Submitted:
03 November 2023
Posted:
06 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Psoriasis Pathogenesis
3. Phosphodiesterase 4 and Inhibition
4. Phosphodiesterase 4 Inhibitors
4.1. Apremilast
| PDE4 Inhibitor |
Formulation | Sponsor | Condition | Phases | NCT Number (Year completed; Bolded randomized control trials) |
|---|---|---|---|---|---|
| Roflumilast (ARQ-151) | Topical | Arcutis Biotherapeutics, Inc. |
Psoriasis, plaque psoriasis, scalp psoriasis | III | NCT05684744 (2023); NCT05763082 (2023); NCT05028582 (2022) [46]; NCT04211389 (2020) [47]; NCT04211363 (2020) [47] |
| II | NCT05684744 (2023); NCT04746911 (2022); NCT04549870 (2022) [48]; NCT04746911 (2022); NCT04655313 (2022); NCT03764475 (2020 [49]); NCT04128007 (2020); NCT03638258 (2019) [50]; NCT03392168 (2018) [51] | ||||
| I | NCT04279119 (2021); NCT03392168 (2018) [51] | ||||
| Crisaborole (AN2728) | Topical | Pfizer | Plaque-type psoriasis; Psoriasis |
II |
NCT01300052 (2011); NCT01029405 (2010) NCT00759161 (2008 [52]); NCT00755196 (2008) |
| I |
NCT01258088 (2010); NCT00763204 (2008) NCT00762658 (2007) |
||||
| PF-07038124 | Topical | Pfizer | Plaque Psoriasis | II | NCT05375955 (2023); NCT04664153 (2021) |
| Leo-29102 | Topical | Leo Pharma | Psoriasis vulgaris |
II |
NCT00875277 (2009) |
| I | NCT01466478 (2011) | ||||
| MK-0873 | Topical | Merck Sharp & Dohme LL | Psoriasis; plaque psoriasis | I | NCT01140061 (2011); NCT01235728 (2011) |
| DRM02 | Topical | Dermira | Plaque psoriasis | II | NCT01993433 (2014) |
| PDE4 Inhibitor |
Apremilast [54] (CC-10004) Otezla ® |
Roflumilast [55] (ARQ-151) Zoryve ® |
Crisaborole [56] (AN2728) Eucrisa ® |
| Molecular formula and 2D structure | C22H24N2O7S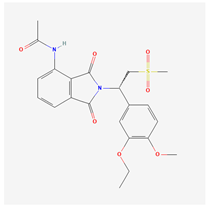
|
C17H14Cl2F2N2O3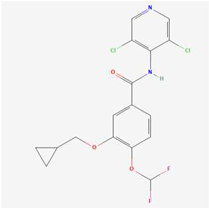
|
C14H10BNO3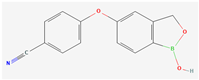
|
| Molecular Weight | 460.5 g/mol | 403.2 g/mol | 251.05 g/mol |
| Formulation, dose | Oral: 30 mg twice daily | Topical: 0.3% once daily to affected areas Oral: 500 mcg once daily |
Topical: 0.5% or 2% once daily to affected areas |
| Adverse events | Nausea, vomiting, diarrhea, headache, nasopharyngitis, dyspepsia, upper respiratory tract infection, loss of appetite, weight loss [28,29,30,31,33,34,36,37,38,39,42] | Application site erythema/pain, nasopharyngitis, upper respiratory tract infection, muscle strain, gastrointestinal symptoms, weight loss, headache, insomnia [46,47,48,50,51] | Mild application site reaction [57] |
4.2. Roflumilast
4.3. Crisaborole
4.4. Other PDE4 Inhibitors Investigated
5. Discussion
6. Limitations
7. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raychaudhuri, S.K.; Maverakis, E.; Raychaudhuri, S.P. Diagnosis and Classification of Psoriasis. Autoimmun. Rev. 2014, 13, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Chronic Plaque Psoriasis: Causes, Symptoms, and Treatment. Available online: https://dermnetnz.org/topics/chronic-plaque-psoriasis (accessed on 19 October 2023).
