General information
A total of about 900 adult females of Trionymus aberrans were collected, most of them (620) from the post-industrial wastelands. About 40% of all of the collected females were parasitised. The highest degree of parasitisation was characteristic of the females that had been collected from the different habitats between August and October.
The females had been found on the leaves or in the leaf sheaths of different grass species. In the post-industrial wastelands, about 90% of the specimens were collected from Deschampsia caespitosa (L.) Palisot de Beauvois. Other grass species, e.g., Agrostis capillaris L., Elymus repens (L.) Gould, Phleum pratense L. and Poa compressa L. were other common host plants in the degraded habitats. The adult females formed colonies consisting of four to ten specimens only on Deschampsia caespitosa in the post-industrial wastelands. In the present study, adult females of T. aberrans were often collected from Brachypodium pinnatum (L.) Palisot de Beauvois and Phleum sp. from the undegraded habitats. On the undegraded habitats, e.g., psammophilous or xerothermic grasslands, the females most often occurred singly on their host plants.
Intraspecific variability in adult females of Trionymus aberrans
Material examined:
Type material:
Trionymus aberrans n. sp. / Hem. Coccidae / ♀ / Avena / Marseille / 1.VIII.1936 / 806 /a / Type / Holotype (MNHN)
Trionymus aberrans ovalis/ Type / Colmars (Basses-Alpes) / L. Goux coll / 19/VII/1939/ 1013 (MNHN)
3 adult females deposited in MNHN
225 adult female specimens were involved in the morphological examination, out of the ca. 900
T. aberrans adult females that were collected during the present studies and deposited in the entomological collections of the Zoology Research Group, Institute of Biology, Biotechnology and Environmental Protection, Faculty of Life Sciences, University of Silesia, Poland (
supplementary material, Table S1).
Instar diagnosis – Diagnosed by the following combination of features: (i) horseshoe-shaped anal ring; (ii) presence of simple tubular ducts of two types (dorsal ducts larger than those of venter); (iii) multilocular pores present on both body surfaces; (iv) presence of 2 pairs of cerarii; (v) each anal lobe cerarius (C18) with 2 conical setae plus 6–16 trilocular pores and 3–4 auxiliary hair-like setae; (vi) circulus absent or rarely present and (vii) antennae 8–or rarely 9–segmented.
Description of a slide-mounted adult female – Body elongate oval, 4.25–4.92 mm long, 2.74 –0.64 mm wide. Eye marginal, 31–32 μm wide. Antennae 8 (rarely 9) segmented, 560–610 μm long; first segment 58.13–64.20 μm long; second 50.05–55.8 μm; third 36.42–41.13 μm long; fourth 26.58–36.77 μm long; fifth 30.68–34.16 μm long; sixth 27.40–31.03 μm long; seventh 31.84–38.03 μm long and apical segment 81.77–93.18 μm long. Number of sensory setae on antennal segments: first segment: 3, second: 4, third: 4, fourth: 3, fifth: 5, sixth: 4–5, seventh: 4, apical segment: 16–17. Apical seta 30.71–32.1 μm long, subapical seta 24.17–26.15 μm long. Clypeolabral shield 180.22–196.61 μm long, wide 143.63–160.77 μm. Labium 3–segmented, 74.25–82.32 μm long, 79.78–80.75 μm wide. Stylet loop reaches line between anterior spiracles. Anterior spiracles 55.52–65.39 μm long, 28.90–32.58 μm wide across atrium with 3–4 associated trilocular pores and one multilocular pore located nearby; posterior spiracles 67.27–78.35 μm long, 42.53–44.23 μm wide across atrium with 5–6 associated trilocular pores and 3–4 multilocular pores located nearby. Circulus absent or rarely small oval circulus present. Legs well developed; hind trochanter + femur 230.55–239.72 μm long; hind tibia + tarsus 266.15–283.66 μm long; hind claw 27.81–29.06 μm long. Hind coxae with translucent pores. Tarsal digitules setose, subequal in length, each 46.45–48.33 μm long. Claw digitules subequal in length, each 27.29–28.46 μm long, both capitate and thicker than tarsal digitules. Ostioles: both pairs present; each anterior ostiole with 4–8 trilocular pores and 1 or 2 setae (total for both lips); each posterior ostiole with 8-12 trilocular pores and 2 setae (total for both lips). Horse-shoe-shaped anal ring 90–110 μm wide, with 6 anal-ring setae, each setae 76.87–91.22 μm long.
