Submitted:
04 November 2023
Posted:
07 November 2023
You are already at the latest version
Abstract
Keywords:
1. Background Information
1.1. Liver to the gut pathway
1.2. Association of metabolic disorder and IBD
2. IBD Pathophysiology
2.1. Intestinal permeability and barrier
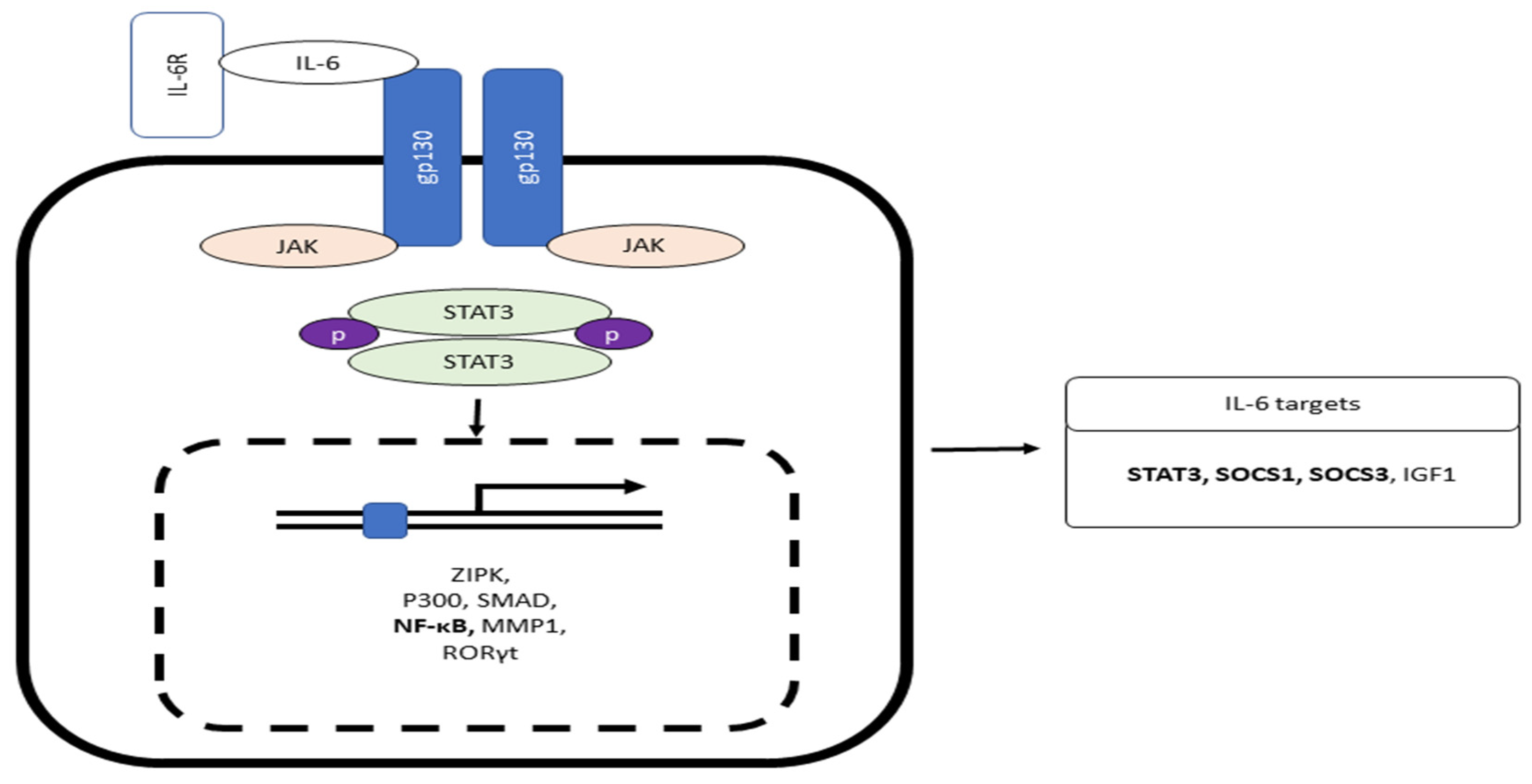
2.2. Inflammatory mediators
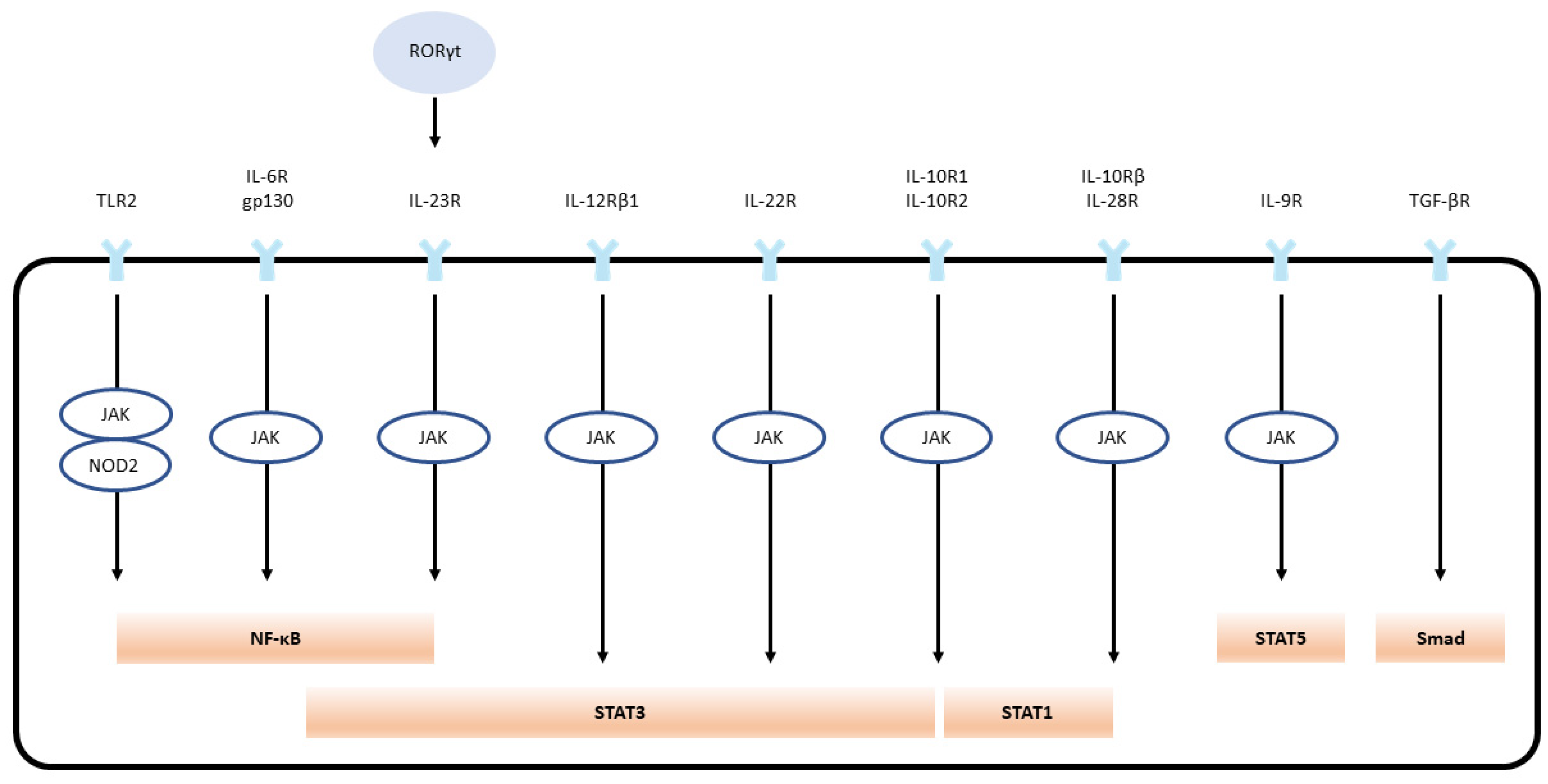
2.3. Immune mechanisms
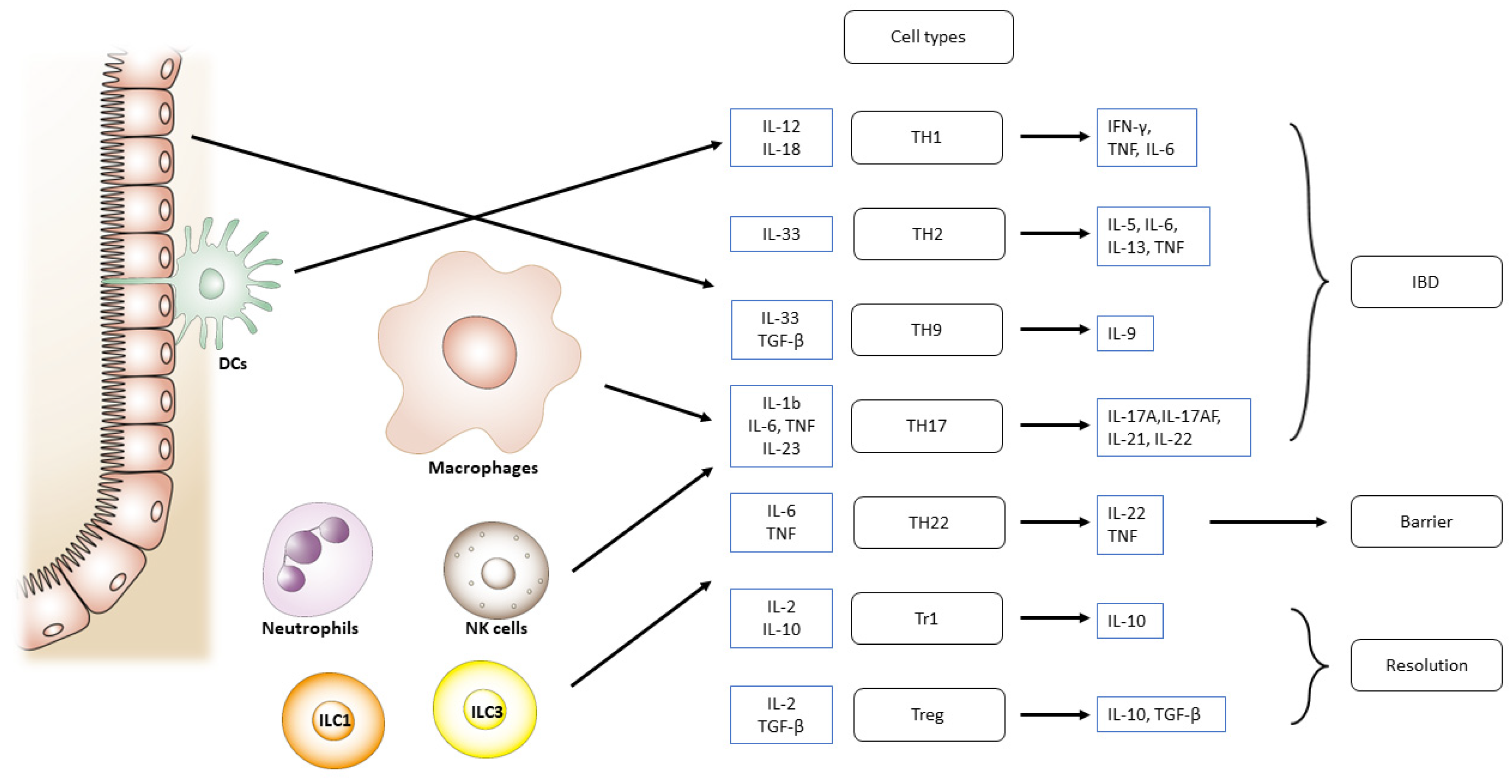
2.4. Lipids and inflammation
2.5. Intestinal stem cell niche and cell signaling
2.6. Role of extracellular matrix in intestinal regeneration
3. Dietary Lipids and IBD progression
4. Therapeutic Approaches and New Candidates
- It has been shown that IL-6 was raised in mice treated with 5% DSS-induced acute colitis, while IL-6 was reduced after treatment with SM934 (artemisinin analog) and ameliorated experimental colitis [179]. The result of the tocilizumab trial on 36 patients with CD has been reported with clinical significance [180]
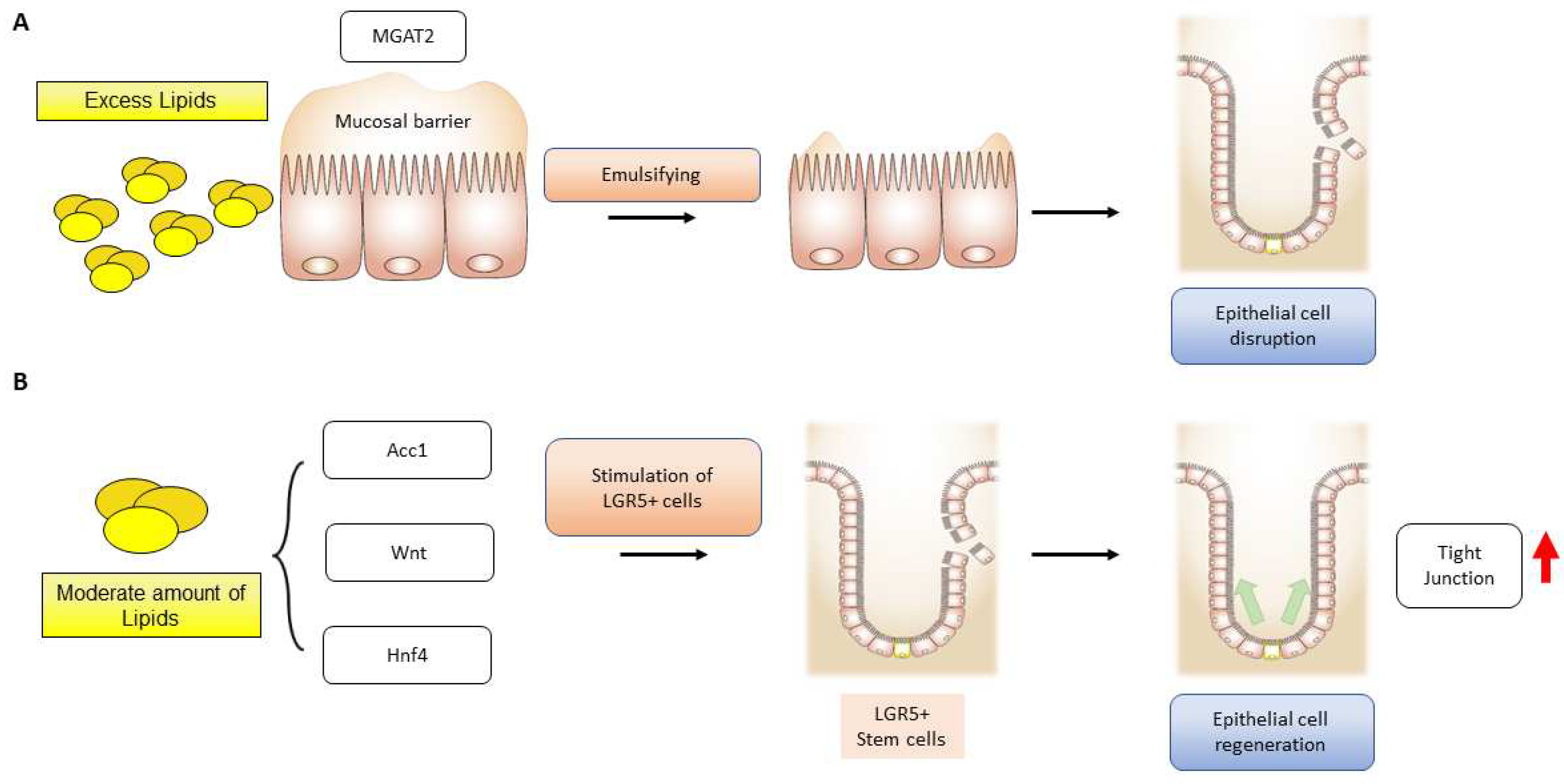
- In experimental models, it has been observed that deficiency of monoacylglycerol acyltransferase (MGAT) 2 provides protection against obesity. Moreover, the specific deletion of MGAT2 deters fat accumulation in the intestine [181]. In another study, monoacylglycerol lipase (MAGL) inhibition enhances the 2-arachidonoglycerol levels and results in decreased macroscopic and histological colon alterations, lowering cytokine levels [182]. MGAT2 deficiency in the intestine safeguards mice from metabolic disorders induced by high-fat feeding [183]. JTP-103237, currently in the preclinical stage, is an inhibitor of MGAT2 and impairs the absorption of luminal lipids in mice [184]. TNBS-induced murine colitis was reversed by the potent MAGL inhibitor JZL184 [182], and another MAGL inhibitor URB602 significantly repressed whole gut transient [185].