- Lebwohl, M.G.; Bachelez, H.; Barker, J.; Girolomoni, G.; Kavanaugh, A.; Langley, R.G.; Paul, C.F.; Puig, L.; Reich, K.; van de Kerkhof, P.C.M. Patient Perspectives in the Management of Psoriasis: Results from the Population-Based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J. Am. Acad. Dermatol. 2014, 70, 871–881.e30. [Google Scholar] [CrossRef] [PubMed]
- Michalek, I.M.; Loring, B.; John, S.M. A Systematic Review of Worldwide Epidemiology of Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.A.; Ilchef, R.; Cooper, A.J. Psychiatric Morbidity in Psoriasis: A Review. Australas. J. Dermatol. 2004, 45, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.I.R.C.; Abreu, J.L.P.D.C.; Reis, J.P.G.D.; Figueiredo, A.M.D.C. Psoriasis and Associated Psychiatric Disorders. J. Clin. Aesthetic Dermatol. 2016, 9, 36–43. [Google Scholar]
- Farley, E.; Menter, A. Psoriasis: Comorbidities and Associations. G. Ital. Dermatol. E Venereol. Organo Uff. Soc. Ital. Dermatol. E Sifilogr. 2011, 146, 9–15. [Google Scholar]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, M.; Helliwell, P.S. Phosphodiesterase 4 Inhibition in the Treatment of Psoriasis, Psoriatic Arthritis and Other Chronic Inflammatory Diseases. Dermatol. Ther. 2013, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Apremilast: A Review in Psoriasis and Psoriatic Arthritis. Drugs 2015, 75, 1393–1403. [Google Scholar] [CrossRef]
- Milakovic, M.; Gooderham, M.J. Phosphodiesterase-4 Inhibition in Psoriasis. Psoriasis Targets Ther. 2021, 11, 21–29. [Google Scholar] [CrossRef]
- Zeng, J.; Luo, S.; Huang, Y.; Lu, Q. Critical Role of Environmental Factors in the Pathogenesis of Psoriasis. J. Dermatol. 2017, 44, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of Psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, M.; Helliwell, P.S. Phosphodiesterase 4 Inhibition in the Treatment of Psoriasis, Psoriatic Arthritis and Other Chronic Inflammatory Diseases. Dermatol. Ther. 2013, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The Role of the Transcription Factor CREB in Immune Function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef] [PubMed]
- Lugnier, C. Cyclic Nucleotide Phosphodiesterase (PDE) Superfamily: A New Target for the Development of Specific Therapeutic Agents. Pharmacol. Ther. 2006, 109, 366–398. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.F.; Faucz, F.R.; Bimpaki, E.; Horvath, A.; Levy, I.; de Alexandre, R.B.; Ahmad, F.; Manganiello, V.; Stratakis, C.A. Clinical and Molecular Genetics of the Phosphodiesterases (PDEs). Endocr. Rev. 2014, 35, 195–233. [Google Scholar] [CrossRef]
- Wang, H.; Peng, M.-S.; Chen, Y.; Geng, J.; Robinson, H.; Houslay, M.D.; Cai, J.; Ke, H. Structures of the Four Subfamilies of Phosphodiesterase-4 Provide Insight into the Selectivity of Their Inhibitors. Biochem. J. 2007, 408, 193–201. [Google Scholar] [CrossRef]
- Ke, H.; Wang, H. Crystal Structures of Phosphodiesterases and Implications on Substrate Specificity and Inhibitor Selectivity. Curr. Top. Med. Chem. 2007, 7, 391–403. [Google Scholar] [CrossRef]
- Fertig, B.A.; Baillie, G.S. PDE4-Mediated cAMP Signalling. J. Cardiovasc. Dev. Dis. 2018, 5, 8. [Google Scholar] [CrossRef]
- Schafer, P. Apremilast Mechanism of Action and Application to Psoriasis and Psoriatic Arthritis. Biochem. Pharmacol. 2012, 83, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Bäumer, W.; Hoppmann, J.; Rundfeldt, C.; Kietzmann, M. Highly Selective Phosphodiesterase 4 Inhibitors for the Treatment of Allergic Skin Diseases and Psoriasis. Inflamm. Allergy Drug Targets 2007, 6, 17–26. [Google Scholar] [CrossRef]
- Padda, I.S.; Tripp, J. Phosphodiesterase Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Liu, Z.; Liu, M.; Cao, Z.; Qiu, P.; Song, G. Phosphodiesterase-4 Inhibitors: A Review of Current Developments (2013–2021). Expert Opin. Ther. Pat. 2022, 32, 261–278. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ (accessed on 4 September 2023).
