1. Introduction
Allergic rhinitis (AR) is one of the most common diseases worldwide. In China, the prevalence of AR in children has increased from 9.1% in 2001 to 15.4% in 2010 [
1], and Dermatophagoides pteronyssinus (
Der p) has been found to be one of the most prevalent allergens [
2]. A growing concern about the impact of AR in children is that it affects contiguous organs such as the sinuses, ears and chest and causes sleep problems that result in reduced quality of life and work performance [
3]. Meanwhile, AR can progress to asthma or worsen the control of existing asthma with significant comorbidities, leading to decreased involvement in outdoor activities and aggravated socioeconomic burden [
4].
Dermatophagoides pteronyssinus (
Der p) is an inhalational allergen and often acts as a trigger for perennial AR in children. It has been reported that the immunoglobulin E (IgE) antibodies against
Der p 1 and
Der p 2 allergens present a dominant prevalence [
5] and represent the
Der p allergens with the greatest clinical significance [
6].
Der p 1 and
Der p 2 are allergen components purified from their native sources or produced as recombinant proteins. Detection of
Der p 1- or
Der p 2-specific IgE (sIgE) has attracted increasing attention as having sufficient diagnostic accuracy for use in the diagnosis of
Der p IgE sensitization [7, 8].
Der p allergen-specific immunotherapy (AIT) has been used as a desensitizing therapy for more than 100 years, has been widely used in China since 2006 [
9] and can be used for children over 3 years old with safety and effectiveness [
10]. AIT has shown potential to alter the natural course of allergic response and the potential to be preferred to a treatment option for preventing new aeroallergen sensitizations, avoiding prolonged use and side effects from medications and reducing the risk of developing asthma in children [11-13], but a subset of children receive little benefit from long-term treatment because of no or little response to AIT [
14].
Currently, there are no biomarkers that can accurately predict clinical outcomes in AIT. The evaluation of the efficacy of AIT relies on the main complaints of patients, and the clinical therapeutic effect is judged by symptom scores such as the rhinitis quality of life questionnaire (RQLQ) and visual analog scale (VAS). It is difficult to evaluate quantitatively and objectively the outcomes of specific immunotherapy AR, so it is very important to find some biomarkers that can be easily detected to predict the clinical outcomes of AR therapy, furthermore, enable clinicians to formulate a personalized therapy. Therefore, there is an urgent need for some generally accepted biomarkers that may predict and monitor the clinical response to AIT to eventually meet the requirements of precision medicine [
15].
Many results raise concerns about the diagnostic value of
Der p 1 and
Der p 2 sIgE [
16]; however, little is currently known about the association of molecular diagnosis with AIT clinical efficacy. In this study, we evaluated the effects and correlation of biomarkers and clinical indices on improvement in AR on SCIT, with the specific aim of determining the association between
Der p 2 sIgE and clinical symptoms score.
2. Materials and Methods
A prospective, randomized, open-label clinical trial was approved by the Ethics Committee of the First Affiliated Hospital of Ningbo University, Ningbo, China (nos. KY20171118). All subjects’ parents provided informed consent before enrollment in the study.
2.1. Clinical data
Children with a clinical history of perennial
Der p-induced AR for more than one year were enrolled in this study. All children were randomly selected and recruited from the First Affiliated Hospital of Ningbo University from January 2018 to December 2018. The clinical manifestations mainly include the following: ① The symptoms are paroxysmal sneezing, watery nose, nasal itching and nasal congestion and may be accompanied by eye symptoms. The allergens are mainly
Der p, and the symptoms are perennial.② The signs are perennial bilateral nasal mucosa that is pale and swollen, lower turbinate edema, and a large amount of watery secretions in the nasal cavity. Inclusion criteria: All were in line with the diagnostic criteria of the Chinese Guidelines on Allergen Immunotherapy for Allergic Rhinitis: The 2022 Update [
17]. Patients were sensitive to
Der p allergens, and serum sIgE concentrations in all patients were > 0.7 KUA/L (sIgE grade Ⅱ or above). Exclusion criteria: asthma, FEV1<80%, neoplasms, chronic diseases, autoimmune disorders and patients who failed to complete the questionnaire and serum administration on time
2.2. Intervention medication
All children were treated with Der p allergen preparation (Alutard@SQ), an internationally standardized allergen preparation (ALK-ABELIJ6, Denmark). This preparation contains Der p allergen extract. According to the concentration and dose of the desensitization vaccine, it was divided into 4 specifications: l (concentration 100 SQ-U/mL), 2 (concentration 1000 SQ-U/mL), 3 (concentration 10000 SQ-U/mL) and 4 (concentration 100000 SQ-U/mL). The treatment of Der p allergens is divided into two stages, namely, the initiation stage and the maintenance stage. Specific immunotherapy using an internationally standardized allergen preparation for Der p allergens (Alutard SQ). Using the standardized treatment regimen recommended by the Department of Allergy at the University of Copenhagen, treatment began at 20 SQ-U and was increased once a week according to the following regimen: 20, 40, 80, 200, 400, 800, 2 000, 4 000, 8 000, 10 000, 20 000, 40 000, 60 000, 80 000 and 100 000 SQ-U were given a total of 15 injections in the initial phase. Follow the regular schedule for each visit. Maintenance dose: In the maintenance phase, the concentration of vaccine was 100 000 SQ-U each time, and the injection interval was extended from 2 weeks to 4 weeks. The vaccine was then injected once every 4 weeks, with a total course of 26-30 months.