Venter. Body setae slender, each 52.8–61.14 μm long, longest setae present medially on the head; apical setae of anal lobe 147.95–181.34 μm long. Multilocular pores each 6–8 μm wide, numerous on the abdomen, forming transverse rows and bands on four last abdominal segments; 1–3 on the head; present irregularly along body margins. Trilocular pores each 4–5 μm in diameter, scattered on the entire surface. Unusual 4-locular pores are present on the three last abdominal segments in some specimens. Simple tubular ducts of two sizes. Larger tubular ducts each 10.4–12.2 μm long and 3.94–4.71 μm wide at distal form transverse bands on the four last abdominal segments, group on the body margin on the sixth abdominal segment, present occasionally on the cephalothorax. Smaller tubular ducts 4.95–6.56 μm long and 2.62–3.1 μm wide scattered on the entire surface.
Dorsum. Derm membranous, with 2 pairs of cerarii (C17 and C18) on the penultimate and last abdominal segments; C17 with 2 cerarian setae; 3–6 trilocular pores between cerarian setae, and 1–2 auxiliary setae; anal lobe cerarii (C18) each with 2 conical setae, 6–16 trilocular pores and 3–4 auxiliary hair-like setae. Dorsal body setae hair-like, each 25.2–32.46 μm long, scattered on the head and the thorax, in rows on abdominal segments. Multilocular pores each 6.68–8.22 μm wide, forming transverse rows on the last three abdominal tergites. Trilocular pores each 3.5–4.25 μm in diameter, scattered on the entire surface. Simple tubular ducts of two sizes are scattered throughout the body surface, present on the head and thorax. Ducts of two sizes are slightly larger than ventral ducts, each 10.7–12.8 μm long and 3.94–4.9 μm wide at distal.
Comments:
The holotype of Trionymus aberrans (MNHN) had the following characteristic features: horseshoe-shaped anal ring, hind coxae with many translucent pores, simple tubular ducts of two types on both body surfaces (dorsal ducts larger than those of venter), numerous trilocular pores on dorsal and ventral body surfaces, multilocular pores present on both body surfaces, 2 pairs of cerarii, each penultimate cerarius (C17) with 2 conical setae plus 2 trilocular pores and 1 auxiliary seta, each anal lobe cerarius (C18) with 2 conical setae plus 8 trilocular pores and 4 auxiliary hair-like setae, circulus absent and antennae 8-segmented.
Morphological intraspecific variability in adult females of
Trionymus aberrans were observed. The vast majority of females had 8-segmented antennae (
Figure 2 (a)), but 11 of the collected specimens had 9-segmented antennae. The presence of 8- or 9-segmented antennae was not associated with the habitat. The type specimen of
Trionymus aberrans ovalis (MNHN) had 9-segmented antennae.
