1. Introduction
The thoracic duct originates in the abdomen from the cisterna chyli sac, which confluences the right and left lumbar trunks and the intestinal trunk, collecting lipid products of digestion. It courses cranially, entering the right chest cavity through the aortic aperture in the diaphragm between the azygos vein, esophagus, and aorta. It drains into the posterior confluence of the left subclavian and internal jugular veins. The thoracic duct is typically 35–45 cm long and has an average diameter of about 2-3 mm [
1]. It collects most of the lymph in the body other than from the right thorax, right arm, right part of the head, and neck, as well as liver convexity. The above-described course is found in 65% of the population. Variations are common, consisting mainly of duplications of the duct, left-sided course, and bilateral course [
2]. The primary function of the thoracic duct is the transport of chyle, a liquid containing both lymph and emulsified fats, from the intestines into the venous circulation.
Chylothorax is an accumulation of lymph in the thoracic cavity commonly due to loss of thoracic duct integrity. The most common reason is iatrogenic thoracic duct injury during thoracic surgical procedures. It typically occurs during esophageal resection. Chylothorax incidence after esophageal surgery is 4 - 10% [
3,
4]. The intimate relation of the esophagus and thoracic duct, the presence of collateral lymphatic ducts, and the variable course of the thoracic duct are the main reasons for a high incidence of injuries during the surgery. It is proposed that prophylactic ligation of the thoracic duct during esophagectomy should be considered an effective preventative measure to reduce the incidence of postoperative chylothorax [
5]. Radical mediastinal lymphadenectomy results in chylothorax in 3-5% of patients. Other surgical procedures potentially causing chylothorax are aortic surgery, pneumonectomy, and removal of posterior mediastinal tumors [
6]. Even non-surgical procedures like subclavian vein catheterization can cause vein thrombosis and bilateral chylothorax with chylopericardium [
7]. Chylothorax occurs after penetrating, rarely after blunt thoracic injuries. The mechanism of blunt injury is spine hyperextension with thoracic duct overstretching over vertebral bodies, leading to duct rupture, usually just above the diaphragm. Dislocation of costovertebral joints of lower ribs and their migration anteriorly can also damage the thoracic duct [
8]. Chylothorax can rarely occur as a result of birth trauma [
9]. Nontraumatic chylothorax occurs in only 20% of cases. The most common cause is malignant thoracic duct obstruction, with lymphoma as a leading malignancy in 70% of patients [
10].
Leakage of lymphatic fluid into the chest leads to severe depletion of proteins, immunoglobulins, fats, vitamins, electrolytes, and water. Massive chylothorax can cause hypovolemia due to extensive circulation volume loss [
1,
4,
6]. The dynamics of decompensation depend on the amount, speed, and duration of leakage. In the early period, patients might be symptom-free. In the advanced stage, malnutrition, hyponatremia, hypocalcemia, and acidosis occur.
2. Patients and methods
We have conducted a retrospective study of all consecutive patients treated at the Department of Thoracic Surgery at Zadar General Hospital, during the ten-year period. Our hospital is a secondary to tertiary referring and teaching center, providing elective and emergency thoracic surgery services to the estimated population of 200,000, which rises during the summer season up to half a million due to a marked tourist influx.
The Hospital’s Ethics Committee approved our study (No. 02-7908/20-2/20).
Between January 2010 to December 2019, all patients with chylothorax records were reviewed. The patients' age, gender, history, the underlying cause of the chylous effusion, treatment outcome, and complications were collected retrospectively using operations logs, and electronic and paper medical records. The collected data were analyzed using descriptive statistical methods.
All of the patients were diagnosed after pleural fluid sampling obtained by insertion of a thoracic drain. The milky appearance of the pleural effusion was usually macroscopically evident, but all the samples were sent for biochemical analysis of the triglyceride level along with the comparison of the serum triglyceride levels which can help determine the diagnosis of chylothorax. All patients in our series had pathognomonic levels of triglyceride >1.24 mmol/L (110 mg/dL). All of the patients had imaging with a contrast-enhanced thorax CT to confirm the pleural effusion and to rule out other pathology that may interfere with the treatment plan.
