1. Introduction
By definition, collision tumors display two distinct cell neoplastic populations developed in juxtaposition to one another without areas of intermingling. Most collision tumors occur in the crania, lung, gastro-oesophageal junction, liver, rectum, bladder, uterus, and testes with two or more independent tumor components without transitional morphology [
1]. Not long ago, a complex inventory of possible collision tumors was carried out, including their differentiation from composite / mixed tumors, on the organ with the largest surface of the body, the skin [
2]. Recently, another case report focused on breast as the same site for an invasive ductal carcinoma to collide with malignant melanoma (metastatic to breast tissue) was published [
3]. Within their systematic review of the literature on collision tumors of the gastrointestinal tract, Schizas et al. inventoried 53 cases over a 10-year period [
4]. They evaluated the location, neoplastic types, biological behaviour, and therapeutic strategy after anatomopathological diagnosis for each individual case. Most of the reported cases were in the stomach (20 cases), followed by the oesophagus (17 cases), large intestine (14 cases) and the small intestine, as expected, with only 2 cases.
Collision tumors are part of a wider category of mixed neoplasms together with composite tumors, carcinosarcomas, or tumors with epithelial-mesenchymal transdifferentiation, respectively, amphicrine tumors. Although there are enough criteria for differential diagnosis between these entities, to which is added the temporal component in the treatment of the oncological patient, the terms are often used mixing the histopathological aspects with the anatomical ones and temporal consecutiveness or iatrogenic induction.
At this point, it is necessary to clear up terms that sometimes are used interchangeably, as well as to list the possible combinations between histological entities and to mention some hypothesis regarding the explanation of such complex pathology:
Terms: collision tumors = two or more morphologically different tumors arising in the same organ, at the same time; synchronous tumors refer to two (or more) independent primary malignancies, when the second malignancy arose within 6 months of the diagnosis of the first malignancy, in the same, or in different organs; metachronous tumors, in which the cancers follow in sequence, that is, more than six months apart; composite / mixed / heterologous tumors, morphological distinct tumors with cellular intermingling, in the same organ;
Histological entities for collision and composite tumors are diverse: both benign or both malignant (primary or secondary – “tumor in tumor”) or one benign, the other malignant (also, primary or secondary); for synchronous and metachronous tumors malignant proliferation is a rule;
Hypothesis: (a) coincidental occurrence of two primary neoplasms within a common location; (b) a common carcinogenic stimulus that altered the cellular microenvironment within the proximity of which two distinct neoplasms arise from; (c) the first tumor may have changed the microenvironment within the organ and increased the likelihood of developing another primary tumor or facilitated metastatic seeding within the vicinity [
5].
To date, within the gastro-intestinal tract, in the English literature, gastric adenocarcinomas have been described that collide with lymphomas, gastrointestinal stromal tumors, squamous cell carcinomas, and neuroendocrine tumors [
6], as well as colorectal carcinomas coexisting with neuroendocrine tumors, leiomyosarcomas, lymphomas, Schwann cell hamartoma, gastrointestinal stromal tumors, or metastatic gastric adenocarcinoma [
7,
8,
9].
The present paper highlights the coexistence in the right colon of an aggressive signet-ring carcinoma with multiple granular cell tumor nodules (Abrikossoff tumor), the latter clinically mistaken for celomic / peritoneal dissemination (carcinomatosis). To our knowledge, this is the first time in literature to report such a neoplastic association.
2. Case Report
We present the case of a 60-year-old man who accuses incomplete cessation of intestinal transit for a couple of days, pain in the fossa and right abdominal flank, associated with vomiting, loss of appetite, and moderate weight loss. He addresses the Gastroenterology Clinic of the Timisoara Emergency Clinical County Hospital, where he has computerised abdominal imaging and colonoscopy. Computer tomography highlights the thickening at the level of the ascending colon and cecum, and the colonoscopy a stenosing proliferative process of the right colon, the hepatic angle not being endoscopically passable.
Local examination shows a soft, depressible abdomen that is mobile with respiratory movements, but at the level of the right iliac fossa, a hard consistency tumor mass, fixed in the deep planes, can be palpated. Because the patient's condition worsened rapidly, he had a surgical indication, so an emergency laparotomy was decided. Intraoperatively, the cecum and ascending colon tumor is found up to the hepatic angle of the colon, with serosa retraction and posterior penetration into the retroperitoneal space. At the same time, regional adenopathies near the origin of the right and middle colic veins were identified. Right hemicolectomy with end-to-end ileotransverse anastomosis is performed.
The resected intestinal specimen - terminal ileum (10 cm), cecum, and ascending colon (together 18 cm) is orientated in the pathological anatomy laboratory. A cauliflower tumor mass is identified from the ileocecal valve, extending for a length of 11 cm in the ascending colon, which completely stenoses the intestinal lumen and infiltrates the muscularis propria, associated with retraction of the serosa. Regardless of the ascending colon tumor, at the cecal submucosal level and in the periintestinal adipose atmosphere, multiple, whitish, firm, and fascicular nodules are observed and processed in the cut section.
In addition to intestinal pathology, it should be mentioned that the patient's pathological history records gastroduodenal ulcer with conservative treatment and lung sequelae after tuberculous infection.
After the final histopathological report, the patient received adjuvant therapy, without postoperative complications or recurrence at clinical follow-up according to oncological protocols.
The completion of the surgical procedure and the patient's compliance to adjuvant therapy manage to prolong his survival by almost 2 years.
