1. Introduction
Primary hyperparathyroidism (PHPT) is a clinical condition characterized by hypercalcemia caused by excessive parathyroid hormone (PTH) secretion from the parathyroid gland and the most prevalent cause of hypercalcemia in outpatient clinics (1, 2). It has been detected more frequently in the last 40 years due to more frequent measurement of serum calcium (Ca) levels (3). Therefore, PHPT is a relatively common endocrine disease with an incidence of 1/1000 among patients (4). Recently, there has been an increasing interest in cardiac evaluation in patients with PHPT because various studies showed that PHPT elevates cardiac morbidity and mortality (5).
Coronary flow reserve (CFR) is calculated by dividing the absolute values of hyperemic and resting myocardial blood flows and primarily refers to coronary microvascular function. A decrease in CFR is an indicator of atherosclerosis and, therefore, early coronary artery disease (CAD) with impaired endothelial functions. It was previously shown to have a prognostic value for cardiovascular events in different systemic diseases (6-8). Transthoracic echocardiography can be used to measure CFR; it is preferred because of its high diagnostic accuracy, versatility, low cost, and especially not being exposed to radiation (9)
Although conventional atherogenic lipid parameters are used to assess the risk of CAD, many extensive epidemiological studies revealed that novel lipid indices, such as the plasma atherogenic index (PAI), have better predictive value for atherosclerotic CAD risk assessment than conventional ones (10-12)
PAI is a newly popular lipid index obtained by logarithmic conversion of triglyceride (TRG) / high-density lipoprotein cholesterol (HDL-C) ratio. Relevant literature host studies having shown that it is associated with atherosclerosis and subclinical coronary artery disease and may be a marker for cardiovascular mortality (13, 14).
Ultimately, we aimed to determine CFR, an indicator of subclinical atherosclerosis, and PAI levels for risk assessment of CAD in patients with PHPT. We also explored whether PAI can be used for the early detection of CAD.
2. Methods
Sample
We prospectively carried out the study with 44 PHPT patients who were followed up in the Endocrinology Clinic of Kayseri City Training and Research Hospital between September and March 2021. We noted down physical examination findings, medical records, and laboratory findings of the patient and control groups. The control group consisted of age- and sex-matched 33 individuals with normal blood PTH levels who were not considered to have coronary artery disease upon their medical records, physical examination, ECG, and echocardiography findings.
Yet, we excluded those under 18 years of age, those with a history of stroke, congestive heart failure, CAD, dilated/hypertrophic or restrictive cardiomyopathies, severe valve disease, hypertension (HT), diabetes/impaired glucose tolerance, hypothyroidism and hyperthyroidism, smoking, obstructive sleep apnea, dyslipidemia, morbid obesity (body mass index >35 kg/m2), excessive alcohol consumption (>120 g/day), and diseases that may affect coronary blood flow such as kidney or liver failure, and those with concomitant systemic disorders. In addition, we excluded asthmatic patients for safety reasons and those with suboptimal image quality or arrhythmias that would preclude the acquisition of adequate images to measure CFR.
We conducted the study strictly complying with the principles of the Declaration of Helsinki and ensured all participants sign an informed consent form before enrollment. Moreover, the local ethics committee granted the relevant approval for our study.
Biochemical parameters and atherogenic index of plasma
Venous blood samples were taken from both the patient and control groups in the morning after 10-12 hours of fasting. We noted down their fasting blood sugar, total cholesterol, HDL-C, total cholesterol (TC), and TRG levels and obtained their complete blood counts, basic biochemistry parameters, and total calcium (Ca), albumin-adjusted Ca, phosphorus, thyroid-stimulating hormone, and PTH levels. Then, we measured their plasma levels of high sensitivity C-reactive protein (hsCRP). Low-density lipoprotein (LDL) cholesterol levels were calculated using the Friedewald formula (TC = LDL + HDL + TRG / 5), while PAI values were obtained using the log10 TRG/HDL formula. Non-HDL cholesterol was reached by subtracting HDL from TC. We calculated Castelli risk indices (CRI) I and II as TC/HDL and LDL/HDL, respectively. The atherogenic coefficient (AC) was calculated by dividing non-HDL by LDL. When calculating PAI, we first converted TRG and HDL values into their molar equivalents and then used the log (TRG / HDL-C) formula.
Echocardiographic and coronary flow reserve assessment
We performed imaging on the basis of second-harmonic imaging using the Vivid-6 (GE Medical Systems, Horten, Norway) ultrasound device. All findings were stored digitally and analyzed by an experienced cardiologist blind to the clinical and laboratory data. Conventional echocardiographic examinations of the patients and healthy volunteers were performed according to the standards defined by the American Society of Echocardiography. We calculated left ventricular mass with the Deveraux formula using end-diastolic left ventricular wall thickness and left ventricular diameter. Left ventricular ejection fraction was calculated through the apical windows using the modified Simpson's method.