3. Results
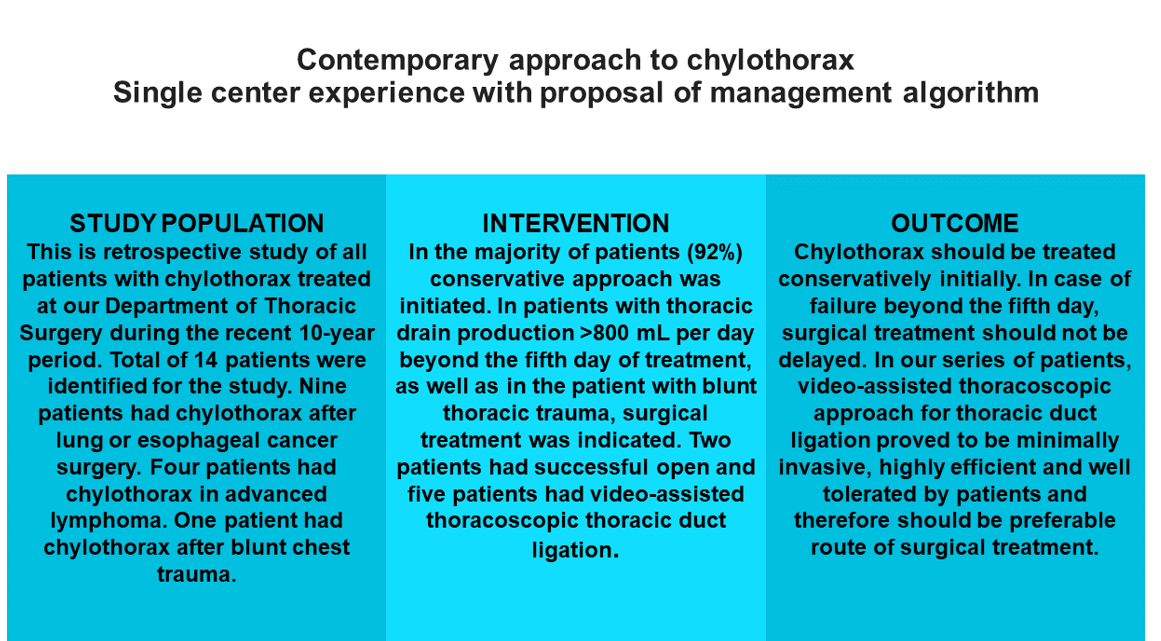
During the ten years (January 2010 to December 2019), a total of 14 patients with chylothorax were treated at our Department of Thoracic Surgery. There were five women (35%) and nine men (65%). The age span was 23 to 81 years (mean 65 years).
Five patients developed chylothorax after lung cancer surgery and mediastinal lymph node dissection. Four patients had chylothorax after radical resection of esophageal cancer. One patient had chylothorax after blunt chest trauma. Malignant chylothorax was noticed in four patients, caused by advanced lymphoma.
Ten patients (71%) had right-sided chylothorax, two patients (14.5%) had left-sided, and two patients (14.5 %) had bilateral chylothorax.
In most patients (13 patients, 92%), a conservative approach was initiated, including pleural drainage, nil per mouth regime, total parenteral nutrition, and octreotide 0.1 mg bid subcutaneously. Although treatment with octreotide, a somatostatin analog is of unproven value, its use was described in the successful treatment of several off-label indications, including chylothorax [
11,
12].
Surgical treatment was indicated relatively early in patients with thoracic drain production >800 mL per day beyond the fifth day of treatment and in a patient with blunt thoracic trauma.
All patients had surgery under endotracheal bi-luminal intubation, allowing selective lung ventilation. Two earlier patients had an open right-sided thoracotomy, while more recent five patients had a video-assisted thoracoscopic surgical approach (VATS). This important shift in the surgical approach over time is related to our growing experience with the use of VATS for various thoracic pathology including lung resections. We have used the EndoCAMeleon™, Karl Storz, 10-mm thoracoscope with the variable direction of view (0
o-90
0) for a minimally invasive approach to thoracic duct identification and ligation [
13].
The distribution and characteristics of 14 analyzed patients are presented in
Table 1.
In seven patients (50%), conservative treatment was successful. The rest had a daily production of >800 mL of fluid beyond the fifth day of conservative treatment, which was the threshold for the surgical approach. It is significant that in all patients with malignant etiology of chylothorax, conservative therapy failed, and they all had to be operated on. The patient with blunt thoracic trauma developed chylothorax on the second day after the injury and had a high daily lymph production of more than 1,500 mL. Considering the mechanism of injury and high output leakage, early surgery was indicated three days after the injury.