- Recent research suggests that ketogenic diets (KD) can increase the levels of circulating ketone bodies and have an anti-inflammatory effect [186]. However, the effects of this particular diet on colitis are still not well-understood. Animal studies have been conducted using KD, a low-carbohydrate diet, and a normal diet [186]. Following colitis, KD was found to protect intestinal barrier function and reduce inflammatory cytokines. Thus, KD may alleviate colitis by modifying microbiota.
- IBD frequently leads to liver injury. Milk fat globule membrane (MFGM) has been shown to mitigate colitis and liver injury [187]. Prophylactic MFGM therapy was found to be effective against colitis, improving weight loss, disease activity index, and pathological scores. Moreover, MFGM reduced levels of inflammatory mediators with an increase in IL-10 levels. MFGM thus alleviated DSS-induced injury, enhancing the mucosal barrier. It appears that MFGM may decrease oxidative stress in the liver [187].
- Signaling agents, including Wnt, EGF, Notch, and BMP ligands, promote the proliferation of Lgr5+ stem cells [188].
- Sphingosine-1-phosphate (S1P) is a signaling molecule involved in physiological processes. In IBD, the excessive infiltration of immune cells into the intestinal tissue is a significant contributor to the pathogenesis of the disease. Studies have shown that targeting S1P receptors could be a viable therapeutic strategy for IBD. Monoclonal antibodies directed to S1P have been tested in preclinical models of prostate and kidney cancer, but no studies have been done in IBD [189,190,191]. However, S1P receptor modulators have shown promise in preclinical studies [192] and are currently being evaluated in clinical trials for inflammatory disorders. These agents work downstream of S1P receptors to limit lymphocyte recruitment to inflammatory areas, reducing immune cell infiltration and mitigating inflammation in the intestine.