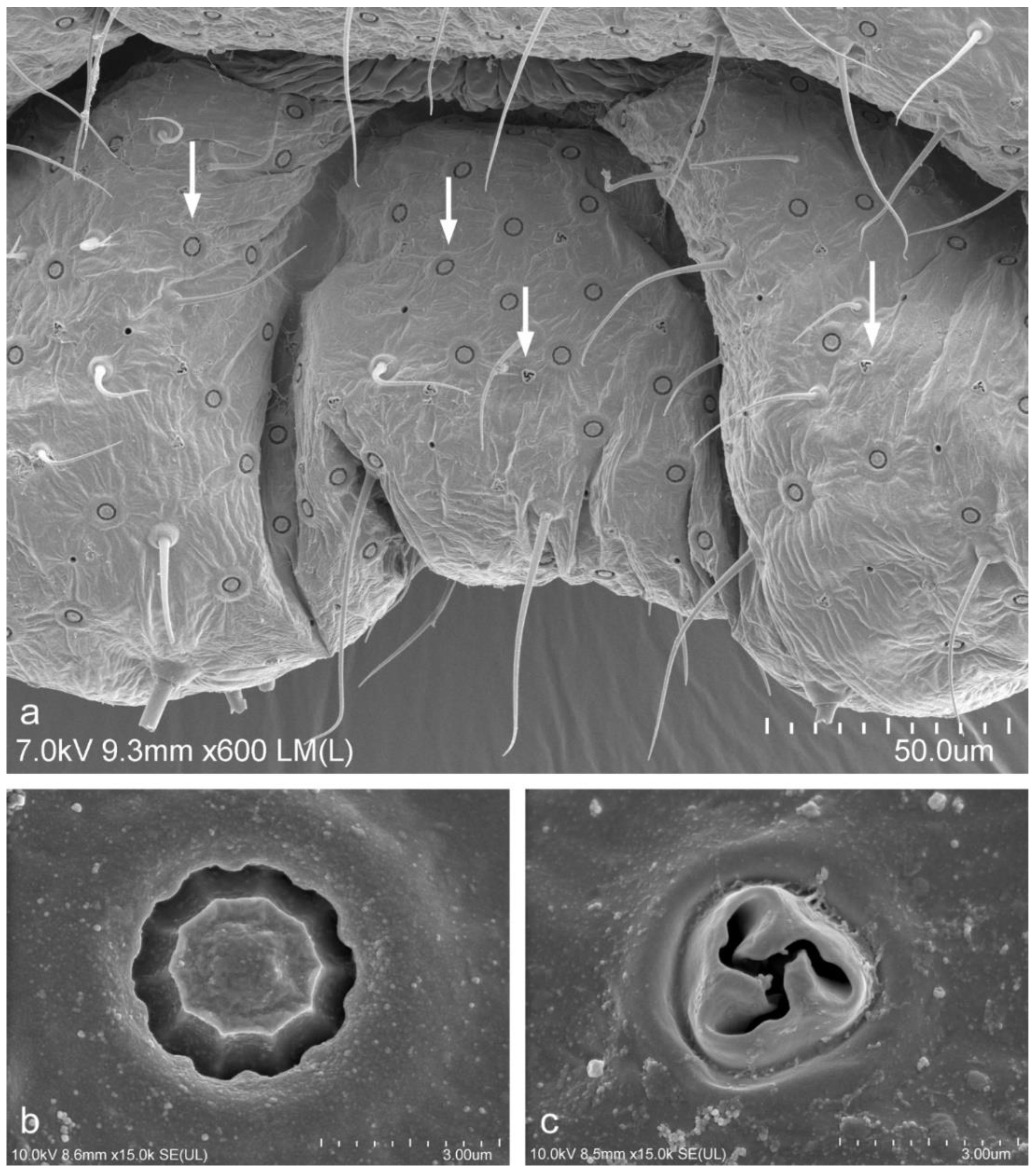
Trilocular pores were present on both body surfaces in all specimens (
Figure 3a,c). Multilocular pores were observed on dorsal and ventral abdominal segments (
Figure 3a,b).
Morphological anomalies were observed in females collected in four of the examined post-industrial wastelands, namely zinc heaps (site no. 1, 2 and 3) and degraded area near the production plant (site no. 5). Specimens collected in coal spoil heap (site no. 4) possess only fewer number of translucent pores in hind coxae compared to specimens found in undegraded habitats.
Unusual pores were observed in seven females out of fifteen examined specimens that had been collected from one of the post-industrial wastelands, namely, the degraded area near the production plant in Piekary Śląskie (site no.5). Females with unusual pores were collected in 2017, 2018 and 2020. The detailed structure of these unusual pores were accurately observed using a scanning electron microscope (SEM). These pores were characterised by the presence of four excretory openings (loculi) (
Figure 4a,b). These unusual pores were scattered on the ventral surface of the last three abdominal segments. These pores were somewhat similar in appearance to the trilocular pores but had one more excretory opening. No pores with four loculi were observed in females that had been collected from the undegraded habitats or in the females that had been deposited in the collections in MNHN and IBBEP.