2.3. Laboratory analysis
Serum samples were taken at baseline and at the primary efficacy endpoint (4-8 months), secondary efficacy endpoint (9-14 months), tertiary efficacy endpoint (18-22 months), and fourth efficacy endpoint (26-30 months) after SCIT and frozen at -80 °C after collection and thawed immediately before use for a total of 1-2 freeze‒thaw cycles. Serum periostin, IL-5, Der p sIgE, Der p 1 sIgE, Der p 2 sIgE, HDM sIgG4, Der p 1 sIgG4, and Der p 2 sIgG4 levels were measured with a sandwich ELISA kit and Labsystems Multiskan MS (Finland).
2.4. Efficacy evaluation criteria
2.4.1. VAS
The VAS score is recognized as a very useful tool for assessing the subjective perception of the overall discomfort of AR at baseline and 4 efficacy endpoints. The score of nasal congestion, itching, sneezing, and runny nose symptoms varies from 0 (no symptoms) to 10 (most severe symptoms). Patients were asked to grade their symptoms retrospectively at baseline and 4 efficacy endpoints, and the average of each item was analyzed.
2.4.2. RQLQ
The patients were asked to complete quality of life questionnaires regarding rhinoconjunctivitis (RQLQ). The RQLQ consists of 28 items in 7 domains, with each score on a 7-point scale. The 7 domains were activities, sleep, nonnose and noneye symptoms, practical problems, nasal symptoms, eye symptoms, and emotional. The average of each item is analyzed.
2.5. Statistical method
This study used SPSS Statistics version 22.0 (IBM/SPSS, Inc., Chicago, IL, USA) for statistical analysis. Mean and SD (mean ± SD) were, respectively deployed for analyses of quantitative variables. Student’s t test was used to calculate statistical significance. Pearson’s correlation analysis helped assess the serum periostin, IL-5 and VAS, RQLQ, Der p sIgE, Der p 1 sIgE, Der p 2 sIgE, Der p sIgG4, Der p 1 sIgG4, and Der p 2 sIgG4 correlations. Statistical significance was set at p < 0.05.