3. Material and methods
Tissue material (from submitted surgical specimen submitted - orientated biopsies: tumor mass and regional lymph nodes) was processed using the standard method: 10% buffered formalin fixation, followed by paraffin embedding. The sections were stained with hematoxylin – eosin (H&E), completed with the immunohistochemical profile (IHC) (see the following
Table 1): anti-cytokeratin 7, anti-cytokeratin 20, CDX2, S100 protein, CD117 (c-kit) and CD68, visualisation with the Polymer system, using DAB (diaminobenzidine) chromogen and hematoxylin counterstain. All imunohistochemical reactions included control tissue sections, either internal or external.
4. Results
Histopathological findings
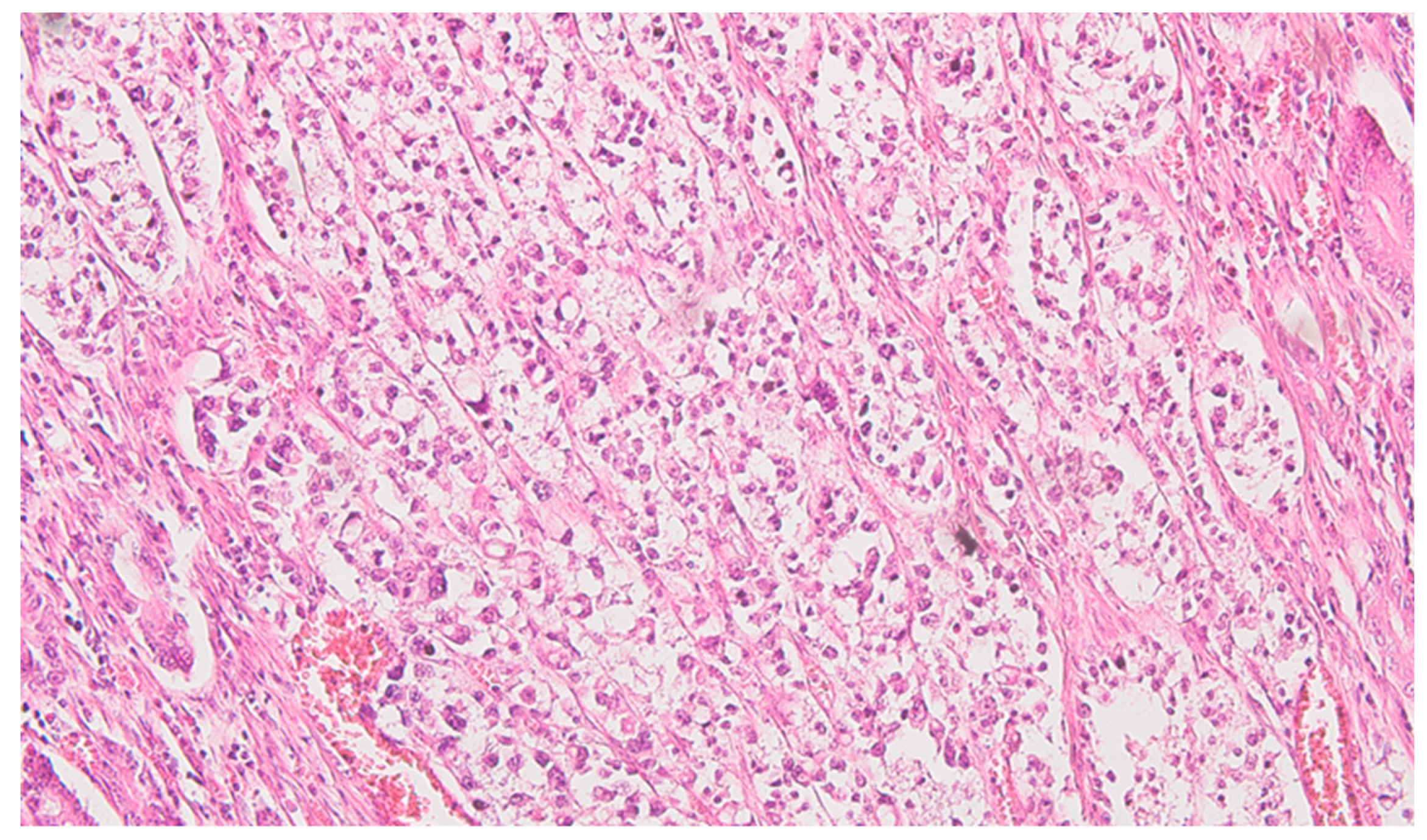
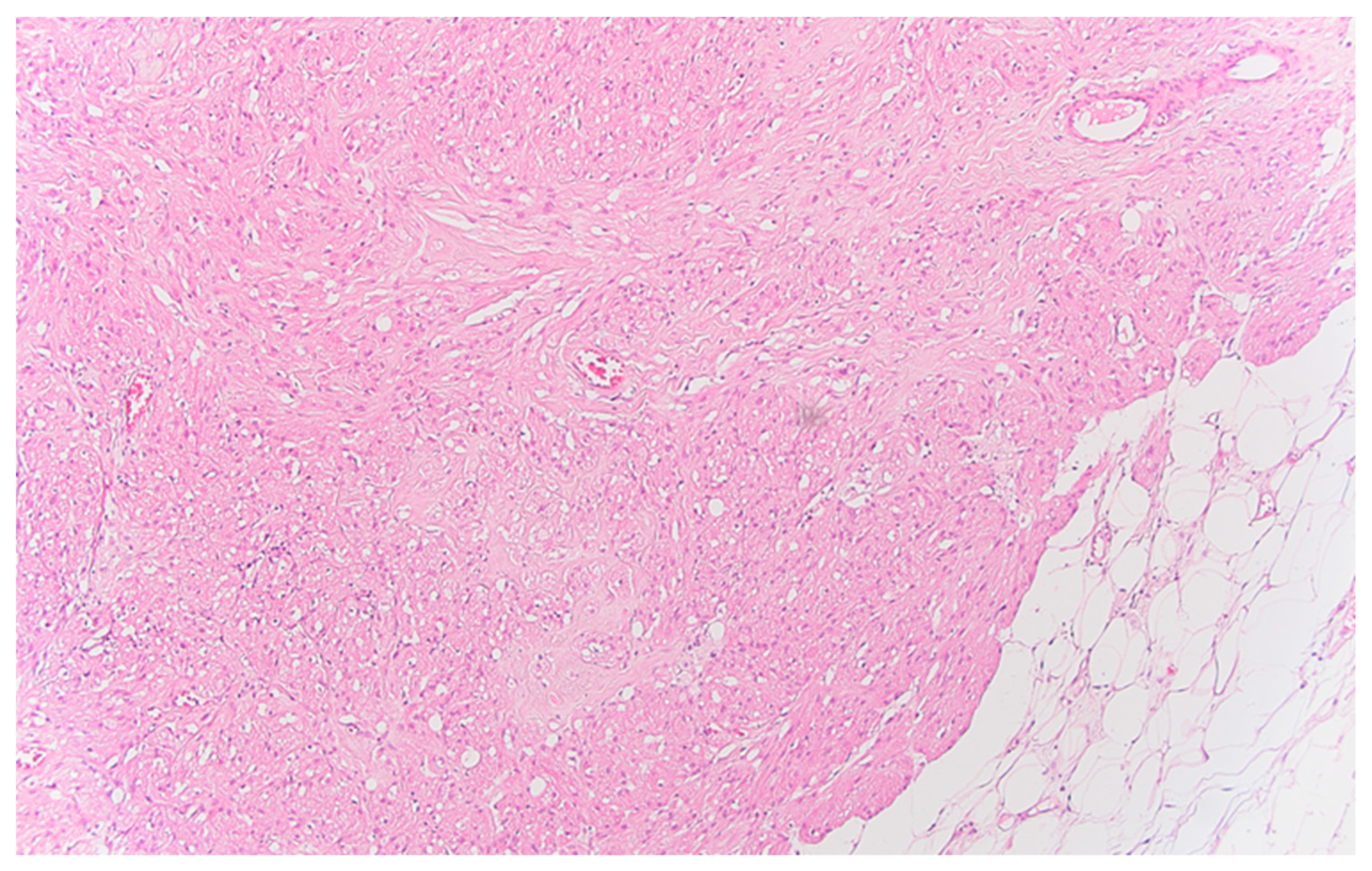
Serial sections of the ascending colon tumor reveal massive infiltration of a diffuse, mucinous carcinoma, with predominantly intracellular secretion of mucin ("signet ring" cells); some isolated aspects of mucinous tubular adenocarcinoma are also observed. The tumor is ulcerated on the surface, extensively invades the submucosa (