We assessed CFR with the patient lying in the left lateral position, using a modified two- or four-chamber window to visualize the left anterior descending (LAD) artery by positioning the transducer in the fourth and fifth intercostal spaces, close to the mid-clavicular line. We continuously monitored the patients' ECG and heart rates. Transducer frequencies for B-mode and Doppler imaging were set to 8 MHz and 1.00 - 2.50 kHz, respectively. All subjects received 0.56 mg/kg of dipyridamole infusion for 4 minutes, and we administered an additional dose of 0.28 mg/kg to the subjects when the target heart rate was not reached. CFR was measured using the pulse-wave Doppler method in the patient and control groups, considering baseline diastolic flow velocity and peak flow velocity after dipyridamole infusion. We averaged at least three cycles of measurements (at rest, during maximum dipyridamole infusion, and three minutes after dipyridamole was discontinued) to obtain diastolic peak flow velocity (DPFV). We defined CFR as the ratio of the hyperemic diastolic peak velocity to the baseline diastolic peak velocity, and a CFR ≥ 2.0 was considered normal. All echocardiographic procedures were performed by a single researcher, and the intraclass correlation coefficient for CFR measurement was determined to be 0.903 (15).
Statistical analysis
We performed all statistical analyses on the SPSS package program (version 26, Chicago, IL, USA). Kolmogorov-Smirnov test was used to evaluate whether the data showed a homogenous distribution. For the homogeneously distributed variables, we compared the groups using the Student's T-test and presented the results as mean ± standard deviation. On the other hand, we used the Mann-Whitney U test to compare the groups where the variables did not show a homogeneous distribution, and the findings were given as minimum-maximum values.
We used Pearson's correlation analysis to reveal the associations between the variables and accepted a p-value below 0.05 as statistically significant. For bivariate correlation analyses, an r-value of <0.30 indicates no or very weak correlation. A value of 0.30 < r < 0.50 indicates a weak correlation, while a value of r ≥ 0.50 indicates a moderate to good correlation between the variables.
3. Results
Baseline clinical, demographic, and laboratory characteristics of the groups are presented in
Table 1. Accordingly, we could not find any significant difference between the groups by gender, age, smoking, diabetes, and hypertension (p> 0.05). Both groups were similar in terms of systolic and diastolic blood pressure measurements. Yet, PHPT patients had significantly higher CRP, total Ca, albumin-adjusted Ca, and PTH levels than the control group, while they had significantly lower phosphorus levels (p <0.001). Other blood parameters were also similar between groups.
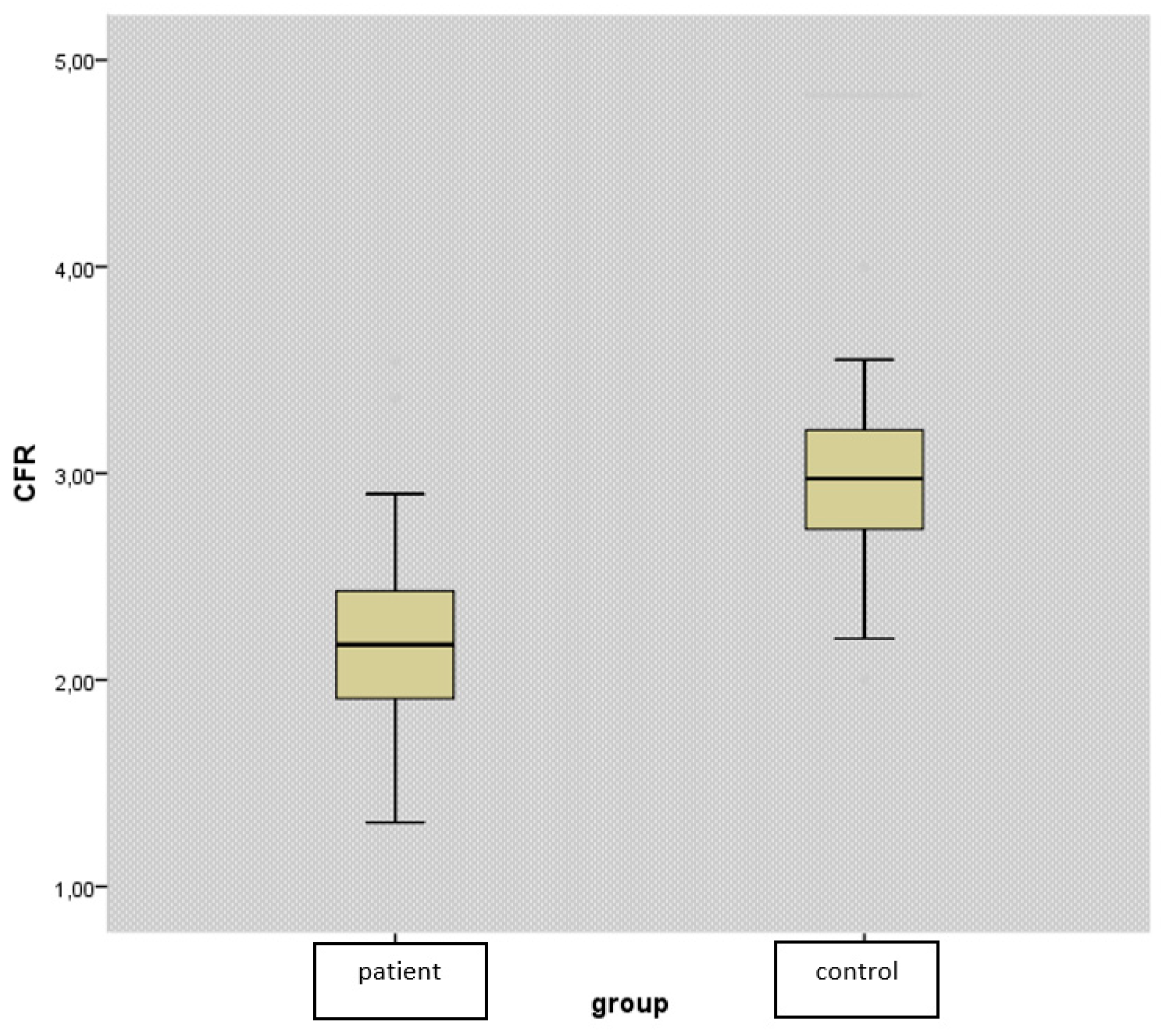
CFR levels were found to be significantly lower in the patient group (2.21 ±0.45 vs. 3.01±0.5, p<0.001) (
Figure 1). While there was no difference between the groups in terms of conventional lipid parameters such as TC, HDL, LDL, and non-HDL (p>0.05), we detected TRG level to be significantly higher in patients with PHPT (p=0.012) (
Table 1).