3. Results
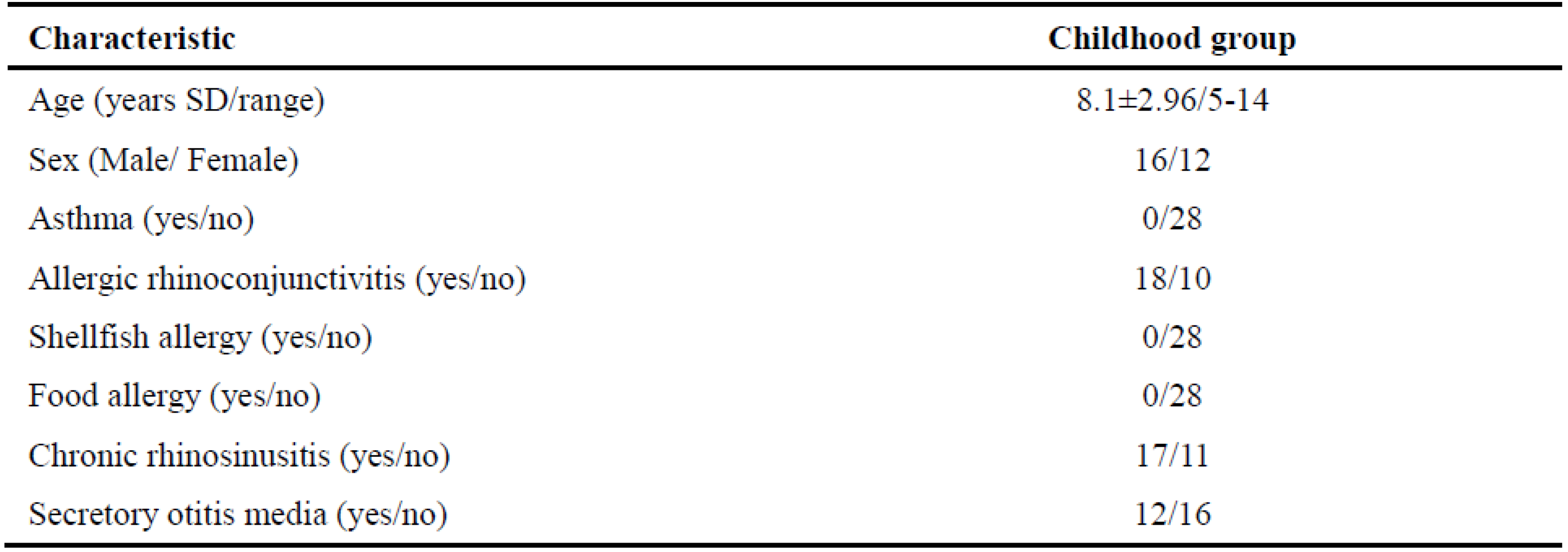
Table 1 summarizes the demographics and survey information of AR childhood at baseline. A total of 45 qualified children with AR were included in this study, and 28 subjects were finally included in the statistical analysis, including 16 males and 12 females. The average age was 8.1±2.96 years, ranging from 5 to 14 years. A total of 37.8% (17/45) of children dropped out during the course of SCIT because of being out of touch, ineffective treatment, and intolerance to long-term treatment.
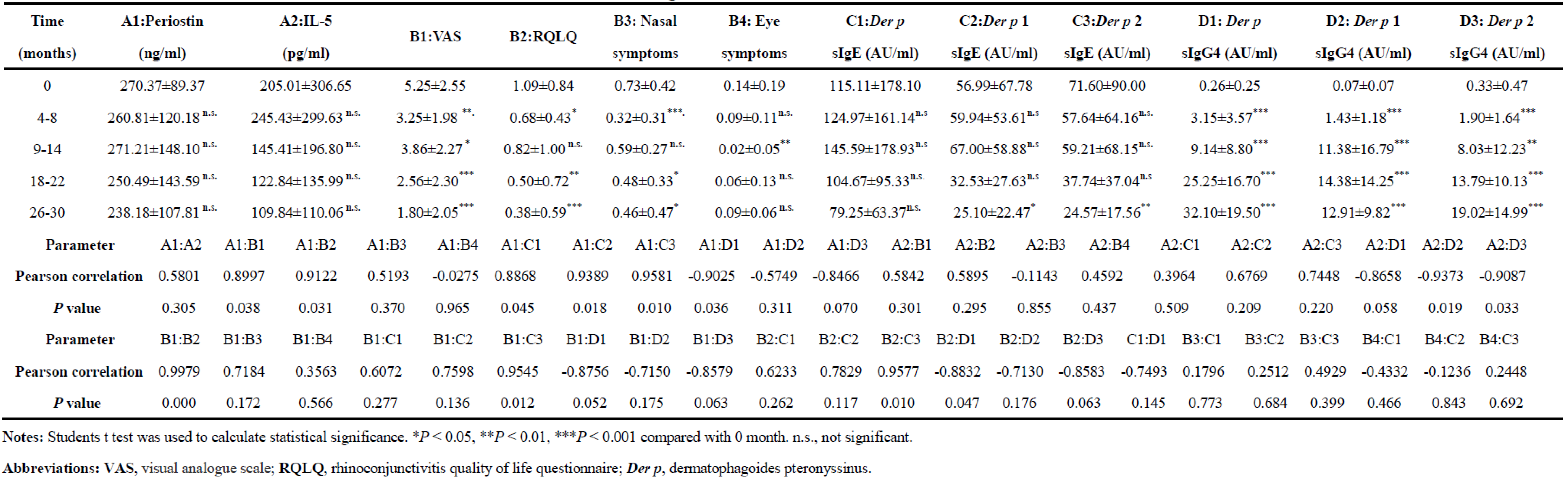
Table 2 shows the correlations between the changes in clinical indices in childhood with AR treatment with SCIT. The analysis revealed that VAS, RQLQ, and concentrations of
Der p 1,
Der p 2 sIgE and
Der p sIgG4 at the fourth efficacy endpoint were significantly lower or higher than those at baseline. The concentrations of serum periostin, IL-5, and
Der p sIgE and the RQLQ nasal and eye symptom scores showed a decreasing trend but did not reach statistical significance with SCIT treatment. Furthermore, a positive linear correlation was found between serum periostin and VAS (Pearson correlation=0.8997, P=0.038), BQLQ (Pearson correlation=0.9122, P=0.013),
Der p sIgE (Pearson correlation=0.8868, P=0.045),
Der p 1 sIgE (Pearson correlation=0.9389, P=0.018), and
Der p sIgG4 (Pearson correlation=-0.9025, P=0.036) at 5 different efficacy endpoints along whole SCIT peaks. Importantly, the concentration of serum
Der p 2 sIgE showed a positive linear correlation with VAS (Pearson correlation=0.9545, P=0.012), RQLQ (Pearson correlation=0.9577, P=0.010) and serum periostin (Pearson correlation=0.9581, P=0.010). Nevertheless, no correlations were found between VAS and RQLQ nasal or eye symptoms, and no correlation was found between serum periostin, IL-5,
Der p sIgE and RQLQ nasal or eye symptoms.
4. Discussion
In this study, our findings confirmed a statistically significant reduction in Der p 1 sIgE and Der p 2 sIgE levels and clinical symptom scores, such as VAS and RQLQ, and confirmed a statistically significant increase in Der p 1 IgG4 and Der p 2 IgG4 levels with SCIT for 26-30 months. Importantly, our findings observed that the changes in the expression of Der p 2 sIgE correlated noteworthily with the improvements in both serum periostin and allergy symptom scores. Meanwhile, this study has shown a marked decline in the expression of serum IL-5 and periostin during SCIT. Although a downward trend of serum IL-5 was observed during AIT, serum IL-5 levels do not correlate with treatment efficacy.