Figure 1), and dissociates the muscular layer
(muscularis propria), being found massively in the subserosa (
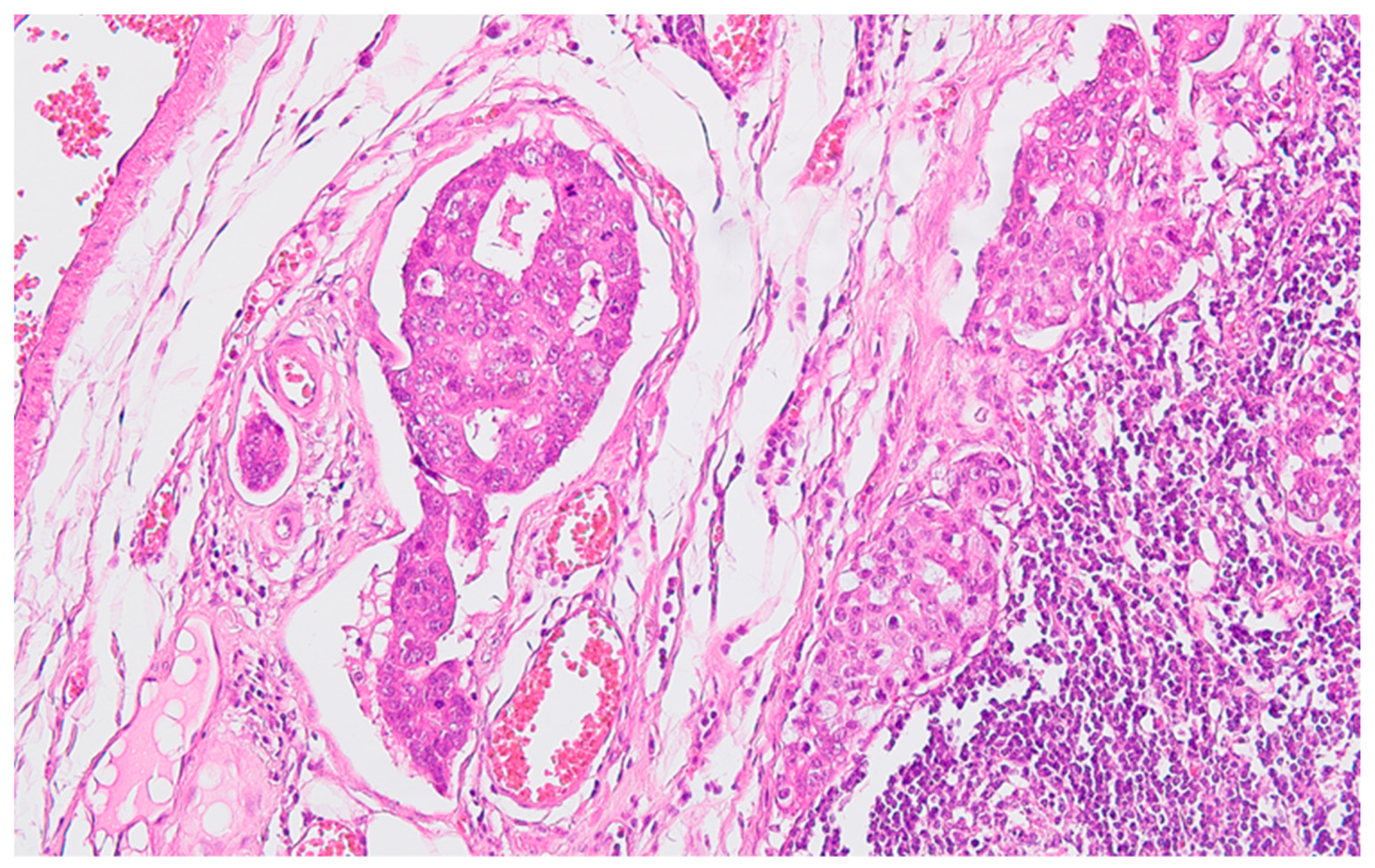
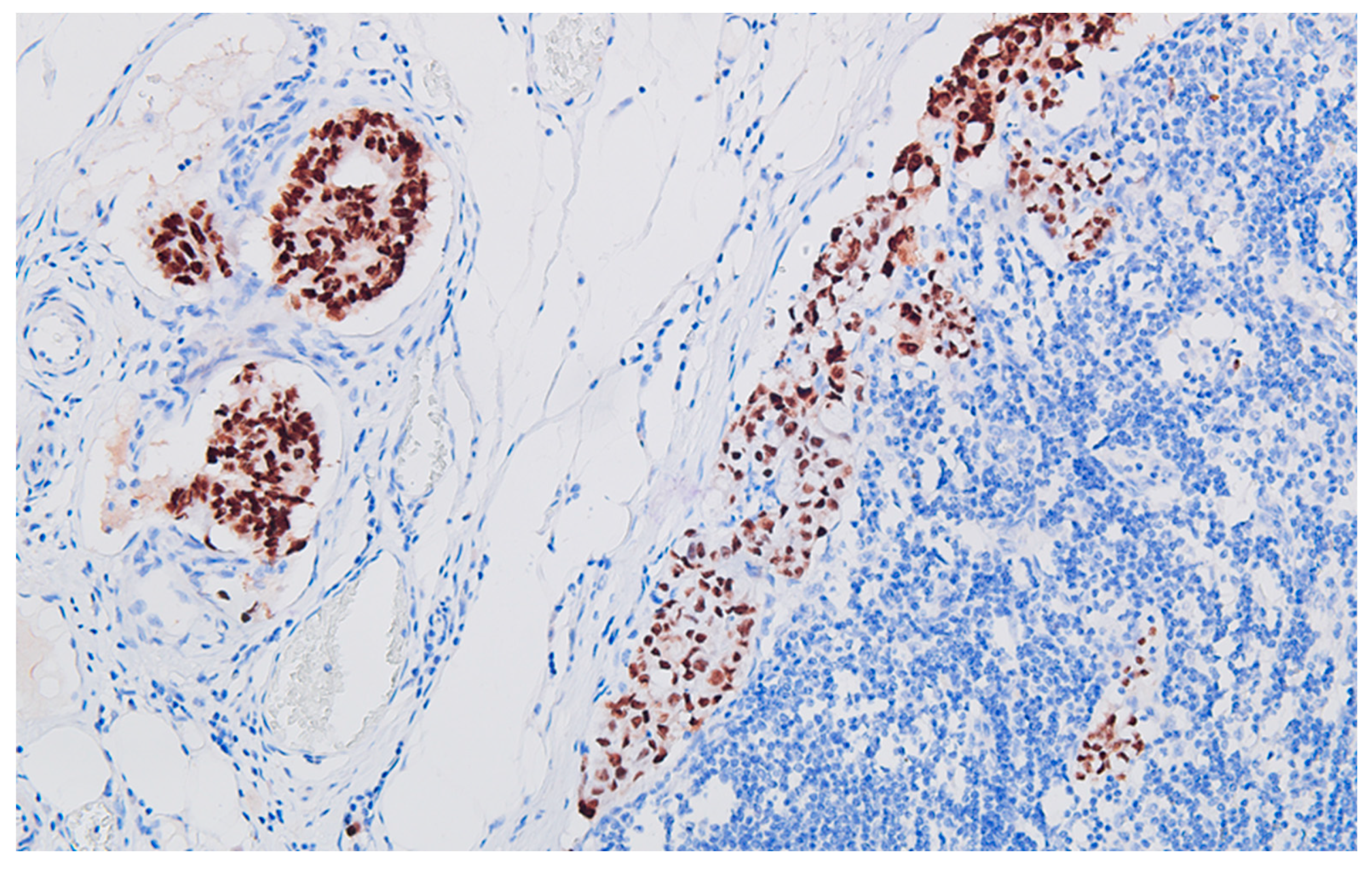
Figure 2), with perforation of the visceral peritoneum: tumor cells in ink or less than 1 mm from the inked serosa (pT4a); numerous lymphatic tumor emboli, frequent aspects of perineural invasion. Of 26 lymph nodes, 24 show massive carcinomatous metastases (adenocarcinoma type,
Figure 3) - pN2b.