VATS approach was successfully used for thoracic duct identification and ligation using Weck
® Hem-o-Lok
® non-absorbable polymer locking clips (Video 1). Resected proximal and distal thoracic duct ends were routinely sent for pathology verification. A total of seven patients underwent surgical therapy. Two of them had massive ligation of tissue between the aorta, azygos vein, esophagus, and spine approached through right thoracotomy. Five patients had video-assisted thoracoscopic precise ligation of the thoracic duct through the right chest. None of the patients required parietal pleurectomy. All operated patients stopped leaking chyle within the 24 hours following surgery which is contrary to the results of the literature review conducted by Kakamad et al on 39 published case reports [
14]. Exact thoracic duct ligation facilitated with the thoracoscopy might provide another reason for the immediate stoppage of chylous leakage.
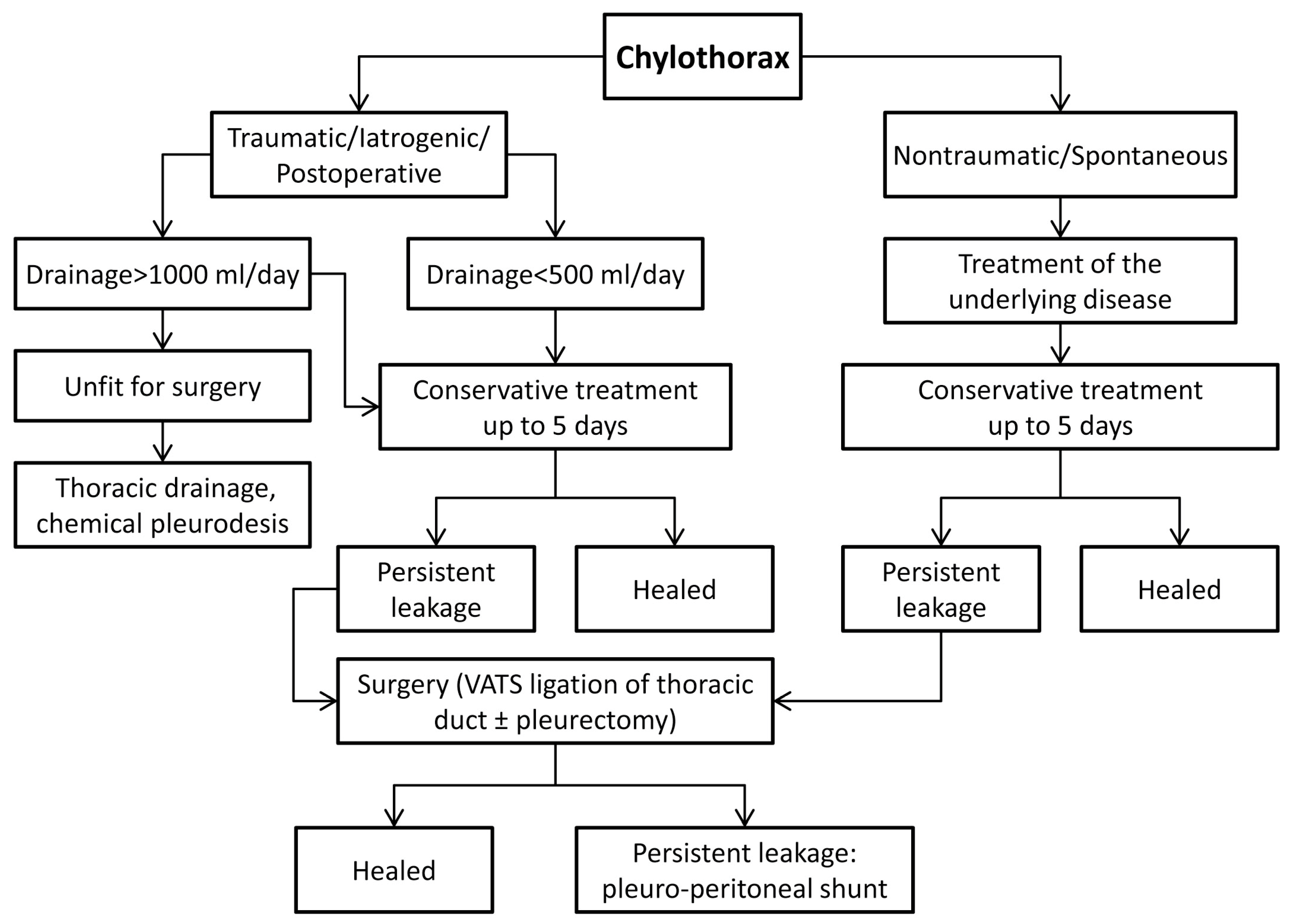
Oral nutrition was introduced on postoperative day one and advanced as tolerated. Based on our experience and along with the reviewed literature, we propose an algorithm for chylothorax management, as shown in
Figure 1.