CFR: Coronary flow reserve, PHPT: Primary hyperparathyroidism
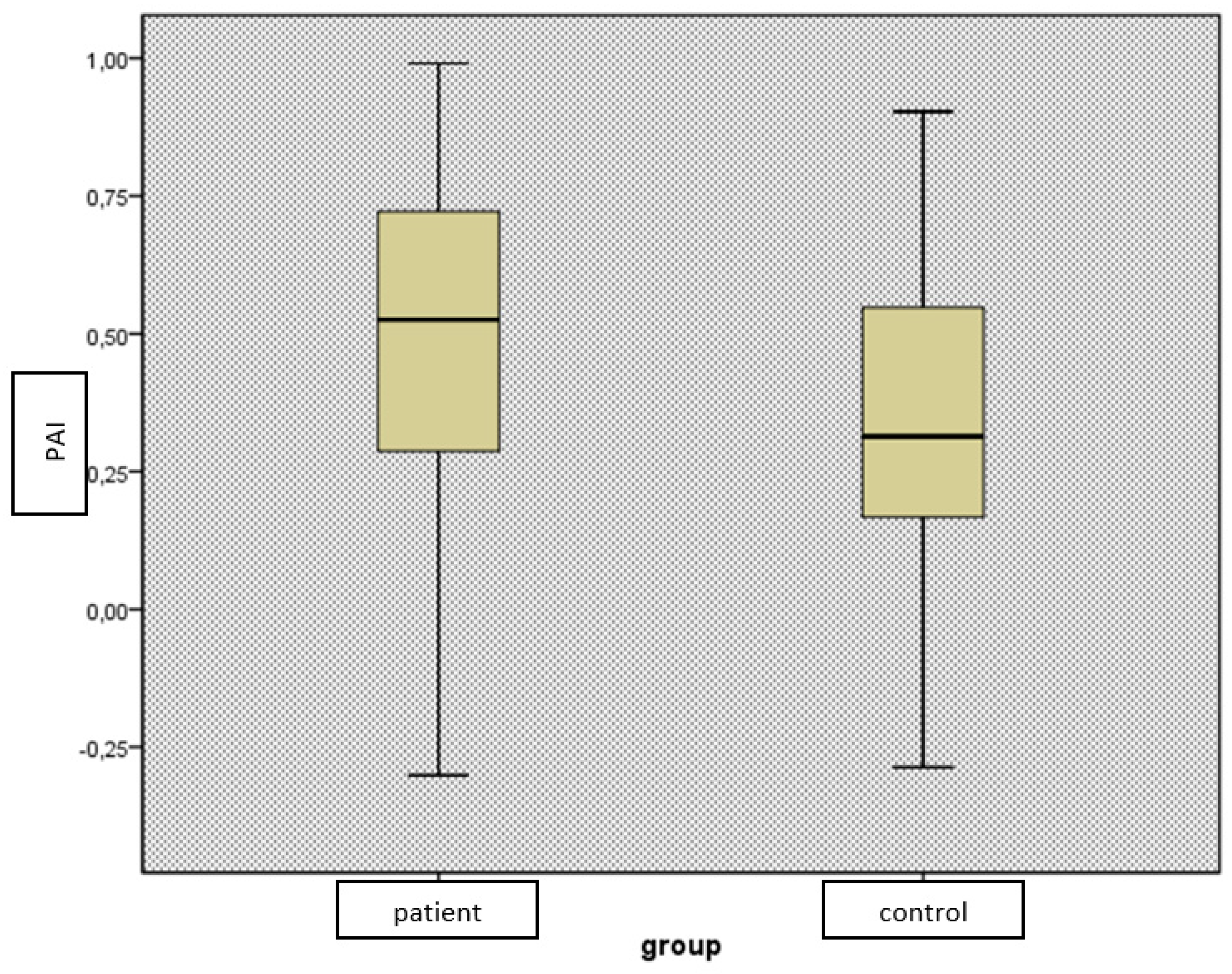
In terms of novel lipid indices, we found no difference between the groups by CRI-1, CRI-2, and AC (p>0.05), while PAI levels were found to be significantly higher in the patient group (p=0.01) (
Table 1).