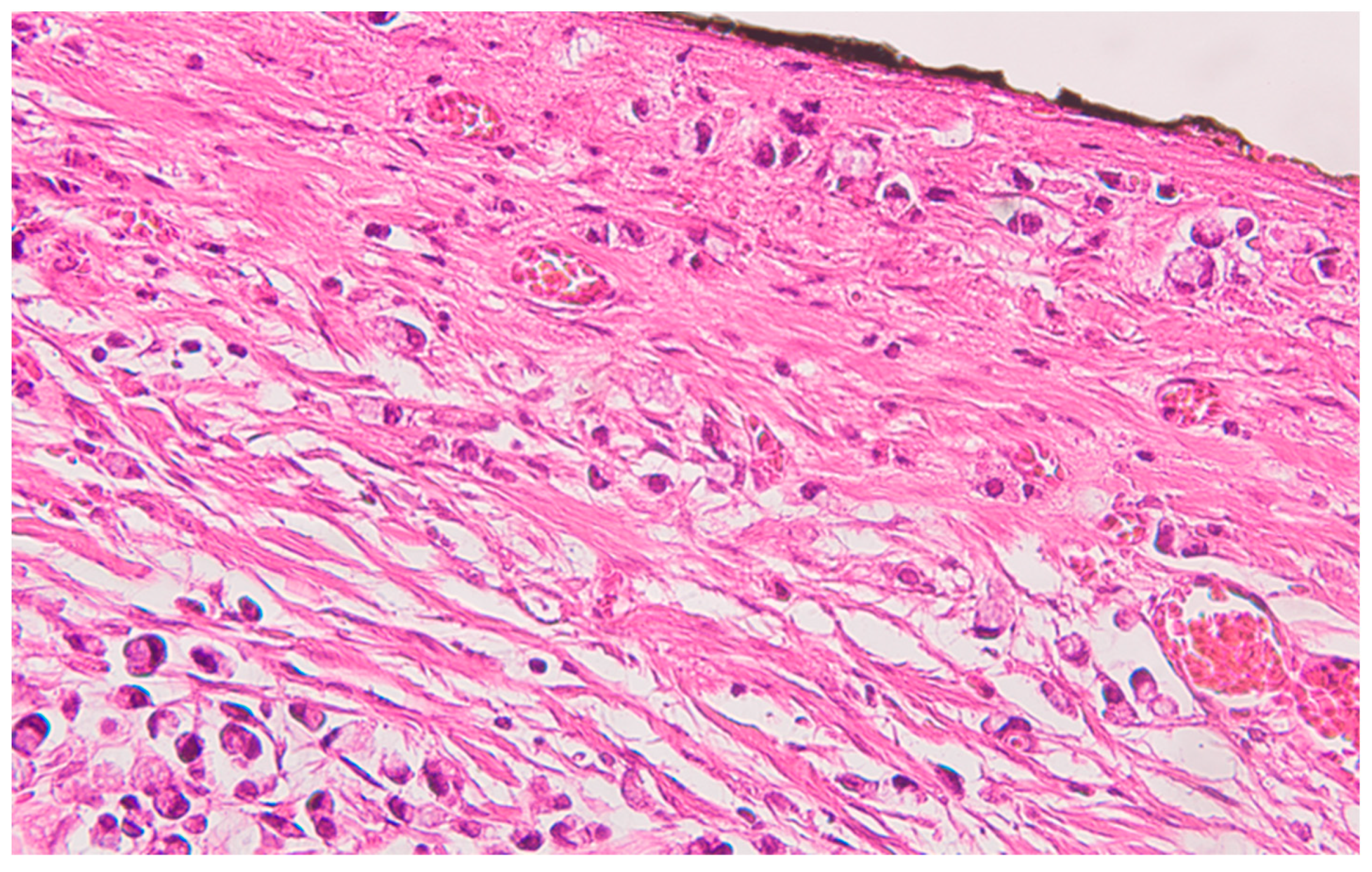
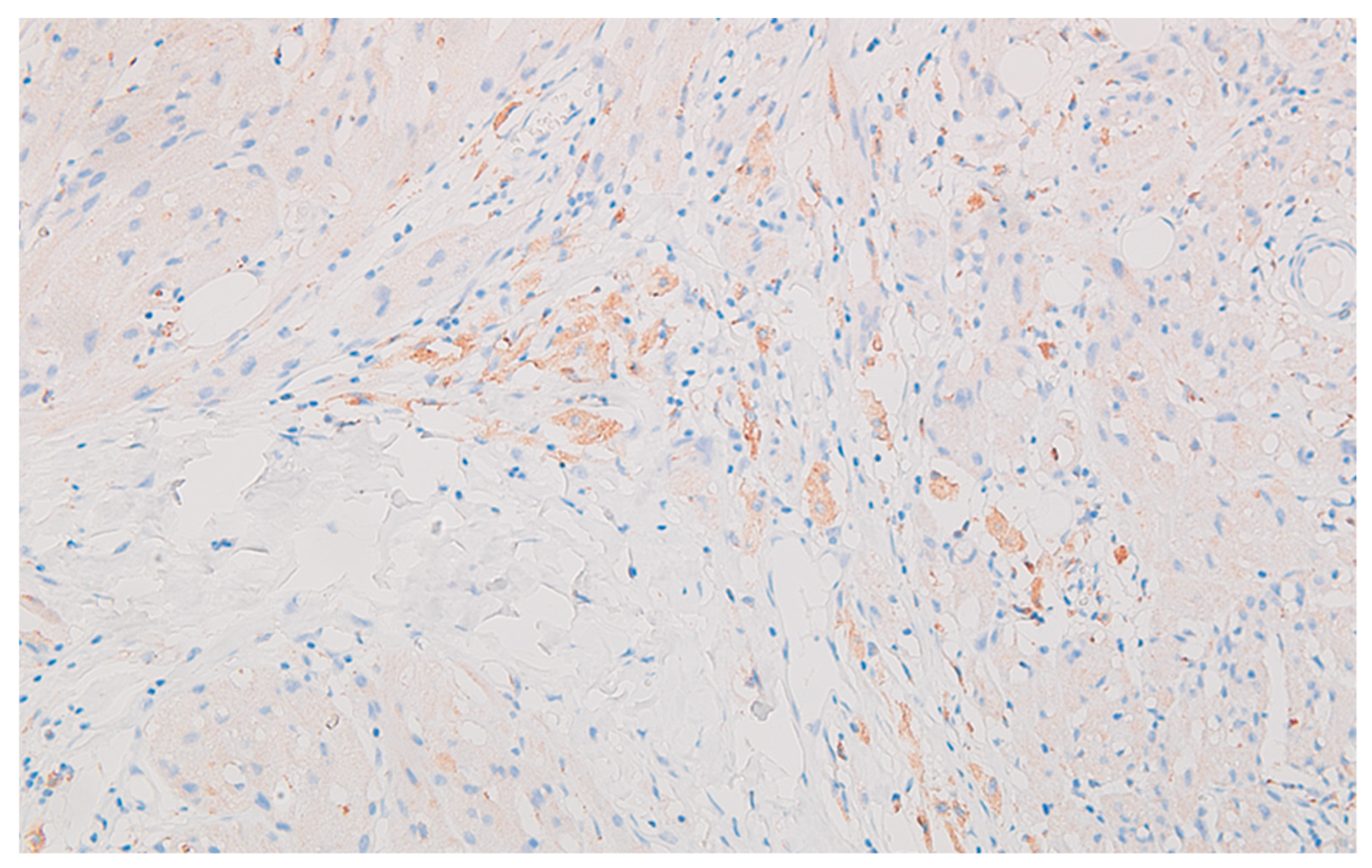
Well delineated from the previously described malignant epithelial-glandular tumor, large polygonal cell nodules, with abundant, eosinophilic, granular cytoplasm, hyperchromatic or vesicular nucleolated nuclei are found in the submucosa of the cecum and the visceral peritoneum of the ascending colon (
Figure 4); without cyto-nuclear atypia, without tumor necrosis. These tumor nodules develop in the vicinity of nerve fibres.
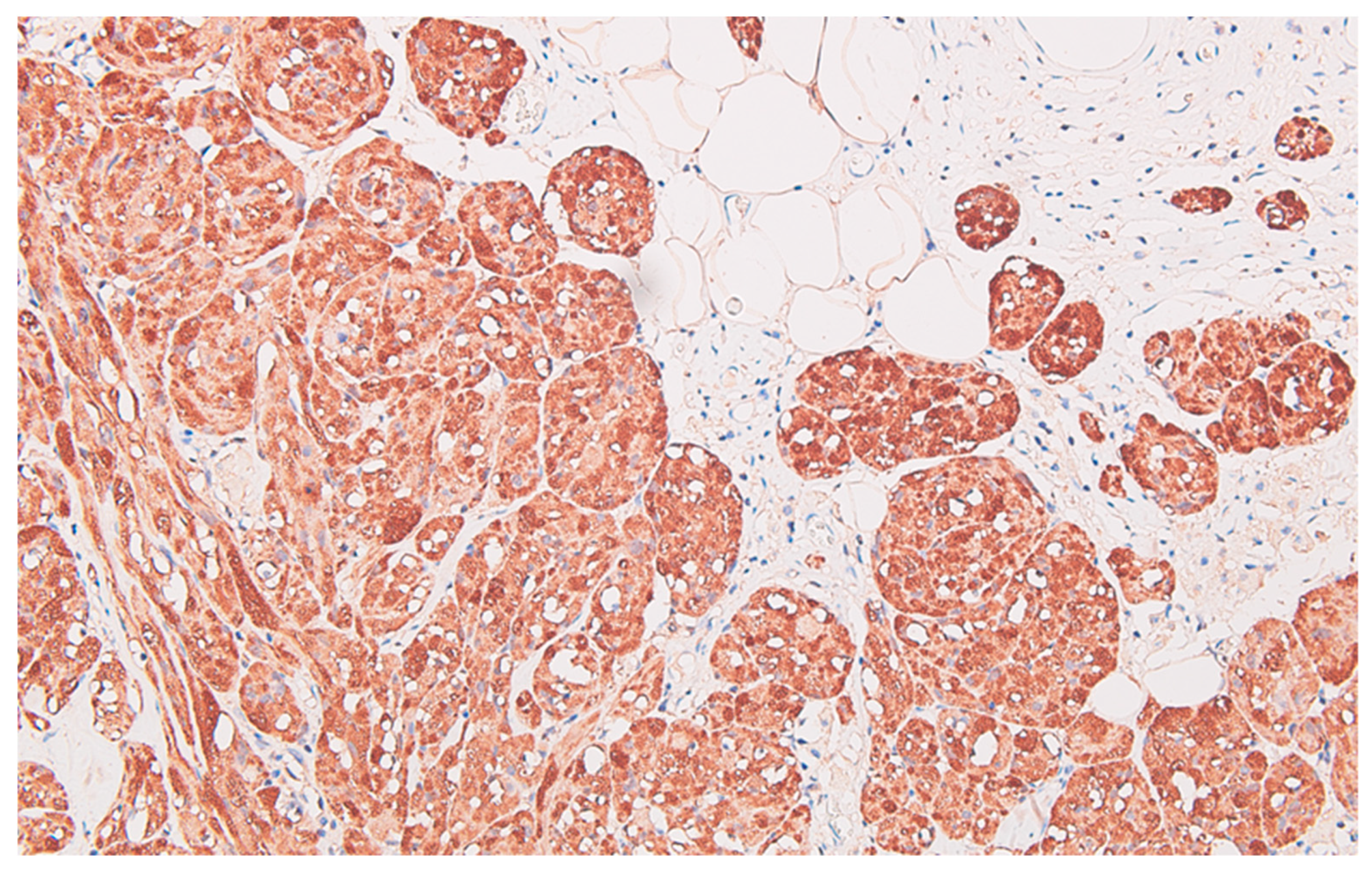
The immunohistochemical profile of the carcinoma CK7 negative, patchy CK20 positive (
Figure 5), diffuse CDX2 positive (
Figure 6), corresponds to a primary colorectal carcinoma, and for the peritoneal tumor nodules the diffuse S100 protein positivity (
Figure 7), patchy CD68 positive (
Figure 8), together with CD117 (c-kit) negative reaction, leads to the diagnosis of granular cell tumor / granular cell nerve sheath tumor / Abrikossoff tumor and exclude a possible association of a gastrointestinal stromal tumor with the colonic carcinoma.
