Submitted:
06 November 2023
Posted:
08 November 2023
You are already at the latest version
Abstract
Keywords:

1. Introduction
1.1. Acute Ischemic Stroke: A Brief Overview
1.2. The Debate Surrounding Medical Foods and Supplements
- N06BX - Other psycho-stimulants and nootropics, a subgroup under the wider category of psycho-stimulants (N06B).
- N06BX06 - The specific code for citicoline as an individual nootropic agent.
1.3. Relevance and Role of the Triadic Conceptual Graph
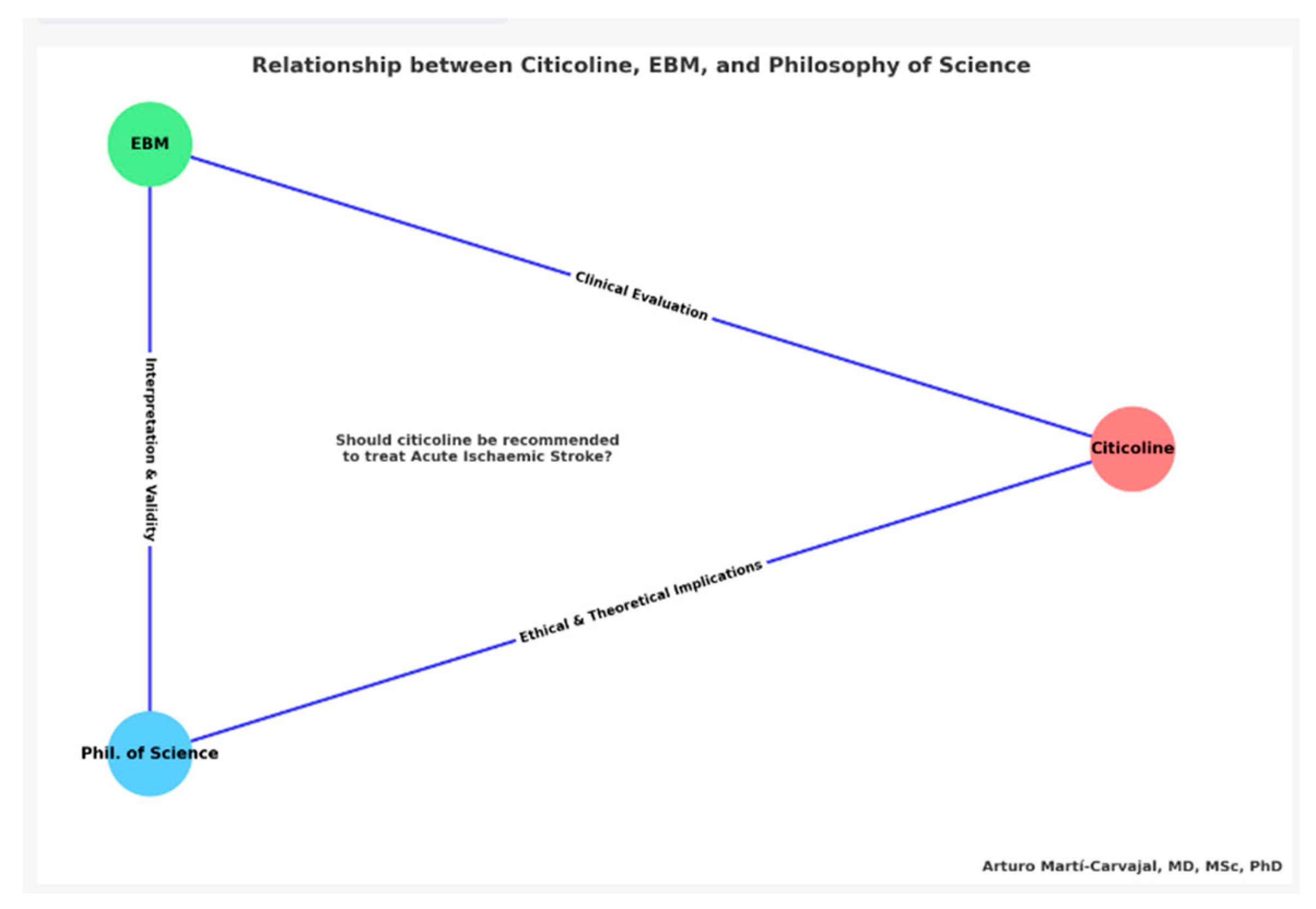
1.3.1. The Bedrock and Cornerstone: Evidence-Based Medicine (EBM)
1.3.2. The Prism: Philosophy of Science
1.3.3. The Exemplar: Citicoline
1.3.4. The Unifying Query
2. Research Question
3. Objective