- Therapeutic agents that enhance insulin sensitivity, such as GLP-1, SGLT-2, and PPAR-γ ligands, have shown benefits for IBD patients by improving insulin-sensitized supplies of fuel and building block sources [193]. However, the potential impact of obesity on IBD treatment efficacy is still not well understood. Studies on various autoimmune diseases suggest that obesity can significantly affect therapeutic efficacy, leading to suboptimal treatment outcomes due to rapid clearance and decreased trough concentrations of medications. Therefore, further investigation is needed to better understand the interplay between obesity and IBD treatment outcomes.
5. Perspectives
- IBD results from the dysregulated immune system and the release of inflammatory mediators and lipotoxicity. Since inflammatory cytokines and lipotoxicity contribute to insulin resistance generation, the patients with IBD and those with metabolic disorders have common characteristics in the context of proinflammatory cytokines and oxidative stress.
- Inhibition of de novo FAS affects intestinal stem cell function and regeneration capacity, so intake of dietary lipids should be carefully interpreted to understand epithelial tissue repair and regeneration for IBD patients.
- Anti-inflammatory agents and insulin-sensitizing drugs are therapeutically beneficial to patients with IBD due to the inhibition of inflammatory injury, efficient cellular fuel oxidation, and increased tissue regeneration capacity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IBD | Inflammatory bowel diseases |
| Lgr5 | Leucine-rich repeat-containing G-protein coupled receptor 5 |
| IEC | Intestinal epithelial cell |
| UC | Ulcerative colitis |
| CD | Crohn’s disease |
| NAFLD | Non-alcoholic fatty liver disease |
| MAFLD | Metabolic dysfunction-associated fatty liver disease |
| T2D | Type 2 diabetes |
| HFD | High-fat diet |
| Tslp | Thymic stromal lymphopoietin |
| TNF | Tumor necrosis factor |
| TRADD | Tumor necrosis factor receptor type 1-associated death domain protein |
| TRAF2 | TNF receptor-associated factor 2 |
| RIPK1 | Receptor-interacting serine/threonine-protein kinase 1 |
| cIAP | Calf intestinal alkaline phosphatase |
| IFN | Interferon |
| TH17 | T helper 17 cells |
| JAK | Janus kinase |
| STAT | Signal transducer and activator of transcription proteins |
| PI3K | Phosphoinositide 3-kinases |
| AKT | Protein kinase B (PKB), also known as Akt |
| mTOR | Mammalian target of rapamycin |
| RAS | Renin–angiotensin system |
| RAF | Rapidly accelerated fibrosarcoma |
| MEK | MAPK/ERK kinase |
| ERK | Extracellular signal-regulated kinases |
| ADAM | A Disintegrin and metalloproteinase domain-containing protein |
| SOCS3 | Suppressors of cytokine signaling 3 |
| AP-1 | Activator protein 1 |
| NLRP3 | NLR family pyrin domain containing 3 |
| DSS | Dextran sodium sulfate |
| TNBS | 2,4,6-Trinitrobenzene sulfonic acid |
| RORγt | RAR-related orphan receptor gamma |
| MAPK | Mitogen-activated protein kinases |
| NOD | Nucleotide-binding oligomerization domain-containing protein |
| TLR2 | Toll-like receptor 2 |
| PMNs | Polymorphonuclear leukocyte |
| ICAM1 | Intercellular adhesion molecule 1 |
| S1Ps | Sphingosine-1-phosphate |
| WNT | Wingless-related integration site |
| EGF | Epidermal growth factor |
| DLL4 | Delta like canonical notch ligand 4 |
| PCs | Paneth cells |
| Gli1 | Glioma-associated oncogene family zinc finger 1 |
| Rspo1 | R-Spondin 1 |
| Pdgfra | Platelet derived growth factor receptor alpha |
| BMP | Bone morphogenetic protein |
| ECM | Extracellular matrix |
| YAP | Yes-associated protein |
| TAZ | Transcriptional coactivator with PDZ-binding motif |
| FAK | Focal adhesion kinase |
| CF | Cystic fibrosis |
| FAS | Fatty acid synthesis |
| 2-MAG | 2-Monoacyglycerol |
| FFA | Free fatty acids |
| PBA | Primary bile acids |
| CA | Cholic acid |
| CDCA | Chenodeoxycholic acid |
| LCA | Lithocholic acid |
| DCA | Deoxycholic acid |
| SBA | Secondary bile acids |
| MAGL | Monoacylglycerol lipase |
| MFGM | Milk fat globule membrane |
| S1P | Sphingosine-1-phosphate |
| GLP-1 | Glucagon-Like Peptide 1 |
| SGLT-2 | Sodium-Glucose Cotransporter 2 |
References
- Yilmaz, B.; Juillerat, P.; Øyås, O.; Ramon, C.; Bravo, F.D.; Franc, Y.; Fournier, N.; Michetti, P.; Mueller, C.; et al. Microbial network disturbances in relapsing refractory Crohn’s disease. Nat Med 2019, 25, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Cleynen, I.; Boucher, G.; Jostins, L.; Schumm, L.P.; Zeissig, S.; Ahmad, T.; Andersen, V.; Andrews, J.M.; Annese, V.; et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: a genetic association study. The Lancet 2016, 387, 156–167. [Google Scholar] [CrossRef]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Philip Schumm, L.; Sharma, Y.; et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, C.; Salleron, J.; Savoye, G.; Fumery, M.; Merle, V.; Laberenne, J.-E.; Vasseur, F.; Dupas, J.-L.; Cortot, A.; et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut 2014, 63, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Kappelman, M.D.; Rifas–Shiman, S.L.; Kleinman, K.; Ollendorf, D.; Bousvaros, A.; Grand, R.J.; Finkelstein, J.A. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol 2007, 5, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Langner, C.; Driessen, A.; Ensari, A.; Geboes, K.; Mantzaris, G.; Villanacci, V.; Becheanu, G.; Nunes, P.B.; et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis 2013, 7, 827–851. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, D.; Zhuo, J.; Lin, Z.; Yang, M.; Xu, X. The gut-liver axis in immune remodeling: new insight into liver diseases. Int J Biol Sci 2020, 16, 2357. [Google Scholar] [CrossRef]
- Yoneyama, H.; Matsuno, K.; Zhang, Y.; Murai, M.; Itakura, M.; Ishikawa, S.; Hasegawa, G.; Naito, M.; Asakura, H.; et al. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract–associated lymphoid tissue, in a granulomatous liver disease. J Exp Med 2001, 193, 35–50. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Adams, D.H. Gut–liver immunity. J Hepatol 2016, 64, 1187–1189. [Google Scholar] [CrossRef] [PubMed]
- Principi, M.; Iannone, A.; Losurdo, G.; Mangia, M.; Shahini, E.; Albano, F.; Rizzi, S.F.; La Fortezza, R.F.; Lovero, R.; et al. Nonalcoholic fatty liver disease in inflammatory bowel disease: prevalence and risk factors. Inflamm Bowel Dis 2018, 24, 1589–1596. [Google Scholar] [CrossRef]
- Shama, S.; Jang, H.; Wang, X.; Zhang, Y.; Shahin, N.N.; Motawi, T.K.; Kim, S.; Gawrieh, S.; Liu, W. Phosphatidylethanolamines Are Associated with Nonalcoholic Fatty Liver Disease (NAFLD) in Obese Adults and Induce Liver Cell Metabolic Perturbations and Hepatic Stellate Cell Activation. Int J Mol Sci 2023, 24, 1034. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-alcoholic fatty liver disease. The Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Khan, M.S.; Lee, C.; Kim, S.G. Non-alcoholic fatty liver disease and liver secretome. Arch Pharm Res 2022, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Hagström, H.; Nasr, P.; Fredrikson, M.; Stål, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Moran, G.W.; Dubeau, M.-F.; Kaplan, G.G.; Panaccione, R.; Ghosh, S. The increasing weight of Crohn’s disease subjects in clinical trials: a hypothesis-generatings time-trend analysis. Inflamm Bowel Dis 2013, 19, 2949–2956. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Ananthakrishnan, A.N.; Konijeti, G.G.; Higuchi, L.M.; Fuchs, C.S.; Richter, J.M.; Chan, A.T. Measures of obesity and risk of Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 2015, 21, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Harpsøe, M.C.; Basit, S.; Andersson, M.; Nielsen, N.M.; Frisch, M.; Wohlfahrt, J.; Nohr, E.A.; Linneberg, A.; Jess, T. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol 2014, 43, 843–855. [Google Scholar] [CrossRef]