- Stein Gold, L.F.; Bagel, J.; Tyring, S.K.; Hong, H.C.-H.; Pavlovsky, L.; Vender, R.; Pinter, A.; Reich, A.; Drogaris, L.; Wu, T.; et al. Comparison of Risankizumab and Apremilast for the Treatment of Adult Patients with Moderate Plaque Psoriasis Eligible for Systemic Therapy: Results from a Randomised, Open-Label, Assessor-Blinded Phase IV (IMMpulse) Study. Br. J. Dermatol. 2023, ljad252. [Google Scholar] [CrossRef] [PubMed]
- Mrowietz, U.; Barker, J.; Conrad, C.; Jullien, D.; Gisondi, P.; Flower, A.; Reddy, J.; Paris, M.; Picard, H.; Jardon, S.; et al. Efficacy and Safety of Apremilast in Patients with Limited Skin Involvement, Plaque Psoriasis in Special Areas and Impaired Quality of Life: Results from the EMBRACE Randomized Trial. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Haydey, R.; Rosoph, L.A.; Lynde, C.W.; Bukhalo, M.; Fowler, J.F.; Delorme, I.; Gagné-Henley, A.; Gooderham, M.; Poulin, Y.; et al. Apremilast for the Treatment of Moderate-to-Severe Palmoplantar Psoriasis: Results from a Double-Blind, Placebo-Controlled, Randomized Study. J. Eur. Acad. Dermatol. Venereol. JEADV 2018, 32, 403–410. [Google Scholar] [CrossRef]
- Stein Gold, L.; Bagel, J.; Lebwohl, M.; Jackson, J.M.; Chen, R.; Goncalves, J.; Levi, E.; Duffin, K.C. Efficacy and Safety of Apremilast in Systemic- and Biologic-Naive Patients With Moderate Plaque Psoriasis: 52-Week Results of UNVEIL. J. Drugs Dermatol. JDD 2018, 17, 221–228. [Google Scholar] [PubMed]
- Strober, B.; Bagel, J.; Lebwohl, M.; Stein Gold, L.; Jackson, J.M.; Chen, R.; Goncalves, J.; Levi, E.; Callis Duffin, K. Efficacy and Safety of Apremilast in Patients With Moderate Plaque Psoriasis With Lower BSA: Week 16 Results from the UNVEIL Study. J. Drugs Dermatol. JDD 2017, 16, 801–808. [Google Scholar]
- Fiorillo, L.; Becker, E.; Lucas, R. de; Belloni-Fortina, A.; Maes, P.; Oberoi, R.K.; Paris, M.; Zhang, W.; Zhang, Z.; Arkin, L. 42163 Apremilast in Pediatric Patients With Moderate to Severe Plaque Psoriasis: 16-Week Efficacy and Safety Results From the Phase 3, Randomized, Double-Blind, Placebo-Controlled SPROUT Study. J. Am. Acad. Dermatol. 2023, 89, AB122. [Google Scholar] [CrossRef]
- Merola, J.F.; Parish, L.C.; Guenther, L.; Lynde, C.; Lacour, J.-P.; Staubach, P.; Cheng, S.; Paris, M.; Picard, H.; Deignan, C.; et al. Efficacy and Safety of Apremilast in Patients with Moderate-to-Severe Genital Psoriasis: Results from Discreet, a Phase 3 Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Acad. Dermatol. 2023. [Google Scholar] [CrossRef]
- Stein Gold, L.; Papp, K.; Pariser, D.; Green, L.; Bhatia, N.; Sofen, H.; Albrecht, L.; Gooderham, M.; Chen, M.; Paris, M.; et al. Efficacy and Safety of Apremilast in Patients with Mild-to-Moderate Plaque Psoriasis: Results of a Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Acad. Dermatol. 2022, 86, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Takahashi, H.; Hino, R.; Endo, K.; Kikuchi, S.; Ozeki, Y.; Nakamura, T.; Paris, M.; Abe, M. Efficacy and Safety of Apremilast in the Treatment of Patients with Mild-to-Moderate Psoriasis in Japan: Results from PROMINENT, A Phase 3b, Open-Label, Single-Arm Study. Dermatol. Ther. 2022, 12, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Van Voorhees, A.S.; Stein Gold, L.; Lebwohl, M.; Strober, B.; Lynde, C.; Tyring, S.; Cauthen, A.; Sofen, H.; Zhang, Z.; Paris, M.; et al. Efficacy and Safety of Apremilast in Patients with Moderate to Severe Plaque Psoriasis of the Scalp: Results of a Phase 3b, Multicenter, Randomized, Placebo-Controlled, Double-Blind Study. J. Am. Acad. Dermatol. 2020, 83, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Gooderham, M.; Green, L.; Bewley, A.; Zhang, Z.; Khanskaya, I.; Day, R.M.; Goncalves, J.; Shah, K.; Piguet, V.; et al. The Efficacy and Safety of Apremilast, Etanercept and Placebo in Patients with Moderate-to-Severe Plaque Psoriasis: 52-Week Results from a Phase IIIb, Randomized, Placebo-Controlled Trial (LIBERATE). J. Eur. Acad. Dermatol. Venereol. 2017, 31, 507–517. [Google Scholar] [CrossRef]