4. Methodology: Navigating the Sea of Evidence
4.1. Sources of Data and Document Evaluation
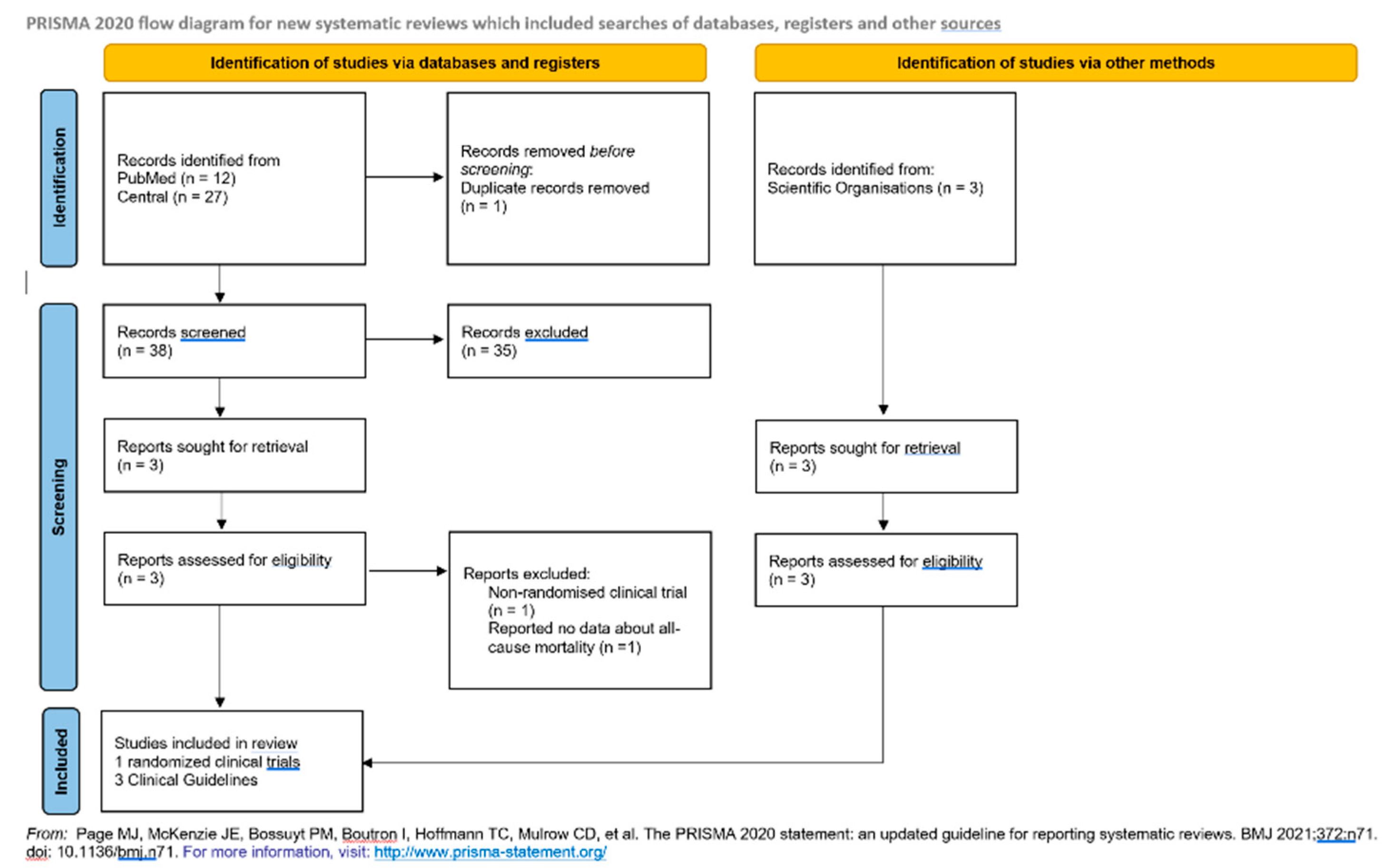
4.2. Application of Cochrane Collaboration Recommendations
4.3. Critical Analysis of Biomedical Literature
4.4. Trial Sequential Analysis
5. The Art of Soluble and Navigating the Mystery of Citicoline
5.1. Medawar's Meditations in the Context of Citicoline
5.2. The Hypothesis-Driven Exploration of Citicoline's Efficacy
6. The Scaffold of Scientific Trust: Deciphering the Hierarchy of Evidence
6.1. The Essence of Evidence-Based Medicine (EBM)
6.2. The Cochrane Review: A Gold Standard in Medical Research
7. Diving into the Cochrane Systematic Review
7.1. The Cochrane Gold Standard
7.2. Unraveling the Findings