As in
Table 2, baseline echocardiographic parameters did not differ significantly between the groups
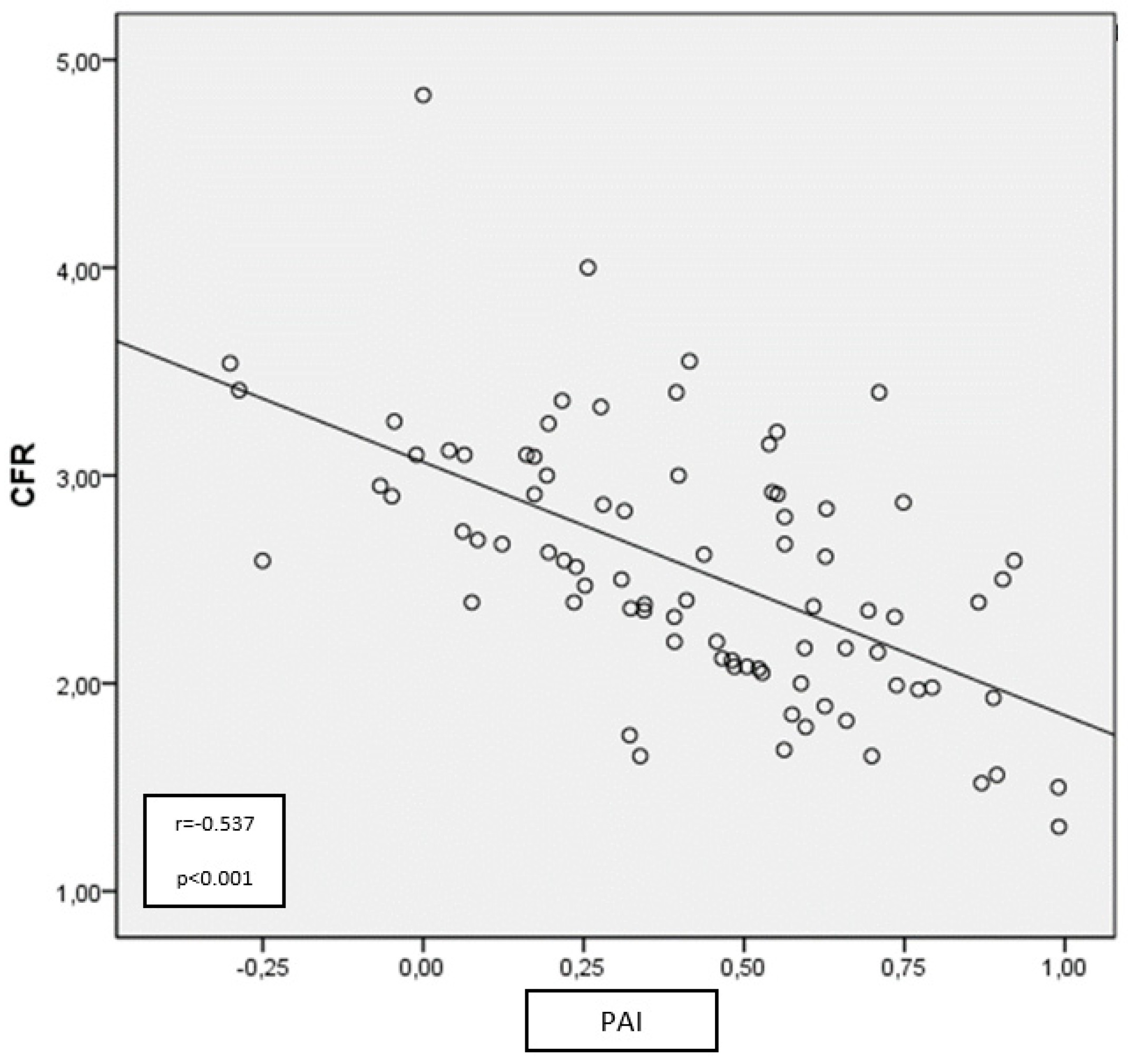
The results of the correlation analysis revealed a negative correlation between CFR and novel lipid indices (except CRI-2), including TRG and PAI (p<0.0001, r=-0.537 for PAI; p=0.026, r=-0.244 for CRI-1; p=0.023, r=-0.250 for AC; p>0.05 for CRI-2) in PHPT patients. However, there was no correlation between CFR and other conventional lipid parameters other than TRG (p>0.05 for all) (
Table 3). Besides, CFR was negatively correlated with PTH and albumin-adjusted Ca levels, while positively correlated with phosphorus (p<0.001, r=-0.610 for Ca; p<0.001, r=-0.494 for PTH; p<0.001, r=0.553 for phosphorus).
Table 3.
Correlation analysis of non-CFR parameters between CFR in PHPT patients.
Table 3.