- Paul, C.; Cather, J.; Gooderham, M.; Poulin, Y.; Mrowietz, U.; Ferrandiz, C.; Crowley, J.; Hu, C.; Stevens, R. m.; Shah, K.; et al. Efficacy and Safety of Apremilast, an Oral Phosphodiesterase 4 Inhibitor, in Patients with Moderate-to-Severe Plaque Psoriasis over 52 Weeks: A Phase III, Randomized Controlled Trial (ESTEEM 2). Br. J. Dermatol. 2015, 173, 1387–1399. [Google Scholar] [CrossRef]
- Papp, K.; Reich, K.; Leonardi, C.L.; Kircik, L.; Chimenti, S.; Langley, R.G.B.; Hu, C.; Stevens, R.M.; Day, R.M.; Gordon, K.B.; et al. Apremilast, an Oral Phosphodiesterase 4 (PDE4) Inhibitor, in Patients with Moderate to Severe Plaque Psoriasis: Results of a Phase III, Randomized, Controlled Trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J. Am. Acad. Dermatol. 2015, 73, 37–49. [Google Scholar] [CrossRef]
- Paller, A.S.; Hong, Y.; Becker, E.M.; de Lucas, R.; Paris, M.; Zhang, W.; Zhang, Z.; Barcellona, C.; Maes, P.; Fiorillo, L. Pharmacokinetics and Safety of Apremilast in Pediatric Patients with Moderate to Severe Plaque Psoriasis: Results from a Phase 2 Open-Label Study. J. Am. Acad. Dermatol. 2020, 82, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.; Cather, J.C.; Rosoph, L.; Sofen, H.; Langley, R.G.; Matheson, R.T.; Hu, C.; Day, R.M. Efficacy of Apremilast in the Treatment of Moderate to Severe Psoriasis: A Randomised Controlled Trial. Lancet Lond. Engl. 2012, 380, 738–746. [Google Scholar] [CrossRef]
- Papp, K. a.; Kaufmann, R.; Thaçi, D.; Hu, C.; Sutherland, D.; Rohane, P. Efficacy and Safety of Apremilast in Subjects with Moderate to Severe Plaque Psoriasis: Results from a Phase II, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Dose-Comparison Study. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e376–e383. [Google Scholar] [CrossRef]
- Gottlieb, A.B.; Strober, B.; Krueger, J.G.; Rohane, P.; Zeldis, J.B.; Hu, C.C.; Kipnis, C. An Open-Label, Single-Arm Pilot Study in Patients with Severe Plaque-Type Psoriasis Treated with an Oral Anti-Inflammatory Agent, Apremilast. Curr. Med. Res. Opin. 2008, 24, 1529–1538. [Google Scholar] [CrossRef]
- Warren, R.B.; Strober, B.; Silverberg, J.I.; Guttman, E.; Andres, P.; Felding, J.; Tutkunkardas, D.; Kjøller, K.; Sommer, M.O.A.; French, L.E. Oral Orismilast: Efficacy and Safety in Moderate-to-Severe Psoriasis and Development of Modified Release Tablets. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Zhang, J.; Wang, L.; Dai, X.; Wang, H.; Bi, X.; Duan, X.; Meng, Z.; Tian, Z.; Xu, A.; et al. A Double-Blind, Placebo-Parallel Controlled Phase III Clinical Study of the Efficacy and Safety of Hemay005 Tablets in Patients with Moderate to Severe Chronic Plaque Psoriasis in China. Arthritis Rheumatol ACR Converg. 2023, 75. [Google Scholar]
- Arcutis Presents Positive Patient-Reported Outcome Data from the Pivotal ARRECTOR Phase 3 Trial in Scalp and Body Psoriasis at European Academy of Dermatology and Venereology (EADV) Congress - Arcutis Biotherapeutics. Available online: https://www.arcutis.com/arcutis-presents-positive-patient-reported-outcome-data-from-the-pivotal-arrector-phase-3-trial-in-scalp-and-body-psoriasis-at-european-academy-of-dermatology-and-venereology-eadv-congress/ (accessed on 18 October 2023).