4. Discussion
Before Lampson reported thoracic duct ligation in 1948, the standard of care in the treatment of traumatic chylothorax was conservative, including nil per mouth, parenteral nutrition, and repeated thoracentesis, which was burdened with devastating 50% mortality [
13]. Nontraumatic (malignant) chylothorax at the time had a mortality of 100% [
1,
6]. Introducing thoracic duct ligation lowered mortality towards a contemporary rate of 7 - 10%. Bilateral and malignant chylothorax harbor the highest mortality rate even today.
The case of death in untreated or inappropriately treated patients is mainly wasting due to malnutrition, gradual immunodeficiency, and sometimes heart failure because of fluid accumulation and compression. Mortality is increased when the condition is unrecognized or treated inappropriately. High output depletion of proteins, immunoglobulins, fats, vitamins, electrolytes, and water leads to rapid malnutrition and immunodeficiency. Therefore, early recognition of this subgroup of patients and timely indication for surgery is essential to prevent the irreversible phase of metabolic and immunologic demise. Continuous loss of immunoglobulins and lymphocytes induces severe immunosuppression with opportunistic infections [
6]. Nevertheless, infection of pleural fluid is rare due to the bacteriostatic properties of lymphatic fluid [
1].
Early diagnosis and appropriate management increase the chances for successful treatment and decrease the complication rate. The milky or murky macroscopic appearance of pleural effusion should raise suspicion of chylothorax [
7]. Clear pleural effusion is expected in the early postoperative period because patients traditionally have reduced oral intake or are fasting. Daily return of the chyle via the thoracic duct is estimated at 1.5 - 2.5 liters. Drainage of more than 400 mL of pleural fluid per day deserves further work-up. Biochemical analysis of pleural fluid in chylothorax is positive if shows high triglyceride content. Triglyceride level >1.24 mmol/L (110 mg/dL) is pathognomonic for chylous effusion. Triglyceride level <0.56 mmol/L (50 mg/dL) rules out chylothorax [
1,
15]. The levels in between the upper and lower range might present diagnostic challenges, but the level of the serum triglyceride level might help confirm or exclude the diagnosis. Also, the cholesterol level in the pleural effusion should be lower than the serum cholesterol value, which is also a prerequisite for a positive diagnosis of chylothorax.
Non-traumatic chylothorax is more challenging to diagnose, sometimes needing magnetic resonance lymphangiography (MRL), which allows noninvasive detection of the source of the chylous leak and selection of the appropriate management approach.
Chylothorax can be treated conservatively, using thoracic duct embolization or with surgery. Medical care aims to lower chyle production by dietetic measures and drainage of pleural space, hopefully leading to spontaneous occlusion of the leaking site. Symptomatic patients need tube thoracostomy, which alleviates respiratory distress caused by excessive fluid accumulation, and lung and heart compression. Drainage allows lung re-expansion, which reduces available space for fluid accumulation. The apposition of the pleurae also contributes to the compression of the leaking site thus reducing the flow and fluid accumulation.
The patient refrains from eating and receives a total parenteral replacement of calories, proteins, fluid, and electrolytes. Several authors reported successful off-label treatment with somatostatin or its synthetic analog octreotide, which inhibits the synthesis of growth hormone, glucagon, and insulin and decreases lymphatic production [
11,
18,
19].