Correlation analysis of non-CFR parameters between CFR in PHPT patients.
| |
CFR |
| |
r values |
p values |
| PAI |
-0,537 |
<0.001 |
| AC |
-0,250 |
0.023 |
| CRI-1 |
-0,244 |
0.026 |
| CRI-2 |
-0,120 |
0.282 |
| hsCRP (mg/dl) |
-0.409 |
<0.001 |
| TC |
-0,077 |
0.488 |
| TRG (mg/dl) |
-0,363 |
0.001 |
| HDL (mg/dl) |
0,135 |
0,223 |
| LDL (mg/dl) |
0.080 |
0.473 |
| Non-HDL (mg/dl) |
-0.133 |
0.231 |
| Albumin-corrected calcium |
-0.610 |
<0.001 |
| Fosfor |
0.553 |
<0.001 |
| PTH |
-0,494 |
<0.001 |
When CFR measurements were divided into two groups (CFR < 2 and CFR ≥ 2 and above; CFR = 2 is considered the cut-off value for being a predictor of atherosclerosis), CRP, PTH, Ca, and TRG levels were significantly higher in the group with lower CFR levels (p=0.032, p= 0.011, p=0.023, and p=0.019, respectively) (
Table 4). Yet, among the novel lipid indices, only PAI level was significantly higher in this group (p=0.001) (
Figure 2). The groups did not differ by other demographics, examination findings, and lipid parameters and indices (
Table 4). Then, we found a negative correlation between CFR and PAI (
Figure 3).
Table 4.
Comparison of demographic, clinical and laboratory values between subgroups with low and high CFR levels (cut-off value 2 for CFR).
Table 4.
Comparison of demographic, clinical and laboratory values between subgroups with low and high CFR levels (cut-off value 2 for CFR).
| |
CFR <2
(n=19) |
CFR ≥2
(n=25) |
p |
| CFR |
1.7±0.2 |
2.39±0.33 |
<0.001 |
| Age (years) |
56.6±11.2 |
55.6±11.5 |
0.768 |
| BMI (kg/m2) |
25.9±3.7 |
25.8±2.8 |
0.94 |
| SBP (mmHg) |
129.6±8.2 |
131.3±5.6 |
0.459 |
| DBP (mmHg) |
78.2±4.1 |
80.3±4.2 |
0.130 |
| TC (mg/dl) |
186.5±37.1 |
182.4±37.5 |
0.706 |
| TRG (mg/dl) |
170.4±76.3 |
120.4±65.6 |
0.019 |
| HDL (mg/dl) |
42.3±17.1 |
43.2±10.6 |
0.836 |
| LDL (mg/dl) |
105.8±33.9 |
113±26.6 |
0.416 |
| Non-HDL (mg/dl) |
145.1±37.8 |
139.2±40.4 |
0.607 |
| PAI |
0.66±0.17 |
0.40±0.25 |
0.001 |
| AC |
3.78±1.3 |
3.48±1,5 |
0.484 |
| CRI-1 |
4.75±1.3 |
4.4±1.54 |
0.521 |
| CRI-2 |
2.74±0.9 |
2.78±1.04 |
0.892 |
| hsCRP (mg/dl) |
6.6±4.4 |
3.97±3.7
|
0.032 |
| PTH |
296.7±214 |
161.5±105.5 |
0.011 |
| Albumin-corrected calcium |
11.3±0.74 |
10.9±0.46 |
0.023 |
| Fosfor |
2.44±0.4 |
2.57±0.4 |
0.315 |
Figure 2.
Comparison of PAI levels of PHPT patients and control groups.
Figure 2.
Comparison of PAI levels of PHPT patients and control groups.
PAI: Plasma atherogenic index, PHPT: Primary hyperparathyroidism
Figure 3.
Relationship between PAI and CFR in patients with PHPT.
Figure 3.
Relationship between PAI and CFR in patients with PHPT.
CFR: Coronary flow reserve, PAI: Plasma atherogenic index, PHPT: Primary hyperparathyroidism
We analyzed the role of some risk factors for the reduction in CFR levels using multivariate analysis. In the univariate analysis, we found a correlation between CFR levels and increased PAI, PTH, albumin-adjusted calcium, and hsCRP levels. The multivariate logistic regression analysis, on the other hand, showed that only a high PAI level was an independent predictor of reduction in CFR levels in PHPT patients (OR: 151.6, 95% confidence interval (CI): 4.1-5480, p=0.006) (
Table 5).
4. Discussion
In this study, we investigated the association between CFR, which can be easily measured echocardiographically, and various lipid parameters and novel lipid indices, which have become increasingly popular in recent years, in PHPT patients. The main results of our study can be listed as follows: 1) we found significantly higher PAI values in PHPT patients compared to healthy controls; 2) we showed that CFR levels were significantly lower in PHPT patients compared to the control group; 3) we found a negative correlation between CFR and PAI, AC, and CRI-1 levels. There was a similar correlation between CFR and hs-CRP, PTH, and Ca levels; 4) PHPT patients with CFR <2.0 had significantly higher serum hs-CRP, PAI, PTH, Ca levels compared to those with CFR ≥2.0; 5) PAI was the parameter with the best predictive value in showing a change in CFR in PHPT patients; 6) Our results suggest that PAI may be an indicator of subclinical atherosclerosis in PHPT patients.
Some studies previously showed that mortality rates due to all-cause and cardiovascular events are higher in PHPT patients. In addition, serum Ca and PTH levels were determined to be independent predictors of mortality and CAD (16, 17). A wealth of evidence from experimental and clinical studies suggests that both Ca and PTH levels may be causally involved in cardiovascular disease processes through the development of vascular dysfunction, atherosclerosis, and inflammation (18-21). In their study, Hagström et al. (22) showed that increased PTH levels were associated with atherogenesis both directly through its receptors on the vessels and indirectly with vascular calcification and vascular remodeling.