- Flores, A.; Burstein, E.; Cipher, D.J.; Feagins, L.A. Obesity in inflammatory bowel disease: a marker of less severe disease. Dig Dis Sci 2015, 60, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Mendall, M.A.; Jensen, C.B.; Sørensen, T.I. A.; Ängquist, L.H.; Jess, T. Body mass index in young men and risk of inflammatory bowel disease through adult life: a population-based Danish cohort study. Sci Rep 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Chan, S.S. M.; Chen, Y.; Casey, K.; Olen, O.; Ludvigsson, J.F.; Carbonnel, F.; Oldenburg, B.; Gunter, M.J.; Tjønneland, A.; et al. Obesity is associated with increased risk of Crohn’s disease, but not ulcerative colitis: a pooled analysis of five prospective cohort studies. Clin Gastroenterol Hepatol 2022, 20, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Alizadeh-Tabari, S.; Singh, S.; Loomba, R. Meta-analysis: prevalence of, and risk factors for, non-alcoholic fatty liver disease in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2022, 55, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Lonardo, A.; Byrne, C.D. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev Endocrinol 2018, 14, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C. Ulceration of the colon with a much enlarged fatty liver. Trans Pathol Soc Phil 1873, 4, 87–88. [Google Scholar]
- Bargiggia, S.; Maconi, G.; Elli, M.; Molteni, P.; Ardizzone, S.; Parente, F.; Todaro, I.; Greco, S.; Manzionna, G.; et al. Sonographic prevalence of liver steatosis and biliary tract stones in patients with inflammatory bowel disease: study of 511 subjects at a single center. J Clin Gastroenterol 2003, 36, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Sourianarayanane, A.; Garg, G.; Smith, T.H.; Butt, M.I.; McCullough, A.J.; Shen, B. Risk factors of non-alcoholic fatty liver disease in patients with inflammatory bowel disease. J Crohns Colitis 2013, 7, e279–e285. [Google Scholar] [CrossRef] [PubMed]
- Blain, A.; Cattan, S.; Beaugerie, L.; Carbonnel, F.; Gendre, J.; Cosnes, J. Crohn’s disease clinical course and severity in obese patients. Clin Nutr 2002, 21, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, D.; da Rocha, S.D. C.; Herfindal, A.M.; Bøhn, S.K.; Carlsen, H. A high-fat western diet attenuates intestinal changes in mice with DSS-induced low-grade inflammation. J Nutr 2022, 152, 758–769. [Google Scholar] [CrossRef]
- Knoop, K.A.; Newberry, R.D. Goblet cells: multifaceted players in immunity at mucosal surfaces. Mucosal Immunol 2018, 11, 1551–1557. [Google Scholar] [CrossRef]
- Neutra, M.R.; O’Malley, L.J.; Specian, R.D. Regulation of intestinal goblet cell secretion. II. A survey of potential secretagogues. Am J Physiol Gastrointest 1982, 242, G380–G387. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Van Es, J.H.; Snippert, H.J.; Stange, D.E.; Vries, R.G.; Van Den Born, M.; Barker, N.; Shroyer, N.F.; Van De Wetering, M.; et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011, 469, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Worthington, J.J.; Reimann, F.; Gribble, F. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol 2018, 11, 3–20. [Google Scholar] [CrossRef]
- Piccand, J.; Vagne, C.; Blot, F.; Meunier, A.; Beucher, A.; Strasser, P.; Lund, M.L.; Ghimire, S.; Nivlet, L.; et al. Rfx6 promotes the differentiation of peptide-secreting enteroendocrine cells while repressing genetic programs controlling serotonin production. Mol Metab 2019, 29, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Birchenough, G.M.; Nyström, E.E.; Johansson, M.E.; Hansson, G.C. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science 2016, 352, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Haber, A.L.; Biton, M.; Rogel, N.; Herbst, R.H.; Shekhar, K.; Smillie, C.; Burgin, G.; Delorey, T.M.; Howitt, M.R.; et al. A single-cell survey of the small intestinal epithelium. Nature 2017, 551, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Petersson, J.; Schreiber, O.; Hansson, G.C.; Gendler, S.J.; Velcich, A.; Lundberg, J.O.; Roos, S.; Holm, L.; Phillipson, M. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest 2011, 300, G327–G333. [Google Scholar] [CrossRef]
- Johansson, M.E.; Gustafsson, J.K.; Sjöberg, K.E.; Petersson, J.; Holm, L.; Sjövall, H.; Hansson, G.C. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PloS one 2010, 5, e12238. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, M.M.; Shen, L.; Rajapakse, H.; Raleigh, D.R.; Wang, Y.; Wang, Y.; Lingaraju, A.; Zha, J.; Abbott, E.; et al. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol Biol Cell 2013, 24, 3056–3068. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Holmes, J.; Bridges, A.; Gookin, J.L.; Coccaro, M.R.; Proctor, W.; Colegio, O.R.; Anderson, J.M. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci 2008, 121, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Present, D.H.; Rutgeerts, P.; Targan, S.; Hanauer, S.B.; Mayer, L.; Van Hogezand, R.A.; Podolsky, D.K.; Sands, B.E.; Braakman, T.; et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 1999, 340, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Puimège, L.; Libert, C.; Van Hauwermeiren, F. Regulation and dysregulation of tumor necrosis factor receptor-1. Cytokine Growth Factor Rev 2014, 25, 285–300. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Nakabayashi, O.; Nakano, H. FLIP the Switch: Regulation of Apoptosis and Necroptosis by cFLIP. Int J Mol Sci 2015, 16, 30321–30341. [Google Scholar] [CrossRef] [PubMed]
- Targan, S.R.; Hanauer, S.B.; Van Deventer, S.J.; Mayer, L.; Present, D.H.; Braakman, T.; DeWoody, K.L.; Schaible, T.F.; Rutgeerts, P.J. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn’s disease. N Engl J Med 1997, 337, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Plevy, S.E.; Landers, C.J.; Prehn, J.; Carramanzana, N.M.; Deem, R.L.; Shealy, D.; Targan, S.R. A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn’s disease. J Immunol 1997, 159, 6276–6282. [Google Scholar] [CrossRef]
- Colombel, J.-F.; Sands, B.E.; Rutgeerts, P.; Sandborn, W.; Danese, S.; D’Haens, G.; Panaccione, R.; Loftus, E.V.; Sankoh, S.; et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017, 66, 839–851. [Google Scholar] [CrossRef]
- Glassman, C.R.; Mathiharan, Y.K.; Jude, K.M.; Su, L.; Panova, O.; Lupardus, P.J.; Spangler, J.B.; Ely, L.K.; Thomas, C.; et al. Structural basis for IL-12 and IL-23 receptor sharing reveals a gateway for shaping actions on T versus NK cells. Cell 2021, 184, 983–999. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev 2019, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Becker, C.; Blumberg, R.; Galle, P.R.; Neurath, M.F. Treatment of T cell-dependent experimental colitis in SCID mice by local administration of an adenovirus expressing IL-18 antisense mRNA. J Immunol 2002, 168, 411–420. [Google Scholar] [CrossRef]
- Dmitrieva-Posocco, O.; Dzutsev, A.; Posocco, D.F.; Hou, V.; Yuan, W.; Thovarai, V.; Mufazalov, I.A.; Gunzer, M.; Shilovskiy, I.P.; et al. Cell-type-specific responses to interleukin-1 control microbial invasion and tumor-elicited inflammation in colorectal cancer. Immunity 2019, 50, 166–180.e7. [Google Scholar] [CrossRef]
- Mantovani, A.; Dinarello, C.A.; Molgora, M.; Garlanda, C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity 2019, 50, 778–795. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Pohin, M.; Jackson, M.A.; Korsunsky, I.; Bullers, S.J.; Rue-Albrecht, K.; Christoforidou, Z.; Sathananthan, D.; Thomas, T.; et al. IL-1-driven stromal–neutrophil interactions define a subset of patients with inflammatory bowel disease that does not respond to therapies. Nat Med 2021, 27, 1970–1981. [Google Scholar] [CrossRef]
- Veenbergen, S.; Li, P.; Raatgeep, H.C.; Lindenbergh-Kortleve, D.J.; Simons-Oosterhuis, Y.; Farrel, A.; Costes, L.M. M.; Joosse, M.E.; van Berkel, L.A.; et al. IL-10 signaling in dendritic cells controls IL-1β-mediated IFNγ secretion by human CD4+ T cells: relevance to inflammatory bowel disease. Mucosal Immunol 2019, 12, 1201–1211. [Google Scholar] [CrossRef]
- Aschenbrenner, D.; Quaranta, M.; Banerjee, S.; Ilott, N.; Jansen, J.; Steere, B.; Chen, Y.-H.; Ho, S.; Cox, K.; et al. Deconvolution of monocyte responses in inflammatory bowel disease reveals an IL-1 cytokine network that regulates IL-23 in genetic and acquired IL-10 resistance. Gut 2021, 70, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Coelho, T.; Mossotto, E.; Gao, Y.; Haggarty, R.; Ashton, J.J.; Batra, A.; Stafford, I.S.; Beattie, R.M.; Williams, A.P.; et al. Immunological profiling of paediatric inflammatory bowel disease using unsupervised machine learning. J Pediatr Gastroenterol Nutr 2020, 70, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-C.; He, S.-H. IL-10 and its related cytokines for treatment of inflammatory bowel disease. World J Gastroenterol 2004, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Baird, A.W.; Parsons, M.J.; Fan, K.; Skerrett-Byrne, D.A.; Nair, P.M.; Makanyengo, S.; Chen, J.; Neal, R.; et al. Platelet activating factor receptor acts to limit colitis-induced liver inflammation. FASEB J 2020, 34, 7718–7732. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, M.; Wu, W.; Yang, W.; Huang, X.; Xiao, Y.; Ma, C.; Xu, L.; Yao, S.; et al. Microbiota metabolite butyrate differentially regulates Th1 and Th17 cells’ differentiation and function in induction of colitis. Inflamm Bowel Dis 2019, 25, 1450–1461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, W.; Yu, T.; Yu, Y.; Cui, X.; Zhou, Z.; Yang, H.; Yu, Y.; Bilotta, A.J.; et al. Th17 cell-derived amphiregulin promotes colitis-associated intestinal fibrosis through activation of mTOR and MEK in intestinal myofibroblasts. Gastroenterology 2023, 164, 89–102. [Google Scholar] [CrossRef]