Chemical pleurodesis with bleomycin, minocycline, tetracycline, or, most often, talk, is another therapeutic option aiming to obliterate pleural space, thus preventing fluid accumulation. Pleurodesis can be combined with thoracic duct ligation or as a stand-alone procedure [
19,
20]. When daily fluid production is <500 mL, the reported success rate of conservative treatment is 70 - 90%.
Itkin and colleagues report a series of 109 patients successfully treated with thoracic duct catheter embolization after traumatic thoracic duct leakage. They conclude that this novel approach is a safe, feasible, and minimally invasive method for the treatment of traumatic chylothorax. Nevertheless, this is a technically challenging procedure that needs appropriate facilities as much as highly trained interventional radiologists available which might be reserved for selected tertiary trauma centers or large teaching hospitals [
21].
Persistent leakage of more than 1000 mL per day indicates high-output chylothorax with poor chances for spontaneous resolution, especially if the etiology is iatrogenic (postoperative). Indications for surgical treatment of chylothorax are daily production of >1000 mL in adults or >600 mL in children for four days; persistent leakage for more than two weeks despite conservative treatment; metabolic complications such as electrolyte or immunologic disbalance [
1,
4,
10,
15]. Surgical options are direct ligation of the thoracic duct approached through thoracotomy or VATS; mass ligation of the thoracic duct through thoracotomy or VATS; pleurectomy with pleurodesis or implantation of a pleuro-peritoneal shunt. If the patient has already had a thoracic surgery, the approach through the postoperative wound is reasonable, but it does not preclude the minimally invasive (VATS) approach, depending on local expertise and the choice of a surgeon. Placement of pledgeted sutures or local sealants such as fibrin glue or blood patch was not necessary in our series of patients although advocated by some as an ancillary means of stopping the leakage [
22,
23].
Intraoperative identification of a leaking spot might be challenging despite a clear anatomical position. Several techniques help surgeons identify the site of injury. Administration of olive oil or full-fat milk one hour before surgery per os or via a nasogastric tube can help visualize the leaking spot due to enhanced production of milky effusion [
4,
6]. If it still cannot be identified, the thoracic duct should be ligated directly or
en masse above the diaphragm. The right pleural space is approached through thoracotomy or VATS.
En masse ligation encompasses a bundle of tissue between the aorta, azygos vein, esophagus, and spine [
25,
26]. Isolated ligature requires precise identification and thoracic duct ligation. Traditional suture ligature is usually replaced with titanium or polymeric clips, especially in the era of VATS [
27,
28,
29]. Parietal pleurectomy might be added after the ligation when lymph leakage control is uncertain [
1,
6]. If thoracic duct ligation remains unsuccessful with persistent symptomatic chylothorax, the ultimate option in recurring cases is a pleuro-peritoneal shunt with the rationale that the peritoneum will absorb the excessive fluid [
30,
31]. The method should be used only as a last resort because of the high complication rate, such as catheter obstruction with fibrin, infection, and pneumoperitoneum [
32].
5. Conclusion
In our experience, chylothorax should be initially treated conservatively, although the indication for surgery should not be delayed for more than five days, especially in non-traumatic (malignant) chylothorax and in postoperative (iatrogenic) cases of chylothorax. Due to the advantages of a minimally invasive thoracoscopic approach, high success rate of operative treatment as well as low morbidity and mortality after surgical ligation of the thoracic duct, the threshold for surgery seems to be lower than ever before.
In our series of patients, the VATS approach for thoracic duct ligation proved to be minimally invasive, highly efficient, and well tolerated by patients. It confirms the golden standard in the treatment of chylothorax with a high success rate. Alternative options such as thoracic duct embolization might need dedicated staff and available facilities which might preclude the wider spread of this interesting but demanding technique. Although recommended by several authors, the need for parietal pleurectomy was not encountered since the therapeutic goal was achieved with thoracic duct ligation only.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Algorithm of chylothorax management; Table S1: Distribution and characteristics of 14 consecutive patients with chylothorax; Video S1: Thoracoscopic (VATS) ligation of thoracic duct.
Author Contributions
Conceptualization, I.B. and J.M.; methodology, I.B. and D.M.; software, D.M. and I.K..; validation, I.K., D.V. and Ž.Č.; formal analysis, I.B. and J.M.; investigation, D.M., I.K. and D.V.; resources, D.M., I.K. and D.V.; data curation, D.M., I.K. and D.V.; writing—original draft preparation, I.B.; writing—review and editing, I.B., J.M.; visualization, D.M.; supervision, Ž.Č.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Hospital’s Ethics Committee approved our study (02-7908/20-2/20).