Moreover, recent studies found independent predictors of atherosclerosis, such as HT, hyperlipidemia, and glucose metabolism disorder, to be more prevalent in PHPT patients (23). In addition to all these variables, Stamatelopoulos et al. (24) found higher levels of CRP and Interleukin-6 (IL-6) in patients with PHPT, which contributes to the atherogenic process in such patients. Several small-scale studies with PHPT patients showed an association between various atherosclerotic mediators, such as a decrease in brachial vasoreactivity, an increase in carotid intima-media thickness (IMT), and an increase in abdominal aortic IMT, reinforcing the claim of endothelial dysfunction and early atherogenesis in these patients (22, 25, 26). CFR is used to evaluate microvascular endothelial functions and, previously, was found to be a more decisive test than other early atherosclerosis predictors used (27). CFR can be used in the evaluation of moderately severe coronary artery lesions, as well as in the evaluation of coronary blood flow regulation and prognosis in conditions such as post stent placement and post-acute myocardial infarction (28, 29). We could not find a study investigating CFR levels in patients with PHPT in the literature. In our study, we reported impaired CFR in patients with PHPT. We also found a negative correlation between CFR and Ca and PTH levels, which was consistent with the results of previous studies in the literature showing the link between increased Ca and PTH levels and the development of atherosclerosis. Our result also suggests that PHPT patients are at risk for CAD.
Although dyslipidemia or hypercholesterolemia is reported to be more prevalent in PHPT patients, there are still conflicting results regarding the levels of conventional lipid parameters (TC, LDL-C, HDL-C, and TRG) in studies conducted so far (23, 30). In our study, TRG was found to be significantly higher in the patient group with PAI, which we can call atherogenic dyslipidemia, and PAI was negatively correlated with CFR. In recent studies, it is claimed that newly created lipid indices, such as PAI, Framingham risk scoring, CRI I-II, and AC, are better than conventional lipid parameters (TC, LDL, TRG, and decreased HDL) in predicting cardiovascular events (31-33). Substantial evidence suggests that changes in serum lipid levels cause deterioration in CFR and that treatment of dyslipidemia may restore CFR (15, 34, 35). Two mechanisms come to mind while explaining why PAI, the logarithmic value of TRG/HDL, causes endothelial dysfunction. In the first mechanism, small-density LDL (sdLDL) was previously shown to be a more robust predictor of atherosclerosis than LDL, associated with coronary events, and its clinical use is now suggested (36, 37). Since sdLDL is an expensive and complicated method to measure, and because it was found to be well correlated with PAI in recent studies, the idea that PAI can be used instead of sdLDL is now popular. In the literature, it was reported that PAI can be a good marker for the early detection of subclinical atherosclerosis in diseases such as Behçet's disease, rheumatoid arthritis, and systemic lupus erythematosus (38). Increased systemic inflammatory activity in PHPT patients was shown in previous studies, and it is not far from mind to expect more LDL oxidation in environments with inflammation (23). As a result, it can be speculated that oxidized LDL is associated with a decrease in CFR (39). Regarding the other mechanism, increased TRG levels were shown in previous studies to impair endothelium-dependent vasodilation. Increased PAI levels due to elevated serum TRG may directly affect CFR (40, 41). Kul et al. (36) found an inverse correlation between PAI and CFR in their study in which they used CFR to evaluate patients with inflammatory bowel disease. Uslu et al. (42) showed that high PAI levels were associated with increased carotid IMT in SLE patients. In our study, we found a negative correlation between CFR and AC, CRI-1, and PAI in patients with PHPT. Since CFR ≥ 2 was considered normal in previous research, we divided the patient group into 2 groups by this cut-off level and found PAI levels to be significantly higher in the group with low CFR, although there was no difference by other novel lipid indices. In the multivariate analysis, we found that only PAI levels affected CFR. Our results suggest that PAI, which can be detected by simple laboratory testing, may be a much better predictor of CFR, which is used to evaluate early atherosclerosis in PHPT patients.
Our study bears a few limitations. The first is the low number of participating patients. Second, although CFR is predictive for coronary artery disease, the lack of long-term follow-up of our patients creates uncertainty about how much of our findings will be projected in daily practice. Third, we measured CFR only in the LAD artery. Other vessels may have led to lower CFR measurements, but we may have been considered them normal. Fourth, we used only CRP as a marker of inflammation, but it may not represent the full spectrum of inflammatory activity.
5. Conclusion
A high atherogenic plasma index may be useful both to identify PHPT patients at high risk for adverse cardiovascular events and allow early detection of subclinical atherosclerosis. However, further studies are needed to elucidate the precise mechanisms of early atherogenesis in PHPT patients and understand the full impact of atherogenic dyslipidemia on cardiovascular outcomes in this subset of patients.
Funding
The authors declare that there is no financial support for this article.
Acknowledgments
Study design and original idea by YY, ZC, and SK. Inclusion of participants by ZC, YY, SK and FK.Collection of data by YY and SK. YY and SK wrote the first draft, but all authors contributed to the final manuscript. SK critically reviewed the manuscript for intellectual content. YY and SK are the guarantors of the work, and as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that there is no conflict of interest associated with this manuscript.