- Xu, Z.-S.; Zhang, H.-X.; Li, W.-W.; Ran, Y.; Liu, T.-T.; Xiong, M.-G.; Li, Q.-L.; Wang, S.-Y.; Wu, M.; et al. FAM64A positively regulates STAT3 activity to promote Th17 differentiation and colitis-associated carcinogenesis. Proc Natl Acad Sci USA 2019, 116, 10447–10452. [Google Scholar] [CrossRef]
- Coccia, M.; Harrison, O.J.; Schiering, C.; Asquith, M.J.; Becher, B.; Powrie, F.; Maloy, K.J. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4+ Th17 cells. J Exp Med 2012, 209, 1595–1609. [Google Scholar] [CrossRef] [PubMed]
- Namai, F.; Shigemori, S.; Ogita, T.; Sato, T.; Shimosato, T. Microbial therapeutics for acute colitis based on genetically modified Lactococcus lactis hypersecreting IL-1Ra in mice. Exp Mol Med 2020, 52, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Wennerås, C.; Hagberg, L.; Andersson, R.; Hynsjö, L.; Lindahl, A.; Okroj, M.; Blom, A.M.; Johansson, P.; Andreasson, B.; et al. Distinct inflammatory mediator patterns characterize infectious and sterile systemic inflammation in febrile neutropenic hematology patients. PloS one 2014, 9, e92319. [Google Scholar] [CrossRef] [PubMed]
- Comini, L.; Pasini, E.; Bachetti, T.; Dreano, M.; Garotta, G.; Ferrari, R. Acute haemodynamic effects of IL-6 treatment in vivo: involvement of vagus nerve in NO-mediated negative inotropism. Cytokine 2005, 30, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat Immunol 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Ohshima, S.; Saeki, Y.; Mima, T.; Sasai, M.; Nishioka, K.; Nomura, S.; Kopf, M.; Katada, Y.; Tanaka, T.; et al. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci USA 1998, 95, 8222–8226. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-H.; Hsiao, H.-F.; Yeh, Y.-C.; Chen, T.-W.; Li, T.-K. Inflammatory interferon activates HIF-1α-mediated epithelial-to-mesenchymal transition via PI3K/AKT/mTOR pathway. J Exp Clin Cancer Res 2018, 37, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Baran, P.; Hansen, S.; Waetzig, G.H.; Akbarzadeh, M.; Lamertz, L.; Huber, H.J.; Ahmadian, M.R.; Moll, J.M.; Scheller, J. The balance of interleukin (IL)-6, IL-6· soluble IL-6 receptor (sIL-6R), and IL-6· sIL-6R· sgp130 complexes allows simultaneous classic and trans-signaling. J Biol Chem 2018, 293, 6762–6775. [Google Scholar] [CrossRef]
- Yan, I.; Schwarz, J.; Lücke, K.; Schumacher, N.; Schumacher, V.; Schmidt, S.; Rabe, B.; Saftig, P.; Donners, M.; et al. ADAM17 controls IL-6 signaling by cleavage of the murine IL-6Rα from the cell surface of leukocytes during inflammatory responses. J Leukoc Biol 2016, 99, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.I.; Alhayyani, S.; McLeod, L.; Yu, L.; Alanazi, M.; Deswaerte, V.; Tang, K.; Jarde, T.; Smith, J.A.; et al. ADAM 17 selectively activates the IL-6 trans-signaling/ERK MAPK axis in KRAS-addicted lung cancer. EMBO Mol Med 2019, 11, e9976. [Google Scholar] [CrossRef] [PubMed]
- Li, H.S.; Liu, C.; Xiao, Y.; Chu, F.; Liang, X.; Peng, W.; Hu, J.; Neelapu, S.S.; Sun, S.-C.; et al. Bypassing STAT3-mediated inhibition of the transcriptional regulator ID2 improves the antitumor efficacy of dendritic cells. Sci Signal 2016, 9, ra94–ra94. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Gadina, M.; Siegel, R.M. Cytokines and cytokine receptors. In Clinical immunology, Elsevier: 2019; pp 127-155.
- Guo, H.; Zhuang, K.; Ding, N.; Hua, R.; Tang, H.; Wu, Y.; Yuan, Z.; Li, T.; He, S. High-fat diet induced cyclophilin B enhances STAT3/lncRNA-PVT1 feedforward loop and promotes growth and metastasis in colorectal cancer. Cell Death Dis 2022, 13, 883. [Google Scholar] [CrossRef] [PubMed]
- Samavati, L.; Rastogi, R.; Du, W.; Hüttemann, M.; Fite, A.; Franchi, L. STAT3 tyrosine phosphorylation is critical for interleukin 1 beta and interleukin-6 production in response to lipopolysaccharide and live bacteria. Mol Immunol 2009, 46, (8–9), 1867-1877. [CrossRef]
- Gupta, S.C.; Phromnoi, K.; Aggarwal, B.B. Morin inhibits STAT3 tyrosine 705 phosphorylation in tumor cells through activation of protein tyrosine phosphatase SHP1. Biochem Pharmacol 2013, 85, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Schust, J.; Sperl, B.; Hollis, A.; Mayer, T.U.; Berg, T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol 2006, 13, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zeng, K.; Ma, X.; Song, F.; Jiang, Y.; Tu, P.; Wang, X. Resokaempferol-mediated anti-inflammatory effects on activated macrophages via the inhibition of JAK2/STAT3, NF-κB and JNK/p38 MAPK signaling pathways. Int Immunopharmacol 2016, 38, 104–114. [Google Scholar] [CrossRef]
- Garg, A.D.; Kaczmarek, A.; Krysko, O.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. ER stress-induced inflammation: does it aid or impede disease progression? Trends Mol Med 2012, 18, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.; Hirota, K.; Stieglitz, B.; Van Snick, J.; Tolaini, M.; Lahl, K.; Sparwasser, T.; Helmby, H.; Stockinger, B. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol 2011, 12, 1071–1077. [Google Scholar] [CrossRef]
- Nalleweg, N.; Chiriac, M.T.; Podstawa, E.; Lehmann, C.; Rau, T.T.; Atreya, R.; Krauss, E.; Hundorfean, G.; Fichtner-Feigl, S.; et al. IL-9 and its receptor are predominantly involved in the pathogenesis of UC. Gut 2015, 64, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Hong, W.; Li, S.; Chen, Z.; Zhou, W.; Dai, J.; Deng, X.; Zhou, H.; Li, B.; et al. Wood smoke particulate matter (WSPM2. 5) induces pyroptosis through both Caspase-1/IL-1β/IL-18 and ATP/P2Y-dependent mechanisms in human bronchial epithelial cells. Chemosphere 2022, 307, 135726. [Google Scholar] [CrossRef] [PubMed]
- Rauch, I.; Deets, K.A.; Ji, D.X.; von Moltke, J.; Tenthorey, J.L.; Lee, A.Y.; Philip, N.H.; Ayres, J.S.; Brodsky, I.E.; et al. NAIP-NLRC4 inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of caspase-1 and-8. Immunity 2017, 46, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Ten Hove, T.; Corbaz, A.; Amitai, H.; Aloni, S.; Belzer, I.; Graber, P.; Drillenburg, P.; Van Deventer, S.J. H.; Chvatchko, Y.; et al. Blockade of endogenous IL-18 ameliorates TNBS-induced colitis by decreasing local TNF-α production in mice. Gastroenterology 2001, 121, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Nowarski, R.; Jackson, R.; Gagliani, N.; de Zoete, M.R.; Palm, N.W.; Bailis, W.; Low, J.S.; Harman, C.C. D.; Graham, M.; et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 2015, 163, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, K.; Backert, I.; Wirtz, S.; Hueber, A.; Schett, G.; Vieth, M.; Probst, H.C.; Bopp, T.; Neurath, M.F.; et al. IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut 2017, 66, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.L.; Abo, H.; Maxim, E.; Harusato, A.; Geem, D.; Medina-Contreras, O.; Merlin, D.; Gewirtz, A.T.; Nusrat, A.; et al. A cytokine network involving IL-36γ, IL-23, and IL-22 promotes antimicrobial defense and recovery from intestinal barrier damage. Proc Natl Acad Sci USA 2018, 115, E5076–E5085. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, K.; Kersten, C.; Schmied, A.; Vieth, M.; Primbs, T.; Carlé, B.; Knieling, F.; Claussen, J.; Klimowicz, A.C.; et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology 2019, 156, 1082–1097. [Google Scholar] [CrossRef] [PubMed]
- Imaeda, H.; Takahashi, K.; Fujimoto, T.; Kasumi, E.; Ban, H.; Bamba, S.; Sonoda, H.; Shimizu, T.; Fujiyama, Y.; et al. Epithelial expression of interleukin-37b in inflammatory bowel disease. Clin Exp Immunol 2013, 172, 410–416. [Google Scholar] [CrossRef] [PubMed]