Informed Consent Statement
Patient consent was waived by the Hospital’s Ethics Committee due to the retrospective design of the study. However, the written informed consent has been obtained from the single patient whose video was presented and publish with this paper.
Data Availability Statement
All data details generated during study can be obtained at the request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Johnstone, DW. Anatomy of the Thoracic Duct and Chylothorax in Shields TW, LoCicero III J, Reed CE (eds). General thoracic surgery 7th edition. Lippincott Williams & Wilkins 2009.
- Cha EM, Sirijintakarn P. Anatomic variation of the thoracic duct and visualization of mediastinal lymph nodes: a lymphographic study. Radiology. 1976 Apr;119(1):45-8. [CrossRef] [PubMed]
- Shah RD, Luketich JD, Schuchert MJ, Christie NA, Pennathur A, Landreneau RJ, Nason KS. Postesophagectomy chylothorax: incidence, risk factors, and outcomes. Ann Thorac Surg. 2012 Mar;93(3):897-903; discussion 903-4. [CrossRef] [PubMed] [PubMed Central]
- Merigliano S, Molena D, Ruol A, Zaninotto G, Cagol M, Scappin S, Ancona E. Chylothorax complicating esophagectomy for cancer: a plea for early thoracic duct ligation. J Thorac Cardiovasc Surg. 2000 Mar;119(3):453-7. [CrossRef] [PubMed]
- Lei Y, Feng Y, Zeng B, Zhang X, Chen J, Zou J, Su C, Liu Z, Luo H, Zhang S. Effect of Prophylactic Thoracic Duct Ligation in Reducing the Incidence of Postoperative Chylothorax during Esophagectomy: A Systematic Review and Meta-analysis. Thorac Cardiovasc Surg. 2018 Aug;66(5):370-375. [CrossRef] [PubMed]
- Servelle M, Noguès C, Soulié J, Andrieux JB, Terhedebrugge R. Spontaneous, post-operative and traumatic chylothorax. J Cardiovasc Surg (Torino). 1980 Jul-Aug;21(4):475-86. [PubMed]
- Bender B, Murthy V, Chamberlain RS. The changing management of chylothorax in the modern era. Eur J Cardiothorac Surg. 2016 Jan;49(1):18-24. [CrossRef] [PubMed]
- Nair SK, Petko M, Hayward MP. Aetiology and management of chylothorax in adults. Eur J Cardiothorac Surg. 2007 Aug;32(2):362-9. [CrossRef] [PubMed]
- Attar MA, Donn SM. Congenital chylothorax. Semin Fetal Neonatal Med. 2017 Aug;22(4):234-239. [CrossRef] [PubMed]
- McGrath EE, Blades Z, Anderson PB. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med. 2010 Jan;104(1):1-8. [CrossRef] [PubMed]
- Sharkey AJ, Rao JN. The successful use of octreotide in the treatment of traumatic chylothorax. Tex Heart Inst J. 2012;39(3):428-30. [PubMed] [PubMed Central]
- Blagus L, Mihanović J, Dijan E, Grbić Pavlović P, Pavić I, Ćoza I et al. High-volume post-obstructive choleresis (biliary hyperproduction) with acute kidney injury after choledochotomy, gallstones extraction, and T-tube drainage, successfully treated with octreotide - Report of a case. Med Jad. 2023;53(1):41-45. [CrossRef]
- Bačić I, Morović D, Sulen N, Petani B, Čulina Ž, Kovačić I. VATS lobectomy at the Thoracic Surgery Ward of Zadar General Hospital. Med Jad. 2014;44(3-4):115-118.