The ethics committee
Kayseri City Hospital, Clinical research ethics committee; 09/2020-146.
References
- Boonen S, Bouillon R, Fagard K, Mullens A, Vlayen J, Vanderschueren D. Primary hyperparathyroidism: pathophysiology, diagnosis and indications for surgery. Acta Oto-Rhino-Laryngol Belg.. 2001; 55: 119–127.
- Lundgren E, Rastad J, Thrufjell E, Akerström G, Ljunghall S. Population-based screening for primary hyperparathyroidism with serum calcium and parathyroid hormone values in menopausal women. Surgery. 1997; 121:287–294. [CrossRef]
- Palmér M, Jakobsson S, Akerström G, Ljunghall S. Prevalence of hypercalcemia in a health survey: a 14-year follow-up study of serum calcium values. Eur J Clin Invest. 1998; 18:39–46. [CrossRef]
- Silverberg SJ, Bilezikian JP. Hyperparathyroidism. In: Becker KL, editor. Principles and practice of endocrinology and metabolism. 3rd edition. Philadelphia: JB Lipincott; 2001. p. 564-573.
- Walker MD, Silverberg SJ. Cardiovascular aspects of primary hyperparathyroidism. J Endocrinol Invest. 2008; 31(10): 925–931. [CrossRef]
- Caiati C, Zedda N, Montaldo C, Montisci R, Iliceto S. Contrast enhanced transthoracic second harmonic echo Doppler with adenosine: a noninvasive, rapid and effective method for coronary flow reserve assessment. J Am Coll Cardiol. 1999; 34:122–130. [CrossRef]
- Britten MB, Zeiher AM, Schachinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coron Artery Dis.2004; 15:259–264. [CrossRef]
- Montisci R, Marchetti MF, Ruscazio M, et al. Non-invasive coronary flow velocity reserve assessment predicts adverse outcome in women with unstable angina without obstructive coronary artery stenosis. J Public Health Res. 2023 Jun 10;12(2):22799036231181716. [CrossRef]
- Picano E. Stress echocardiography: a historical perspective. Am J Med 2003; 114:126–30.). [CrossRef]
- Won KB, Heo R, Park HB, et al. Atherogenic index of plasma and the risk of rapid progression of coronary atherosclerosis beyond traditional risk factors. Atherosclerosis. 2021 May;324:46-51. [CrossRef]
- Hong SP, Kim CY, Jung HW. The Comparison of the Associations of Lipoprotein(a) and the Atherogenic Index of Plasma With Coronary Artery Calcification in Patients Without High LDL-C: A Comparative Analysis. J Lipid Atheroscler. 2023 May;12(2):152-63. [CrossRef]
- Zheng Y, Li C, Yang J, Seery S, Qi Y, Wang W, Zhang K, Shao C, Tang YD. Atherogenic index of plasma for non-diabetic, coronary artery disease patients after percutaneous coronary intervention: a prospective study of the long-term outcomes in China. Cardiovasc Diabetol. 2022;21(1):29.). [CrossRef]
- Onat A, Can G, Kaya H, Hergenç G. "Atherogenic index of plasma" (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J Clin Lipidol. 2010; 4(2):89-98. [CrossRef]
- Edwards MK, Blaha MJ, Loprinzi PD. Atherogenic index of plasma and triglyceride/high-density lipoprotein cholesterol ratio predict mortality risk better than individual cholesterol risk factors, among an older adult population. Mayo Clin Proc. 2017; 92(4):680–681. [CrossRef]
- Caliskan M, Erdogan D, Gullu H, et al. Effects of atorvastatin on coronary flow reserve in patients with slow coronary flow. Clin Cardiol 2007 Sep;30(9):475-479. [CrossRef]
- Yu N, Donnan PT, Flynn RW, et al. Increased mortality and morbidity in mild primary hyperparathyroid patients. The Parathyroid Epidemiology and Audit Research Study (PEARS). Clin Endocrinol (Oxf). 2010; 73(1):30-34. [CrossRef]
- Leifsson BG, Ahren B. Serum calcium and survival in a large health screening program. J Clin Endocrinol Metab.1996; 81:2149–2153. [CrossRef]
- Nilsson IL, Aberg J, Rastad J, Lind L. Endothelial vasodilatory dysfunction in primary hyperparathyroidism is reversed after parathyroidectomy, Surgery. 1999; 126: 1049-1055. [CrossRef]
- Nilsson IL, Rastad J, Johansson K, Lind L. Endothelial vasodilatory function and blood pressure response to local and systemic hypercalcemia, Surgery. 2001; 130: 986-990. [CrossRef]
- Rashid G, Bernheim J, Green J, Benchetrit S. Parathyroid hormone stimulates endothelial expression of atherosclerotic parameters through protein kinase pathways, Am J Physiol Ren Physiol. 2007; 292: 1215-1218. [CrossRef]
- Wareham NJ, Byrne CD, Carr D, Day NE, Boucher BJ, Hales CN. Glucose intolerance is associated with altered calcium homeostasis: a possible link between increased serum calcium concentration and cardiovascular disease mortality. Metabolism. 1997; 46: 1171-1177. [CrossRef]