Consistent with previous results [18, 19], we confirmed that SCIT has an important advantage of long-term efficacy to become the main basis for evidence-based treatment of childhood with AR, but no significant correlation was found between clinical efficacy and levels of
Der p sIgE, which may suggest that allergen sIgE levels are not constant during the course of SCIT. The clinical utility of measuring the levels of
Der p 2 sIgE has been validated as a diagnostic tool for
Der p IgE sensitization detection [
20]. However, whether the long-term efficacy of SCIT and the alleviation of allergy symptoms are related to the decrease in
Der p 2 sIgE has rarely been published. In this study, we confirmed that
Der p 2 sIgE was significantly higher at baseline than at the fourth efficacy endpoint and was closely related to allergy symptoms and serum periostin. These findings identified a close relationship between
Der p 2 sIgE and clinical efficacy and periostin in AR childhood undergoing SCIT.
AR is a type I allergic inflammation and IgE - mediated by sensitization to inhaled allergens, involving a variety of immunoactive cells and type 2 (T2) cytokines [
21]. AIT is currently the only treatment to modify the natural history of allergic diseases characterized by the induction of regulatory Treg and Breg cells [22, 23], which can release regulatory cytokines such as IL-10 and transforming growth factor-β to induce immune tolerance against
Der p allergen [
24]. Meanwhile, AIT can increase the production of specific IgG4 antibodies [
25], which can inhibit the binding of the allergen-IgE compound to the receptor on the surface of B cells and reduce the inhibition of the allergen presented to specific T cells, thereby blocking type I hypersensitivity [
26]. Consistent with previous results [
27], this study shows that the expression of IgG4 was significantly upregulated with SCIT, suggesting that the increase in IgG4 expression may be closely related to the improvement of patient symptoms and the efficacy of SCIT.