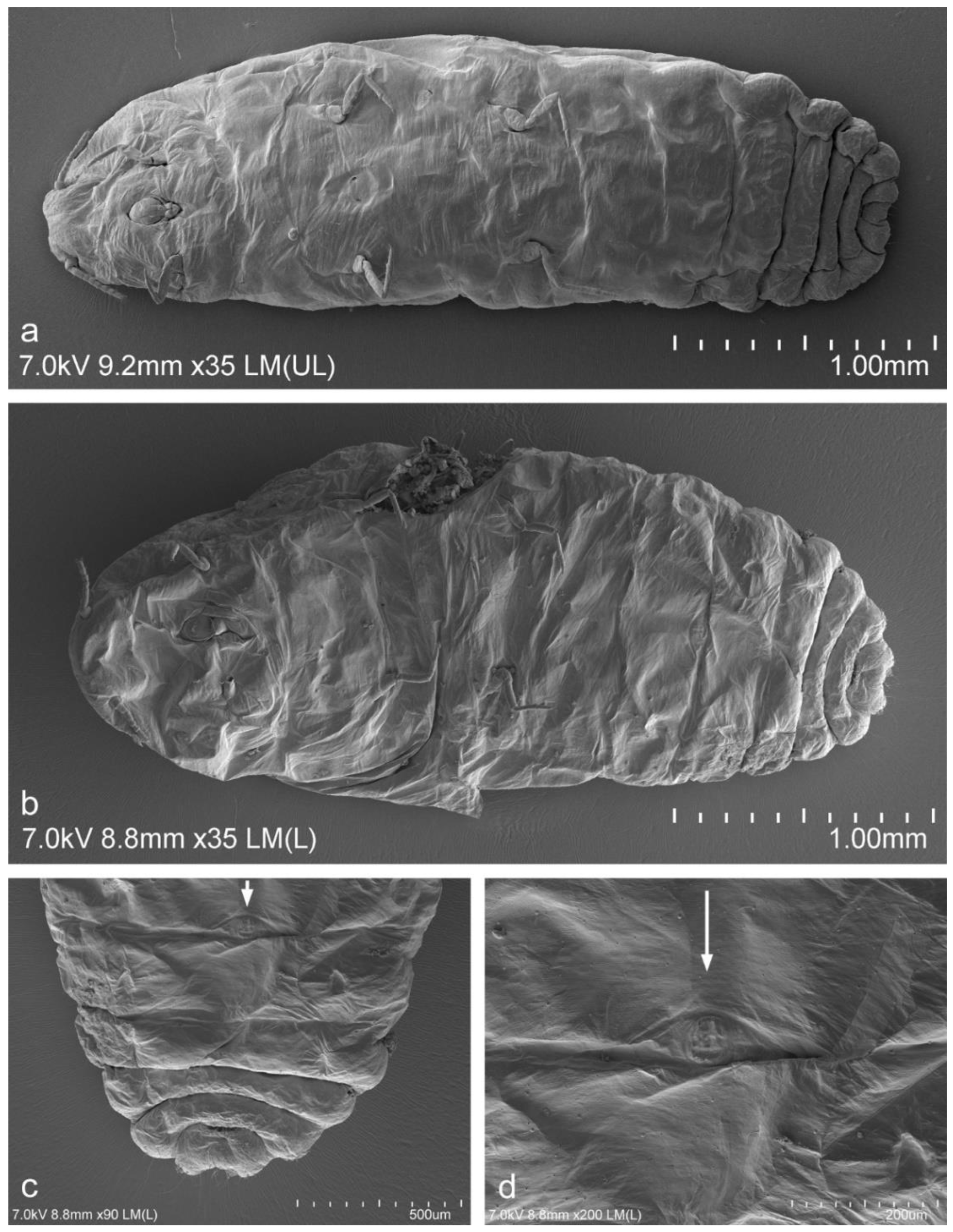
Five adult females out of fifteen examined specimens that had been collected in the degraded area near a production plant in Piekary Śląskie (site no. 5) had a small and oval circulus between the third and fourth abdominal segments (
Figure 2b–d). The females with circulus were collected in 2015, 2017 and 2020. The circulus was observed in a type specimen of
Trionymus aberrans ovalis. The circulus was absent in all of the females that had been collected from the undegraded habitats and in almost all of the females deposited in the examined collections in MNHN and IBBEP. The circulus was also absent in the vast majority of specimens that had been collected from post-industrial habitats.