- McNamee, E.N.; Masterson, J.C.; Jedlicka, P.; McManus, M.; Grenz, A.; Collins, C.B.; Nold, M.F.; Nold-Petry, C.; Bufler, P.; et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci USA 2011, 108, 16711–16716. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-q.; Dong, K.; Zhou, L.; Jiao, G.-h.; Zhu, C.-z.; Li, W.-w.; Yu, G.; Wu, W.-t.; Chen, S.; et al. IL-37b gene transfer enhances the therapeutic efficacy of mesenchumal stromal cells in DSS-induced colitis mice. Acta Pharmacol Sin 2015, 36, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Duchmann, R.; Kaiser, I.; Hermann, E.; Mayet, W.; Ewe, K.; BÜSCHENFELDE, K.H. M. Z. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol 1995, 102, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Mow, W.S.; Vasiliauskas, E.A.; Lin, Y.-C.; Fleshner, P.R.; Papadakis, K.A.; Taylor, K.D.; Landers, C.J.; Abreu-Martin, M.T.; Rotter, J.I.; et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn’s disease. Gastroenterology 2004, 126, 414–424. [Google Scholar] [CrossRef]
- Mannon, P.J.; Fuss, I.J.; Mayer, L.; Elson, C.O.; Sandborn, W.J.; Present, D.; Dolin, B.; Goodman, N.; Groden, C.; et al. Anti–interleukin-12 antibody for active Crohn’s disease. N Engl J Med 2004, 351, 2069–2079. [Google Scholar] [CrossRef]
- Sawada, K.; Kusugami, K.; Suzuki, Y.; Bamba, T.; Munakata, A.; Hibi, T.; Shimoyama, T. Leukocytapheresis in ulcerative colitis: results of a multicenter double-blind prospective case-control study with sham apheresis as placebo treatment. Am J Gastroenterol 2005, 100, 1362–1369. [Google Scholar] [CrossRef]
- Lichtiger, S.; Present, D.H.; Kornbluth, A.; Gelernt, I.; Bauer, J.; Galler, G.; Michelassi, F.; Hanauer, S. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 1994, 330, 1841–1845. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B.; Hoentjen, F. Proinflammatory cytokines and signaling pathways in intestinal innate immune cells. Mucosal Immunol 2005, 30, 681–701. [Google Scholar] [CrossRef]
- Eshleman, E.M.; Shao, T.-Y.; Woo, V.; Rice, T.; Engleman, L.; Didriksen, B.J.; Whitt, J.; Haslam, D.B.; Way, S.S.; et al. Intestinal epithelial HDAC3 and MHC class II coordinate microbiota-specific immunity. J Clin Invest 2023. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Diao, J.; Liu, H.; Liu, S.; Liu, J.; Yuan, J.; Lin, J. The pathogenicity and synergistic action of Th1 and Th17 cells in inflammatory bowel diseases. Inflamm Bowel Dis 2022. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Di Giovangiulio, M.; Stolfi, C.; Franzè, E.; Fehling, H.-J.; Carsetti, R.; Giorda, E.; Colantoni, A.; Ortenzi, A.; et al. RORγt-Expressing Tregs Drive the Growth of Colitis-Associated Colorectal Cancer by Controlling IL6 in Dendritic CellsRORγt+ Tregs Drive CAC by Controlling IL6 in DCs. Cancer Immunol Res 2018, 6, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, H.K.; Bording-Jorgensen, M.; Santer, D.M.; Zhang, Z.; Valcheva, R.; Rieger, A.M.; Kim, J.S.-H.; Dijk, S.I.; Mahmood, R.; et al. Unfermented β-fructan fibers fuel inflammation in select inflammatory bowel disease patients. Gastroenterology 2023, 164, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.H.; Ferrero, R.L.; Philpott, D.J.; Girardin, S.E. Nod-like proteins in immunity, inflammation and disease. Nat Immunol 2006, 7, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Cho, J.H. Functional consequences of NOD2 (CARD15) mutations. Inflamm Bowel Dis 2006, 12, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat Immunol 2004, 5, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Reaves, T.A.; Chin, A.C.; Parkos, C.A. Neutrophil transepithelial migration: role of toll-like receptors in mucosal inflammation. Mem Inst Oswaldo Cruz 2005, 100, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Smythies, L.E.; Sellers, M.; Clements, R.H.; Mosteller-Barnum, M.; Meng, G.; Benjamin, W.H.; Orenstein, J.M.; Smith, P.D. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Investig 2005, 115, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.A.; Goldsby, J.S.; Callaway, E.S.; Shah, M.S.; Barker, N.; Chapkin, R.S. Alteration of colonic stem cell gene signatures during the regenerative response to injury. Biochim Biophys Acta Mol Basis Dis 2012, 1822, 1600–1607. [Google Scholar] [CrossRef]
- Andersson-Rolf, A.; Zilbauer, M.; Koo, B.-K.; Clevers, H. Stem cells in repair of gastrointestinal epithelia. Physiology 2017, 32, 278–289. [Google Scholar] [CrossRef]
- Duncan, M.; Grant, G. Oral and intestinal mucositis—causes and possible treatments. Aliment Pharmacol Ther 2003, 18, 853–874. [Google Scholar] [CrossRef]
- Miyoshi, H.; Ajima, R.; Luo, C.T.; Yamaguchi, T.P.; Stappenbeck, T.S. Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science 2012, 338, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Seno, H.; Miyoshi, H.; Brown, S.L.; Geske, M.J.; Colonna, M.; Stappenbeck, T.S. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci USA 2009, 106, 256–261. [Google Scholar] [CrossRef]
- Cairnie, A.B.; Millen, B.H. Fission of crypts in the small intestine of the irradiated mouse. Cell Prolif 1975, 8, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Dekaney, C.M.; Gulati, A.S.; Garrison, A.P.; Helmrath, M.A.; Henning, S.J. Regeneration of intestinal stem/progenitor cells following doxorubicin treatment of mice. Am J Physiol Gastrointest 2009, 297, G461–G470. [Google Scholar] [CrossRef] [PubMed]
- Bruens, L.; Ellenbroek, S.I. J.; van Rheenen, J.; Snippert, H.J. In vivo imaging reveals existence of crypt fission and fusion in adult mouse intestine. Gastroenterology 2017, 153, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Van Es, J.H.; Kuipers, J.; Kujala, P.; Van Den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Montgomery, R.K.; Carlone, D.L.; Richmond, C.A.; Farilla, L.; Kranendonk, M.E. G.; Henderson, D.E.; Baffour-Awuah, N.Y.; Ambruzs, D.M.; Fogli, L.K.; et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci USA 2011, 108, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef]
- Tian, H.; Biehs, B.; Warming, S.; Leong, K.G.; Rangell, L.; Klein, O.D.; De Sauvage, F.J. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 2011, 478, 255–259. [Google Scholar] [CrossRef]
- Metcalfe, C.; Kljavin, N.M.; Ybarra, R.; de Sauvage, F.J. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 2014, 14, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, C.; Klein, A.M.; Simons, B.D.; Winton, D.J. Intestinal stem cell replacement follows a pattern of neutral drift. Science 2010, 330, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Snippert, H.J.; Van Der Flier, L.G.; Sato, T.; Van Es, J.H.; Van Den Born, M.; Kroon-Veenboer, C.; Barker, N.; Klein, A.M.; Van Rheenen, J.; et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 2010, 143, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ritsma, L.; Ellenbroek, S.I. J.; Zomer, A.; Snippert, H.J.; De Sauvage, F.J.; Simons, B.D.; Clevers, H.; Van Rheenen, J. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature 2014, 507, 362–365. [Google Scholar] [CrossRef]
- Koch, U.; Lehal, R.; Radtke, F. Stem cells living with a Notch. Development 2013, 140, 689–704. [Google Scholar] [CrossRef] [PubMed]
- Durand, A.; Donahue, B.; Peignon, G.; Letourneur, F.; Cagnard, N.; Slomianny, C.; Perret, C.; Shroyer, N.F.; Romagnolo, B. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1). Proc Natl Acad Sci USA 2012, 109, 8965–8970. [Google Scholar] [CrossRef]
- Kim, T.-H.; Escudero, S.; Shivdasani, R.A. Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci USA 2012, 109, 3932–3937. [Google Scholar] [CrossRef]
- Rodríguez-Colman, M.J.; Schewe, M.; Meerlo, M.; Stigter, E.; Gerrits, J.; Pras-Raves, M.; Sacchetti, A.; Hornsveld, M.; Oost, K.C.; et al. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature 2017, 543, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Farin, H.F.; Van Es, J.H.; Clevers, H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology 2012, 143, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, Z.; Greicius, G.; Madan, B.; Biechele, S.; Zhong, Z.; Zaribafzadeh, H.; Aliyev, J.; Wu, Y.; Bunte, R.; et al. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development 2014, 141, 2206–2215. [Google Scholar] [CrossRef]
- Powell, D.W.; Pinchuk, I.V.; Saada, J.I.; Chen, X.; Mifflin, R.C. Mesenchymal cells of the intestinal lamina propria. Annual review of physiology 2011, 73, 213–237. [Google Scholar] [CrossRef] [PubMed]