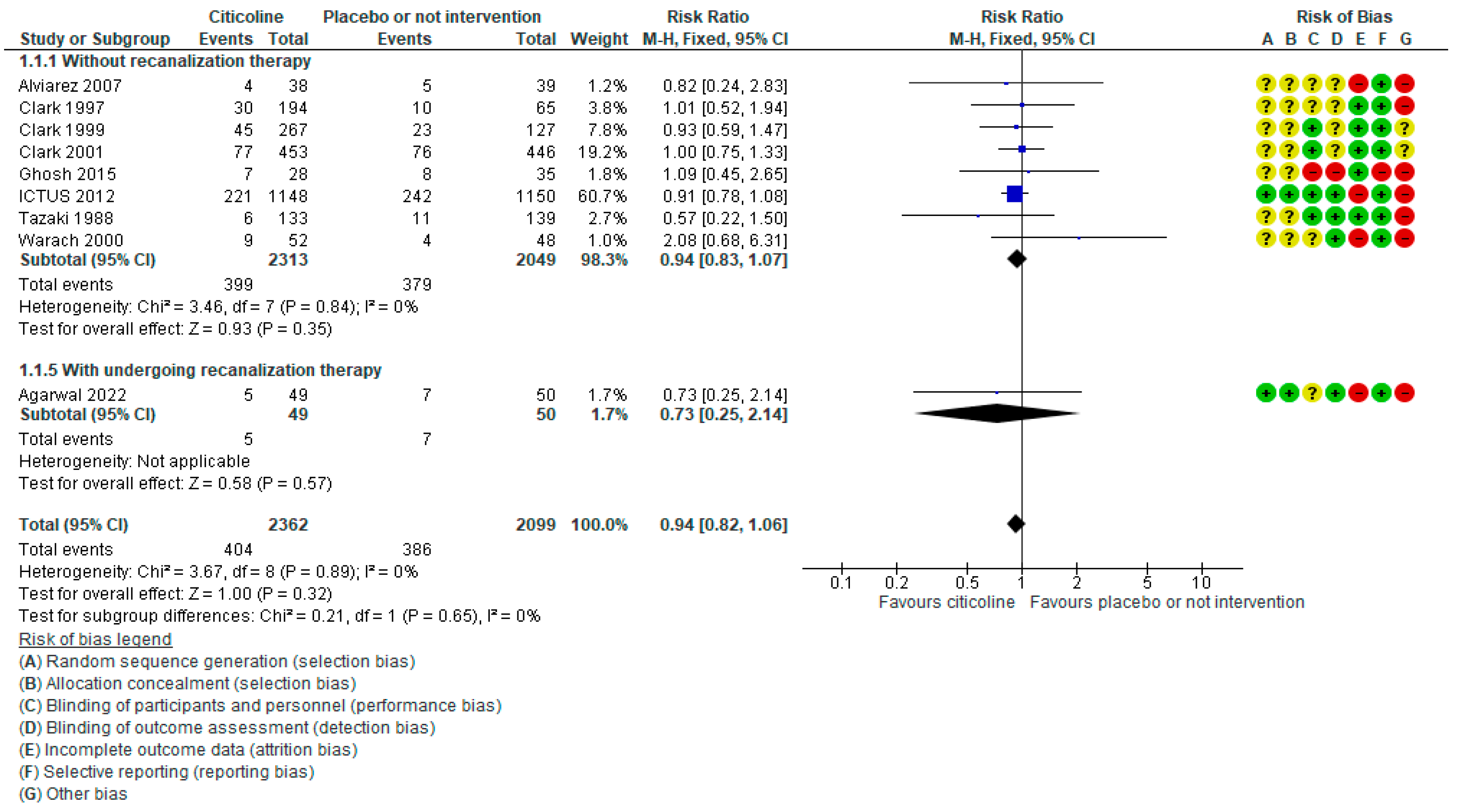
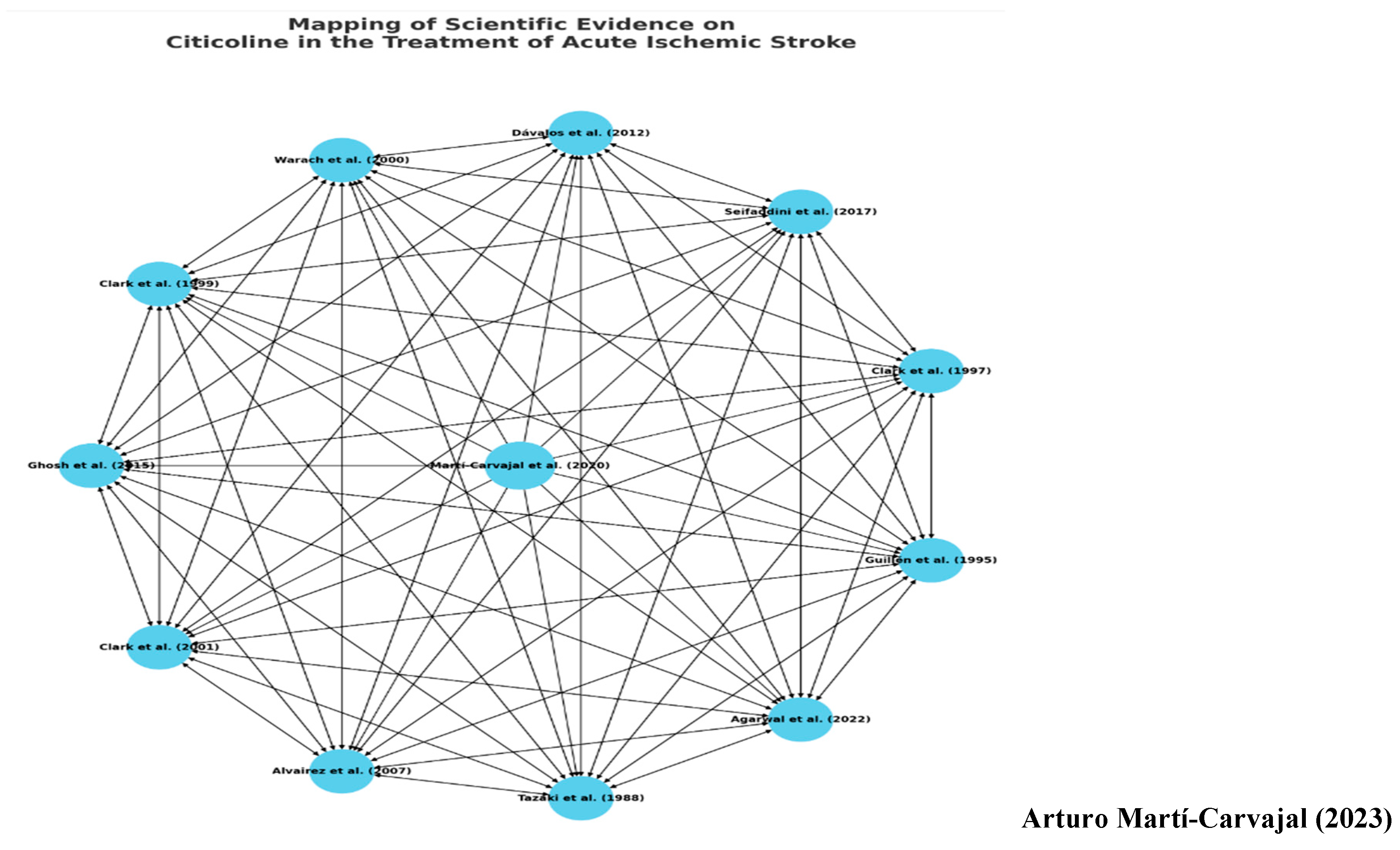
7.3. Beyond Numbers - Implications for Practice
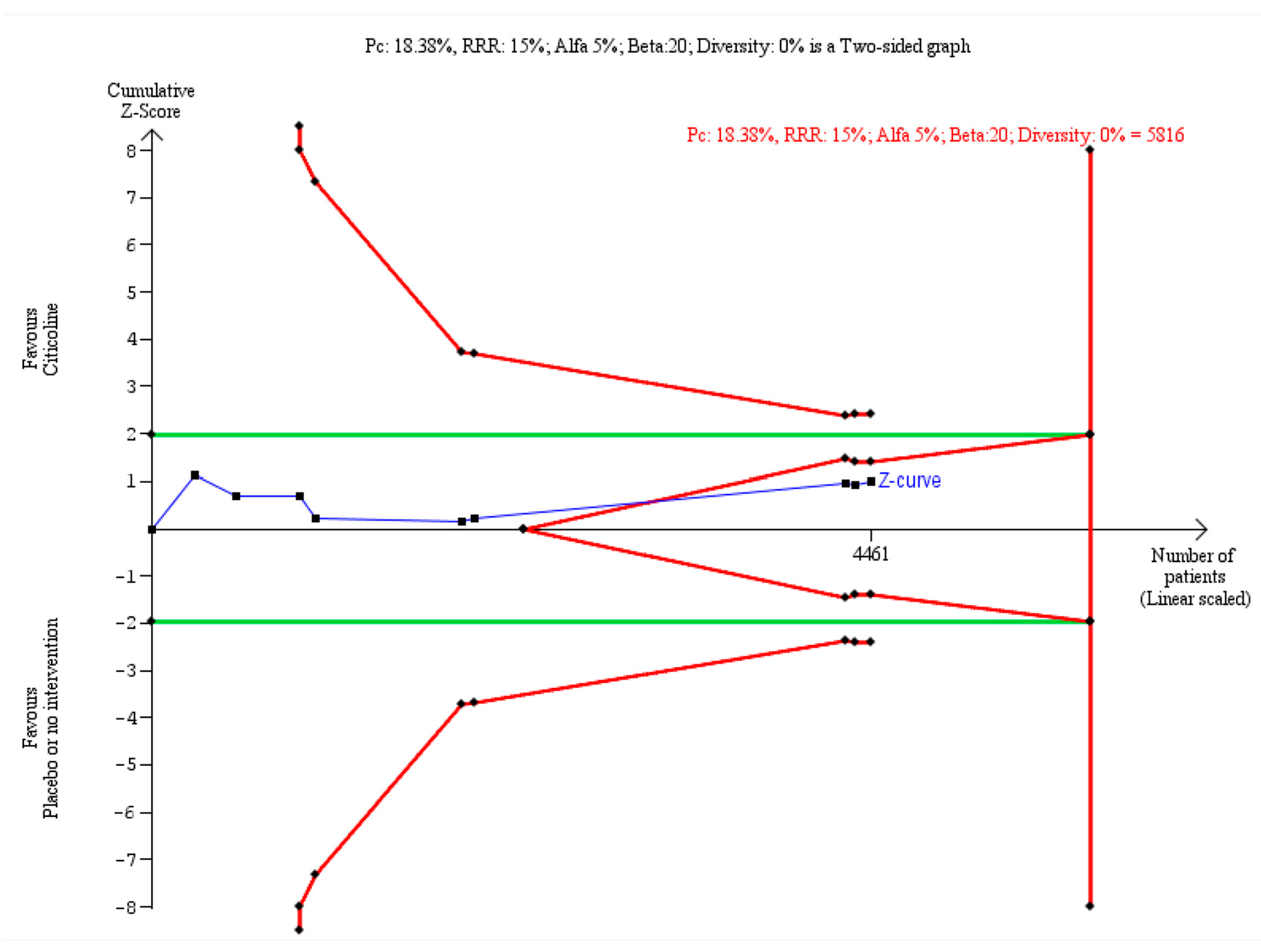
7.4. Trial Sequential Analysis (TSA) - The Final Seal
7.5. Concluding Thoughts
8. Probing the Citicoline Controversy: A Discourse Analysis through Popper, Medawar and Foucault.
8.1. Bridging the Divide: A Comparative Evaluation of Current Evidence on Citicoline for Acute Ischemic Stroke
8.2. Philosophy of Science and Evidence-Based Medicine
8.1.1. Bridging Two Domains
8.1.2. Popperian Falsification and the Citicoline Debate
8.1.3. Foucauldian Analysis - Beyond the Surface
8.1.4. Concluding Thoughts
Colophon
Coda
Funding
Conflicts of Interest