Translucent pores were present on the hind coxae in all of the adult females, but there are differences in the number of these pores between the females that had been collected from the post-industrial wastelands and the undegraded habitats. Generally, 60 females collected in post-industrial wastelands (site no. 1-5) had fewer translucent pores than the specimens from the undegraded habitats.
All of the adult females, both those that had been collected during the present study and those that had been deposited in the collections in MNHN and IBBEP, had 2 pairs of abdominal cerarii (C17 and C18). There were differences in the morphology of the two pairs of cerarii in the examined specimens. The penultimate cerarii (C17) most often consisted of 2 conical setae, 1 or 2 auxiliary hair-like setae and a diverse number of trilocular pores that ranged from 3 to 8. Most of the specimens that had been collected from both the post-industrial wastelands and the other habitats had 5 trilocular pores in C17. Twelve females of fifteen examined specimens collected from the spoil heap in Bolesław (site no. 3) possessed 3 trilocular pores and two of them had 4 trilocular pores in C17. Ten specimens with 6 trilocular pores in C17 were collected in undegraded habitats.
Each of the anal lobe cerarius (C18) most often consisted of 2 conical setae, 2 auxiliary hair-like setae and 6-16 trilocular pores. The number of trilocular pores in C18 differed in the females living in different habitats. Most of the females that had been collected from three of the examined zinc heaps in Ruda Śląska (site no. 1), Piekary Śląskie (site no. 2) and Bolesław (site no. 3) and also in site no. 5 near production plant had 6-7 trilocular pores in each anal lobe cerarius. Ten females collected in site no. 1 had 6 trilocular pores in C18, whereas two of them possessed 7 trilocular pores in C18. Thirteen females collected in site no. 2 had 6 trilocular pores in C18. Eleven specimens collected in site no. 3 had 6 trilocular pores in C18 and three specimens had 7 trilocular pores in C18. Ten females collected in site no. 5 had 6 trilocular pores in C18 (these females did not have circulus). The females that were collected during the present study from coal spoil heap (site no. 4) and undegraded habitats had anal lobe cerarii with 8-14 trilocular pores each, most of them had 10 trilocular pores in C18. The females that were deposited in the collections in MNHN and IBBEP had 8-16 trilocular pores in C18, but 13 specimens with 15-16 pores were only collected in psammophilous grasslands in Austria. In four specimens from the post-industrial wasteland (Bolesław, site no.3), there was an additional third conical seta in C18.
In specimens collected in undegraded psammophilous grasslands in Ruda Śląska, Piekary Śląskie and Bolesław morphological anomalies were not observed.
Simple tubular ducts of two sizes were present in all of the examined specimens of
Trionymus aberrans (
Figure 4a,c).
Description of a second-instar nymph of Trionymus aberrans
Material examined:
2261 / Trionymus aberrans / ♀ / larva / Graminae vagina / det. Koteja // 5.9.1967 / 6 / Las Wolski Kraków / leg. Koteja // SC28-249-1-004-DZUS;
Trionymus aberrans / nymph / Phleum sp. / Olsztyn near Częstochowa / 12.09.2018 // leg. et det. M. Kalandyk-Kołodziejczyk;
Trionymus aberrans / nymph / Deschampsia caespitosa / Piekary Śląskie, Lotników / 12.09.2020 // leg. et det. M. Kalandyk-Kołodziejczyk.
Instar diagnosis – Diagnosed by the following combination of features: (i) horseshoe-shaped anal ring; (ii) presence of a few simple tubular ducts of one type only on the last segments of the abdomen (dorsal ducts larger than those on venter); (iii) absence of multilocular pores; (iv) presence of 2 pairs of cerarii; (v) each anal lobe cerarius (C18) with 2 conical setae, 5 trilocular pores and 2 auxiliary hair-like setae; (vii) circulus absent and (viii) antennae 6-segmented.