- Stzepourginski, I.; Nigro, G.; Jacob, J.-M.; Dulauroy, S.; Sansonetti, P.J.; Eberl, G.; Peduto, L. CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Natl Acad Sci USA 2017, 114, E506–E513. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Shoshkes-Carmel, M.; Gao, N.; Shin, S.; May, C.L.; Golson, M.L.; Zahm, A.M.; Ray, M.; Wiser, C.L.; et al. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell Mol Gastroenterol Hepatol 2016, 2, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Shoshkes-Carmel, M.; Wang, Y.J.; Wangensteen, K.J.; Tóth, B.; Kondo, A.; Massasa, E.E.; Itzkovitz, S.; Kaestner, K.H. Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature 2018, 557, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Kosinski, C.; Li, V.S. W.; Chan, A.S. Y.; Zhang, J.; Ho, C.; Tsui, W.Y.; Chan, T.L.; Mifflin, R.C.; Powell, D.W.; et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci USA 2007, 104, 15418–15423. [Google Scholar] [CrossRef]
- McCarthy, N.; Manieri, E.; Storm, E.E.; Saadatpour, A.; Luoma, A.M.; Kapoor, V.N.; Madha, S.; Gaynor, L.T.; Cox, C.; et al. Distinct mesenchymal cell populations generate the essential intestinal BMP signaling gradient. Cell Stem Cell 2020, 26, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Sachs, N.; Wiebrands, K.; Ellenbroek, S.I. J.; Fumagalli, A.; Lyubimova, A.; Begthel, H.; van den Born, M.; van Es, J.H.; et al. Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc Natl Acad Sci USA 2016, 113, E5399–E5407. [Google Scholar] [CrossRef] [PubMed]
- Degirmenci, B.; Valenta, T.; Dimitrieva, S.; Hausmann, G.; Basler, K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 2018, 558, 449–453. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.-l. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordóñez-Morán, P.; Clevers, H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.-X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012, 150, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Trappmann, B.; Gautrot, J.E.; Connelly, J.T.; Strange, D.G. T.; Li, Y.; Oyen, M.L.; Cohen Stuart, M.A.; Boehm, H.; Li, B.; et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater 2012, 11, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Seminerio, J.L.; Koutroubakis, I.E.; Ramos-Rivers, C.; Hashash, J.G.; Dudekula, A.; Regueiro, M.; Baidoo, L.; Barrie, A.; Swoger, J.; et al. Impact of obesity on the management and clinical course of patients with inflammatory bowel disease. Inflamm Bowel Dis 2015, 21, 2857–2863. [Google Scholar] [CrossRef] [PubMed]
- De Lisle, R.C.; Borowitz, D. The cystic fibrosis intestine. Cold Spring Harb Perspect Med 2013, 3, a009753. [Google Scholar] [CrossRef] [PubMed]
- Smyth, R.L.; Croft, N.M.; O’Hea, U.; Marshall, T.G.; Ferguson, A. Intestinal inflammation in cystic fibrosis. Arch Dis Child 2000, 82, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, C.-W.; Diem, E.C.; Li, W.; Guderian, M.; Lindenberg, M.; Kruse, F.; Buettner, M.; Floess, S.; et al. Acetyl-CoA-Carboxylase 1-mediated de novo fatty acid synthesis sustains Lgr5+ intestinal stem cell function. Nat Commun 2022, 13, 3998. [Google Scholar] [CrossRef] [PubMed]
- Shiau, Y.F.; Popper, D.A.; Reed, M.; Umstetter, C.; Capuzzi, D.; Levine, G.M. Intestinal triglycerides are derived from both endogenous and exogenous sources. Am J Physiol Gastrointest 1985, 248, G164–G169. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.-A. L. S.; Skinner, J.R.; Shew, T.M.; Abumrad, N.A.; Wolins, N.E. Monoacylglycerol disrupts Golgi structure and perilipin 2 association with lipid droplets. BioRxiv 2021, 2021–07. [Google Scholar] [CrossRef]
- Phan, C.T.; Tso, P. Intestinal lipid absorption and transport. Front Biosci 2001, 6, D299–D319. [Google Scholar] [CrossRef]
- Venuti, E.; Shishmarev, D.; Kuchel, P.W.; Dutt, S.; Blumenthal, C.S.; Gaskin, K.J. Bile salt stimulated lipase: Inhibition by phospholipids and relief by phospholipase A2. J Cyst Fibros 2017, 16, 763–770. [Google Scholar] [CrossRef]
- Fiorucci, S.; Distrutti, E.; Carino, A.; Zampella, A.; Biagioli, M. Bile acids and their receptors in metabolic disorders. Prog Lipid Res 2021, 82, 101094. [Google Scholar] [CrossRef] [PubMed]
- Gälman, C.; Arvidsson, I.; Angelin, B.; Rudling, M. Monitoring hepatic cholesterol 7α-hydroxylase activity by assay of the stable bile acid intermediate 7α-hydroxy-4-cholesten-3-one in peripheral blood. J Lipid Res 2003, 44, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Pullinger, C.R.; Eng, C.; Salen, G.; Shefer, S.; Batta, A.K.; Erickson, S.K.; Verhagen, A.; Rivera, C.R.; Mulvihill, S.J.; et al. Human cholesterol 7α-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Investig 2002, 110, 109–117. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, L.; Jiang, C.; Xie, Y.; Cheng, X.; Krausz, K.W.; Qi, Y.; Sun, L.; Shah, Y.M.; et al. PPARα-UGT axis activation represses intestinal FXR-FGF15 feedback signalling and exacerbates experimental colitis. Nat Commun 2014, 5, 4573. [Google Scholar] [CrossRef] [PubMed]
- Krupa, Ł.; Staroń, R.; Dulko, D.; Łozińska, N.; Mackie, A.R.; Rigby, N.M.; Macierzanka, A.; Markiewicz, A.; Jungnickel, C. Importance of bile composition for diagnosis of biliary obstructions. Molecules 2021, 26, 7279. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, P.; Powell, N.; Vincent, R.P.; Ehrlich, D.; Bjarnason, I.; Hayee, B. Systematic review: bile acids and intestinal inflammation-luminal aggressors or regulators of mucosal defence? Aliment Pharmacol Ther 2015, 42, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.I.; Tenreiro, M.F.; Martinez-Santamaria, L.; Guerrero-Aspizua, S.; Gisbert, J.P.; Alves, P.M.; Serra, M.; Baptista, P.M. Hallmarks of the human intestinal microbiome on liver maturation and function. J Hepatol 2022, 76, 694–725. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S.; Sim, D.; Jarr, K.; Spear, E.T.; et al. Dysbiosis-induced secondary bile acid deficiency promotes intestinal inflammation. Cell Host Microbe 2020, 27, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Funabashi, M.; Grove, T.L.; Wang, M.; Varma, Y.; McFadden, M.E.; Brown, L.C.; Guo, C.; Higginbottom, S.; Almo, S.C.; et al. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature 2020, 582, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Pasolli, E.; Asnicar, F.; Manara, S.; Zolfo, M.; Karcher, N.; Armanini, F.; Beghini, F.; Manghi, P.; Tett, A.; et al. Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell 2019, 176, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, J.; Ren, X.; Zhang, Y.; Ke, Z.; Zhou, J.; Wang, Y.; Zhang, Y.; Liu, Y. Cholecystectomy-induced secondary bile acids accumulation ameliorates colitis through inhibiting monocyte/macrophage recruitment. Gut Microbes 2022, 14, 2107387. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhou, N.; Li, Z.; Fu, D.; Guo, Y.; Gao, X.; Liu, X. Co-occurrence of gut microbiota dysbiosis and bile acid metabolism alteration is associated with psychological disorders in Crohn’s disease. FASEB 2022, 36, e22100. [Google Scholar] [CrossRef] [PubMed]
- Bernbäck, S.; Bläckberg, L.; Hernell, O. Fatty acids generated by gastric lipase promote human milk triacylglycerol digestion by pancreatic colipase-dependent lipase. Biochim Biophys Acta Mol Cell Biol Lipids 1989, 1001, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Hamosh, M.; Scanlon, J.W.; Ganot, D.; Likel, M.; Scanlon, K.B.; Hamosh, P. Fat digestion in the newborn: characterization of lipase in gastric aspirates of premature and term infants. J Clin Investig 1981, 67, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.B. Lipid digestion and absorption. Pediatrics 1985, 75, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Guryn, K.; Hubert, N.; Frazier, K.; Urlass, S.; Musch, M.W.; Ojeda, P.; Pierre, J.F.; Miyoshi, J.; Sontag, T.J.; et al. Small intestine microbiota regulate host digestive and absorptive adaptive responses to dietary lipids. Cell Host Microbe 2018, 23, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Dal Buono, A.; Gabbiadini, R.; Solitano, V.; Vespa, E.; Parigi, T.L.; Repici, A.; Spinelli, A.; Armuzzi, A. Critical Appraisal of Filgotinib in the Treatment of Ulcerative Colitis: Current Evidence and Place in Therapy. Clin Exp Gastroenterol 2022, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Danese, S.; Loftus, E.V.; Vermeire, S.; Schreiber, S.; Ritter, T.; Fogel, R.; Mehta, R.; Nijhawan, S.; et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. The Lancet 2021, 397, 2372–2384. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Grisham, M.; Hodge, J.; Telliez, J.-B. JAK inhibition using tofacitinib for inflammatory bowel disease treatment: a hub for multiple inflammatory cytokines. Am J Physiol Gastrointest Liver Physiol 2016, 310, G155–G162. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’Haens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017, 376, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Peyrin-Biroulet, L.; Sharara, A.I.; Su, C.; Modesto, I.; Mundayat, R.; Gunay, L.M.; Salese, L.; Sands, B.E. Efficacy and safety of tofacitinib in ulcerative colitis based on prior tumor necrosis factor inhibitor failure status. Clin Gastroenterol Hepatol 2022, 20, 591–601.e8. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W.; Bar–Meir, S.; Feher, J.; Mickisch, O.; Mlitz, H.; Faszczyk, M.; Chowers, Y.; Lengyele, G.; Kovacs, A.; et al. The optimal dose of 5-aminosalicylic acid in active ulcerative colitis: a dose-finding study with newly developed mesalamine. Clin Gastroenterol Hepatol 2003, 1, 36–43. [Google Scholar] [CrossRef]