- Lebwohl, M.G.; Kircik, L.H.; Moore, A.Y.; Stein Gold, L.; Draelos, Z.D.; Gooderham, M.J.; Papp, K.A.; Bagel, J.; Bhatia, N.; Del Rosso, J.Q.; et al. Effect of Roflumilast Cream vs Vehicle Cream on Chronic Plaque Psoriasis: The DERMIS-1 and DERMIS-2 Randomized Clinical Trials. JAMA 2022, 328, 1073. [Google Scholar] [CrossRef] [PubMed]
- Gyldenløve, M.; Meteran, H.; Sørensen, J.A.; Fage, S.; Yao, Y.; Lindhardsen, J.; Nissen, C.V.; Todberg, T.; Thomsen, S.F.; Skov, L.; et al. Efficacy and Safety of Oral Roflumilast for Moderate-to-Severe Psoriasis—a Randomized Controlled Trial (PSORRO). Lancet Reg. Health – Eur. 2023, 30. [Google Scholar] [CrossRef]
- Lebwohl, M.; Gold, L.S.; Gooderham, M.; Papp, K.A.; Ferris, L.K.; Adam, D.N.; Hong, H.C.H.; Kircik, L.H.; Zirwas, M.; Burnett, P.; et al. CO45 Durability of Efficacy and Safety of Roflumilast Cream 0.3% in Adults with Chronic Plaque Psoriasis from a 52-Week, Phase 2 Open-Label Safety Trial. Present. Innov. Dermatol. Virtual Spring Conf. 2021 March 16-20 2021 Value Health 2023, 26, S22–S23. [Google Scholar] [CrossRef]
- Lebwohl, M.G.; Papp, K.A.; Stein Gold, L.; Gooderham, M.J.; Kircik, L.H.; Draelos, Z.D.; Kempers, S.E.; Zirwas, M.; Smith, K.; Osborne, D.W.; et al. Trial of Roflumilast Cream for Chronic Plaque Psoriasis. N. Engl. J. Med. 2020, 383, 229–239. [Google Scholar] [CrossRef]
- Papp, K.A.; Gooderham, M.; Droege, M.; Merritt, C.; Osborne, D.W.; Berk, D.R.; Thurston, A.W.; Smith, V.H.; Welgus, H. Roflumilast Cream Improves Signs and Symptoms of Plaque Psoriasis: Results from a Phase 1/2a Randomized, Controlled Study. J. Drugs Dermatol. JDD 2020, 19, 734–740. [Google Scholar] [CrossRef]
- Beutner, K.; Lathrop, A.; Marti, D.; Zane, L.; Toledo, M. AN2728 Demonstrates Significant Safety and Efficacy in a Phase IIa Double-Blind Trial in Plaque Type Psoriasis. J. Am. Acad. Dermatol. 2009, 60, AB168. [Google Scholar] [CrossRef]
- Gao, J.C.; Wu, A.G.; Contento, M.N.; Maher, J.M.; Cline, A. Apremilast in the Treatment of Plaque Psoriasis: Differential Use in Psoriasis. Clin. Cosmet. Investig. Dermatol. 2022, 15, 395–402. [Google Scholar] [CrossRef]
- PubChem Apremilast. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/11561674 (accessed on 4 September 2023).
- PubChem Roflumilast. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/449193 (accessed on 4 September 2023).