Description of slide-mounted second-instar (
Figure 5) – Body elongate oval, 1.1–1.3 mm long, 0.4–0.5 mm wide.
Eyes present marginally, 20.18 μm wide. Antennae 6–segmented; first segment 33.94–38.37 μm long; second 35.40–36.48 μm long; third 39.83–41.80 μm long; fourth 21.17–21.80 μm long; fifth 25.16–25.68 μm long and apical segment 70.51–71.88 μm long. Number of sensory setae on antennal segments: first segment: 3, second: 3, third: 3, fourth: 3, fifth: 3, apical segment: 13. Apical seta 25.58–26.21 μm long, subapical seta 14.11–15.45 μm long. Clypeolabral shield 137.94–142.62 μm long and 99.53–100.79 μm wide. Labium 54.09–54.82 μm long and 57.51–56.58 μm wide. Stylet loop reaches line between anterior spiracles. Anterior spiracles 44.12–44.43 μm long, 16.32–18 μm wide across atrium; posterior spiracles 44.12–45.8 μm long, 20.18–21.5 μm wide across atrium. Both anterior and posterior spiracles associated with 2 trilocular pores each. Circulus absent. Legs well developed: hind trochanter + femur 148.11–151.65 μm long, hind tibia + tarsus 167.04–168.30 μm long, hind claw 14.74–16.80 μm long. Hind coxae with a few translucent pores. Claw without denticle. Tarsal digitules slightly capitate, subequal in length, each 30.87–31.64 μm long. Claw digitules clubbed, subequal in length, each 20.16 μm long, slightly thicker than tarsal digitules. Ostioles: both pairs present; each anterior ostiole with 4 trilocular pores and 1 seta (total for both lips); each posterior ostiole with 8 trilocular pores and 2 setae (total for both lips). Anal ring horseshoe-shaped, 42.46 μm 43.88 μm wide, with 6 anal-ring setae, each setae 64.05–67.63 μm long.
Venter. Body setae slender, hair-like, 21.53–25.96 μm long, longest setae present medially on the head; apical seta of anal lobe 107.15–109.14 μm long. Multilocular pores absent. Trilocular pores each scattered evenly on the entire surface. Very few simple tubular ducts of one type only on the lateral margin of the VII abdominal segment. Each duct 4.87¬–5.00 μm long and 3.65–3.85 μm wide at the distal end.
Dorsum. Derm membranous, with 2 pairs of cerarii on body margin, C17 with 1 conical seta, 2 trilocular pores and 2 hair-like setae. Anal lobe cerarii (C18) each with 2 conical setae, each seta 8.56–8.82 μm long, 5 trilocular pores and 2 auxiliary hair-like setae. Dorsal body setae hair-like, shorter than ventral setae, each 13.6–16.24 μm long. Trilocular pores evenly scattered on dorsum. A few simple tubular ducts of one type present only on VI-VIII abdominal segments; on VI and VII segment, very few ducts on lateral margins of each segment; on VIII abdominal segment, a few ducts on lateral margin and in the middle. Each duct longer than ventral ducts, 5.82–5.88 μm long and 3.5–3.66 μm wide at distal end.
Comments: Species identification of second-instar nymph was based on the presence of a horseshoe-shaped anal ring. According to Gullan et al. [
18], the sex of the second-instar nymph can only be determined for specimens that have the pharate third-instar male or female. Several authors [
17,
19,
20] report that the second instar males have more dorsal tubular ducts than the second instar females.
Translucent pores were observed on the hind coxae in all of the examined second-instar nymphs of
Trionymus aberrans. These pores have so far been observed almost solely in adult females [
14].
One of the examined nymphs had been collected from a post-industrial wasteland in Piekary Śląskie (site no. 5). Further studies with more specimens are necessary to investigate the existence of the intraspecific variability in the nymphs from post-industrial wastelands.