Statement
Invitation
| 1 | EFSA denotes The European Food Safety Authority. |
References
- Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJB, Culebras A; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition; Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44(7):2013 Jul;44(7):2064-89. [CrossRef]
- Adams Jr HP, Bendixen BH, L J KappelleL J, Biller J, Love BB, Gordon Dl, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993 Jan;24(1):35-41;24(1):35-41. [CrossRef]
- Feske, SK. Ischemic stroke. Am. J. Med. 2021, 134, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carlson AP, et al. Heart disease and stroke statistics-2020 Update: a report from the American Heart Association. Circulation 2020;141(9):e139-e596. [CrossRef]
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K; American Heart Association Stroke Council. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50:12: e46-e99. [CrossRef]
- National Institute of Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. Available online: http://www.nice.org.uk/guidance/NG128 (accessed on 27 September 2023).
- Qiu S, Xu Y. Guidelines for acute ischemic stroke treatment. Neuroscience Bulletin. 2020;36(10):1229-32. [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2013. Scientific Opinion on the safety of “citicoline” as a Novel Food ingredient. EFSA Journal 2013; 11 (10):3421, 22 pp. [CrossRef]
- Food and Drug Administration. Frequently asked questions about medical foods; third edition guidance for industry. Silver Spring, MD: Food and Drug Administration; 2023 Mar.
- European Commission. Commission implementing decision of 1 July 2014 authorising the placing on the market of citicoline as a novel food ingredient under regulation (EC) No 258/97 of the European Parliament and of the Council (notified under document C(2014) 4252) (2014/423/EU). Off. J. Eur. Union 2014, L196, 24–26. [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology. Available online: https://www.whocc.no/atc_ddd_index/?code=N06BX06 (accessed on 30 October 2023).
- Cochrane, A.L. Effectiveness and efficiency: random reflections on health services. First edition. London: Nuffield Provincial Hospitals Trust, 1972.