- PubChem Crisaborole. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/44591583 (accessed on 4 September 2023).
- Stein Gold, L.F.; Spelman, L.; Spellman, M.C.; Hughes, M.H.; Zane, L.T. A Phase 2, Randomized, Controlled, Dose-Ranging Study Evaluating Crisaborole Topical Ointment, 0.5% and 2% in Adolescents With Mild to Moderate Atopic Dermatitis. J. Drugs Dermatol. 2015, 14, 1394–1399. [Google Scholar]
- Rich, P.; Gooderham, M.; Bachelez, H.; Goncalves, J.; Day, R.M.; Chen, R.; Crowley, J. Apremilast, an Oral Phosphodiesterase 4 Inhibitor, in Patients with Difficult-to-Treat Nail and Scalp Psoriasis: Results of 2 Phase III Randomized, Controlled Trials (ESTEEM 1 and ESTEEM 2). J. Am. Acad. Dermatol. 2016, 74, 134–142. [Google Scholar] [CrossRef]
- Oba, Y.; Lone, N.A. Efficacy and Safety of Roflumilast in Patients with Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Ther. Adv. Respir. Dis. 2013, 7, 13–24. [Google Scholar] [CrossRef]
- Presbyla, M. FDA Approves Arcutis’ ZORYVE® (Roflumilast) Cream 0.3% for Treatment of Psoriasis in Children Ages 6 to 11. Arcutis Biotherapeutics 2023.
- Food and Drug Administration ZORYVE® (Roflumilast) Cream, for Topical Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215985s002lbl.pdf.
- O’Toole, A.; Gooderham, M. Topical Roflumilast for Plaque Psoriasis. Skin Ther. Lett. 2023, 28, 1. [Google Scholar]
- Arcutis Biotherapeutics Topical Roflumilast Cream. Available online: https://www.arcutis.com/pipeline/topical-roflumilast-cream/ (accessed on 18 October 2023).
- Dong, C.; Virtucio, C.; Zemska, O.; Baltazar, G.; Zhou, Y.; Baia, D.; Jones-Iatauro, S.; Sexton, H.; Martin, S.; Dee, J.; et al. Treatment of Skin Inflammation with Benzoxaborole PDE Inhibitors: Selectivity, Cellular Activity, and Effect on Cytokines Associated with Skin Inflammation and Skin Architecture Changes. J. Pharmacol. Exp. Ther. 2016, 358. [Google Scholar] [CrossRef]
- Lebwohl, M.; Gold, L.S.; Gooderham, M.; Papp, K.; Ferris, L.; Adam, D.; Hong, H.C.; Kircik, L.; Zirwas, M.; Burnett, P.; et al. Durability of Efficacy and Safety of Roflumilast Cream 0.3% in Adults With Chronic Plaque Psoriasis From a 52-Week, Phase 2 Open-Label Safety Trial. SKIN J. Cutan. Med. 2023, 7, s111–s111. [Google Scholar] [CrossRef]
- Yazdanian, N.; Mozafarpoor, S.; Goodarzi, A. Phosphodiesterase Inhibitors and Prostaglandin Analogues in Dermatology: A Comprehensive Review. Dermatol. Ther. 2021, 34, e14669. [Google Scholar] [CrossRef]
- US Food and Drug Administration. EUCRISA® (Crisaborole). Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/207695s012lbl.pdf.
- Fishbein, A.B.; Silverberg, J.I.; Wilson, E.J.; Ong, P.Y. Update on Atopic Dermatitis: Diagnosis, Severity Assessment, and Treatment Selection. J. Allergy Clin. Immunol. Pract. 2020, 8, 91–101. [Google Scholar] [CrossRef]
- Hashim, P.W.; Chima, M.; Kim, H.J.; Bares, J.; Yao, C.J.; Singer, G.; Chen, T.; Genece, J.; Baum, D.; Kimmel, G.W.; et al. Crisaborole 2% Ointment for the Treatment of Intertriginous, Anogenital, and Facial Psoriasis: A Double-Blind, Randomized, Vehicle-Controlled Trial. J. Am. Acad. Dermatol. 2020, 82, 360–365. [Google Scholar] [CrossRef]
- Lee, E.B.; Lebwohl, M.G.; Wu, J.J. Treatment of Psoriasis with Crisaborole. J. Dermatol. Treat. 2019, 30, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Robbins, A.B.; Gor, A.; Bui, M.R. Topical Crisaborole—A Potential Treatment for Recalcitrant Palmoplantar Psoriasis. JAMA Dermatol. 2018, 154, 1096–1097. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, W. Successful Treatment with Crisaborole for Facial Lesions Refractory to Adalimumab in a Man with Psoriasis: A Case Report. Dermatol. Ther. 2022, 35, e15424. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, W. Crisaborole Ointment as Treatment for Genital Psoriasis. J. Cosmet. Dermatol. 2022, 21, 4080–4081. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.; Armstrong, A.; Koresawa, T.; Otake, K.; Hirata, M.; Kano, A. Efficacy and Safety of ME3183 Administered Orally in Patients with Moderate to Severe Plaque Psoriasis: A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Phase 2a Study. Presented at the the 32nd European Academy of Dermatology and Venereology, 2023.