The microsatellite stability / instability status was not evaluated in the presented case because the patient died before this expertise was validated in our health care system’ area. However, there were enough clinical and microscopic features that did not match microsatellite instability-high (MSI-high) signet ring cell carcinomas of the colorectum: gender, absence of Crohn-like reaction or numerous tumor-infiltrating lymphocytes (TILs).
5. Discussion
Even if collision tumors were rarely reported, they always raise difficulties in diagnosis and therapeutic strategies for the patient. Not to mention the huge amount of time, skill, and anatomoclinical correlation of the medical team.
Why does this event occur? There are few explanations:
- a)
from a coincidental neoplastic change [
10], in other words, as Purdy et al., cited by [
10] described it: may simply be the chance apposition of two unrelated tumors;
- b)
a double clonal origin for both components of the collision tumor [
11];
- c)
one tumor develops and changes the local microenvironment to promote the development of the second tumor [
12] or
- d)
the two types of tumors share a common origin of pluripotent precursor stem cells that differentiate into the components of tumor cell types [
12].
In the current case, signet-ring carcinoma by its ability to invade the perineural spaces certainly determines the change in their microenvironment, which could trigger another clonal mutation, this time in the sheaths of nerve twigs. As Brahmania et al. suggested in their paper [
12] this change in the atmosphere of small nerves may contribute to the proliferation of Schwann cells from the myelinated sheaths of peripheral nerves at the level of the colon and its peritoneal covering.
Another particularity of the presenting case is the unexpected malignant epithelial contingent that spreads through the lymphatics: the adenocarcinomatous one, despite that in the colonic wall there was the 'minor' carcinoma type as area of involvement and the fact that it is more histologically differentiated compared to the signet-ring cell type of caecinoma. As a rule in oncology, the more differentiated the malignancy, it is less eager to invade lympho-vascular spaces. And here it was the other way around: signet-ring cell carcinoma (the least differentiated type) was aggressive locally (visceral peritoneum perforation), and adenocarcinoma (more differentiated type) spreads to the vast majority of regional lymph nodes (24 positive for metastases out of 26 resected lymph nodes resected). Exactly this paradoxical behaviour could lead to the disturbance of the microenvironment in the invasion front of the tumor with signet ring cells and allowed or triggered the initiation and development of the Schwann cells tumor derived from the nerve fibres in that area.
The histopathological report ruled out the clinical supposition of peritoneal carcinomatosis by unequivocally demonstration (usual H&E stain and immune profile: S100+, CD68+) that the peritoneal nodules are composed of granular cells, without cyto-nuclear atypia, originating in the peripheral nerve sheath: granular cell nerve sheath tumor (Abrikossoff tumor). In addition, immunohistochemical profiling excluded the gastro-intestinal tumor, a very similar aspect on routine (H&E) staining with granular cell tumor, by negative reaction to CD117 (c-kit) and positive S100, combined with patchy CD68 positive.
Between these two collided tumors, there were no intervening intermediate cell population or intermingling areas between carcinoma and granular cell tumor, the sine qua non condition for final diagnosis of collision tumor.
The consequences of peritoneal granular cell tumor diagnosis, lacking histopathological criteria for malignancy, could be more of an academic interest or as an incidental finding after sampling due to, e.g. a puzzling abdominal imaging. But it’s collision with colon signet-ring cell carcinoma, in an advanced stage (IIIC / pT4aN2b), definitively changed the therapeutic management and the prognosis of the patient.
Even in this stage, the patient benefits from chemotherapy, especially because the surgical procedure was successful, as it is mentioned in several studies [
13,
14], but, of course, the signet-ring cell carcinoma histology
per se signifies a dismal evolution [
15,
16].
He was followed-up imagistic and surgically for near 2 years after the operation without local recurrence.
The patient profile parallels what was observed in the meta-analysis performed by the already mentioned Greek pathology and surgery team of researchers [
4], that is, male gender and 7
th decade of life. But what is completely different in the presented case is the uncommon association: signet-ring cell carcinoma with a minor adenocarcinoma component and granular cell tumor (a typical one). The meta-analysis stresses that the most common histological component was adenocarcinoma (78.6% of those 14 cases of collision tumors found published in the literature for a decade), in collision with gastro-intestinal stromal tumors or neuroendocrine tumors [
4].
With regard to locally advanced epithelial malignancy, similar to the previously mentioned meta-analysis, it seems that without any connection with the size / volume of the adenocarcinoma component, precisely this component is found in regional lymph node metastases.