- Wallis, R.S. Tumour necrosis factor antagonists: structure, function, and tuberculosis risks. Lancet Infect Dis 2008, 8, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, G.; Samaan, M.A.; Irving, P.M. Golimumab in the treatment of ulcerative colitis. Therap Adv Gastroenterol 2019, 12, 1756284818821266. [Google Scholar] [CrossRef] [PubMed]
- Arora, Z.; Shen, B. Biological therapy for ulcerative colitis. Gastroenterol Rep 2015, 3, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, O.H.; Steenholdt, C.; Juhl, C.B.; Rogler, G. Efficacy and safety of methotrexate in the management of inflammatory bowel disease: a systematic review and meta-analysis of randomized, controlled trials. EClinicalMedicine 2020, 20. [Google Scholar] [CrossRef]
- Pithadia, A.B.; Jain, S. Treatment of inflammatory bowel disease (IBD). Pharmacol Rep 2011, 63, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Travis, S. Conventional medical management of inflammatory bowel disease. Gastroenterology 2011, 140, 1827–1837. [Google Scholar] [CrossRef]
- Yan, Y.-x.; Shao, M.-j.; Qi, Q.; Xu, Y.-s.; Yang, X.-q.; Zhu, F.-h.; He, S.-j.; He, P.-l.; Feng, C.-l.; et al. Artemisinin analogue SM934 ameliorates DSS-induced mouse ulcerative colitis via suppressing neutrophils and macrophages. Acta Pharmacol Sin 2018, 39, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Takazoe, M.; Fukuda, Y.; Hibi, T.; Kusugami, K.; Andoh, A.; Matsumoto, T.; Yamamura, T.; Azuma, J.; et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology 2004, 126, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.W.; Gao, Y.; Yen, M.-I.; Yen, C.-L. E. Intestine-specific deletion of acyl-CoA: monoacylglycerol acyltransferase (MGAT) 2 protects mice from diet-induced obesity and glucose intolerance. J Biol Chem 2014, 289, 17338–17349. [Google Scholar] [CrossRef] [PubMed]
- Alhouayek, M.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D.; Muccioli, G.G. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J 2011, 25, 2711–2721. [Google Scholar] [CrossRef]
- Yen, C.-L. E.; Cheong, M.-L.; Grueter, C.; Zhou, P.; Moriwaki, J.; Wong, J.S.; Hubbard, B.; Marmor, S.; Farese Jr, R.V. Deficiency of the intestinal enzyme acyl CoA: monoacylglycerol acyltransferase-2 protects mice from metabolic disorders induced by high-fat feeding. Nat Med 2009, 15, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Okuma, C.; Ohta, T.; Tadaki, H.; Hamada, H.; Oda, T.; Taniuchi, H.; Yamanaka, K.; Ishii, Y.; Ohe, Y.; et al. JTP-103237, a novel monoacylglycerol acyltransferase inhibitor, modulates fat absorption and prevents diet-induced obesity. Eur J Pharmacol 2015, 758, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.; Thomas, A.D.; Cluny, N.L.; Patel, A.; Patel, K.D.; Lutz, B.; Piomelli, D.; Alexander, S.P. H.; Sharkey, K.A. Distribution and function of monoacylglycerol lipase in the gastrointestinal tract. Am J Physiol Gastrointest 2008, 295, G1255–G1265. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Yan, X.; Liu, Y.; Huang, L.; Zhu, Y.; He, J.; Gao, R.; Kalady, M.F.; Goel, A.; et al. Ketogenic diet alleviates colitis by reduction of colonic group 3 innate lymphoid cells through altering gut microbiome. Signal Transduct Target Ther 2021, 6, 154. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, X.; Huang, S.; Li, T.; Zhang, X.; Pang, J.; Zhao, J.; Chen, L.; Zhang, B.; et al. Milk fat globule membrane attenuates acute colitis and secondary liver injury by improving the mucus barrier and regulating the gut microbiota. Front Immunol 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Gehart, H.; Clevers, H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol 2019, 16, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Bullock, A.J.; Callea, M.; Shah, H.; Song, J.; Moreno, K.; Visentin, B.; Deutschman, D.; et al. Anti-S1P Antibody as a Novel Therapeutic Strategy for VEGFR TKI-Resistant Renal CancerS1P Inhibition as a New Treatment for RCC. Clin Cancer Res 2015, 21, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Cuvillier, O.; Ader, I.; Bouquerel, P.; Sabbadini, R.A.; Malavaud, B. Effect of a therapeutic sphingosine 1-phosphate antibody on intratumoral hypoxia and standard chemotherapy in a preclinical model of prostate cancer. J Clin Oncol 2012, 30, (5_suppl), 223-223. [CrossRef]
- Sabbadini, R.A. Sphingosine-1-phosphate antibodies as potential agents in the treatment of cancer and age-related macular degeneration. Br J Pharmacol 2011, 162, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Mao-Draayer, Y.; Sarazin, J.; Fox, D.; Schiopu, E. The sphingosine-1-phosphate receptor: A novel therapeutic target for multiple sclerosis and other autoimmune diseases. Clin Immunol 2017, 175, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, Z.; Jing, D.; Huang, X.; Ren, D.; Shao, Z.; Zhang, Z. SGLT2 inhibitor activates the STING/IRF3/IFN-β pathway and induces immune infiltration in osteosarcoma. Cell Death Dis 2022, 13, 523. [Google Scholar] [CrossRef] [PubMed]

| Number of Patients and Characteristics | Results | References |
|---|---|---|
| 153 Crohn’s disease (CD) and 229 ulcerative colitis (UC) patients, respectively | The risk of developing CD is 2.3 times higher in obese women (18 years) and no significant association UC patients with obesity | [15] |
| CD (n = 138) and UC (n = 394) | The risk of CD was found to be increased by 1.9 times in obese non-pregnant women, but no significant association was reported for UC | [16] |
| CD (n = 75) and UC (n = 177) | According to the findings of the study, obesity alone is not enough to trigger the development of either CD or UC | [17] |
| CD (n = 297) and UC (n = 284), respectively | No statistically significant association was found between obesity and either UC or CD in the studied patient populations | [18] |
| 377,597 men with increased BMI have associative risk in developing CD and UC | The results of the COX regression analysis showed a positive correlation between BMI and CD in the group of 1523 patients, while an inverse correlation was observed in UC patients (n = 3323) | [19] |
| A pooled analysis of cohort studies inlcuding CD (n = 563) and UC (n = 1047) patients | The findings suggest that there is a significant association between CD and obese patients with BMI ≥30 kg/m2, while not significance relation between UC and obese patients | [20] |
| Systemic studies comprising 14,947 IBD subjects | Notably, 13.6% of IBD patients with NAFLD were found to have liver fibrosis | [21] |
| Drugs | Mechanisms of Action | Doses | References |
|---|---|---|---|
| Filgotinib | JAK1 inhibitor | 100 mg O.D. While 200 mg resulted in a primary embolism |
[167,168] |
| Tofacitinib | JAK1, JAK3 inhibitor | 5 or 10 mg B.I.D. for moderately to severe UC | [169,170,171] |
| Mesalazine (5-ASA) | Anti-inflammatory effect on colonic epithelial cells | 0.5 g and can be increased to 1 g 5-aminosalicylic acid T.I.D. against ulcerative colitis | [172] |
| Infliximab | Monoclonal antibody to TNF-α | Highest blood concentration via intravenous infusion 80-100 µg/ml and not less than 5µg/in 4-6 weeks |
[173] |
| Adalimumab | Monoclonal antibody to TNF-α | Subcutaneous injection 5-10 µg/ml leads to reduced TNF-α levels |
[173] |
| Golimumab | Monoclonal antibody to TNF-α | Initial starting with 200 mg and reduced to 100 mg after 2 weeks, and the dose is maintained by either 50 mg or 100 mg administered at intervals of 4 weeks for UC treatment | [174] |
| Vedolizumab | Anti-α4β7 integrin | 300 mg within 2 weeks | [175] |
| Methotrexate | inhibit enzymes responsible for nucleotide synthesis | 12.5–25 mg/week p.o or i.p. | [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





