a Convenient Dynamic Indentation System and Procedure. Mater Today Proc 2022, 57, 464–468. [Google Scholar] [CrossRef]
- Koruk, H.; Besli, A.; Koc, H.O.; Yurdaer, S.B. Identification of Material Viscoelastic Properties Using the Motion of a Rigid Sphere Located at Tissue-Mimicking Material Interface in Response to a Dynamic Force. Materials Science Forum 2022, 73–78. [Google Scholar] [CrossRef]
- Qiang, B.; Greenleaf, J.; Oyen, M.; Zhang, X. Estimating Material Elasticity by Spherical Indentation Load-Relaxation Tests on Viscoelastic Samples of Finite Thickness. IEEE Trans Ultrason Ferroelectr Freq Control 2011, 58, 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Boots, J.N.M.; Fokkink, R.; van der Gucht, J.; Kodger, T.E. Development of a Multi-Position Indentation Setup: Mapping Soft and Patternable Heterogeneously Crosslinked Polymer Networks. Review of Scientific Instruments 2019, 90, 15108. [Google Scholar] [CrossRef] [PubMed]
- Chim, Y.H.; Mason, L.M.; Rath, N.; Olson, M.F.; Tassieri, M.; Yin, H. A One-Step Procedure to Probe the Viscoelastic Properties of Cells by Atomic Force Microscopy. Sci Rep 2018, 8, 14462. [Google Scholar] [CrossRef]
- Huth, S.; Sindt, S.; Selhuber-Unkel, C. Automated Analysis of Soft Hydrogel Microindentation: Impact of Various Indentation Parameters on the Measurement of Young’s Modulus. PLoS One 2019, 14, e0220281. [Google Scholar]
- Orikasa, K.; Bacca, N.; Agarwal, A. Meso/Macro-Scale Ultra-Soft Materials’ Mechanical Property Evaluation Device and Testbed. Review of Scientific Instruments 2021, 92, 73904. [Google Scholar] [CrossRef]
- Koruk, H.; Koc, H.O.; Yurdaer, S.B.; Besli, A.; Pouliopoulos, A.N. A New Approach for Measuring Viscoelastic Properties of Soft Materials Using the Dynamic Response of a Spherical Object Placed at the Sample Interface. Exp Mech, 2023; under revision. [Google Scholar]
- Koruk, H.; Pouliopoulos, A.N. Investigation of the Motion of a Spherical Object Located at Soft Elastic and Viscoelastic Material Interface for Identification of Material Properties. Applied Science and Engineering Progress 2023. Accepted. [Google Scholar] [CrossRef]
- Karakatsani, M.E.; Pouliopoulos, A.N.; Liu, M.; Jambawalikar, S.R.; Konofagou, E.E. Contrast-Free Detection of Focused Ultrasound-Induced Blood-Brain Barrier Opening Using Diffusion Tensor Imaging. IEEE Trans Biomed Eng 2021, 68, 2499–2508. [Google Scholar] [CrossRef]
- Maccabi, A.; Shin, A.; Namiri, N.K.; Bajwa, N.; St. John, M.; Taylor, Z.D.; Grundfest, W.; Saddik, G.N. Quantitative Characterization of Viscoelastic Behavior in Tissue-Mimicking Phantoms and Ex Vivo Animal Tissues. PLoS One 2018, 13, e0191919. [Google Scholar] [CrossRef]
- Woodard, H.Q.; White, D.R. The Composition of Body Tissues. Br J Radiol 1986, 59, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Urban, M.W.; Pislaru, C.; Kinnick, R.; Zheng, Y.; Yao, A.; Greenleaf, J.F. Shearwave Dispersion Ultrasound Vibrometry (SDUV) for Measuring Tissue Elasticity and Viscosity. IEEE Trans Ultrason Ferroelectr Freq Control 2009, 56, 55–62. [Google Scholar] [CrossRef]
- Koruk, H.; Sanliturk, K.Y. Identification and Removal of Adverse Effects of Non-Contact Electromagnetic Excitation in Oberst Beam Test Method. Mech Syst Signal Process 2012, 30, 274–295. [Google Scholar] [CrossRef]
- Koruk, H.; Dreyer, J.T.; Singh, R. Modal Analysis of Thin Cylindrical Shells with Cardboard Liners and Estimation of Loss Factors. Mech Syst Signal Process 2014, 45, 346–359. [Google Scholar] [CrossRef]

| Particle Used | Particle Location | Excitation Method | Monitoring Method | Target Material | Identified Property | Reference |
|---|---|---|---|---|---|---|
| bubble | inside medium | ultrasonic | ultrasonic | gelation phantom | Young’s modulus | Erpelding et al. 2005 [20] |
| bubble | inside medium | ultrasonic | ultrasonic | human lenses | Young’s modulus | Hollman et al. 2007 [35] |
| bubble | inside medium | ultrasonic | ultrasonic | bovine and porcine crystalline lenses | Young’s modulus and viscosity | Yoon et al. 2013 [39] |
| sphere | inside medium | ultrasonic | ultrasonic | gelation phantom | shear modulus | Karpiouk et al. 2009 [41] |
| sphere | inside medium | ultrasonic | ultrasonic | porcine blood clots | shear modulus and viscosity | Huang et al. 2011 [43] |
| sphere | inside medium | magnetic | ultrasonic | gelation phantom | shear modulus and viscosity | Cebrecos et al. 2021 [45] |
| cloud of bubbles | at medium interface | ultrasonic | optical | gelation phantom | shear modulus | Koruk et al. 2015 [23] |
| bubble | at medium interface | ultrasonic | optical | hydrogel | shear modulus and viscosity | Bezer et al. 2020 [24] |
| sphere | at medium interface | magnetic | optical | gelation phantom | shear modulus and viscous damping ratio | Koruk et al. 2022 [57] |
| sphere | at medium interface | magnetic | optical | gelation phantom | shear modulus and viscosity | Koruk et al. 2022 [64] & Koruk and Pouliopoulos [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