- Kakamad FH, Salih RQM, Mohammed SH, HamaSaeed AG, Mohammed DA, Jwamer VI, Ali PG, M Mikael TMS, Hassan MN, Ali RA, Salih AM. Chylothorax caused by blunt chest trauma: a review of literature. Indian J Thorac Cardiovasc Surg. 2020 Nov;36(6):619-624. [CrossRef] [PubMed] [PubMed Central]
- Lampson, RS. Traumatic chylothorax; a review of the literature and report of a case treated by mediastinal ligation of the thoracic duct. J Thorac Surg. 1948 Dec;17(6):778-91. [PubMed]
- Agrawal V, Doelken P, Sahn SA. Pleural fluid analysis in chylous pleural effusion. Chest. 2008 Jun;133(6):1436-1441. [CrossRef] [PubMed]
- Nadolski, G. Nontraumatic Chylothorax: Diagnostic Algorithm and Treatment Options. Tech Vasc Interv Radiol. 2016 Dec;19(4):286-290. [CrossRef] [PubMed]
- Foo NH, Hwang YS, Lin CC, Tsai WH. Congenital chylothorax in a late preterm infant and successful treatment with octreotide. Pediatr Neonatol. 2011 Oct;52(5):297-301. [CrossRef] [PubMed]
- Roehr CC, Jung A, Proquitté H, Blankenstein O, Hammer H, Lakhoo K, Wauer RR. Somatostatin or octreotide as treatment options for chylothorax in young children: a systematic review. Intensive Care Med. 2006 May;32(5):650-7. [CrossRef] [PubMed]
- Mares DC, Mathur PN. Medical thoracoscopic talc pleurodesis for chylothorax due to lymphoma: a case series. Chest. 1998 Sep;114(3):731-5. [CrossRef] [PubMed]
- Itkin M, Kucharczuk JC, Kwak A, Trerotola SO, Kaiser LR. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg. 2010 Mar;139(3):584-89; discussion 589-90. [CrossRef] [PubMed]
- Paul S, Altorki NK, Port JL, Stiles BM, Lee PC. Surgical management of chylothorax. Thorac Cardiovasc Surg. 2009 Jun;57(4):226-8. [CrossRef] [PubMed]
- Sesti J, Luker J, Decker J, Paul S. Modified Blood Patch Used to Treat a High Output Chyle Leak After McKeown Esophagectomy. Ann Thorac Surg. 2020 Jun;109(6):e401-e402. [CrossRef] [PubMed]
- Huang PM, Lee YC. A new technique of continuous pleural irrigation with minocycline administration for refractory chylothorax. Thorac Cardiovasc Surg. 2011 Oct;59(7):436-8. [CrossRef] [PubMed]
- Paul S, Altorki NK, Port JL, Stiles BM, Lee PC. Surgical management of chylothorax. Thorac Cardiovasc Surg. 2009 Jun;57(4):226-8. [CrossRef] [PubMed]
- Patterson GA, Todd TR, Delarue NC, Ilves R, Pearson FG, Cooper JD. Supradiaphragmatic ligation of the thoracic duct in intractable chylous fistula. Ann Thorac Surg. 1981 Jul;32(1):44-9. [CrossRef] [PubMed]
- Graham DD, McGahren ED, Tribble CG, Daniel TM, Rodgers BM. Use of video-assisted thoracic surgery in the treatment of chylothorax. Ann Thorac Surg. 1994 Jun;57(6):1507-11; discussion 1511-2. [CrossRef] [PubMed]
- Kent RB 3rd, Pinson TW. Thoracoscopic ligation of the thoracic duct. Surg Endosc. 1993 Jan-Feb;7(1):52-3. [CrossRef] [PubMed]
- Christodoulou M, Ris HB, Pezzetta E. Video-assisted right supradiaphragmatic thoracic duct ligation for non-traumatic recurrent chylothorax. Eur J Cardiothorac Surg. 2006 May;29(5):810-4. [CrossRef] [PubMed]
- Little AG, Kadowaki MH, Ferguson MK, Staszek VM, Skinner DB. Pleuro-peritoneal shunting. Alternative therapy for pleural effusions. Ann Surg. 1988 Oct;208(4):443-50. [CrossRef] [PubMed] [PubMed Central]
- Gupta D, Ross K, Piacentino V 3rd, Stepnowski D, McClurken JB, Furukawa S, Dempsey DT. Use of LeVeen pleuroperitoneal shunt for refractory high-volume chylothorax. Ann Thorac Surg. 2004 Jul;78(1):e9-12. [CrossRef] [PubMed]
- Foroulis CN, Desimonas NA. Massive pneumoperitoneum: a late complication of the Denver pleuroperitoneal shunt. Ann Thorac Surg. 2005 Oct;80(4):e13. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).