6. Conclusions
To our knowledge, is the first time when such juxtaposition of different tumors (as biological behaviour and histogenesis), that of the signet-ring cell carcinoma and granular cell tumor is brought to the attention of medical and surgical specialists. The rarity of colon signet-ring cell carcinoma and it’s collision with a similar rare tumor, the granular cell, made it difficult to achieve an accurate histopathological diagnosis. Although there are still controversies regarding the microsatellite instability status as a significant predictor of survival in signet ring cell carcinoma of the colorectum, further studies are necessary to finalise this aspect, knowing that approximately 1/3 of colorectal signet ring cell carcinoma cases have MSI-high. However, all professional efforts and multidisciplinary collaboration were rewarded with the application of the appropriate therapy to the patient, increasing his disease-free survival to almost 2 years.
Funding
This research received no external funding.
Institutional Review Board Statement
The reported case was edited according to ethical standards of the "Pius Brinzeu" Emergency County Clinical Hospital Timisoara, RI-SCJUPBT-01/2022.
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ting Qian: Feng Gao, Mao-Zhen Chen, Fan-Hua Meng, Xiao-Jing Li, Yong-Juan Liu, Hua-Bin Yin - Collision tumor of the esophagus: Report of a case with mixed squamous cell carcinoma and gastrointestinal stromal tumor, Int J Clin Exp Pathol 2014;7(3):1206-1211. www.ijcep.com /ISSN:1936-2625/IJCEP1312077.
- Bulte CA, Hoegler KM, Khachemoune, A. Collision tumors: A review of their types, pathogenesis, and diagnostic challenges. Dermatol Ther. 2020 Nov;33(6):e14236. [CrossRef]
- Grove J, Komforti M K, Craig-Owens L; et al. (March 28, 2022) A Collision Tumor in the Breast Consisting of Invasive Ductal Carcinoma and Malignant Melanoma. Cureus 14(3): e23588. [CrossRef]
- Brandwein-Gensler M, Urken M, Wang B (2004) Collision tumor of the thyroid: A case report of metastatic liposarcoma plus papillary thyroid carcinoma. Head Neck 26(7):637–641. [CrossRef]
- Adamantios Michalinos, Anastasia Constantinidou, Michael Kontos - Gastric Collision Tumors: An Insight into Their Origin and Clinical Significance Gastroenterology Research and Practice Volume 2015, Article ID 314158, 8 pages. [CrossRef]
- Rajekar, Harshal; Bhoje, Amol; Vaiphei, Kim - Rectal adenocarcinoma coexisting with gastro-intestinal stromal tumor: A case report and literature review. Journal of Cancer Research and Therapeutics 9(1):p 138-140, Jan–Mar 2013. |. [CrossRef]
- Dimitrios Schizas, Ioannis Katsaros, Adamantios Michalinos, Christos Damaskos, Nikolaos Garmpis, Vasileia Ntomi, George Agrogiannis, Spyridon Stergiopoulos, Alexandra, K. Tsaroucha - Collision Tumors of the Gastrointestinal Tract: A Systematic Review of the Literature, Anticancer Research November 2018, 38 (11) 6047-6057. [CrossRef]
- Bhattacharya A, Saha R, Biswas J, Biswas, J., Ghosh, B. - Collision tumors in the gastrointestinal tract: A rare case series. Int Med Case Rep, J. 2012 Oct 25;5:73-7. [CrossRef]
- Miyamoto R, Kikuchi K, Uchida A; et al. Collision tumor consisting of a colorectal adenocarcinoma and dissemination of a gastric adenocarcinoma. SAGE Open Medical Case Reports. 2018;6. [CrossRef]
- Sung, C.T., Shetty, A., Menias, C.O. et al. Collision and composite tumors; radiologic and pathologic correlation. Abdom Radiol 42, 2909–2926 (2017). [CrossRef]
- Gonzalez L, Sanz-Esponera J, Saez C, Alvarez T, Sierra E and Sanz-Ortega, J. Case report: Esophageal collision tumor (oat cell carcinoma and adenocarcinoma) in Barrett’s esophagus: Immunohistochemical, electron microscopy and LOH analysis. Histol Histopathol 2003; 18: 1-5.
- Brahmania M, Kanthan C and Kanthan, R. Collision tumor of the colon-colonic adenocarcinoma and ovarian granulosa cell tumor. World J Surg Oncol 2007; 5: 118. [CrossRef]
- Jingxu Sun, Xin Wang, Peng Gao, Yongxi Song, Xiaowan Chen, Yu Sun, Dehao Yu, Xinger Lv, Zhenning Wang - Prognosis and efficiency of adjuvant therapy in resected colon signet-ring cell carcinoma, Transl Cancer Res 2018;7(4):1006-1025.
- Zhao Z, Yan N, Pan S, Wang DW, Li ZW. The value of adjuvant chemotherapy in stage II/III colorectal signet ring cell carcinoma. Sci Rep. 2020 Aug 24;10(1):14126. [CrossRef]
- Hugen N, Verhoeven RH, Lemmens VE, van Aart CJ, Elferink MA, Radema SA, Nagtegaal ID, de Wilt JH. Colorectal signet-ring cell carcinoma: Benefit from adjuvant chemotherapy but a poor prognostic factor. Int J Cancer. 2015 Jan 15;136(2):333-9. [CrossRef]
- Alfredo Annicchiarico, Andrea Morini, Andrea Romboli, Matteo Riccò, Francesco Leonardi, Pellegrino Crafa, Edoardo Virgilio, Paolo Dell’abate, Renato Costi - Stage III and Metastatic Lymph Node Ratio Are the only Independent Prognostic Factors in Colorectal Signet-ring Cell Carcinoma Patients, Anticancer Research Dec 2020, 40 (12) 7127-7134. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).