Periostin and IL-5 result in eosinophilic airway infiltration and then contribute to T2 airway inflammation. Periostin is an extracellular matrix protein downstream of IL-4 and IL-13, and it has been recognized as an inflammatory medium that is an important cytokine involved in the T2 immune response; its expression level is significantly increased in AR patients [
28], and it has been shown to be involved in many aspects of allergic inflammation [
29]. In previous studies, serum periostin levels were associated with the treatment response to lebrikizumab and anti-IL-13 therapy [
30]. Krasilnikova et al [
31] further found that the severity level of AR symptoms is correlated with the increase in periostin levels in nasal secretions. Therefore, the expression level of nasal periostin can be used as a biomarker of local allergic inflammation in the nasal cavity in patients with AR. Consistent with previous results [
32], our findings confirmed that SCIT can inhibit T2 cytokine expression. Periostin is closely related to clinical outcomes, such as VAS and BQLQ, but not to nasal and eye symptoms. The possible factors were that not all children have sinusitis or conjunctivitis comorbidities, which may suggest that the suppression of the T2 response is related to the mechanisms of SCIT.
We found that
Der p sIgE and periostin increase at the early stage of SCIT and decrease thereafter, which have a positive linear correlation, but they do not correlate with SCIT efficacy. This phenomenon may suggest that the suppression of the T2 response is related to the mechanisms of SCIT. Although our previous findings indicated that serum periostin can be used as a biomarker to evaluate T2-driven airway inflammation [
33], the results of this study may not be used as evidence for considering periostin as a biomarker to evaluate SCIT clinical efficacy. Importantly, a positive linear correlation has been shown between the level of
Der p 2 sIgE and serum periostin and SCIT efficacy, which suggests that
Der p 2 sIgE may be applied as a molecular biomarker evaluation because it can be feasibly implemented in the clinic. This will also ensure that
Der p 2 sIgE has the potential to be beneficial in the treatment, which can improve the correlation of biomarkers to treatment efficacy. Meanwhile, evaluating sensitization to
Der p 2 sIgE is also considered an important step in the selection of patients suitable for SCIT.
Although there are important discoveries revealed by this study, there are also limitations. Posttreatment dropouts were relatively high, and the limited sample size did not enable further stratified analysis of children and may have resulted in statistical bias. The accuracy of complaints of subjective symptoms in children should be taken into account because inaccurate questionnaires being collected can lead to an inability to evaluate biomarkers accurately and thus to realize personalized therapy.
Author Contributions
Conceptualization and methodology: M.X. and Y.H.; experimental procedures: B.X. and J.W.; statistical analysis: X.J.; writing-original draft preparation: J.W., B.X. and M.X.; writing-review and editing: J.W., B.X. and M.X.; supervision: M.X. resource: B.J. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Ningbo Health Science and Technology Project, China (nos. 2022Y19), to J.W.; and by the Ningbo Natural Science Foundation, China (nos. 2022J281), to M.X.
Hospital Ethics Committee Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the First Affiliated Hospital of Ningbo University, Ningbo, China (nos. KY20171118).
Informed Consent Statement
Written informed consent was obtained from the children’s patient(s) at the time of recruitment.
Data availability statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aalberse, R.C., Stapel, S.O., Schuurman, J., and Rispens, T. Immunoglobulin G4: an odd antibody. Clin. Exp. Allergy. 2009, 39, 469-477. [CrossRef]