- Hagström E, Ahlström T, Ärnlöv J, et al. Parathyroid hormone and calcium are independently associated with subclinical vascular disease in a community-based cohort. Atherosclerosis. 2015; 238(2):420-426. [CrossRef]
- Luboshitzky R, Chertok-Schaham Y, Lavi I, Ishay A. Cardiovascular risk factors in primary hyperparathyroidism. J Endocrinol Invest 2009; 32(4):317-321. [CrossRef]
- Stamatelopoulos K, Athanasouli F, Pappa T, et al. Hemodynamic markers and subclinical atherosclerosis in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2014; 99(8):2704-2711. [CrossRef]
- Kosch M, Hausberg M, Vormbrock K, et al. Impaired flow-mediated vasodilation of the brachial artery in patients with primary hyperparathyroidism improves after parathyroidectomy. Cardiovasc Res. 2000; 47(4):813-818. [CrossRef]
- Sumbul HE, Koc AS. The Abdominal Aortic Intima-Media Thickness Increases in Patients with Primary Hyperparathyroidism. Exp Clin Endocrinol Diabetes. 2019; 127(6):387-395. [CrossRef]
- Gullu H, Erdogan D, Caliskan M, et al. Interrelationship between noninvasive predictors of atherosclerosis: transthoracic coronary flow reserve, flow-mediated dilation, carotid intima-media thickness, aortic stiffness, aortic distensibility, elastic modulus, and brachial artery diameter. Echocardiography.2006; 23(10):835-842. [CrossRef]
- Haude M, Baumgart D, Verna E, et al. Intracoronary Doppler- and quantitative coronary angiography-derived predictors of major adverse cardiac events after stent implantation. Circulation. 2001;103:1212–1217. [CrossRef]
- Serruys PW, di Mario C, Piek J, et al. Prognostic value of intracoronary flow velocity and diameter stenosis in assessing the short- and long-term outcomes of coronary balloon angioplasty: the DEBATE Study (Doppler Endpoints Balloon Angioplasty Trial Europe). Circulation. 1997; 96:3369–3377. [CrossRef]
- Lundgren E, Ljunghall S, Akerström G, Hetta J, Mallmin H, Rastad J. Case-control study on symptoms and signs of "asymptomatic" primary hyperparathyroidism. Surgery. 1998; 124(6):980-985. [CrossRef]
- Fernández-Macías JC, Ochoa-Martínez AC, Varela-Silva JA, Pérez-Maldonado IN. Atherogenic Index of Plasma: Novel Predictive Biomarker for Cardiovascular Illnesses. Arch Med Res. 2019; 50(5):285-294. [CrossRef]
- Ulloque-Badaracco JR, Hernandez-Bustamante EA, Alarcon-Braga EA, Mosquera-Rojas MD, et al. Atherogenic index of plasma and coronary artery disease: A systematic review. Open Med (Wars). 2022 Dec 6;17(1):1915-1926. [CrossRef]
- Shui X, Chen Z, Wen Z, et al. Association of Atherogenic Index of Plasma With Angiographic Progression in Patients With Suspected Coronary Artery Disease. Angiology. 2022 Nov-Dec;73(10):927-935. [CrossRef]
- Yokoyama I, Ohtake T, Momomura S, Nishikawa J, Sasaki Y,Omata M. Reduced coronary flow reserve in hypercholesterolemic patients without overt coronary stenosis. Circulation. 1996; 94:3232–3238. [CrossRef]
- Lee CH, Hwang J, Kim IC, et al. Effect of Atorvastatin on Serial Changes in Coronary Physiology and Plaque Parameters. JACC Asia. 2022 Nov 1;2(6):691-703. [CrossRef]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002; 106: 3143–3421.
- Kul Ş, Çalışkan Z, Güvenç TS, Güvenç RÇ, Çalışkan M. Plasma lipids in patients with inflammatory bowel disease: Observations on the associations between lipid indices and coronary flow reserve. Wien Klin Wochenschr. 2020; 132(11-12):283-294. [CrossRef]
- Cure E, Icli A, Uslu AU, et al. Atherogenic index of plasma: a useful marker for subclinical atherosclerosis in ankylosing spondylitis: AIP associate with cIMT in AS. Clin. Rheumatol. 2018; 37(5):1273-1280. [CrossRef]
- Frohlich J, Dobiasova M. Fractional esterification rate of cholesterol and triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin Chem. 2003; 4911: 1873–1880. [CrossRef]
- Lundman P, Eriksson M, Schenck-Gustafsson K, Karpe F, Tornvall P. Transient triglyceridemia decreases vascular reactivity in young, healthy men without risk factors for coronaryheartdisease. Circulation. 1997; 96(10):3266–3268. [CrossRef]
- Hozumi T, Eisenberg M, Sugioka K, et al. Change in coronary flow reserve on transthoracic Doppler echocardiography after a single high-fat meal in young healthy men. Ann Intern Med. 2002; 136(7):523–528. [CrossRef]
- Uslu AU, Kucuk A, Icli A, et al. Plasma Atherogenic Index is an Independent Indicator of Subclinical Atherosclerosis in Systemic Lupus Erythematosus. Eurasian J Med. 2017; 49(3):193-197. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).