- Sackett DL, Rosenberg WM. The need for evidence-based medicine. J R Soc Med. 1995; 88:620–4. [CrossRef]
- Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn't. BMJ. 1996; 312:71–2. [CrossRef]
- Spade PV, Panaccio C. "William of Ockham", The Stanford Encyclopedia of Philosophy (Spring 2019 Edition), Zalta EN. (ed.). Available online: http://plato.stanford.edu/archives/spr2019/entries/ockham (accessed on 15 October 2023).
- Simon GL, Rios JC. Ockham's razor revisited. Archives of Internal Medicine 1984;144(71371-2). [CrossRef]
- Popper, K.R. The logic of scientific discovery. London and New York: Routledge Classics, 2002. [Other: ISBN 0-415-27844-9]. [Other.
- Uebel, T. "Vienna Circle", The Stanford Encyclopedia of Philosophy (Fall 2022 Edition), Zalta EN, Nodelman U. (eds.). Available online: http://plato.stanford.edu/archives/fall2022/entries/vienna-circle/> (accessed on 30 October 2023).
- Martí-Carvajal_AJ, Valli_C, Martí-Amarista_CE, Solà_I, Martí-Fàbregas_J, Bonfill Cosp_X. Citicoline for treating people with acute ischemic stroke. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No.: CD013066. [CrossRef]
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons, 2019.
- Agarwal A, Vishnu VY, Sharma J, Bhatia R, Garg A, Dwivedi S, et al. Citicoline in acute ischemic stroke: A randomized controlled trial. PLoS ONE 2022;17(5):e0269224. [CrossRef]
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence--imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [PubMed: 21839614] Copenhagen Trial Unit. TSA - Trial Sequential Analysis. Available online: http://ctu.dk/tsa/ (accessed on 25 May 2017).
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). Available online: http://ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed on 6 October 202023).
- Medawar, P. Pluto's Republic: Incorporating the art of the soluble and induction and intuition in scientific thought. Oxford: Oxford University Press, 1984. [Other: ISBN 9780192830395].
- Blunt, C. Hierarchies of evidence in Evidence -Based Medicine. The London School of Economics and Political Science. A thesis submitted to the Department of Philosophy, Logic & Scientific Method of the London School of Economics for the degree of Doctor of Philosophy, London, September 2015. Available online: http://etheses.lse.ac.uk/id/eprint/3284.
- Cassels, A. The Cochrane Collaboration Medicine's Best-Kept Secret. Gabriola, BC, Canada: Agio Publishing House, 2015. [Other: ISBN 97819277545303].
- Sestine, P. Epistemology and ethics of evidence-based medicine: putting goal-setting in the right place. Journal of Evaluation of Clinical Practice 2010;16(2):301-5. [CrossRef]
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Medical Research Methodology 2017;17(1):39. [CrossRef]
- Sagaro GG, Amenta F. Choline-containing phospholipids in stroke treatment: A systematic review and meta-analysis. Journal of Clinical Medicine 2023;12(8):2875. [CrossRef]
- Gonzales NR, Grotta JC. Pharmacologic modification of acute cerebral ischemia. In: Grotta JC, Albers GW, Broderick JP, Day AL, Kasner SE, Lo EH, Sacco RL, Wong LKS, editor(s). Stroke. Pathophysiology, Diagnosis, and Management. Seventh edition. Philadelphia, PA: Elsevier, 2022:831-51.e6. [Other: ISBN: 978-0-323-69424-7].
- Foucault, M. La arqueología del saber. 1ra reimpresión. 1ra edición. Buenos Aires, Argentina: Siglo XXI Editores, S.A de C.V, 2004. [Other: ISBN 987-1105-07-X].
- Foucault, M. ¿Qué es la crítica? 1era edición. Buenos Aires, Argentina: Siglo XXI Editores Argentina, 2018. [Other: ISBN 978-987-629-869-8].
- Dávalos A, Alvarez-Sabín J, Castillo J, Díez-Tejedor E, Ferro J, Martínez-Vila E, International Citicoline Trial on acute Stroke (ICTUS) trial investigators. Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial). Lancet 2012;380(9839):349-57. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