- Drakos, A.; Vender, R. A Review of the Clinical Trial Landscape in Psoriasis: An Update for Clinicians. Dermatol. Ther. 2022, 12, 2715–2730. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.A.; Adam, D.N.; Gooderham, M.J.; Green, L.J.; Lebwohl, M.; Moore, A.Y.; Pariser, D.M.; Feng, A.; Higham, R.C.; Burnett, P.; et al. 33467 Long-Term Safety and Efficacy of Roflumilast Cream 0.3% in Patients with Chronic Plaque Psoriasis: Interim Results from a 24-Week, Phase 3 Open-Label Study. J. Am. Acad. Dermatol. 2022, 87, AB180. [Google Scholar] [CrossRef]
- Andrews, S.N.; Jeong, E.; Prausnitz, M.R. Transdermal Delivery of Molecules Is Limited by Full Epidermis, Not Just Stratum Corneum. Pharm. Res. 2013, 30, 1099–1109. [Google Scholar] [CrossRef]
- Zane, L.T.; Hughes, M.H.; Shakib, S. Tolerability of Crisaborole Ointment for Application on Sensitive Skin Areas: A Randomized, Double-Blind, Vehicle-Controlled Study in Healthy Volunteers. Am. J. Clin. Dermatol. 2016, 17, 519–526. [Google Scholar] [CrossRef]
| PDE4 inhibitor |
Formulation | Sponsor | Condition | Phases | NCT Number (Year completed; Bolded randomized control trials) |
|---|---|---|---|---|---|
| Apremilast (CC-10004) | Oral | Amgen | Psoriasis; Plaque psoriasis; Nail psoriasis; Palmo-plantar psoriasis | IV | NCT06032858 (2023); NCT04908475 (2023) [27] NCT03774875 (2021) [28]; NCT03082729 (2021); NCT03022617 (2020); NCT03000309 (2018); NCT03441789 (2018); NCT02412644 (2016); NCT02400749 (2016) [29]; NCT02425826 (2016) [30,31] |
| III | NCT03701763 (2023) [32]; NCT06084663 (2022); NCT03777436 (2022) [33]; NCT03930186 (2020); NCT03721172 (2020) [34] ; NCT03930186 (2020) [35]; NCT03123471 (2019) [36]; NCT01690299 (2016) [37]; NCT01232283 (2016) [38]; NCT01194219 (2016) [39] | ||||
| II | NCT03442088 (2021); NCT02576678 (2019); NCT02576678 (2017) [40]; NCT01988103 (2014); NCT00773734 (2009) [41]; NCT00521339 (2009); NCT00606450 (2007) [42]; NCT00604682 (2005) [43] |
||||
| Orismilast (LEO-32731) | Oral | LEO Pharma; Union therapeutics |
Psoriasis; Psoriasis vulgaris |
II | NCT05190419 (2022); NCT02888236 (2017) [44] |
| I |
NCT03231124 (2017); NCT02126371 (2015) NCT02514694 (2015); NCT02888236 (2017) [44] |
||||
| Mufemilast (Hemay005) | Oral | Tianjin Hemay Pharmaceutical Co., Ltd | Psoriasis | II | NCT04102241 (2021) [45] |
| I | NCT03007810 (2018) | ||||
| ME3183 | Oral | Meiji Pharma USA Inc. | Plaque psoriasis | II | NCT05268016 (2023) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