- Akdis, M., and Akdis, C.A. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J. Allergy. Clin. Immunol. 2014,133, 621-631. [CrossRef]
- Bousquet, J., Schünemann, H.J., Togias, A., Bachert, C., Erhola, M., Hellings, P.W., Klimek, L., Pfaar, O., Wallace, D., Ansotegui, I., et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J. Allergy. Clin. Immunol. 2020,145, 70-80.e73. [CrossRef]
- Chen, K.W., Blatt, K., Thomas, W.R., Swoboda, I., Valent, P., Valenta, R., and Vrtala, S. Hypoallergenic Der p 1/Der p 2 combination vaccines for immunotherapy of house dust mite allergy. J. Allergy. Clin. Immunol. 2012,130, 435-443.e434. [CrossRef]
- Cheng, L., and Zhou, W.C. Sublingual immunotherapy of house dust mite respiratory allergy in China. Allergol. Immunopathol. 2019,47, 85-89. [CrossRef]
- Corren, J., Lemanske, R.F., Hanania, N.A., Korenblat, P.E., Parsey, M.V., Arron, J.R., Harris, J.M., Scheerens, H., Wu, L.C., Su, Z., et al. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 2011,365, 1088-1098. [CrossRef]
- Drazdauskaitė, G., Layhadi, J.A., and Shamji, M.H. Mechanisms of Allergen Immunotherapy in Allergic Rhinitis. Curr. Allergy. Asthma. Rep. 2020,21, 2. [CrossRef]
- Eifan, A.O., Akkoc, T., Yildiz, A., Keles, S., Ozdemir, C., Bahceciler, N.N., and Barlan, I.B. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: an open randomized controlled trial. Clin. Exp. Allergy. 2010,40, 922-932. [CrossRef]
- Giannetti, A., Ricci, G., Procaccianti, M., Santoro, A., and Caffarelli, C. Safety, Efficacy, and Preventive Role of Subcutaneous and Sublingual Allergen Immunotherapy for the Treatment of Pediatric Asthma. J. Asthma. Allergy. 2020, 13, 575-587. [CrossRef]
- Hoshino, M., Akitsu, K., Kubota, K., and Ohtawa, J. Association between biomarkers and house dust mite sublingual immunotherapy in allergic asthma. Clin. Exp. Allergy. 2020, 50, 1035-1043. [CrossRef]
- Hoshino, M., Akitsu, K., Kubota, K., and Ohtawa, J. Serum Periostin as a Biomarker for Predicting Clinical Response to House Dust Mite Sublingual Immunotherapy in Allergic Rhinitis. J. Allergy. Clin. Immunol. Pract. 2021,9, 1864-1870. [CrossRef]
- Incorvaia, C., Cavaliere, C., Schroeder, J.W., Leo, G., Nicoletta, F., Barone, A., and Ridolo, E. Safety and adverse reactions in subcutaneous allergen immunotherapy: a review. Acta. Biomed. 2023,94, e2023172. [CrossRef]
- Kappen, J.H., Durham, S.R., Veen, H.I., and Shamji, M.H. Applications and mechanisms of immunotherapy in allergic rhinitis and asthma. Ther. Adv. Respir. Dis. 2017,11, 73-86. [CrossRef]
- Krasilnikova, S.V., Tush, E.V., Frolov, P.A., Ovsyannikov, D.Y., Terentyeva, A.B., Kubysheva, N.I., and Eliseeva, T.I. Periostin as a Biomarker of Allergic Inflammation in Atopic Bronchial Asthma and Allergic Rhinitis (a Pilot Study). Sovrem. Tekhnologii. Med. 2021, 12, 37-45. [CrossRef]
- Li, H., Chen, S., Cheng, L., Guo, Y., Lai, H., Li, Y., Lin, X., Liu, Z., Qiu, Q., Shao, J., et al. Chinese guideline on sublingual immunotherapy for allergic rhinitis and asthma. J. Thorac. Dis. 2019,11, 4936-4950. [CrossRef]
- Li, J., Sun, B., Huang, Y., Lin, X., Zhao, D., Tan, G., Wu, J., Zhao, H., Cao, L., and Zhong, N. A multicentre study assessing the prevalence of sensitizations in patients with asthma and/or rhinitis in China. Allergy. 2009, 64,1083-1092. [CrossRef]
- Li, P., Li, Q., Huang, Z., Chen, W., Lu, Y., and Tian, M. Efficacy and safety of house dust mite sublingual immunotherapy in monosensitized and polysensitized children with respiratory allergic diseases. Int. Forum. Allergy. Rhinol. 2014,4, 796-801. [CrossRef]
- Li, W., Gao, P., Zhi, Y., Xu, W., Wu, Y., Yin, J., and Zhang, J. Periostin: its role in asthma and its potential as a diagnostic or therapeutic target. Respir. Res. 2015,16,57. [CrossRef]
- Minami, T., Fukutomi, Y., Lidholm, J., Yasueda, H., Saito, A., Sekiya, K., Tsuburai, T., Maeda, Y., Mori, A., Taniguchi, M., et al. IgE Abs to Der p 1 and Der p 2 as diagnostic markers of house dust mite allergy as defined by a bronchoprovocation test. Allergol. Int. 2015,64, 90-95. [CrossRef]
- Nolte, H., Bernstein, D.I., Nelson, H.S., Kleine-Tebbe, J., Sussman, G.L., Seitzberg, D., Rehm, D., Kaur, A., Li, Z., and Lu, S. Efficacy of house dust mite sublingual immunotherapy tablet in North American adolescents and adults in a randomized, placebo-controlled trial. J. Allergy. Clin. Immunol. 2016,138, 1631-1638. [CrossRef]
- Ozceker, D., Yucel, E., Sipahi, S., Dilek, F., Ozkaya, E., Guler, E.M., Kocyigit, A., Guler, N., and Tamay, Z. Evaluation of periostin level for predicting severity and chronicity of childhood atopic dermatitis. Postepy. Dermatol. Alergol. 2019, 36, 616-619. [CrossRef]
- Parashar, S., Pandya, A., and Portnoy, J.M. Pediatric subcutaneous allergen immunotherapy. Allergy. Asthma. Proc. 2022, 43, 286-291. [CrossRef]
- Roberts, G., Pfaar, O., Akdis, C.A., Ansotegui, I.J., Durham, S.R., Gerth van Wijk, R., Halken, S., Larenas-Linnemann, D., Pawankar, R., Pitsios, C., et al. EAACI Guidelines on Allergen Immunotherapy: Allergic rhinoconjunctivitis. Allergy. 2018, 73, 765-798. [CrossRef]
- Scadding, G.K., Smith, P.K., Blaiss, M., Roberts, G., Hellings, P.W., Gevaert, P., Mc Donald, M., Sih, T., Halken, S., Zieglmayer, P.U., et al. Allergic Rhinitis in Childhood and the New EUFOREA Algorithm. Front. Allergy. 2021, 2, 706589. [CrossRef]
- Shamji, M.H., Sharif, H., Layhadi, J.A., Zhu, R., Kishore, U., and Renz, H. Diverse immune mechanisms of allergen immunotherapy for allergic rhinitis with and without asthma. J. Allergy. Clin. Immunol. 2022, 149, 791-801. [CrossRef]
- Shao, J., Cui, Y.X., Zheng, Y.F., Peng, H.F., Zheng, Z.L., Chen, J.Y., Li, Q., and Cao, L.F. Efficacy and safety of sublingual immunotherapy in children aged 3-13 years with allergic rhinitis. Am. J. Rhinol. Allergy. 2014, 28, 131-139. [CrossRef]
- Tan, T.J., Layhadi, J.A., and Shamji, M.H. Mechanisms and biomarkers of subcutaneous immunotherapy and sublingual immunotherapy in allergen immunotherapy. Allergy. Asthma. Proc. 2022, 43, 254-259. [CrossRef]
- Tian, M., Zhou, Y., Zhang, W., and Cui, Y. Der p 1 and Der p 2 specific immunoglobulin E measurement for diagnosis of Dermatophagoides pteronyssinus allergy: A systematic review and meta-analysis. Allergy. Asthma. Proc. 2017, 38, 333-342. [CrossRef]
- Wang, C., Bao, Y., Chen, J., Chen, X., Cheng, L., Guo, Y.S., Hao, C., Lai, H., Li, H., Li, J., et al. Chinese Guideline on Allergen Immunotherapy for Allergic Rhinitis: The 2022 Update. Allergy. Asthma. Immunol. Res. 2022, 14, 604-652. [CrossRef]
- Wang, H.Y., Gao, Z.S., Zhou, X., Dai, Y., Yao, W., Zhang, X.F., Yang, Z.W., Wu, S.D., Yu, C.H., Yang, X.Y., et al. Evaluation of the Role of IgE Responses to Der p 1 and Der p 2 in Chinese House Dust Mite-Allergic Patients. Int. Arch. Allergy. Immunol. 2015, 167, 203-210. [CrossRef]
- Xu, M., Zhang, W., Chen, D., Zhou, H., and Chen, L. Diagnostic significance of serum periostin in eosinophilic chronic sinusitis with nasal polyps. Acta. Otolaryngol. 2018, 138, 387-391. [CrossRef]
- Yang, X., Fan, G., and Li, J. Diagnostic value of Der p 1 and Der p 2 specific IgE in Dermatophagoides pteronyssinus IgE sensitization. Ann. Allergy. Asthma. Immunol. 2016,116, 295-301. [CrossRef]
- Zielen, S., Devillier, P., Heinrich, J., Richter, H., and Wahn, U. Sublingual immunotherapy provides long-term relief in allergic rhinitis and reduces the risk of asthma: A retrospective, real-world database analysis. Allergy. 2018, 73, 165-177. [CrossRef]
Table 1.
Demographic and survey information at baseline of AR childhood
Table 1.
Demographic and survey information at baseline of AR childhood
Table 2.
Correlations between the changes in clinical indices in childhood with AR treatment with SCII
Table 2.
Correlations between the changes in clinical indices in childhood with AR treatment with SCII
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).